Abstract
Purpose
Hypoxia-inducible factor 2α (HIF-2α) overexpression leads to activation of angiogenic pathways. However, little is known about the association between HIF-2α expression and anti-tumor immunity in clear cell renal cell carcinoma (ccRCC). We aimed to explore how HIF-2α influenced the microenvironment and the underlying mechanisms.
Experimental design
We immunohistochemically evaluated immune cells infiltrations and prognostic value of HIF-2α expression in a retrospective Zhongshan Hospital cohort of 280 ccRCC patients. Fresh tumor samples, non-tumor tissues and autologous peripheral blood for RT-PCR, ELISA and flow cytometry analyses were collected from patients who underwent nephrectomy in Zhongshan Hospital from September 2017 to April 2018. The TCGA KIRC cohort and SATO cohort were assessed to support our findings.
Results
We demonstrated that ccRCC patients with HIF-2αhigh tumors exhibited reduced overall survival (p = 0.025) and recurrence-free survival (p < 0.001). Functions of CD8+ T cells were impaired in HIF-2αhigh patients. In ccRCC patients, HIF-2α induced the expression of stem cell factor (SCF), which served as chemoattractant for mast cells. Tumor infiltrating mast cells impaired anti-tumor immunity partly by secreting IL-10 and TGF-β. HIF-2α mRNA level adversely associated with immunostimulatory genes expression in KIRC and SATO cohorts.
Conclusions
HIF-2α contributed to evasion of anti-tumor immunity via SCF secretion and subsequent recruitment of mast cells in ccRCC patients.
Electronic supplementary material
The online version of this article (10.1007/s00262-019-02314-y) contains supplementary material, which is available to authorized users.
Keywords: Clear cell renal cell carcinoma, Hypoxia-inducible factor 2α, Immune evasion, Tumor infiltrating mast cells, Stem cell factor
Introduction
Renal cell carcinoma (RCC) represents 2–3% of all malignancies in adults with around 271,000 newly diagnosed cases estimated worldwide annually [1, 2]. The most common histological subtype is clear cell renal cell carcinoma (ccRCC), accounting for 70–80% of all RCC [3]. Approximately one-third of the patients develop recurrences or metastases after curative surgeries [4]. Additionally, ccRCC is historically one of the first malignancies to respond to immunotherapy and continues to be among the most responsive [5, 6].
The lack of functional Von Hippel-Lindau (VHL) protein (pVHL) is a key oncogenic event in majority of patients with ccRCC [7]. As a component of the ubiquitin-mediated proteolysis pathway, pVHL is important for degradation of hypoxia-inducible factors (HIFs; HIF-1α and HIF-2α) [8]. In ccRCCs, hypoxia-inducible factor 2α (HIF-2α) is tumorigenic while HIF-1α has anti-tumorigenic functions [9]. Genome-wide analysis reveals that at least 500–1000 genes regulating angiogenesis and metabolism are under the control of HIF [10, 11]. There is an upregulation of HIFs when VHL is inactivated, resulting in a metabolic shift to anaerobic glycolysis, increased secretion of pro-angiogenic factors, remodeling of the extracellular matrix, resistance to apoptosis and increased mobility. The tumorigenic role of HIF-2α was exhibited in a mouse ccRCC-xenograft model, wherein HIF-2α overexpression led to an increased tumor burden [12]. Knockdown of HIF-2α reduced tumor burden in the same model. HIF-2α expression associated with worse ccRCC patient survival in two independent studies [13, 14]. Two specific antagonists of HIF-2α, PT2385 and PT2399 are under preclinical development [15, 16].
Tumors are organized tissues with reciprocal local and systemic connections with immune cell populations of both the myeloid and lymphoid lineages [17]. The interplay between tumor cells and tumor microenvironment plays profound roles in tumor progression [18]. However, although the contribution of VHL-HIF-2α signaling pathway is well defined, the signaling cross talk between high HIF-2α-expressing RCC cells and the tumor microenvironment is just beginning to be investigated [19–21]. Specifically, whether HIF-2α expression in ccRCC can initiate signaling pathways that facilitate an immunosuppressive microenvironment remains unknown. Here, we sought to identify the mechanism by which high HIF-2α-expressing ccRCC cells interacted with the microenvironment.
Methods
Patients and specimens
A cohort of 280 ccRCC patients who received partial or radical nephrectomy at the Department of Urology, Zhongshan Hospital, Fudan University (Shanghai, China) between Jan 2005 and June 2007 were studied. Patients with former malignant tumors, perioperative mortalities or multi-primary cancers were excluded. Formalin-fixed, paraffin-embedded tissue samples were constructed into tissue microarrays. Fresh tumor tissues, non-tumor tissues and autologous peripheral blood for RT-PCR, ELISA, flow cytometry analyses were collected from patients who underwent nephrectomy in Zhongshan Hospital from September 2017 to April 2018.
Data sets
The RNA-seq data for The Cancer Genome Atlas (TCGA) kidney clear cell carcinoma (KIRC) cohort was downloaded from the UCSC Xena (https://xenabrowser.net/heatmap/). The Sato et al. Agilent microarray gene expression dataset was downloaded from ArrayExpress (http://www.ebi.ac.uk/arrayexpress/experiments/E-MTAB-1980/) [22]. We referred to this cohort of 101 Japanese ccRCC samples as the SATO dataset from here on. To estimate the immune cell compositions for KIRC and SATO cohort, we used analytical platform CBERSORT (https://cibersort.stanford.edu/) that can quantify relative levels of distinct cell types in a mixed cell populations [23]. Analyses were performed with 100 permutations with default statistical parameters. The activated and resting statuses of the same type of immune cells were analyzed as a whole. Differential gene expression between groups of samples was determined using limma package with R software. The protein–protein interaction network was identified by the STRING database and visualized by Cytoscape [24, 25]. Funrich version 3.0 was applied for gene ontology (GO) analyses [26]. Gene set enrichment analyses (GSEA) were carried out to identify significant gene sets down regulated in high tumor infiltrating mast cells (TIMs) patients, with FDR < 0.25 and P value < 0.05 [27]. Gene sets were downloaded from the Broad Institute’s MSigDB (http://www.broadinstitute.org/gsea/index.jsp).
Tissue microarrays and Immunohistochemistry
Formalin-fixed paraffin-embedded tissue block were sliced and stained with hematoxylin-eosin for pathologic and diagnostic confirmation. Representative area was selected before construction of tissue microarrays. Tissue microarrays were constructed as previously described [28]. Mouse monoclonal antibodies for HIF-2α (NB100-122, Novus), IgG isotype control (NBP2-45266, Novus), CD8 (clone C8/144B, DAKO), tryptase (ab2378, Abcam), FOXP3 (ab20034, Abcam), CD68 (clone KP1, DAKO), and CD66b (clone G10F5, BD) were used for immunohistochemistry (IHC). IHC staining was conducted as previously described [29]. Staining results were recorded with Olympus CDD camera, Nikon eclipse Ti-s microscope (200× magnification) and NIS-Elements F3.2 software. Scores (0 = negative, 1 = weak staining, 2 = moderate staining, and 3 = strong staining) were assigned for cytoplasmic staining by two pathologists who were blinded to patient statistics. CD8+ T cells, mast cells, macrophages and Tregs were calculated as cells/mm2. Neutrophils were recorded as presence or absence. The slides were reviewed by the two observers until a consensus was reached in case of disagreement.
Quantitative RT-PCR
Extraction of RNA of fresh tumor tissues was performed using TRIzol (Invitrogen) according to the manufacturer’s instructions. Reverse transcription was done by QuantiTect reverse transcription kits (QIAGEN). Primers used were: HIF-2α primers ACCTGAAGATTGAAGTGATTGAG and GTGGCTGGAAGATGTTTGTC; Glyceraldehyde-3-phosphate dehydrogenase (GADPH) primers AAGGTCGGAGTCAACGGATT and TGGTGGCTGGAAGATGTTTGTC. The mRNA level of HIF-2α was normalized by GADPH.
ELISA
Supernatants of tumor tissue conditioned media were collected. ELISA assays to quantify stem-cell factor (SCF) in tumor tissue supernatants with or without PT2385 (HY-12867, MCE), a HIF-2α specific antagonist, were performed using human SCF ELISA kit (ab100636, Abcam) according to the manufacturer’s instructions.
Flow cytometry
Single cells were isolated from fresh tumor tissues or normal tissues with collagenase IV and then stained with appropriate monoclonal antibodies for 30 min at 4 degree centigrade. Intracellular protein was performed with Fixation/Permeabilization Solution Kit (BD Biosciences). Cell suspensions from tumor and non-tumor tissues were stained with fluorochrome-labeled antibodies specific for human CD45 (557748, BD), CD8 (555366, BD), IFNG (562988, BD), GZMB (560212, BD), CD117 (562094, BD), IL-10 (556013, BD), TGF-β (562962, BD) and Fc epsilon RI alpha (FAB6678A, R&D). Flow cytometry was performed with a BD FACScelesta and cells were analyzed using Flowjo Software (Tree Star).
Statistical analysis
The differences between two groups were analyzed by Student’s t test, paired t test or Mann–Whitney U test. Correlations between HIF-2α expression and patient characteristics were assessed by Pearson’s Chi-square test or Cochran–Mantel–Haenszel χ2 test. Pearson correlation coefficient and linear regression analysis were used to determine the correlation between two parameters. We used Kaplan–Meier analysis and log rank test to demonstrate survival curves between different groups. Overall survival and recurrence-free survival were calculated from the date of surgery to time of death and recurrence, respectively. Data analyses were performed using SPSS Statistics 21.0 (SPSS Inc., IL, Chicago, USA) and R 3.2.3 (R Foundation for Statistical Computing). All statistical tests were two-sided and P < 0.05 was considered statistically significant.
Results
HIF-2α expression associates with worse prognosis and impaired anti-tumor immunity in ccRCC patients
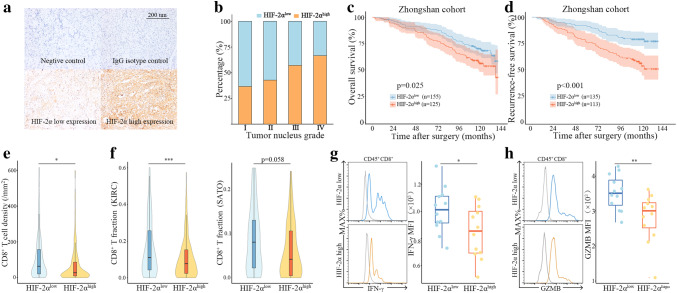
Baseline clinicopathological characteristics of 280 ccRCC patients were exhibited in Table S1. For further analysis and easier clinical use, there were 155 patients grouped as low HIF-2α expression (score = 0 or 1) and 125 patients grouped as high HIF-2α expression (score = 2 or 3) (Fig. 1a, Figure S1a). Representative images of HIF-2α expression and corresponding negative control as well as isotype control were shown in Fig. 1a. HIF-2α expression was spilt at median in KIRC and SATO cohort (Figure S1b, S1c). HIF-2α expression was positively correlated with HIF-2α expression (P = 0.048) (Fig. 1b). Other clinicopathological characteristics listed in table S1 did not show a clear association (Table S1). Kaplan–Meier analyses demonstrated that patients with HIF-2αhigh tumors had significantly worse overall survival (P = 0.025) (Fig. 1c). A total of 248 localized ccRCC patients with detailed follow-up information were evaluated for recurrence-free survival. Patients with HIF-2αhigh tumors exhibited reduced recurrence-free survival as well (P < 0.001) (Fig. 1d). Interestingly, we noticed that the number of tumor infiltrating CD8+ T cells was significantly lower in HIF-2αhigh tumors (P = 0.016) (Fig. 1e). We then evaluated CD8+ T-cell infiltration in KIRC and SATO cohorts with CIBERSORT, a computational approach for inferring leukocyte representation in bulk tumor transcriptomes [23]. There was an inverse correlation between HIF-2α expression and CD8+ T-cell infiltration in both KIRC cohort (P < 0.001) and SATO cohort (P = 0.058) (Fig. 1f). Since CD8+ T-cell infiltration alone was not informative enough about the anti-tumor immunity of CD8+ T cells, we next investigated the functions of CD8+ T cells in fresh tumor samples. HIF-2α mRNA expression of fresh tumor tissues was split at median as well. CD8+ T cells in HIF-2αlow tumors exhibited heightened expression of IFN-γ and GZMB, effector molecules prominently associated with T-cell response (Fig. 1g, h). These results suggested that HIF-2α was implicated in establishment of an immune-suppressive microenvironment.
Fig. 1.
HIF-2α expression associates with worse prognosis and impaired anti-tumor immunity in ccRCC patients. a Representative immunohistochemistry images of hypoxia-inducible factor 2α (HIF-2α) expression. b Percentage of patients with HIF-2α high expression in different tumor nucleus grades. c Kaplan–Meier plots of overall survival in Zhongshan cohort. d Kaplan–Meier plots of recurrence-free survival in Zhongshan cohort. e Quantification of CD8+ T cells in HIF-2αlow tumors and HIF-2αhigh tumors in Zhognshan cohort. f CD8+ T-cell fraction in HIF-2αlow tumors and HIF-2αhigh tumors in KIRC and SATO cohorts. g Representative images of flow cytometry analysis and IFN-γ expression in CD8+ T cells. h Representative images of flow cytometry analysis and GZMB expression in CD8+ T cells. *P < 0.05, **P < 0.01, ***P < 0.001
Mast cells accumulate in HIF-2αhigh tumors and correlate with reduced immunosurveillance in ccRCC patients
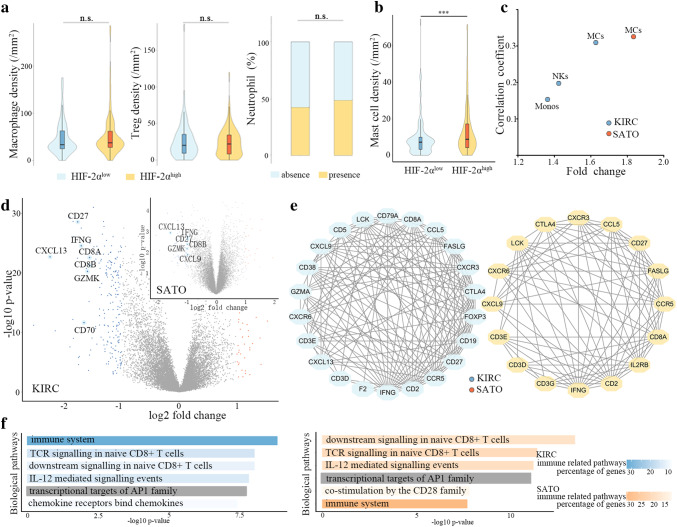
Next, we sought to explore whether the tumor microenvironment imparted different features on the composition of immune cells in HIF-2αhigh and HIF-2αlow tumors. We analyzed macrophages, regulatory T cells, neutrophils and mast cells infiltrations with immunohistochemistry in Zhongshan cohort (Fig. 2a, Figure S2a, S2b, S2c). We observed significantly elevated number of TIMs in HIF-2αhigh tumors (Fig. 2b, Figure S2d). CIBERSORT analysis also revealed that among all the 18 types of immune cells, only mast cells accumulated in HIF-2αhigh tumors (KIRC: P < 0.001, fold change = 1.67; SATO: P = 0.005, fold change = 1.85) and moderately associated with HIF-2α expression in both KIRC (P < 0.001, r = 0.309) and SATO (P = 0.001, r = 0.325) cohorts (Fig. 2c). Mast cells have been associated with the promotion of immune tolerance. We identified some differentially expressed genes in tumors with high TIMs versus tumors with low TIMs. In particular, genes encoding components of CD8+ T receptor (CD8A, CD8B, CD27), related to cytotoxic activities mediated by CD8+ T (IFNG, GZMK) and suggesting a cytokine-rich microenvironment (CXCL9, CXCL13) were among the top 30 down-regulated genes in two cohorts (Fig. 2d). We then performed protein–protein interaction analysis with top 200 down-regulated genes in high TIMs group. Genes related to anti-tumor immunity (IFNG, GZMA, GZMK, CCL5, CD8A, CD3E, CD3D, CXCL9, CCR5, IL2RG) were in center of both KIRC and SATO protein–protein interaction network (Fig. 2e). In short, expressions of genes characteristic of adaptive immune system and cytotoxic functions were markedly down-regulated in tumors with high TIMs.
Fig. 2.
Mast cells accumulate in HIF-2αhigh tumors and correlate with reduced immunosurveillance in ccRCC patients. a Quantification of macrophages, Tregs and neutrophils in HIF-2αlow and HIF-2αhigh tumors in Zhongshan cohort. b Quantification of mast cells in HIF-2αlow and HIF-2αhigh tumors in Zhongshan cohort. c Mast-cell fraction in HIF-2αlow tumors and HIF-2αhigh tumors in KIRC and SATO cohorts. d Volcano plots of differentially expressed genes between tumors with high tumor infiltrating mast cells (TIM) and low TIMs in KIRC (adjusted p < 0.05, log2 fold change > 1.5) and SATO (p < 0.05, log2 fold change > 1) cohorts. e Protein–protein interaction networks of the top 200 down-regulated genes in tumors with high TIMs in KIRC and SATO cohorts (interaction score ≥ 0.4, node degree ≥ 10). f Gene ontology analysis of top 200 down-regulated genes in tumors with high TIMs versus tumors with low TIMs in KIRC and SATO cohorts. ***P < 0.001
IL-10 and TGF-β contribute to immunosuppressive properties of tumor infiltrating mast cells in ccRCC patients
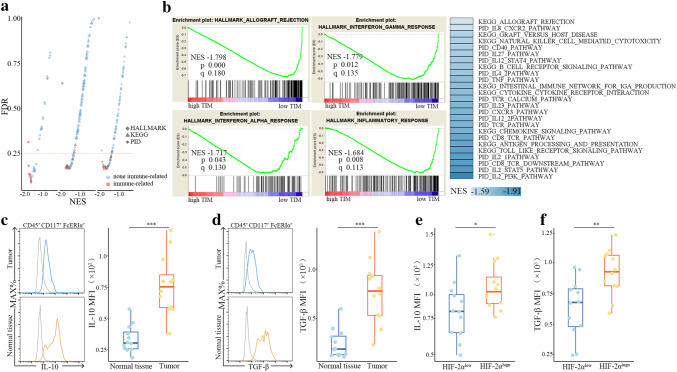
Biological pathways of immune system, TCR signaling in naïve CD8+ T cells, IL-12 mediated signaling events in both cohorts were significantly down-regulated in tumor with high TIMs (Fig. 2f). Additional comparison of gene expression profile of tumors with high TIMs and low TIMs was performed by GSEA. Most of the down-regulated gene sets associated with immune parameters reached statistical significance in KIRC cohort (Fig. 3a). Allograft rejection [Normalized enrichment score (NES) = − 1.798], interferon gamma response (NES = − 1.779), interferon gamma response (NES = − 1.717) and inflammatory response (NES = − 1.684) were among the top five down-regulated HALLMARK pathways. Analyses of KEGG and PID pathways revealed down-regulation of pathways related to anti-tumor immunity including IL-2 pathway, IL-12 pathway, CD8-TCR pathway, natural killer cell-mediated cytotoxicity, etc (Fig. 3b). Similar results were observed in SATO cohort (Figure S1d). These down-regulated pathways demonstrated an association between TIMs and immune suppression. Both IL-10 and TGF-β levels were found to be significantly up-regulated in TIMs as compared to mast cells from normal tissues, thus TIMs could at least partly suppress T-cell immunity via secretion of IL-10 and TGF-β in ccRCC patients (Fig. 3c, d). Concomitantly, IL-10 and TGF-β expressions in TIMs significantly elevated in HIF-2αhigh tumors, hinting that HIF-2α high expression contributed to mast-cell activation (Fig. 3e, f).
Fig. 3.
IL-10 and TGF-β contribute to immunosuppressive properties of tumor infiltrating mast cells in ccRCC patients. a Gene set enrichment analysis (GSEA) (HALLMARK, KEGG and PID) pathway distribution for tumors with high TIMs versus tumors with low TIMs in KIRC cohort. Horizontal line denotes FDR significance. b Immune-related gene sets down-regulated in tumors with high TIMs in KIRC cohort. c Flow cytometric detection of IL-10 on mast cells in tumor tissues and normal tissues. d Flow cytometric detection of TGF-β on mast cells in tumor tissues and normal tissues. e Protein expression of IL-10 on mast cells from HIF-2αlow and HIF-2αhigh tumors. f Protein expression of TGF-β on mast cells from HIF-2αlow and HIF-2αhigh tumors. *P < 0.05, **P < 0.01, ***P < 0.001
HIF-2α expression induces SCF secretion in ccRCC patients
To pursue mechanism underlying increased mast-cell infiltration, we analyzed KIRC and SATO RNA-seq data focusing on chemokines implicated in mast cells trafficking to tumors. Significance Analysis for Microarray identified three differentially expressed genes (delta value > 2) in KIRC cohort and one gene in SATO cohort. Significant up-regulation of SCF in HIF-2αhigh tumors was observed in KIRC and SATO cohorts (Fig. 4a, b). In KIRC cohort, SCF mRNA levels moderately correlated with HIF-2α expression while in SATO cohort, they had a strong correlation (Fig. 4c). Similar to data at the mRNA levels, analysis of supernatants of fresh tumor tissue from ccRCC patients revealed that SCF increased greatly in HIF-2αhigh tumors (Fig. 4c). Analysis of circulating SCF with blood samples showed that there was also a systemic increase in the level of SCF (Fig. 4e). The identification of SCF as a product of HIF-2α expression was consistent with the finding that the secretion of SCF was regulated in a HIF-2α-dependent manner [30]. To determine the role of HIF-2α expression in production of SCF, we treated ccRCC suspensions with PT2385, a selective HIF-2α antagonist. The antagonist significantly down-regulated SCF secretion (Fig. 4f).
Fig. 4.
HIF-2α expression induces SCF secretion in ccRCC patients. a Heat-maps displaying cytokines able to recruit mast cells in KIRC and SATO cohorts. b Significance analysis for Microarray of cytokines in heat-maps in KIRC and SATO cohorts. c Correlations between stem cell factor (SCF) and HIF-2α mRNA levels in KIRC and SATO cohorts. d Stem cell factor (SCF) protein levels in supernatants of HIF-2αlow tumors and HIF-2αhigh tumors. e SCF protein levels in serum of patients with HIF-2αlow tumors and HIF-2αhigh tumors. (f) SCF protein levels in suspensions of ccRCC treated with PT2385 (10 µmol/ml) for 12 h and DMSO (paired t test). *P < 0.05, **P < 0.01
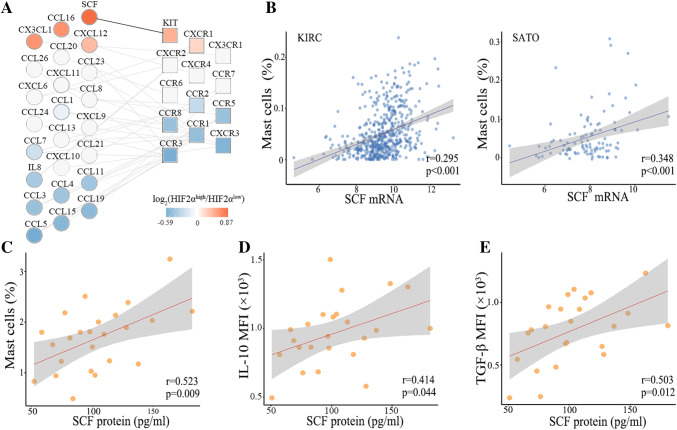
SCF recruits tumor infiltrating mast cells and associates with increased IL-10 and TGF-β secretion in ccRCC patients
SCF plays a major role in recruitment of mast cells to the tumor site [31]. The receptor of SCF, c-Kit, was up-regulated in HIF-2αhigh tumors as well in both KIRC and SATO cohorts (Fig. 5a, Figure S1e). In this study, SCF levels were positively correlated with mast-cell infiltrations in all three cohorts (Fig. 5b, c). Together, these results indicated that increased mast-cell recruitment into the microenvironment of HIF-2αhigh tumors was at least in part, due to increased production of SCF. We found that SCF protein levels were positively correlated with IL-10 and TGF-β expression in TIMs (Fig. 5d, e).
Fig. 5.
SCF recruits tumor infiltrating mast cells and associates with IL-10 and TGF-β secretion in ccRCC patients. a Interaction network analysis of chemokine ligand gene expression and corresponding receptor gene expression detected in HIF-2αhigh versus HIF-2αlow tumors in KIRC cohort. Genes are ordered vertically by fold change. Black lines connect up-regulated pairs. b Correlations between SCF and HIF-2α mRNA levels in KIRC and SATO cohorts. c Correlation between SCF protein levels in tumor supernatants and number of TIMs. d Correlation between SCF protein levels and IL-10 expression in TIMs. e Correlation between SCF protein levels and TGF-β expression in TIMs
HIF-2α mRNA levels adversely associate with immune-stimulatory genes expression in ccRCC patients
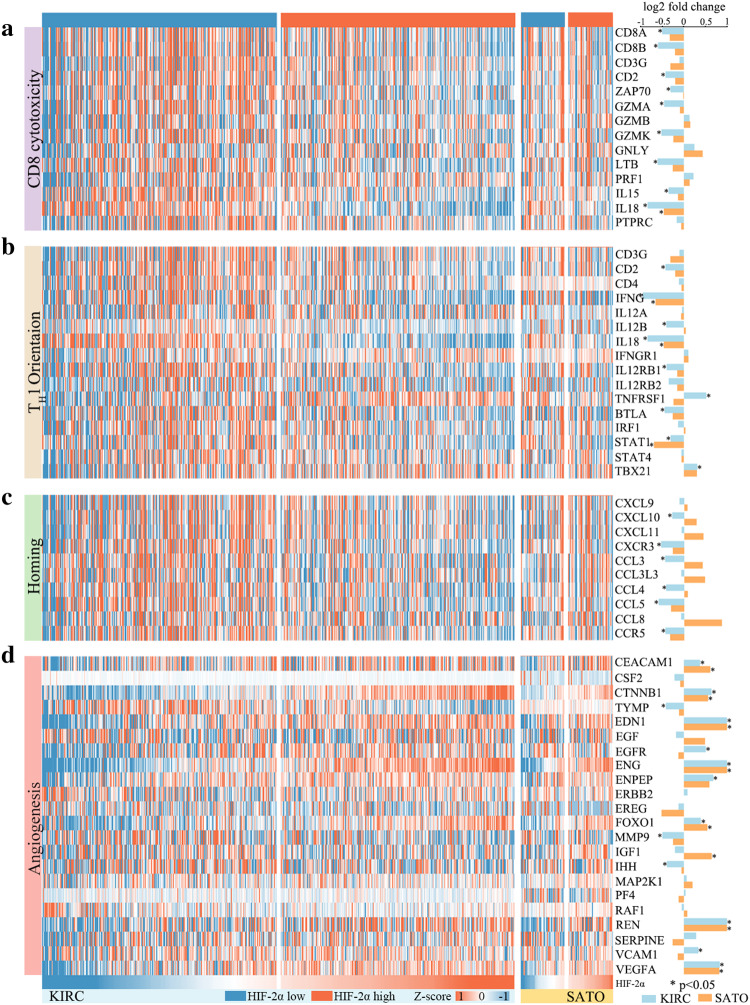
To confirm the immunosuppressive roles of HIF-2α,we performed a correlation analysis across the TCGA KIRC tumors for HIF-2α expression and immune gene signatures including CD8+ T-cell cytotoxicity, TH1 orientation, homing of T cells and angiogenesis. Heat-maps showed that high HIF-2α expression was associated with the downregulation of the clusters referring to CD8+ T-cell cytotoxicity, TH1 orientation and homing of T cells (Fig. 6a–c). Nine genes of the CD8 cytotoxicity cluster (CD8A, CD8B, CD2, ZAP70, GZMA, GZMK, LTB, IL15, IL18), seven genes of the TH1 orientation cluster (CD2, IFNG, IL12B, IL18, IL12RB1, BTLA, STAT1) and six homing of T-cell cluster genes (CXCL10, CXCR3, CCL3, CCL4, CCL5, CCR5) were significantly differentially expressed between patient groups. The dominant angiogenesis-promoting role of HIF-2α was reflected in angiogenesis gene cluster, with ten signature genes (CEACAM1, CTNNB1, EDN1, EGFR, ENG, ENPEP, FOXO1, REN, VCAM1, VEGFA) significantly upregulated in HIF-2αhigh tumors (Fig. 6d). Meanwhile, we observed similar trends for gene clusters representing CD8+ T-cell cytotoxicity, TH1 orientation and angiogenesis in SATO cohort, but few genes reached statistical significance, probably due to limited sample size. These data further confirmed the immunosuppressed state in HIF-2αhigh tumors. HIF-2α linked oncogenic activation to the evasion of anti-tumor immunity.
Fig. 6.
HIF-2α mRNA levels adversely associate with immune-stimulatory genes expression in ccRCC patients. a Heat-map displaying CD8 cytotoxicity gene cluster in KIRC and SATO cohorts. b Heat-map displaying TH1 orientation gene cluster in KIRC and SATO cohorts. c Heat-map displaying homing and migration gene cluster in KIRC and SATO cohorts. d Heat-map displaying angiogenesis gene cluster in KIRC and SATO cohorts. Log2 fold changes of genes in HIF-2αhigh tumors compared with genes in HIF-2αlow tumors are displayed as bar graphs on the right. *P < 0.05
Discussion
It is increasingly recognized that host immunity plays a critical role in regulating tumor progression [32]. The efforts of immune cells that populate the tumor microenvironment to mount an effective anti-tumor response usually fail, partly due to the immune-suppressing cells [33]. The current understanding is that HIF-2α promotes tumor progression by regulating metabolism, angiogenesis, extracellular matrix and apoptosis [34]. However, the immune-modulating capabilities of HIF-2α are poorly understood. In this study, we implicated that HIF-2α high expression restrained anti-tumor immunity through recruitment of mast cells and the subsequent suppression of CD8+ T-cell function. We reported that HIF-2α expression in ccRCC cells induced the expression of SCF and HIF-2α antagonist significantly inhibited SCF secretion. SCF acted as a chemoattractant for mast cells. Recruited TIMs were immunosuppressive and blunted immunosurveillance via secretion of IL-10 and TGF-β.
The increase in SCF protein level was corroborated by an increase in SCF transcripts level. Chromatin immunoprecipitation and luciferase assay have proved that HIF-2α induces the transcription of SCF gene through the hypoxia response element in SCF promoter [30]. SCF is a major chemoattractant for mast cells and mast-cell progenitors [31]. In accordance with this, SCF protein level is positively correlated with the number of TIMs in our study. SCF promotes not only recruitment of mast cells into tumors, but also their maturation and activation. Growing evidence has indicated a more regulatory role for mast cells in immune system. Mast cells are able to produce a long list of cytokines, among which IL-10 and TGF-β receive particular attention due to their known role as immunosuppressive mediators [35]. Several lines of evidence in our study proved that mast cells accumulated in response to SCF production by high HIF-2α-expressing ccRCC cells had immunosuppressive properties. Thus we observed reduced CD8+ T-cell infiltration and impaired CD8+ T-cell function in fresh tumor samples with high HIF-2α expression. In accordance with this, HIF-2α mRNA expression inversely correlated with gene clusters reflective of CD8 cytotoxicity, TH1 orientation and homing of T cells. Taken together, these findings suggested that HIF-2α was an important regulator of immune suppression within the ccRCC tumor microenvironment. The roles of mast cells and SCF in tumor immunosuppression have already been elucidated [36]. However, the role of HIF-2α-SCF-mast cell cascade has not been reported and these results extend the oncogenic functions of the HIF-2α from a cancer cell-intrinsic to a systemic level.
Two HIF-2α selective inhibitors both show greater anti-tumor activity than sunitinib in mouse models [15, 31]. HIF-2α has been mainly implicated in angiogenesis while our work provides another rationale for the development of HIF-2α inhibitors. Targeting a molecular pathway that is upregulated in cancer cells may provide tumor specificity and help to overcome some of the potential issues with severe autoimmunity. Besides, the tight link between HIF-2α expression, mast-cell accumulation and impaired immunosurveillance highlight the opportunities for immunotherapy in ccRCC patients with HIF-2αhigh tumors. The use of neutralizing antibodies of SCF or pharmacological agents modulating mast cells functions might be a new way to prevent progression of ccRCC. Effective and safe antagonists that target SCF/c-kit axis or mast cells are in development, which might be repurposed for treatment of HIF-2αhigh ccRCC patients [38].
Conclusions
Disruption of CD8+ T-cell immunity by elements of the tumor microenvironment is thought to be a major mechanism of tumor immune evasion [39]. Mast cells accumulated in ccRCC with high HIF-2α expression. Our data for the first time demonstrated that HIF-2α induced expression of SCF, which promote the formation of an immunosuppressive, tumorigenic microenvironment by inducing mast-cell accumulation and subsequent suppression of CD8+ T-cell function.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- ccRCC
Clear cell renal cell carcinoma
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- GO
Gene ontology
- GSEA
Gene set enrichment analyses
- HIF
Hypoxia-inducible factors
- KIRC
Kidney clear cell carcinoma
- pVHL
Von Hippel-Lindau (VHL) protein
- RCC
Renal cell carcinoma
- SCF
Stem cell factor
- TCGA
The Cancer Genome Atlas
- TIM
Tumor infiltrating mast cell
- VHL
Von Hippel-Lindau
Author contributions
Acquisition of data, analysis and interpretation of data, statistical analysis and drafting of the manuscript were carried out by YX, LL and YX; YQ, YC, LC, PZ, YK, YQ, ZW, ZL, XC, ZX, JW, QB, WZ and YY provided technical and material support; JG and JX were responsible for the study concept and design, analysis and interpretation of data, drafting of the manuscript, obtained funding and study supervision. All authors read and approved the final manuscript.
Funding
This work was supported by grants from National Natural Science Foundation of China (81471621, 81472227, 81472376, 81671628, 31770851, 81702496, 81702497, 81702805, 81772696, 81871306), Shanghai Municipal Natural Science Foundation (17ZR1405100), Shanghai Municipal Commission of Health and Family Planning (20174Y0042), and Zhongshan Hospital Science Foundation (2016ZSQN30, 2017ZSQN18, 2017ZSYQ26). All these study sponsors have no roles in design of the study or collection, analysis, and interpretation of data.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Ethical approval and ethical standards
The study was approved by the Clinical Research Ethics Committee of Zhongshan Hospital, Fudan University with the approval number B2015-030. Our study followed the Helsinki declaration.
Informed consent
Informed consent to use clinical samples and information was obtained from each patient.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ying Xiong, Li Liu and Yu Xia contributed equally to this work.
Contributor Information
Jianming Guo, Email: guo.jianming@zs-hospital.sh.cn.
Jiejie Xu, Email: jjxufdu@fudan.edu.cn.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med. 2005;353:2477–2490. doi: 10.1056/NEJMra043172. [DOI] [PubMed] [Google Scholar]
- 4.Gupta K, Miller JD, Li JZ, Russell MW, Charbonneau C. Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): a literature review. Cancer Treat Rev. 2008;34:193–205. doi: 10.1016/j.ctrv.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Escudier B. Emerging immunotherapies for renal cell carcinoma. Ann Oncol. 2012;23(Suppl 8):viii35–40. doi: 10.1093/annonc/mds261. [DOI] [PubMed] [Google Scholar]
- 6.Atkins MB, Regan M, McDermott D. Update on the role of interleukin 2 and other cytokines in the treatment of patients with stage IV renal carcinoma. Clin Cancer Res. 2004;10:6342S–6342S6S. doi: 10.1158/1078-0432.CCR-040029. [DOI] [PubMed] [Google Scholar]
- 7.Nyhan MJ, O’Sullivan GC, McKenna SL. Role of the VHL (von Hippel-Lindau) gene in renal cancer: a multifunctional tumour suppressor. Biochem Soc Trans. 2008;36:472–478. doi: 10.1042/BST0360472. [DOI] [PubMed] [Google Scholar]
- 8.Farber LJ, Furge K, Teh BT. Renal cell carcinoma deep sequencing: recent developments. Curr Oncol Rep. 2012;14:240–248. doi: 10.1007/s11912-012-0230-3. [DOI] [PubMed] [Google Scholar]
- 9.Schodel J, Grampp S, Maher ER, Moch H, Ratcliffe PJ, Russo P, Mole DR. Hypoxia, Hypoxia-inducible Transcription Factors, and Renal Cancer. Eur Urol. 2016;69:646–657. doi: 10.1016/j.eururo.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schodel J, Oikonomopoulos S, Ragoussis J, Pugh CW, Ratcliffe PJ, Mole DR. High-resolution genome-wide mapping of HIF-binding sites by ChIP-sEq. Blood. 2011;117:e207–e217. doi: 10.1182/blood-2010-10-314427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mole DR, Blancher C, Copley RR, Pollard PJ, Gleadle JM, Ragoussis J, Ratcliffe PJ. Genome-wide association of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha DNA binding with expression profiling of hypoxia-inducible transcripts. J Biol Chem. 2009;284:16767–16775. doi: 10.1074/jbc.M901790200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raval RR, Lau KW, Tran MG, et al. Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol Cell Biol. 2005;25:5675–5686. doi: 10.1128/MCB.25.13.5675-5686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kroeger N, Seligson DB, Signoretti S, Yu H, Magyar CE, Huang J, Belldegrun AS, Pantuck AJ. Poor prognosis and advanced clinicopathological features of clear cell renal cell carcinoma (ccRCC) are associated with cytoplasmic subcellular localisation of Hypoxia inducible factor-2alpha. Eur J Cancer. 2014;50:1531–1540. doi: 10.1016/j.ejca.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 14.Biswas S, Charlesworth PJ, Turner GD, et al. CD31 angiogenesis and combined expression of HIF-1alpha and HIF-2alpha are prognostic in primary clear-cell renal cell carcinoma (CC-RCC), but HIFalpha transcriptional products are not: implications for antiangiogenic trials and HIFalpha biomarker studies in primary CC-RCC. Carcinogenesis. 2012;33:1717–1725. doi: 10.1093/carcin/bgs222. [DOI] [PubMed] [Google Scholar]
- 15.Wallace EM, Rizzi JP, Han G, et al. A small-molecule antagonist of HIF2alpha is efficacious in preclinical models of renal cell carcinoma. Cancer Res. 2016;76:5491–5500. doi: 10.1158/0008-5472.CAN-16-0473. [DOI] [PubMed] [Google Scholar]
- 16.Cho H, Kaelin WG. Targeting HIF2 in clear cell renal cell carcinoma. Cold Spring Harb Symp Quant Biol. 2016;81:113–121. doi: 10.1101/sqb.2016.81.030833. [DOI] [PubMed] [Google Scholar]
- 17.Palucka AK, Coussens LM. The basis of oncoimmunology. Cell. 2016;164:1233–1247. doi: 10.1016/j.cell.2016.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 19.Vandyke K, Zeissig MN, Hewett DR, et al. HIF-2alpha promotes dissemination of plasma cells in multiple myeloma by regulating CXCL12/CXCR4 and CCR1. Cancer Res. 2017;77:5452–5463. doi: 10.1158/0008-5472.CAN-17-0115. [DOI] [PubMed] [Google Scholar]
- 20.Yamamura K, Uruno T, Shiraishi A, et al. The transcription factor EPAS1 links DOCK8 deficiency to atopic skin inflammation via IL-31 induction. Nat Commun. 2017;8:13946. doi: 10.1038/ncomms13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Messai Y, Noman MZ, Hasmim M, Escudier B, Chouaib S. HIF-2alpha/ITPR1 axis: a new saboteur of NK-mediated lysis. Oncoimmunology. 2015;4:e985951. doi: 10.4161/2162402X.2014.985951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato Y, Yoshizato T, Shiraishi Y, et al. Integrated molecular analysis of clear-cell renal cell carcinoma. Nat Genet. 2013;45:860–867. doi: 10.1038/ng.2699. [DOI] [PubMed] [Google Scholar]
- 23.Gentles AJ, Newman AM, Liu CL, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21:938–945. doi: 10.1038/nm.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szklarczyk D, Franceschini A, Wyder S, et al. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43:D447–D452. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benito-Martin A, Peinado H. FunRich proteomics software analysis, let the fun begin! Proteomics. 2015;15:2555–2556. doi: 10.1002/pmic.201500260. [DOI] [PubMed] [Google Scholar]
- 27.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J, Liu L, Qu Y, et al. Prognostic value of SETD2 expression in patients with metastatic renal cell carcinoma treated with tyrosine kinase inhibitors. J Urol. 2016;196:1363–1370. doi: 10.1016/j.juro.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 29.Liu H, Xu J, Zhou L, Yun X, Chen L, Wang S, Sun L, Wen Y, Gu J. Hepatitis B virus large surface antigen promotes liver carcinogenesis by activating the Src/PI3K/Akt pathway. Cancer Res. 2011;71:7547–7557. doi: 10.1158/0008-5472.CAN-11-2260. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Dong J, Jia L, et al. HIF-2-dependent expression of stem cell factor promotes metastasis in hepatocellular carcinoma. Cancer Lett. 2017;393:113–124. doi: 10.1016/j.canlet.2017.01.032. [DOI] [PubMed] [Google Scholar]
- 31.Meininger CJ, Yano H, Rottapel R, Bernstein A, Zsebo KM, Zetter BR. The c-kit receptor ligand functions as a mast cell chemoattractant. Blood. 1992;79:958–963. [PubMed] [Google Scholar]
- 32.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 33.Disis ML. Immune regulation of cancer. J Clin Oncol. 2010;28:4531–4538. doi: 10.1200/JCO.2009.27.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baldewijns MM, van Vlodrop IJ, Vermeulen PB, Soetekouw PM, van Engeland M, de Bruine AP. VHL and HIF signalling in renal cell carcinogenesis. J Pathol. 2010;221:125–138. doi: 10.1002/path.2689. [DOI] [PubMed] [Google Scholar]
- 35.Khazaie K, Blatner NR, Khan MW, et al. The significant role of mast cells in cancer. Cancer Metastasis Rev. 2011;30:45–60. doi: 10.1007/s10555-011-9286-z. [DOI] [PubMed] [Google Scholar]
- 36.Marech I, Gadaleta CD, Ranieri G. Possible prognostic and therapeutic significance of c-Kit expression, mast cell count and microvessel density in renal cell carcinoma. Int J Mol Sci. 2014;15:13060–13076. doi: 10.3390/ijms150713060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen W, Hill H, Christie A, et al. Targeting renal cell carcinoma with a HIF-2 antagonist. Nature. 2016;539:112–117. doi: 10.1038/nature19796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ribatti D. Mast cells as therapeutic target in cancer. Eur J Pharmacol. 2016;778:152–157. doi: 10.1016/j.ejphar.2015.02.056. [DOI] [PubMed] [Google Scholar]
- 39.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.