Abstract
Immunotherapy of non-small cell lung cancer (NSCLC), by immune checkpoint inhibitors, has profoundly improved the clinical management of advanced disease. However, only a fraction of patients respond and no effective predictive factors have been defined. Here, we discuss the prospects for identification of such predictors of response to immunotherapy, by fostering an in-depth analysis of the immune landscape of NSCLC. The emerging picture, from several recent studies, is that the immune contexture of NSCLC lesions is a complex and heterogeneous feature, as documented by analysis for frequency, phenotype and spatial distribution of innate and adaptive immune cells, and by characterization of functional status of inhibitory receptor+ T cells. The complexity of the immune landscape of NSCLC stems from the interaction of several factors, including tumor histology, molecular subtype, main oncogenic drivers, nonsynonymous mutational load, tumor aneuploidy, clonal heterogeneity and tumor evolution, as well as the process of epithelial–mesenchymal transition. All these factors contribute to shape NSCLC immune profiles that have clear prognostic significance. An integrated analysis of the immune and molecular profile of the neoplastic lesions may allow to define the potential predictive role of the immune landscape for response to immunotherapy.
Keywords: Non-small cell lung cancer, Immune landscape, Immune checkpoint blockade, Immunotherapy, NIBIT 2016
Introduction
Immunotherapy of NSCLC based on antibodies directed at the PD-1/PD-L1 axis has profoundly changed the treatment of advanced disease. Between 2015 and 2017, different immune checkpoint inhibitors have been approved for second-line treatment, or for first-line treatment in high-PD-L1 expressing tumors [1]. Meta-analysis of published studies has indicated that immunotherapy, compared to chemotherapy, significantly improves progression-free survival (PFS) in squamous cell carcinomas (SCC), but not in non-SCC NSCLC, while overall survival (OS) is significantly improved by immune checkpoint blockade (ICB) in both the main NSCLC subsets [2]. In spite of these remarkable results, only a fraction of patients respond and there is a strong need for definition of predictive factors to understand which patients may achieve a long lasting clinical benefit. Most clinical trials have investigated the predictive role of PD-L1, expressed on tumor or on immune cells, as a main predictive factor, but at the present time, no general consensus has emerged [3], since not all PD-L1+ patients respond, while a fraction of PD-L1-negative patients do respond to immunotherapy.
A potentially relevant way forward, discussed in this paper, is based on the comprehensive analysis of the immune landscape of NSCLC. Several recent studies are built on the notion that the goal of predicting how patients may respond to immunotherapy may require a much deeper understanding of frequency, phenotype, function and spatial distribution of innate and adaptive immune cells present in the lesion. These efforts require the integration of several experimental approaches (multicolor flow and mass cytometry, multiplex digital pathology, computational inference on the tumor immune profile by analysis of immune-related gene signatures, etc.). The emerging picture, that will be reviewed here, is that the immune landscape of NSCLC is the result of a complex cross-talk between the tumor and the immune system. To understand this cross-talk, investigators need to evaluate several factors, including the comparison of the immune profile of the tumor and of the adjacent non-tumoral tissue, the differences in immune landscape of the lesions associated with histological and molecular NSCLC subsets, mutational load, tumor aneuploidy and the role of innate and adaptive immune cells with anti- or pro-tumoral function. Moreover, since ICB therapeutic efficacy is strongly dependent on re-activation of functionally impaired T cells [4] expressing inhibitory receptors (IR), a critical area of research is aimed at understanding the true meaning of IR expression on tumor-infiltrating lymphocytes. Different experimental studies, as well as results of our group [5], in fact suggest that primary NSCLC lesions may contain not only IR+-exhausted T cells, but even functionally competent and recently activated IR+ effectors.
The emerging complexity of the immune landscape of primary NSCLC
NSCLC induces a strong immune infiltration [6], as characterized by the higher percentage of CD45+ leukocytes in the tumor compared to normal lung tissue (nLung). The strong infiltration of lung tumors by immune cells is due to enhanced frequency of up to 37 different immune cell subsets including B and T cell subsets as shown by Kargl et al. [6]. In this study, cells of the myeloid lineage were the most frequent cell type accounting for 50% of the infiltrating CD45+ cells. Neutrophils represented the most frequent subset (20% of CD45+) and showed a negative correlation with CD8+ tumor content. Monocyte frequency was not associated with CD4+ or CD8+ presence with the exception of a negative association with CD4+ cells in adenocarcinomas (ADC). NSCLC tumors frequently contain cells with a CD45+ CD14− CD68− CD66b− CD33+ profile, possibly “early” MDSCs (eMDSCs), representing 10% of the CD45+ cells in the tumor tissue. Human eMDSCs, characterized by a Lin− (i.e., CD3−, CD14−, CD15−, CD19−, CD56−) HLA-DR− CD33+ phenotype, are thought to represent immature MDSCs, distinct from M-MDSCs (CD14+ CD11b+ CD15− HLA-DR−) and from PMN-MDSCs (CD11b+ CD14− CD15+). Their relevance/role in the NSCLC immune contexture remains undefined as no significant differences were found between NSCLC histological subtypes, relationship with other immune subsets and tumor size [6]. ADC and SCC subtypes showed a strong difference in the immune landscape: SCC tumors contained twice the amount of Tregs as ADCs, associated with concomitant reduction in TH1 and TH17 cells, but were enriched in CD8EMRA (CCR7− CD45RA+) and in CD8+ PD-1+ cells.
Comparison of tumor and adjacent or distant nLung is strongly informative. Del Mar Valenzuela Membrives et al. [7] in tissues from 61 NSCLC patients found a higher frequency of T and B cells and a lower frequency of NK cells in tumor compared to distant nLung. The tumor tissue was enriched for memory (CD45RO+) and activated (HLA-DR+) T cells, but even for T cells with immunosuppressive function. Lizotte et al. [8] found an increased frequency of CD8+ T cells, and particularly CD45RO+ memory CD8+ T, as well as CD19+ B cells and FOXP3+ Tregs in the tumor, compared to nLung. IRs (such as PD-1, TIM-3, LAG-3 and CTLA-4) showed enhanced expression in T cells from tumor compared to nLung and in SCC compared to ADC subtype. In the same paper, Lizotte et al. [8] used the recently developed t-distributed stochastic neighbor embedding (t-SNE) algorithm [9], a computational tool allowing reduction of high-dimensional datasets and visualization of data complexity in a two-dimensional color-coded map. By such approach, they found that NSCLC lesions clustered into distinct immunophenotype subsets: an immunologically “hot” cluster, a “cold” cluster and a third small cluster, characterized by strong granulocytic infiltrate. The hot cluster showed strong signals for CD8+ T cells, high expression of PD-1 and TIM-3 IRs on CD8+ T cells, but even infiltration of FOXP3+ Tregs and expression of PD-L1 on tumor cells and immune cells. The hot cluster was enriched in SCC lesions, while ADCs were equally distributed in the hot and in the cold clusters. By transcriptomic analysis, the hot cluster showed high expression of genes as CXCL9, CXCL10, IDO1, granzyme B, IFN-γ, and STAT1, all contributing to define the well-known “IFN-γ signature”. Interestingly, the “IFN-γ signature” (that contains genes as IFNG, STAT1, CCR5, CXCL9, CXCL10, CXCL11, IDO1, PRF1, GZMA, and HLA-DRA) has been associated with response to immunotherapy targeting PD-1 [10].
The distinction of NSCLC lesions based on the “hot/cold” classifier emerges even in other studies where RNAseq and gene expression data were analyzed. Karasaki et al. [11] plotted selected and normalized gene expression data in a radar chart with eight axes (the immunogram). Each axis of the immunogram (see Fig. 1 for representative immunogram profiles) describes the “level of intensity” of one of the eight main steps in the cancer immunity cycle (1: T-cell infiltration, existence of T-cell immunity in the tumor; 2: neoantigens, tumor antigenicity; 3: DCs, priming and activation; 4: chemokines, trafficking and infiltration; 5: HLA molecules, recognition of tumor antigens; 6: Tregs, MDSCs, suppressive cells; 7: PD-1, PD-L1, IRs and ligands; 8: IDO1, ARG1, inhibitory molecules). Three main immunogram patterns were found: hot/T cell rich, cold/T cell poor, and intermediate. The hot/T cell-rich immunogram pattern was characterized by gene expression signatures of T cells, Tregs, MDSCs, IRs and inhibitory molecules in the tumor, indicating an active immune response being counteracted by immunosuppressive mechanisms. The cold immunogram indicated lack of anti-tumor T-cell-mediated immunity, defective DC activation, impaired antigen presentation and strong immunosuppressive mechanisms. The immunogram approach is a promising way forward for the design of clinically useful prognostic and predictive tools based on the characterization of neoplastic tissues by a large set of immune-related features.
Fig. 1.
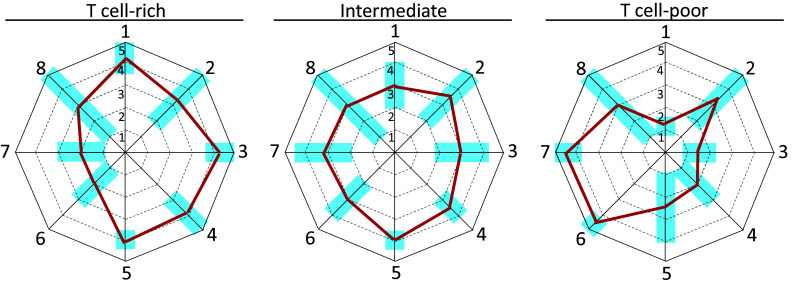
Representative immunograms of T-cell-rich-, intermediate and T-cell-poor neoplastic lesions. Eight-axes immunograms for the cancer immunity cycle, designed according to criteria defined by Karasaki et al. [11], show three representative profiles (thick blue lines) of T-cell-rich (left), intermediate (middle), and T-cell-poor lesions (right). Light blue rectangles: range of immunogram scores, for each axis, observed in NSCLC lesions as reported in ref. 11. Axis 1: T-cell infiltration; axis 2: neoantigens; axis 3: DCs, priming and activation; axis 4: Chemokines, trafficking and infiltration; axis 5: HLA molecules, recognition of tumor antigens; axis 6: Tregs, MDSCs, suppressive cells; axis 7: PD-1, PD-L1, IRs and ligands; axis 8: IDO1, ARG1, inhibitory molecules
The introduction of mass cytometry coupled to multiplexed digital pathology characterization of tissues is fostering a significant leap forward in the dissection of the NSCLC immune landscape. Lavin et al. [12], using mass cytometry on primary ADCs, confirmed the selective enrichment in tumor tissue, compared to nLung, for T cells (as well as for monocytes and B cells) and a reduced presence of NK cells in the tumor.
Moreover, the tumor lesions in many patients were associated with tertiary lymphoid structures (TLS), close to the invasive tumor margin. TLS are cellular aggregates organized as a multicellular lymphoid structure [13] and were initially named “tumor-induced bronchus-associated lymphoid tissues” (Ti-BALT). They contain mature dendritic cells associated with a T-cell zone that is placed in close proximity to a B-cell follicle, the latter including a germinal center (GC). These structures are thought to represent active sites of generation of T-cell-mediated adaptive immunity to the tumor. In agreement, TLS presence has been associated with improved clinical outcome in NSCLC and other tumors [13]. Figure 2a–e shows representative immunohistochemistry stainings of an ADC primary lesion containing associated TLS. In agreement with similar findings in other tumors [14], T cells in the TLS of NSCLC tissues often express PD-1 at high (Hi) or intermediate (Int) levels (Fig. 2d). PD-1Hi T cells are found in the GC of the B-cell follicle and possibly represent CD4+ T follicular helper cells [14], while PD-1Int T cells are found in the T-cell zone of the TLS (Fig. 2d). The NSCLC-associated TLS also contains PD-L1+ cells (Fig. 2e).
Fig. 2.
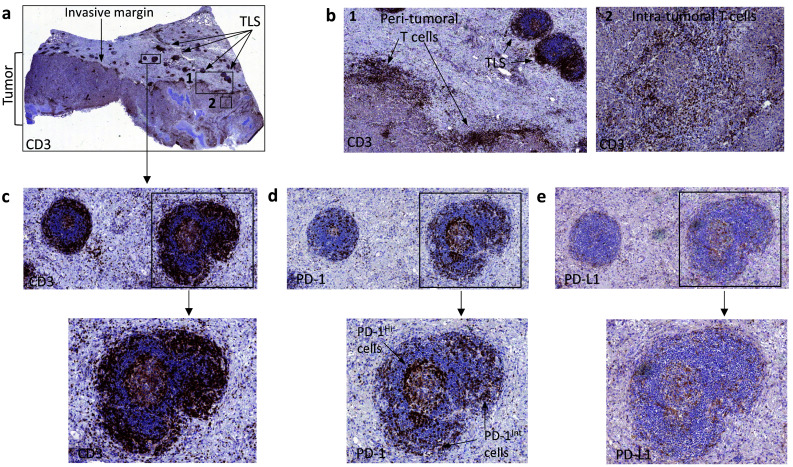
TLS associated with a primary adenocarcinoma lesion. a, b A poorly differentiated lung adenocarcinoma (a) showing peri-tumoral and intratumoral CD3+ T cells (b) is associated with several tertiary lymphoid structures (TLS) close to the tumor-invasive margin. c–e TLS shows a CD3+ T cell zone surrounding a GC (c). TLS may contain both PD-1Hi and PD-1Int lymphocytes (d), as well as PD-L1+ cells (e)
Several additional findings in the Lavin et al. study [12] pointed to an early impairment of adaptive immunity in ADCs. They found reduced frequency of CD8+ T cells in the tumor compared to nLung. In the tumor, the CD8+ T cells were characterized by PD-1 expression and evidence of functional impairment (reduced granzyme B and IFN-γ expression upon stimulation). Moreover, the tumor tissue contained an enhanced frequency of Tregs and of proinflammatory macrophages. Combined analysis for Tregs and CD8+ T cells indicated a reduced Treg/Teffector ratio in the tumor lesions compared to nLung. Tumor-associated macrophages showed a distinct phenotypic signature, characterized by higher expression of PPARβ, CD64, CD14, and CD11c and lower levels of CD86 and CD206 compared to macrophages in nLung. PD-L1 was expressed at high levels in macrophages and mast cells and, when expressed in macrophages, it negatively correlated with T-cell infiltration and was found in clusters of myeloid cells at the invasive margin of the lesions. Moreover, CD141+ DCs, thought to play a key role in cross-presentation of antigens to CD8+ T cells [15], were selectively reduced in the tumor compared to nLung [12].
Collectively, these studies shed new light on the complexity and heterogeneity of the immune landscape in NSCLC and point to at least four issues that may have a prognostic role and a potential predictive relevance for response to immunotherapy: (a) “hot” tumors indicate a strong immune reaction to the neoplastic lesion, but represent only a fraction of the cases; (b) the two main NSCLC subtypes do not share a similar immune landscape nor a similar fraction of “hot” tumors; (c) “hot” tumors show not only the evidence of T-cell activation but also presence of immunosuppressive cells (Tregs, MDSCs and, possibly, M2-polarized pro-tumoral macrophages); (d) when present, T cells upregulate IRs and appear to be functionally compromised.
The meaning of IR expression on T cells at tumor site in NSCLC
Upregulation of several IRs by T cells is conventionally interpreted as a marker of exhaustion, the functional impairment of T cells subjected to chronic antigen stimulation that develops in the context of some viral infections and in cancer [16]. T cell exhaustion at tumor site, associated with multiple IR expression of T cells, has been demonstrated in NSCLC. Thommen et al. [17] found that expression of PD-1 and of TIM-3 on CD8+ T cells at tumor site increases with tumor stage. Cumulative expression of several IRs (PD-1, Tim-3, CTLA-4, LAG-3, and BTLA) on tumor-associated T cells was significantly linked to advanced tumor stage and nodal-positive cancers. A significant but negative correlation was found between the “IR score” (an index of co-expression of multiple IRs by tumor-infiltrating T cells) and the ability of T cells, from the same tumor samples, to produce IL2, IFN-γ, TNFα, and to express CD25 and granzyme B upon polyclonal stimulation with anti-CD3 plus anti-CD28. T-cell function could be rescued by blocking the PD-1 receptor with the therapeutic antibody Nivolumab, but PD-1Hi T cells were less responsive to functional rescue compared to PD-1int T cells. Therefore, co-expression of multiple IR may be associated with functional impairment in T cells at tumor site in NSCLC, but the degree of T cell exhaustion is also related to the level of expression of PD-1.
The true meaning of multiple IR expressed on tumor-associated T cells is likely more complex and does not uniquely point to exhaustion. As shown by Wherry et al. [18] IRs as PD-1 are not only upregulated in a constitutive fashion in T cells subjected to chronic antigen stimulation but are also transiently upregulated even in recently activated T cells, early on after priming. In the instance of PD-1, this receptor is transiently upregulated in T cells within a few hours after activation, as shown by Lu et al. [19]. Therefore, as pointed out by Fuertes Marraco et al. [20], a tumor T-cell profile characterized by expression of multiple IRs, does not mean only functional impairment. By developing these concepts, we recently provided a new view of the adaptive immune profile in early NSCLC [5]. We focused on frequency and functional phenotype of T cells in early stage, primary ADCs and SCCs from 87 patients. Compared to nLung, we found an enhanced frequency in the tumor of activated (HLA-DR+) T cells with a TEM phenotype (CCR7−CD45RA−). This T-cell phenotype was a common feature across patients’ subsets defined by tumor stage, histotype, smoking status and neoadjuvant chemotherapy. Functional assays indicated that the tumor lesions contained a low frequency of functional tumor-reactive T cells producing IL2 and upregulating CD107a in response to autologous tumor, although IFN-γ production was observed only after pre-incubation of T cells with an anti-PD-L1 mAb. Analysis for IR expression indicated higher levels of PD-1, TIM-3, LAG-3, TIGIT on T cells from tumor compared to nLung. Interestingly, these IR+ T cells also expressed Ki-67 (i.e., they were proliferating cells) as well as CD137 and CD38 (all markers associated with recent activation) and were negative for the exhaustion-related transcription factor Eomes. Moreover, PD-1+ T cells from tumor site retained functional competence (IFN-γ and IL-2 production as well as CD107a upregulation upon polyclonal stimulation). These data suggested that IR upregulation by T cells at tumor site does not only uniquely signal for exhaustion, but can also mark a subset of recently activated, tumor-reactive T cells that retain functional competence. Accordingly, IR+ T cells at tumor site contained a low frequency of FOXP3+ CD8+ “early effector cells” (EEC), a subset that we previously identified in melanoma lesions [21]. These FOXP3+ EECs expressed all the markers of functional T cells at the earliest stage of differentiation after priming. In fact, they showed a KLRG1− CD127− profile. This enabled us to distinguish these cells from CD127+ KLRG1− naïve T lymphocytes as well as from more advanced short-lived effector cells (SLEC, CD127−KLRG1+) or memory precursors (MPEC, CD127+ KLRG1−). Moreover, the FOXP3+ EECs were also antigen experienced (CCR7−CD45RA−), expressed a profile consistent with recent activation (Ki-67+, t-bet+, CD38+, HLA-DR+) and expressed several IRs (CTLA-4+, PD-1+ TIM-3+ TIGIT+ LAG-3+), but not markers of senescence (CD57−). Finally, these CD8+ EECs produced IL-2 as well as IFN-γ in response to autologous tumor.
Taken together, a possible model emerging from these studies is that tumor lesions may contain a high frequency of multiple IR+ T cells, but these lymphocyte populations are in fact a mixture of functionally impaired, truly exhausted T cells as well as of recently activated, functionally competent T cells. The key question that remains to be answered is whether the relative proportion of IR+-functional T lymphocytes and of IR+-impaired T cells has a prognostic significance and/or a predictive value for response to immunotherapy in NSCLC.
The immune landscape in NSCLC is “molecular subtype” related
Several NSCLC subtypes, in addition to the standard ADC and SCC histological subsets, have been identified by mining of whole genome gene expression datasets. Each of these “expression subtypes” may be associated with a distinct immune contexture. In 2012 [22], the Cancer Genome Atlas Research Network identified four expression subtypes (primitive, classical, secretory, and basal) in SCCs. In 2014, three expression subtypes were identified in ADCs and were defined proximal inflammatory (PI), proximal proliferative (PP) and terminal respiratory unit (TRU), respectively [23]. These seven different expression subtypes are characterized by distinct immune contextures (Table 1). To this end, Faruki et al. [24] exploited the “immunome compendium” previously developed by Bindea et al. [25]. The “immunome compendium” is a carefully selected set of immune-related genes that enables the unambiguous identification of 24 immune cell subsets, including all main cellular mediators of innate and adaptive immunity. Interrogation of publicly available NSCLC datasets, containing gene expression data for 1190 patients, revealed that among ADCs, the PP subtype showed the lowest expression of most immune cells investigated (Table 1). Compared to PI tumors, the TRU subtype showed enhanced expression of DCs, CD56bright NK cells, mast cells and eosinophils, as well as of B cells, TFH cells, TCM cells, TH17 cells, and CD8 T cells [24]. In contrast, a higher expression of TH1 and TH2 cells, Treg cells, cytotoxic T cells, and NK CD56dim cells characterized the PI subtype (Table 1). Among SCCs, the lowest and the highest immune cell expressions, for both innate and adaptive immunity, were found in the classical and secretory subsets, respectively (Table 1). Two relevant immune checkpoints, CTLA-4 and PD-L1, also showed differential expression according to the expression subtype, with higher expression in the ADC PI subtype. A significant implication of this study is that expression subtypes of NSCLC are likely associated with different types of immune response and this may have an impact both on disease progression as well as on response to immunotherapy.
Table 1.
Relationship of TCGA-defined NSCLC molecular subtypes with adaptive and innate immune cell signatures of the lesions
| NSCLC histotype | NSCLC molecular subtype | Immune-related gene expression signatures | Reference |
|---|---|---|---|
| SCC | Primitive (P) | TH2 signature similar to S subtype | [24] |
| SCC | Classical (C) | Lowest immune cell-related signatures of all SCC subtypes | [24] |
| SCC | Secretory (S) | Higher immune expression for adaptive and innate immune cells compared to other SCC subtypes. TH2 signature comparable to P subtype | [24] |
| SCC | Basal (B) | Increased representation, compared to C subtype, of signatures related to T, TH1, TH2, TH17, Tregs, CD8, CD56dim NK cells, DC subsets, eosinophils, macrophages and neutrophils | [24] |
| ADC | Proximal inflammatory (PI) | Increased representation of signatures related to TH1, TH2 cells, Tregs, cytotoxic T cells, and CD56dim NK cells compared to TRU subtype | [24] |
| ADC | Proximal proliferative (PP) | Lower immune expression compared to PI and TRU subtype for most cells evaluated | [24] |
| ADC | Terminal respiratory unit (TRU) | Increased representation of signatures related to DCs, CD56bright NK cells, mast cells, eosinophils, B, TFH, TCM, TH17 and CD8 T cells, compared to PI subtype | [24] |
A further recent approach, developed by Chen et al. [26], has allowed to identify multiple NSCLC molecular subsets with distinctive immune-related gene expression signatures. These authors used multiplatform classification of NSCLC lesion (including DNA methylation and copy number, and RNA and protein expression) on 1023 NSCLC cases from the TCGA SCC and ADC studies and identified nine genomic subtypes (three within the SCC subset, named SQ.1, SQ.2a, SQ.2b and six within the AD subset, named AD.1, AD.2, AD.3, AD.4, AD.5a and AD.5b). These nine subtypes have complex relationships with the seven expression subtypes identified in the TCGA studies. In detail, the TCGA-defined basal/secretory, classical and primitive SCC subtypes had significant correspondence with SQ.1, SQ.2a/SQ.2b and SQ.2/AD.1 subtypes, respectively. The TCGA-defined PP, PI and TRU ADC subtypes had correspondence with AD.1, AD.2/AD.3 and AD.4/AD.5a/AD.5b subtypes, respectively. The nine subtypes identified by Chen et al. [26] show distinctive immune profiles (Table 2): for example, a high immune cell infiltrate was found in AD.2, AD.3 and AD.4 as well as in SQ.1 and SQ.2a. Immune checkpoint activation was higher in AD.2, AD.3 and AD.4, but AD.4 showed the lowest neoepitope count. Tumor-expressed PDL1, lymphocyte-expressed PD1, as well as CD3 and CD8 signals, were higher in AD.2, AD.3, AD.4 subtypes compared to AD.1, AD.5a, AD.5b (Table 2).
Table 2.
Relationships of multiplatform-defined NSCLC molecular subtypes with adaptive and innate immune cell signatures of the lesions
| NSCLC Histotype | NSCLC molecular subtype | Immune-related gene expression signatures | Reference |
|---|---|---|---|
| SCC | SQ.1 | Predicted high immune cell infiltrate. Increased expression of genes encoding cancer testis antigens | [26] |
| SCC | SQ.2a | Predicted high immune cell infiltrate. Increased expression of genes encoding cancer testis antigens | [26] |
| SCC | SQ.2b | Increased expression of genes encoding cancer testis antigens | [26] |
| ADC | AD.1 | Increased expression of genes encoding cancer testis antigens. Tumor PD-L1, lymphocyte PD-1, CD3 and CD8 expressed at lower levels compared to AD.2 / AD.3 / AD.4 subtypes | [26] |
| ADC | AD.2 | Predicted high immune cell infiltrate. Higher expression, compared to other subtypes, of genes encoding PD-1, CD3, PD-L1, PD-L2, CTLA-4, CD137, CD134 and TLR9 | [26] |
| ADC | AD.3 | Predicted high immune cell infiltrate. Higher expression, compared to other subtypes, of genes encoding PD-1, CD3, PD-L1, PD-L2, CTLA-4, CD137, CD134 and TLR9. Increased expression of genes encoding cancer testis antigens | [26] |
| ADC | AD.4 | Predicted high immune cell infiltrate. Higher expression, compared to other subtypes, of genes encoding PD-1, CD3, PD-L1, PD-L2, CTLA-4, CD137, CD134 and TLR9 | [26] |
| ADC | AD.5a | Lower PD-1 expression compared to AD.2, AD.3, AD.4. Tumor PD-L1, lymphocyte PD-1, CD3 and CD8 expressed at lower levels compared to AD.2 / AD.3 / AD.4 subtypes | [26] |
| ADC | AD.5b | Lower PD-1 expression compared to AD.2, AD.3, AD.4. Tumor PD-L1, lymphocyte PD-1, CD3 and CD8 expressed at lower levels compared to AD.2 / AD.3 / AD.4 subtypes | [26] |
Main NSCLC oncogenic drivers and genome-wide genetic alterations shape the tumor immune profile
Specific genetic alterations present in the tumor shape the immune profile of NSCLC lesions. Pre-clinical mouse models of lung ADC showed that tumors driven by KRAS, P53 or EGFR have distinct immune contextures [27]. EGFR-mutant tumors display robust myeloid recruitment, but defective CD8+ T cells, while KRAS-mutant tumors show expansion of several immune subsets including CD8+ T cells and Tregs (Table 3). In the human setting, the immune profile appears to be shaped by co-occurring genetic events, and not only by presence of a single major oncogenic driver. This was documented by analysis of KRAS+ adenocarcinoma lesions from NSCLC patients, where further subsets based on alterations of p53, LKB11/STK1 or CDKN2A/B may be distinguished [28]. Tumors with concurrent KRAS and p53 alterations (“KP” tumors) show enhanced T cell infiltration associated with upregulation of immune checkpoint molecules and increased mutational load [28]. In contrast, KRAS+ LKB11-deficient tumors (“KL” tumors) show reduced T cell infiltration and reduced PD-L1 expression. KRAS+ CDKN2A/B deficient tumors (“KC” tumors) show a mixed pattern of immune involvement (Table 3). Deletion of LKB11 in a mouse model of KRAS-driven NSCLC has been shown to significantly alter the immune landscape of the lesions [29]. Compared to KRAS-mutant, but LKB11-proficient controls (Table 3), the LKB11-deleted tumors showed strong chemokine-dependent recruitment of suppressive neutrophils, reduction of frequency of infiltrating T cells (but with enhanced expression of IRs), higher Treg/Teff ratio, reduced expression of IFN-γ and of Ki-67 in the T cells and reduced tumor PD-L1 expression (independent of IFN-γ). Interestingly, in the same study the authors found that LKB11-deficient human tumors showed a reduced CD8+ T cell infiltration and interrogation of the ATLAS NSCLC dataset revealed that human LKB11-mutant tumors express less PD-L1.
Table 3.
Impact of oncogenic drivers and of genome-wide genetic alterations on the tumor immune profile
| Setting | Tumor histotype | Type of genetic alteration | Associated immune-related features of the tumors | References |
|---|---|---|---|---|
| Mouse model | ADC | EGFRL858R | Strong macrophage recruitment, defective CD8+ immune response | [27] |
| Mouse model | ADC | KRASG12D | Strong macrophage recruitment, NK cell decrease over time, B and T cell increase, expansion of CD8+ cells, Tregs, IL-17A–producing lymphocytes and myeloid cells | [27] |
| Mouse model | ADC | KRASG12D; p53fl/fl | Strong macrophage recruitment, B cell, T cell, Tregs, CD8+ cell increase | [27] |
| Mouse model | ADC,SCC,LCC | KRASG12D; LKB1−/− | Promotion of neutrophil recruitment, reduced T cell infiltration, high Treg/Teff ratio, reduced tumor PD-L1 expression | [29] |
| Human | ADC | Concurrent KRAS and LKB11 alteration (“KL”) | Defective immune system engagement compared to “KP” and “KC” tumors | [28] |
| Human | ADC | Concurrent KRAS and CDKN2a alteration (“KC”) | Mixed profile for immune system engagement compared to “KL” and “KP” tumors | [28] |
| Human | ADC | Concurrent KRAS and p53 alteration (“KP”) | Enhanced T cell infiltration associated with upregulation of immune checkpoint molecules (PD-1, PD-L1, CTLA-4) compared to “KL” and “KC” subtypes | [28] |
| Human | ADC | ALK rearrangement | Higher tumor PD-L1 expression, associated with intratumoral PD-1+ CD8+ cells, compared to EGFR-mutant or to wt ADC | [31] |
| Human | ADC,SCC | High arm- or chromosome-level aneuploidy (SCNA) |
Significant decrease in the adaptive immunity-related gene signatures Decrease in the ratio between the mRNA levels of CD8+ T cell–specific genes vs. Treg-specific genes and of M1 vs. M2 macrophage-specific genes |
[33] |
The specific genetic make-up of the NSCLC lesions impacts even on the expression of PD-L1 on the neoplastic cells. Major NSCLC oncogenic drivers as EGFR and KRAS are known for activating the AKT-mTOR pathway and the latter signaling cascade has been shown to promote PD-L1 expression in neoplastic cells [30]. In contrast, in the subset of ALK-rearranged ADC, Roussel et al. [31] found that tumor PD-L1 expression correlated with tumor infiltrating CD8+ cells (Table 3). ALK-positive tumors also showed a more frequent occurrence of PD-L1 positivity associated with infiltrating PD-1+ T cells, compared to EGFR-mutated or WT cancers. Collectively, these studies indicate that in some NSCLC subsets (depending on the specific genetic make-up of the tumor) PD-L1 expression may be an “oncogene-induced”, tumor cell-autonomous process. In other instances PD-L1 expression may reflect the well-known adaptive resistance mechanism secondary to IFN-γ production by the adaptive T cell-mediated immune response [32].
The type of tumor aneuploidy (also known as SCNA, somatic copy number alterations) contributes to shaping of the immune landscape of human cancers, including NSCLC. Davoli et al. [33] evaluated three standardized SCNA level scores (chromosome SCNA level, arm SCNA level, and focal SCNA level) and found that immune evasion markers mainly correlated with chromosome- and arm-level SCNA. Tumors with high aneuploidy showed decreased expression of several classes of immune related genes including those encoding the TCR complex, the B cell receptor, those related to cytotoxic activity of CD8+ cells and to the IFN-γ pathway, as well as several cytokine and chemokine genes (Table 3). High aneuploidy tumors showed reduced Tcytotox/Treg and M1/M2 ratios, indicating that a high SCNA level shapes an immunosuppressive microenvironment.
The epithelial mesenchymal transition impacts on the immune contexture of the NSCLC lesions
The epithelial mesenchymal transition (EMT) is a main biological process involved in the acquisition of invasive and metastatic ability by neoplastic cells. The EMT phenotype (low E-cadherin, high N-cadherin, high integrin αβvβ6 and activation of transcription factors as ZEB1) is frequent in both SCC and ADC [34]. Interestingly, in ADC, an EMT profile has been associated with a specific immune contexture independent of tumor mutational burden [35]. Tumors with a “mesenchymal” profile, compared to those with an “epithelial” phenotype, showed elevated expression of several immune checkpoint molecules, chemokines and enhanced infiltration by regulatory T cells. The EMT process may even contribute to shaping the immune response in NSCLC lesions by compromising the antigen presentation pathway. Tripathi et al. [36] found that NSCLC lesions with defective expression of immunoproteasome subunits PSMB8 and PSMB9, showed a mesenchymal phenotype. The immunoproteasome plays a key role in generation of suitable peptides for binding to HLA class I antigens, therefore immunoproteasome-deficient mesenchymal tumors may escape recognition by CD8+ CTLs, due to defective tumor antigen expression.
Prognostic significance of specific immune cell subsets in NSCLC
Development of adaptive immunity, documented by presence of tumor infiltrating lymphocytes (TIL), has a protective effect against tumor progression across different histologies. Specifically, TIL are associated with a positive clinical outcome in different solid tumors including melanoma, head and neck, breast, bladder, ovarian, colorectal, renal, prostatic and lung cancer [37]. In these tumors, the immune cells with the most consistent positive prognostic impact are the T cells, mainly those with a cytotoxic, or memory, or TH1 phenotypic profile [38]. In NSCLC, in a large study in 797 stage I–III patients [39], the immunoscore, as defined by density of stromal CD8+ T cells, has been shown to be an independent prognostic factor, regardless of endpoint (DFS and OS), and to add prognostic impact in each pathological stage. For example, in Stage IA, 5-year OS rate is 59 and 79%, respectively, in patients with “low” vs “high” immunoscore, but in stage IIIA, 5-year OS increases from 17 to 55%, respectively, in subsets with “low” vs “high” immunoscore. Further evidence points to distinct prognostic roles of TILs depending on the NSCLC histological subset. In a study in 320 stage IIIA (N2) patients [40], TILs where shown to exert a positive prognostic role mainly in patients with SCC and TIL+ SCC patients showed a significant increase in 3-year distant metastasis-free survival and overall survival compared to TIL− patients. In a different study [41] the combination of low CD4/CD8/CD68-positive cell density and high PD-L1 score on tumor cells identified patients with the worse clinical outcome, but only in the ADC subtype. In addition to T cells, broadly classified as CD4+ or CD8+, even specific T cell subsets, defined by distinct activation/differentiation markers, may have prognostic significance in NSCLC. Djenidi et al. [42] found that a subset of CD8+ CD103+ T cells, representing a type of tissue-resident memory cells, correlated with improved survival in early stage NSCLC patients and with increased lymphocyte infiltration of the neoplastic tissue.
Additional immune cell subsets, including B cells, NK cells, and mature dendritic cells may exert a positive prognostic role in NSCLC. Tumor associated macrophages have been reported to have contrasting prognostic relevance in different studies, often with relationship to the type of markers used to identify them (such as CD14, CD68, CD163, CD204, see ref. [43] for review). A high Foxp3+ Tregs infiltration is associated with poor OS in NSCLC as documented in a meta-analysis on 11 studies involving 1303 patients [44]. The prognostic significance of the distinct immune subsets is often related not only to their density in the lesion, but also to their specific spatial location, for example in the stroma, rather than in the tumor tissue. In a recent meta-analysis on the prognostic role of macrophages in NSCLC by Mei et al. [45] the pooled hazard ratio (HR) of four studies indicated a better overall survival (OS) associated with high density of CD68+ TAMs in the tumor, while the pooled HR of six studies indicated poor OS associated with high density of CD68+ TAMs in the tumor stroma. In addition, high density of M1 TAMs in the tumor was associated with better survival, while a high density of M2 TAMs in the tumor stroma was associated with poor OS. The role of immune cell location, in addition to density, in shaping the prognostic role of distinct subsets, has been confirmed even for neutrophils. Rakaee et al. [46] in 563 NSCLC patients found that neutrophils had contrasting prognostic significance in different histological subsets. A high intratumoral density of CD66+ neutrophils was an independent positive predictor of disease-specific survival in SCC, but a negative prognostic factor in ADC. An increasingly significant prognostic role in NSCLC is emerging for the TLS and the associated B cells and dendritic cells. Germain et al. [47] found that B cells in the TLS associated with NSCLC lesions exhibit features of a developing immune response, as they show all stages of B cell differentiation associated with activation of somatic hypermutation and class switch recombination. Interestingly, a high density of follicular B cells was associated with improved survival in both early and advanced stage patients. Goc et al. [48] in 458 NSCLC lesions found that a high density of mature DC (DC-lamp+) in the TLS correlated with infiltration of the lesions by T cells at the TEM stage and with expression of immune-related genes indicating T-cell activation, TH1 phenotype and cytotoxic differentiation. A high density of TLS-associated DCs was also associated with improved survival.
The potential predictive role of the NSCLC immune landscape in the context of immunotherapy-targeting immune checkpoints
The most significant associations with clinical benefit, after immunotherapy targeting the PD-1/PD-L1 axis, have been found for a high-tumor mutational load/neoantigen burden [49], a low level of intra-tumor heterogeneity for neoantigens [50] and a low level of chromosome- or arm-level aneuploidy [33]. The analysis on the predictive role of different immune cells in the pre-therapy lesions has provided strong evidence for CD8+ T cells, when present at the invasive tumor margin, in melanoma [51], while studies in NSCLC have not provided concordant results. In an early study involving patients with different tumor types, including NSCLC, Taube et al. [52] found that PD-L1 expression (on tumor or on immune cells) in the pre-therapy lesions was significantly associated with presence of infiltrating lymphocytes. Moreover, PD-1 expression by TIL was significantly associated with PD-L1 expression by tumor cells and by immune cells. However, the immune infiltrate and the PD-1+ TIL failed to show a significant association with objective responses or with clinical benefit after anti-PD-1 therapy. More recent studies have begun to shed a different light on the potential predictive role of immune subsets, mainly T cells, as evaluated by different methods in pre-therapy lesions. By exploiting immune-related signatures, Prat et al. [53] found that PD-1 gene expression and 12 signatures related to CD8 and CD4 T-cell activation, NK cells and Interferon activation were significantly associated with non progressive disease and with progression-free survival after anti-PD-1 therapy in patients with different tumor types, including NSCLC. In the study on the relevance of clonal architecture of neoantigens [50], McGranahan et al. found that NSCLC tumors (ADC subtype) with a high, but clonal neoantigen burden, were characterized by significantly higher expression of CD8A and CD8B genes, as well as of genes associated with antigen presentation, T-cell migration and effector T-cell function. In these tumors, neoantigen-specific T cells were isolated and were shown to express both PD-1 and LAG-3 IRs. In the same study, clinical benefit from targeting of PD-1 was shown to be associated with the presence of a high but homogeneous neoantigen load. Taken together, these results suggest that the response to immunotherapy is likely dependent on re-activation of neo-antigen-specific CD8+ T cells present in the pre-therapy lesions (in agreement with the findings in melanoma). However, such tumor-reactive T cells are mainly present in NSCLC lesions characterized by a high but clonal neoantigen load. Additional recent studies indicate that factors associated with response to immunotherapy can be discovered by looking not only at the tumor tissue, but even at markers of immunity evaluated in peripheral blood. Huang et al. [54] in melanoma patients, found that clinical response to anti-PD-1 treatment correlates with exhausted T-cell (Tex) reinvigoration to tumor burden ratio. In this study, T cell reinvigoration was measured as increase in expression of markers as Ki67, CD38 and HLA-DR by PD-1+ Tex cells during immunotherapy. Interestingly, Kamphorst et al. [55] found that 80% of NSCLC patients responding to PD-1-targeted immunotherapy had an increase in circulating PD-1+ CD8+ T cells expressing Ki-67, CD38 and HLA-DR. This change was not detected in 70% of non-responding patients. The relevance of specific surface markers of circulating T cells, as immune correlates of response to immunotherapy, has been corroborated by additional findings in NSCLC patients. Kamphorst et al. [56] recently found that the circulating PD-1+ T cells, reinvigorated by PD-1 therapy (= expressing Ki67, HLA-DR and CD38) were CD28+. These results underscore the relevance of CD28 expression, as a main target of the PD-1 signaling pathway in T cells, for the efficacy of immune checkpoint blockade.
Conclusions
The in-depth analysis of the immune landscape of NSCLC, carried out over the past 5 years by a variety of emerging investigational approaches, has provided evidence for an extremely high degree of complexity and heterogeneity. The immune profile of the lesions shows specific features depending on tumor stage, histological subset and molecular subtypes. This complexity appears to reflect the action of some of the most powerful biological and molecular processes that control tumor biology as a whole. The epithelial mesenchymal transition, the presence of main oncogenic drivers, the level and extent of SCNA, the nonsynonymous mutational load, as well as clonal heterogeneity and tumor molecular evolution, are all involved in shaping the NSCLC immune contexture and in determining its complexity and heterogeneity. Facing this heterogeneity is at the same time an urgent need and a way forward for improving NSCLC treatment. However, we must be aware that the goal of disentangling the immune landscape complexity in NSCLC, with the aim of identifying predictive factors of response to immunotherapy, is a strenuous effort that will require an integrated approach based on extensive molecular, genetic and immunological characterization of tumor lesions from large sets of patients enrolled in immunotherapy trials.
Acknowledgements
The authors gratefully acknowledge the excellent technical contribution of Mrs. Claudia Vegetti, Alessandra Molla, Ilaria Bersani and Paola Baldassari to the work mentioned in this paper.
Abbreviations
- ADC
Adenocarcinoma
- DFS
Disease-free survival
- EEC
Early effector cell
- eMDSC
Early MDSC
- EMT
Epithelial mesenchymal transition
- GC
Germinal center
- ICB
Immune checkpoint blockade
- IR
Inhibitory receptor
- MDSC
Myeloid-derived suppressor cell
- M-MDSC
Monocytic MDSC
- nLung
Non-neoplastic lung tissue
- NSCLC
Non-small cell lung cancer
- OS
Overall survival
- PFS
Progression-free survival
- PI
Proximal inflammatory
- PMN-MDSC
Polymorphonuclear MDSC
- PP
Proximal proliferative
- SCC
Squamous cell carcinoma
- SCNA
Somatic copy number alteration
- TCR
T cell receptor
- TEM
T effector memory
- TEMRA
T effector memory RA
- Tex
Exhausted T cell
- TH1
Type 1 T Helper cell
- TH2
Type 2 T Helper cell
- TH17
T Helper 17 cell
- Ti-BALT
Tumor-induced bronchus-associated lymphoid tissues
- TIL
Tumor-infiltrating lymphocyte
- TLS
Tertiary lymphoid structure
- Treg
Regulatory T cell
- TRU
Terminal respiratory unit
- t-SNE
t-distributed stochastic neighbor embedding
Author contributions
AA designed the structure of the review and took the lead in writing the paper. ET and GG contributed to select and review the mentioned literature and to the final revision of the text. RM contributed to design and writing of the paper and to selecting and reviewing all of the mentioned literature.
Funding
The work mentioned in this paper was supported by Grant #17431 from Associazione Italiana per la Ricerca sul Cancro (A. I. R. C.) to Andrea Anichini. Elena Tassi was supported by a fellowship from Fondazione Beretta-Berlucchi. Giulia Grazia was supported by a fellowship from Fondazione Italiana per la Ricerca sul Cancro (FIRC).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
This paper is a Focussed Research Review based on a presentation given at the Fourteenth Meeting of the Network Italiano per la Bioterapia dei Tumori (NIBIT) on Cancer Bio-Immunotherapy, held in Siena, Italy, 13th–15th October 2016. It is part of a series of Focussed Research Reviews and meeting report in Cancer Immunology, Immunotherapy.
References
- 1.Malhotra J, Jabbour SK, Aisner J. Current state of immunotherapy for non-small cell lung cancer. Transl Lung Cancer Res. 2017;6:196–211. doi: 10.21037/tlcr.2017.03.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim BJ, Kim JH, Kim HS. Survival benefit of immune checkpoint inhibitors according to the histology in non-small-cell lung cancer: a meta-analysis and review. Oncotarget. 2017;8:51779–51785. doi: 10.18632/oncotarget.17213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tibaldi C, Lunghi A, Baldini E. Use of programmed cell death protein ligand 1 assay to predict the outcomes of non-small cell lung cancer patients treated with immune checkpoint inhibitors. World J Clin Oncol. 2017;8:320–328. doi: 10.5306/wjco.v8.i4.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei SC, Levine JH, Cogdill AP, et al. Distinct cellular mechanisms underlie anti-CTLA-4 and anti-PD-1 checkpoint blockade. Cell. 2017;170:1120–1133. doi: 10.1016/j.cell.2017.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tassi E, Grazia G, Vegetti C, et al. Early effector T lymphocytes coexpress multiple inhibitory receptors in primary non-small cell lung cancer. Cancer Res. 2017;77:851–861. doi: 10.1158/0008-5472.CAN-16-1387. [DOI] [PubMed] [Google Scholar]
- 6.Kargl J, Busch SE, Yang GH, et al. Neutrophils dominate the immune cell composition in non-small cell lung cancer. Nat Commun. 2017;8:14381. doi: 10.1038/ncomms14381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Del Mar Valenzuela-Membrives M, Perea-García F, Sanchez-Palencia A, et al. Progressive changes in composition of lymphocytes in lung tissues from patients with non-small-cell lung cancer. Oncotarget. 2016;7:71608–71619. doi: 10.18632/oncotarget.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lizotte PH, Ivanova EV, Awad MM, et al. Multiparametric profiling of non-small-cell lung cancers reveals distinct immunophenotypes. JCI Insight. 2016;1:e89014. doi: 10.1172/jci.insight.89014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Der Maaten L. Accelerating t-SNE using Tree-based Algorithms. J Mach Learn Res. 2014;15:3221–3245. [Google Scholar]
- 10.Ayers M, Lunceford J, Nebozhyn M, et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest. 2017;127:2930–2940. doi: 10.1172/JCI91190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karasaki T, Nagayama K, Kuwano H, et al. An immunogram for the cancer-immunity cycle: towards personalized immunotherapy of lung cancer. J Thorac Oncol. 2017;12:791–803. doi: 10.1016/j.jtho.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Lavin Y, Kobayashi S, Leader A, et al. Innate immune landscape in early lung adenocarcinoma by paired single-cell analyses. Cell. 2017;169:750–765. doi: 10.1016/j.cell.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dieu-Nosjean MC, Giraldo NA, Kaplon H et al (2016) Tertiary lymphoid structures, drivers of the anti-tumor responses in human cancers. Immunol Rev 271:260 – 75. 10.1111/imr.12405 [DOI] [PubMed]
- 14.Solinas C, Garaud S, De Silva P, et al. Immune checkpoint molecules on tumor-infiltrating lymphocytes and their association with tertiary lymphoid structures in human breast cancer. Front Immunol. 2017;8:1412. doi: 10.3389/fimmu.2017.01412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts EW, Broz ML, Binnewies M, et al. Critical role for CD103(+)/CD141(+) dendritic cells bearing CCR7 for tumor antigen trafficking and priming of T cell immunity in melanoma. Cancer Cell. 2016;30:324–336. doi: 10.1016/j.ccell.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15:486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thommen DS, Schreiner J, Müller P, et al. Progression of lung cancer is associated with increased dysfunction of T cells defined by coexpression of multiple inhibitory receptors. Cancer Immunol Res. 2015;3:1344–1355. doi: 10.1158/2326-6066.CIR-15-0097. [DOI] [PubMed] [Google Scholar]
- 18.Wherry EJ, Ha SJ, Kaech SM, et al. Molecular signature of CD8 + T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Lu P, Youngblood BA, Austin JW et al (2014) Blimp-1 represses CD8 T cell expression of PD-1 using a feed-forward transcriptional circuit during acute viral infection. J Exp Med 211:515 – 27. 10.1084/jem.20130208 [DOI] [PMC free article] [PubMed]
- 20.Fuertes Marraco SA, Neubert NJ, Verdeil G, et al. Inhibitory receptors beyond T cell exhaustion. Front Immunol. 2015;6:310. doi: 10.3389/fimmu.2015.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anichini A, Molla A, Vegetti C, et al. Tumor-reactive CD8 + early effector T cells identified at tumor site in primary and metastatic melanoma. Cancer Res. 2010;70:8378–8387. doi: 10.1158/0008-5472.CAN-10-2028. [DOI] [PubMed] [Google Scholar]
- 22.Cancer Genome Atlas Research Network Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489:519–525. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cancer Genome Atlas Research Network Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511:543–550. doi: 10.1038/nature13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faruki H, Mayhew GM, Serody JS, et al. Lung adenocarcinoma and squamous cell carcinoma gene expression subtypes demonstrate significant differences in tumor immune landscape. J Thorac Oncol. 2017;12:943–953. doi: 10.1016/j.jtho.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bindea G, Mlecnik B, Tosolini M, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39:782–795. doi: 10.1016/j.immuni.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Chen F, Zhang Y, Parra E, et al. Multiplatform-based molecular subtypes of non-small-cell lung cancer. Oncogene. 2017;36:1384–1393. doi: 10.1038/onc.2016.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Busch SE, Hanke ML, Kargl J, et al. Lung cancer subtypes generate unique immune responses. J Immunol. 2016;197:4493–4503. doi: 10.4049/jimmunol.1600576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skoulidis F, Byers LA, Diao L, et al. Co-occurring genomic alterations define major subsets of KRAS-mutant lung adenocarcinoma with distinct biology, immune profiles, and therapeutic vulnerabilities. Cancer Discov. 2015;5:860–877. doi: 10.1158/2159-8290.CD-14-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koyama S, Akbay EA, Li YY, et al. STK11/LKB1 Deficiency promotes neutrophil recruitment and proinflammatory cytokine production to suppress T-cell activity in the lung tumor microenvironment. Cancer Res. 2016;76:999–1008. doi: 10.1158/0008-5472.CAN-15-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lastwika KJ, Wilson W, 3rd, Li QK, et al. Control of PD-L1 expression by oncogenic activation of the AKT-mTOR pathway in non-small cell lung cancer. Cancer Res. 2016;76:227–738. doi: 10.1158/0008-5472.CAN-14-3362. [DOI] [PubMed] [Google Scholar]
- 31.Roussel H, De Guillebon E, Biard L, et al. Composite biomarkers defined by multiparametric immunofluorescence analysis identify ALK-positive adenocarcinoma as a potential target for immunotherapy. Oncoimmunology. 2017;6:e1286437. doi: 10.1080/2162402X.2017.1286437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ribas A. Adaptive immune resistance: how cancer protects from immune attack. Cancer Discov. 2015;5:915–919. doi: 10.1158/2159-8290.CD-15-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davoli T, Uno H, Wooten EC et al (2017) Tumor aneuploidy correlates with markers of immune evasion and with reduced response to immunotherapy. Science 355(6322). pii: eaaf8399. 10.1126/science.aaf8399. [DOI] [PMC free article] [PubMed]
- 34.Prudkin L, Liu DD, Ozburn NC, et al. Epithelial-to-mesenchymal transition in the development and progression of adenocarcinoma and squamous cell carcinoma of the lung. Mod Pathol. 2009;22:668–678. doi: 10.1038/modpathol.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lou Y, Diao L, Cuentas ER, et al. Epithelial–mesenchymal transition is associated with a distinct tumor microenvironment including elevation of inflammatory signals and multiple immune checkpoints in lung adenocarcinoma. Clin Cancer Res. 2016;22:3630–3642. doi: 10.1158/1078-0432.CCR-15-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tripathi SC, Peters HL, Taguchi A, et al. Immunoproteasome deficiency is a feature of non-small cell lung cancer with a mesenchymal phenotype and is associated with a poor outcome. Proc Natl Acad Sci USA. 2016;113:E1555-64. doi: 10.1073/pnas.1521812113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fridman WH, Pages F, Sautes-Fridman C, et al. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 38.Bremnes RM, Busund LT, Kilvær TL, et al. The role of tumor-infiltrating lymphocytes in development, progression, and prognosis of non-small cell lung cancer. J Thorac Oncol. 2016;11:789–800. doi: 10.1016/j.jtho.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 39.Donnem T, Hald SM, Paulsen EE, et al. Stromal CD8 + T-cell density—a promising supplement to TNM staging in non-small cell lung cancer. Clin Cancer Res. 2015;21:2635–2643. doi: 10.1158/1078-0432.CCR-14-1905. [DOI] [PubMed] [Google Scholar]
- 40.Feng W, Li Y, Shen L, et al. Prognostic value of tumor-infiltrating lymphocytes for patients with completely resected stage IIIA(N2) non-small cell lung cancer. Oncotarget. 2016;7:7227–7240. doi: 10.18632/oncotarget.6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parra ER, Behrens C, Rodriguez-Canales J, et al. Image analysis-based assessment of PD-L1 and tumor-associated immune cells density supports distinct intratumoral microenvironment groups in non-small cell lung carcinoma patients. Clin Cancer Res. 2016;22:6278–6289. doi: 10.1158/1078-0432.CCR-15-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Djenidi F, Adam J, Goubar A, et al. CD8+ CD103+ tumor-infiltrating lymphocytes are tumor-specific tissue-resident memory T cells and a prognostic factor for survival in lung cancer patients. J Immunol. 2015;194:3475–3486. doi: 10.4049/jimmunol.1402711. [DOI] [PubMed] [Google Scholar]
- 43.Remark R, Becker C, Gomez JE, et al. The non-small cell lung cancer immune contexture. A major determinant of tumor characteristics and patient outcome. Am J Respir Crit Care Med. 2015;191:377–390. doi: 10.1164/rccm.201409-1671PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao S, Jiang T, Zhang L, et al. Clinicopathological and prognostic significance of regulatory T cells in patients with non-small cell lung cancer: a systematic review with meta-analysis. Oncotarget. 2016;7:36065–36073. doi: 10.18632/oncotarget.9130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mei J, Xiao Z, Guo C, et al. Prognostic impact of tumor-associated macrophage infiltration in non-small cell lung cancer: a systemic review and meta-analysis. Oncotarget. 2016;7:34217–34228. doi: 10.18632/oncotarget.9079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rakaee M, Busund LT, Paulsen EE, et al. Prognostic effect of intratumoral neutrophils across histological subtypes of non-small cell lung cancer. Oncotarget. 2016;7:72184–72196. doi: 10.18632/oncotarget.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Germain C, Gnjatic S, Tamzalit F, et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am J Respir Crit Care Med. 2014;189:832–844. doi: 10.1164/rccm.201309-1611OC. [DOI] [PubMed] [Google Scholar]
- 48.Goc J, Germain C, Vo-Bourgais TK, et al. Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. Cancer Res. 2014;74:705–715. doi: 10.1158/0008-5472.CAN-13-1342. [DOI] [PubMed] [Google Scholar]
- 49.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McGranahan N, Furness AJ, Rosenthal R, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351:1463–1469. doi: 10.1126/science.aaf1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20:5064–5074. doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prat A, Navarro A, Paré L, et al. Immune-related gene expression profiling after PD-1 blockade in Non-Small Cell Lung Carcinoma, head and neck squamous cell carcinoma, and melanoma. Cancer Res. 2017;77:3540–3550. doi: 10.1158/0008-5472.CAN-16-3556. [DOI] [PubMed] [Google Scholar]
- 54.Huang AC, Postow MA, Orlowski RJ, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature. 2017;545:60–65. doi: 10.1038/nature22079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kamphorst AO, Pillai RN, Yang S, et al. Proliferation of PD-1 + CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci USA. 2017;114:4993–4998. doi: 10.1073/pnas.1705327114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kamphorst AO, Wieland A, Nasti T, et al. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science. 2017;355:1423–1427. doi: 10.1126/science.aaf0683. [DOI] [PMC free article] [PubMed] [Google Scholar]


