Abstract
Myeloid-derived suppressor cells (MDSCs) have been shown to contribute to tumor escape from host immune surveillance and to cancer progression by production of tumor-promoting soluble factors. Granulocyte colony-stimulating factor (G-CSF) is a principle cytokine controlling granulocyte number. Recombinant human G-CSF (rhG-CSF) has become the main therapeutic agent for the treatment of neutropenia and prophylaxis of febrile neutropenia in cancer patients. However, we show here that rhG-CSF triggers accumulation of granulocytic and monocytic subsets. Consequently, we discuss the pharmacological use of granulopoiesis stimulating factors not only in the context of febrile neutropenia but also from the perspective of MDSC-dependent and MDSC-independent mechanisms of immunosuppression and cancer angiogenesis.
Electronic supplementary material
The online version of this article (10.1007/s00262-018-2166-4) contains supplementary material, which is available to authorized users.
Keywords: Myeloid-derived suppressor cells, Granulocyte colony-stimulating factor, Cancer, Prophylaxis of febrile neutropenia, CITIM 2017
Introduction
Myeloid-derived suppressor cells (MDSCs) are myeloid cells with the capacity to inhibit immune reactions through multiple mechanisms. Under some pathological conditions, MDSC subsets represent an inhibitory component of immune response that upon sensing cytokines produced by inflamed tissue, namely GM-CSF, G-CSF, VEGF, and/or IL-6, are recruited to the inflammation site to suppress T cells which in normal physiologic immune responses reduces the risk of collateral tissue damage or a potentially lethal cytokine-induced shock. Within the tumor microenvironment, MDSC-inducing cytokines are produced by tumor cells and tumor stroma cells.
Although the existence of MDSCs was definitively proven by immunologists only in the last two decades, their activity was reported in 1946 as an ‘endotoxin tolerance’ leading to abolition of the fever response of rabbits undergoing repeated daily injection of the same dose of typhoid vaccine [1]. As of now, several MDSC subtypes have been identified that share their myeloid characteristics, immunosuppressive function and their origin from STAT3-regulated pathologic myeloid cell activation [2–4].
In recent years, MDSCs were studied in the context of tumor growth, where they contribute to tumor escape from the host immune surveillance and to tumor progression by the production of several tumor-promoting soluble factors [5]. Associations between increased circulating MDSC numbers and worse prognosis have been reported across various cancer types (reviewed in [6]). With expanding knowledge on the contribution of MDSCs to immune evasion and subsequent cancer progression, targeting these cells is currently being considered as an anti-cancer therapeutic approach [6]. In animal models and in vitro studies, several agents have been shown to inhibit the levels and/or suppressive action of MDSCs, including pyrimidine analogues such as gemcitabine and 5-FU, differentiation agents such ATRA and vitamin D3, taxanes, signaling kinase inhibitors such as vemurafenib, sunitinib, and COX-2/PGE2 inhibitors such as celecoxib and aspirin (summarized and referenced in [7]). On the other hand, therapeutic management of carcinoma patients includes pharmacologically enforced myelopoiesis that results in MDSC accumulation. Here, we focus on clinically relevant issues pertaining of granulocytic and monocytic MDSC subsets in the context of pharmacologically administered G-CSF.
MDSC and cancer: a bidirectional connection
MDSCs: subsets, phenotype and origin
In humans, MDSCs are defined as myeloid cells with immunosuppressive capacity. MDSCs are heterogeneous populations that can be subdivided into three main groups: (1) monocytic MDSCs (M-MDSCs), that are phenotypically and morphologically comparable to monocytes; (2) granulocytic polymorphonuclear MDSCs (PMN-MDSCs) comparable to low-density neutrophils; and (3) early MDSCs (e-MDSCs) comprising various immature progenitors capable of immunosuppression [8, 9]. M-MDSCs and PMN-MDSCs originate from common myeloid progenitors and are driven in a two-signal process by myeloid growth factors that control normal myelopoiesis, such as GM-CSF, G-CSF, and M-CSF representing ‘signal 1’ [10, 11] and persistent and/or low levels of inflammatory cytokines and danger-associated molecular patterns (DAMPs) representing ‘signal 2’ that governs abnormal myelopoiesis and immunosuppressive MDSC activation [2].
MDSCs represent a heterogeneous group of cells and the immunophenotypic characterization of human MDSC subsets has proven difficult and is still debated. Therefore, published data on human “MDSCs” describe various myeloid cell subsets and must be interpreted and generalized with reference to the particular method of MDSC identification.
The least enigmatic MDSC cell subsets are M-MDSCs that can be detected in both whole blood and gradient-separated PBMCs as CD11b+ CD14+ HLA-DR−/lo CD15− cells [9]. In principle, M-MDSCs overlap with HLA-DR− monocytes that have been and still are recognized as part of compensatory anti-inflammatory response syndrome (CARS) by the intensive care community. In this context, the term ‘immunodepression’ and its most severe form ‘immunoparalysis’ are used for reduced expression of HLA-DR on monocytes [12]. Despite the traditional view of MDSCs as immature cells, CD14+ HLA-DRlo/− M-MDSCs have mature morphology and immunophenotype and likely originate from an alternative pathway of myeloid differentiation or reprogramming of monocytes. The latter mode of M-MDSC accumulation predominates in pro-inflammatory states and reprogrammed anti-inflammatory CARS monocytes are in principle (by function and immunophenotype) M-MDSCs [12, 13]. M-MDSCs can be generated from mature monocytes upon low-strength TLR4 activation (Fig. 1b). The accumulation of M-MDSCs that results in endotoxin tolerance does not represent just a simple loss of MHC class II (MHC-II) molecules to discontinue antigen presentation in the inflammatory state; instead, monocyte reprogramming to M-MDSC/CARS monocytes is a complex process resulting in type 2 monocyte (M2) polarization accompanied by immunosuppressive/tolerogenic cytokine and chemokine production and pro-angiogenic properties including VEGF production [13, 14]. Accordingly, M-MDSCs have been shown to differentiate into tumor-associated M2 macrophages once they migrate to tumor tissue [15].
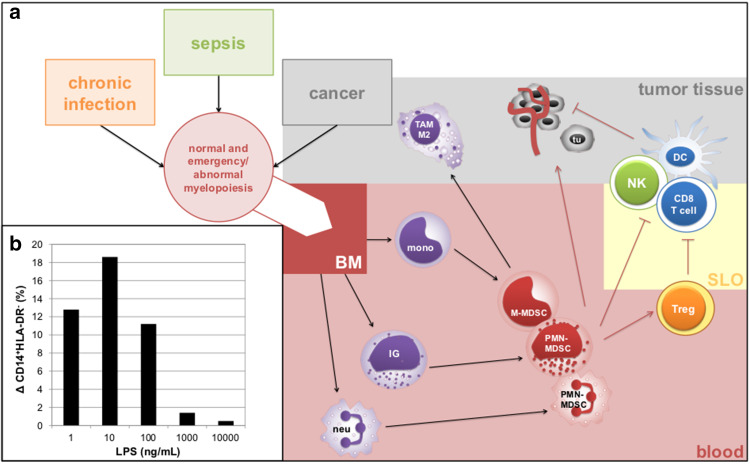
Fig. 1.
Summary of MDSC nature and their immunosuppressive and tumor-promoting actions. a Acute-phase conditions, such as chronic infection, sepsis or cancer lead to normal and abnormal/emergency myelopoiesis that is controlled by the production of granulocytic or monocytic growth factors (GM-CSF, G-CSF and M-CSF) leading to the production of mature and immature myeloid cells from precursors in the bone marrow (BM). MDSCs may act in secondary lymphoid organs (SLO) by various immunosuppressive mechanisms (e.g., by the production of IDO, NOS, arginase, IL-10 or TGFβ) that stimulate Treg expansion and inhibit cytotoxic T cell and NK cell function or dendritic cell differentiation and maturation. Upon entering tumor tissue, M-MDSCs rapidly differentiate into tumor-associated M2 macrophages (TAM M2). Furthermore, MDSCs support tumor growth by production of pro-angiogenic factors or factors that promote metastatic spread (e.g., VEGF, bFGF, MMP-9). b To address the issue of strength of TLR4 activation on monocyte reprogramming, we examined dose-dependent change in M-MDSC proportion in monocytes upon 24 h stimulation of whole blood with LPS. Here, we show that low, but not high, endotoxin concentrations result in the increase of M-MDSC/CARS proportion in CD14+ cells. BM bone marrow, NOS nitric oxide synthase, SLO secondary lymphoid organ, tu tumor cell
The nature and subsequently the methodology to detect PMN-MDSCs are complex. In a broad sense, a granulocytic fraction of MDSCs has been defined as CD11b+ CD14− CD15+ or CD11b+ CD14− CD66b+ cells detected in PBMCs separated by gradient centrifugation using 1.077 g/mL density due to their low-density properties [9]. This approach allows identification of PMN-MDSCs as a heterogeneous group of myeloid cells including (1) immature granulocytes (myeloblasts, promyelocytes, and neutrophilic myelocytes) as these cells have low density and are CD11b+ and CD15+ and (2) a rare subset of CD11b+ CD33hi CD14− CD15+ PMN cells that have been identified as PMN-MDSCs by several groups [16, 17] (Supplementary Fig. 1, Supplementary Fig. 2). The nature of the immature myeloid cells is clear; they are a product of emergency myelopoiesis and, therefore, are present to a certain extent in the periphery under multiple pathological conditions, such as infection, allergy, sepsis, and cancer. Consequently, the key question is whether immature granulocytic cells possess immunosuppressive properties. Several recent studies have indicated that immature PMN-MDSCs are truly suppressive [18]. CD33hi CD15+ cells represent a PMN-MDSC subset that possesses immunosuppressive properties such as arginase I production and correspond to activated neutrophils [17]. This MDSC subset likely arises in the periphery upon stimulation with pro-inflammatory cytokines and can be distinguished phenotypically from other leukocyte subsets without the need for gradient centrifugation.
In summary, there are several granulocytic and monocytic MDSC subsets with immature character originating from myeloid progenitors or mature MDSCs that likely arise in the periphery. All MDSC subsets are generated under conditions that overlap with acute phase with emergency myelopoiesis (Fig. 1a).
MDSCs accumulation is induced by tumor-derived factors
In general, MDSCs are generated in a two-signal manner [10]. ‘Expansion’ signal 1 is typically represented by CSFs produced by hematopoietic and non-hematopoietic cells in bone marrow or peripheral tissues upon sensing of pathogen-associated molecular pattern (PAMP) [3]. Besides CSFs, signal 1 can be provided by VEGF [19] and polyunsaturated fatty acids [20]. The main transcriptional factors/regulators downstream of signal 1 are STAT3, STAT5, IRF8 (interferon-regulatory factor 8), C/EBPβ (CCAAT/enhancer-binding protein beta), and NOTCH. Signal 2 is provided by inflammatory cytokines and DAMPs, including IFNγ, IL-1β, IL-4, IL-6, IL-13, TNF, PGE2, and TLR agonists including LPS (low strength) and HMGB1 (high-mobility group box 1). The role of specific factors in accumulation of MDSCs depends on the particular MDSC subtype of interest.
PMN-MDSCs are generated during skewed emergency granulopoiesis
In cancer, emergency myelopoiesis is driven by elevated granulopoietic factors, most notably G-CSF, and occurs in a STAT3-dependent manner to enhance and prolong expansion of bone-marrow-derived immature myeloid cells [4]. In cancer patients, emergency granulopoiesis is triggered directly by CSFs produced by tumor cells [11, 21] or indirectly by CSFs produced by hematopoietic and non-hematopoietic cells upon sensing of tumor-derived DAMP or inflammatory cytokines [22]. Various factors produced by a reactive and/or immunosuppressive tumor environment, such as the alarmins S100A8/A9, VEGF, IL-10, and COX-2/PGE2, have been shown to block differentiation of immature myeloid cells and thus result in accumulation/activation of PMN-MDSCs. As a consequence, immature PMN-MDSCs as well as CD33hi PMN-MDSCs are generated during emergency granulopoiesis as a response to CSFs in the context of tumor-modified micro- and macroenvironments [3, 16].
M-MDSCs are generated by monocyte reprogramming induced by tumor-derived DAMP
Mature M-MDSC/CART monocytes are generated by reprogramming of differentiated monocytes triggered by microbial signatures/PAMPs of low strength (Fig. 1b) and by the persistent presence of pro-inflammatory cytokines and pro-inflammatory factors such as prostaglandins and S100A8/A9 alarmins [23]. In cancer, the TLR receptors are activated by tumor-derived DAMPs providing low strength and persistent signals that, in combination with factors such as IL-10 and PGE2, lead to monocyte reprogramming [24].
MDSCs: immunosuppressive and cancer-promoting actions
Cancer development is caused by stepwise accumulation of intrinsic cellular changes that are shaped by host-related factors such as inflammatory cells and cytokine/chemokine levels in the tumor macro- and microenvironment. Chronic inflammation supports cancer angiogenesis, glycolytic phenotype, and Th2 immune response and thus contributes to cancer progression.
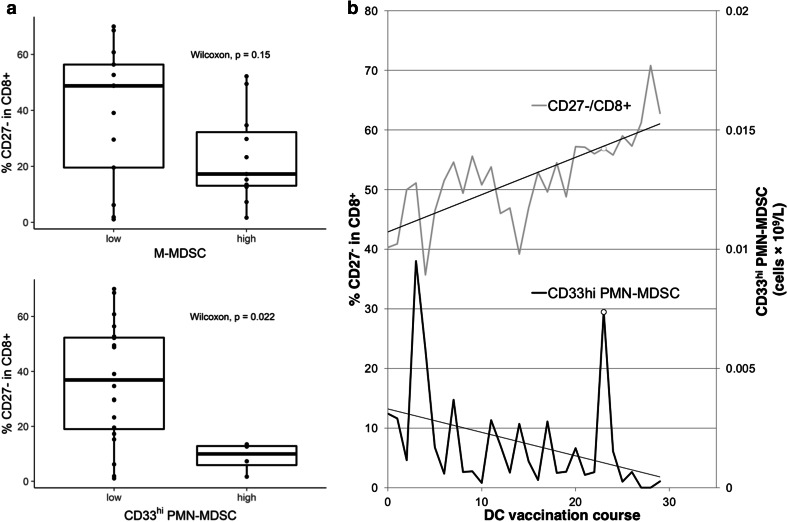
MDSCs can employ multiple immunosuppressive pathways and the employment of a particular pathway and its suppressive efficacy is related to MDSC subtype. In general, MDSCs elicit their immunosuppressive functions through various mechanisms including production of (1) immunoregulatory enzymes, such as arginase-1 depleting arginine required for T-cell activation, indoleamine 2,3 dioxygenase and nitric oxide synthase 2, (2) reactive oxygen species (ROS) that damage cells and interfere with cellular functions, and (3) suppressive cytokines, such as TGFβ, PGE2, IL-10, and IL-8. The latter is associated with the tolerogenic action of MDSCs leading to a T-cell tolerogenic phenotype or expansion of immunosuppressive Treg cells [25]. MDSCs can inhibit antigen presenting cell (APC) properties of macrophages and dendritic cells (DC) [26]. In pediatric patients with high-risk tumors, we observed substantially reduced proportions of effector CD27− T cells in the circulating CD8+ cytotoxic compartment in patients with a high frequency of CD33hi PMN-MDSCs compared to those with low CD33hi PMN-MDSC frequency (Fig. 2a). A similar trend, although not achieving statistical significance, was observed for M-MDSC (Fig. 2a). Evaluating the frequencies of CD33hi PMN-MDSCs in an individual cancer patient in the course of anti-cancer DC vaccination, we observed a gradual decrease in frequency of CD33hi PMN-MDSCs and a gradual increase in circulating effector cytotoxic T cells (Fig. 2b). Data from ex vivo settings with renal cell carcinoma patients have shown that depletion of CD15+ cells from PBMC (corresponding to PMN-MDSC) resulted to the recovery of normal T-cell functions [27].
Fig. 2.
Inverse correlation between effector CD27− cytotoxic CD8+ T cells and number of MDSCs in pediatric cancer patients. We perform immunomonitoring of circulating immune cells in high-risk pediatric cancer patients in the clinical trial evaluating anti-cancer vaccine based on dendritic cells loaded with autologous tumor lysate (EudraCT: 2014-003388-39). a Patients with low MDSC counts before DC vaccination have high proportions of effector CD27− CD8+ T cells compared to patients with high MDSC counts. A low MDSC count was defined as ≤ 0.01 109/L for CD33hi PMN-MDSCs and ≤ 0.2 109/L for M-MDSCs. b Case of early relapsing Burkitt lymphoma with PI3K-delta subunit germline mutation treated with long-term anti-cancer DC vaccination (29 doses) is shown. We observed opposing dynamics of CD27− CD8+ effector T cells and CD33hi PMN-MDSCs during the course of vaccination. The peak at dose 23 (depicted with an empty circle) overlaps with respiratory infection resolved by amoxicillin
MDSC-derived IL-10, VEGF, matrix metallopeptidases (MMPs) and TGFβ have been shown to contribute to tumor angiogenesis and promote metastasis [28, 29]. Taken together, MDSC possess immunosuppressive and pro-angiogenic functions to limit inflammation and to stimulate wound healing. In cancer, however, these mechanisms contribute to cancer progression and metastasis.
rhG-CSF: a chemotherapy delivery perspective
G-CSF as a key regulator of (not only) granulopoiesis
G-CSF is the principal cytokine controlling neutrophil development and function. It is produced at low levels under steady-state conditions with serum concentration of 101 ng/L in healthy individuals [30]. In acute conditions, such as bacterial infection, pro-inflammatory stimuli such as IL-1β, LPS, or TNFα enhance production of G-CSF with serum concentrations 102–103 ng/L in humans [30]. G-CSF directly stimulates proliferation of granulocytic progenitor cells by shortening their passage through the cell cycle [31] leading to ‘emergency’ or ‘demand-driven’ granulopoiesis, or generally myelopoiesis, and sustained output of circulating neutrophils during infection.
Being a key cytokine controlling granulocyte numbers in peripheral blood, recombinant human G-CSF (rhG-CSF) has become the main therapeutic agent for the treatment of neutropenia and primary and secondary prophylaxis of febrile neutropenia in cancer patients. Besides its use for the stimulation of granulocytes/granulocyte progenitors, rhG-CSF is clinically used (1) to increase endometrial thickness in women during in vitro fertilization [32], (2) as a therapy for stroke [33] or (3) to promote spermatogenic regeneration in fertility-restoring therapy [34].
rhG-CSF in the management of febrile neutropenia in carcinoma patients
Febrile neutropenia (FN) is a common dose-limiting toxic complication of many chemotherapy regimens that can prompt dose reductions or treatment delays. FN is defined as an oral temperature of > 38.3 °C or two consecutive readings of > 38.0 °C for 2 h and an absolute neutrophil count of < 0.5 × 109/L, or expected to fall below 0.5 × 109/L. About 25–40% of treatment-naive patients develop FN with common chemotherapy regimens [35]. There is a clear relationship between the intensity of the regimen and the severity of neutropenia. Thus, the overall FN risk assessment is based on (1) the type of chemotherapy, namely its myelosuppressive potential, (2) the intensity (high-dose, dose-dense, or standard-dose therapy), and (3) the intent (curative/adjuvant/neoadjuvant vs. palliative) of the chemotherapy regimen. Currently, the different regimens are classified as producing a high risk (> 20%), an intermediate risk (10–20%), or a low risk (< 10%) of FN. Current guidelines begin with an evaluation of risk for chemotherapy-induced FN prior to the first cycle of chemotherapy and the risk should be re-evaluated prior to each subsequent cycle to determine the risk categorization and treatment intent. If the patient experienced a previous episode of FN or a dose-limiting neutropenic event during the previous treatment cycle, this patient would then be in the high-risk group. Moreover, patient-related factors increasing the risk of FN, such as age over 65 years prior to chemotherapy or radiotherapy, pre-existing neutropenia or tumor involvement in the bone marrow, poor performance status, or comorbidities including renal or liver dysfunction must be considered [36].
rhG-CSFs are currently used for primary and secondary prophylaxis of FN in patients treated with established cytotoxic chemotherapy regimens. International and national guidelines recommend that G-CSF be administered prophylactically if the risk of FN is > 20% for all planned cycles of treatment.
There are two major derivatives of recombinant G-CSF currently available clinically, filgrastim and its pegylated form, pegfilgrastim, each with specific pharmacokinetics parameters. European Medicines Agency (EMA) or Food and Drug Administration (FDA)-approved biosimilars can also be used according to their summary of product characteristics.
Filgrastim is rhG-CSF with a short half-life that requires repeated applications per chemotherapy cycle. Following subcutaneous administration of the recommended daily dose of 5 µg/kg, absorption results in maximum serum concentrations with Tmax of 4.5 ± 0.9 h that remained above 104 ng/L for 8–16 h. Filgrastim is primarily eliminated by the kidney and neutrophils/neutrophil precursors; the latter presumably involves binding to the G-CSF receptor, internalization of the growth factor–receptor complexes via endocytosis, and subsequent degradation inside the cells [37]. Once the nadir of neutrophils has passed, the daily dose of filgrastim should be titrated against the neutrophil response [38].
Pegfilgrastim is a sustained duration form of filgrastim, a covalent conjugate of filgrastim with a polyethylene glycol (PEG) molecule. After a single subcutaneous dose of pegfilgrastim, the peak serum concentration is maintained from 16 to 120 h after administration, and therefore, one injection of pegfilgrastim is required during one cycle of chemotherapy. Pegylation of filgrastim renders renal clearance insignificant, and therefore, renal impairment, including end-stage renal disease, had no impact on the pharmacokinetics of pegfilgrastim. Neutrophil-mediated clearance is the predominant elimination pathway for pegfilgrastim, meaning that clearance from the circulation is self-regulating and pegfilgrastim elimination is enhanced after neutrophil numbers begin to recover.
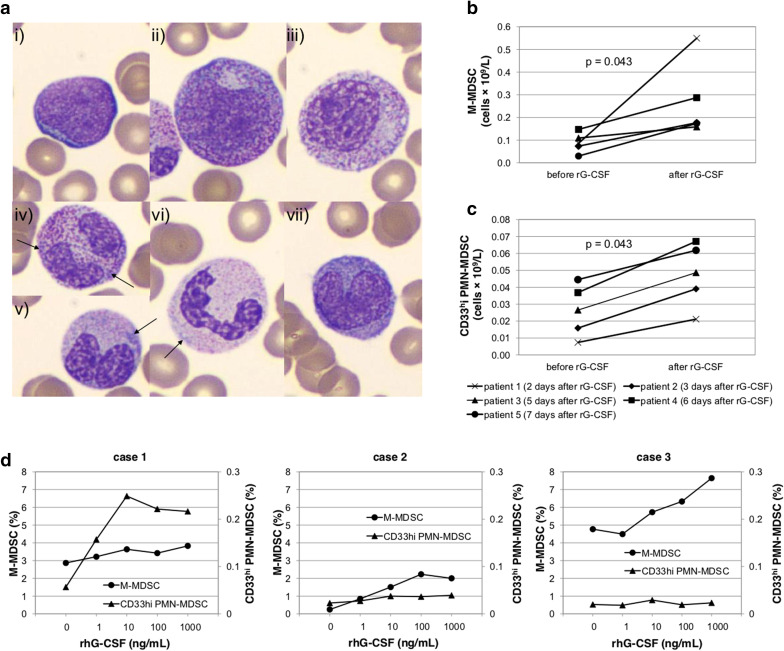
From the pharmacodynamic perspective, both compounds, filgrastim and pegfilgrastim, increase the proliferation and differentiation of neutrophils from committed progenitor cells, induce maturation, and enhance the survival and function of mature neutrophils, resulting in dose-dependent increases in neutrophil numbers. Moreover, after rhG-CSF administration, an immediate activation of mature neutrophils can be observed leading to a rapid, but a transient decrease in absolute neutrophil count as a large number of neutrophils is marginated via increased adherence to endothelial cells [39]. Subsequently, the myeloid mitotic compartment of bone marrow is expanded by rhG-CSF activity, with significant increases in promyelocytes and myelocytes and a relative depletion of mature neutrophils in peripheral blood. rhG-CSF-induced myelopoiesis results in phenotypic changes parallel to emergency granulopoiesis (Fig. 3a).
Fig. 3.
rhG-CSF triggers ‘emergency’ myelopoiesis and the accumulation of granulocytic and monocytic MDSCs in carcinoma patients. a Typical morphological changes in myeloid cells after rhG-CSF administration are shown. rhG-CSF caused release of immature granulocytes (IG) in peripheral blood: myeloblasts (i), promyelocytes (ii). Dysplastic changes with nucleo-cytoplasmic maturation asynchrony are apparent in IG (iii). Neutrophilic granulocytes have hyposegmented nuclei, contain Döhle bodies (arrows) (iv–vi), are hypergranulated (iv), less typically hypogranulated (vi) and/or show nucleo-cytoplasmic asynchrony (v). Monocytes of younger morphology have basophilic cytoplasm and lack vacuolization (vii). WBC differential count from blood smear was: band neutrophils: 15.7%, segmented neutrophils: 29.8%, eosinophils: 1.7%, basophils: 0.0%, lymphocytes: 8.3%, monocytes: 14.0%, promyelocytes: 4.1%, myelocytes: 13.2%, metamyelocytes: 11.6%, blasts: 1.7%. b, c Dynamics of circulating MDSC subsets after rhG-CSF administration. Numbers of circulating b M-MDSCs and c CD33hi PMN-MDSCs were examined in breast carcinoma patients before and 2–7 days after rhG-CSF (filgrastim) administration. d Effect of rhG-CSF on MDSC level ex vivo in peripheral blood samples of cancer patients. Whole blood specimens were incubated (37 °C, 5% CO2) with raising concentrations of rhG-CSF (filgrastim) (0–10,000 ng/mL) for 17 up to 20 h. Case 1—metastatic brain lesion of Grawitz tumor with bronchopneumonia under corticotherapy (CRP = 15 mg/L, increased IG in peripheral blood: 11%), case 2—inoperable cholangiocarcinoma treated with gemcitabine (CRP = 0.8 mg/L), case 3—colorectal cancer 1 day after surgery (sigmoid colectomy, CRP = N/A). Concentration of rhG-CSF of 10 ng/mL, that is reached in patients receiving filgrastim for neutropenia, led in all cases to accumulation of M-MDSCs as well as CD33 PMN-MDSCs ex vivo compared to baseline level. IG immature granulocyte
Short-term adverse events of filgrastim and derivative products are bone pain, headache, myalgias, fatigue, nausea, insomnia, and redness at the injection site [40]. These are usually mild-to-moderate and self-limiting. Far less common events include splenic rupture, acute lung injury, vascular events, and exacerbation of autoimmune or inflammatory conditions. Even though rare, these events can be clinically serious and life-threatening [40]. Recently, potential connections of rhG-CSF with leukemia and myelodysplasia have been reported [41–43].
rhG-CSF: a cancer immunobiology perspective
rhG-CSF drives emergency/abnormal myelopoiesis and accumulation of MDSCs
CSFs drive emergency granulopoiesis/myelopoiesis to meet an increased need for myeloid cells. Mature and immature myeloid cells migrate from the bone marrow in response to CSF inflammatory signals. This process often includes extramedullary myelopoiesis in organs outside of the bone-marrow compartment and is seen in ‘acute’ conditions such as trauma, sepsis, and cancer [3, 44]. As a consequence, administration of G-CSF and GM-CSF may result in splenomegaly [45, 46].
In an animal model, recombinant G-CSF has been shown to induce MDSCs and angiogenesis and in turn reduces responsiveness to anti-VEGF therapy [47]. It has been shown in humans that rhG-CSF used for stem-cell mobilization in human donors induces expansion of MDSCs with the capacity to suppress alloreactive T-cell responses in vitro [48].
In cancer patients, the role of rhG-CSF in MDSC accumulation is obvious for immature PMN-MDSCs, since rhG-CSF is by its nature a stimulator of bone marrow and extramedullary granulopoiesis. Here, we show that CD33hi PMN-MDSCs (presumably activated granulocytes) are elevated by rh-GSF in vivo in breast cancer patients as well as ex vivo in an experimental setting, mimicking peripheral MDSC generation by rhG-CSF in whole blood of cancer patients (Fig. 3c, d). Regarding M-MDSCs, we observed their consistent accumulation in breast cancer patients who received rhG-CSF as prophylaxis of FN in a process that may be related to de novo myelopoiesis stimulation (Fig. 3b). Interestingly, however, we also observed ex vivo rhG-CSF-induced dose-dependent M-MDSC elevation (Fig. 3d), suggesting that granulopoietic growth factors may induce monocyte reprogramming towards M-MDSC, presumably in an STAT3-dependent manner [27].
Immunosuppressive and cancer-promoting actions of G-CSF: beyond MDSCs
It must be noted that rhG-CSF-stimulated MDSCs are not the only potential vector of G-CSF-induced action that might support tumor growth. Several types of solid tumor cells have receptors for G-CSF and, therefore, may proliferate upon stimulation with rhG-CSF [49–51]. G-CSF possesses several modes of T-cell tolerance induction under pathophysiological conditions that is partially MDSC independent [52]. rhG-CSF has been shown to exert an enhancing effect on IL-10, IL-8, and IL-4 production while reducing IL-12, TNFα, and subsequently IFNγ, enhancing the effect on regulatory T cells, and may induce a transient prothrombotic or hypercoagulable state, substantially enhancing endothelial progenitor cells and MMP-9 [53]. Hypercoagulable state and platelet activation are followed by the release of cytokines and angiogenic factors (e.g., VEGF, bFGF, or PDGF) from platelet α-granules that contribute to tumor angiogenesis [54]. Finally, rhG-CSF-activated and stimulated neutrophils may contribute to cancer-promoting chronic inflammation [55].
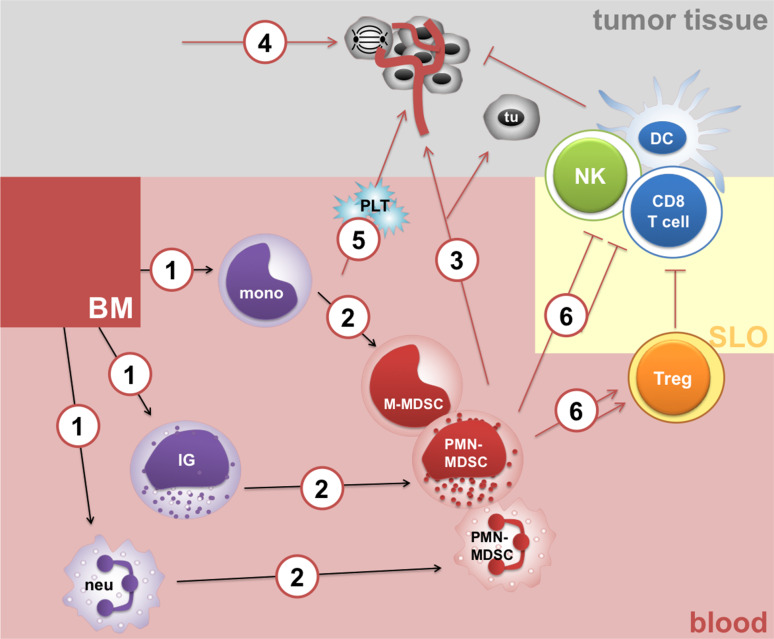
Taken together, rhG-CSF-triggered emergency granulopoiesis is believed to provide a protective innate immune response by replenishing myeloid cells. Nevertheless, a highly expanded myeloid cell compartment skewed towards suppressive myeloid populations may jeopardize host immunity, thereby promoting diseases such as sepsis or cancer (Fig. 4).
Fig. 4.
Mechanisms of rhG-CSF immunosuppressive and tumor-promoting actions. rhG-CSF stimulates production of mature and immature myeloid cells (1), release of immature PMN-MDSCs and peripheral reprogramming of mature myeloid cells into M-MDSCs and mature PMN-MDSCs (2). In turn, rhG-CSF may contribute to MDSC-dependent cancer angiogenesis and metastasis (3), may directly stimulate the proliferation of tumor cells (tu) through their G-CSF receptors (4) and may contribute to a hypercoagulable state implicated in cancer progression (5). Finally, rhG-CSF inhibits T-cell function in MDSC-dependent and partially MDSC-independent manners and promotes Treg (6). BM bone marrow, SLO secondary lymphoid organ, tu tumor cell, PLT platelets
rhG-CSF in cancer patients: ...no free lunch?
In light of the current knowledge on the key role of G-CSF in MDSC induction and the importance of MDSCs in tumor growth and spread, medical oncologists must ask an uncomfortable question: do rhG-CSF-induced MDSCs contribute to tumor progression via immune suppression and/or stimulation of tumor angiogenesis? It is notable that clinical trials evaluating rhG-CSF for FN prophylaxis in cancer patients have not been designed to answer this question. rhG-CSF improves the delivery of full dose-intensity chemotherapy on schedule, although this has not been shown to lead to better responses or higher overall survival in most studies [56]. To date, rhG-CSF administration is a rigid part of cancer patient management, and therefore, its possible effect on protracted immunosuppression and enhanced angiogenesis is hard to detect in retrospective studies or in clinical trials evaluating novel anti-cancer agents. Nevertheless, in patients with stage III and IV unresectable oro- and hypopharyngeal carcinomas treated by chemoradiotherapy in a multicentric randomized trial, recombinant G-CSF and GM-CSF resulted in reduced local control and prognosis in both sites of head-and-neck cancers examined [57, 58].
Anti-cancer therapy (chemotherapy or concomitant chemoradiotherapy) that may be accompanied by pharmacological rhG-CSF is applied in (1) adjuvant settings where possibly dormant micrometastases in lymph nodes or distant organs are present; (2) in neoadjuvant settings with a clinically advanced immunosuppressive tumor present plus possibly dormant micrometastases; or (3) in palliative settings where metastases that evaded the immune system and developed aberrant cancer angiogenesis are present. Considering the possible contribution of MDSCs to cancer progression, transient induction of MDSCs by rhG-CSF could interfere with anti-cancer treatment in neoadjuvant and palliative settings.
In general, rhG-CSFs are perceived by medical oncologists as well-tolerated and safe with cost-effectiveness being the main consideration for their use in patients with intermediate risk of FN. Consequently, there might to be pharmacological overuse of rhG-CSF for “primary” prevention of febrile neutropenia in cancer patients [59], as has been pointed out in the 2012 ASCO “Choosing Wisely” recommendations [60]. rhG-CSFs assist in maintaining the chemotherapy dose density and intensity and this modality is often translated from patients with high risk of FN to patients with intermediate risk of FN. This occurs mainly for practical reasons, such as to reduce the need for frequent WBC count monitoring between cycles, and to be certain of achieving sufficient numbers of neutrophils at each chemotherapy cycle. However, with respect to possible interference of rhG-CSF with anti-cancer therapy, prophylactic rhG-CSF should not be given to patients who are not at high-risk of FN, in adherence to the current guidelines.
Conclusions
In recent years, scientific interest in the role of MDSCs in cancer has moved to clinical research and is approaching clinical practice. Detection of human MDSCs requires multicolor flow cytometry to distinguish MDSC subsets that have different time-dependent dynamics in human cancers.
Endogenous G-CSF produced directly by tumor tissues or as a response to factors derived from tumor tissue stimulates granulocytic and monocytic suppressor cells [21] and G-CSF and VEGF are major players in an MDSC—tumor progression positive feedback loop [47]. Emergency myelopoiesis induced by endogenous or iatrogenic G-CSF leads to, at least transient, up-regulation of both MDSC subsets. This may in turn interfere with current attempts to define patients with elevated MDSCs and target them as principle inducers of existing immunosuppression. In practice, MDSCs are elevated in cancer patients with ongoing infection or receiving rhG-CSF irrespective of the frequency of MDSCs induced by the tumor.
rhG-CSFs are used for their pleiotropic effects in various clinical conditions. Emerging data indicate that, at least in certain cancer types, growth factors that regulate myelopoiesis may contribute in both MDSC-dependent and MDSC-independent manners to immunosuppression and cancer angiogenesis which may in turn contribute to tumor progression and cancer spread.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- ASCO
American Society of Clinical Oncology
- ATRA
All-trans-retinoic acid
- bFGF
Basic fibroblast growth factor
- C/EBPβ
CCAAT/enhancer-binding protein beta
- CARS
Compensatory anti-inflammatory response syndrome
- CRP
C-reactive protein
- CSF
Colony-stimulating factor
- DAMP
Danger-associated molecular pattern
- DC
Dendritic cell
- EMA
European Medicines Agency
- e-MDSC
Early myeloid-derived suppressor cell
- FDA
Food and Drug Administration
- FN
Febrile neutropenia
- HMGB1
High-mobility group box 1
- IRF
Interferon-regulatory factor
- M-MDSC
Monocytic myeloid-derived suppressor cell
- MMP
Matrix metallopeptidase
- PAMP
Pathogen-associated molecular pattern
- PDGF
Platelet-derived growth factor
- PEG
Polyethylene glycol
- PGE2
Prostaglandin E 2
- PMN-MDSC
Polymorphonuclear myeloid-derived suppressor cell
- rhG-CSF
Recombinant human granulocyte colony-stimulating factor
- ROS
Reactive oxygen species
- TAM
Tumor-associated macrophage
Author contributions
KP performed or supervised laboratory testing, contributed to data interpretation, prepared figures, and drafted the manuscript. BB referred patients and drafted the manuscript. RD contributed to data interpretation and drafted the manuscript. DV drafted and edited the manuscript. LZ-D conceived of the presented idea, designed the experiment and laboratory testing, and drafted and finalized the manuscript. All authors discussed the results and contributed to the final manuscript.
Funding
The work was supported by the Czech Ministry of Health (projects AZV 16-31966A and DRO 00209805) and the Czech Ministry of Education, Youth and Sports (projects LO1413, LM2015089, and LM2015090).
Compliance with ethical standards
Ethical approval
For pediatric patients treated by anti-cancer DC vaccines, academic clinical trial (EudraCT: 2014-003388-39) was approved by Czech national authority (State Institute for Drug Control).
Ethical standards
For adult cancer patients, the study was approved by Ethical Board of Masaryk Memorial Cancer Institute, Brno, Czech Republic.
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- 1.Beeson PB. Development of tolerance to typhoid bacterial pyrogen and its abolition by reticulo-endothelial blockade. Proc Soc Exp Biol Med. 1946;61:248–250. doi: 10.3181/00379727-61-15291P. [DOI] [PubMed] [Google Scholar]
- 2.Veglia F, Perego M, Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat Immunol. 2018;19:108–119. doi: 10.1038/s41590-017-0022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manz MG, Boettcher S. Emergency granulopoiesis. Nat Rev Immunol. 2014;14:302–314. doi: 10.1038/nri3660. [DOI] [PubMed] [Google Scholar]
- 4.Zhang H, Nguyen-Jackson H, Panopoulos AD, Li HS, Murray PJ, Watowich SS. STAT3 controls myeloid progenitor growth during emergency granulopoiesis. Blood. 2010;116:2462–2471. doi: 10.1182/blood-2009-12-259630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nat Rev Cancer. 2013;13:739–752. doi: 10.1038/nrc3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shipp C, Speigl L, Janssen N, Martens A, Pawelec G. A clinical and biological perspective of human myeloid-derived suppressor cells in cancer. Cell Mol Life Sci. 2016;73:4043–4061. doi: 10.1007/s00018-016-2278-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Najjar YG, Finke JH. Clinical perspectives on targeting of myeloid derived suppressor cells in the treatment of cancer. Front Oncol. 2013;3:49. doi: 10.3389/fonc.2013.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peranzoni E, Zilio S, Marigo I, Dolcetti L, Zanovello P, Mandruzzato S, Bronte V. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol. 2010;22:238–244. doi: 10.1016/j.coi.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 9.Bronte V, Brandau S, Chen SH, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. 2016;7:12150. doi: 10.1038/ncomms12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bronte V, Chappell DB, Apolloni E, Cabrelle A, Wang M, Hwu P, Restifo NP. Unopposed production of granulocyte-macrophage colony-stimulating factor by tumors inhibits CD8 + T cell responses by dysregulating antigen-presenting cell maturation. J Immunol. 1999;162:5728–5737. [PMC free article] [PubMed] [Google Scholar]
- 11.Waight JD, Hu Q, Miller A, Liu S, Abrams SI. Tumor-derived G-CSF facilitates neoplastic growth through a granulocytic myeloid-derived suppressor cell-dependent mechanism. PloS One. 2011;6:e27690. doi: 10.1371/journal.pone.0027690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adib-Conquy M, Cavaillon JM. Compensatory anti-inflammatory response syndrome. Thromb Haemost. 2009;101:36–47. doi: 10.1160/TH08-07-0421. [DOI] [PubMed] [Google Scholar]
- 13.Pena OM, Pistolic J, Raj D, Fjell CD, Hancock RE. Endotoxin tolerance represents a distinctive state of alternative polarization (M2) in human mononuclear cells. J Immunol. 2011;186:7243–7254. doi: 10.4049/jimmunol.1001952. [DOI] [PubMed] [Google Scholar]
- 14.Sakuta T, Matsushita K, Yamaguchi N, et al. Enhanced production of vascular endothelial growth factor by human monocytic cells stimulated with endotoxin through transcription factor SP-1. J Med Microbiol. 2001;50:233–237. doi: 10.1099/0022-1317-50-3-233. [DOI] [PubMed] [Google Scholar]
- 15.Corzo CA, Condamine T, Lu L, et al. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207:2439–2453. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strauss L, Sangaletti S, Consonni FM, et al. RORC1 regulates tumor-promoting “emergency” granulo-monocytopoiesis. Cancer Cell. 2015;28:253–269. doi: 10.1016/j.ccell.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, Sierra R, Ochoa AC. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69:1553–1560. doi: 10.1158/0008-5472.CAN-08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Solito S, Falisi E, Diaz-Montero CM, et al. A human promyelocytic-like population is responsible for the immune suppression mediated by myeloid-derived suppressor cells. Blood. 2011;118:2254–2265. doi: 10.1182/blood-2010-12-325753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Umansky V, Sevko A. Tumor microenvironment and myeloid-derived suppressor cells. Cancer Microenviron. 2013;6:169–177. doi: 10.1007/s12307-012-0126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan D, Yang Q, Shi M, Zhong L, Wu C, Meng T, Yin H, Zhou J. Polyunsaturated fatty acids promote the expansion of myeloid-derived suppressor cells by activating the JAK/STAT3 pathway. Eur J Immunol. 2013;43:2943–2955. doi: 10.1002/eji.201343472. [DOI] [PubMed] [Google Scholar]
- 21.Abrams SI, Waight JD. Identification of a G-CSF-granulocytic MDSC axis that promotes tumor progression. Oncoimmunology. 2012;1:550–551. doi: 10.4161/onci.19334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allen MD, Jones LJ. The role of inflammation in progression of breast cancer: friend or foe? (Review) Int J Oncol. 2015;47:797–805. doi: 10.3892/ijo.2015.3075. [DOI] [PubMed] [Google Scholar]
- 23.Dorhoi A, Du Plessis N. Monocytic myeloid-derived suppressor cells in chronic infections. Front Immunol. 2017;8:1895. doi: 10.3389/fimmu.2017.01895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mao Y, Poschke I, Wennerberg E, et al. Melanoma-educated CD14+ cells acquire a myeloid-derived suppressor cell phenotype through COX-2-dependent mechanisms. Cancer Res. 2013;73:3877–3887. doi: 10.1158/0008-5472.CAN-12-4115. [DOI] [PubMed] [Google Scholar]
- 25.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Markowitz J, Wang J, Vangundy Z, et al. Nitric oxide mediated inhibition of antigen presentation from DCs to CD4(+) T cells in cancer and measurement of STAT1 nitration. Sci Rep. 2017;7:15424. doi: 10.1038/s41598-017-14970-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen PA, Ko JS, Storkus WJ, et al. Myeloid-derived suppressor cells adhere to physiologic STAT3- vs STAT5-dependent hematopoietic programming, establishing diverse tumor-mediated mechanisms of immunologic escape. Immunol Investig. 2012;41:680–710. doi: 10.3109/08820139.2012.703745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shojaei F, Ferrara N. Refractoriness to antivascular endothelial growth factor treatment: role of myeloid cells. Cancer Res. 2008;68:5501–5504. doi: 10.1158/0008-5472.CAN-08-0925. [DOI] [PubMed] [Google Scholar]
- 29.Ben-Baruch A. The tumor-promoting flow of cells into, within and out of the tumor site: regulation by the inflammatory axis of tnfalpha and chemokines. Cancer Microenviron. 2012;5:151–164. doi: 10.1007/s12307-011-0094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watari K, Asano S, Shirafuji N, Kodo H, Ozawa K, Takaku F, Kamachi S. Serum granulocyte colony-stimulating factor levels in healthy volunteers and patients with various disorders as estimated by enzyme immunoassay. Blood. 1989;73:117–122. [PubMed] [Google Scholar]
- 31.Lord BI, Bronchud MH, Owens S, Chang J, Howell A, Souza L, Dexter TM. The kinetics of human granulopoiesis following treatment with granulocyte colony-stimulating factor in vivo. Proc Natl Acad Sci USA. 1989;86:9499–9503. doi: 10.1073/pnas.86.23.9499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarvi F, Arabahmadi M, Alleyassin A, Aghahosseini M, Ghasemi M. Effect of increased endometrial thickness and implantation rate by granulocyte colony-stimulating factor on unresponsive thin endometrium in fresh in vitro fertilization cycles: a randomized clinical trial. Obstet Gynecol Int. 2017;2017:3596079. doi: 10.1155/2017/3596079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang X, Liu Y, Bai S, Peng L, Zhang B, Lu H. Granulocyte colony stimulating factor therapy for stroke: a pairwise meta-analysis of randomized controlled trial. PloS One. 2017;12:e0175774. doi: 10.1371/journal.pone.0175774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kotzur T, Benavides-Garcia R, Mecklenburg J, Sanchez JR, Reilly M, Hermann BP. Granulocyte colony-stimulating factor (G-CSF) promotes spermatogenic regeneration from surviving spermatogonia after high-dose alkylating chemotherapy. Reprod Biol Endocrinol. 2017;15:7. doi: 10.1186/s12958-016-0226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dale DC. Colony-stimulating factors for the management of neutropenia in cancer patients. Drugs. 2002;62(Suppl 1):1–15. doi: 10.2165/00003495-200262001-00001. [DOI] [PubMed] [Google Scholar]
- 36.Lyman GH, Kuderer NM, Crawford J, Wolff DA, Culakova E, Poniewierski MS, Dale DC. Predicting individual risk of neutropenic complications in patients receiving cancer chemotherapy. Cancer. 2011;117:1917–1927. doi: 10.1002/cncr.25691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang BB, Kido A. Pharmacokinetics and pharmacodynamics of pegfilgrastim. Clin Pharmacokinet. 2011;50:295–306. doi: 10.2165/11586040-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 38.Kuwabara T, Kobayashi S, Sugiyama Y. Pharmacokinetics and pharmacodynamics of a recombinant human granulocyte colony-stimulating factor. Drug Metab Rev. 1996;28:625–658. doi: 10.3109/03602539608994020. [DOI] [PubMed] [Google Scholar]
- 39.Carulli G. Effects of recombinant human granulocyte colony-stimulating factor administration on neutrophil phenotype and functions. Haematologica. 1997;82:606–616. [PubMed] [Google Scholar]
- 40.Tigue CC, McKoy JM, Evens AM, Trifilio SM, Tallman MS, Bennett CL. Granulocyte-colony stimulating factor administration to healthy individuals and persons with chronic neutropenia or cancer: an overview of safety considerations from the Research on Adverse Drug Events and Reports project. Bone Marrow Transplant. 2007;40:185–192. doi: 10.1038/sj.bmt.1705722. [DOI] [PubMed] [Google Scholar]
- 41.Socie G, Mary JY, Schrezenmeier H, et al. Granulocyte-stimulating factor and severe aplastic anemia: a survey by the European Group for Blood and Marrow Transplantation (EBMT) Blood. 2007;109:2794–2796. doi: 10.1182/blood-2006-07-034272. [DOI] [PubMed] [Google Scholar]
- 42.Rosenberg PS, Alter BP, Bolyard AA, et al. The incidence of leukemia and mortality from sepsis in patients with severe congenital neutropenia receiving long-term G-CSF therapy. Blood. 2006;107:4628–4635. doi: 10.1182/blood-2005-11-4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hershman D, Neugut AI, Jacobson JS, Wang J, Tsai WY, McBride R, Bennett CL, Grann VR. Acute myeloid leukemia or myelodysplastic syndrome following use of granulocyte colony-stimulating factors during breast cancer adjuvant chemotherapy. J Natl Cancer Inst. 2007;99:196–205. doi: 10.1093/jnci/djk028. [DOI] [PubMed] [Google Scholar]
- 44.Kim CH. Homeostatic and pathogenic extramedullary hematopoiesis. J Blood Med. 2010;1:13–19. doi: 10.2147/JBM.S7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Platzbecker U, Prange-Krex G, Bornhauser M, et al. Spleen enlargement in healthy donors during G-CSF mobilization of PBPCs. Transfusion. 2001;41:184–189. doi: 10.1046/j.1537-2995.2001.41020184.x. [DOI] [PubMed] [Google Scholar]
- 46.Picardi M, De Rosa G, Selleri C, Scarpato N, Soscia E, Martinelli V, Ciancia R, Rotoli B. Spleen enlargement following recombinant human granulocyte colony-stimulating factor administration for peripheral blood stem cell mobilization. Haematologica. 2003;88:794–800. [PubMed] [Google Scholar]
- 47.Shojaei F, Wu X, Qu X, Kowanetz M, Yu L, Tan M, Meng YG, Ferrara N. G-CSF-initiated myeloid cell mobilization and angiogenesis mediate tumor refractoriness to anti-VEGF therapy in mouse models. Proc Natl Acad Sci USA. 2009;106:6742–6747. doi: 10.1073/pnas.0902280106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luyckx A, Schouppe E, Rutgeerts O, et al. G-CSF stem cell mobilization in human donors induces polymorphonuclear and mononuclear myeloid-derived suppressor cells. Clin Immunol. 2012;143:83–87. doi: 10.1016/j.clim.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 49.Morris KT, Khan H, Ahmad A, Weston LL, Nofchissey RA, Pinchuk IV, Beswick EJ. G-CSF and G-CSFR are highly expressed in human gastric and colon cancers and promote carcinoma cell proliferation and migration. Br J Cancer. 2014;110:1211–1220. doi: 10.1038/bjc.2013.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aliper AM, Frieden-Korovkina VP, Buzdin A, Roumiantsev SA, Zhavoronkov A. A role for G-CSF and GM-CSF in nonmyeloid cancers. Cancer Med. 2014;3:737–746. doi: 10.1002/cam4.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gay AN, Chang S, Rutland L, Yu L, Byeseda S, Naik-Mathuria B, Cass DL, Russell H, Olutoye OO. Granulocyte colony stimulating factor alters the phenotype of neuroblastoma cells: implications for disease-free survival of high-risk patients. J Pediatr Surg. 2008;43:837–842. doi: 10.1016/j.jpedsurg.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rutella S, Zavala F, Danese S, Kared H, Leone G. Granulocyte colony-stimulating factor: a novel mediator of T cell tolerance. J Immunol. 2005;175:7085–7091. doi: 10.4049/jimmunol.175.11.7085. [DOI] [PubMed] [Google Scholar]
- 53.Anderlini P. Effects and safety of granulocyte colony-stimulating factor in healthy volunteers. Curr Opin Hematol. 2009;16:35–40. doi: 10.1097/MOH.0b013e328319913c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pilatova K, Greplova K, Demlova R, Bencsikova B, Klement GL, Zdrazilova-Dubska L. Role of platelet chemokines, PF-4 and CTAP-III, in cancer biology. J Hematol Oncol. 2013;6:42. doi: 10.1186/1756-8722-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dumitru CA, Moses K, Trellakis S, Lang S, Brandau S. Neutrophils and granulocytic myeloid-derived suppressor cells: immunophenotyping, cell biology and clinical relevance in human oncology. Cancer Immunol Immunother. 2012;61:1155–1167. doi: 10.1007/s00262-012-1294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lyman G, Reiner M, Morrow P, Crawford J. The effect of filgrastim or pegfilgrastim on survival outcomes of patients with cancer receiving myelosuppressive chemotherapy. Ann Oncol. 2015;26:1452–1458. doi: 10.1093/annonc/mdv174. [DOI] [PubMed] [Google Scholar]
- 57.Staar S, Rudat V, Stuetzer H, Dietz A, Volling P, Schroeder M, Flentje M, Eckel HE, Mueller RP. Intensified hyperfractionated accelerated radiotherapy limits the additional benefit of simultaneous chemotherapy—results of a multicentric randomized German trial in advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;50:1161–1171. doi: 10.1016/S0360-3016(01)01544-9. [DOI] [PubMed] [Google Scholar]
- 58.Gutschalk CM, Herold-Mende CC, Fusenig NE, Mueller MM. Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor promote malignant growth of cells from head and neck squamous cell carcinomas in vivo. Cancer Res. 2006;66:8026–8036. doi: 10.1158/0008-5472.CAN-06-0158. [DOI] [PubMed] [Google Scholar]
- 59.Fishman ML, Kumar A, Davis S, Shimp W, Hrushesky WJ. Guideline-based peer-to-peer consultation optimizes pegfilgrastim use with no adverse clinical consequences. J Oncol Pract. 2012;8:e14s–e17s. doi: 10.1200/JOP.2012.000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith TJ, Bohlke K, Lyman GH, et al. Recommendations for the use of wbc growth factors: american society of clinical oncology clinical practice guideline update. J Clin Oncol. 2015;33:3199–3212. doi: 10.1200/JCO.2015.62.3488. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.