Abstract
The presence of immune infiltrates in the tumor microenvironment has been documented in many types of cancer. Moreover, the preexistent or endogenous immunity which consists of interactions between intratumoral lymphocytes and tumor cells is mostly relevant for the successful application of various anticancer therapies, including standard chemotherapy, immune checkpoint inhibition-based immunotherapy and targeted therapies. The immunoscore defines densities of intratumoral immune infiltrates which determine poor or favorable prognosis depending on their quantity and quality in the tumor compartments. Results from large clinical studies have demonstrated an association between high densities of cytotoxic and memory TILs in the tumor compartments with improved prognosis. Importantly, we have demonstrated that differential combined densities of immune infiltrates jointly analyzed in the tumor center (TC) and the invasive margin (IM) have a significant prognostic value in breast cancer patients with poor clinicopathological parameters.
Keywords: Breast cancer, TILs, CD163+ cells, CD8+ T cells, Biomarkers, TIMO XIV
TILs and the endogenous antitumor immunity
Malignant cell transformation alters the structure of cell membrane proteins and induces antitumor responses against tumor antigens which eliminate the developing tumor cells, controlling tumor growth rates. However, as postulated in the “immunoediting hypothesis”, this immune surveillance may be overridden by various acquired resistance mechanisms employed by the tumor or other cells in the tumor microenvironment including fibroblasts and MDSCs, leading to immune evasion and clinical manifestation of cancer [1–3]. Thus, this inherent capacity of the immune system to respond to the tumor and to control cancer cell growth (referred to as endogenous or preexisting immunity) has a major impact on the balance between tumor immune surveillance and escape. TILs are key to our understanding of tumor immunogenicity and results from clinical studies revealed that these cells have a leading role on the clinical course of several cancers, with CTLs providing the most consistent positive prognostic impacts [4]. These findings underscore the relationship between the endogenous antitumor immunity and cancer, and also highlight the potential role of patients’ immune system for the management of malignant disease. At this point we are not aware at which levels the adaptive immune response generated within the tumor microenvironment may hinder tumor progress, namely if there is a complete destruction of the tumor or, due to tumor heterogeneity, only the immunogenic clones will be recognized and eliminated, whereas “the rest” will continue growing. In the latter case, the immunoediting process will ensure that the tumor-specific TILs, by eliminating most of tumor cells, will “shape” the tumor to be more clonal than heterogeneous, and thus more accessible to reinforced immune attack via immunotherapies [5].
TILs as biomarkers
The complex process of interactions among TILs having different densities and function, will variously impact tumor growth depending on the strength by which they mediate opposite or synergistic antitumor effects. The magnitude of the immune response generated will be further influenced by the molecular characteristics of the tumor and its microenvironment, rendering this dynamic interaction of the tumor and immune system an indispensable parameter for clinical outcome. Indeed, in many cancer types there is an association of immune-cell infiltrates with prognosis and prediction which confirms the capacity of the immune system to influence clinical outcome and the ability to predict therapeutic responses [6].
The role of TILs as favorable prognostic biomarkers has been identified in multiple types of malignant diseases, whereby the densities of certain TIL subpopulations were indicative for the magnitude of the endogenous antitumor immunity [7]. In general terms, strong lymphocytic infiltration, mainly consisting of CD45RO+ (memory) and CD8+ (cytotoxic) TILs (comprising the “immunoscore”), has been associated with clinical benefits in patients with colon, lung, breast, ovarian, prostate, and head and neck cancer [8]. For colorectal cancer it has been established that the densities and type of CD8+ TILs correlate with tumor progression regardless of patients’ tumor stage, rendering the immunoscore a significant prognostic factor, complementing or even outperforming standard pathological criteria alone [9].
In contrast to CD8+, the prognostic value of CD4+ immune infiltrates are rather debatable. This is mostly due to the fact that the CD4+ T-cell pool comprises at least 4 lineages [e.g., T-helper (Th)1, Th2, Th17 and regulatory T cells (Tregs)] with distinct functional programs also characterized by functional plasticity [10]. Accumulating evidence suggests that CD4+ Tregs have the ability to inhibit host-derived adaptive antitumor immunity mostly by suppressing tumor-specific cytotoxicity [11, 12]. The transcription factor FOXp3 plays an important role for Tregs development and function, and is considered as a reliable marker for Tregs. Reports concerning the role of CD4+ Tregs TILs ascribe a functional dichotomy in this particular subset: on one hand Tregs appear to support cancer progression by suppressing antitumor immunity, possibly representing a potential unfavorable prognostic factor which could be valuable as a therapeutic target. Indeed, this relation has been reported in a wide range of localized or metastatic human carcinomas, including ovarian, hepatocellular, lung, gastric and cervical cancers [13]. The capacity of Tregs to support a hostile tumor microenvironment promoting tumor progression has been highlighted also from reports in breast cancer supporting the role of these cells both as prognostic as well as predictive biomarkers. In comprehensive retrospective analyses, increased densities of FoxP3+ immune infiltrates were associated with poor OS which highlighted their role as unfavorable prognosticator [14, 15]. Because the endogenous tumor-reactive immunity in addition to regulating the outcome of immunotherapies also influences responses to other type of therapies, intratumoral Tregs have been also reported to function as predictive biomarkers. Thus, the density balance between CTLs and Tregs significantly associated with clinical responses after neoadjuvant chemotherapies in breast cancer with high complete pathological responses reported in patients having high CD8+/FOXp3+ ratios [16–18].
Besides adjuvant chemotherapies, densities of CD8+ T-lymphocytes have additionally been demonstrated to correlate with the outcome of neoadjuvant endocrine therapies in breast cancer patients [19]. This correlation may be linked to the tumor mutational burden (TMB)—CD8+ T infiltrates axis [5, 20]. For instance, Luminal B breast tumors are immunologic “hot” tumors characterized by high TMB and are less responsive to aromatase inhibitors [21, 22] presumably because their growth is regulated by alternate oncogenic pathways induced by activating mutations. On the other hand, tumors responsive to aromatase inhibitors should be dependent mainly on the estrogen receptor (ER) signaling for their growth meaning that such tumors have less activating oncogenic mutations during their evolution. However, irrespective of the molecular subtypes of breast cancer, the tumor microenvironment negatively impacts the antitumor immunity by downregulating tumor reactivity mediated by CD8+ CTLs mainly through induction of suppressive mechanisms [23]. In breast cancer, hormonal signaling can variously enhance tumor progression [24] which provides a serious obstacle for the successful outcome of immunotherapies in this type of cancer. This necessitates the development of combinatorial modalities to boost immunotherapeutic responses. For instance, blocking of immune checkpoints combined with hormonal therapies represents a promising therapeutic modality; however, the frequently emerging acquired immune resistance during immunotherapies with antibodies that block immune checkpoints provide a serious hurdle for its successful application. Another problem that obviously prevents the use of combined therapies is the lack of knowledge about the appropriate time for their initiation as well as their sequencing. Despite these problems, immunotherapies targeting negative pathways of immune regulation seem to hold promise for inducing durable clinical responses through the generation of tumor-specific memory which can be even improved in the presence of conventional hormone therapies, thereby significantly prolonging patients’ survival.
Besides gene mutations, oncogenic pathways in breast cancer can be activated via gene overexpression. For instance, overexpression of the cyclin D/ cyclin-dependent kinase 4 and 6 (CDK4/6) complex has been associated with oncogenes in various types of cancers with a much higher incidence in Luminal B vs Luminal A breast cancers [25] which may suggest a link between CDK4/6 overexpression with CD8+ T lymphocyte tumor infiltration. Moreover, there are studies to demonstrate modulation of the tumor microenvironment by CDK4/6 inhibitors in favor of antitumor immunity. Thus, CDK4/6 inhibitors have been reported to potentiate tumor-specific T-cell immunity by downregulating immunosuppressive tumor cell infiltrates including Tregs and myeloid populations, and also to augment antitumor CD8+ T cell-mediated antitumor cytotoxicity via increase of tumor immunogenicity [26]. Such immunoenhancing effects may pave the way for the design of novel therapeutic modalities combining CDK4/6 inhibitors with immune checkpoint blockade.
On the other hand, Tregs appear as inhibiting cancer progression by suppressing cancer-promoting inflammatory processes [27]. To this end, recent studies reported that increased frequencies of FOXp3+ Tregs were associated with improved prognosis in some tumors, such as colorectal cancer and bladder cancer [28]. So far there are no results to provide a mechanistic explanation for the functional dichotomy of Tregs. One reason could be that the prognostic effect of FOXp3+ Tregs is determined by the tumor microenvironment which is unique for each tumor depending on various parameters including tumor biology, the anatomic site where the tumor grows and host factors (e.g., microbiota, local cytokine milieu). Second, there are no phenotypic markers fully restricted to Tregs which provide a serious obstacle when applying methodologies for their isolation and functional analysis. For instance, FOXp3 and CD25 which are widely being used as phenotypic markers for Tregs, are also expressed on activated CD4+ T-helper cells which could contaminate Tregs preparations and leading to misinterpretations. Moreover, the prognostic value of Tregs is correlated with tumor stage or molecular subtype. Therefore, it will be essential to further improve our understanding of the biology and functional programs of Tregs in different human cancers so to be able to use these cells as useful biomarkers, but also as targets for immunotherapy.
During the past decade results from various studies have ascribed a multifaceted role for macrophages within the tumor microenvironment. Based on phenotype analyses there are various subsets of tumor-associated macrophages (TAMs) which play a significant role for tumor initiation and progression. In general terms, TAMs can be classified in M1 inflammatory and M2 anti-inflammatory subsets with distinct phenotypes and functions regulating tumor proliferation, progression and angiogenesis through the release of cytokines, chemokines and growth factors [29, 30]. In particular, M2 TAMs have been demonstrated to exert pro-tumoral functions in various types of solid malignancies but also in hematological cancers [29]. In breast cancer, CD163 positive M2 TAMs have been associated with tumor progression to invasive carcinomas [31]. Moreover, there are studies to suggest that the spatial distribution of CD163+ cells intratumorally may determine their clinical relevance [32, 33].
Although the central role of the immune system and particularly of T lymphocytes for controlling tumor growth has been widely accepted by the scientific community, still it remains a challenging issue why TILs cannot successfully eradicate tumors. Τhis kind of controversy results from the fact that TILs are usually identified and characterized based on their phenotypic profiles and not their functional status. Accumulating data show that TILs have become functionally silent via a plethora of immunosuppressive and immune resistance mechanisms emerging in the tumor microenvironment. Such inert TILs should appear at the late stages of the escape phase under the immunoediting process during which the tumor microenvironment becomes quite hostile for TILs. Thus, we can hypothesize that by the time human cancers become clinically detectable, at least at the early phase of their detection, the adaptive immune response is still active playing a significant role in slowing down tumor growth rates. TILs may still be capable of controlling tumor growth in a way that prolongs OS, thus delaying progression to the advanced stages of the disease. This has been recently shown by us for patients with advanced, non-metastatic, invasive ductal carcinoma who had similar frequencies of relapse and OS with early stage patients provided they had a favorable composition of TILs [32]. It is also plausible that tumor infiltration by antitumor effectors (e.g., CD8+ CTLs) and consequently the levels of intratumoral antitumor immunity, could be determined by a combination of several parameters including, TMB, MHC and tumor antigen expression, and cytokines/chemokines released by the tumor. Therefore, the strength of the antitumor endogenous immunity exerted by TILs during the early stages of the disease could be a major parameter significantly controlling tumor evolution. The proposed immune classification of colorectal cancer patients based on the density, type and localization of TILs (comprising the “immunoscore”) has a prognostic value that is superior to the standard TNM classification [9]. Considering that the immunoscore reflects to a great extend the magnitude of the anticancer endogenous immune response, it is imperative to identify cellular immune signatures functioning not only as reliable prognostic factors, but also predicting responses to therapeutic treatments as this has been shown in neoadjuvant and adjuvant chemotherapies in breast and colorectal cancer.
TILs in breast cancer
Among the various molecular subtypes in breast cancer, those characterized as triple-negative or HER2-positive, in their majority have dense immune infiltrates [34]. There are studies to show that strong infiltration of lymphocytes in the tumor tissue (occasionally designated as lymphocyte-predominant breast cancer) may predict response to neoadjuvant therapy and also may have a significant prognostic value after adjuvant chemotherapy [35]. This is an important issue which is in line with recent clinical reports showing that chemotherapies can induce clinical benefit if successfully activating the endogenous TIL-mediated antitumor immunity. There is now an increasing body of reports to show that chemotherapy can exert beneficial effects within the tumor immune microenvironment through various mechanisms including the increase of (i) CTLs and NK cells function; (ii) Th1 cytokine release; (iii) tumor cells immunogenicity; and (iv) macrophages/DCs antigen presenting capacity. Chemotherapies may also promote antitumor immunity via mechanisms decreasing the densities of Tregs and MDSCs, as well as the release of suppressor enzymes [36–39]. In particular, chemotherapeutic drugs applied for breast cancer treatment, such as anthracyclines and taxanes, are efficient in triggering immunogenic tumor cell death, thereby triggering an immune response in TILs via cross-presentation of tumor antigens by mature DCs [37]. In similar lines, Denkert et al. [40] studied intratumoral lymphocytes in pretherapy core biopsies of breast cancer patients receiving anthracyclines/taxane-based neoadjuvant chemotherapy and reported a dependence of pathologic complete responses on lymphocytic infiltrates. The authors proposed a re-activation of the endogenous antitumor immunity via presentation of tumor protein antigenic epitopes released by chemotherapy-induced tumor cell death. This suggested that chemotherapy may function as an immunopotentiator in patients whose tumors have triggered endogenously antitumor immune responses.
Cyclophosphamide and gemcitabine, which are also being used for breast cancer treatment, indirectly stimulate TIL-mediated adaptive immune responses via reduction of immunosuppressive elements [41]. Hence, chemotherapy, besides being tumoricidal, may additionally have an immunotherapeutic effect via stimulation of immune responses in TILs leading to complete clinical responses [42]. Consequently, it is conceivable that the endogenous antitumor immunity in the form of TILs confirms the presence of underlying immune pathways in breast cancer supporting the notion that this type of cancer is immunogenic and amenable to immunotherapeutic regimens. Because different immune-cell infiltrates may induce variations in clinical response rates during systemic therapies, it is important to analyze quantitatively as well as qualitatively TILs in the various compartments of breast tumors to be able to use them as potential prognostic and predictive biomarkers. Nevertheless, clinical studies are needed with a special focus on combinatorial treatments to determine the sequence and dosing of chemoimmunotherapies based on the assumption that the kinetics of development of antitumor responses are critical for the magnitude of clinical responses. To this end, we should keep in mind that despite robust clinical efficacy such combinatorial modalities may induce significant toxicity. Therefore, it is imperative to design trials to define the minimal dosage of combined chemoimmunotherapeutics causing maximal immune activation with minimal toxicity.
Differential distribution of immune infiltrates in the tumor compartments
The complex interactions between immune infiltrates and the tumor microenvironment is very crucial for determining tumor progression and clinical outcome. In various types of cancer, the type and densities of immune subsets have been associated with clinical outcomes. In the majority of human cancers increased densities of CD3+, CD8+ and memory CD45RO+ T cells in the various tumor compartments are associated with favorable prognosis. In colorectal cancer, densities of CD8+ T cells were decreased along with tumor progression, suggesting that intratumoral immune signatures can function as potential markers predicting tumor relapses and overall survival [9]. Nevertheless, this concept may not be unambiguous since in some malignancies, including B-cell lymphoma, Hodgkin lymphoma and clear cell renal cell carcinoma, high densities of tumor-infiltrating CD8+ T cells have been associated with poor prognosis [6]. Certainly, the prognostic value of CD8+ T cells may be influenced by other types of immune-cell infiltrates capable of negatively regulating their functional program. This could also represent another type of acquired immune resistance whereby tumor cells, under immune pressure by high density cytotoxic CD8+ T cells, attract intratumorally suppressor elements to counteract such an immune attack, or become immune-resistant by altering their phenotype. Therefore, detailed information concerning not only the abundance and type of TILs, but also their functional programs as well as their spatial locations is needed to have a more accurate information of their role as prognostic biomarkers [43]. For instance, in colorectal cancer, high densities of CD3+ T cells at the invasive margin were significantly associated with disease-free survival, whereas CD8+ T cells infiltrating the tumor stroma could predict breast cancer-specific survival. Moreover, a high degree of stromal immune infiltration was found to be associated with increased disease-specific survival in ER-negative/HER2-negative breast cancer patients [44, 45]. Notably, some groups reported that this favorable prognosis, due to the abundance of immune infiltrates, was dependent on the levels of “hotspots” defined as the co-localization of cancer and immune cells. In this way they could identify groups of patients with low hotspot scores who, regardless of high or low immune infiltrates, had a similar poor prognosis [46]. More recently, Miyan et al. studied T-cell infiltration in the various molecular subtypes of breast cancer and found low ratios of CD8+ and FOXp3+ T cells in TC and IM which were associated with poor prognosis [47].
One reasonable question then is what causes such differential distributions of immune cells among the tumor compartments and how this is linked to clinical outcome. A crucial factor that could account for this concerns the biological properties of the tumor. This is supported by a broad range of inflammatory gene and protein signatures within the tumor as determined by high throughput technologies. Such signatures include information for (i) factors related to Th1 or Th2 adaptive immunity; (ii) immune effector or cytotoxic factors; (iii) chemokine ligands, chemokines, and adhesion molecules and (iv) regulatory molecules. These signatures are heterogeneous among tumor types and may contribute to differences in vascularization, high endothelial venule (HEV) density, tumor antigenicity, tumor suppressor potential and chemokine/cytokine profiles, all of which can influence to various levels the density and function of intratumoral immune cells as well as their spatial localization within tumor regions predicting patient survival [48].
To this end, of particular interest is the association of CD8+ T-cell infiltration with the immunogenic ΤΜΒ. Accumulating evidence suggests that patients’ tumors with somatic nonsynonymous coding mutations have elevated expression of tumor-specific neoantigens and elevated counts of CD8+ TIL [49, 50]. Among the dense CD8+ T-cell infiltrates, are tumor-reactive clones recognizing tumor expressed neoantigens [5, 51]. Nevertheless, it appears that missense mutations generating endogenous tumor reactivity are relatively rare. This is due to the fact that only few mutations per tumor are immunogenic, and tumors with a higher mutational burden have increased chances of expressing immunogenic neoantigens. However, there are many tumors having high CD8+ infiltrates albeit with few or no predicted immunogenic missense mutations suggesting that in these tumors, immunogenic neoantigenic epitopes attracting CD8+ T cells have been generated by other conditions of genetic hypermutability, mainly including that of microsatellite instability [52]. Alternatively, during the process of immune editing, tumor clones with immunogenic missense mutations may have been selectively recognized and deleted resulting in the accumulation of CD8+ T cells. Irrespective of the mechanism(s) that may lead to imbalances between CD8+ T-cell densities and mutational load, tumor areas overpopulated by tumor cells may attract tumor-reactive CD8+ T cells resulting in the generation of an inflammatory milieu with release of MIP-3b attracting DCs and thus completing the picture of a sustainable local antitumor immune response [53, 54]. DCs, in turn, may facilitate the accumulation of TILs at specific tumor compartments through generation and maintenance of HEV intratumorally [55]. In breast cancer, HEV number and density are highly correlated with the extravasation of immune effector and memory T lymphocytes into the tumor, which was correlated with improved clinical outcomes [56]. Moreover, patients with high lymphatic vessels in the IM of their tumors had high levels of cytotoxicity and reduced metastases [57] ascribing antitumor properties to lymphatic vascularization via facilitated immune-cell infiltration and prevention of metastatic invasions at the IM.
Intratumoral immune infiltrates and signatures in patients with breast cancer
As outlined above, the spatiotemporal tumor infiltration by immune cells is a dynamic process which depends on multiple factors released by various cells, also including tumor cells, which attract TILs at distinct tumor areas followed by the generation of pro-inflammatory or immunosuppressive pathways. With TILs migrating to the different tumor areas, considerable levels of heterogeneity, both in terms of cell-types and function, are generated which suggests that their separate and combined evaluation in well-defined tumor regions would be valuable [32]. To this end, there are reports to show that combined density analyses for memory (CD45RO+) and cytotoxic markers in TC and IM could be useful for precisely predicting tumor recurrences and survival for different patient groups in colorectal cancer [58, 59]. Although the concept of evaluating immune infiltration in breast cancer and making correlations with clinical outcomes is not new, yet there have been few consistent data on the differential distribution of immune cells in the tumor areas and their combined evaluation as reliable prognosticators. The presence of TILs in either the stromal or intratumoral compartment was valuable for the outcome of neoadjuvant therapies [60]. Spatial heterogeneity in the co-localization of tumor cells and TILs was found to play a prominent role for clinical outcome in ER-negative breast cancer [43] regardless the abundance of TILs infiltration, underlying the need to study heterogeneity of spatial distribution in addition to measuring abundance of microenvironmental components. Further to this, Miyan et al. demonstrated differential densities of CD3+ and CD8+ cells in the TC and IM among the different molecular subtypes of BCa, but they did not address their clinical relevance [47].
Our retrospective analyses of CD8+ and CD163+ cells in TC and IM as single subpopulations or jointly, provided first information regarding the role of spatial heterogeneity of immune infiltrates on the frequency of relapses and overall survival in patients with breast cancer [32]. Our study was designed to answer novel questions. First, can combined analyses of immune infiltrates (CD8+ or CD163+ cells) in the tumor regions (TC and IM) provide advantages over single region analyses for patient prognosis? Second, can the combined evaluation of CD8+ and CD163+ cell densities jointly in TC and IM improve prognosis? Third, how does the immune infiltration in the tumor compartments compare with clinicopathological parameters regarding clinical prognosis? Starting with single CD8+ or CD163+ cell analyses in either TC or IM, we could hardly find statistical correlations with the frequency of tumor recurrence or survival [except in the case of low vs high CD8+ cell densities in the IM which correlated with disease-free survival (DFS)]. As expected, both DFS and OS were correlated with standard TNM staging, grade, tumor size and invaded lymph nodes. This first part of our analyses demonstrated that neither CD8+ nor CD163+ cell densities could contribute to prognosis when separately analyzed in either TC or IM. By analyzing each one of these subsets in TC and IM, we found that high densities of CD8+ cells in the TC concomitantly with low ones in the IM (referred to as HL) predict significantly better DFS and OS compared with the inverse densities of CD8+ cells in the combined tumor regions (i.e., CD8+ low in the TC and CD8+ high in the IM; referred to as LH) [32]. This suggests that CTL (assuming that in our case the CD8+ cells are cytotoxic) represent a prognostic biomarker, and further delineates the importance of their spatial distribution in high or low numbers, which can help refining their predictive capability on patients’ clinical outcome. Interestingly, when DFS and OS were assessed based on CD163+ cell densities, patients with LH densities (i.e., TC low and IM high densities) had significantly increased DFS or prolonged OS compared to patients with the inverse densities of CD163+ cells, namely high in TC and low in IM (HL). Next, we assessed these CD8+ and CD163+ cell density profiles for complementarity. The patients were separated into 2 groups. The 1st group included patients with CD8HL or CD163LH (also including those patients with CD8HL and CD163LH); this constituted the favorable combined immune signature (FCIS). In the 2nd group we have included patients with CD8LH or CD163HL (and patients with CD8LH and CD163HL, but not CD8HL or CD163 LH); this constituted the unfavorable combined immune signature (UCIS) (Fig. 1). We found that the combined immune signatures had a much higher prognostic significance for both DFS and OS as compared to the single ones. Given that this type of analyses was performed with the total patient population, we were keen to analyze the prognostic value of FCIS vs UCIS in subgroups of patients based on their clinicopathologic characteristics. Our data consistently showed that the FCIS had a robust favorable prognostic value in patients with poor clinicopathologic parameters: patients having the FCIS and poor clinicopathological parameters (i.e., undifferentiated grade 3 tumors, large T2,3 tumors, invaded lymph nodes, and advanced disease) had similar DFS and OS with patients having favorable prognosis (i.e., grade 1,2/T1 tumors, non-invaded lymph nodes and early disease) [32]. Ongoing studies in our laboratory show that, in the vast majority of these patients, high CD8+ cell densities in the tumor compartments (TC and/or IM) are accompanied by high densities of FoxP3+ cells which could be explained as a mechanism of acquired immune resistance. This was supported by data showing that low densities of CD8+ cells in these compartments did not attract high numbers of FoxP3+ cells and this was correlated with favorable clinical outcomes.
Fig. 1.
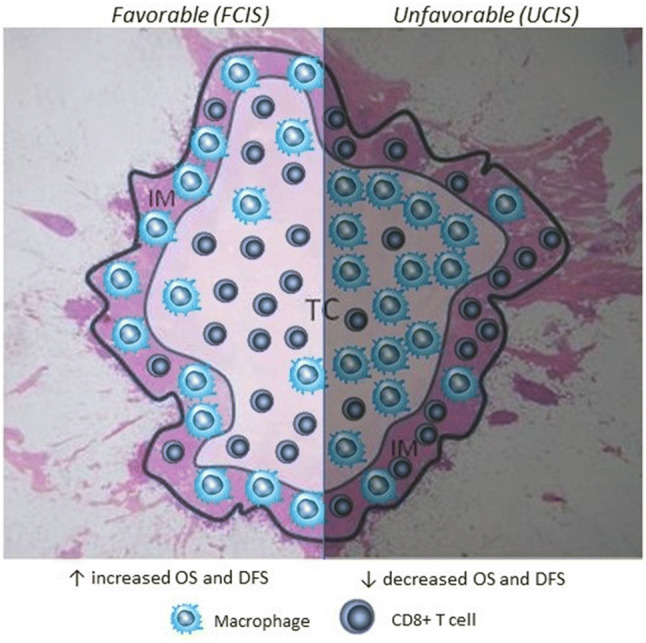
A Schematic representation of intratumoral FCIS and UCIS. The former is characterized by high CD8+ cell densities and low CD163+ ones within the TC combined with inverse densities for the same subsets in the IM. Mechanistically this could be explained via chemical gradients consisting of chemokines released by the tumor cells which attract CD8+ cells into the TC leaving only sparse CD8+ cells in the IM. In contrast, CD163+ cells are not affected by such gradients thus remaining in the IM and allowing the CD8+ cells in the TC to develop efficient antitumor reactivity resulting in improved clinical outcomes. In the UCIS the intratumoral cellular densities are inverse (i.e., low CD8+ and high CD163+ cell densities in the TC combined with high CD8+ and low CD163+ cell densities in the IM) which may be explained by different chemokine gradients through different tumor-released chemokine patterns
Future perspectives
Advances in tumor biology along with improvements in our understanding of the dynamic interplay between the immune system and tumor cells are of pivotal importance for disease evolution. Reports from various clinical trials offer now accumulating evidence to propose a link between divergent tumor biology with the composition of tumor microenvironment and activation of tumor-specific adaptive immunity. The majority of cancers at diagnosis may have evaded immune surveillance, but nevertheless, residual anticancer immune responses in the form of preexisting immunity could have significant prognostic and predictive roles. Although this has been explored in many cancer types, including breast cancer, ongoing investigations on the contribution of the tumor microenvironment to prediction and prognosis will surely provide additional fundamental information. So far, knowledge derived from the immune contexture of a tumor yields information on the spatial distribution of immune infiltrates which undoubtedly improves standard clinicopathological criteria for clinical outcomes. Our studies have an important contribution to this, demonstrating for the first time that differential densities of immune infiltrates jointly assessed in the TC and IM have a robust prognostic value in breast cancer patients with poor clinicopathological parameters. To this end we have shown that favorable prognostic features include the dense presence of T-lymphocyte subsets supporting cytotoxic responses (e.g., CD8+ cells) in TC along with low densities of immunosuppressive elements (e.g., CD163+ cells) combined with the inverse cell densities in IM (i.e., low CD8+ and high CD163+ cell densities). Of course, our findings do not preclude other types of cells with prognostic significance in the tumor microenvironment. Thus, clinical research should focus to further evaluation of the potential prognostic and predictive role of cells constituting the preexisting immunity in tumors. Through this, we may have better chances to select patients who most likely will benefit from therapeutic modalities targeting immunomodulatory molecules to reinforce the endogenous antitumor immunity.
Another important aspect that should be explored for biomarker discovery is that of T-cell functionality. It will be namely essential to identify CD8+ T cells capable of mediating antitumor cytotoxic responses and not being anergic or exhausted. T-cell functionality depends on the expression of immune checkpoints mostly as a result of increased tumor mutational load and expression of neoantigens [50, 61]. The latter represent another factor which strongly impacts the outcome of intratumoral immune responses. Depending on the modus of their expression, neoantigens may influence such responses in opposite directions: tumors exhibiting clonal expression of neoantigens have an increased likelihood of responding to checkpoint blockade and highly clonal tumors are more immunogenic than their more heterogeneous counterparts [5]. The latter only poorly respond to immunotherapies. Nevertheless, mutational load leading to the formation of neoantigens, is not the only factor which may determine the outcome of an anticancer immune response. Several studies suggested that additional biomarkers, including copy number alterations affecting particular genes and signaling pathways, correlate with poor T-lymphocyte tumor infiltrates, and may function as valuable predictive biomarkers for clinical outcomes after immune checkpoint therapies [20, 62, 63]. Given that lack of clinical responses after immune checkpoint inhibition in heterogeneous tumors correlates with absence or low T-cell infiltration, it is conceivable that lack of T-cell-based immunoselection during immunoediting results in tumors that escape immunosurveillance. Thus, combining the mutational load with the copy number alterations, may predict more accurately clinical response to immune checkpoint inhibition. This intratumoral network which applies for the majority of cancers has led to the application of combined therapeutic modalities including standard chemotherapy, targeted agents and immunotherapies with impressive clinical achievements in some patients [64]. Moreover, distinct immunotherapies aiming at reversing immune suppression in the tumor microenvironment induced prolonged clinical responses [23].
Conclusions
There is now compelling evidence on the prognostic and predictive role of TILs. It is a general consensus that higher numbers of TILs are linked to higher probability to achieve clinical responses. In the light of the impressive clinical results with immune checkpoint inhibitors based on the re-activation of the endogenous antitumor immunity, the presence and type of TILs will be used as a guide to inform decisions on systemic therapy. However, there is a serious obstacle which may hinder the field moving forward, namely tumor heterogeneity may prove to be a confounding factor towards validation of TIL prognostic and predictive biomarkers. Inventing therapeutic modalities to generate robust host immune response and enable tumor control are therefore required to circumvent this problem. For this it will be mandatory to improve our knowledge about the immune circuits which regulate antitumor T-cell reactivity in the tumor microenvironment to develop strategies for further improving clinical results from cancer immunotherapies.
Abbreviations
- BCa
Breast cancer
- CDK4/6
Cyclin-dependent kinase 4 and 6
- DFS
Disease-free survival
- ER
Estrogen receptor
- FCIS
Favorable combined immune signature
- HEV
High endothelial venule
- IM
Invasive margin
- TAM
Tumor-associated macrophages
- TC
Tumor center
- Th
T-helper
- TMB
Tumor mutational burden
- Tregs
Regulatory T cells
- UCIS
Unfavorable combined immune signature
Author Contributions
Constantin N. Baxevanis conceived the manuscript. All authors wrote and edited the manuscript and approved its final version.
Funding
No relevant funding.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, Adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168(4):707–723. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21(2):137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 3.Zhu J, Petit PF, Van den Eynde BJ. Apoptosis of tumor-infiltrating T lymphocytes: a new immune checkpoint mechanism. Cancer Immunol Immunother. 2018 doi: 10.1007/s00262-018-2269-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bindea G, Mlecnik B, Fridman WH, Pages F, Galon J. Natural immunity to cancer in humans. Curr Opin Immunol. 2010;22(2):215–222. doi: 10.1016/j.coi.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Spranger S. Tumor heterogeneity and tumor immunity: a chicken-and-egg problem. Trends Immunol. 2016;37(6):349–351. doi: 10.1016/j.it.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Fridman WH, Zitvogel L, Sautes-Fridman C, Kroemer G. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol. 2017;14(12):717–734. doi: 10.1038/nrclinonc.2017.101. [DOI] [PubMed] [Google Scholar]
- 7.Baxevanis CN, Perez SA. Cancer dormancy: a regulatory role for endogenous immunity in establishing and maintaining the tumor Dormant state. Vaccines (Basel) 2015;3(3):597–619. doi: 10.3390/vaccines3030597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vano YA, Petitprez F, Giraldo NA, Fridman WH, Sautes-Fridman C. Immune-based identification of cancer patients at high risk of progression. Curr Opin Immunol. 2018;51:97–102. doi: 10.1016/j.coi.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Church SE, Galon J. Regulation of CTL infiltration within the tumor microenvironment. Adv Exp Med Biol. 2017;1036:33–49. doi: 10.1007/978-3-319-67577-0_3. [DOI] [PubMed] [Google Scholar]
- 10.Fang D, Zhu J. Dynamic balance between master transcription factors determines the fates and functions of CD4 T cell and innate lymphoid cell subsets. J Exp Med. 2017;214(7):1861–1876. doi: 10.1084/jem.20170494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shang B, Liu Y, Jiang SJ, Liu Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: a systematic review and meta-analysis. Sci Rep. 2015;5:15179. doi: 10.1038/srep15179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whiteside TL. Regulatory T cell subsets in human cancer: are they regulating for or against tumor progression? Cancer Immunol Immunother. 2014;63(1):67–72. doi: 10.1007/s00262-013-1490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whiteside TL. The role of regulatory T cells in cancer immunology. Immunotargets Ther. 2015;4:159–171. doi: 10.2147/ITT.S55415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shou J, Zhang Z, Lai Y, Chen Z, Huang J. Worse outcome in breast cancer with higher tumor-infiltrating FOXP3+ Tregs: a systematic review and meta-analysis. BMC Cancer. 2016;16:687. doi: 10.1186/s12885-016-2732-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whiteside TL. Disarming suppressor cells to improve immunotherapy. Cancer Immunol Immunother. 2012;61(2):283–288. doi: 10.1007/s00262-011-1171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asano Y, Kashiwagi S, Goto W, Kurata K, Noda S, Takashima T, Onoda N, Tanaka S, Ohsawa M, Hirakawa K. Tumour-infiltrating CD8 to FOXP3 lymphocyte ratio in predicting treatment responses to neoadjuvant chemotherapy of aggressive breast cancer. Br J Surg. 2016;103(7):845–854. doi: 10.1002/bjs.10127. [DOI] [PubMed] [Google Scholar]
- 17.Miyashita M, Sasano H, Tamaki K, Chan M, Hirakawa H, Suzuki A, Tada H, Watanabe G, Nemoto N, Nakagawa S, Ishida T, Ohuchi N. Tumor-infiltrating CD8+ and FOXP3+ lymphocytes in triple-negative breast cancer: its correlation with pathological complete response to neoadjuvant chemotherapy. Breast Cancer Res Treat. 2014;148(3):525–534. doi: 10.1007/s10549-014-3197-y. [DOI] [PubMed] [Google Scholar]
- 18.Nabholtz JM, Abrial C, Mouret-Reynier MA, Dauplat MM, Weber B, Gligorov J, Forest AM, Tredan O, Vanlemmens L, Petit T, Guiu S, Van Praagh I, Jouannaud C, Dubray-Longeras P, Tubiana-Mathieu N, Benmammar KE, Kullab S, Bahadoor MR, Radosevic-Robin N, Kwiatkowski F, Desrichard A, Cayre A, Uhrhammer N, Chalabi N, Chollet P, Penault-Llorca F. Multicentric neoadjuvant phase II study of panitumumab combined with an anthracycline/taxane-based chemotherapy in operable triple-negative breast cancer: identification of biologically defined signatures predicting treatment impact. Ann Oncol. 2014;25(8):1570–1577. doi: 10.1093/annonc/mdu183. [DOI] [PubMed] [Google Scholar]
- 19.Chan MS, Wang L, Felizola SJ, Ueno T, Toi M, Loo W, Chow LW, Suzuki T, Sasano H. Changes of tumor infiltrating lymphocyte subtypes before and after neoadjuvant endocrine therapy in estrogen receptor-positive breast cancer patients—an immunohistochemical study of Cd8+ and Foxp3+ using double immunostaining with correlation to the pathobiological response of the patients. Int J Biol Markers. 2012;27(4):e295–e304. doi: 10.5301/JBM.2012.10439. [DOI] [PubMed] [Google Scholar]
- 20.McGranahan N, Furness AJ, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, Jamal-Hanjani M, Wilson GA, Birkbak NJ, Hiley CT, Watkins TB, Shafi S, Murugaesu N, Mitter R, Akarca AU, Linares J, Marafioti T, Henry JY, Van Allen EM, Miao D, Schilling B, Schadendorf D, Garraway LA, Makarov V, Rizvi NA, Snyder A, Hellmann MD, Merghoub T, Wolchok JD, Shukla SA, Wu CJ, Peggs KS, Chan TA, Hadrup SR, Quezada SA, Swanton C. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351(6280):1463–1469. doi: 10.1126/science.aaf1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Creighton CJ. The molecular profile of luminal B breast cancer. Biologics. 2012;6:289–297. doi: 10.2147/BTT.S29923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Budczies J, Bockmayr M, Denkert C, Klauschen F, Lennerz JK, Gyorffy B, Dietel M, Loibl S, Weichert W, Stenzinger A. Classical pathology and mutational load of breast cancer—integration of two worlds. J Pathol Clin Res. 2015;1(4):225–238. doi: 10.1002/cjp2.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munn DH, Bronte V. Immune suppressive mechanisms in the tumor microenvironment. Curr Opin Immunol. 2016;39:1–6. doi: 10.1016/j.coi.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rothenberger Natalie, Somasundaram Ashwin, Stabile Laura. The Role of the Estrogen Pathway in the Tumor Microenvironment. International Journal of Molecular Sciences. 2018;19(2):611. doi: 10.3390/ijms19020611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J, Barretina J, Boehm JS, Dobson J, Urashima M, Mc Henry KT, Pinchback RM, Ligon AH, Cho YJ, Haery L, Greulich H, Reich M, Winckler W, Lawrence MS, Weir BA, Tanaka KE, Chiang DY, Bass AJ, Loo A, Hoffman C, Prensner J, Liefeld T, Gao Q, Yecies D, Signoretti S, Maher E, Kaye FJ, Sasaki H, Tepper JE, Fletcher JA, Tabernero J, Baselga J, Tsao MS, Demichelis F, Rubin MA, Janne PA, Daly MJ, Nucera C, Levine RL, Ebert BL, Gabriel S, Rustgi AK, Antonescu CR, Ladanyi M, Letai A, Garraway LA, Loda M, Beer DG, True LD, Okamoto A, Pomeroy SL, Singer S, Golub TR, Lander ES, Getz G, Sellers WR, Meyerson M. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463(7283):899–905. doi: 10.1038/nature08822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teh JLF, Aplin AE. Arrested Developments: CDK4/6 Inhibitor Resistance and Alterations in the Tumor Immune Microenvironment. Clin Cancer Res. 2018 doi: 10.1158/1078-0432.CCR-18-1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whiteside TL. Induced regulatory T cells in inhibitory microenvironments created by cancer. Expert Opin Biol Ther. 2014;14(10):1411–1425. doi: 10.1517/14712598.2014.927432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.deLeeuw RJ, Kost SE, Kakal JA, Nelson BH. The prognostic value of FoxP3+ tumor-infiltrating lymphocytes in cancer: a critical review of the literature. Clin Cancer Res. 2012;18(11):3022–3029. doi: 10.1158/1078-0432.CCR-11-3216. [DOI] [PubMed] [Google Scholar]
- 29.Ruffell B, Coussens LM. Macrophages and therapeutic resistance in cancer. Cancer Cell. 2015;27(4):462–472. doi: 10.1016/j.ccell.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santoni M, Massari F, Amantini C, Nabissi M, Maines F, Burattini L, Berardi R, Santoni G, Montironi R, Tortora G, Cascinu S. Emerging role of tumor-associated macrophages as therapeutic targets in patients with metastatic renal cell carcinoma. Cancer Immunol Immunother. 2013;62(12):1757–1768. doi: 10.1007/s00262-013-1487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoskoppal D, Reisenbichler ES. Can tumor-associated macrophages in ductal carcinoma in situ (DCIS) on biopsy predict invasive carcinoma on excision? Hum Pathol. 2018 doi: 10.1016/j.humpath.2018.07.023. [DOI] [PubMed] [Google Scholar]
- 32.Fortis SP, Sofopoulos M, Sotiriadou NN, Haritos C, Vaxevanis CK, Anastasopoulou EA, Janssen N, Arnogiannaki N, Ardavanis A, Pawelec G, Perez SA, Baxevanis CN. Differential intratumoral distributions of CD8 and CD163 immune cells as prognostic biomarkers in breast cancer. J Immunother Cancer. 2017;5:39. doi: 10.1186/s40425-017-0240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu SQ, Xu R, Li XF, Zhao XK, Qian BZ. Prognostic roles of tumor associated macrophages in bladder cancer: a system review and meta-analysis. Oncotarget. 2018;9(38):25294–25303. doi: 10.18632/oncotarget.25334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pfitzner BM, Lederer B, Lindner J, Solbach C, Engels K, Rezai M, Dohnal K, Tesch H, Hansmann ML, Salat C, Beer M, Schneeweiss A, Sinn P, Bankfalvi A, Darb-Esfahani S, von Minckwitz G, Sinn BV, Kronenwett R, Weber K, Denkert C, Loibl S. Clinical relevance and concordance of HER2 status in local and central testing-an analysis of 1581 HER2-positive breast carcinomas over 12 years. Mod Pathol. 2018;31(4):607–615. doi: 10.1038/modpathol.2017.171. [DOI] [PubMed] [Google Scholar]
- 35.Denkert C, Wienert S, Poterie A, Loibl S, Budczies J, Badve S, Bago-Horvath Z, Bane A, Bedri S, Brock J, Chmielik E, Christgen M, Colpaert C, Demaria S, Van den Eynden G, Floris G, Fox SB, Gao D, Ingold Heppner B, Kim SR, Kos Z, Kreipe HH, Lakhani SR, Penault-Llorca F, Pruneri G, Radosevic-Robin N, Rimm DL, Schnitt SJ, Sinn BV, Sinn P, Sirtaine N, O’Toole SA, Viale G, Van de Vijver K, de Wind R, von Minckwitz G, Klauschen F, Untch M, Fasching PA, Reimer T, Willard-Gallo K, Michiels S, Loi S, Salgado R. Standardized evaluation of tumor-infiltrating lymphocytes in breast cancer: results of the ring studies of the international immuno-oncology biomarker working group. Mod Pathol. 2016;29(10):1155–1164. doi: 10.1038/modpathol.2016.109. [DOI] [PubMed] [Google Scholar]
- 36.Zheng H, Zeltsman M, Zauderer MG, Eguchi T, Vaghjiani RG, Adusumilli PS. Chemotherapy-induced immunomodulation in non-small-cell lung cancer: a rationale for combination chemoimmunotherapy. Immunotherapy. 2017;9(11):913–927. doi: 10.2217/imt-2017-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8(1):59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- 38.Liljenfeldt L, Gkirtzimanaki K, Vyrla D, Svensson E, Loskog AS, Eliopoulos AG. Enhanced therapeutic anti-tumor immunity induced by co-administration of 5-fluorouracil and adenovirus expressing CD40 ligand. Cancer Immunol Immunother. 2014;63(3):273–282. doi: 10.1007/s00262-013-1507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fritzell S, Sanden E, Eberstal S, Visse E, Darabi A, Siesjo P. Intratumoral temozolomide synergizes with immunotherapy in a T cell-dependent fashion. Cancer Immunol Immunother. 2013;62(9):1463–1474. doi: 10.1007/s00262-013-1449-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Denkert C, Loibl S, Noske A, Roller M, Muller BM, Komor M, Budczies J, Darb-Esfahani S, Kronenwett R, Hanusch C, von Torne C, Weichert W, Engels K, Solbach C, Schrader I, Dietel M, von Minckwitz G. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28(1):105–113. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- 41.Vacchelli E, Enot DP, Pietrocola F, Zitvogel L, Kroemer G. Impact of pattern recognition receptors on the prognosis of breast cancer patients undergoing adjuvant chemotherapy. Cancer Res. 2016;76(11):3122–3126. doi: 10.1158/0008-5472.CAN-16-0294. [DOI] [PubMed] [Google Scholar]
- 42.Menard C, Martin F, Apetoh L, Bouyer F, Ghiringhelli F. Cancer chemotherapy: not only a direct cytotoxic effect, but also an adjuvant for antitumor immunity. Cancer Immunol Immunother. 2008;57(11):1579–1587. doi: 10.1007/s00262-008-0505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nawaz S, Heindl A, Koelble K, Yuan Y. Beyond immune density: critical role of spatial heterogeneity in estrogen receptor-negative breast cancer. Mod Pathol. 2015;28(12):1621. doi: 10.1038/modpathol.2015.133. [DOI] [PubMed] [Google Scholar]
- 44.Dushyanthen S, Beavis PA, Savas P, Teo ZL, Zhou C, Mansour M, Darcy PK, Loi S. Relevance of tumor-infiltrating lymphocytes in breast cancer. BMC Med. 2015;13:202. doi: 10.1186/s12916-015-0431-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wein L, Savas P, Luen SJ, Virassamy B, Salgado R, Loi S. Clinical validity and utility of tumor-infiltrating lymphocytes in routine clinical practice for breast cancer patients: current and future directions. Front Oncol. 2017;7:156. doi: 10.3389/fonc.2017.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McIntire PJ, Irshaid L, Liu Y, Chen Z, Menken F, Nowak E, Shin SJ, Ginter PS. Hot spot and whole-tumor enumeration of CD8(+) tumor-infiltrating lymphocytes utilizing digital image analysis is prognostic in triple-negative breast cancer. Clin Breast Cancer. 2018 doi: 10.1016/j.clbc.2018.04.019. [DOI] [PubMed] [Google Scholar]
- 47.Miyan M, Schmidt-Mende J, Kiessling R, Poschke I, de Boniface J. Differential tumor infiltration by T-cells characterizes intrinsic molecular subtypes in breast cancer. J Transl Med. 2016;14(1):227. doi: 10.1186/s12967-016-0983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Galon J, Angell HK, Bedognetti D, Marincola FM. The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures. Immunity. 2013;39(1):11–26. doi: 10.1016/j.immuni.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 49.Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, Sucker A, Hillen U, Foppen MHG, Goldinger SM, Utikal J, Hassel JC, Weide B, Kaehler KC, Loquai C, Mohr P, Gutzmer R, Dummer R, Gabriel S, Wu CJ, Schadendorf D, Garraway LA. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015;350(6257):207–211. doi: 10.1126/science.aad0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Popovic A, Jaffee EM, Zaidi N. Emerging strategies for combination checkpoint modulators in cancer immunotherapy. J Clin Invest. 2018;128(8):3209–3218. doi: 10.1172/JCI120775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gajewski TF, Corrales L, Williams J, Horton B, Sivan A, Spranger S. Cancer immunotherapy targets based on understanding the T cell-inflamed versus non-T cell-inflamed tumor microenvironment. Adv Exp Med Biol. 2017;1036:19–31. doi: 10.1007/978-3-319-67577-0_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mlecnik B, Bindea G, Angell HK, Maby P, Angelova M, Tougeron D, Church SE, Lafontaine L, Fischer M, Fredriksen T, Sasso M, Bilocq AM, Kirilovsky A, Obenauf AC, Hamieh M, Berger A, Bruneval P, Tuech JJ, Sabourin JC, Le Pessot F, Mauillon J, Rafii A, Laurent-Puig P, Speicher MR, Trajanoski Z, Michel P, Sesboue R, Frebourg T, Pages F, Valge-Archer V, Latouche JB, Galon J. Integrative analyses of colorectal cancer show immunoscore is a stronger predictor of patient survival than microsatellite instability. Immunity. 2016;44(3):698–711. doi: 10.1016/j.immuni.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 53.Tokalov SV, Abolmaali ND. Protection of p53 wild type cells from taxol by nutlin-3 in the combined lung cancer treatment. BMC Cancer. 2010;10:57. doi: 10.1186/1471-2407-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Middel P, Brauneck S, Meyer W, Radzun HJ. Chemokine-mediated distribution of dendritic cell subsets in renal cell carcinoma. BMC Cancer. 2010;10:578. doi: 10.1186/1471-2407-10-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moussion C, Girard JP. Dendritic cells control lymphocyte entry to lymph nodes through high endothelial venules. Nature. 2011;479(7374):542–546. doi: 10.1038/nature10540. [DOI] [PubMed] [Google Scholar]
- 56.Martinet L, Garrido I, Filleron T, Le Guellec S, Bellard E, Fournie JJ, Rochaix P, Girard JP. Human solid tumors contain high endothelial venules: association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res. 2011;71(17):5678–5687. doi: 10.1158/0008-5472.CAN-11-0431. [DOI] [PubMed] [Google Scholar]
- 57.Mlecnik B, Bindea G, Kirilovsky A, Angell HK, Obenauf AC, Tosolini M, Church SE, Maby P, Vasaturo A, Angelova M, Fredriksen T, Mauger S, Waldner M, Berger A, Speicher MR, Pages F, Valge-Archer V, Galon J. The tumor microenvironment and Immunoscore are critical determinants of dissemination to distant metastasis. Sci Transl Med. 2016;8(327):327ra326. doi: 10.1126/scitranslmed.aad6352. [DOI] [PubMed] [Google Scholar]
- 58.Pages F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, Lagorce C, Wind P, Marliot F, Bruneval P, Zatloukal K, Trajanoski Z, Berger A, Fridman WH, Galon J. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 2009;27(35):5944–5951. doi: 10.1200/JCO.2008.19.6147. [DOI] [PubMed] [Google Scholar]
- 59.Mlecnik B, Tosolini M, Kirilovsky A, Berger A, Bindea G, Meatchi T, Bruneval P, Trajanoski Z, Fridman WH, Pages F, Galon J. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol. 2011;29(6):610–618. doi: 10.1200/JCO.2010.30.5425. [DOI] [PubMed] [Google Scholar]
- 60.Denkert C, von Minckwitz G, Brase JC, Sinn BV, Gade S, Kronenwett R, Pfitzner BM, Salat C, Loi S, Schmitt WD, Schem C, Fisch K, Darb-Esfahani S, Mehta K, Sotiriou C, Wienert S, Klare P, Andre F, Klauschen F, Blohmer JU, Krappmann K, Schmidt M, Tesch H, Kummel S, Sinn P, Jackisch C, Dietel M, Reimer T, Untch M, Loibl S. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol. 2015;33(9):983–991. doi: 10.1200/JCO.2014.58.1967. [DOI] [PubMed] [Google Scholar]
- 61.Miao D, Margolis CA, Vokes NI, Liu D, Taylor-Weiner A, Wankowicz SM, Adeegbe D, Keliher D, Schilling B, Tracy A, Manos M, Chau NG, Hanna GJ, Polak P, Rodig SJ, Signoretti S, Sholl LM, Engelman JA, Getz G, Janne PA, Haddad RI, Choueiri TK, Barbie DA, Haq R, Awad MM, Schadendorf D, Hodi FS, Bellmunt J, Wong KK, Hammerman P, Van Allen EM. Genomic correlates of response to immune checkpoint blockade in microsatellite-stable solid tumors. Nat Genet. 2018;50(9):1271–1281. doi: 10.1038/s41588-018-0200-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kato S, Goodman A, Walavalkar V, Barkauskas DA, Sharabi A, Kurzrock R. Hyperprogressors after immunotherapy: analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res. 2017;23(15):4242–4250. doi: 10.1158/1078-0432.CCR-16-3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miao D, Margolis CA, Gao W, Voss MH, Li W, Martini DJ, Norton C, Bosse D, Wankowicz SM, Cullen D, Horak C, Wind-Rotolo M, Tracy A, Giannakis M, Hodi FS, Drake CG, Ball MW, Allaf ME, Snyder A, Hellmann MD, Ho T, Motzer RJ, Signoretti S, Kaelin WG, Jr, Choueiri TK, Van Allen EM. Genomic correlates of response to immune checkpoint therapies in clear cell renal cell carcinoma. Science. 2018;359(6377):801–806. doi: 10.1126/science.aan5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019;18(3):197–218. doi: 10.1038/s41573-018-0007-y. [DOI] [PubMed] [Google Scholar]


