Abstract
Antitumor strategies based on positive modulation of the immune system currently represent therapeutic options with prominent acceptance for cancer patients’ treatment due to its selectivity and higher tolerance compared to chemotherapy. Racotumomab is an anti-idiotype (anti-Id) monoclonal antibody (mAb) directed to NeuGc-containing gangliosides such as NeuGcGM3, a widely reported tumor-specific neoantigen in many human cancers. Racotumomab has been approved in Latin American countries as an active immunotherapy for advanced non-small cell lung cancer (NSCLC) treatment. In this work, we evaluated the induction of Ab-dependent cell-mediated cytotoxicity (ADCC) in NSCLC patients included in a phase III clinical trial, in response to vaccination with racotumomab. The development of anti-NeuGcGM3 antibodies (Abs) in serum samples of immunized patients was first evaluated using the NeuGcGM3-expressing X63 cells, showing that racotumomab vaccination developed antigen-specific Abs that are able to recognize NeuGcGM3 expressed in tumor cell membranes. ADCC response against NeuGcGM3-expressing X63 (target) was observed in racotumomab-treated- but not in control group patients. When target cells were depleted of gangliosides by treatment with a glucosylceramide synthase inhibitor, we observed a significant reduction of the ADCC activity developed by sera from racotumomab-vaccinated patients, suggesting a target-specific response. Our data demonstrate that anti-NeuGcGM3 Abs induced by racotumomab vaccination are able to mediate an antigen-specific ADCC response against tumor cells in NSCLC patients.
Keywords: Racotumomab, Active immunotherapy, ADCC, Non-small cell lung cancer, NeuGcGM3 ganglioside
Introduction
Lung cancer is one of the most frequently diagnosed malignancies and also the leading cause of cancer deaths among males and females [1]. In particular, NSCLC represents 85% of total lung cancer and is usually diagnosed in an advanced or metastatic stage [2]. Therapeutic options for NSCLC treatment include surgical removal of the primary tumor, chemo- and radiotherapy [3] and molecular-targeted therapies [4]. Most recently, immunotherapy has also become a standard of care therapy for a selected group of NSCLC patients [5]. However, lung cancer prognosis remains poor and overall survival rate is still less than 20%. In this scenario, where new, safe and specific therapeutic approaches are needed, immunotherapeutic agents are promising drugs that overcome the limitations of conventional strategies.
Immunotherapy has been used for the treatment of cancer patients for many years, with clinically proven benefits. The development of successful immunotherapeutic products is the result of decades of scientific research in tumor antigens and Abs, tumor-relevant signaling pathways and the interaction between cancer cells and the immune system [6–9]. Nonetheless, one of the main challenges in this field is the improvement in the efficacy of Ab-based therapies, such as tumor-specific Ab (passive immunotherapy) and cancer vaccines (active immunotherapy) [10, 11].
Immunotherapeutic strategies are based on the modulation of the immune system, by activating either humoral or cellular immunity, or both. Cellular immune response quantification is mainly focused on the evaluation of cytotoxic T lymphocytes, cytokine production, macrophages and natural killer (NK) cell activation. While antitumor activity of antigen-specific T lymphocytes and inflammatory cytokine production have been extensively reported, the role of NK cells in cancer immunotherapy has been just recently highlighted [12]. Ab-dependent cell-mediated cytotoxicity (ADCC) is believed to play an important role in antitumor immunotherapy-based treatments. Both monocytes/macrophages and NK cells are described as the main effector cells in this mechanism [12–14]. In particular, NK cells play a key role in the induction of ADCC since they highly express the activating receptors FcγIIIa [15] and FcγIIa/c [16], and they do not co-express the inhibitory receptor FcγRIIb [17]. ADCC mechanism is triggered when target cells are first coated with specific Abs that are then recognized by Fc receptors present on cytotoxic effector cells. The killing of Ab-coated cells occurs through a non-phagocytic process, characterized by granule exocytosis containing perforin and granzymes, activation of TNF family death receptors signaling and recruitment of nearby immune cells by INFγ release [12]. Clinical efficacy of many tumor antigen-directed mAbs used in cancer treatment as passive immunotherapy is mediated by NK cells through ADCC induction [18, 19]. However, there are only a few reports describing the induction of ADCC as an antitumor mechanism in active immunotherapies.
Active immunotherapies are therapeutic strategies intended to activate the endogenous immune system against cancer antigens. Anti-Id mAbs are defined as the mirror image of an Ab that specifically reacts with an antigen. Anti-Id mAbs directed to tumor immunogens are able to induce an antitumor response against the molecule they mimic acting as active immunotherapy effectors. Racotumomab-alum (Vaxira®) is an anti-NeuGc-containing gangliosides anti-Id mAb [20] that has been approved in Latin American countries as active immunotherapy for advanced NSCLC treatment [21, 22]. In particular, NeuGcGM3 ganglioside has been described as a tumor neoantigen in NSCLC in humans [23–25]. Preclinical studies have demonstrated that racotumomab exerts its antitumor activity by increasing CD8+ tumor-infiltrating T lymphocytes and tumor apoptosis [26]. In addition, Diaz and colleagues also reported that racotumomab reduced the number of tumor blood vessels [27]. Moreover, we demonstrated the induction of NeuGcGM3-specific Abs in the sera of tumor-bearing mice vaccinated with racotumomab [28] and Hernandez and colleagues showed the presence of anti-NeuGcGM3 cytotoxic Abs induced by racotumomab in NSCLC patients [29].
Despite these studies regarding the mechanism of action of racotumomab, the induction of cell-mediated immunity based on ADCC has not been described for this active immunotherapy. Considering the growing evidence that supports ADCC as one of the most successful approaches for induction of tumor cell killing by Ab-based therapies, the purpose of our study was to evaluate whether racotumomab vaccination is able to induce an ADCC response against NeuGcGM3-expressing cells using serum samples from NSCLC patients included in a phase III study for racotumomab.
Materials and methods
Cell lines
NeuGcGM3-positive P3 × 63 Ag8.653 (X63) murine myeloma cell line was obtained from the American Type Culture Collection (CRL-1580). Tumor cells were grown in Dulbecco’s Modified Eagle Media (DMEM) culture medium (Gibco by Life Technologies. California, United States) containing 10% heat-inactivated fetal bovine serum (FBS) (Gibco by Life Technologies. California, United States). Cells were subcultured three times a week, in suspension culture, at 37 °C in a humidified atmosphere of 5% CO2. Cell viability was assessed using the trypan blue exclusion technique.
Monoclonal antibodies
We used the specific anti-NeuGcGM3 mouse IgG1 mAb14F7 [30], produced and kindly provided by the Center of Molecular Immunology, La Habana, Cuba. Mouse and human IgG1 Ab (Dako by Agilent. Santa Clara, United States) were used as isotype controls. Polyclonal R-phycoerythrin-conjugated goat anti-mouse Igs, polyclonal rabbit anti-mouse Igsfluorescein isotiocyanate (FITC)-conjugated (Dako by Agilent. Santa Clara, United States) and polyclonal goat anti-human IgG, IgM, IgA FITC-conjugated (Abcam. Cambridge, United Kingdom) were utilized as secondary Ab in flow cytometry assays.
Immune response evaluation
Humoral immune response against NeuGcGM3 induced by racotumomab was evaluated in serum samples from advanced NSCLC patients included in a prospective, randomized, multicenter, open label phase III study of active specific immunotherapy with racotumomab plus best supportive care vs best support care (NCT01460472). Patients were 18 years or older, with histologically or cytologically confirmed stage IIIB–IV NSCLC according to the American Joint Committee on Cancer (AJCC). All patients in the study received best supportive care according to institutional standards including any subsequent onco-specific therapies. Vaccinated patients were immunized intradermally and received 15 doses of racotumomab-alum (1 mg). Immunization protocol consists of an induction phase of five doses administered every 2 weeks, followed by a maintenance phase consisting of ten more doses of racotumomab injected every 4 weeks. Patients in the best supportive care (control group) did not receive racotumomab. Serum samples were collected previously (basal samples) and at the third and sixth months post vaccine treatment (hyperimmune samples). For patients in the control group, serum samples were obtained previous (basal samples) and at the third month post treatment.
The presence of anti-NeuGcGM3 IgG and IgM Abs in serum samples from racotumomab-treated patients was first determined by enzyme-linked immunosorbent assay (ELISA). Plates were coated with 20 ng of NeuGcGM3 per well in methanol high performance liquid chromatography grade. Control wells treated with NeuAcGM3 were included. After solvent evaporation, plates were blocked with 4% phosphate-buffered saline (PBS) human serum albumin. Serum samples were used in two-fold dilution series starting at 1:200 for titration. Alkaline phosphatase-conjugated goat anti-human IgG or IgM (Jackson ImmunoResearch Laboratories) were used to detect bound Abs. Specific anti-NeuGcGM3 reactivity was calculated by subtraction of the absorbance of the control wells. Ab titers were defined as the inverse of the dilution yielding an absorbance of 0.25.
Antibody-binding assay
The detection of anti-NeuGcGM3 Abs present in serum samples from vaccinated patients was confirmed using X63 cells. Briefly, 5 × 105 cells were incubated with serum samples diluted 1:20 with PBS for 30 min at room temperature. After washing with PBS, cells were incubated for 30 min on ice with a goat FITC-labeled anti-human Igs (Dako by Agilent. Santa Clara, United States). Acquisition was achieved by a FACSCalibur flow cytometer (Becton Dickinson. New Jersey, United States) and data were analyzed by the FlowJo software. 14F7 mAb was used as positive NeuGcGM3 staining control.
ADDC assay
Cytotoxic activity induced by anti-NeuGcGM3 Abs was analyzed by a colorimetric lactate dehydrogenase (LDH)-release assay. For 30 min, 8 × 104 X63 cells (target) were incubated with a 1:50 dilution of serum samples from patients in DMEM medium supplemented with 2% FBS. Then, 2 × 106 peripheral blood mononuclear cells (PBMCs) from healthy donors (effector cells) were co-incubated (test cell mix) in DMEM1% FBS for 4 h. Both incubations were done at 37 °C and 5% CO2. PBMC were isolated using Ficoll density centrifugation (Lobov. Buenos Aires, Argentina), according to standard procedures. After co-incubation, 50 µl of the supernatant was collected and cell lysis was detected by CytoTox 96 kit (Promega. Wisconsin, United States) measuring absorbance at 490 nm in an ELISA reader (Asys VVM340), according to manufacturer’s instructions. Maximum LDH release was determined in target cells treated with 1% Triton X-100 (Max lysis), while minimum release (low control) was determined for each patient incubating target cells with serum samples, without adding effector cells. Spontaneous release control was determined in target cells incubated with effector cells without serum samples. The percentage of specific lysis was calculated according to the following formula:
To make a comparison between ADCC activity from various patients and their own responses observed after racotumomab therapy, specific lysis data were expressed in terms of percentage of basal sample, considering that 100% of the value is obtained with the basal sample from each patient.
d-threo-PDMP treatment
X63 cells with impaired ganglioside synthesis were obtained by adding d-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol (PDMP) to the culture medium at 10 µM for 4 days. X63-treated cells were used as negative controls in ADDC assays as previously described.
Evaluation of NeuGcGM3 specificity
Serum samples were diluted in PBS 1% BSA (the working dilution used in the ADCC assay) and incubated in coated-NeuGcGM3 during 2 h at 37 °C. Coating with NeuGcGM3 and plate blocking were done as previously described (see Immune response evaluation). Pre-incubated sera were used in ADCC assays to evaluate NeuGcGM3-dependent cytotoxic response. Control samples were equally diluted and incubated in a non-coated plate under the same conditions. Pre-absorbed and control serum samples were then used in ADCC assay as described.
Statistical analysis
Results described in this work are expressed as mean values ± standard deviation. Statistical analyses were carried out using Prism 6.0 statistical software (GraphPad, Inc., CA, United States). The normal distribution of data was evaluated using the D’Agostino and Pearson omnibus normality test. For multiple comparisons between experimental groups, we used one-way ANOVA or Kruskal Wallis followed by Dunnet post hoc test, and two-way ANOVA followed by mean 95% confidence interval comparison post hoc test. For repeated measures ANOVA, the Geisser–Greenhouse correction was used for assumption of sphericity. Correlation of data was evaluated using Pearson r test. Significant levels were defined as p < 0.05.
Results
Racotumomab induces anti-NeuGcGM3 antibodies
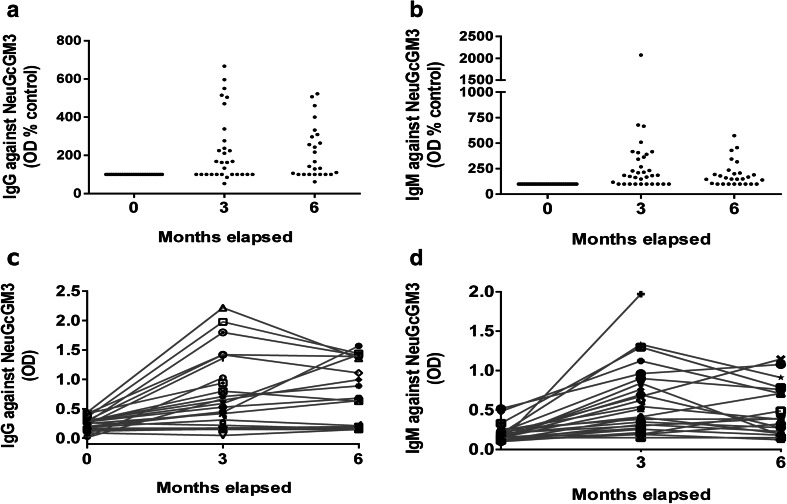
To evaluate if vaccination with racotumomab was able to induce specific anti-NeuGcGM3 Ig Abs, serum samples were collected as previously described and analyzed by ELISA. Figure 1 shows that racotumomab vaccination induced a significant Ab response against NeuGcGM3, reaching the maximum IgG titer at the end of the induction phase (third month samples) and sustaining it during the maintenance phase (sixth month samples) (Fig. 1a). Patients showed a two-fold increase in the median IgG titer against NeuGcGM3 at the third month post vaccination compared to basal samples, with titers that increased up to six-fold. NeuGcGM3-specific IgM Abs were also induced after vaccination. Similar to the IgG response, the maximum title was obtained at the third month samples when the induction phase was completed (Fig. 1b). Figure 1c, d represent the development of anti-NeuGcGM3 IgG and IgM Abs, respectively, after vaccination with racotumomab for all analyzed patients.
Fig. 1.
IgG and IgM Abs against NeuGcGM3 in NSCLC patients vaccinated with racotumomab. ELISA assays were performed to identify anti-NeuGcGM3 Abs induced by racotumomab treatment in hyperimmune serum samples. Optical density (OD) values were used to follow Ab levels. IgG (a) and IgM (b) titers against the ganglioside are depicted as percentage of OD values obtained for basal samples (indicated as 0 month). Kruskal–Wallis followed by Dunnett’s multiple comparison test was used to analyze differences between groups; p < 0.001 basal vs month 3 and basal vs month 6; p > 0.05 month 3 vs month 6, for both IgG and IgM titers. IgG (c) and IgM (d) humoral immune response against NeuGcGM3 developed in vaccinated patients
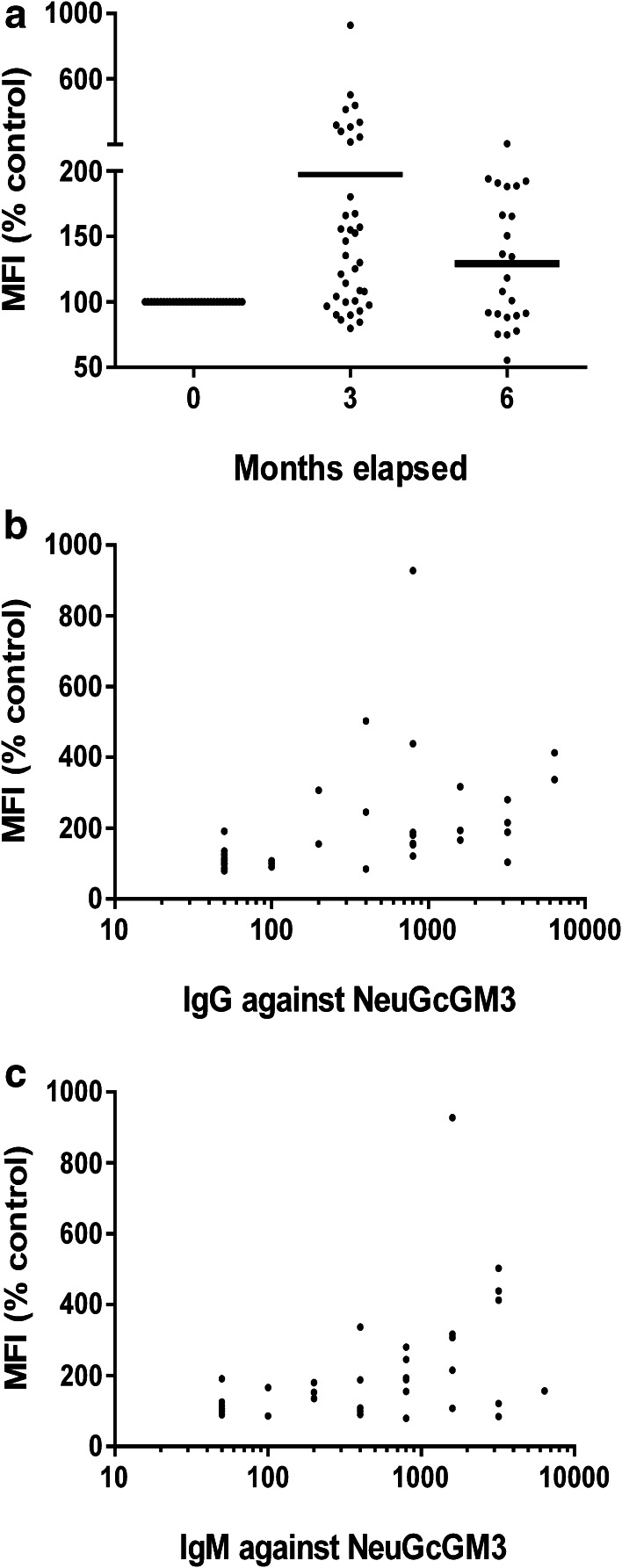
We then evaluated whether Abs induced by racotumomab were able to recognize the antigen as part of the tumor cell membrane. Reactivity of Abs present in basal and hyperimmune samples to X63 myeloma cell line was evaluated by flow cytometry. These cells are known to have a predominant expression of NeuGcGM3 ganglioside and, to a much lesser extent, its precursor NeuAcGM3 [31]. As shown in Fig. 2a, the binding of Abs present in the third month post-vaccination sera to X63 cells was significantly higher in comparison with basal sera, with a median fluorescence intensity value (MFI) that reaches twice the value of the basal samples. No significant binding differences were found between hyperimmune serum samples, as it was observed in the ELISA assay. Data analysis demonstrated a moderate but still significant positive correlation between NeuGcGM3-specific IgG (Fig. 2b) and IgM Abs (Fig. 2c) and the binding to tumor cells. These results confirm that racotumomab vaccination develops antigen-specific Abs that are able to recognize purified NeuGcGM3as well as NeuGcGM3 expressed in tumor cell membranes, measured by ELISA and flow cytometry, respectively.
Fig. 2.
Binding of basal and hyperimmune sera from vaccinated patients to NeuGcGM3-expressing X63 cells. Serum samples from vaccinated patients were incubated with target cells and analyzed by flow cytometry. a Binding to X63 cells are expressed as percentage of MFI obtained wit basal samples for each patient. Kruskal–Wallis followed by Dunnett’s multiple comparison test was used to analyze differences between groups; p < 0.001 basal vs month 3. b, c Direct correlation exhibited between MFI, depicted as percentage of basal samples, and anti-NeuGcGM3 IgG titer (b) or and anti-NeuGcGM3 IgM titer (c). Pearson r test was used to analyze the results; p < 0.01, r = 0.4339, MFI vs log IgG titer; r = 0.4454, MFI vs log IgM titer
Antibodies induced by racotumomab mediate a cytotoxic activity based on ADCC
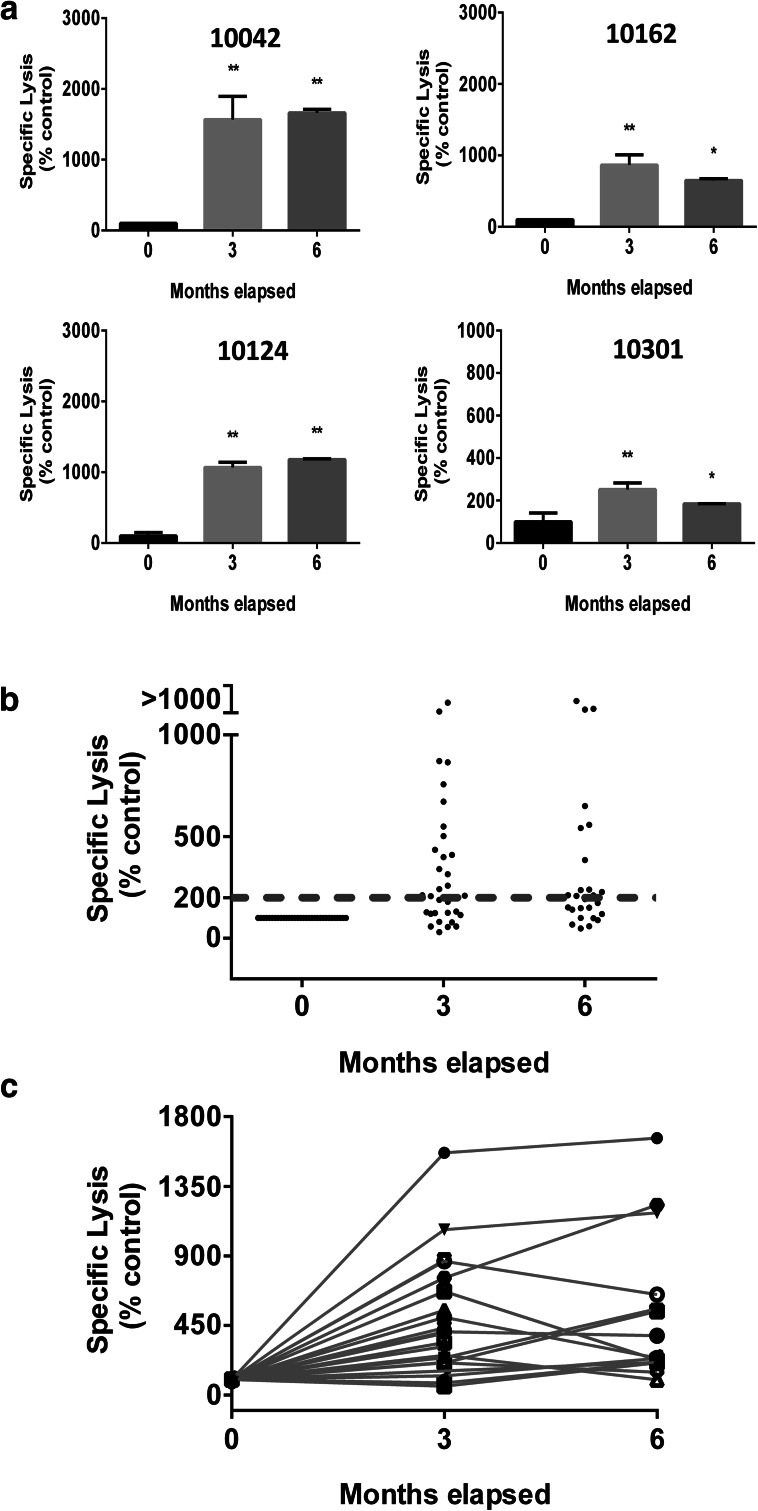
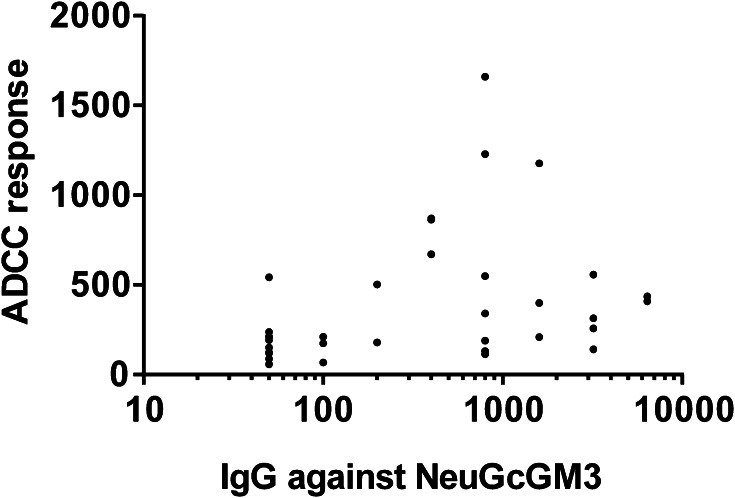
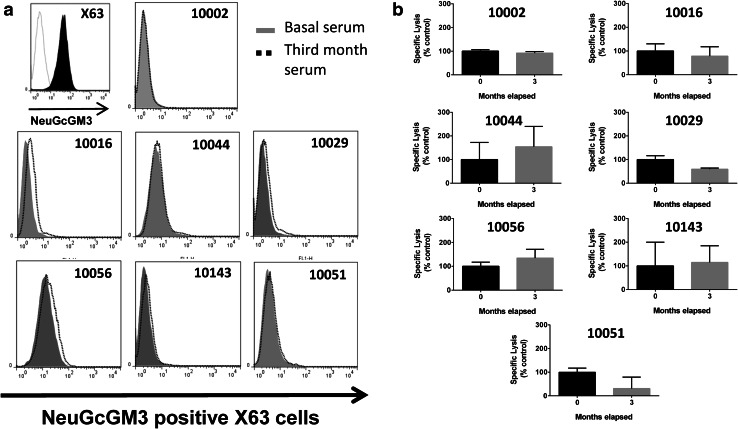
To assess if anti-NeuGcGM3 Abs induced by racotumomab were able to mediate an ADCC activity against X63 cells, we performed a LDH-release assay using serum samples from 36 patients treated with racotumomab and PMNC from healthy donors. Figure 3a shows the ADCC response obtained when serum samples from four selected patients were analyzed. The development of specific ADCC response was then evaluated in all racotumomab-treated patients (Fig. 3b). ADCC response obtained with hyperimmune samples showed a significant increase compared to basal samples. No significant differences were observed between ADCC responses obtained with hyperimmune sera corresponding to the third or the sixth month post vaccination. We considered that a patient develops a positive ADCC response when a two-fold increase of the cytotoxic activity obtained with its own basal sample was reached. The development of ADCC was demonstrated in 22 out of the 36 evaluated patients (61%) (Fig. 3c). As expected, a moderate but still significant correlation was found between ADCC response and anti-NeuGcGM3 IgG titer (Fig. 4). Serum samples from seven control group patients were also analyzed, showing no differences between the binding to X63 cells obtained with basal and third month post-treatment samples (Fig. 5a). As shown in Fig. 5b, specific lysis values exhibited no significant differences between samples collected at the beginning of the clinical study and at the third month, demonstrating that the ADCC response observed in racotumomab-treated patients is related to vaccination.
Fig. 3.
ADCC induced by anti-NeuGcGM3 Ab present in serum samples from racotumomab-vaccinated patients. Specific lysis against X63 cells was analyzed using a LDH-release assay and effector cells from healthy donors. a ADCC activity (indicated as specific lysis) obtained with hyperimmune serum samples corresponding to the third and the sixth months post-vaccination from four representative patients. Bars indicate mean values ± standard deviation. Patients’ identification numbers are indicated on each graph. ANOVA followed by Dunnett’s multiple comparison test was used to analyze differences between groups; *p < 0.05, **p < 0.01. b ADCC response obtained for 36 racotumomab vaccinated NSCLC patients using basal and hyperimmune serum samples. Results are expressed as percentage respect to basal samples (0 month). Dotted line indicates the minimum specific lysis value to be considered a positive ADCC response (threshold value). Kruskal–Wallis followed by Dunnett’s multiple comparison test was used to analyze differences between groups; p < 0.001 basal vs month 3 and basal vs month 6. c ADCC activity developed by anti-NeuGcGM3 Ab present in basal and hyperimmune samples
Fig. 4.
Direct correlation exhibited between ADCC response and anti-NeuGcGM3 IgG titer. Pearson r test was used to analyze the results; p < 0.05, r = 0.3424, ADCC response vs log IgG titer
Fig. 5.
Binding to NeuGcGM3-expressing X63 cells and cell-mediated cytotoxicity developed by serum samples from control group patients. Binding and ADCC response were measured as previously described. a Histograms of the flow cytometric analysis showing post-treatment (3 month) sera binding to X63 cells. Positive NeuGcGM3 staining control using 14F7 mAb was included. b ADCC response (shown as specific lysis values) obtained with post-treatment serum samples for each control patient. Results are depicted as percentage respect to basal samples (0 month) and bars indicate mean values ± standard deviation. Patients’ identification numbers are indicated on each individual graph
ADCC induced by racotumomab depends on NeuGcGM3 expression
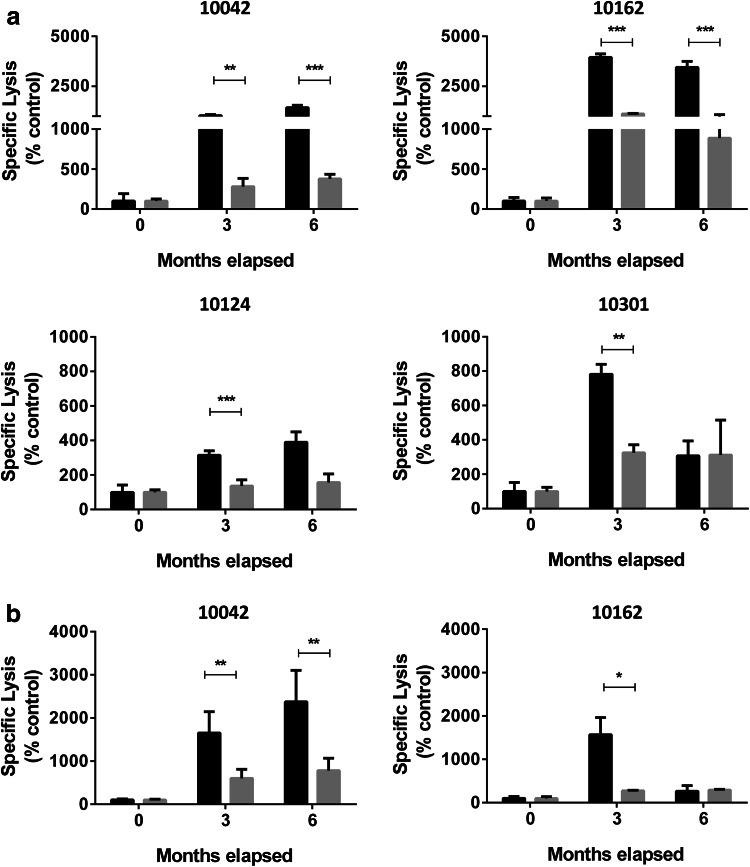
Finally, we explored whether the induction of ADCC is dependent on the expression of NeuGcGM3 in target cells. ADCC activity was evaluated on X63 cells incubated with PDMP, a well-described inhibitor of the glucosylceramide synthase that blocks the formation of glucosylceramide from ceramide, decreasing cell surface glycosphingolipids [32]. Ganglioside expression was significantly reduced in PDMP-treated X63 cells, showing a decrease of 65% in comparison to non-treated cells (p < 0.01, Umpired T test; data not shown). X63 cells with impaired ganglioside synthesis were then used as target cells in ADCC assay using the same hyperimmune sera. A significant reduced ADCC response against PDMP-treated X63 cells was observed in this set of experiments (Fig. 6a). To confirm ADCC specificity, we also evaluated the cytotoxic response developed against X63 using serum samples pre-absorbed by NeuGcGM3. ADCC was significantly reduced when pre-absorbed sera was used, in contrast to nonabsorbed samples. As expected, there was no difference between the responses developed by pre- and nonabsorbed basal samples (Fig. 6b). These results confirm the participation of NeuGcGM3 as target ganglioside in the cellular antitumor response developed by racotumomab vaccination.
Fig. 6.
NeuGcGM3-specific ADCC response. a ADCC against NeuGcGM3-expressing (black bars) or NeuGcGM3-depleted (gray bars) X63 cells was evaluated as previously described. Two-way ANOVA followed by mean 95% confidence interval comparison post hoc test was used to analyze differences between groups; **p < 0.01, ***p < 0.001. Results are shown as percentage respect to the control and bars indicate mean values ± standard deviation. b Evaluation of ADCC response using pre-absorbed with NeuGcGM3 serum samples (gray bars). Controls indicate ADCC response induced by nonabsobed sera (black bars). Paired T test was used to analyze differences between non and pre-absorbed sera for the same month; **p < 0.01; *p < 0.05. Results are shown as percentage respect to the control and bars indicate mean values ± standard error. ADCC responses are expressed as specific lysis. Basal samples are indicated as 0 month. Patients’ identification numbers are indicated on each graph
Discussion
Lung tumor microenvironment has been traditionally characterized as highly immunosuppressive. Nevertheless, the understanding of tumor immunosurveillance and the recent approval of several immune checkpoints inhibitors as therapeutic options for NSCLC, some of them as first-line treatments, has led to a new era in the treatment of this pathology. In this scenario, immunotherapy for NSCLC has recently become an accurate treatment modality for this type of tumor. Cancer vaccines have demonstrated the induction of an enhanced immune response, evolving into promising therapeutic alternatives characterized by the ability to elicit an immune response against specific tumor antigens and showing minor adverse effects than other strategies. Combination strategies based on checkpoint inhibitors and cancer vaccines are currently being evaluated in several clinical trials [33–35].
Normal human tissues do not express NeuGc-containing gangliosides due to a deletion in the CMP-N-acetylneuraminic acid hydroxylase (CMAH) gene. CMAH catalyzes NeuGc synthesis [36]. In particular, NeuGcGM3 ganglioside has been widely reported as a specific tumor antigen not only in NSCLC [23, 25] but also in many other human cancers such as melanoma [30], breast [37], retinoblastoma [38] and neuroectodermal tumors [39] among others. Although there are several theories that have been postulated to explain the expression of NeuGcGM3 in tumor cells [40, 41], the most accepted mechanism proposes a metabolic incorporation form dietary sources [42]. In fact, studies with tumor cells demonstrate that sialin, a sialic acid transporter, is overexpressed under hypoxic conditions promoting the incorporation of NeuGc from culture medium [43]. The upregulation of sialin allows tumor cells to increase the amount of NeuGcGM3 on their membranes, which confers advantages over cells than do not express it. In line with this evidence, we have previously reported that murine B16 melanoma cells with NeuGcGM3 expression exhibit an increased proliferation and adhesion in vitro [44]. Moreover, tumor cells can uptake NeuGc from culture medium increasing their ability to disseminate when they are injected in mice [28, 45]. In addition, the presence of NeuGcGM3 has been associated with a down-modulation of CD4 molecule in tumor infiltrating T cells [46] without altering CD4+CD25+ regulatory T-cell inhibitory functions. Tumor-associated NeuGcGM3 also reduces the antigen-presenting function of dendritic cells [47]. These experimental evidences support the relevance of NeuGcGM3 ganglioside as an important target for lung cancer immunotherapy.
Despite the differential expression observed in tumors over normal tissues, NeuGcGM3 as well as glycosphingolipids in general are poorly immunogenic due to their carbohydrate nature. However, an anti-id mAb that acts as NeuGcGM3 surrogate was developed to overcome the immune tolerance and to elicit an effective immune response against this tumor antigen. Racotumomab is an anti-NeuGc-containing gangliosides anti-id mAb, approved in Latin American countries as active immunotherapy for NSCLC treatment. Racotumomab exerts its mechanism of action by inducing tumor-infiltrating CD8+T lymphocytes and tumor apoptosis. Moreover, it reduces the number of tumor blood vessels and augments the presence of anti-NeuGcGM3 cytotoxic Abs in vaccinated NSCLC patients. Hernández and co-workers also described that the cytotoxic activity developed by antigen-specific Abs involves a mechanism independent of complement activation and the formation of membrane lesions that resembles an oncotic necrosis process [29]. In the present work, we demonstrate for the first time the induction of ADCC in NSCLC patients vaccinated with the active immunotherapy racotumomab.
Serum samples from 36 racotumomab-vaccinated patients were first analyzed to confirm the presence of NeuGcGM3 specific Abs. As it was previously reported in different clinical trials of racotumomab, we were able to confirm the induction of an Ab response of both IgG and IgM isotypes against the ganglioside using hyperimmune sera corresponding to either the third or the sixth month post vaccination. Those patients who completed the induction phase of the immunization achieve the highest Ab response against NeuGcGM3 ganglioside, maintaining the Ab titer after 6 months of racotumomab treatment. Same results were also described in breast and melanoma cancer patients included in two phase I clinical trials for racotumomab. In both clinical studies, evaluation of the IgG subclasses represented in the humoral response showed the induction of Abs predominantly of IgG2 subclass, with very low representation of other IgG subclasses in most patients [48, 49]. On the contrary, basal samples show no reaction in the ELISA assay, confirming the immunogenicity of racotumomab in this group of patients as it was reported [48–52]. In addition, anti-NeuGcGM3 Abs present in hyperimmune serum samples show a significant binding to X63 myeloma cells demonstrating they can recognize the ganglioside when it is expressed in tumor cell membranes. This result confirms findings previously reported by Alfonso and co-workers, who evaluated the binding of patients´ sera to a different cell line, the murine lymphocytic leukemia L1210, using serum samples from patients enrolled in a phase II/III clinical trial of racotumomab vaccine [21].
Abs mediate tumor cell killing by inducing one of these three mechanisms: direct action on tumor cells, immune-mediated cell killing and specific action on tumor microenvironment cells. An increasing body of evidence suggests that immune-mediated cell killing mechanisms are important contributors to the anti-tumor activity of many Ab-based therapies. In particular, the induction of ADCC activity has been extensively reported in response to the treatment with passive immunotherapies based on the administration of rituximab and trastuzumab among others mAbs mainly directed against protein antigens [12, 53]. With the approval of dinutuximab, a chimeric anti-GD2 therapeutic Ab, the ganglioside GD2 became the first glycosphingolipid proven as an effective target antigen for passive immunotherapy against neuroblastoma [54]. Dinutuximab induces ADCC response against GD2-expressing cell lines [55]. On the other hand, the induction of ADCC in cancer patients treated with active immunotherapies has been less described. To our knowledge, Snijdewint and co-workers described for the first time the induction of ADCC response in breast cancer patients vaccinated with MUC1 peptide in a phase I clinical trial [56]. Even though two anti-Id mAbs, mimicking carcinoembryonic antigen (3H1) and GD2 (gangliodimab), have demonstrated the induction of ADCC in murine tumor models [57, 58], no further clinical trials reporting cell cytotoxic response in cancer patients have been described for these Abs. Moreover, the induction of ADCC in response to immunotherapies directed against glycan antigens has not been reported so far.
ADCC against target cells is triggered when tumor antigen-specific Abs belonging to IgG, IgA and IgE classes bind to Fc receptors present on effector cells. Both therapeutic Abs themselves (passive immunotherapy) and Abs induced in response to active immunotherapies are able to bind to Fc receptors and trigger ADCC against tumor cells. It was previously reported that racotumomab induces a ganglioside-specific cellular response in breast cancer patients that were included in a year-long vaccination scheme. PBMCs from these vaccinated patients secreted INFγ when incubated with dendritic cells and NeuGcGM3 [50]. In addition, Abs elicited by vaccination can directly kill target cells with NeuGcGM3 expression [21]. However, the evaluation of cell cytotoxic activity developed by racotumomab-induced Abs has not been demonstrated to date. Here, we describe that anti-NeuGcGM3 Abs present in serum samples from NSCLC-vaccinated patients are able to mediate the lysis of NeuGcGM3-expressing X63 cells in the presence of PBMCs from healthy donors by an ADCC mechanism. This Ab-induced cytotoxic response is antigen dependent because specific lysis was significantly reduced when both, X63 cells lacking ganglioside expression and sera pre-absorbed with purified NeuGcGM3, were used. In agreement with previous clinical studies, NSCLC patients analyzed in this work developed specific IgG Abs against NeuGcGM3 in response to the vaccination with racotumomab. Furthermore, our data indicate that there is a correlation between anti-ganglioside IgG titer and the ADCC activity against X63cells.
In summary, we report here that therapeutic active immunization with racotumomab induces anti-NeuGcGM3-specific Abs capable of mediating a cell-mediated cytotoxic response based on ADCC against tumor cells in NSCLC patients.
Acknowledgements
The authors gratefully acknowledge Diego Mengual Gomez for his valuable support with the blood samples from healthy donors. The authors also thank Marina Pifano and Nazareno Gonzalez for their valuable contribution during the statistical analysis of the data. Valeria I Segatori and Héctor A Cuello are research fellows of ANPCyT (Argentina). Marina Albertó is a research fellow and Mariano R Gabri and Daniel F Alonso are members of the National Research Council (CONICET, Argentina).
Abbreviations
- ADCC
Antibody-dependent cell-mediated cytotoxicity
- Anti-id
Anti-idiotype
- PDMP
d-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol
Author contributions
Valeria I. Segatori and Mariano R. Gabri participated in the conception and design of the experiments, the analysis and interpretation of data and in the process of manuscript writing. Héctor A. Cuello, Cynthia A. Gulino and Marina Albertó also contributed to the final version of the manuscript. Valeria I. Segatori, Héctor A. Cuello, Cynthia A. Gulino and Marina Albertó worked in development of methodology and acquisition of data. Cecilia Venier was involved in the ELISA assays. Marcelo D. Guthmann and Ignacio A. Demarco were responsible for patients’ samples. Review of the manuscript was done by Daniel F. Alonso and Mariano R. Gabri.
Funding
This study was partially funded by National University of Quilmes (Grant No. 1398/15) and ELEA Laboratories.
Compliance with ethical standards
Conflict of interest
Marcelo D. Guthmann and Ignacio A. Demarco are full employees of Elea Laboratories. They have no conflict of interest to declare. All other authors declare that they have no potential conflict of interest.
Ethical approval and ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584–594. doi: 10.1016/S0025-6196(11)60735-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tabchi S, Blais N, Campeau MP, Tehfe M. Single-center comparison of multiple chemotherapy regimens for concurrent chemoradiotherapy in unresectable stage III non-small cell lung cancer. Cancer Chemother Pharmacol. 2017;79(2):381–387. doi: 10.1007/s00280-016-3226-0. [DOI] [PubMed] [Google Scholar]
- 4.Jackman DM, Miller VA, Cioffredi L-A, Yeap BY, Jänne PA, Riely GJ, Ruiz MG, Giaccone G, Sequist LV, Johnson BE. Impact of epidermal growth factor receptor and KRAS mutations on clinical outcomes in previously untreated non-small cell lung cancer patients: results of an online tumor registry of clinical trials. Clin Cancer Res. 2009;15(16):5267. doi: 10.1158/1078-0432.CCR-09-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Somasundaram A, Burns TF. Pembrolizumab in the treatment of metastatic non-small-cell lung cancer: patient selection and perspectives. Lung Cancer. 2017;8:1–11. doi: 10.2147/LCTT.S105678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pillay V, Allaf L, Wilding AL, Donoghue JF, Court NW, Greenall SA, Scott AM, Johns TG. The plasticity of oncogene addiction: implications for targeted therapies directed to receptor tyrosine kinases. Neoplasia. 2009;11(5):448–458. doi: 10.1593/neo.09230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pillay V, Gan HK, Scott AM. Antibodies in oncology. N Biotechnol. 2011;28(5):518–529. doi: 10.1016/j.nbt.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 8.Konitzer JD, Sieron A, Wacker A, Enenkel B. Reformatting rituximab into human IgG2 and IgG4 isotypes dramatically improves apoptosis induction in vitro. PLoS One. 2015;10(12):e0145633. doi: 10.1371/journal.pone.0145633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gong Q, Hazen M, Marshall B, Crowell SR, Ou Q, Wong AW, Phung W, Vernes JM, Meng YG, Tejada M, Andersen D, Kelley RF. Increased in vivo effector function of human IgG4 isotype antibodies through afucosylation. MAbs. 2016;8(6):1098–1106. doi: 10.1080/19420862.2016.1189049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lesterhuis WJ, Haanen JBAG, Punt CJA. Cancer immunotherapy—revisited. Nat Rev Drug Discov. 2011;10(8):591–600. doi: 10.1038/nrd3500. [DOI] [PubMed] [Google Scholar]
- 11.Galluzzi L, Vacchelli E, Bravo-San Pedro JM, Buque A, Senovilla L, Baracco EE, Bloy N, Castoldi F, Abastado JP, Agostinis P, Apte RN, Aranda F, Ayyoub M, Beckhove P, Blay JY, Bracci L, Caignard A, Castelli C, Cavallo F, Celis E, Cerundolo V, Clayton A, Colombo MP, Coussens L, Dhodapkar MV, Eggermont AM, Fearon DT, Fridman WH, Fucikova J, Gabrilovich DI, Galon J, Garg A, Ghiringhelli F, Giaccone G, Gilboa E, Gnjatic S, Hoos A, Hosmalin A, Jager D, Kalinski P, Karre K, Kepp O, Kiessling R, Kirkwood JM, Klein E, Knuth A, Lewis CE, Liblau R, Lotze MT, Lugli E, Mach JP, Mattei F, Mavilio D, Melero I, Melief CJ, Mittendorf EA, Moretta L, Odunsi A, Okada H, Palucka AK, Peter ME, Pienta KJ, Porgador A, Prendergast GC, Rabinovich GA, Restifo NP, Rizvi N, Sautes-Fridman C, Schreiber H, Seliger B, Shiku H, Silva-Santos B, Smyth MJ, Speiser DE, Spisek R, Srivastava PK, Talmadge JE, Tartour E, Van Der Burg SH, Van Den Eynde BJ, Vile R, Wagner H, Weber JS, Whiteside TL, Wolchok JD, Zitvogel L, Zou W, Kroemer G. Classification of current anticancer immunotherapies. Oncotarget. 2014;5(24):12472–12508. doi: 10.18632/oncotarget.2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang W, Erbe AK, Hank JA, Morris ZS, Sondel PM. NK cell-mediated antibody-dependent cellular cytotoxicity in cancer immunotherapy. Front Immunol. 2015;6:368. doi: 10.3389/fimmu.2015.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uchida J, Hamaguchi Y, Oliver JA, Ravetch JV, Poe JC, Haas KM, Tedder TF. The innate mononuclear phagocyte network depletes B lymphocytes through Fc receptor-dependent mechanisms during anti-CD20 antibody immunotherapy. J Exp Med. 2004;199(12):1659–1669. doi: 10.1084/jem.20040119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biburger M, Aschermann S, Schwab I, Lux A, Albert H, Danzer H, Woigk M, Dudziak D, Nimmerjahn F. Monocyte subsets responsible for immunoglobulin G-dependent effector functions in vivo. Immunity. 2011;35(6):932–944. doi: 10.1016/j.immuni.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Lanier LL, Ruitenberg JJ, Phillips JH. Functional and biochemical analysis of CD16 antigen on natural killer cells and granulocytes. J Immunol. 1988;141(10):3478–3485. [PubMed] [Google Scholar]
- 16.Metes D, Manciulea M, Pretrusca D, Rabinowich H, Ernst LK, Popescu I, Calugaru A, Sulica A, Chambers WH, Herberman RB, Morel PA. Ligand binding specificities and signal transduction pathways of Fc gamma receptor IIc isoforms: the CD32 isoforms expressed by human NK cells. Eur J Immunol. 1999;29(9):2842–2852. doi: 10.1002/(SICI)1521-4141(199909)29:09<2842::AID-IMMU2842>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 17.Veri MC, Gorlatov S, Li H, Burke S, Johnson S, Stavenhagen J, Stein KE, Bonvini E, Koenig S. Monoclonal antibodies capable of discriminating the human inhibitory Fcgamma-receptor IIB (CD32B) from the activating Fcgamma-receptor IIA (CD32A): biochemical, biological and functional characterization. Immunology. 2007;121(3):392–404. doi: 10.1111/j.1365-2567.2007.02588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer. 2012;12(4):278–287. doi: 10.1038/nrc3236. [DOI] [PubMed] [Google Scholar]
- 19.Seidel UJ, Schlegel P, Lang P. Natural killer cell mediated antibody-dependent cellular cytotoxicity in tumor immunotherapy with therapeutic antibodies. Front Immunol. 2013;4:76. doi: 10.3389/fimmu.2013.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vazquez AM, Perez A, Hernandez AM, Macias A, Alfonso M, Bombino G, Perez R. Syngeneic anti-idiotypic monoclonal antibodies to an anti-NeuGc-containing ganglioside monoclonal antibody. Hybridoma. 1998;17(6):527–534. doi: 10.1089/hyb.1998.17.527. [DOI] [PubMed] [Google Scholar]
- 21.Alfonso S, Valdes-Zayas A, Santiesteban ER, Flores YI, Areces F, Hernandez M, Viada CE, Mendoza IC, Guerra PP, Garcia E, Ortiz RA, de la Torre AV, Cepeda M, Perez K, Chong E, Hernandez AM, Toledo D, Gonzalez Z, Mazorra Z, Crombet T, Perez R, Vazquez AM, Macias AE. A randomized, multicenter, placebo-controlled clinical trial of racotumomab-alum vaccine as switch maintenance therapy in advanced non-small cell lung cancer patients. Clin Cancer Res. 2014;20(14):3660–3671. doi: 10.1158/1078-0432.CCR-13-1674. [DOI] [PubMed] [Google Scholar]
- 22.Gabri MR, Cacciavillano W, Chantada GL, Alonso DF. Racotumomab for treating lung cancer and pediatric refractory malignancies. Expert Opin Biol Ther. 2016;16(4):573–578. doi: 10.1517/14712598.2016.1157579. [DOI] [PubMed] [Google Scholar]
- 23.van Cruijsen H, Ruiz MG, van der Valk P, de Gruijl TD, Giaccone G. Tissue micro array analysis of ganglioside N-glycolyl GM3 expression and signal transducer and activator of transcription (STAT)-3 activation in relation to dendritic cell infiltration and microvessel density in non-small cell lung cancer. BMC Cancer. 2009;9:180. doi: 10.1186/1471-2407-9-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blanco R, Rengifo CE, Cedeno M, Frometa M, Rengifo E, Carr A. Immunoreactivity of the 14F7 Mab (Raised against N-glycolyl GM3 ganglioside) as a positive prognostic factor in non-small-cell lung cancer. Patholog Res Int. 2012;2012:235418. doi: 10.1155/2012/235418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blanco R, Dominguez E, Morales O, Blanco D, Martinez D, Rengifo CE, Viada C, Cedeno M, Rengifo E, Carr A. Prognostic significance of N-Glycolyl GM3 ganglioside expression in non-small cell lung carcinoma patients: new evidences. Patholog Res Int. 2015;2015:132326. doi: 10.1155/2015/132326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fuentes D, Avellanet J, Garcia A, Iglesias N, Gabri MR, Alonso DF, Vazquez AM, Perez R, Montero E. Combined therapeutic effect of a monoclonal anti-idiotype tumor vaccine against NeuGc-containing gangliosides with chemotherapy in a breast carcinoma model. Breast Cancer Res Treat. 2010;120(2):379–389. doi: 10.1007/s10549-009-0399-9. [DOI] [PubMed] [Google Scholar]
- 27.Diaz Y, Gonzalez A, Lopez A, Perez R, Vazquez AM, Montero E. Anti-ganglioside anti-idiotypic monoclonal antibody-based cancer vaccine induces apoptosis and antiangiogenic effect in a metastatic lung carcinoma. Cancer Immunol Immunother. 2009;58(7):1117–1128. doi: 10.1007/s00262-008-0634-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Segatori VI, Vazquez AM, Gomez DE, Gabri MR, Alonso DF. Preclinical evaluation of racotumomab, an anti-idiotype monoclonal antibody to N-glycolyl-containing gangliosides, with or without chemotherapy in a mouse model of non-small cell lung cancer. Front Oncol. 2012;2:160. doi: 10.3389/fonc.2012.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hernandez AM, Rodriguez N, Gonzalez JE, Reyes E, Rondon T, Grinan T, Macias A, Alfonso S, Vazquez AM, Perez R. Anti-NeuGcGM3 antibodies, actively elicited by idiotypic vaccination in nonsmall cell lung cancer patients, induce tumor cell death by an oncosis-like mechanism. J Immunol. 2011;186(6):3735–3744. doi: 10.4049/jimmunol.1000609. [DOI] [PubMed] [Google Scholar]
- 30.Carr A, Mullet A, Mazorra Z, Vazquez AM, Alfonso M, Mesa C, Rengifo E, Perez R, Fernandez LE. A mouse IgG1 monoclonal antibody specific for N-glycolyl GM3 ganglioside recognized breast and melanoma tumors. Hybridoma. 2000;19(3):241–247. doi: 10.1089/02724570050109639. [DOI] [PubMed] [Google Scholar]
- 31.Muthing J, Steuer H, Peter-Katalinic J, Marx U, Bethke U, Neumann U, Lehmann J. Expression of gangliosides GM3 (NeuAc) and GM3 (NeuGc) in myelomas and hybridomas of mouse, rat, and human origin. J Biochem. 1994;116(1):64–73. doi: 10.1093/oxfordjournals.jbchem.a124504. [DOI] [PubMed] [Google Scholar]
- 32.Fedoryszak-Kuska N, Panasiewicz M, Domek H, Pacuszka T. Glucosylceramide synthase inhibitors D-PDMP and D-EtDO-P4 decrease the GM3 ganglioside level, differ in their effects on insulin receptor autophosphorylation but increase Akt1 kinase phosphorylation in human hepatoma HepG2 cells. Acta Biochim Pol. 2016;63(2):247–251. doi: 10.18388/abp.2014_930. [DOI] [PubMed] [Google Scholar]
- 33.Wei XX, Fong L, Small EJ. Prostate cancer immunotherapy with sipuleucel-T: current standards and future directions. Expert Rev Vaccines. 2015;14(12):1529–1541. doi: 10.1586/14760584.2015.1099437. [DOI] [PubMed] [Google Scholar]
- 34.van den Eertwegh AJM, Versluis J, van den Berg HP, Santegoets SJAM, van Moorselaar RJA, van der Sluis TM, Gall HE, Harding TC, Jooss K, Lowy I, Pinedo HM, Scheper RJ, Stam AGM, von Blomberg BME, de Gruijl TD, Hege K, Sacks N, Gerritsen WR. Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13(5):509–517. doi: 10.1016/S1470-2045(12)70007-4. [DOI] [PubMed] [Google Scholar]
- 35.Le DT, Jaffee EM. Next-generation cancer vaccine approaches: integrating lessons learned from current successes with promising biotechnologic advances. J Natl Compr Canc Netw. 2013;11(7):766–772. doi: 10.6004/jnccn.2013.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Irie A, Koyama S, Kozutsumi Y, Kawasaki T, Suzuki A. The molecular basis for the absence of N-glycolylneuraminic acid in humans. J Biol Chem. 1998;273(25):15866–15871. doi: 10.1074/jbc.273.25.15866. [DOI] [PubMed] [Google Scholar]
- 37.Oliva JP, Valdés Z, Casacó A, Pimentel G, González J, Álvarez I, Osorio M, Velazco M, Figueroa M, Ortiz R, Escobar X, Orozco M, Cruz J, Franco S, Díaz M, Roque L, Carr A, Vázquez AM, Mateos C, Rubio MC, Pérez R, Fernández LE. Clinical evidences of GM3 (NeuGc) ganglioside expression in human breast cancer using the 14F7 monoclonal antibody labelled with 99mTc. Breast Cancer Res Treat. 2006;96(2):115–121. doi: 10.1007/s10549-005-9064-0. [DOI] [PubMed] [Google Scholar]
- 38.Torbidoni AV, Scursoni A, Camarero S, Segatori V, Gabri M, Alonso D, Chantada G, de Davila MT. Immunoreactivity of the 14F7 Mab raised against N-Glycolyl GM3 ganglioside in retinoblastoma tumours. Acta Ophthalmol. 2015;93(4):e294–e300. doi: 10.1111/aos.12578. [DOI] [PubMed] [Google Scholar]
- 39.Scursoni AM, Galluzzo L, Camarero S, Lopez J, Lubieniecki F, Sampor C, Segatori VI, Gabri MR, Alonso DF, Chantada G, de Dávila MTG. Detection of N-glycolyl GM3 ganglioside in neuroectodermal tumors by immunohistochemistry: an attractive vaccine target for aggressive pediatric cancer. Clin Dev Immunol. 2011;2011:245181. doi: 10.1155/2011/245181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vamecq J, Mestdagh N, Henichart JP, Poupaert J. Subcellular distribution of glycolyltransferases in rodent liver and their significance in special reference to the synthesis of N-glycolylneuraminic acid. J Biochem. 1992;111(5):579–583. doi: 10.1093/oxfordjournals.jbchem.a123800. [DOI] [PubMed] [Google Scholar]
- 41.Malykh YN, Schauer R, Shaw L. N-Glycolylneuraminic acid in human tumours. Biochimie. 2001;83(7):623–634. doi: 10.1016/S0300-9084(01)01303-7. [DOI] [PubMed] [Google Scholar]
- 42.Bardor M, Nguyen DH, Diaz S, Varki A. Mechanism of uptake and incorporation of the non-human sialic acid N-glycolylneuraminic acid into human cells. J Biol Chem. 2005;280(6):4228–4237. doi: 10.1074/jbc.M412040200. [DOI] [PubMed] [Google Scholar]
- 43.Yin J, Hashimoto A, Izawa M, Miyazaki K, Chen GY, Takematsu H, Kozutsumi Y, Suzuki A, Furuhata K, Cheng FL, Lin CH, Sato C, Kitajima K, Kannagi R. Hypoxic culture induces expression of sialin, a sialic acid transporter, and cancer-associated gangliosides containing non-human sialic acid on human cancer cells. Cancer Res. 2006;66(6):2937–2945. doi: 10.1158/0008-5472.CAN-05-2615. [DOI] [PubMed] [Google Scholar]
- 44.Segatori VI, Otero LL, Fernandez LE, Gomez DE, Alonso DF, Gabri MR. Antitumor protection by NGcGM3/VSSP vaccine against transfected B16 mouse melanoma cells overexpressing N-glycolylated gangliosides. In Vivo. 2012;26(4):609–617. [PubMed] [Google Scholar]
- 45.Gabri MR, Otero LL, Gomez DE, Alonso DF. Exogenous incorporation of neugc-rich mucin augments N-glycolyl sialic acid content and promotes malignant phenotype in mouse tumor cell lines. J Exp Clin Cancer Res. 2009;28:146. doi: 10.1186/1756-9966-28-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Leon J, Fernandez A, Mesa C, Clavel M, Fernandez LE. Role of tumour-associated N-glycolylated variant of GM3 ganglioside in cancer progression: effect over CD4 expression on T cells. Cancer Immunol Immunother. 2006;55(4):443–450. doi: 10.1007/s00262-005-0041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de León J, Fernández A, Clavell M, Labrada M, Bebelagua Y, Mesa C, Fernández LE. Differential influence of the tumour-specific non-human sialic acid containing GM3 ganglioside on CD4+ CD25—effector and naturally occurring CD4+ CD25+ regulatory T cells function. Int Immunol. 2008;20(4):591–600. doi: 10.1093/intimm/dxn018. [DOI] [PubMed] [Google Scholar]
- 48.Alfonso M, Diaz A, Hernandez AM, Perez A, Rodriguez E, Bitton R, Perez R, Vazquez AM. An anti-idiotype vaccine elicits a specific response to N-glycolyl sialic acid residues of glycoconjugates in melanoma patients. J Immunol. 2002;168(5):2523–2529. doi: 10.4049/jimmunol.168.5.2523. [DOI] [PubMed] [Google Scholar]
- 49.Díaz A, Alfonso M, Alonso R, Saurez G, Troche M, Catalá M, Díaz RM, Pérez R, Vázquez AM. Immune responses in breast cancer patients immunized with an anti-idiotype antibody mimicking NeuGc-containing gangliosides. Clin Immunol. 2003;107(2):80–89. doi: 10.1016/S1521-6616(03)00036-6. [DOI] [PubMed] [Google Scholar]
- 50.Guthmann MD, Castro MA, Cinat G, Venier C, Koliren L, Bitton RJ, Vázquez AM, Fainboim L. Cellular and humoral immune response to N-glycolyl-GM3 elicited by prolonged immunotherapy with an anti-idiotypic vaccine in high-risk and metastatic breast cancer patients. J Immunother. 2006;29(2):215–223. doi: 10.1097/01.cji.0000188502.11348.34. [DOI] [PubMed] [Google Scholar]
- 51.Alfonso S, Díaz RM, de la Torre A, Santiesteban E, Aguirre F, Pérez K, Rodríguez JL, Barroso MC, Hernández AM, Toledo D, Gabri MR, Alonso DF, Viada C, Gómez RE, Pestana E, Suarez E, Vázquez AM, Perez R, Macías A. 1E10 anti-idiotype vaccine in non-small cell lung cancer: experience in stage IIIb/IV patients. Cancer Biol Ther. 2007;6(12):1847–1852. doi: 10.4161/cbt.6.12.5000. [DOI] [PubMed] [Google Scholar]
- 52.Neninger E, Diaz RM, de la Torre A, Rives R, Diaz A, Saurez G, Gabri MR, Alonso DF, Wilkinson B, Alfonso AM, Combet T, Perez R, Vázquez AM. Active immunotherapy with 1E10 anti-idiotype vaccine in patients with small cell lung cancer: report of a phase I trial. Cancer Biol Ther. 2007;6(2):145–150. doi: 10.4161/cbt.6.2.3574. [DOI] [PubMed] [Google Scholar]
- 53.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6(4):443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 54.Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, Smith M, Anderson B, Villablanca JG, Matthay KK, Shimada H, Grupp SA, Seeger R, Reynolds CP, Buxton A, Reisfeld RA, Gillies SD, Cohn SL, Maris JM, Sondel PM. Anti-GD2 Antibody with GM-CSF, Interleukin-2, and Isotretinoin for Neuroblastoma. N Engl J Med. 2010;363(14):1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barker E, Mueller BM, Handgretinger R, Herter M, Yu AL, Reisfeld RA. Effect of a chimeric anti-ganglioside GD2 antibody on cell-mediated lysis of human neuroblastoma cells. Cancer Res. 1991;51(1):144–149. [PubMed] [Google Scholar]
- 56.Snijdewint FGM, von Mensdorff-Pouilly S, Karuntu-Wanamarta AH, Verstraeten AA, Livingston PO, Hilgers J, Kenemans P. Antibody-dependent cell-mediated cytotoxicity can be induced by MUC1 peptide vaccination of breast cancer patients. Int J Cancer. 2001;93(1):97–106. doi: 10.1002/ijc.1286. [DOI] [PubMed] [Google Scholar]
- 57.Pervin S, Chakraborty M, Bhattacharya-Chatterjee M, Zeytin H, Foon KA, Chatterjee SK. Induction of antitumor immunity by an anti-idiotype antibody mimicking carcinoembryonic antigen. Cancer Res. 1997;57(4):728–734. [PubMed] [Google Scholar]
- 58.Lode HN, Schmidt M, Seidel D, Huebener N, Brackrock D, Bleeke M, Reker D, Brandt S, Mueller H-P, Helm C, Siebert N. Vaccination with anti-idiotype antibody ganglidiomab mediates a GD2-specific anti-neuroblastoma immune response. Cancer Immunol Immunother. 2013;62(6):999–1010. doi: 10.1007/s00262-013-1413-y. [DOI] [PMC free article] [PubMed] [Google Scholar]