Abstract
Immune checkpoint inhibitors (ICIs) are becoming a standard therapy for non-small-cell lung cancer in the advanced stage. As these ICIs become widely available in clinical practice, immune-related adverse effects will become more common. Here we report a patient with lung adenocarcinoma who was treated with nivolumab and developed obstruction because of biliary inflammation. A 63-year-old Japanese man having lung adenocarcinoma with pleural dissemination complained of epigastric pain on the fifth cycle of nivolumab. Computed tomography showed wall thickening at the lower part of the bile duct and cholecystitis. Endoscopic retrograde cholangiopancreatography was repeatedly performed for drainage and stenting of the bile duct. Biopsies did not show obvious malignancy. Laboratory data on day 85 demonstrated grade 3 elevation of serum alkaline phosphatase, transaminase, and amylase levels. We initiated high-dose oral prednisone, resulting in gradual improvement of symptoms and laboratory data. Follow-up magnetic resonance cholangiopancreatography demonstrated no progression of duct obstruction, which confirmed the absence of biliary malignancy. Combined with results from previous reports, nivolumab may cause extrahepatic cholangitis.
Keywords: Immune-related adverse event, Nivolumab, Lung adenocarcinoma, Cholangitis, Cholecystitis
Introduction
Programmed death receptor ligand 1 (PD-L1), which is often expressed on tumor cells, binds to programmed death receptor-1 (PD-1) on activated cytotoxic T cells. This binding is known to induce cancer immunotolerance and is one of the targets of cancer immunotherapy [1]. The anti-PD-1 antibody nivolumab has been shown to improve overall survival when compared to that with docetaxel in non-small-cell lung carcinoma (NSCLC) patients [2, 3].
Immune checkpoint inhibitors (ICIs) induce inflammatory reactions against cancer tissue, and normal organs can be involved in a similar mechanism, which is referred to as immune-related adverse effects (irAEs). Biliary system complications are considered to be rare manifestations of irAEs, and the management strategy remains unclear. Here, we report a patient with lung adenocarcinoma treated with nivolumab, who developed immune-related bile duct obstruction. High-dose prednisone and stenting resolved the patient’s symptoms.
Case report
A 63-year-old Japanese man was diagnosed with lung adenocarcinoma with pleural dissemination. The patient had a remote history of peptic ulcer disease but no evidence of metastatic disease below the diaphragm. No hepatic, pancreatic, or biliary disease was pointed out. He was treated with five cycles of cisplatin and pemetrexed as first-line chemotherapy; however, the disease progressed.
Thereafter, he received second-line treatment with nivolumab (3 mg/kg every 2 weeks). No adverse events were observed, and his liver and pancreatic enzyme levels were normal on day 1 of the fourth cycle (aspartate aminotransferase (AST): 26 U/L, alanine transferase (ALT): 15 U/L, alkaline phosphatase (ALP): 443 U/L, amylase (Amy) 97 U/L). On day 1 of the fifth cycle, computed tomography (CT) for a routine checkup of adverse events showed cholecystitis; however, no gallstone was detected and he had no symptoms at that time. He was, therefore, carefully followed up without treatment until day 24 of cycle five, when he was admitted to our hospital with complains of epigastric pain and soft stool. Blood test revealed elevation in AST (88 U/L), ALT (92 U/L), and ALP (1543 U/L) levels. Obstruction at the lower part of the common bile duct was noted on CT and magnetic resonance cholangiopancreatography (MRCP) (Fig. 1). We postponed the sixth cycle of nivolumab. We started with ampicillin/sulbactam empirically and performed endoscopic retrograde cholangiopancreatography (ERCP) on the day of admission, and inserted a stent via the ampulla of Vater. No gastric hemorrhage was observed. A biliary biopsy showed atypical epithelium with neutrophil infiltration. However, front formation was not observed; therefore, no evident malignancy was confirmed pathologically. Abdominal pain was noted the next day of admission; therefore, ERCP was performed again, and an endoscopic nasobiliary drainage (ENBD) tube was inserted. The symptoms regressed after the second ERCP. The ENBD tube was changed to a biliary stent on day six of hospital stay. Repeated biopsies of the bile duct obtained at that time did not show biliary malignancy, and only interstitial fibrosis and neutrophil-infiltrated atypical mucosa were noted. He was discharged on day 9 of hospital stay. However, 4 days later, he again presented to our hospital with fever and epigastric pain. Ultrasound showed wall thickening of the gall bladder. He was admitted, an ENBD tube was inserted again and we started on sulbactam/cefoperazone. A biliary biopsy showed atypical mucosa with neutrophil infiltration, while immunostaining of p53 was negative. Fever temporarily abated on day 6 of the hospital stay, but recurred on day 11. Bile and blood culture were examined, and we started on piperacillin/tazobactam and vancomycin. Bile culture showed small amount of Pseudomonas aeruginosa and Enterococcus faecalis isolates, while blood culture showed negative results. Considering the sensitivity test and renal function, the antibiotics were de-escalated to cefepime and metronidazole. The ENBD tube was changed to a stent with ERCP. Fever persisted, whereas abdominal pain and elevation in enzyme levels were absent. We assumed that the fever might be due to the use of antibiotics and so discontinued the antibiotics; thereafter, the fever gradually declined. He was discharged on day 33 of the second hospital stay. Laboratory data obtained in the outpatient ward on day 20 after discharge demonstrated grade 3 elevation of serum ALP (5058 U/L), Amy (159 U/L), and aminotransferase levels (AST: 121 U/L, ALT: 142 U/L). We suspected that these abnormalities stem from biliary obstruction and hepatitis along with pancreatitis. We assumed that this obstruction was nivolumab-induced irAEs because it recurred even though infection was treated and drainage was placed; therefore, high-dose prednisone (1 mg/kg/day) was initiated on the same day. The elevation of pancreatic and hepatic enzyme levels did not resolve after 2 weeks; therefore, we doubled the dose of prednisone to 2 mg/kg/day. We tapered the dose to 10 mg/day (0.25 mg/kg/day) as the fall in enzyme levels gradually peaked. CT on day 49 after the initiation of prednisone and MRCP on day 66 showed no bile duct obstruction or wall thickening of the common bile duct (Fig. 1c, d). Pleural dissemination progressed; therefore, we started docetaxel (55 mg/m2) and ramucirumab (7.5 mg/kg) from day 141 after prednisone introduction, that is, day 226 of cycle five of nivolumab treatment. In total, four cycles of docetaxel and ramucirumab were administrated and no enzyme elevation is observed.
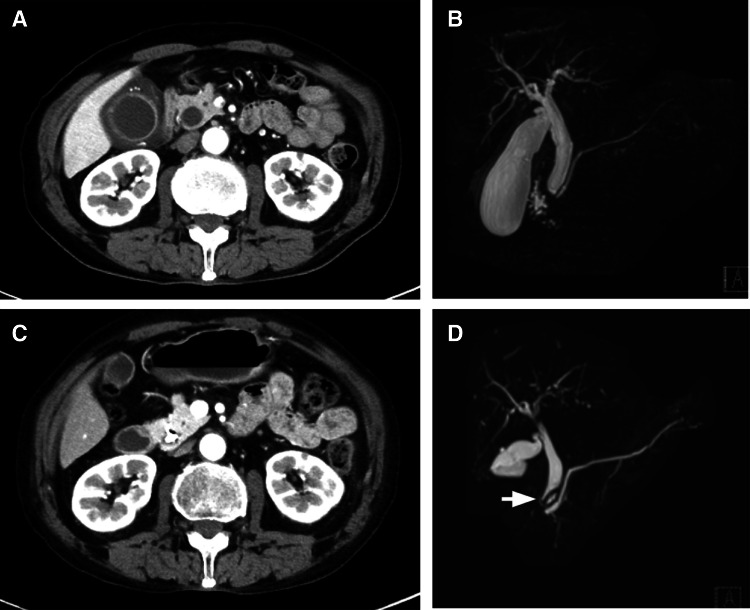
Fig. 1.
Computed tomography (CT) image obtained on day 24 shows wall thickening of the gallbladder and obstruction of the lower portion of the bile duct (a). Magnetic resonance cholangiopancreatography (MRCP) image obtained on the same day shows narrowing of the bile duct (b). Follow-up CT image on day 134 (c) and MRCP image obtained on day 151 (d) show resolution of bile duct dilation. The stent is observed as a signal defect (arrow)
Discussion
We treated bile duct obstruction secondary to nivolumab administration using bilio-papillary stenting and high-dose prednisone administration. No progression was observed during the cessation of chemotherapy, which indicated that this phenomenon did not stem from biliary cancer.
Pathologically, none of the biopsies performed at three different points showed obvious tumor tissue. Additionally, CT revealed regression of the thickening of the lower part of the bile duct without prednisone or anticancer reagent. These findings cannot exclude the possibility of malignancy considering sampling error; however, the obstruction resolved after high-dose prednisone administration and bile duct stenting. No gallstone was detected on MRCP or ERCP. He had no history of hepatic/biliary/pancreatic diseases or abdominal surgery. There was bacterial infection during his second hospital stay; however, biliary obstruction persisted after treatment with antibiotics and elevation of AST, ALT, and ALP levels also recurred until initiation of prednisone. Another etiology is possible, but the clinical course indicates that the symptoms were irAEs related to nivolumab.
Non-specific neutrophil infiltration in the epithelium and interstitial tissue was observed. Autoimmune-associated cholangitis and cholecystitis were previously reported. Cholangitis includes IgG4-related sclerosing cholangitis, which responds well to prednisone treatment [4]. In many cases, the lower part of the bile duct is affected. These clinical features are similar to those in our case. Histologically, periductal localization of IgG4-positive plasma cells and fibrosis characterize IgG4-related cholangitis. However, biopsies obtained from our case showed non-specific neutrophil-dominant inflammation. These findings may imply that a similar disease phenotype is provoked by different mechanisms from IgG4-related cholangitis.
A bile duct stent was placed at the first admission; however, the symptoms recurred. We initiated prednisone and increased the dose to 2 mg/kg/day. Prednisone at 1–4 mg/kg/day has been recommended according to the grade of inflammation for the treatment of other irAEs [5]. We assumed that the present bile duct obstruction was an irAE and needed an anti-inflammatory agent for treatment. One of the options for refractory nivolumab-related bile duct obstruction is infliximab, similar to the option for steroid-resistant irAEs of pneumonitis.
Although this case demonstrated elevation of amylase, MRCP and other imaging modalities showed no abnormalities in the pancreas, such as a narrow pancreatic duct and pancreatic swelling. We previously reported a patient with nivolumab-related pancreatitis [6]. Unlike acute pancreatitis, the symptoms of the reported case were not so severe, and it resembled autoimmune pancreatitis. Grade 4 amylase and lipase level elevations were observed, and the pancreas appeared swollen. The etiology of this case appeared to be different from that of the previously reported case.
Theoretically, all organs can be involved in nivolumab-induced irAEs. In phase II and III trials of nivolumab for advanced NSCLC, diarrhea (8–10%), rash (4–11%), pruritus (6–8%), hypothyroidism (4–7%), hepatitis (1–3%), and renal injury (0–4%) were commonly reported complications; however, grade 3/4 irAEs comprised only 0–3% of all complications [7]. Four cases of nivolumab-associated cholangitis were already reported [8, 9]. Case series of three patients from Japan demonstrated that extrahepatic bile ducts were involved [8]; however, in another reported case, the intrahepatic bile duct was mainly affected and was confirmed by liver biopsy [9]. The former case series presented the features of nivolumab-related cholangitis as follows: (1) localized extrahepatic bile duct dilation, (2) diffuse hypertrophy of the extrahepatic bile duct wall, (3) a dominant increase in biliary tract enzymes relative to hepatic enzymes, (4) normal or reduced levels of serum immunological markers indicating other hepatobiliary diseases, (5) biliary tract CD8-positive T cell infiltration from liver biopsy, and (6) a moderate to poor response to steroid therapy. Our case showed extrahepatic dilation with narrowing of the lower part of the duct, and no stones or tumors were found. Biliary images are also similar to those of previous report. The patient needed as much as 2 mg/kg of prednisone until resolution of cholangitis. These findings seem consistent with those of a previous report [8]. Meanwhile, biliary tract enzyme levels were extremely elevated, whereas liver enzyme levels were also increased. We did not perform a survey of serum for autoimmune markers such as anti-neutrophil antibodies or liver biopsy. We cannot completely exclude other autoimmune-related cholangitis and confirm the involvement of the intrahepatic bile duct. Instead, we repeatedly biopsied the extrahepatic bile duct which turned out to be non-specific neutrophil infiltration. These findings may contradict those of previous reports; however, because there are few cases of cholangitis reported as irAE, we suspect that these features represent one of the phenotypes of irAE. The latest report on the number of sclerosing cholangitis induced by nivolumab, including this case, is 0.05% (10 case/18562 case of estimated treated patients for metastatic melanoma, NSCLC, renal cell carcinoma, classical Hodgkin lymphoma, and head and neck) according to the post-marketing surveillance from 2014 to 2017 of Ministry of Health, Labour and Welfare (MHLW) in Japan.
It is known that irAEs persist. The terminal half-life (t1/2) of nivolumab has been reported to be 17–25 days [5, 10]. It is also known to take about five times of the t1/2 for an antibody reagent to become ineffective. Therefore, we should be careful even after treatment for irAEs. Pembrolizumab, which was recently verified as a first-line therapy for advanced NSCLC, is one of the PD-1 antibody reagents. The t1/2 of pembrolizumab is 23 days [11], which is longer than that of nivolumab, indicating that the irAEs of pembrolizumab persist longer than those of nivolumab and that the interval of administration can be prolonged.
Conclusion
We presented a rare case of bile duct obstruction due to biliary inflammation during nivolumab treatment. The diagnosis was confirmed using CT and MRCP. Repeated biopsy did not show malignant stenosis of the bile duct. High-dose prednisone and stenting via ERCP may be required in such cases.
Acknowledgement
The authors would like to thank Enago (https://www.enago.jp) for the English language review.
Abbreviations
- ALP
Alkaline phosphatase
- ALT
Alanine aminotransferase
- Amy
Amylase
- AST
Aspartate aminotransferase
- CT
Computed tomography
- ENBD
Endoscopic nasobiliary drainage
- ERCP
Endoscopic retrograde cholangiopancreatography
- irAEs
Immune-related adverse effects
- MRCP
Magnetic resonance cholangiopancreatography
- NSCLC
Non-small-cell lung carcinoma
- PD-1
Programmed death receptor-1
- PD-L1
Programmed death receptor ligand 1
Compliance with ethical standards
Conflict of interests
The authors declare that they have no conflict of interest.
Funding sources
This report did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
For this type of study, formal consent is not required.
Informed consent
Informed consent was obtained from the patient presented in this article.
References
- 1.Melosky B, Chu Q, Juergens R, Leighl N, McLeod D, Hirsh V. Pointed progress in second-line advanced non-small-cell lung cancer: the rapidly evolving field of checkpoint inhibition. J Clin Oncol. 2016;34(14):1676–1688. doi: 10.1200/JCO.2015.63.8049. [DOI] [PubMed] [Google Scholar]
- 2.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohara H, Okazaki K, Tsubouchi H, Inui K, Kawa S, Kamisawa T, Tazuma S, Uchida K, Hirano K, Yoshida H, Nishino T. Clinical diagnostic criteria of IgG4-related sclerosing cholangitis 2012. J Hepatobiliary Pancreat Sci. 2012;19(5):536–542. doi: 10.1007/s00534-012-0521-y. [DOI] [PubMed] [Google Scholar]
- 5.U.S.FDA. Administration (2014) OPDIVO (nivolumab) Injection label. http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/125554lbl.pdf. Accessed 8 Apr 2017
- 6.Ikeuchi K, Okuma Y, Tabata T. Immune-related pancreatitis secondary to nivolumab in a patient with recurrent lung adenocarcinoma: A case report. Lung Cancer. 2016;99:148–150. doi: 10.1016/j.lungcan.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Champiat S, Lambotte O, Barreau E, Belkhir R, Berdelou A, Carbonnel F, Cauquil C, Chanson P, Collins M, Durrbach A, Ederhy S. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol. 2016;27(4):559–574. doi: 10.1093/annonc/mdv623. [DOI] [PubMed] [Google Scholar]
- 8.Kawakami H, Tanizaki J, Tanaka K, Haratani K, Hayashi H, Takeda M, Kamata K, Takenaka M, Kimura M, Chikugo K, Sato T, Kudo M, Ito A, Nakagawa K. Imaging and clinicopathological features of nivolumab-related cholangitis in patients with non-small cell lung cancer. Invest New Drugs. 2017;35:529–536. doi: 10.1007/s10637-017-0453-0. [DOI] [PubMed] [Google Scholar]
- 9.Gelsomino F, Vitale G, D’Errico A, Bertuzzi C, Andreone P, Ardizzoni A. Nivolumab-induced cholangitic liver disease: a novel form of serious liver injury. Ann Oncol. 2017;28(3):671–672. doi: 10.1093/annonc/mdw649. [DOI] [PubMed] [Google Scholar]
- 10.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, Gilson MM. Phase I study of single-agent anti–programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.U.S.FDA. Administration (2014) KEYTRUDA (pembrolizumab) Injection label. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/125514lbl.pdf. Accessed 8 Apr 2017