Abstract
Background
To investigate the association between pretreatment blood neutrophil-to-lymphocyte ratio (NLR) and clinical outcomes for advanced-stage cancer patients treated with immunotherapy.
Methods
We conducted a comprehensive literature search to assess the relationship between pretreatment blood NLR and overall survival (OS) or progression-free survival (PFS) in advanced-stage cancer patients treated with immunotherapy. Published data including hazard ratios (HRs) and related 95% confidence interval (CI) were extracted. Pooled estimates of treatment outcomes were calculated using RevMan 5.3.5.
Results
Twenty-seven studies with 4647 patients were included in the current study. The pooled results suggested that high pretreatment blood NLR was correlated with significant shorter OS (HR = 1.98, 95% CI 1.66–2.36, P < 0.001) and PFS (HR = 1.78, 95% CI 1.48–2.15, P < 0.001). Subgroup analysis stratified by study targets revealed that anti-VEGF/VEGFR therapy (HR = 2.04, 95% CI 1.61–2.60, P < 0.001) and immune checkpoints blockade (HR = 2.16, 95% CI 1.86–2.51, P < 0.001) were significantly associated with inferior OS while other targets (HR = 1.63, 95% CI 0.89–2.99, P = 0.120) were not associated with OS. There was no correlation between distinct NLR cutoff values and OS ( = 0.218, P = 0.329) or PFS benefit ( = − 0.386, P = 0.140). Of note, HRs of PFS showed significant correlation with HRs of OS ( = 0.656, P = 0.015).
Conclusion
Elevated pretreatment blood NLR was a promising prognostic and predictive biomarker for advanced-stage cancer patients treated with immunotherapy.
Electronic supplementary material
The online version of this article (10.1007/s00262-018-2126-z) contains supplementary material, which is available to authorized users.
Keywords: Cancer, Neutrophil-to-lymphocyte ratio, Immunotherapy, Biomarker, Immune microenvironment
Introduction
Cancer still remains the most threatening disease to human health worldwide [1]. Although we have a deeper understanding to cancer with the completion of genomic sequence, the effective therapeutic strategies are still limited and long-term survival rate remains disappointing. Recently, with the improvement in the understanding of the role of immune system in the tumor development, and immune response to cancer, immunotherapy has experienced a rapid development and plays a critical role in the current cancer therapy [2, 3]. The advent of cancer immunotherapy, especially the immune checkpoints blockade, has brought about a paradigm shift in the landscape of advanced-stage cancer treatment [4].
The ultimate aim of immunotherapy was to effectively establish or enhance the immune response to cancer, which can be achieved via distinct strategies including tumor vaccination, adoptive immune cells transfer, and blockade of inhibitory signal pathways in TME [5–7]. The most successful case refers to the immune checkpoints inhibitors anti-PD-1 and CTLA-4 that have recently obtained huge success in several types of solid tumors including melanoma, renal cell carcinoma, NSCLC, etc. [4, 6, 8, 9]. In addition, another effective strategy was against VEGF and its receptor, VEGFR. The activation of VEGF/VEGFR could promote the angiogenesis in TME, which is one of the significant hallmarks of cancers [10]. Blockade of VEGF/VEGFR via antibody or small-molecule kinase inhibitors also showed the encouraging anti-tumor effect in several solid tumors [11–15]. However, both blockade of immune checkpoints and VEGF/VEGFR were confronted with the same obstacle to further expand the survival benefit: lack of the reliable biomarkers. For immune checkpoints inhibitors, published data suggested that the response rate to anti-PD-1/PD-L1 monotherapy was approximately 30% [16, 17]. Although several factors including PD-L1 expression, tumor infiltrating lymphocytes, tumor mutation load, neoantigen and so on, showed the predictive value in preclinical or clinical studies [4, 18–20], the optimal predictive biomarkers still remain undetermined. For anti-VEGF/VEGFR, researchers have put so much effort into the exploration of predictive biomarkers to anti-VEGF/VEGFR over these years, but the results were disappointing [21, 22]. To date, there is no study to report the reliable biomarkers of anti-VEGF/VEGFR therapy in advanced cancers.
Emerging evidence suggested that tumor-associated inflammation plays a significant role in the distinct stages of cancer development, including initiation, promotion, invasion and distant metastasis [23, 24]. Inflammation could also influence the host immune response to cancers and could be applied to cancer immunotherapy [24–26]. Several studies attempted to utilize the inflammatory mediators and the measurable parameters of systemic inflammatory response to predict the therapeutic effect or survival in patients with advanced cancers. The latter category includes albumin, C-reactive protein and neutrophil-to-lymphocyte ratio (NLR) that have been incorporated in prognostic scores for different types of cancer [27]. NLR was defined as neutrophil counts divided by lymphocyte counts. Theoretically, lymphopenia reflects the impaired cell-mediated immunity, whereas neutrophilia represents the response to systematic inflammation [23]. Elevated NLR would be associated with poor response to immunotherapy in patients with advanced cancers. Recently, several studies investigated the predictive value of pretreatment blood NLR in advanced cancer patients treated with immunotherapy [28–31], but the results remain inconsistent. Therefore, we conducted this meta-analysis to systematically and comprehensively evaluate both the predictive and prognostic significance of pretreatment blood NLR for advanced-stage cancer patients treated with immunotherapy. The pooled results could be used on daily clinical practice and help physicians to stratify patients in future clinical trials of immunotherapy.
Methods
Search strategy
We carried out a comprehensive online search to select the potential studies on PubMed, Web of Science, EMBASE, and Cochrane Library up to May 2017 without language restrictions. The main keywords used for the online search were “neoplasms”, “tumor”, “cancer”, “neutrophil”, “lymphocyte”, “ratio”, “survival” and “prognosis”. The full search strategies are listed in Supplementary Table S1. Abstracts from conference proceedings of the American Society of Clinical Oncology (ASCO), the European Society for Medical Oncology (ESMO), and the World Lung Cancer Conference (WCLC) were searched to identify unpublished studies. We also manually searched the reference lists of the selected articles until no additional potential articles could be identified.
Inclusion criteria
The following items were the inclusion criteria for each eligible publication: (1) studies investigated the patients with advanced cancer treated with immunotherapy (targeting tumor immune microenvironment including VEGF, VEGFR, CTLA-4, PD-1, PD-L1, etc.); (2) studies reported the predictive and/or prognostic value of pretreatment NLR; (3) data were presented for OS and/or PFS and related hazard ratio (HR) with 95% confidence interval (CI); (4) if two or more studies used the same population, only the study with the largest sample size and latest information was included; (5) the full text was available. Case report, reviews, comments, editorials, letters or articles unrelated with our topics were excluded. First, the titles and abstracts were screened to assess the eligibility and then the full text of articles were reviewed. According to the inclusion criteria, two reviewers (Meng Qiao and Chunxia Su) conducted the selection of all included publications independently. The third reviewer resolved the discrepancy on whether an article should be included.
Data extraction
Two reviewers independently carried out the data extraction on the basis of Preferred Reporting Items for Systematic Reviews and Meta-analyses statement (PRISMA). The following items were extracted from each study: first author’s name, published year, inclusion period, study design, tumor type and stage, country of origin study, number of patients, average ages, treatment, main targets, cut-off value of NLR, time of NLR assessment, follow-up period and study endpoints. Two researchers (Tao Jiang and Shengxiang Ren) also independently extracted the HRs and the associated 95% CIs for PFS and OS outcomes to assess the therapeutic efficacy. HRs from multivariate analyses were preferentially extracted. Where available, we included the most updated survival data.
Quality assessment
As the previous studies reported [32, 33], two reviewers independently investigated the risk of bias of the included studies using a set of modified predefined criteria: (1) Representativeness of population; (2) Non exposed cohort; (3) Ascertainment of exposure; (4) Outcome not present at start of study; (5) Appropriate confounding measurement and account; (6) Sufficient measurement of outcomes; (7) Completeness of follow-up. Studies with a score of 7 or higher were considered as high quality and with a score of less than 7 defining low quality. Any disagreement was resolved by discussion.
Statistical analysis
Pretreatment blood NLR was defined as the ratio of the number of neutrophils to the number of lymphocytes in the peripheral blood before any treatment. OS was calculated from the date of initial diagnosis to the time of death from any cause or was censored at the last follow-up. PFS was defined as the time from the date of first-line treatment initiation to the date of cancer progression or death or was censored at the last tumor assessment. Cochran’s Q test and I2 statistic were used to test the heterogeneity of different studies. For time-to-event data, the HRs with 95% CIs were directly extracted from the research article or calculated using previously published methods proposed by Tierney et al. [34]. The I2 test was used to test for statistical heterogeneity and the I2 statistic was used to assess the extent of variability attributable to statistical heterogeneity across studies. I2 < 25% was interpreted as signifying low-level heterogeneity. When there was no statistically significant heterogeneity, a pooled effect was calculated with a fixed-effects model; otherwise, a random-effect model was used. PFS and OS were calculated using effect variables. Publication bias was assessed using funnel plots, Begg’s and Egger’s tests. A sensitivity analysis was conducted by excluding the studies with the low-quality score. P values were two-sided and considered significant if less than 0.05. All data were analyzed using the Statistical Package for Social Sciences (SPSS) software (version 20.0 for Windows). Meta-analyses were performed using RevMan 5.3.5 (http://tech.cochrane.org/revman).
Results
Selection of eligible studies
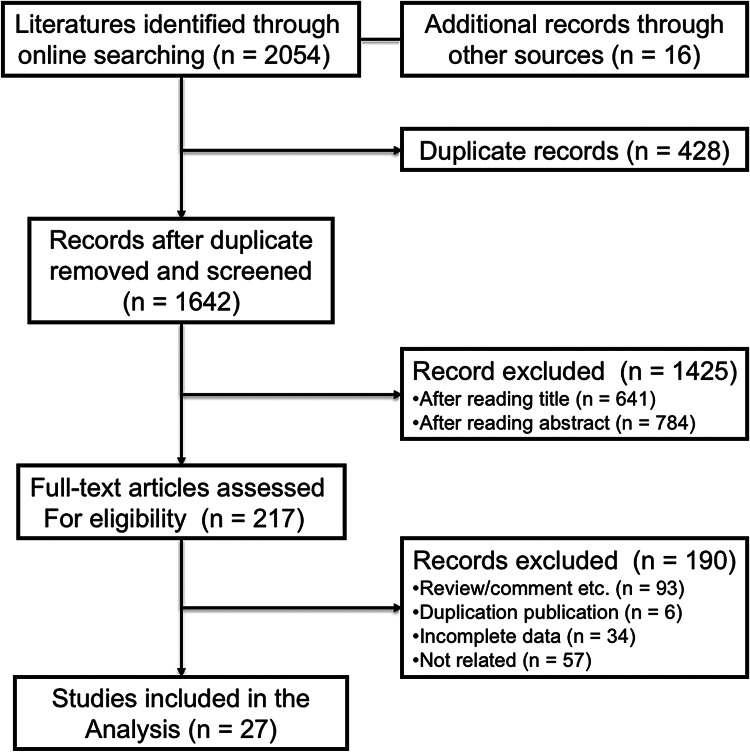
Totally, we identified 2054 studies that met the inclusion criteria after searching the relevant online databases; 428 of them were excluded due to duplicate records. By verifying related terms in the titles and abstracts, we excluded 1425 irrelevant articles, and another 190 articles were excluded after the assessment of full text. Finally, 27 studies were selected for the present meta-analysis [28–31, 35–57]. A flowchart describing the eligible study selection was shown in Fig. 1.
Fig. 1.
Flow chart of the eligible studies in the current meta-analysis
Characteristics of included studies and quality assessment
A total of 4647 patients with six different kinds of advanced cancers were included in the current study. The baseline characteristics of the included studies are summarized in Table 1. In summary, all studies were published between 2012 and 2017. 26 of the included studies were retrospective studies and only one was prospective study. Fifteen studies focused on metastatic renal cell carcinoma and 4 of them studied metastatic melanoma. Other types of cancer included advanced NSCLC, hepatocellular carcinoma, gastric cancer and metastatic colorectal cancer. Ten studies were conducted in Asia, 6 in America, 10 in Europe and one covered multiple countries. VEGF/VEGFR and CTLA-4 were the main targets. The most common cut-off value of NLR was 3 and median cut-off value was 3.02. Twenty-one studies investigated the association between pretreatment NLR and OS for patients with advanced cancer, whereas 15 studies reported PFS outcome. Of note, two studies recorded the Kaplan–Meier curve of PFS and OS. To avoid the selection bias, we did not extract the HRs with 95% CIs from the published figures. According to the risk assessment scale, we evaluated the eligible studies using the aspects mentioned above [32]. The results of quality assessment are listed in Supplemental Table S2. Twelve studies had quality scores of 7 or less, and 15 studies had a score of more than 7.
Table 1.
Baseline characteristics of included studies (n = 27)
| Authors | Year | Inclusion period | Study design | Tumor type and stage | Country of origin | Number of cases | Age (years) | Treatment | Main targets | NLR cutoff | Time of assessment | Follow-up period (months) | Study endpoints | Quality score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Keizman et al. | 2012 | 2004–2011 | Retrospective | Metastatic renal cell carcinoma | USA | 133 | 61 (24–85) | Sunitinib | VEGFR, PDGFR | > 3 | Pretreatment | 37 (5–85) | RR, PFS, OS | 8 |
| Botta et al. | 2013 | 2008–2011 | Retrospective | Advanced non-small cell lung cancer | USA | 112 | 62 | Bevacizumab | VEGF | > 4 | Pretreatment | 15 | RR, PFS, OS | 7 |
| Cetin et al. | 2013 | 2008–2011 | Retrospective | Metastatic renal cell carcinoma | Turkey | 100 | 58 (24–80) | VEGF-TKIs | VEGF | >3.04 | Pretreatment | 15 (1–53) | PFS, OS | 8 |
| Dirican et al. | 2013 | 2006–2011 | Retrospective | Metastatic renal cell carcinoma | Turkey | 23 | 59 (43–76) | Sunitinib | VEGFR, PDGFR | > 3 | Pretreatment | 13 (2–41) | RR, PFS, OS | 8 |
| Zheng et al. | 2013 | 2011–2012 | Retrospective | Hepatocellular carcinoma | China | 65 | 55 | Sorafenib | VEGFR, PDGFR | > 4 | Pretreatment | 9 (2–26) | RR, PFS, OS | 8 |
| Kobayashi et al. | 2013 | 2008–2012 | Retrospective | Metastatic renal cell carcinoma | Japan | 58 | 64 (53–81) | Sorafenib, sunitinib | VEGFR, PDGFR | > 3.32 | Pretreatment | 12 (1–49) | PFS, OS | 8 |
| Fonseca et al. | 2014 | 2009–2013 | Retrospective | Hepatocellular carcinoma | Brazil | 120 | 60 (19–80) | Sorafenib | VEGFR, PDGFR | > 3.5 | Pretreatment | 11 (0.5–27) | OS | 8 |
| Gunduz et al. | 2014 | 2009–2013 | Retrospective | Metastatic renal cell carcinoma | Turkey | 45 | 63 (41–90) | Sunitinib, sorafenib and pazopanib | VEGFR, PDGFR | > 2 | Pretreatment | 24 | PFS, OS | 7 |
| Keizman et al. | 2014 | 2004–2013 | Retrospective | Metastatic renal cell carcinoma | Israel | 278 | 63 (22–87) | Sunitinib | VEGFR, PDGFR | > 3 | Pretreatment | 55 (12–109) | RR, PFS, OS | 8 |
| Dana et al. | 2014 | 2006–2013 | Retrospective | Metastatic renal cell carcinoma | Israel | 145 | 65 | Sunitinib | VEGFR, PDGFR | > 3 | Pretreatment | NR | PFS, OS | 6 |
| Park et al. | 2014 | 2005–2011 | Retrospective | Metastatic celaer cell renal cell carcinoma | Korea | 109 | 61 (49–67) | Sunitinib | VEGFR, PDGFR | > 2.5 | Pretreatment | 24 (10–35) | OS | 8 |
| Wang et al. | 2014 | 2006–2011 | Retrospective | Metastatic renal cell carcinoma | China | 41 | 53 (24–81) | Sorafenib | VEGFR, PDGFR | > 4 | Pretreatment | NR | PFS | 6 |
| Ferrucci et al. | 2015 | 2010–2013 | Retrospective | Metastatic melanoma | Italy | 187 | 62 (33–87) | Ipilimumab | CTLA-4 | > 5 | Pretreatment | NR | RR, PFS, OS | 6 |
| Namikawa et al. | 2015 | 2007–2012 | Retrospective | Gastric cancer | Japan | 190 | 68 (56–80) | Protein-bound polysaccha- ride K | Unknown | > 2.5 | Pretreatment | NR | OS | 6 |
| Santoni et al. | 2015 | 2005–2014 | Retrospective | Metastatic renal cell carcinoma | Italy | 151 | 64 (29–88) | Sunitinib, sorafenib and pazopanib | VEGFR, PDGFR | > 3 | Pretreatment | 52 | PFS, OS | 7 |
| Gardini et al. | 2016 | 2012–2015 | Retrospective | Hepatocellular carcinoma | Italy | 56 | NR | Sorafenib | VEGFR, PDGFR | > 3 | Pretreatment | NR | RR, PFS, OS | 6 |
| Chrom et al. | 2016 | 2010–2014 | Retrospective | Metastatic renal cell carcinoma | Poland | 266 | 61 (22–85) | Sunitinib, sorafenib and pazopanib | VEGFR, PDGFR | > 4 | Pretreatment | NR | OS | 6 |
| Ferrucci et al. | 2016 | 2010–2012 | Prospective | Metastatic melanoma | Italy | 720 | NR | Ipilimumab | CTLA-4 | > 3 | Pretreatment | 17 | PFS, OS | 7 |
| Keizman et al. | 2016 | 2004–2014 | Retrospective | Metastatic Chromophobe Renal Cell Carcinoma | Israel and USA | 72 | 64 (26–87) | Sunitinib | VEGFR, PDGFR | > 3 | Pretreatment | NR | RR, PFS, OS | 6 |
| Passardi et al. | 2016 | 2007–2012 | Retrospective | Metastatic colorectal cancer | Italy | 289 | 65 (33–83) | Bevacizumab | VEGF | > 3 | Pretreatment | 36 (1–65) | PFS, OS | 8 |
| Taipale et al. | 2016 | 2007–2012 | Retrospective | Solid tumors | Finland | 290 | NR | Adenoviral oncolytic immunotherapy | Oncolytic viruses | NR | Pretreatment | NR | PFS, OS | 6 |
| Zhang et al. | 2016 | 2006–2014 | Retrospective | Metastatic renal cell carcinoma | China | 373 | 58 (17–90) | Sunitinib, sorafenib | VEGFR, PDGFR | > 2.2 | Pretreatment | NR | PFS, OS | 6 |
| Bagley et al. | 2017 | 2015–2016 | Retrospective | Advanced non-small cell lung cancer | USA | 175 | 68 (33–88) | Nivolumab | PD-1 | > 5 | Pretreatment | NR | RR, PFS, OS | 6 |
| Cassidy et al. | 2017 | 2006–2011 | Retrospective | Metastatic melanoma | USA | 197 | NR | Ipilimumab | CTLA-4 | > 5 | Pretreatment | 54 (5-109) | PFS, OS | 8 |
| Jung et al. | 2017 | 2014–2015 | Retrospective | Metastatic melanoma | Korea | 104 | 58 (50–66) | Ipilimumab | CTLA-4 | > 5 | Pretreatment | NR | RR, PFS, OS | 6 |
| Kuzman et al. | 2017 | 2003–2012 | Retrospective | Metastatic renal cell carcinoma | USA | 71 | 55 (49–59) | High dose interleukin-2 | Interleukin-2 receptor | > 4 | Pretreatment | NR | RR, PFS, OS | 6 |
| Tanaka, et al. | 2017 | NR | Retrospective | Metastatic renal cell carcinoma | Japan | 277 | 66 (59–73) | Sunitinib, sorafenib, axitinib, and pazopanib | VEGFR, PDGFR | > 3.6 | Pretreatment | 18 (8–30) | OS | 8 |
NR not reported, VEGF vascular endothelial growth factor, PDGF platelet derived growth factor, CTLA-4 cytotoxic T-lymphocyte associated protein 4, PD-1 programmed death 1, RR response rate, PFS progression-free survival, OS overall survival
Association between pretreatment NLR and overall survival
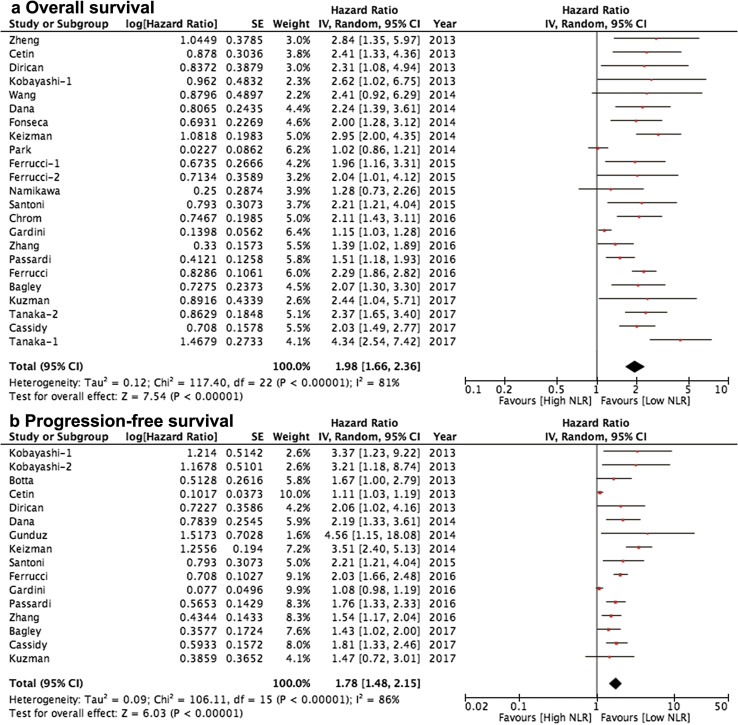
Twenty-one studies with 3891 cases were included in the final analysis of pretreatment NLR and OS. The pooled result showed that high pretreatment NLR was correlated with significantly poorer OS (HR = 1.98, 95% CI 1.66–2.36, P < 0.001; Fig. 2a), among which metastatic renal cell carcinoma (n = 11, HR = 2.18, 95% CI 1.63–2.92, P < 0.001) and metastatic melanoma (n = 3, HR = 2.17, 95% CI 1.85–2.55, P < 0.001) were two common types of cancer involved. We summarized the results of the subgroup analyses by the potential sources of heterogeneity among several related clinical parameters of the included studies for OS in Table 2. The pooled results for most subgroups were not markedly changed by the study features. However, there was only marginally statistical significance in patients with hepatocellular carcinoma when we pooled three studies (HR = 1.72, 95% CI 1.00–2.96, P = 0.050; I2 = 82%, P = 0.004). Significant difference was not indicated in patients with gastric cancer (HR = 1.28, 95% CI 0.73–2.25, P = 0.368). Of note, stratified analysis by study targets of TME suggested that anti-VEGF/VEGFR (n = 14; HR = 2.04, 95% CI 1.61–2.60, P < 0.001; I2 = 84%, P < 0.001) and immune checkpoints blockade (n = 4; HR = 2.16, 95% CI 1.86–2.51, P < 0.001; I2 = 0%, P = 0.960) were significantly correlated with inferior OS while other targets (including IL-2 receptor and oncolytic viruses) (n = 2; HR = 1.63, 95% CI 0.89–2.99, P = 0.120; I2 = 34%, P = 0.220) were not associated with OS, indicating the prognostic value of pretreatment NLR in these studies. Interestingly, the pooled HRs did not significantly alter by pretreatment NLR cutoff value but it can reduce the level of statistical heterogeneity (NLR > 3, I2 = 79%; NLR > 4, I2 = 0%; NLR > 5, I2 = 0%;). We also noted the significant reduction of statistical heterogeneity by median age (≤ 60, I2 = 7%) and main target of immune checkpoints (I2 = 0%).
Fig. 2.
Meta-analysis of the associations between pretreatment blood neutrophil-to-lymphocyte ratio and a overall survival, b progression-free survival
Table 2.
Subgroup analyses of the associations between NLR and survival
| Variables | No of studies | Test of association | Test of heterogeneity | |||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | I 2 | P value | ||
| Overall survival | ||||||
| Total | 21 | 1.98 | 1.66–2.36 | < 0.001 | 81% | < 0.001 |
| Publication year | ||||||
| Before year 2015 | 9 | 2.15 | 1.46–3.16 | < 0.001 | 82% | < 0.001 |
| After year 2015 | 12 | 1.92 | 1.55–2.37 | < 0.001 | 82% | < 0.001 |
| Initial inclusion period | ||||||
| Before year 2010 | 14 | 1.86 | 1.49–2.32 | < 0.001 | 73% | < 0.001 |
| After year 2010 | 6 | 1.94 | 1.37–2.74 | < 0.001 | 87% | < 0.001 |
| Study design | ||||||
| Retrospective | 20 | 1.96 | 1.63–2.34 | < 0.001 | 79% | < 0.001 |
| Prospective | 1 | 2.29 | 1.86–2.82 | < 0.001 | – | – |
| Tumor types | ||||||
| Metastatic renal cell carcinoma | 11 | 2.18 | 1.63–2.92 | < 0.001 | 82% | < 0.001 |
| Advanced non-small cell lung cancer | 1 | 2.07 | 1.30–3.30 | < 0.001 | – | – |
| Hepatocellular carcinoma | 3 | 1.72 | 1.00-2.96 | 0.050 | 82% | 0.004 |
| Gastric cancer | 1 | 1.28 | 0.73–2.25 | 0.368 | – | – |
| Metastatic colorectal cancer | 1 | 1.76 | 1.33–2.32 | 0.001 | – | – |
| Metastatic melanoma | 3 | 2.17 | 1.85–2.55 | < 0.001 | 0% | 0.890 |
| Research region | ||||||
| Asia | 7 | 1.96 | 1.32–2.91 | < 0.001 | 84% | < 0.001 |
| Europe and America | 11 | 1.92 | 1.53–2.41 | < 0.001 | 80% | < 0.001 |
| Others | 3 | 2.06 | 1.49–2.85 | < 0.001 | 66% | 0.030 |
| Sample size | ||||||
| > 100 | 14 | 1.96 | 1.10–2.40 | < 0.001 | 80% | < 0.001 |
| ≤ 100 | 7 | 2.09 | 1.36–3.20 | 0.001 | 71% | 0.002 |
| Median age (years) | ||||||
| > 60 | 11 | 2.03 | 1.56–2.63 | < 0.001 | 81% | < 0.001 |
| ≤ 60 | 7 | 1.85 | 1.51–2.26 | < 0.001 | 7% | 0.370 |
| Main targets | ||||||
| VEGF/VEGFR | 14 | 2.04 | 1.61–2.60 | < 0.001 | 84% | < 0.001 |
| Immune checkpoints | 4 | 2.16 | 1.86–2.51 | < 0.001 | 0% | 0.960 |
| Others | 2 | 1.63 | 0.89–2.99 | 0.120 | 34% | 0.220 |
| NLR cutoff | ||||||
| > 3 | 18 | 2.17 | 1.78–2.63 | < 0.001 | 79% | < 0.001 |
| > 4 | 7 | 2.11 | 1.77–2.53 | < 0.001 | 0% | 1.000 |
| > 5 | 3 | 2.03 | 1.63–2.53 | < 0.001 | 0% | 1.000 |
| Follow-up period (months) | ||||||
| > 24 | 4 | 2.05 | 1.51–2.78 | < 0.001 | 65% | 0.030 |
| ≤ 24 | 7 | 2.20 | 1.49–3.24 | < 0.001 | 88% | < 0.001 |
| NR | 10 | 1.73 | 1.37–2.18 | < 0.001 | 67% | 0.001 |
| Study quality | ||||||
| > 7 | 10 | 2.17 | 1.61–2.92 | < 0.001 | 84% | < 0.001 |
| ≤ 7 | 11 | 1.84 | 1.45–2.33 | < 0.001 | 79% | < 0.001 |
| Progression-free survival | ||||||
| Total | 15 | 1.78 | 1.48–2.15 | < 0.001 | 86% | < 0.001 |
| Publication year | ||||||
| Before year 2015 | 7 | 2.28 | 1.41–3.69 | < 0.001 | 88% | < 0.001 |
| After year 2015 | 8 | 1.60 | 1.26–2.05 | < 0.001 | 85% | < 0.001 |
| Initial inclusion period | ||||||
| Before year 2010 | 12 | 1.97 | 1.51–2.57 | < 0.001 | 84% | < 0.001 |
| After year 2010 | 3 | 1.46 | 0.93–2.28 | 0.010 | 94% | < 0.001 |
| Study design | ||||||
| Retrospective | 14 | 1.74 | 1.44–2.10 | < 0.001 | 83% | < 0.001 |
| Prospective | 1 | 2.03 | 1.66–2.47 | < 0.001 | – | – |
| Tumor types | ||||||
| Metastatic renal cell carcinoma | 9 | 2.11 | 1.47–3.02 | < 0.001 | 85% | < 0.001 |
| Advanced non-small cell lung cancer | 2 | 1.50 | 1.13–1.99 | 0.005 | 0% | 0.620 |
| Hepatocellular carcinoma | 1 | 1.08 | 0.98–1.20 | 0.129 | – | – |
| Metastatic colorectal cancer | 1 | 1.51 | 1.18–1.95 | 0.001 | – | – |
| Metastatic melanoma | 2 | 1.96 | 1.66–2.32 | < 0.001 | 0% | 0.540 |
| Research region | ||||||
| Asia | 2 | 2.14 | 1.21–3.80 | 0.009 | 47% | 0.150 |
| Europe and America | 11 | 1.57 | 1.30–1.89 | < 0.001 | 84% | < 0.001 |
| Others | 2 | 2.36 | 1.52–3.68 | < 0.001 | 76% | 0.020 |
| Sample size | ||||||
| > 100 | 9 | 1.91 | 1.62–2.25 | 0.020 | 51% | 0.040 |
| ≤ 100 | 5 | 1.21 | 1.03–1.42 | 0.020 | 61% | 0.020 |
| Median age (years) | ||||||
| > 60 | 8 | 2.17 | 1.68–2.80 | < 0.001 | 53% | 0.030 |
| ≤ 60 | 4 | 1.37 | 1.03–1.81 | 0.030 | 64% | 0.040 |
| Main targets | ||||||
| VEGF/VEGFR | 10 | 1.80 | 1.43–2.25 | < 0.001 | 85% | < 0.001 |
| Immune checkpoints | 3 | 1.80 | 1.48–2.19 | < 0.001 | 35% | 0.220 |
| NLR cutoff | ||||||
| > 3 | 13 | 1.92 | 1.51–2.44 | < 0.001 | 84% | < 0.001 |
| > 4 | 3 | 1.61 | 1.30-2.00 | < 0.001 | 0% | 0.580 |
| > 5 | 2 | 1.63 | 1.30–2.04 | < 0.001 | 2% | 0.310 |
| Follow-up period (months) | ||||||
| > 24 | 4 | 2.19 | 1.58–3.04 | < 0.001 | 68% | 0.020 |
| ≤ 24 | 6 | 1.54 | 1.22–1.93 | < 0.001 | 87% | < 0.001 |
| NR | 5 | 1.43 | 1.10–1.86 | 0.008 | 72% | 0.007 |
| Study quality | ||||||
| > 7 | 6 | 2.06 | 1.38–3.10 | < 0.001 | 90% | < 0.001 |
| ≤ 7 | 9 | 1.67 | 1.28–2.18 | < 0.001 | 83% | < 0.001 |
Association between pretreatment NLR and progression-free survival
Fifteen studies with 2793 cases were included in the final analysis of pretreatment NLR and PFS. The pooled result suggested that low pretreatment NLR was correlated with significantly longer PFS (HR = 1.78, 95% CI 1.48–2.15, P < 0.001; Fig. 2b), among which metastatic renal cell carcinoma (n = 9, HR = 2.11, 95% CI 1.47–3.02, P < 0.001; I2 = 85%, P < 0.001) was the most common types of cancer involved. Table 2 lists the results of the subgroup analyses by the potential sources of heterogeneity among several related clinical parameters of the included studies for PFS. The pooled results for most subgroups were not markedly changed by the study characteristics. However, there was no statistical significance in patients with hepatocellular carcinoma (HR = 1.08, 95% CI 0.98–1.20, P = 0.129). Most stratified factors cannot reduce the level of statistical heterogeneity. However, stratified analysis by pretreatment NLR cutoff value could significantly reduce the level of statistical heterogeneity (NLR > 3, I2 = 84%; NLR > 4, I2 = 0%; NLR > 5, I2 = 2%;) while the pooled HRs did not significantly alter.
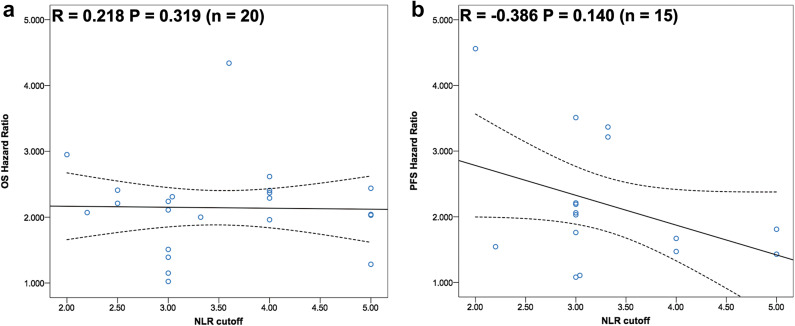
Correlation between distinct NLR cutoff values and clinical outcome
As we previously mentioned, stratified analysis by pretreatment NLR cutoff value could significantly reduce the level of statistical heterogeneity for both OS and PFS. We further investigated the correlation between distinct NLR cutoff values and clinical outcome of advanced cancer patients treated with immunotherapy. As shown in Fig. 3a, the results indicated that there was no correlation between distinct NLR cutoff values and OS benefit ( = 0.218, P = 0.329). Although higher NLR cutoff value seemed to be associated with the decreased HRs of PFS, there was no statistical significance ( = − 0.386, P = 0.140; Fig. 3b). Interestingly, HRs of PFS showed significant correlation with HRs of OS ( = 0.656, P = 0.015), indicating PFS was a potential surrogate for OS in these trials’ designs (Supplemental Figure S1).
Fig. 3.
Correlation analysis between pretreatment blood neutrophil-to-lymphocyte ratio and a overall survival, b progression-free survival
Publication bias
As shown in Supplemental Figure S2, the funnel plots were almost symmetrical and the test results indicated that no publication bias existed regarding the HRs of OS (Begg’s test, P = 0.673; Egger’s test, P = 0.100) or PFS (Begg’s test, P = 0.760; Egger’s test, P = 0.356).
Discussion
To our best knowledge, this is the first time to report that pretreatment blood NLR is associated with outcome of advanced-stage cancer patients treated with immunotherapy. The present study summarized the available evidence from twenty-seven studies with a total of 4647 cases. The pooled results indicated that elevated pretreatment blood NLR was significantly associated with inferior OS (HR = 1.98, P < 0.001) and PFS (HR = 1.78, P < 0.001) in all groups. Subgroup analyses stratified by publication year, initial inclusion period, study design, research region, sample size, median age, follow-up period, main targets, NLR cutoff and quality scores showed that the results remained constant. It is worth mentioning that there was no correlation between distinct NLR cutoff values and OS benefit. Although higher NLR cutoff value seemed to be correlated with the decreased HRs of PFS, there was no statistical significance. Notably, HRs of PFS showed significant correlation with HRs of OS, suggesting PFS was a potential surrogate for OS in these trials’ designs.
There have been two high-quality published meta-analyses to investigate the prognostic value of pretreatment blood NLR in advanced cancer patients. The first study included 100 studies incorporating a total of 40,559 patients and the pooled analyses suggested that a high NLR was associated with an adverse OS in all groups (HR = 1.81, 95% CI 1.67–1.97; P < 0.001) [58]. Furthermore, NLR greater than cutoff was also significantly associated poor PFS and DFS and these effects were observed in all disease subgroups, sites and stages. In another study, the authors included 66 studies involving a total of 24,536 patients for the meta-analysis [32]. Pooled results indicated high pretreatment NLR was correlated with inferior OS (HR = 1.70, 95% CI 1.57–1.84; P < 0.001) and PFS (HR = 1.70, 95% CI 1.57–1.84; P < 0.001) in advanced cancers. Similarly, the results remain constant in the subgroup analyses. However, both studies just investigated the prognostic role of NLR in advanced cancers, whether pretreatment NLR had the predictive value in patients with advanced tumor treated with immunotherapy remains unknown. In our study, we comprehensively demonstrated both the predictive and prognostic value of pretreatment blood NLR in advanced cancer patients treated with immunotherapy. The integrated results elucidated that elevated pretreatment blood NLR was significantly associated with poor OS and PFS in all groups, suggesting pretreatment blood NLR was a promising predictive and prognostic biomarker in advanced cancer patients treated with immunotherapy. Taken together with previous meta-analyses, well-designed, prospective clinical trials are needed to confirm the prominent role of pretreatment blood NLR in advanced cancer patients treated with immunotherapy. In addition, we performed the correlation analysis between NLR cutoff value and OS/PFS benefit. The result showed that different NLR cutoffs were not correlated with OS but higher cutoff seemed to be associated with less PFS benefit. Similarly, Mei et al. reported that higher cutoff value was associated with worse PFS (HR = 2.23, 95% CI 1.54–3.23; P = 0.019). However, the optimal NLR cutoff value remains undetermined and further large-scale prospective studies are warranted.
The relationship between inflammation and cancer has been extensively explored for a long period. In the nineteenth century, Rudolf Virchow had observed the presence of leukocytes within tumors giving the first indication of potential relationship between inflammation and cancer [23]. Subsequently, a series of studies demonstrated that inflammation could promote the tumor initiation by secreting growth factors, cytokines or inducing gene mutations [59–61]. Epidemiological study indicated that about 25% of all cancer cases could ascribe to infection and chronic inflammation [62]. Although the accurate molecular mechanism remains largely unknown, the role of inflammation in cancer initiation, promotion, invasion and distant metastasis has been gradually acknowledged. Hence, several studies attempted to use the inflammatory mediators and measurable parameters of systemic inflammatory response to predict the therapeutic effect or prognosis in patients with advanced cancers. Neutrophils could substantially contribute to cancer progression in multiple ways including direct effect on the tumor cells and indirect effect on the TME [63]. Neutrophils and other immune cells such as MDSC and macrophages could also secrete tumor growth promoting factors including TGF-beta, VEGF, IL-6, IL-8 and matrix metalloproteinases [58]. Furthermore, a recent study revealed a strong negative correlation between neutrophil and CD8+ cellular content in NSCLC [64], suggesting neutrophilia as an inflammatory response to inhibit anti-tumor immune response via suppressing the cytotoxic activity of immune cells especially activated T cells [65]. Lymphocytes play an important role in the anti-tumor immune response. The increased infiltration of lymphocytes in the tumor region has been correlated with better responsiveness to therapy and prognosis in patients with solid tumors [66]. Theoretically, lymphopenia reflects the impaired cell-mediated immunity, whereas neutrophilia represents the response to systematic inflammation. Therefore, elevated NLR would be associated with poor response to immunotherapy in patients with advanced cancers.
The TME is being increasingly recognized as a significant element in cancer progression, immune-escaping and metastatic dissemination [67]. TME-targeting therapies have achieved huge success in the treatment of advanced solid tumors. The most successful cases were immune checkpoints blockade and anti-VEGF/VEGFR-mediated angiogenesis. Nevertheless, these two approaches faced the same dilemma: lack of effective predictive biomarkers. In the current study, our results suggested that elevated NLR was significantly correlated with inferior OS and PFS in patients treated with immune checkpoints inhibitors or anti-VEGF/VEGFR therapy. Whereas high NLR was not associated with OS and PFS in patients treated with other immunotherapies including high-dose IL-2 and adenoviral oncolytic immunotherapy. The exact mechanism that explains these relationships has not been clearly clarified. Emerging evidence suggested that elevated NLR represented the relative reduction of circulating lymphocytes and elevated circulating inflammatory cytokines or mediators. The reduction of circulating lymphocytes would weaken the efficacy of immune checkpoint inhibitors, which mainly unleash the inhibitory signal of T cell function (main subtypes of lymphocytes). The elevated inflammatory cytokines or mediators such as VEGF, TGF-beta and IL-8 would promote angiogenesis [23]. However, these explanations were the potential hypotheses and further research should be performed to uncover the underlying mechanisms.
The present meta-analysis has several limitations that should be acknowledged. First, the number of the eligible studies was relatively small and some of these studies had small sample sizes. Although all of the included studies were well-performed studies, our conclusions should be interpreted with caution due to the overestimation of the treatment effect in smaller studies. Second, NLR cutoff values and main study targets were different among the included studies that make therapy comparisons difficult. For instance, the cutoff value of NLR was 3 in most of eligible studies while other studies used 4 or 5 as the cut-off value. Most of studies reported the data of anti-VEGF/VEGFR and immune checkpoints inhibitors, while two of them studied other targets of TME. Thirdly, it is possible that there may be some degree of publication bias in this area of research. We identified several abstracts describing articles without further detailed publications; hence, we excluded these articles in this meta-analysis. Last but not least, it is not an individual patient data analysis. There was the considerable heterogeneity in the meta-analyses. The pooled results based on published data tend to overestimate treatment effects compared with individual patient data analyses. Herein, clinicians should interpret our findings with caution when applying them in daily clinical practice.
In conclusion, the current study indicated that elevated pretreatment blood NLR was a promising prognostic and predictive biomarker for advanced-stage cancer patients treated with immunotherapy. The optimal NLR cutoff value remains undetermined and further large-scale prospective studies are warranted to confirm this relationship in specific tumor types. In the future, clinical trials in advanced cancer patients are advocated to determine whether pretreatment NLR could be evolved in cancer prognostic risk assessment to help stratify patients who could benefit from immunotherapy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
None.
Abbreviations
- ASCO
American Society of Clinical Oncology
- CI
Confidence interval
- ESMO
European Society for Medical Oncology
- HR
Hazard ratio
- NLR
Neutrophil-to-lymphocyte ratio
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-analyses statement
- SPSS
Statistical Package for Social Sciences
- WCLC
World Lung Cancer Conference
Author contributions
Tao Jiang and Caicun Zhou designed this study; Tao Jiang, Meng Qiao, Xuefei Li, Chao Zhao, Guanghui Gao, Chunxia Su and Shengxiang Ren collected the clinical data; Xuefei Li, Chao Zhao and Guanghui Gao performed the quality assessment; Tao Jiang, Meng Qiao and Shengxiang Ren performed statistical analyses; Caicun Zhou gave critical comments and suggestions; Tao Jiang and Shengxaing Ren drafted the manuscript; all authors approved the final version of the manuscript.
Funding
This study was supported in part by grants from the National Natural Science Foundation of China (No. 81672286 and 81402486), the Fundamental Research Funds for the Central Universities (No. 1511219041), key project of Shanghai Municipal Commission of Health and Family Planning (No. 2013zyjb0401), Shanghai Committee of Science and Technology (134119b1001) and Outstanding Young Doctor Program of Shanghai Municipal Commission of Health and Family Planning (No. XYQ2013097).
Compliance with ethical standards
Conflict of interest
The authors have declared no conflicts of interest.
Ethical approval and ethical standards
None.
Informed consent
None.
Animal source
None.
Cell line authentication
None.
Footnotes
Tao Jiang, Meng Qiao and Chao Zhao are contributed equally to this paper.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Jiang T, Zhou C. The past, present and future of immunotherapy against tumor. Transl Lung Cancer Res. 2015;4(3):253–264. doi: 10.3978/j.issn.2218-6751.2015.01.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farkona S, Diamandis EP, Blasutig IM. Cancer immunotherapy: the beginning of the end of cancer? BMC Med. 2016;14:73. doi: 10.1186/s12916-016-0623-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishino M, Ramaiya NH, Hatabu H, Hodi FS. Monitoring immune-checkpoint blockade: response evaluation and biomarker development. Nat Rev Clin Oncol. 2017;14(11):655–668. doi: 10.1038/nrclinonc.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541(7637):321–330. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 7.Palucka AK, Coussens LM. The Basis of Oncoimmunology. Cell. 2016;164(6):1233–1247. doi: 10.1016/j.cell.2016.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoos A. Development of immuno-oncology drugs—from CTLA4 to PD1 to the next generations. Nat Rev Drug Discov. 2016;15(4):235–247. doi: 10.1038/nrd.2015.35. [DOI] [PubMed] [Google Scholar]
- 10.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Zhou C, Wu YL, Chen G, Liu X, Zhu Y, Lu S, Feng J, He J, Han B, Wang J, Jiang G, Hu C, Zhang H, Cheng G, Song X, Lu Y, Pan H, Zheng W, Yin AY. BEYOND: a randomized, double-blind, placebo-controlled, multicenter, phase III study of first-line carboplatin/paclitaxel plus bevacizumab or placebo in Chinese patients with advanced or recurrent nonsquamous non-small-cell lung cancer. J Clin Oncol. 2015;33(19):2197–2204. doi: 10.1200/JCO.2014.59.4424. [DOI] [PubMed] [Google Scholar]
- 12.Garon EB, Ciuleanu TE, Arrieta O, Prabhash K, Syrigos KN, Goksel T, Park K, Gorbunova V, Kowalyszyn RD, Pikiel J, Czyzewicz G, Orlov SV, Lewanski CR, Thomas M, Bidoli P, Dakhil S, Gans S, Kim JH, Grigorescu A, Karaseva N, Reck M, Cappuzzo F, Alexandris E, Sashegyi A, Yurasov S, Perol M. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384(9944):665–673. doi: 10.1016/S0140-6736(14)60845-X. [DOI] [PubMed] [Google Scholar]
- 13.Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, dos Santos LV, Aprile G, Ferry DR, Melichar B, Tehfe M, Topuzov E, Zalcberg JR, Chau I, Campbell W, Sivanandan C, Pikiel J, Koshiji M, Hsu Y, Liepa AM, Gao L, Schwartz JD, Tabernero J, Investigators RT. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383(9911):31–39. doi: 10.1016/S0140-6736(13)61719-5. [DOI] [PubMed] [Google Scholar]
- 14.Zhu AX, Park JO, Ryoo BY, Yen CJ, Poon R, Pastorelli D, Blanc JF, Chung HC, Baron AD, Pfiffer TE, Okusaka T, Kubackova K, Trojan J, Sastre J, Chau I, Chang SC, Abada PB, Yang L, Schwartz JD, Kudo M, Investigators RT. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16(7):859–870. doi: 10.1016/S1470-2045(15)00050-9. [DOI] [PubMed] [Google Scholar]
- 15.Tabernero J, Yoshino T, Cohn AL, Obermannova R, Bodoky G, Garcia-Carbonero R, Ciuleanu TE, Portnoy DC, Van Cutsem E, Grothey A, Prausova J, Garcia-Alfonso P, Yamazaki K, Clingan PR, Lonardi S, Kim TW, Simms L, Chang SC, Nasroulah F, Investigators RS. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015;16(5):499–508. doi: 10.1016/S1470-2045(15)70127-0. [DOI] [PubMed] [Google Scholar]
- 16.Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016;17(12):e542–e551. doi: 10.1016/S1470-2045(16)30406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sacher AG, Gandhi L. Biomarkers for the clinical use of PD-1/PD-L1 inhibitors in non-small-cell lung cancer: a review. JAMA Oncol. 2016;2(9):1217–1222. doi: 10.1001/jamaoncol.2016.0639. [DOI] [PubMed] [Google Scholar]
- 18.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16(5):275–287. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lesterhuis WJ, Bosco A, Millward MJ, Small M, Nowak AK, Lake RA. Dynamic versus static biomarkers in cancer immune checkpoint blockade: unravelling complexity. Nat Rev Drug Discov. 2017;16(4):264–272. doi: 10.1038/nrd.2016.233. [DOI] [PubMed] [Google Scholar]
- 20.Chae YK, Pan A, Davis AA, Raparia K, Mohindra NA, Matsangou M, Giles FJ. Biomarkers for PD-1/PD-L1 Blockade therapy in non-small-cell lung cancer: Is PD-L1 expression a good marker for patient selection? Clin Lung Cancer. 2016;17(5):350–361. doi: 10.1016/j.cllc.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Funakoshi T, Lee CH, Hsieh JJ. A systematic review of predictive and prognostic biomarkers for VEGF-targeted therapy in renal cell carcinoma. Cancer Treat Rev. 2014;40(4):533–547. doi: 10.1016/j.ctrv.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 22.O’Connor JP, Jayson GC. Do imaging biomarkers relate to outcome in patients treated with VEGF inhibitors? Clin Cancer Res. 2012;18(24):6588–6598. doi: 10.1158/1078-0432.CCR-12-1501. [DOI] [PubMed] [Google Scholar]
- 23.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13(11):759–771. doi: 10.1038/nrc3611. [DOI] [PubMed] [Google Scholar]
- 25.Crusz SM, Balkwill FR. Inflammation and cancer: advances and new agents. Nat Rev Clin Oncol. 2015;12(10):584–596. doi: 10.1038/nrclinonc.2015.105. [DOI] [PubMed] [Google Scholar]
- 26.Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol. 2009;27:83–117. doi: 10.1146/annurev.immunol.021908.132544. [DOI] [PubMed] [Google Scholar]
- 27.McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev. 2013;39(5):534–540. doi: 10.1016/j.ctrv.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Ferrucci PF, Gandini S, Battaglia A, Alfieri S, Di Giacomo AM, Giannarelli D, Cappellini GC, De Galitiis F, Marchetti P, Amato G, Lazzeri A, Pala L, Cocorocchio E, Martinoli C. Baseline neutrophil-to-lymphocyte ratio is associated with outcome of ipilimumab-treated metastatic melanoma patients. Br J Cancer. 2015;112(12):1904–1910. doi: 10.1038/bjc.2015.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrucci PF, Ascierto PA, Pigozzo J, Del Vecchio M, Maio M, Antonini Cappellini GC, Guidoboni M, Queirolo P, Savoia P, Mandala M, Simeone E, Valpione S, Altomonte M, Spagnolo F, Cocorocchio E, Gandini S, Giannarelli D, Martinoli C. Baseline neutrophils and derived neutrophil-to-lymphocyte ratio: prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann Oncol. 2016;27(4):732–738. doi: 10.1093/annonc/mdw016. [DOI] [PubMed] [Google Scholar]
- 30.Kuzman JA, Stenehjem DD, Merriman J, Agarwal AM, Patel SB, Hahn AW, Alex A, Albertson D, Gill DM, Agarwal N. Neutrophil-lymphocyte ratio as a predictive biomarker for response to high dose interleukin-2 in patients with renal cell carcinoma. BMC Urol. 2017;17(1):1. doi: 10.1186/s12894-016-0192-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park YH, Ku JH, Kwak C, Kim HH. Post-treatment neutrophil-to-lymphocyte ratio in predicting prognosis in patients with metastatic clear cell renal cell carcinoma receiving sunitinib as first line therapy. Springerplus. 2014;3:243. doi: 10.1186/2193-1801-3-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mei Z, Shi L, Wang B, Yang J, Xiao Z, Du P, Wang Q, Yang W. Prognostic role of pretreatment blood neutrophil-to-lymphocyte ratio in advanced cancer survivors: a systematic review and meta-analysis of 66 cohort studies. Cancer Treat Rev. 2017;58:1–13. doi: 10.1016/j.ctrv.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 33.Hayden JA, Cote P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med. 2006;144(6):427–437. doi: 10.7326/0003-4819-144-6-200603210-00010. [DOI] [PubMed] [Google Scholar]
- 34.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keizman D, Ish-Shalom M, Huang P, Eisenberger MA, Pili R, Hammers H, Carducci MA. The association of pre-treatment neutrophil to lymphocyte ratio with response rate, progression free survival and overall survival of patients treated with sunitinib for metastatic renal cell carcinoma. Eur J Cancer. 2012;48(2):202–208. doi: 10.1016/j.ejca.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Botta C, Barbieri V, Ciliberto D, Rossi A, Rocco D, Addeo R, Staropoli N, Pastina P, Marvaso G, Martellucci I, Guglielmo A, Pirtoli L, Sperlongano P, Gridelli C, Caraglia M, Tassone P, Tagliaferri P, Correale P. Systemic inflammatory status at baseline predicts bevacizumab benefit in advanced non-small cell lung cancer patients. Cancer Biol Ther. 2013;14(6):469–475. doi: 10.4161/cbt.24425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cetin B, Berk V, Kaplan MA, Afsar B, Tufan G, Ozkan M, Isikdogan A, Benekli M, Coskun U, Buyukberber S. Is the pretreatment neutrophil to lymphocyte ratio an important prognostic parameter in patients with metastatic renal cell carcinoma? Clin Genitourin Cancer. 2013;11(2):141–148. doi: 10.1016/j.clgc.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 38.Dirican A, Kucukzeybek Y, Erten C, Somali I, Demir L, Can A, Payzin KB, Bayoglu IV, Akyol M, Yildiz Y, Koeseoglu M, Alacacioglu A, Tarhan MO. Prognostic and predictive value of hematologic parameters in patients with metastatic renal cell carcinoma: second line sunitinib treatment following IFN-alpha. Asian Pac J Cancer Prev. 2013;14(3):2101–2105. doi: 10.7314/APJCP.2013.14.3.2101. [DOI] [PubMed] [Google Scholar]
- 39.Kobayashi M, Kubo T, Komatsu K, Fujisaki A, Terauchi F, Natsui S, Nukui A, Kurokawa S, Morita T. Changes in peripheral blood immune cells: their prognostic significance in metastatic renal cell carcinoma patients treated with molecular targeted therapy. Med Oncol. 2013;30(2):556. doi: 10.1007/s12032-013-0556-1. [DOI] [PubMed] [Google Scholar]
- 40.Zheng YB, Zhao W, Liu B, Lu LG, He X, Huang JW, Li Y, Hu BS. The blood neutrophil-to-lymphocyte ratio predicts survival in patients with advanced hepatocellular carcinoma receiving sorafenib. Asian Pac J Cancer Prev. 2013;14(9):5527–5531. doi: 10.7314/APJCP.2013.14.9.5527. [DOI] [PubMed] [Google Scholar]
- 41.da Fonseca LG, Barroso-Sousa R, Bento Ada S, Blanco BP, Valente GL, Pfiffer TE, Hoff PM, Sabbaga J. Pre-treatment neutrophil-to-lymphocyte ratio affects survival in patients with advanced hepatocellular carcinoma treated with sorafenib. Med Oncol. 2014;31(11):264. doi: 10.1007/s12032-014-0264-5. [DOI] [PubMed] [Google Scholar]
- 42.Gunduz S, Mutlu H, Uysal M, Coskun HS, Bozcuk H. Prognostic value of hematologic parameters in patients with metastatic renal cell carcinoma using tyrosine kinase inhibitors. Asian Pac J Cancer Prev. 2014;15(8):3801–3804. doi: 10.7314/APJCP.2014.15.8.3801. [DOI] [PubMed] [Google Scholar]
- 43.Keizman D, Gottfried M, Ish-Shalom M, Maimon N, Peer A, Neumann A, Hammers H, Eisenberger MA, Sinibaldi V, Pili R, Hayat H, Kovel S, Sella A, Boursi B, Weitzen R, Mermershtain W, Rouvinov K, Berger R, Carducci MA. Active smoking may negatively affect response rate, progression-free survival, and overall survival of patients with metastatic renal cell carcinoma treated with sunitinib. Oncologist. 2014;19(1):51–60. doi: 10.1634/theoncologist.2012-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Livne-Segev D, Gottfried M, Maimon N, Peer A, Neumann A, Hayat H, Kovel S, Sella A, Mermershtain W, Rouvinov K, Boursi B, Weitzen R, Berger R, Keizman D. Experience with sunitinib treatment for metastatic renal cell carcinoma in a large cohort of Israeli patients: outcome and associated factors. Isr Med Assoc J. 2014;16(6):347–351. [PubMed] [Google Scholar]
- 45.Wang HK, Zhang HL, Zhu Y, Yao XD, Zhang SL, Dai B, Shen YJ, Zhu YP, Shi GH, Qin XJ, Ma CG, Lin GW, Xiao WJ, Ye DW. A Phase II trial of dosage escalation of sorafenib in Asian patients with metastatic renal cell carcinoma. Future Oncol. 2014;10(12):1941–1951. doi: 10.2217/fon.14.131. [DOI] [PubMed] [Google Scholar]
- 46.Namikawa T, Fukudome I, Ogawa M, Munekage E, Munekage M, Shiga M, Maeda H, Kitagawa H, Kobayashi M, Hanazaki K. Clinical efficacy of protein-bound polysaccharide K in patients with gastric cancer undergoing chemotherapy with an oral fluoropyrimidine (S-1) Eur J Surg Oncol. 2015;41(6):795–800. doi: 10.1016/j.ejso.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 47.Santoni M, Buti S, Conti A, Porta C, Procopio G, Sternberg CN, Bracarda S, Basso U, De Giorgi U, Rizzo M, Derosa L, Ortega C, Massari F, Milella M, Bersanelli M, Cerbone L, Muzzonigro G, Burattini L, Montironi R, Santini D, Cascinu S. Prognostic significance of host immune status in patients with late relapsing renal cell carcinoma treated with targeted therapy. Target Oncol. 2015;10(4):517–522. doi: 10.1007/s11523-014-0356-3. [DOI] [PubMed] [Google Scholar]
- 48.Casadei Gardini A, Scarpi E, Faloppi L, Scartozzi M, Silvestris N, Santini D, de Stefano G, Marisi G, Negri FV, Foschi FG, Valgiusti M, Ercolani G, Frassineti GL. Immune inflammation indicators and implication for immune modulation strategies in advanced hepatocellular carcinoma patients receiving sorafenib. Oncotarget. 2016;7(41):67142–67149. doi: 10.18632/oncotarget.11565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chrom P, Stec R, Semeniuk-Wojtas A, Bodnar L, Spencer NJ, Szczylik C. Fuhrman grade and neutrophil-to-lymphocyte ratio influence on survival in patients with metastatic renal cell carcinoma treated with first-line tyrosine kinase inhibitors. Clin Genitourin Cancer. 2016;14(5):457–464. doi: 10.1016/j.clgc.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 50.Keizman D, Sarid D, Lee JL, Sella A, Gottfried M, Hammers H, Eisenberger MA, Carducci MA, Sinibaldi V, Neiman V, Rosenbaum E, Peer A, Neumann A, Mermershtain W, Rouvinov K, Berger R, Yildiz I. Outcome of patients with metastatic chromophobe renal cell carcinoma treated with sunitinib. Oncologist. 2016;21(10):1212–1217. doi: 10.1634/theoncologist.2015-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Passardi A, Scarpi E, Cavanna L, Dall’Agata M, Tassinari D, Leo S, Bernardini I, Gelsomino F, Tamberi S, Brandes AA, Tenti E, Vespignani R, Frassineti GL, Amadori D, De Giorgi U. Inflammatory indexes as predictors of prognosis and bevacizumab efficacy in patients with metastatic colorectal cancer. Oncotarget. 2016;7(22):33210–33219. doi: 10.18632/oncotarget.8901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taipale K, Liikanen I, Koski A, Heiskanen R, Kanerva A, Hemminki O, Oksanen M, Gronberg-Vaha-Koskela S, Hemminki K, Joensuu T, Hemminki A. Predictive and prognostic clinical variables in cancer patients treated with adenoviral oncolytic immunotherapy. Mol Ther. 2016;24(7):1323–1332. doi: 10.1038/mt.2016.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang GM, Zhu Y, Gu WJ, Zhang HL, Shi GH, Ye DW. Pretreatment neutrophil-to-lymphocyte ratio predicts prognosis in patients with metastatic renal cell carcinoma receiving targeted therapy. Int J Clin Oncol. 2016;21(2):373–378. doi: 10.1007/s10147-015-0894-4. [DOI] [PubMed] [Google Scholar]
- 54.Bagley SJ, Kothari S, Aggarwal C, Bauml JM, Alley EW, Evans TL, Kosteva JA, Ciunci CA, Gabriel PE, Thompson JC, Stonehouse-Lee S, Sherry VE, Gilbert E, Eaby-Sandy B, Mutale F, DiLullo G, Cohen RB, Vachani A, Langer CJ. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer. 2017;106:1–7. doi: 10.1016/j.lungcan.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 55.Cassidy MR, Wolchok RE, Zheng J, Panageas KS, Wolchok JD, Coit D, Postow MA, Ariyan C. Neutrophil to lymphocyte ratio is associated with outcome during ipilimumab treatment. EBioMedicine. 2017;18:56–61. doi: 10.1016/j.ebiom.2017.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jung M, Lee J, Kim TM, Lee DH, Kang JH, Oh SY, Lee SJ, Shin SJ. Ipilimumab real-world efficacy and safety in korean melanoma patients from the korean named-patient program cohort. Cancer Res Treat. 2017;49(1):44–53. doi: 10.4143/crt.2016.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tanaka N, Mizuno R, Yasumizu Y, Ito K, Shirotake S, Masunaga A, Ito Y, Miyazaki Y, Hagiwara M, Kanao K, Mikami S, Nakagawa K, Momma T, Masuda T, Asano T, Oyama M, Oya M. Prognostic value of neutrophil-to-lymphocyte ratio in patients with metastatic renal cell carcinoma treated with first-line and subsequent second-line targeted therapy: a proposal of the modified-IMDC risk model. Urol Oncol. 2017;35(2):39.e19–39.e28. doi: 10.1016/j.urolonc.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 58.Templeton AJ, McNamara MG, Seruga B, Vera-Badillo FE, Aneja P, Ocana A, Leibowitz-Amit R, Sonpavde G, Knox JJ, Tran B, Tannock IF, Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 59.Kraus S, Arber N. Inflammation and colorectal cancer. Curr Opin Pharmacol. 2009;9(4):405–10. doi: 10.1016/j.coph.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 60.Okazaki IM, Kotani A, Honjo T. Role of AID in tumorigenesis. Adv Immunol. 2007;94:245–73. doi: 10.1016/S0065-2776(06)94008-5. [DOI] [PubMed] [Google Scholar]
- 61.Oguma K, Oshima H, Aoki M, Uchio R, Naka K, Nakamura S, Hirao A, Saya H, Taketo MM, Oshima M. Activated macrophages promote Wnt signalling through tumour necrosis factor-alpha in gastric tumour cells. EMBO J. 2008;27(12):1671–1681. doi: 10.1038/emboj.2008.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121(11):2373–2380. doi: 10.1002/ijc.23173. [DOI] [PubMed] [Google Scholar]
- 63.Treffers LW, Hiemstra IH, Kuijpers TW, van den Berg TK, Matlung HL. Neutrophils in cancer. Immunol Rev. 2016;273(1):312–28. doi: 10.1111/imr.12444. [DOI] [PubMed] [Google Scholar]
- 64.Kargl J, Busch SE, Yang GH, Kim KH, Hanke ML, Metz HE, Hubbard JJ, Lee SM, Madtes DK, McIntosh MW, Houghton AM. Neutrophils dominate the immune cell composition in non-small cell lung cancer. Nat Commun. 2017;8:14381. doi: 10.1038/ncomms14381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Souto JC, Vila L, Bru A. Polymorphonuclear neutrophils and cancer: intense and sustained neutrophilia as a treatment against solid tumors. Med Res Rev. 2011;31(3):311–63. doi: 10.1002/med.20185. [DOI] [PubMed] [Google Scholar]
- 66.Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011;105(1):93–103. doi: 10.1038/bjc.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen F, Zhuang X, Lin L, Yu P, Wang Y, Shi Y, Hu G, Sun Y. New horizons in tumor microenvironment biology: challenges and opportunities. BMC Med. 2015;13:45. doi: 10.1186/s12916-015-0278-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.