Abstract
It has been shown that protein tyrosine phosphatase non-receptor type (PTPN) 3 inhibits T-cell activation. However, there is no definitive conclusion about how the inhibition of PTPN3 in lymphocytes affects immune functions in human lymphocytes. In the present study, we showed that PTPN3 inhibition significantly contributes to the enhanced activation of activated human lymphocytes. The PTPN3 expression of lymphocytes was significantly increased through the activation process using IL-2 and anti-CD3 mAb. Interestingly, inhibiting the PTPN3 expression in activated lymphocytes significantly augmented the proliferation, migration, and cytotoxicity through the phosphorylation of zeta-chain-associated protein kinase 70 (ZAP-70), lymphocyte-specific protein tyrosine kinase (LCK), and extracellular signal-regulated kinases (ERK). Lymphocyte activation by PTPN3 inhibition was observed only in activated CD3+ T cells and not in NK cells or resting T cells. In therapy experiments using autologous tumors and lymphocytes, PTPN3 inhibition significantly augmented the number of tumor-infiltrated lymphocytes and the cytotoxicity of activated lymphocytes. Our results strongly imply that PTPN3 acts as an immune checkpoint in activated lymphocytes and that PTPN3 inhibitor may be a new non-antibody-type immune checkpoint inhibitor for cancer therapy.
Electronic supplementary material
The online version of this article (10.1007/s00262-019-02403-y) contains supplementary material, which is available to authorized users.
Keywords: PTPN3, Activated lymphocyte, Immune checkpoint, Cancer immunology, Immunotherapy
Introduction
Recently, remarkable progress has been made in immunotherapy. Many authors have reported the effectiveness of immune check point inhibitors [1, 2]. However, the response rate is still limited, and many severe adverse effects are reported, while the treatment remains costly. Therefore, new methodologies, including combination therapy with existing antibody agents, are still urgently needed.
Many molecules that act as immune checkpoint inhibitors have been discovered and investigated, including programmed cell death (PD)-1, programmed cell death ligand-1 (PD-L1), PD-L2, cytotoxic T-lymphocyte associated antigen (CTLA)-4, T-cell immunoglobulin and mucin domain (TIM)-3, and lymphocyte activation gene (LAG)-3 [1–4]. However, all of these molecules are antibody-type immune checkpoint inhibitors and no non-antibody-type immune checkpoint inhibitors are available at the present time.
The actions of protein tyrosine phosphatase non-receptor type (PTPN) 3, which removes phosphorylation from protein tyrosyl residues, may be the opposite of those of protein tyrosine kinase [5]. The role of PTPN3 in lymphocytes is still controversial. PTPN3 has been shown to inhibit T-cell activation [5]. On the other hand, some authors have demonstrated that PTPN3 is dispensable for the negative regulation of T-cell activation using PTPN3 knockout mice [6]. However, how the inhibition of PTPN3 affects the immune function against cancer in human activated lymphocytes remains unclear. The development of new immunotherapy is significantly important for solving problems of the response rate, treatment cost, and adverse effects of existing antibody agents. In the present study, we focused on PTPN3 in activated lymphocytes as a target for non-antibody-type immune checkpoint inhibitors and investigated the functions of lymphocytes by inhibiting PTPN3.
Materials and methods
Induction of activated lymphocytes
In vitro and in vivo experiments used peripheral blood mononuclear cells (PBMCs) collected from the heparinized peripheral blood of ten healthy volunteers and three cancer patients via HISTOPAQUE-1077 (Merck KGaA, Darmstadt, Germany) density gradient centrifugation. PBMCs were cultured in RPMI-1640 (Nacalai Tesque, Kyoto, Japan) supplemented with 0.5% human serum, 2000 units/ml penicillin (Meijiseika, Tokyo, Japan), 10 µg/ml streptomycin (Meijiseika), and 200 U/ml IL-2 (Primmune, San Diego, CA, USA) in a 2.5 µg/ml anti-CD3 monoclonal antibody (OKT3, JANSSEN PHARMACEUTICAL K.K., Osaka, Japan)-coated T-75/T-25 flask or 6-well plate for 7 days. Non-adherent fractions were collected as activated lymphocytes. In the microarray analysis, activated lymphocytes from two patients with pancreatic cancer and lung cancer were used. In in vivo therapy experiments, tumor cells and autologous-activated lymphocytes from a patient with ovarian cancer were used.
For CD3+CD4+NKG2D− T cell and CD3+CD8+NKG2D+ T-cell selection, a magnetic cell separation system was used (Mojosort Human CD4 T-cell isolation kit and Mojosort Human CD8 T-cell isolation kit, respectively; Biolegend, San Diego, CA, USA).
Induction of dendritic cell (DC)-stimulated activated lymphocytes (DALs)
DC-stimulated activated lymphocytes were induced as described previously [7]. PBMCs were incubated in RPMI supplemented with 2000 units/ml penicillin, 10 mg/ml streptomycin, 0.5% human serum, and 200 U/ml IL-2. After 30–60 min, the supernatant was discarded, and adherent cells were cultured for 4–7 days in new medium supplemented with 50 ng/ml IL-4 (Primmune) and 100 ng/ml GM-CSF (Primmune). Following this, 200 µg of SUIT-2 tumor lysate was added to the culture. After 4–12 h, TNF-α (500 U/ml; Gentaur Molecular Products, Brussels, Belgium) and 500 U/ml IFN-α (MSD, Kenilworth, NJ, USA) were added to the culture. After 1 day of incubation, lymphocytes were added to the culture and cultured for 4 days. Non-adherent fractions were collected and cultured in RPMI medium supplemented with 200 U/ml IL-2 in 2.5 µg/ml OKT3-coated culture plates for 5 days. Cells were then collected as DALs.
Cancer cell culture
The human pancreatic ductal adenocarcinoma cell line SUIT-2 and human tongue squamous cell carcinoma cell line SCC-9 were maintained in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS; Thermo Fisher Scientific, Waltham, MA, USA) and antibiotics (2000 units/ml of penicillin and 10 µg/ml of streptomycin).
RNA interference
Nucleotide sequences for short hairpin RNA (shRNA) targeting PTPN3 were as follows: shPTPN3#1, CAATCAGAAGCAGAATCCTGCTATA; and shPTPN3#2, GACAGCTACTTAGTCTTGATCCGTA. These oligonucleotides were ligated in the plasmid vector (pcDNA™6.2-GW/Em-miR,#K4934-00; Thermo Fisher Scientific). This plasmid vector was then co-transfected with the ViraPower™ Packaging Mix (included in BLOCK-iT™ Lentiviral Poll l miR RNAi Expression Systems; Thermo Fisher Scientific) into the 293FT cell line to produce the lentiviral stock. PTPN3 transfection using lentivirus in lymphocytes was performed from culture days 3–6.
DNA microarray
The methodology of the DNA microarray which we used has been described previously [8]. Intensity-based Z-scores and ratios (nonlog-scale fold-change) were calculated from the normalized signal intensities of each probe to compare lymphocytes before and after activation. The registration numbers are GSE86293 and GSE89328.
Reverse transcription (RT)-polymerase chain reaction (PCR)
Total RNA was extracted using a High Pure RNA Isolation kit (Roche, Basel, Switzerland) and quantified by spectrophotometry (Ultrospec 2100 Pro; Amersham Pharmacia Biotech, Piscataway, NJ, USA). For RT-PCR, 1.0 μg of RNA was reverse transcribed to cDNA with the Verso cDNA Synthesis Kit (Thermo Fisher Scientific) according to the manufacturer’s protocol. RT-PCR reactions were performed using MasterMix kit (Qiagen, Valencia, CA, USA). All primer sets used were as follows: PTPN3 forward, 5′-TGGTATCGTG GAAGGACTCA-3′; reverse, 5′-AGCTCCACCTA GAAGCACAGAA-3′; and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) forward, 5′-CCACCCATGGCAAATTCCATGGCA-3′; reverse, 5′-TCTAGACGGCAGGTCAGGTCCACC-3′.
Real-time RT-PCR
RNA (1 µg) was treated with DNase and reverse transcribed using the Quantitect Reverse Transcription kit (Qiagen). Reactions were run on a DNA Engine Opticon 2 System (Bio-Rad, Hercules, CA, USA) using SYBR Premix Ex Taq II (Takara, Otsu, Japan) as previously described in [9]. Human PTPN3 real-time primer was purchased from Bio-Rad. β-actin forward, 5′-TTGTTACAGGAAGTCCCTTGCC-3′; reverse, 5′-ATGCTATCACCTCCCCTGTGTG-3′. The amount of each target gene in a given sample was normalized to the level of β-actin in that sample.
Fluorescence-activated cell sorting (FACS)
Cells were stained with fluorescein isothiocyanate (FITC)-conjugated anti-CD3, CD4, and monoclonal antibodies (mAbs), or phycoerythrin (PE)-conjugated anti-NKG2D, CD8 and CD69 mAbs (BD Pharmingen, San Diego, CA, USA). Mouse IgG was used as an isotype control (BD Pharmingen). In the proliferation experiment, cells were labeled with CFSE and the Far Red cell Proliferation Kit (Thermo Fisher Scientific) according to the manufacturer’s protocol. The labeled cells were incubated 48 h before the FACS analysis.
After washing with PBS, the fluorescence intensity or percent positive cells were measured using an FACSCalibur flow cytometer (BD Pharmingen) and analyzed with the CELLQuest software program (BD Pharmingen).
For cell separation, CD3+ and CD3− T cells were collected separately using an MoFlo XDP system (Beckman Coulter, Brea, CA, USA).
Following the established method, gating strategy for lymphocytes was based on forward and side scatter.
Western blotting
Western blotting was performed as described previously [10]. In brief, the blots were incubated with an anti-PTPN3 (1:200, sc-9789; Santa Cruz Biotechnology, Dallas, TX, USA), anti-ERK1/2 (1:1000, No. 9102; Cell Signaling Technology, Danvers, MA, USA), anti-pERK (1:1000, No. 9101; Cell Signaling Technology), anti-Zap (1:2000, No. 3165; Cell Signaling Technology), anti-pZap (1:1000, No. 2701; Cell Signaling Technology), anti-Lck (1:1000, No. 2657; Cell Signaling Technology), anti-pSrc (1:1000, No. 2101; Cell Signaling Technology), and anti-α tubulin (1:1000; Sigma Aldrich) overnight at 4 °C. The blots were then incubated in HRP-linked secondary antibody (GE Healthcare UK Ltd., Buckinghamshire, UK) at room temperature for 1 h. Immunocomplexes were detected with Amersham ECL Prime Western Blotting Detection Reagent (GE Healthcare UK Ltd.) and visualized with EZ Capture ST (ATTO, Tokyo, Japan). α-Tubulin was used as a protein loading control. Results were quantified using the imageJ software program (USA).
Migration assay
The migration of activated lymphocytes was assessed based on the number of migrated cells using transwell inserts (pore size 3.0 µm; BD Biosciences) and confirmed by time-lapse imaging as previously described in detail in [11].
Cytotoxic assay
Target SUIT-2 cells or SCC-9 cells (1 × 104 cells/well) were seeded into a 96-well flat-bottom plate and incubated for 12 h to allow adherence. Effector cells (activated lymphocytes) were then added to the culture. Target and effector cells were co-incubated for 72 h. Viable adherent cancer cells were quantified as previously described in detail in [11]. Viable cancer cells were detected by subtracting the absorbance of lymphocytes alone from that of co-culture.
To confirm the cytotoxicity of activated lymphocytes, target and effector cells were co-incubated for 15 h at effector/target ratios of 5:1, 10:1, and 20:1. The fluorescent intensity of viable cancer cells was measured by the Image-Pro Analyzer software program (Nippon Roper KK).
Xenograft mouse model
SUIT-2 cells or cells from a patient with ovarian cancer (1 × 106 cells) in 50 µl RPMI medium were injected subcutaneously into the bilateral flank of four NODScid female mice (4–6 weeks old) per group. 1 week after the tumor injection, 50 µl of saline or activated lymphocytes (1 × 106 cells) was injected intraperitoneally two times in 2 weeks (four times total).
The tumor size was determined twice a week, and the tumor volume was calculated with the following formula: A × B2 × 0.5, where A is the larger and B is the smaller of the two perpendicular diameters.
Immunohistochemistry
Paraffin sections of the tumors formed in mice were deparaffinized and rehydrated. The sections were immersed in 3% H2O2 and 10% goat serum, and incubated with a primary antibody for CD3 (Dako, Tokyo, Japan), CD4 (Abcam, Cambridge, MA, USA), CD8 (Dako), and CD56 (Novocastra, Buffalo Grove, IL, USA) at 4 °C overnight. The samples were incubated with HISTOFINE simple stain MAX-PO (R) (Nichirei, Tokyo, Japan) and finally visualized by 3,3′-diaminobenzidine (DAB) with hematoxylin counterstain.
Absolute numbers of labeled tumor-infiltrating cells were counted manually. The total numbers in the ten selected areas were represented as intratumoral CD3+ T cells, CD8+ T cells, CD4+ T cells, and CD56+ NK cells.
Enzyme-linked immunosorbent assay (ELISA)
SCC-9 cells (1 × 104 cells/well) were seeded in a 96-well flat-bottom plate and incubated for 12 h to allow adherence. Activated lymphocytes at a target/effector ratio of 1:10 were added to the culture. After 6 and 48 h, the supernatant was collected, and cytokine production of IFN-γ and perforin was measured using a Biotrak visible plate reader (Amersham Biosciences) with Human IFN-γ ELISA Ready-SET-Go! (Thermo Fisher) and a Perforin ELISA Kit (Diaclone, Besancon Cedex, France).
Statistical analyses
The data are presented as the mean ± standard deviation (SD). Student’s t test was used to compare continuous variables between two groups. p values of < 0.05 were considered statistically significant.
Results
PTPN3 is highly expressed in activated lymphocytes
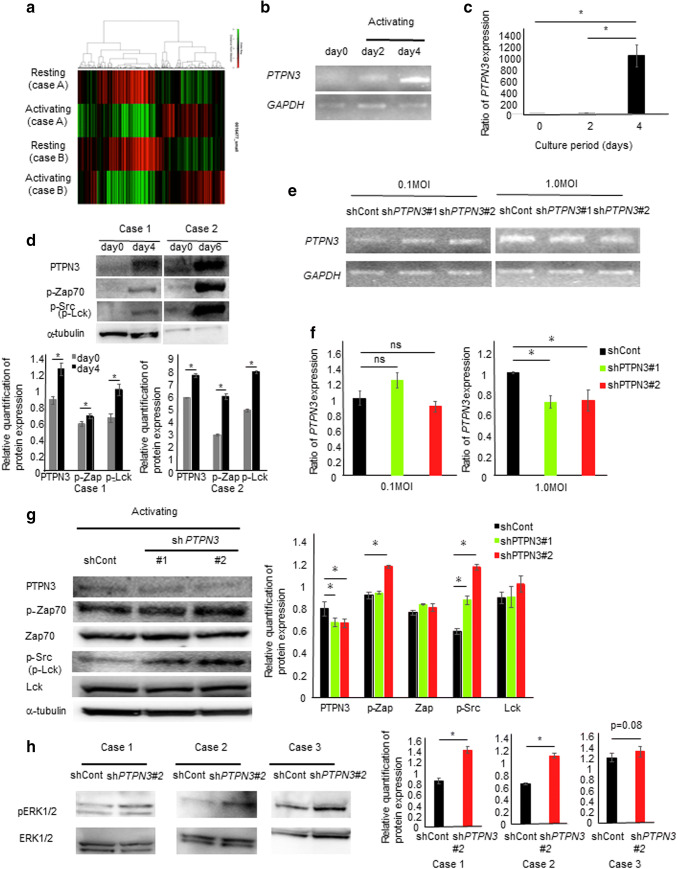
To evaluate the nature of activated lymphocytes, a microarray analysis was performed using lymphocytes before and after activation by anti–anti-CD3 antibody and IL-2 (GSE86293) (Fig. 1a and Supplementary Table 1). Among the elevated genes, protein tyrosine phosphatase non-receptor type (PTPN) 3, which showed the second highest level of expression among all the evaluated genes, was selected for this study, since we considered that PTP, which dephosphorylates protein tyrosyl residues, might exert an activity which is completely opposite from the activity associated with protein tyrosine kinase, as shown previously [5]. Interestingly, among the members of the PTPN family (PTPN1-23), only PTPN3 was found to be dramatically increased by the activation of lymphocytes in a microarray analysis (GSE86293) (Supplementary Table 2).
Fig. 1.
Activated lymphocytes express high levels of PTPN3, which inhibits tyrosine kinase and the MAPK pathway. a A heat map of a microarray analysis in lymphocytes before and after activation by anti-antiCD3 antibody and IL-2 in two different cancer patients. The PTPN3 mRNA expression in lymphocytes from healthy volunteers during the activation process was estimated by conventional RT-PCR (b) and real-time RT-PCR (c). Similar results were obtained in three different healthy volunteers. d The expression of pZap70 and pSrc in lymphocytes from two different healthy volunteers during the activation process was investigated by Western blotting, and the relative quantification of the protein expression is shown. The PTPN3 mRNA expression in lymphocytes from healthy volunteers transfected with PTPN3 shRNA was investigated by RT-PCR (e) and real-time PCR (f). Similar results were obtained in two different healthy volunteers. g The expression of pZap70/pSrc in lymphocytes from healthy volunteers transfected with PTPN3 shRNA was investigated by Western blotting, and the relative quantification of the protein expression is shown. Similar results were obtained in three different healthy volunteers. h The expression of pERK1/2 in lymphocytes from three different healthy volunteers transfected with PTPN3 shRNA was investigated by Western blotting, and the relative quantification of the protein expression is shown
The biological functional role for PTPN3 in activated lymphocytes was also investigated. PTPN3 was shown to be strongly expressed throughout the activation process (Fig. 1b, c). The expression of pZap70 and pSrc was also increased throughout this process (Fig. 1d). To inhibit the PTPN3 expression, shPTPN3 plasmid transfected with lentivirus was used. Although the PTPN3 expression was not inhibited by 0.1 multiplicity of infection (MOI) of lentivirus, its expression was successfully suppressed with 1.0 MOI (Fig. 1e, f). When PTPN3 was inhibited, the expression of pZAP70 and pSrc increased over that noted in controls (Fig. 1g). The MAPK pathway was activated by PTPN3 inhibition (Fig. 1h and Supplementary Fig. 1).
PTPN3 inhibition augmented functions of activated lymphocytes
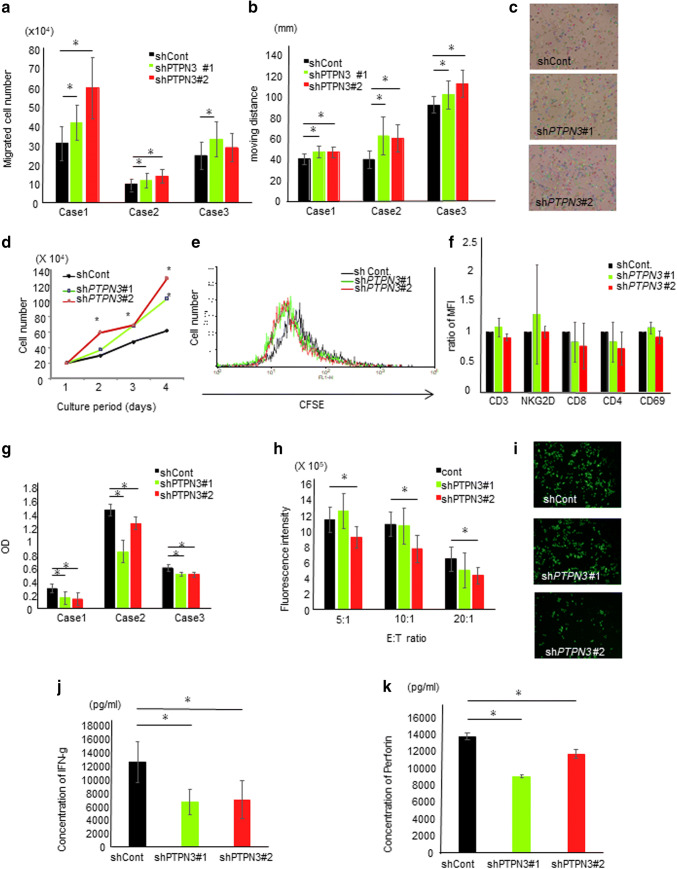
We next assessed the migration ability of activated lymphocytes in two different manners, discovering that the inhibition of PTPN3 significantly augmented the migration ability of activated lymphocytes (Fig. 2a–c). The proliferative capacity was then investigated in two different methods, and the inhibition of PTPN3 was found to significantly increase the proliferative capacity of activated lymphocytes (Fig. 2d, e, and Supplementary Fig. 2). Early cell death was not observed in PTPN3-inhibited lymphocytes during the culture period. Regarding surface antigens, no significant changes were noted in the expression of CD3, CD4, CD8, CD69, or NK-G2D (Fig. 2f). The cytotoxicity against allo-tongue cancer (SCC-9) and pancreatic cancer (SUIT-2) cells was estimated in two different manners, and PTPN3 inhibition was found to significantly augment the cytotoxicity in vitro (Fig. 2g–i). However, unexpectedly, the IFN-γ secretion in PTPN3-inhibited lymphocytes was significantly lower than that in control lymphocytes (Fig. 2j). In addition, the perforin secretion in PTPN3-inhibited lymphocytes was also significantly lower than that in control lymphocytes (Fig. 2k). These results suggest the presence of another mechanism underlying the augmentation of cytotoxicity in PTPN3-inhibited lymphocytes. PTPN3 may, therefore, play a role in the secretion of cytokines, including IFN-γ and perforin.
Fig. 2.
PTPN3 inhibition augments the functions of activated T lymphocytes from healthy volunteers in vitro. a The migration ability of lymphocytes of the day of the 7 day culture was analyzed by the chamber method. b The migration ability of lymphocytes of the day of the 7 day culture was analyzed by time-lapse imaging. The graph shows the moving distance of lymphocytes analyzed by the Image-Pro Analyzer software program. c Representative pictures of time-lapse imaging. The tracking line shows the moving distance. Original magnification ×20. d The proliferation of lymphocytes was estimated. Cell numbers at the indicated days were counted using a light microscope. e 5 days after activation, lymphocytes were labeled by CFSE, and after an additional 2 days of culture, the immunofluorescence intensity of CFSE in lymphocytes was analyzed by FACS. f The surface antigen expression of lymphocytes was analyzed by FACS. g The cytotoxicity of lymphocytes after cancer cells and lymphocytes were co-cultured was analyzed. After WST reagent solution had been added to the well, the absorbance of viable cancer cells was detected by subtracting the absorbance of lymphocytes alone from that of co-culture. OD shows the calculated absorbance of viable cancer cells. h The cytotoxicity of lymphocytes was estimated by time-lapse imaging. After co-culture of lymphocytes and calcein-labeled SCC cells, the fluorescent intensity of viable cancer cells was measured by the Image-Pro Analyzer software program. i Representative pictures of time-lapse imaging of viable cancer cells. Original magnification ×20. The IFN-γ (j) and perforin (k) secretion capacity of lymphocytes was measured by an ELISA. The lymphocytes used in each experiment of a–g were obtained from three different healthy volunteers. Similar results were obtained in two different healthy volunteers (h–k). Data are presented as the mean ± SD. *p < 0.05
Antigen presentation from dendritic cells (DCs) is a very important process prefacing the activation of lymphocytes in vivo. We, therefore, induced allo-pancreatic cancer (SUIT-2) cell-pulsed mature DCs-activated lymphocytes (DALs) and investigated their biological functions. The migration (Supplementary Fig. 3a, b) and proliferation (Supplementary Fig. 3c) abilities in PTPN3sh RNA-transfected DALs were significantly increased compared to the control lymphocytes. Cytotoxicity targeting SUIT-2 cells in PTPN3sh RNA-transfected DAL was also significantly augmented (Supplementary Fig. 3d). These results suggest that lymphocytes stimulated by DCs were also activated by PTPN3 inhibition.
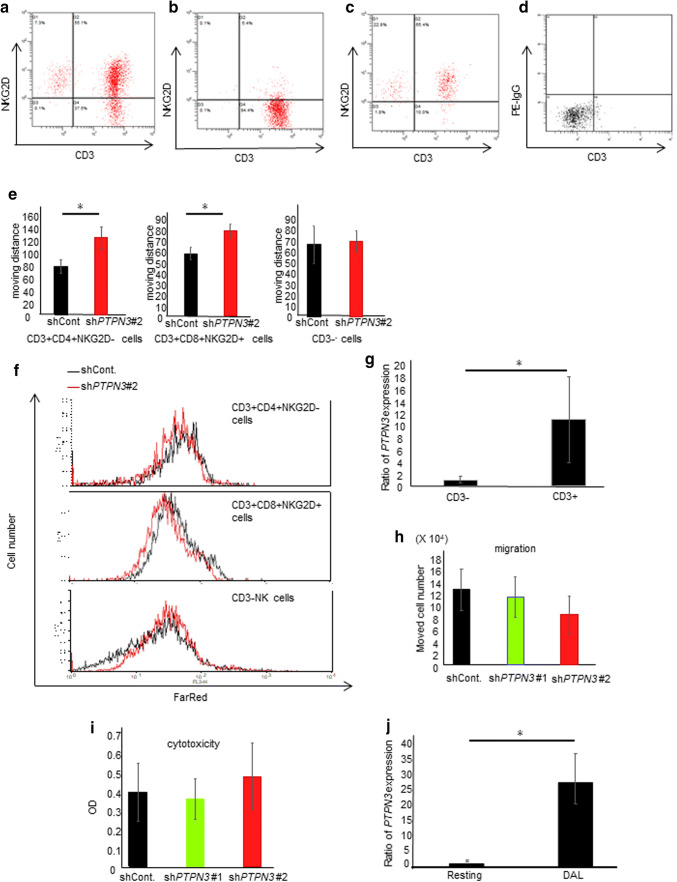
Lymphocyte activation by PTPN3 inhibition was observed only in activated CD3+ T cells
Activated lymphocytes include CD3+ T cells and CD3−NKG2D+ (NK) cells (Fig. 3a). We, therefore, assessed which populations were affected by PTPN3 inhibition. CD3+CD4+NKG2D− cells (Fig. 3b), CD3+CD8+NKG2D+ cells (Fig. 3c), and CD3− (NK) cells (Fig. 3d) were separately collected using microbeads and FACS sorting. The migration (Fig. 3e and Supplementary Fig. 4a) and proliferative abilities (Fig. 3f and Supplementary Fig. 4b) were significantly increased by PTPN3 inhibition in CD3+CD4+NKG2D− cells and CD3+CD8+NKG2D+ cells but not in CD3−NK cells. The PTPN3 expression in CD3+-activated lymphocytes was significantly higher than that in CD3− activated lymphocytes (Fig. 3g). These results suggest the importance of stimulation through CD3 signaling.
Fig. 3.
Lymphocyte activation by PTPN3 inhibition was observed only in CD3+ T cells, not in CD3− cells. a An FACS analysis of activated lymphocytes before selection. CD3+CD4+NKG2D− cells (b) and CD3+CD8+NKG2D+ cells (c) were separately collected using microbeads. CD3− (NK) cells (d) were collected using FACS sorting. e The migration ability of lymphocytes was analyzed by time-lapse imaging. The graph shows the moving distance of lymphocytes analyzed by the Image-Pro Analyzer software program. f The proliferation of FAR red-labeled lymphocytes was estimated by FACS. g The PTPN3 mRNA expression in CD3− cells and CD3+ cells after activation was estimated by real-time RT-PCR. h The migration ability of non-activated lymphocytes was analyzed by the chamber method. i The cytotoxicity of non-activated lymphocytes was investigated. After co-culture of SCC cells and lymphocytes for 72 h, the absorbance of viable cancer cells was measured. j The PTPN3 mRNA expression in lymphocytes stimulated by allo-pancreatic cancer (SUIT-2) cell-pulsed mature DCs without using anti CD3 mAb was estimated by real-time RT-PCR. Similar results were obtained in two different healthy volunteers in all experiments. Data are presented as the mean ± SD. Lymphocytes and DCs from healthy volunteers were used in all experiments. *p < 0.05
The PTPN3 expression is relatively low in resting lymphocytes (Fig. 1b), and the migration and cytotoxicity of lymphocytes before activation were not markedly changed, even after PTPN3 knockdown (Fig. 3h, i). Consistent with our results, Bauler et al. [12] showed that the functions of resting lymphocytes derived from PTPN3 knockout mice were similar to those from control mice. In contrast, the PTPN3 expression in lymphocytes stimulated by DCs without anti-CD3 mAb stimulation was 30-fold increased (Fig. 3j). The migration of DC-activated lymphocytes was significantly greater than that of anti-CD3 Ab-activated lymphocytes (Supplementary Fig. 5a). In contrast, the proliferation of DC-activated lymphocytes was lower than that of anti-CD3 Ab-activated lymphocytes (Supplementary Fig. 5b).
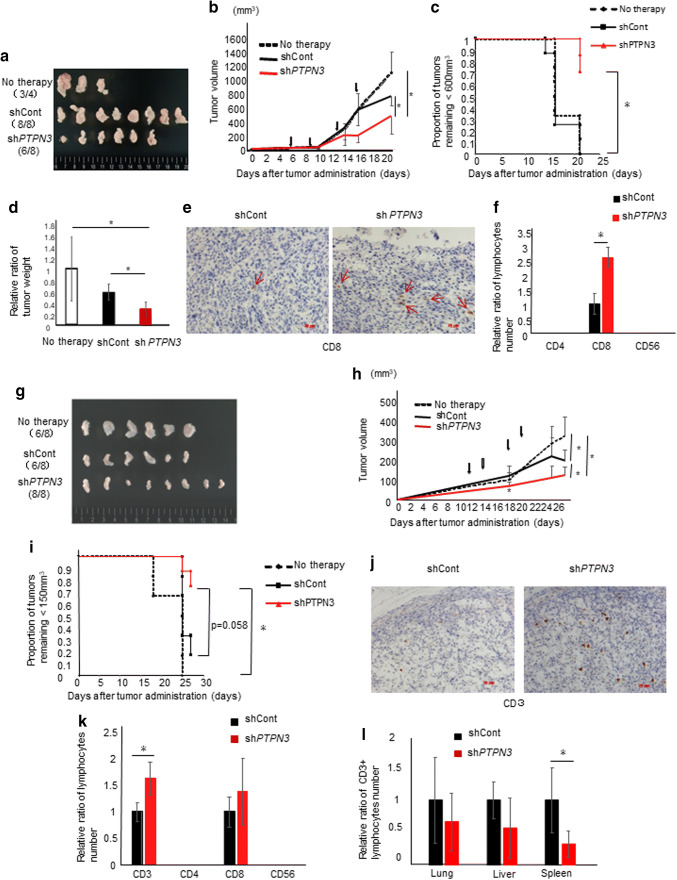
PTPN3 inhibition increased cytotoxicity in vivo
In therapy experiments using allo-activated lymphocytes targeting SUIT-2 cells, activated lymphocytes transfected with control or PTPN3 shRNA were administered intraperitoneally on days 6, 9, 13, and 16 after tumor injection. Tumors formed in six of eight in the PTPN3 shRNA group and in all eight in the control group (Fig. 4a). The tumors that formed on the mice in the PTPN3 shRNA group were significantly smaller (Fig. 4b, c) and lower in weight (Fig. 4d) in comparison to the controls. There were no metastases to the distant organs in any mice.
Fig. 4.
PTPN3 inhibition increases the cytotoxicity against cancer cells in vivo. a–f Therapy experiments using allo-activated lymphocytes targeting SUIT-2 cells from healthy volunteers are shown. Allo-activated lymphocytes transfected with control shRNA or PTPN3#2 shRNA were administered at 6, 9, 13, and 16 days after tumor injection (arrows in b). a Representative photos of formed tumors. b The tumor volume at the indicated days and c Kaplan–Meier plot of the tumor growth are shown. d The tumor weight at 22 days after tumor injection was assessed. e Representative photos of immunohistochemical staining of CD8 in formed tumors. Original magnification ×200. f The numbers of labeled tumor-infiltrating-administrated lymphocytes (TILs) were counted. g–l Therapy experiments using tumor and autologous lymphocytes from a patient with ovarian cancer are shown. Autologous-activated lymphocytes transfected with control shRNA or PTPN3#2 shRNA were administered at 11, 14, 18, and 21 days after tumor injection (arrows in h). g Representative photos of formed tumors. h The tumor volume at the indicated days and i Kaplan–Meier plot of the tumor growth are shown. j Representative photos of immunohistochemical staining of CD3 in formed tumors. Original magnification ×200. k The numbers of non-tumor-tissue-infiltrating-administrated lymphocytes (ntTILs) were counted. l The numbers of ntTILs in the spleen, liver and lung were counted. Data are presented as the mean ± SD. *p < 0.05
Significantly greater infiltration of CD8+ T cells into the cancer tissue was noted in the PTPN3 shRNA group than in the control shRNA group (Fig. 4e, f). CD4+ T cells and CD56+ T cells could not be detected in either group (Fig. 4f). These results suggest that CD8+ T cells contributed to the volume shrinkage of allo-pancreatic cancer.
More interestingly, in therapy experiments using tumors and autologous lymphocytes transfected with control or PTPN3 shRNA from a patient with ovarian cancer, PTPN3 inhibition also significantly augmented the cytotoxicity of activated lymphocytes (Fig. 4g–i). In addition, delayed effect which is a characteristic of immunotherapy was observed (Fig. 4h). The number of CD3+ tumor-infiltrating-administrated lymphocytes (TILs) was significantly greater in the PTPN3 shRNA group than in the control shRNA group (Fig. 4j, k). There were no metastases to the distant organs, including the lung, liver, and spleen, in any mice. The number of non-tumor-tissue-infiltrating-administrated lymphocytes (ntTILs) that infiltrated the spleen in the control group was significantly higher than that in the PTPN3 shRNA group, while the number of ntTILs in the liver and lung was almost the same in the two groups (Fig. 4l).
Discussion
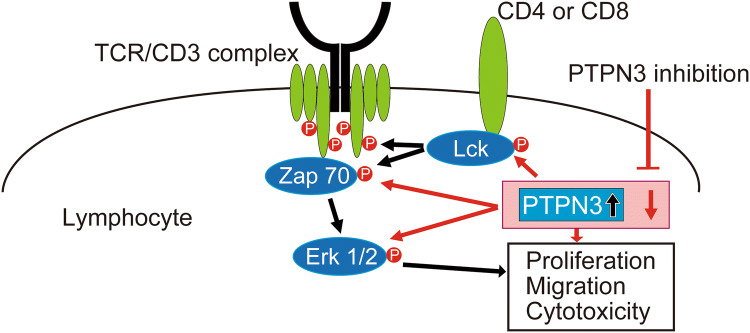
In the present study, we demonstrated—for the first time—that PTPN3 inhibition could be a crucial new treatment for cancer as the first non-antibody-type immune checkpoint inhibitor. Figure 5 is a schematic figure of this study. The novel findings are that the inhibition of PTPN3, the expression of which was greater in activated lymphocytes than in non-activated lymphocytes, leads to further activation of lymphocytes through the enhanced phosphorylation of ZAP70, LCK, and ERK1/2, suggesting that PTPN3 may be a new therapeutic target as an immune checkpoint. Taken together, our results imply that PTPN3 inhibition is effective as adoptive immunotherapy using activated lymphocytes.
Fig. 5.
A schematic illustration of this study. The expression of PTPN3 was increased by signaling from CD3. The inhibition of PTPN3 led to the further activation of lymphocytes, including their proliferation, migration, and cytotoxicity, through the augmentation of phosphorylation of ZAP70, LCK, and ERK1/2
In this study, lymphocyte activation was achieved using anti-CD3 Ab and IL-2. In the activation process using our method, lymphocytes were co-cultured with monocytes, thereby ensuring that lymphocytes would receive stimulation from CD28. There were no significant differences in the migration, proliferation, or cytotoxicity between lymphocytes activated using only anti-CD3 antibody and those activated using anti-CD3 and anti-CD28 antibodies together (Supplementary Fig. 6).
Importantly, our results showed that the activation of lymphocytes by PTPN3 inhibition was only observed in activated CD3+ T cells, and not in CD3− NK cells or resting T cells. Based on our results (Fig. 3g, j), PTPN3 inhibition may only be useful in patients harboring cytotoxic T lymphocytes (CTLs) that have been stimulated through CD3 signaling from antigen-presenting cells, such as DCs. Thus, PTPN3 inhibition may induce few major adverse effects; however, it will be necessary to verify this point in the clinical setting.
Cytokines such as IFN-γ, perforin, and granzyme are thought to contribute to the cytotoxicity of lymphocytes. However, conversely, PTPN3 inhibition led to the decreased secretion of cytokines in our repeated experiments. We also analyzed the cytokine secretion at 8 h after co-culture, because the half-life of cytokines is short. However, no significant change was observed between the control and PTPN3 inhibition group. These results suggest that the mechanism underlying the enhanced cytotoxicity caused by PTPN3 inhibition may not involve the killing of cytokines. PTPN3 is one of the enzymes in lymphocytes that may play a role in the secretion of cytokines including IFN-γ and perforin.
PTPN3 shRNA transfection to lymphocytes using a virus is methodologically difficult, and inhibition rate is not very high. Only after PTPN3 was successfully inhibited at 1.0 MOI could phenotypic changes be observed (Supplementary Fig. 7). We, therefore, believe that our results were not off-target effects. In addition, shRNA is difficult to use clinically. We made an antisense peptide of the PTPN3 activation site and tried to transfect it to lymphocytes using lipofectamine; however, this attempt failed. We then attempted to use the pan-PTPN inhibitor RK-682 (Sigma Aldrich) to investigate the function of activated lymphocytes. However, unexpectedly, RK-682 did not affect the proliferation or migration abilities of activated lymphocytes (Supplementary Fig. 8). This suggests that a PTPN3-specific inhibitor is required to activate lymphocytes. Our finding of a large difference in the cytotoxic effect in vivo despite relatively small phenotypic changes in vitro is quite promising. If an effective specific molecular targeting agent against PTPN3 can be developed, the therapeutic effect could be much higher than was observed in this study.
PTPN11 was recently reported to be a key mediator of programmed cell death 1 (PD-1) and the B- and T-lymphocyte attenuator immune checkpoint pathways [13–15]. Based on these findings, PTPN3 may be related to the PD-1/PD-L1 axis. Clinically, a PTPN3 inhibitor may be co-used with an anti PD-1/PD-L1 Ab, or also the single use of a PTPN3 inhibitor may be effective. If PTPN3 inhibitor is developed as a low-molecular-eight compound, it should reduce treatment costs and may be administered orally in an outpatient setting. If the clinical effect of a PTPN3 inhibitor is equivalent to that of existing antibody agents, then it may be able to replace these existing agents as a new immune checkpoint inhibitor.
Although why the PTPN3 expression is upregulated by activation through CD3 signaling remains unclear, increased PTPN3 expression is thought to induce negative feedback that regulates the over-activation of lymphocytes. It has been shown that PTPN22 inhibition improves the immunosuppressive function of regulatory T (Treg) cells [16, 17]. Some authors have shown the direct tumor suppressive effect of PTPN3 inhibition in cancer cells [18–20]. To develop a PTPN3 inhibitor and use it clinically, we must address many unresolved issues, such as how PTPN3 inhibition affects the Treg function and the cancer biology itself. Furthermore, the application of PTPN3 inhibitors may reduce treatment costs and eliminate adverse effects of immunotherapy. Taken together, our results strongly imply that PTPN3 inhibitors may be a new non-antibody-type immune checkpoint inhibitor for cancer therapy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Ms. Emi Onishi for skillful technical assistance.
Abbreviations
- DALs
DC-stimulated activated lymphocytes
- ERK
Extracellular signal-regulated kinases
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- LCK
Lymphocyte-specific protein tyrosine kinase
- MOI
Multiplicity of infection
- ntTILs
Non-tumor-tissue-infiltrating-administrated lymphocytes
- PTP
Protein tyrosine phosphatase
- PTPN
Protein tyrosine phosphatase non-receptor type
- shRNA
Short hairpin RNA
- Zap-70
Zeta-chain-associated protein kinase 70
Author contributions
AF was involved in the analysis of all experiments. KN, AI, and MK were involved in the gene transfection. YO and SI were involved in the interpretation of data. MU and NK were involved in the acquisition and analysis of data. TM, TN, and HO were involved in the design of the work.
Funding
This study was supported by the Japan Society for the Promotion of Science KAKENHI Grant Numbers JP15K10055, JP17H04283, and JP18K08682.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval and ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Kyushu University Ethics Committee (study approval numbers 29-251 and 28-277) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All procedures performed in studies involving animals were in accordance with the ethical standards of the Animal Care and Use Committee of Kyushu University (study approval numbers A29-376-0 and A29-333-0).
Informed consent
Written informed consent was obtained from all participants included in the study to the use of their PBMC and tumor specimen for research and for publication before blood collection.
Animal source
All mice were obtained from Charles River Laboratories Japan, Yokohama, Japan.
Cell line authentication
SUIT-2 is a human pancreatic ductal adenocarcinoma cell line, and SCC-9 is a human tongue squamous cell carcinoma cell line. Both cell lines were purchased from American Type Culture Collection (ATCC) previously and were stored in liquid nitrogen. All cell lines were cultured for no more than 2–3 weeks after thawing, routinely checked for mycoplasma infection while cultured, and showed consistent phenotypes by microscopy prior to in vitro and in vivo experiments.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Borqhaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhäufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crinò L, Blumenschein GR, Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, Castellano D, Choueiri TK, Gurney H, Donskov F, Bono P, Wagstaff J, Gauler TC, Ueda T, Tomita Y, Schutz FA, Kollmannsberger C, Larkin J, Ravaud A, Simon JS, Xu LA, Waxman IM, Sharma P. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blank CU, Rozeman EA, Fanchi LF, Sikorska K, van de Wiel B, Kvistborg P, Krijgsman O, van den Braber M, Philips D, Broeks A, van Thienen JV, Mallo HA, Adriaansz S, Ter Meulen S, Pronk LM, Grijpink-Ongering LG, Bruining A, Gittelman RM, Warren S, van Tinteren H, Peeper DS, Haanen JBAG, van Akkooi ACJ, Schumacher TN. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat Med. 2018 doi: 10.1038/s41591-018-0198-0. [DOI] [PubMed] [Google Scholar]
- 4.Marcq E, Waele J, Audenaerde JV, Lion E, Santermans E, Hens N, Pauwels P, van Meerbeeck JP, Smits ELJ. Abundant expression of TIM-3, LAG-3, PD-1 and PD-L1 as immunotherapy checkpoint targets in effusions of mesothelioma patients. Oncotarget. 2017;8:88904–88917. doi: 10.18632/oncotarget.21113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han S, Williams S, Mustelin T. Cytoskeletal protein tyrosine phosphatase PTPH1 reduces T cell antigen receptor signaling. Eur J Immunol. 2000;30:1318–1325. doi: 10.1002/(SICI)1521-4141(200005)30:5<1318::AID-IMMU1318>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 6.Bauler TJ, Hendriks WJ, King PD. The FERM and PDZ domain-containing protein tyrosine phosphatases, PTPN4 and PTPN3, are both dispensable for T cell receptor signal transduction. PLoS One. 2008;3:e4014. doi: 10.1371/journal.pone.0004014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Onishi H, Koya N, Kiyota A, Tanaka H, Umebayashi M, Katano M, Morisaki T. A new method for rapid cytotoxic T-lymphocyte induction using a multiple cytokine cocktail. Anticancer Res. 2012;32:2385–2390. [PubMed] [Google Scholar]
- 8.Miyahara E, Nishikawa T, Takeuchi T, Yasuda K, Okamoto Y, Kawano Y, Horiuchi M. Effect of myeloperoxidase inhibition on gene expression profiles in HL-60 cells exposed to 1, 2, 4,-benzenetriol. Toxicology. 2014;317:50–57. doi: 10.1016/j.tox.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Ogino T, Onishi H, Suzuki H, Morisaki T, Tanaka M, Katano M. Inclusive estimation of complex antigen presentation functions of monocyte-derived dendritic cells differentiated under normoxia and hypoxia conditions. Cancer Immunol Immunother. 2012;61:409–424. doi: 10.1007/s00262-011-1112-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suyama K, Onishi H, Imaizumi A, Shinkai K, Umebayashi M, Kubo M, Mizuuchi Y, Oda Y, Tanaka M, Nakamura M, Katano M. CD24 suppresses malignant phenotype by downregulation of SHH transcription through STAT1 inhibition in breast cancer cells. Cancer Lett. 2016;374:44–53. doi: 10.1016/j.canlet.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 11.Onishi H, Morisaki T, Kiyota A, Koya N, Tanaka H, Umebayashi M, Katano M. The Hedgehog inhibitor cyclopamine impairs the benefits of immunotherapy with activated T and NK lymphocytes derived from patients with advanced cancer. Cancer Immunol Immunother. 2013;62:1029–1039. doi: 10.1007/s00262-013-1419-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bauler TJ, Hughes ED, Arimura Y, Mustelin T, Saunders TL, King PD. Normal TCR signal transduction in mice that lack catalytically active PTPN3 protein tyrosine phosphatase. J Immunol. 2007;178:3680–3687. doi: 10.4049/jimmunol.178.6.3680. [DOI] [PubMed] [Google Scholar]
- 13.Gavrieli M, Watanabe N, Loftin SK, Murphy TL, Murphy KM. Characterization of phosphotyrosine binding motifs in the cytoplasmic domain of B and T lymphocyte attenuator required for association with protein tyrosine phosphatase SHP-1 and SHP-2. Biochem Biophys Res Commun. 2003;312:1236–1243. doi: 10.1016/j.bbrc.2003.11.070. [DOI] [PubMed] [Google Scholar]
- 14.Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat Immunol. 2013;14:1212–1218. doi: 10.1038/ni.2762. [DOI] [PubMed] [Google Scholar]
- 15.Chen YN, LaMarche MJ, Chan HM, Fekkes P, Garcia-Fortanet J, Acker MG, Antonakos B, Chen CH, Chen Z, Cooke VG, Dobson JR, Deng Z, Fei F, Firestone B, Fodor M, Fridrich C, Gao H, Grunenfelder D, Hao HX, Jacob J, Ho S, Hsiao K, Kang ZB, Karki R, Kato M, Larrow J, La Bonte LR, Lenoir F, Liu G, Liu S, Majumdar D, Meyer MJ, Palermo M, Perez L, Pu M, Price E, Quinn C, Shakya S, Shultz MD, Slisz J, Venkatesan K, Wang P, Warmuth M, Williams S, Yang G, Yuan J, Zhang JH, Zhu P, Ramsey T, Keen NJ, Sellers WR, Stams T, Fortin PD. Allosteric inhibition of SHP2 phosphatase inhibits cancers driven by receptor tyrosine kinases. Nature. 2016;535:148–152. doi: 10.1038/nature18621. [DOI] [PubMed] [Google Scholar]
- 16.Brownlie RJ, Miosge LA, Vassilakos D, Svensson LM, Cope A, Zamoyska R. Lack of the phosphatase PTPN22 increases adhesion of murine regulatory T cells to improve their immunosuppressive function. Sci Signal. 2012;5:ra87. doi: 10.1126/scisignal.2003365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salmond RJ, Brownlie RJ, Zamoyska R. Multifunctional roles of the autoimmune disease-associated tyrosine phosphatase PTPN22 in regulating T cell homeostasis. Cell Cycle. 2015;14:705–711. doi: 10.1080/15384101.2015.1007018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao Q, Zhao YJ, Wang XY, Guo WJ, Gao S, Wei L, Shi JY, Shi GM, Wang ZC, Zhang YN, Shi YH, Ding J, Ding ZB, Ke AW, Dai Z, Wu FZ, Wang H, Qiu ZP, Chen ZA, Zhang ZF, Qiu SJ, Zhou J, He XH, Fan J. Activating mutations in PTPN3 promote cholangiocarcinoma cell proliferation and migration and are associated with tumor recurrence in patients. Gastroenterology. 2014;146:1397–1407. doi: 10.1053/j.gastro.2014.01.062. [DOI] [PubMed] [Google Scholar]
- 19.Li MY, Lai PL, Chou YT, Chi AP, Mi YZ, Khoo KH, Chang GD, Wu CW, Meng TC, Chen GC. Protein tyrosine phosphatase PTPN3 inhibits lung cancer cell proliferation and migration by promoting EGFR endocytic degradation. Oncogene. 2015;34:3791–3803. doi: 10.1038/onc.2014.312. [DOI] [PubMed] [Google Scholar]
- 20.Li S, Cao J, Zhang W, Zhang F, Ni G, Luo Q, Wang M, Tao X, Xia H. Protein tyrosine phosphatase PTPN3 promotes drug resistance and stem cell-like characteristics in ovarian cancer. Sci Rep. 2016;6:36873. doi: 10.1038/srep36873. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.