Abstract
Purpose
CD8+ T cells are primarily cytotoxic cells that provide immunological protection against malignant cells. Considerable evidence suggests that the T-cell repertoire is closely associated with the host immune response and the development of cancer. In this study, we explored the characteristics of the circulating CD8+ T-cell repertoire and their potential value in predicting the clinical response of breast cancer patients to chemotherapy.
Experimental design
We applied a high-throughput TCR β-chain sequencing method to characterize the CD8+ T-cell repertoire of the peripheral blood from 26 breast cancer patients. In addition, changes in the circulating CD8+ T-cell repertoire during chemotherapy were analyzed.
Results
We found that the HEC ratios of the CD8+ T-cell repertoires from HER2+ breast cancer patients were significantly higher than those of HER2− patients, suggesting that the HER2 protein is released into circulation where it is targeted by CD8+ T cells. Several Vβ and CDR3 motifs preferentially used in HER2+ patients were identified. Besides, we found that the circulating CD8+ T-cell repertoires evolved during chemotherapy and correlated with patient clinical responses to chemotherapy. Increased CD8+ T-cell repertoire heterogeneity during chemotherapy was associated with a better clinical response.
Conclusions
Although functional studies of clonally expanded CD8+ T-cell populations are clearly required, our results suggest that the circulating CD8+ T-cell repertoire reflects the characteristics of the tumor-associated biomolecules released into the blood and correlates with the clinical responses of the patients to chemotherapy which might assist in making treatment decisions.
Electronic supplementary material
The online version of this article (10.1007/s00262-018-2213-1) contains supplementary material, which is available to authorized users.
Keywords: Breast cancer, CD8+ T-cell repertoire, HER2 expression status, Clinical response
Introduction
Breast cancer is the most common cancer among women worldwide; however, breast cancer mortality has decreased over the last two decades due to new concepts and improved strategies for chemotherapy. Chemotherapy, particularly neoadjuvant chemotherapy, has become a standard treatment for patients with locally advanced breast cancer, large inoperable breast tumors, or certain biological breast cancer subtypes, as well as for patients who desire breast conservation [1]. Studies suggest that neoadjuvant chemotherapy benefits patients by shrinking the tumor, reducing the risk of recurrence, prolonging survival and increasing the opportunity for breast-conserving therapy [2, 3]. Despite extensive research, monitoring and predicting the clinical response of a breast cancer patient to chemotherapy remains a formidable challenge.
T cells play an important role in anti-tumor immune responses. The clinical outcomes of chemotherapy in cancer patients are associated with the host T-cell immune status [4]. The T-cell receptor (TCR) is a surface specific molecule present on T cells that is responsible for recognizing antigen peptides bound to the major histocompatibility complex (MHC). Millions of different T-cell clones comprise a diverse TCR repertoire that can be characterized in tissues or in peripheral blood using high-throughput TCR sequencing. Recent studies of the TCR repertoire in various types of cancer or other immune-related diseases indicate that the TCR repertoire could potentially serve as a diagnostic or prognostic biomarker [5–8].
Several studies have been published describing the TCR repertoire in breast cancer patients. For example, a previous study of early-stage breast cancer patients showed that cryoablation plus ipilimumab leads to increased numbers of peripheral blood and intratumoral T-cell clones that expand robustly following treatment compared to monotherapy [9]. Moreover, another study reported that the TCR repertoires of tumor-infiltrating lymphocytes in breast cancer patients who showed a complete response (CR) to chemotherapy showed significantly decreased diversity compared to patients who showed a partial response (PR), stable disease (SD) or progressive disease (PD) [10]. However, no reports have been published that monitor the TCR repertoire at multiple time points during breast cancer treatment despite the fact that such information could potentially provide timely clinical guidance.
The characteristics of circulating TCR repertoires at multiple time points have been investigated in patients with metastatic castration-resistant prostate cancer or metastatic melanoma. The results showed that T-cell repertoires evolve over the course of anti-CTLA-4 treatment and that the maintenance of high-frequency TCR clones is associated with improved overall survival [11]. In addition, given that most CD8+ T cells are cytotoxic and provide immunological protection against malignant and virus-infected cells, monitoring the CD8+ T-cell repertoire might prove beneficial and aid in understanding the patient’s immune capacity to eliminate residual tumor cells.
In the present study, we performed high-throughput TCR sequencing to characterize the circulating CD8+ T-cell repertoires in breast cancer patients before and after preoperative chemotherapy at multiple time points. This study provides comprehensive TCR immunogenetic characterization of CD8+ T cells in the peripheral blood of breast cancer patients. The results show that the circulating CD8+ T-cell repertoires differ between HER2+ (human epidermal growth factor receptor 2-positive) and HER2− patients, which suggests that the biological characteristics of tumors can be revealed by analysis of the circulating CD8+ T-cell repertoires. Interestingly, we also found that obvious changes in Vβ gene usage patterns or increasing CD8+ T-cell repertoire heterogeneity during chemotherapy is associated with patient clinical response.
Materials and methods
Clinical samples
This study was approved by the ethics committee of Affiliated Foshan Hospital of Sun Yat-sen University and was performed according to the Declaration of Helsinki. A total of 26 breast cancer patients were recruited and an informed consent form was signed by each patient before blood samples were taken. All patients were confirmed as having breast cancer by pathologic diagnosis and were treated with standard first-line chemotherapy regimens. In addition, they all had measurable lesions for treatment assessment. None of the 26 patients had received operative treatment, other immune-related diseases such as infectious or autoimmune diseases, undergone a transplant or had other types of cancer. Peripheral blood samples were collected before treatment initiation for all patients, and post-treatment blood samples were collected from 7 of the 26 patients on the day before the next chemotherapy cycle. The blood samples were collected when the corresponding radiographic assessment results were obtained. According to the Response Evaluation Criteria in Solid Tumors, clinical response to chemotherapy was determined using computed tomography (CT) scans and magnetic resonance imaging (MRI).
Immunohistochemistry and patient molecular subtypes
According to the 14th St. Gallen International Breast Cancer Consensus Conference (2015), molecular subtypes of breast cancer were classified as luminal A, luminal B, HER2-positive and triple-negative breast cancer (TNBC) depending on estrogen receptor (ER), progesterone receptor (PR), HER2 and Ki-67 protein expression [12]. ER, PR, HER2 and Ki-67 protein expression was detected via immunohistochemical staining. HER2 overexpression was confirmed by either an immunohistochemistry score of 3+ or a positive result using fluorescence in situ hybridization. If immunohistochemistry was used for the initial test, the specimens with HER2 scores of 2+ were subjected to confirmatory fluorescence in situ hybridization testing.
Immune cell isolation and CD8+ T-cell purification from peripheral blood
Peripheral blood mononuclear cells (PBMCs) were isolated from 20 ml of fresh peripheral blood containing an anticoagulant by density gradient centrifugation using Lymphoprep (Progen, Heidelberg, Germany). CD8+ T cells were then purified from the isolated PBMCs by positive selection using MACS magnetic cell separation (Miltenyi Biotec, Bergisch Gladbach, Germany) following the manufacturer’s protocols. Purified CD8+ T cells were over 95% pure as determined by flow cytometry. The purified cells were lysed with TRIzol® (Invitrogen, USA) and frozen at − 80 °C until further processing.
High-throughput sequencing of TCR β-chains
The total RNA was extracted from CD8+ T-cell lysates using a total RNA Kit (Omega Bio-tek, USA) according to the manufacturer’s instructions. 5′ RACE-ready cDNA of CD8+ T cells was synthesized from approximately 1 µg of the total RNA using a SMARTer PCR cDNA synthesis kit (Clontech Laboratories, USA) via semi-nested PCR amplification. The TCR β-chain-specific primers were as follows: 3′-TCR β outer primer (5′-AGATCTCTGCTTCTGATGGCT-3′) for the first round of PCR, 3′-TCR β inner primer (5′-TGGCTCAAACACAGCGACCT-3′) for the second round of PCR, and the nested universal primer (NUP, Clontech Laboratories, 5′-AAGCAGTGGTATCAACGCAGAGT-3′). Each TCRβ gene was randomly rearranged at its complementary-determining region 3 (CDR3) by combining noncontiguous variable (V), diversity (D), and joining (J) region gene segments of the germline locus. The 3′-TCR β outer and inner primers were homologous to the TCRβ constant (C) region gene segments (including Cβ1 and Cβ2), so that the TCR β sequence mapping of the 5′-V-D-J-3′ partial C region was amplified, including the CDR3 region at the VDJ junctions. The reliability of the nested primers for TCR-specific amplification was validated in our previous studies [13–15]. Finally, 1.5 µg of the objective PCR product that had been purified via a gel extraction kit was used for the TCR sequencing library and was sequenced on the Hiseq 2500 platform.
TCR sequence data analysis
Raw sequence data were stored in the FASTQ format. First, low-quality sequences were filtered out. The remaining sequences were reserved for further filtration based on the TCRβ V, D, J and C gene sequences provided by the IMGT/GeneDB database using BLAT software [16]. Sequences with TCR β-chain V, J and C gene segments were then translated into amino acid (aa) sequences. The TCRβ aa sequences without a terminator were finally selected as productive aa sequences. At the VDJ junctions, the sequences that started with a cysteine and ended with the FGXG motif were defined as CDR3 sequences. CDR3 motifs were identified by clustering and comparing the total CDR3 aa sequences using the CD-HIT program [17]. The motif models were constructed using Weblogo 3.0.
To evaluate changes in the TCR repertoire after each cycle of chemotherapy, we used the pairwise distance metric. The pairwise distance metric was converted from the Morisita–Horn (M–H) similarity index of two different TCR repertoires as described below [18, 19]. The M–H similarity index between two TCR repertoires was calculated based on the number of shared sequences between the two samples and the contribution of the shared sequences to each repertoire, and the index ranged from 0 to 1:
In this formula, i represents the TCRβ CDR3 sequence and xi and yi represent the count of the sequence identified in the two samples, respectively. S is the total number of unique sequences identified in the two samples. X and Y represent the total number of sequences identified in the two samples, respectively.
We then took 1 minus the M–H similarity index to obtain a pairwise distance metric. The pairwise distance metric was higher for less similar TCR repertoires and ranged from 0 to 1.
Statistical analysis
GraphPad Prism version 5.1 (GraphPad Software, Inc., San Diego, CA, USA) and SPSS 20.0 (SPSS, Inc., Chicago, IL, USA) were used for statistical analysis of the data. Comparisons between groups were conducted using the two-tailed Student’s t test, paired t test, Mann–Whitney U tests, or the Kruskal–Wallis test if appropriate and P values < 0.05 were considered statistically significant.
Results
Patient characteristics
The demographic and clinical characteristics of the 26 breast cancer patients included in this study are listed in Table 1 and Supplementary Table 1. The patients were all female with a median age of 52 years (range 38–68 years). According to the American Joint Committee on Cancer/Union for International Cancer Control staging system of breast cancer, 6 (23.1%) patients were stage II, 7 (26.9%) patients were stage III and 13 (50%) patients were stage IV. According to the protein expression levels of ER, PR, HER2 and Ki-67, 2 (7.7%) patients were classified as having luminal A breast cancer, 13 (50.0%) as having luminal B breast cancer, 8 (30.8%) as being HER2 positive, and 3 (11.5%) as having TNBC. While 3 patients (11.5%) were lost during follow-up, 2 patients (7.7%) showed a CR, 11 patients (42.3%) showed a PR, and 10 patients showed a SD or PD.
Table 1.
Patient characteristics
| Characteristics | Number |
|---|---|
| Median age in years (range) | 52 (38–68) |
| Gender | |
| Female | 26 (100%) |
| TNM stage | |
| II a/b | 6 (23.1%) |
| III a/b/c | 7 (26.9%) |
| IV | 13 (50.0%) |
| Biologic subtypes | |
| Luminal A | 2 (7.7%) |
| Luminal B | 13 (50.0%) |
| HER2 | 8 (30.7%) |
| TNBC | 3 (11.5%) |
| ER expressed in tumor | |
| + | 15 (57.7%) |
| – | 11 (42.3%) |
| PR expressed in tumor | |
| + | 11 (42.3%) |
| – | 15 (57.7%) |
| HER2 expressed in tumor | |
| + | 18 (69.2%) |
| – | 8 (30.8%) |
| Ki67 expressed in tumor | |
| ≥ 30% | 14 (53.8%) |
| < 30% | 12 (46.2%) |
| Clinical response | |
| CR | 2 (7.7%) |
| PR | 11 (42.3%) |
| SD/PD | 10 (38.5%) |
| Unknown | 3 (11.5%) |
Profiling of the CD8+ TCRβ sequences in the peripheral blood of breast cancer patients before chemotherapy
A total of 54,345,690 productive aa sequences were obtained from 26 pretreatment samples (Supplementary Table 2), with an average of 2,090,219 productive aa sequences generated per sample. The average number of productive unique aa sequences per sample was 56,859. A total of 65 distinct Vβ and 13 distinct Jβ segments were identified, and the usage frequencies of these segments were analyzed in each patient (Fig. 1a, b). The five most frequent genes detected in almost all the samples were Vβ5.1 (average of 18.53%), Vβ20.1 (15.50%), Vβ9.1 (7.13%), Vβ12.4 (5.45%) and Vβ15 (5.04%), as well as Jβ2.1 (25.53%), Jβ2.7 (19.76%), Jβ1.2 (9.90%), Jβ2.3 (98.9%) and Jβ1.1 (8.76%). Notably, Vβ5.1, Vβ20.1, Jβ2.1, and Jβ2.7 were also the most frequently detected gene segments of the total TCR repertoires in other studies [20–22], indicating that the V/J gene usage pattern of CD8+ T cells is similar to that of the total T-cell population.
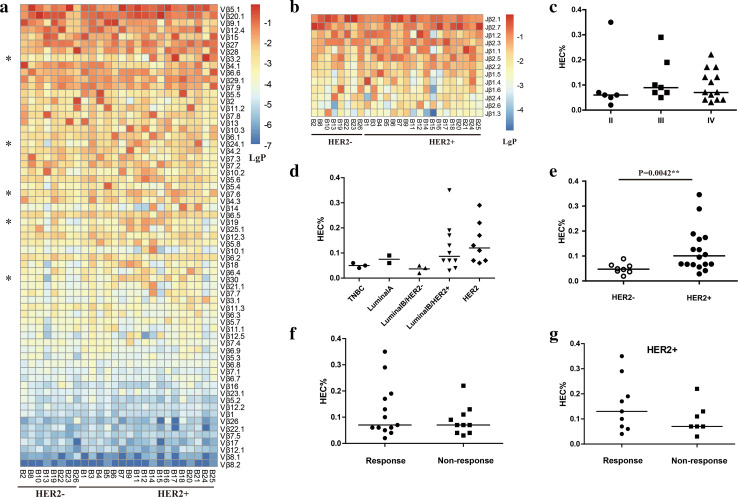
Fig. 1.
Comparison of Vβ gene usage and the HEC ratios in blood samples from different patient subgroups. a Vβ gene usage in blood samples from 26 patients. * Vβ gene usage frequencies in HER+ patients were significantly higher than those of HER− patients. b Jβ gene usage in blood samples from 26 patients. c Variance analysis of the HEC ratios in blood samples from patients with different TNM stages. d Variance analysis of the HEC ratios in blood samples from patients with different biological subtypes. e Variance analysis of HEC ratios comparing the HER+ and HER− patient subgroups. f Variance analysis of HEC ratios comparing the response and non-response subgroups. g Variance analysis of HEC ratios comparing HER2+ patients in the response and non-response subgroups. The bar indicates the median value
HEC ratios of CD8+ T-cell repertoires are influenced by the tumor HER2 expression status
To identify the differential expression profiles of CD8+ TCRβ genes in various samples, we selected all the clones in each sample with a frequency of more than 0.1% (defined as highly expanded clones, HECs) for further analysis, as this has proven to be a stable and effective parameter for the identification of potential biomarkers in other studies [23]. The HEC ratio was calculated by taking the number of HECs and dividing by the number of total clonotypes. The resulting HEC ratios were then used to compare CD8+ TCR repertoire heterogeneity across samples or among different subgroups.
We found that HEC ratios varied significantly between samples, with an average of 0.1% and ranging from 0.02 to 0.35% (Supplementary Table 2). We then compared the HEC ratios of patients with different TNM stages or molecular subtypes. While no significant differences were found, the HEC ratios of the HER2+ and luminal B/HER2+ patient subgroups were slightly higher than those of the other subgroups (Fig. 1c, d), suggesting that the HER2 expression level might influence the immune responses of CD8+ T cells.
As HER2+ and HER2− breast cancer patients are known to exhibit different basal immunologic profiles [24, 25], we then divided the patients into two subgroups based on their HER2 expression status. The HER2+ subgroup consisted of 18 patients while the HER2− subgroup consisted of 8 patients. As expected, the HEC ratios of patients in the HER2+ subgroup were significantly higher than those of the HER2− subgroup (P = 0.0042; Fig. 1e). Importantly, we determined that age did not influence these results (P = 0.27) although age has been proved to influence the clonal distribution of T cells [26].
The different usage patterns of Vβ genes and CDR3 motifs in HER2+ and HER2− patients
Next, we investigated the Vβ genes and CDR3 sequences present in HER2+ and HER2− patients. The usage frequencies of the Vβ3.2 (P = 0.040), Vβ7.6 (P = 0.046), Vβ19 (P = 0.026), Vβ24.1 (P = 0.023) and Vβ30 (P = 0.023) genes were significantly higher in the HER2+ subgroup compared to the HER2− subgroup (Fig. 1a). In contrast, no differences in the usage frequencies of the Jβ gene segments were identified (Fig. 1b). Given that TCRs with similar CDR3 sequences might recognize the same antigenic peptides [27], we identified similar CDR3 motifs based on similarities in their CDR3 aa sequences using the CD-HIT program (90% quantile). We found that 8 CDR3 motifs were significantly associated with HER2-positive breast cancers using the Chi-square test (P < 0.05; Fig. 2), which might prove valuable in immunotherapy.
Fig. 2.
Comparison of the amino acid sequences of 8 CDR3 motifs preferentially used in HER2+ patients. Each logo consists of stacks of symbols, with one stack for each amino acid in the sequence. The overall height of the stack indicates the degree of sequence conservation at that position, while the height of the symbols within the stack indicates the relative frequency of each amino acid at that position. The width of the stack is proportional to the fraction of valid symbols at that position. (Positions with many gaps have thin stacks.) The CDR3 sequences within the motifs are listed below the stacks. *Amino acid is missing
HEC ratios of CD8+ TCR repertoires before chemotherapy weakly correlate with clinical response
To evaluate whether the CD8+ TCR repertoire before chemotherapy has an effect on clinical response, we separated patients into two subgroups based on their clinical responses. Patients that showed a CR or PR (n = 12) at the time of their last follow-up were included in the response subgroup while patients that showed SD or PD (n = 11) were included in the non-response subgroup. The HEC ratios of the CD8+ TCR repertoires between the two subgroups were then compared.
Our results showed that there is no significant difference in the HEC ratios between the response and the non-response subgroups (Fig. 1e). When this analysis was performed in HER2+ patients only, we observed that the HEC ratios of patients with clinical responses were slightly higher than those of patients who did not respond to treatment, although the difference was not statistically significant (Fig. 1f).
The CD8+ TCR repertoire evolves during chemotherapy and correlates with clinical response
To explore the relationship between the CD8+ TCR repertoire and clinical response during chemotherapy treatment, we investigated the CD8+ TCR repertoire in blood samples across multiple chemotherapy cycles from seven breast cancer patients (Patient ID 1–7). Sample information for each patient is listed in Supplementary Table 1. The chemotherapy regimen for each patient was designed according to the 2015 NCCN Clinical Practice Guidelines in Oncology on Breast Cancer, and the therapeutic effect of each chemotherapy cycle was individually assessed.
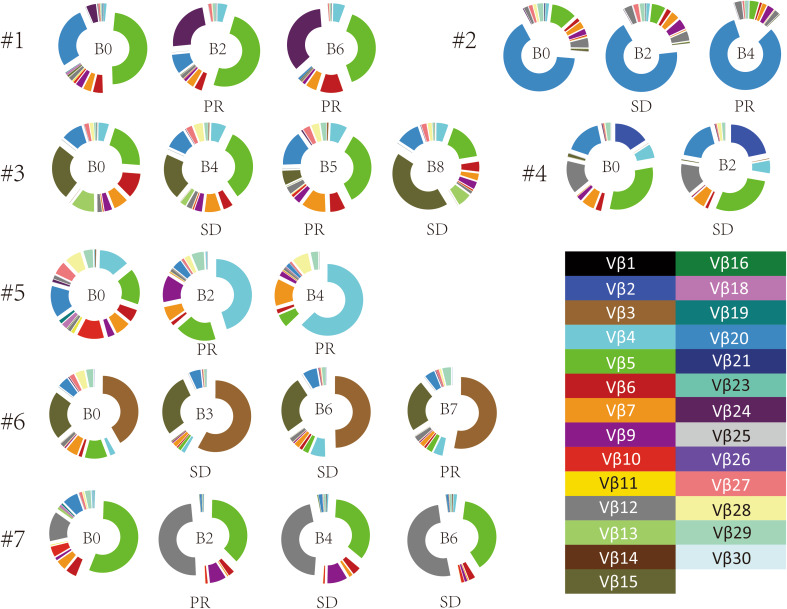
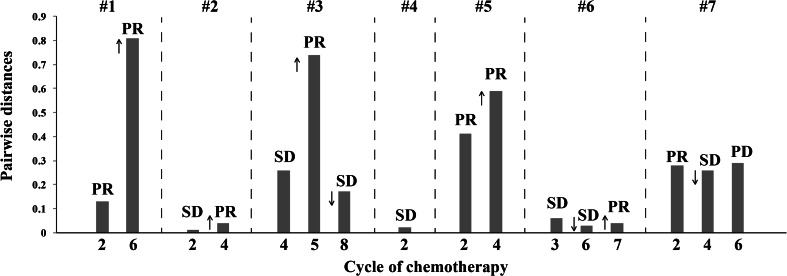
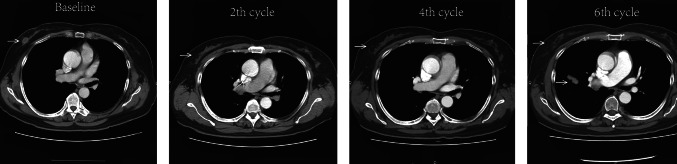
We first focused on changes in the Vβ gene usage pattern during chemotherapy. The 65 distinct Vβ gene segments were merged into 30 distinct Vβ gene families [28]. Gene family usage frequencies were calculated for each sample and then compared across samples from multiple chemotherapy cycles. We found that Vβ gene family usage patterns clearly evolved during chemotherapy (Fig. 3). In addition, we compared CD8+ TCR repertoire heterogeneity between the baseline and each cycle of chemotherapy by calculating the pairwise distance. The TCR repertoire similarity index was converted into a distance metric, with a scale of 0–1, to indicate minimal and maximal distance, respectively [18, 29]. The results showed that the pairwise distances varied differently in different individual patients during chemotherapy, indicating that chemotherapy influences the circulating CD8+ TCR repertoire of breast cancer patients in various degrees (Fig. 4). Interestingly, we observed that increasing pairwise distances during chemotherapy were associated with better clinical responses, while decreasing pairwise distances were associated with worse clinical outcome. Of note, although the clinical response of Patient 7 was determined to be PD after the 6th cycle of chemotherapy due to tumor metastasis, the primary lesion of this patient decreased in size, and the pairwise distances were increased (Fig. 5).
Fig. 3.
Usage patterns of Vβ gene families in blood samples before chemotherapy and after the indicated cycle of chemotherapy from seven patients. The clinical responses after the indicated chemotherapy cycle are shown below
Fig. 4.
Good clinical response during chemotherapy correlates with increased pairwise distance of circulating CD8+ TCR repertoires before and after the indicated chemotherapy cycle. The pairwise distance was recorded at the indicated chemotherapy cycle for seven patients. The clinical responses after the indicated chemotherapy cycle are shown above. ↓ indicates clinical confirmation of PR; ↑ indicates clinical confirmation of SD
Fig. 5.
Representative computed tomography images of tumors from breast cancer patient 7. The white arrows in the CT images indicate the locations of the tumor lesions. The size of the primary lesion decreased during chemotherapy, but metastasis was observed during the 6th cycle of chemotherapy
These results suggest that the obvious changes in the Vβ gene usage patterns and the increasing pairwise distances of CD8+ T-cell repertoires during chemotherapy might provide potential indicators for evaluating the clinical responses of breast cancer patients.
Discussion
To the best of our knowledge, the present study is the first to characterize the circulating CD8+ TCR repertoire in breast cancer patients. We found that the HEC ratio of CD8+ TCR repertoires is associated with the tumor HER2 expression status. Several Vβ genes and CDR3 motifs were identified as being preferentially expressed in HER2+ patients. Furthermore, we investigated the evolution of the CD8+ TCR repertoire during chemotherapy and its potential value in assessing chemotherapy efficacy. No significant differences were found between the CD8+ TCR repertoires of the clinical response and non-response groups before initiation of chemotherapy. Interestingly, we found that CD8+ TCR repertoires evolved during chemotherapy and correlated with clinical response.
CD8+ T cells are primarily cytotoxic T cells that recognize and kill malignant cells. Their efficiency may be influenced by the immune mechanisms developed by the tumor itself, such as the overexpression of HER2 [4, 24]. HER2 is a self-antigen that is expressed by normal epithelial cells and by several types of cancer cells. In addition, it is a known tumor-associated antigen (TAA) in breast cancer [30]. Studies suggest that HER2 is released by cells into the peripheral blood where it can be targeted by circulating CD8+ T cells [31, 32]. Our data show that circulating CD8+ T-cell repertoires are different between HER2+ and HER2− patients as reflected in their disparate HEC ratios, Vβ gene usage profiles and CDR3 motifs. These results confirm our hypothesis that the circulating CD8+ T-cell repertoire can be influenced by the tumor HER2 expression status. Studies also suggest that the ability to stimulate the generation of CD8+ T-cell responses against TAAs is more pronounced in HER2+ patients [25], thus we speculate that the HECs, Vβ genes or CDR3 motifs prominent in HER2+ breast cancer patients might be specific for HER2 and contribute to the immunotherapy of HER2+ cancer patients. However, whether these HECs, Vβ genes or CDR3 motifs are induced by circulating HER2 protein remains to be elucidated.
It has been reported that chemotherapy kills cancer cells, releasing a large amount of tumor-derived antigens, which then elicit an anti-tumor immune response or enhance the susceptibility of tumor cells to CD8+ T-cell immune attack [4]. However, clinical responses to chemotherapy vary between individual patients, suggesting that the efficiency of anti-tumor CD8+ T cells induced by chemotherapy might also vary from patient to patient. Therefore, we attempted to analyze the CD8+ T-cell repertoires of breast cancer patients to better understand the potential mechanisms involved in chemotherapy-induced immunomodulation. Our results show that patients that responded to chemotherapy and those that did not respond had similar HEC ratios prior to chemotherapy, suggesting that the responses of pre-existing CD8+ T cells might not reflect the clinical outcome of chemotherapy. Nevertheless, by investigating the evolution of CD8+ T-cell repertoires during chemotherapy, we found that CD8+ T-cell repertoires change to different degrees for each patient, suggesting that chemotherapy-induced immunomodulation also varies between individuals.
Interestingly, we found that the Vβ gene usage patterns of CD8+ T-cell repertoires clearly evolve during chemotherapy and that the heterogeneity of CD8+ T-cell repertoires increases during chemotherapy compared to the baseline is associated with a better clinical response. These results suggest that changes in the circulating CD8+ T-cell repertoire might serve as an evaluation index for clinical responses to chemotherapy. However, these findings require further validation using a larger number of patients, and the components of the CD8+ T-cell repertoire that correlate with tumor cell death also need clarification. Additional aspects related to the chemotherapeutic regimens, in particular, whether assessment of their therapeutic effects via analysis of the evolving characteristics of the CD8+ T-cell repertoires can contribute to chemotherapeutic regimen decisions needs further research.
In summary, this study provides novel data on the circulating CD8+ TCR repertoires of breast cancer patients, which may improve our understanding of the mechanisms underlying the systemic evolution of CD8+ T cells in cancer. Our findings suggest that the selective expansion of certain circulating CD8+ T-cell clones correlates with the characteristics of the tumor, such as the expression status of tumor-associated antigens that are released into peripheral circulation. Further research that expands on the information obtained from the current study could provide timely guidance and clarify which patients need to adjust their therapeutic regimens. In addition, monitoring of the circulating CD8+ TCR repertoire could be used to prevent the unnecessary exposure of patients to the side effects of an ineffective chemotherapy. Finally, the circulating CD8+ TCR repertoire could serve as a valuable biomarker for assessing the response to chemotherapy in breast cancer patients.
Conclusions
We found that the HEC ratios of the circulating CD8+ TCR repertoires in HER2-positive breast cancer patients were significantly higher than those of HER2-negative breast cancer patients, suggesting that the HER2 protein is released into the blood where it is targeted by circulating CD8+ T cells. Several Vβ and CDR3 motifs that are preferentially used in HER2-positive breast cancer patients were identified, and they might have potential value in immunotherapy. We also found that circulating CD8+ T-cell repertoires evolve during chemotherapy and that increasing CD8+ T-cell repertoire heterogeneity during chemotherapy is associated with a better clinical response. Our results suggest that the circulating CD8+ TCR repertoire could serve as a valuable biomarker and provide valuable information regarding the characteristics of the tumor-associated biomolecules. Furthermore, changes in the circulating CD8+ TCR repertoire during chemotherapy could provide a new method for assessing breast cancer patient clinical responses, which might assist in making treatment decisions.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- aa
Amino acid
- CDR3
Complementarity-determining region 3
- C gene
Constant gene
- CR
Complete response
- CT
Computed tomography
- D gene
Diversity gene
- ER
Estrogen receptor
- HEC
Highly expanded clone
- HER2
Human epidermal growth factor receptor 2
- J gene
Joining gene
- NUP
Nested universal primer
- MHC
Major histocompatibility complex
- MRI
Magnetic resonance imaging
- PBMC
Peripheral blood mononuclear cell
- PD
Progressive disease
- PR
Progesterone receptor
- PR
Partial response
- SD
Stable disease
- TCR
T-cell receptor
- TNBC
Triple-negative breast cancer
- V gene
Variable gene
Author contributions
WL and D-MP conceived and designed the study. D-MP, QH, YXL and H-BD provided the patient samples and clinical data. WL, K-RL and Y-BJ had full access to all the data in this study and take responsibility for the integrity of the data and the accuracy of the data analysis. Y-MP, J-HC and X-PC performed the experiments. K-RL drafted the manuscript. Y-BJ performed the statistical analysis. WL, Y-BJ and X-FM revised the manuscript. WL obtained the funding. All authors read and approved the final manuscript.
Funding
This work was funded by grants from the National Natural Science Foundation of China (81402255), Guangdong Province Natural Science Funds for Distinguished Young Scholar (2016A030306050), Guangdong Province Natural Science Funds (2014A030313803), Science and Technology Innovation Platform in Foshan City (2015AG10002) and the “Guangdong Te Zhi Program” youth science and technology talent project (2015TQ01R462).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethics approval
This study was approved by the Ethics Committee of the Affiliated Foshan Hospital of Sun Yat-sen University.
Informed consent
Written informed consent was obtained from all individual participants included in the study.
Footnotes
Kai-Rong Lin and Dan-Mei Pang contributed equally to this work.
References
- 1.Manguso N, Gangi A, Giuliano AE. Neoadjuvant chemotherapy and surgical management of the axilla in breast cancer: a review of current data. Oncology (Williston Park) 2015;29:733–738. [PubMed] [Google Scholar]
- 2.Golshan M, Cirrincione CT, Sikov WM, et al. Impact of neoadjuvant chemotherapy in stage II-III triple negative breast cancer on eligibility for breast-conserving surgery and breast conservation rates: surgical results from CALGB 40603 (Alliance) Ann Surg. 2015;262:434–439. doi: 10.1097/SLA.0000000000001417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Killelea BK, Yang VQ, Mougalian S, et al. Neoadjuvant chemotherapy for breast cancer increases the rate of breast conservation: results from the National Cancer Database. J Am Coll Surg. 2015;220:1063–1069. doi: 10.1016/j.jamcollsurg.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol. 2011;8:151–160. doi: 10.1038/nrclinonc.2010.223. [DOI] [PubMed] [Google Scholar]
- 5.Ritter J, Zimmermann K, Johrens K, et al. T-cell repertoires in refractory coeliac disease. Gut. 2017;67:644–653. doi: 10.1136/gutjnl-2016-311816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su S, Liao J, Liu J, et al. Blocking the recruitment of naive CD4(+) T cells reverses immunosuppression in breast cancer. Cell Res. 2017;27:461–482. doi: 10.1038/cr.2017.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schneider-Hohendorf T, Mohan H, Bien CG, et al. CD8(+) T-cell pathogenicity in Rasmussen encephalitis elucidated by large-scale T-cell receptor sequencing. Nat Commun. 2016;7:11153. doi: 10.1038/ncomms11153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riaz N, Havel JJ, Makarov V, et al. Tumor and microenvironment evolution during immunotherapy with nivolumab. Cell. 2017;171:934–949 e915. doi: 10.1016/j.cell.2017.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Page DB, Yuan J, Redmond D, et al. Deep sequencing of T-cell Receptor DNA as a biomarker of clonally expanded TILs in breast cancer after immunotherapy. Cancer Immunol Res. 2016;4:835–844. doi: 10.1158/2326-6066.CIR-16-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park JH, Jang M, Tarhan YE, et al. Clonal expansion of antitumor T cells in breast cancer correlates with response to neoadjuvant chemotherapy. Int J Oncol. 2016;49:471–478. doi: 10.3892/ijo.2016.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cha E, Klinger M, Hou Y, et al. Improved survival with T cell clonotype stability after anti-CTLA-4 treatment in cancer patients. Sci Transl Med. 2014;6:238ra270. doi: 10.1126/scitranslmed.3008211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackisch C, Harbeck N, Huober J, et al. 14th St. Gallen International Breast Cancer Conference 2015: evidence, controversies, consensus-primary therapy of early breast cancer: opinions expressed by german experts. Breast Care (Basel) 2015;10:211–219. doi: 10.1159/000433590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo W, Ma L, Wen Q, et al. Analysis of the interindividual conservation of T cell receptor alpha- and beta-chain variable regions gene in the peripheral blood of patients with systemic lupus erythematosus. Clin Exp Immunol. 2008;154:316–324. doi: 10.1111/j.1365-2249.2008.03770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo W, Ma L, Wen Q, et al. Analysis of the conservation of T cell receptor alpha and beta chain variable regions gene in pp65 peptide-specific HLA-A*0201-restricted CD8 + T cells. Cell Mol Immunol. 2009;6:105–110. doi: 10.1038/cmi.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo W, He WT, Wen Q, et al. Changes of TCR repertoire diversity in colorectal cancer after Erbitux (cetuximab) in combination with chemotherapy. Am J Cancer Res. 2014;4:924–933. [PMC free article] [PubMed] [Google Scholar]
- 16.Lefranc MP. IMGT, the International ImMunoGeneTics Information System for Immunoinformatics: methods for querying IMGT databases, tools, and web resources in the context of immunoinformatics. Mol Biotechnol. 2008;40:101–111. doi: 10.1007/s12033-008-9062-7. [DOI] [PubMed] [Google Scholar]
- 17.Li W, Godzik A. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics. 2006;22:1658–1659. doi: 10.1093/bioinformatics/btl158. [DOI] [PubMed] [Google Scholar]
- 18.Chen Z, Zhang C, Pan Y, et al. T cell receptor beta-chain repertoire analysis reveals intratumour heterogeneity of tumour-infiltrating lymphocytes in oesophageal squamous cell carcinoma. J Pathol. 2016;239:450–458. doi: 10.1002/path.4742. [DOI] [PubMed] [Google Scholar]
- 19.Mansfield AS, Ren H, Sutor S, et al. Contraction of T cell richness in lung cancer brain metastases. Sci Rep. 2018;8:2171. doi: 10.1038/s41598-018-20622-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freeman JD, Warren RL, Webb JR, et al. Profiling the T-cell receptor beta-chain repertoire by massively parallel sequencing. Genome Res. 2009;19:1817–1824. doi: 10.1101/gr.092924.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bai X, Zhang Q, Wu S, et al. Characteristics of tumor infiltrating lymphocyte and circulating lymphocyte repertoires in pancreatic cancer by the sequencing of T cell receptors. Sci Rep. 2015;5:13664. doi: 10.1038/srep13664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y, Xu Y, Zhao M, et al. High-throughput T cell receptor sequencing reveals distinct repertoires between tumor and adjacent non-tumor tissues in HBV-associated HCC. Oncoimmunology. 2016;5:e1219010. doi: 10.1080/2162402X.2016.1219010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han Y, Liu X, Wang Y, et al. Identification of characteristic TRB V usage in HBV-associated HCC by using differential expression profiling analysis. Oncoimmunology. 2015;4:e1021537. doi: 10.1080/2162402X.2015.1021537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perez SA, Karamouzis MV, Skarlos DV, et al. CD4+ CD25+ regulatory T-cell frequency in HER-2/neu (HER)-positive and HER-negative advanced-stage breast cancer patients. Clin Cancer Res. 2007;13:2714–2721. doi: 10.1158/1078-0432.CCR-06-2347. [DOI] [PubMed] [Google Scholar]
- 25.Muraro E, Martorelli D, Turchet E, et al. A different immunologic profile characterizes patients with HER-2-overexpressing and HER-2-negative locally advanced breast cancer: implications for immune-based therapies. Breast Cancer Res. 2011;13:R117. doi: 10.1186/bcr3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goronzy JJ, Qi Q, Olshen RA, Weyand CM. High-throughput sequencing insights into T-cell receptor repertoire diversity in aging. Genome Med. 2015;7:117. doi: 10.1186/s13073-015-0242-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glanville J, Huang H, Nau A, et al. Identifying specificity groups in the T cell receptor repertoire. Nature. 2017;547:94–98. doi: 10.1038/nature22976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banerjee JK, Schatz DG. Synapsis alters RAG-mediated nicking at Tcrb recombination signal sequences: implications for the “beyond 12/23” rule. Mol Cell Biol. 2014;34:2566–2580. doi: 10.1128/MCB.00411-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Emerson RO, Sherwood AM, Rieder MJ, et al. High-throughput sequencing of T-cell receptors reveals a homogeneous repertoire of tumour-infiltrating lymphocytes in ovarian cancer. J Pathol. 2013;231:433–440. doi: 10.1002/path.4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dieci MV, Griguolo G, Miglietta F, et al. The immune system and hormone-receptor positive breast cancer: Is it really a dead end? Cancer Treat Rev. 2016;46:9–19. doi: 10.1016/j.ctrv.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 31.Payne RC, Allard JW, Anderson-Mauser L, et al. Automated assay for HER-2/neu in serum. Clin Chem. 2000;46:175–182. [PubMed] [Google Scholar]
- 32.Bailur JK, Gueckel B, Derhovanessian E, et al. Presence of circulating Her2-reactive CD8+ T-cells is associated with lower frequencies of myeloid-derived suppressor cells and regulatory T cells, and better survival in older breast cancer patients. Breast Cancer Res. 2015;17:34. doi: 10.1186/s13058-015-0541-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.