Abstract
Various immune cells are recruited in the tumor microenvironment. It is well established that cellular immune responses, such as cytotoxic or suppressive activities, play an important role in regulating tumor growth and metastasis. However, the contribution of humoral immune responses against tumors is poorly understood. Fc receptors constitute critical elements for the up- or downregulation of immune responses through immune complexes. Here, we examined the potential role of the inhibitory Fc receptor, Fcγ receptor IIB (FcγRIIB), in tumor immunity using a mouse model. Our findings indicated that tumor-specific antibodies are induced in tumor-bearing mice and control tumor immunity. FcγRIIB deletion significantly improved both cellular and humoral immunity against tumors and delayed tumor growth. These findings indicated that spontaneous antibodies against tumors create a suppressive tumor microenvironment through FcγRIIB signaling, thus suggesting an attractive therapeutic target for cancer immunotherapy.
Electronic supplementary material
The online version of this article (10.1007/s00262-019-02413-w) contains supplementary material, which is available to authorized users.
Keywords: Fc receptors, Antibody, Macrophages, Tumor microenvironment, Mouse model
Introduction
It is well established that various immune cells constitute a large fraction of the tumor microenvironment and modulate tumor progression [1–4]. In particular, cellular immunity contributes to tumor development and results in either enhancement or reduction of tumor growth and metastasis [2, 3]. Most tumor cells express specific antigens and can be recognized by T lymphocytes. The activities of tumor-specific CD4 and CD8 T cells are critical for tumor elimination [1, 2, 5–7]. In general, if antigen-specific T cells are induced, then antigen-specific humoral immunity, including the production of appropriate antibodies, is also induced. However, the actual biological and clinical relevance of humoral immunity in tumors has not been well evaluated.
Antibodies are produced by B cells and play an important role in the immune system by neutralizing pathogens, such as bacteria and viruses. They can also influence T-cell function via antigen presentation. Antibodies bind to surface antigens on bacteria or cells and stimulate effector functions against coated targets by cells that recognize their Fc regions via membrane-bound Fc receptors (FcRs). The engagement of a particular antibody with an Fc receptor of a particular cell triggers various effector functions in the cell, such as phagocytosis, degranulation, and release of cytokines and cytotoxic molecules (antibody-dependent cell-mediated cytotoxicity) [8–10]. It is unclear whether such an antibody-mediated immune response functions in the tumor microenvironment.
FcRs constitute immune regulatory systems that participate in maintaining reactivity to foreign antigens and peripheral tolerance to self-antigens [8, 9]. The FcR family is functionally divided into two classes: activating receptors and the inhibitory receptor, FcγRIIB [8, 9, 11]. The activating receptors are multimeric protein complexes composed of a ligand-binding α-chain and dimer of the signal-transducing γ-chain, which contains an immune-receptor tyrosine-based activation motif. The inhibitory receptor consists of a peptide single chain containing an immune-receptor tyrosine-based inhibitory motif in its cytoplasmic domain. Both functionally distinct types of FcR are commonly co-expressed on the same cell; they counteract each other and constitute one of the important regulatory systems [8–12]. It is well established that the impairment of these FcRs in mice models affects the development of various autoimmune diseases and hypersensitivity reactions [13–18]. In particular, FcγRIIB may function in vivo to suppress the development of autoimmunity by negative regulation of immune complex-triggered activation [13–18].
This study examined the potential role of inhibitory FcR, FcγRIIB in tumor microenvironment. Our studies using FcγRIIB knockout (KO) mice revealed enhanced tumor-specific immunity and delayed tumor growth. Consistent with earlier studies, tumor-specific antibodies were spontaneously induced, and tumor antigen-related immune complexes activated macrophages. These results demonstrate that the deletion of FcγRIIB significantly improves both cellular and humoral antitumor immunity and enhances inflammation at the tumor site. Thus, this study established the important contribution of FcγRIIB to the host response to tumors. We therefore propose pharmacological targeting of FcγRIIB as a promising treatment for cancer.
Materials and methods
Animals and tumor cell lines
C57Bl/6 and FcγRIIB KO mice [17] were kept under specific pathogen-free conditions and used at 8–12 weeks of age. All mice were bred and housed at our animal facility. The following cell lines were used: E.G7, which is a CD8+ T-cell line derived from an EL-4 thymoma that has been transfected to express ovalbumin (OVA); Lewis lung carcinoma (LLC), which is derived from C57BL mice; and TC-1, which is a lung epithelial tumor cell line that expresses the E7 oncoprotein from human papilloma virus 16.
In vivo tumor studies
All in vivo experiments with FcγRIIB KO mice were conducted using respective age- and sex-matched wild-type (WT) counterparts or FcγRIIB heterozygous (HT) progeny as controls. Mice were injected s.c. with viable tumor cells (the number of cells varied with the tumor type as described in the figure legends). Tumor size was calculated by the formula (length × width × height)/2 [19]. Tumor size in each animal was measured by technicians or investigators, who were blinded to the genotype of the mice. Tumor growth curves were generated using 3–5 mice per group, and all the results were derived by combining data from two or three independent experiments, because control animals behaved similarly in all experiments. In addition, a couple of more experiments (at least two times) were repeated to confirm the data.
Flow cytometry
Tumor cells for flow cytometry were prepared as described previously [20] Cells were washed with PBS and stained with fluorochrome-conjugated anti-mouse IgG, anti-CD45, anti-CD3, anti-CD4, anti-CD8, anti-Ly6g, anti-Ly6c and/or anti-CD11b Abs for 30 min and fixed in fixation buffer for 10 min. All Abs were obtained from BD Pharmingen (San Diego, CA) or Biolegend (San Diego, CA). Stained cells were analyzed using a BD LSRFortessa cell analyzer (BD Bioscience).
Cell preparation
Tumors were digested in Liberase/DNase I (Roche Molecular Biochemicals, Manheim, Germany/Sigma-Aldrich, St. Louis, MO) solution for 30 min at 37 °C. Cells were then washed with 2% fetal calf serum (FCS) in PBS, stained with fluorochrome-conjugated antibodies for 30 min at 4 °C, and sorted using a BD FACSAria to isolate macrophages, as defined by the following characteristics: CD45+, CD11b+, Ly6c− and Ly6g−.
Detection of tumor-specific antibodies
Sera were diluted 300-fold and incubated with E.G7 tumor cells. The cells were then washed, stained with an Alexa-Fluor-647-conjugated mouse IgG-specific secondary antibody (Invitrogen, Carlsbad, CA) and analyzed by flow cytometry.
Quantitative RT-PCR
Quantitative real-time RT-PCR (qPCR) was performed as described previously [21]. Briefly, total RNA was extracted from cells using TRIzol reagent (Life Technologies, Carlsbad, CA) and reverse transcribed using an iScript cDNA synthesis kit (Bio-Rad, Richmond, CA). qPCR was conducted on cDNA using TaqMan probes and the TaqMan Gene Expression Master Mix kit (Applied Biosystems, Foster City, CA) according to the manufacturer’s instructions. Relative mRNA expression levels were calculated independently after correcting for GAPDH expression.
Preparation of macrophages
Bone marrow-derived macrophages (BMDMs) were prepared from C57Bl/6 and FcγRIIB KO mice as described previously [22]. Briefly, 2 × 106 bone marrow cells obtained from a femur were seeded into a 100 mm Petri dish in 10 ml of RPMI medium supplemented with 10% FCS and 20 ng/ml macrophage colony-stimulating factors (Biolegend). Medium was replaced on day 3, and cells harvested on day 7 by treatment with 3 mM ethylenediaminetetraacetic acid for 5 min.
Histology
Tumors were fixed in 4% paraformaldehyde for 24 h and then embedded in paraffin. Tissue sections were stained with goat anti-mouse IgG-specific secondary antibody (Southern Biotech, Birmingham, AL), followed by the addition of Simple Stain Goat MAX PO (Nichirei, Tokyo, Japan) and diaminobenzidine substrate (DAKO Japan) according to the manufacturers’ instructions.
ELISPOT assay
Single-cell suspensions were prepared from spleens and the H-2Kb restricted peptide from OVA (SIINFEKL) was used for the stimulation of CD8 T cells. A total of 1.5–3.0 × 105 cells per well were stimulated for 12–14 h with 0.1 µg/mL of SIINFEKL peptide in 96-well Immulon II plates (Merck Millipore, Burlington, MA) that had been previously coated with monoclonal rat anti-IFNγ (R4-6A2; BD Biosciences) and the number of spots was determined as previously described [21].
Immunoblotting with serum
Tumor lysate was prepared by repeated freeze–thaw and sonication of E.G7 tumor cells. Proteins were separated under reducing conditions using 15% SDS‐PAGE. Sera were diluted 300-fold in blocking buffer. Bound antibodies were detected using Alexa Flour 680-conjugated goat anti-mouse IgG-specific secondary antibody (Thermo Fisher Scientific, Waltham, MA). The blots were visualized using the Odyssey Infrared Imaging System and software (LI-COR Biosciences, Lincoln, NB).
Statistical analysis
A two-sided unpaired Student’s t test was used to analyze tumor growth and cellular responses. Tests were performed in JMP Pro 13 (SAS institute, Cary, NC). Difference with P < 0.05 was considered statistically significant.
Results
Antitumor antibodies are elicited in tumor-bearing mice
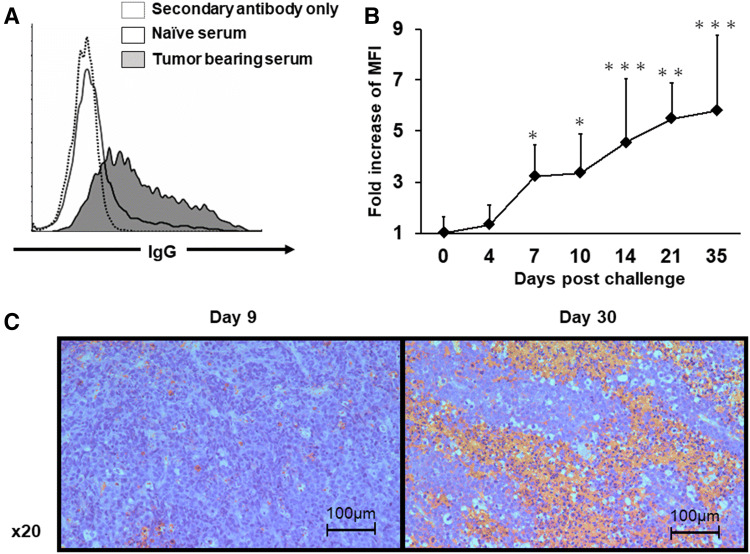
It is well established that autoantibodies to tumor antigens are found in the sera of cancer patients [23–25]. To investigate whether humoral immunity against tumors is induced in tumor-bearing animal models, tumor-specific antibody responses were evaluated in the serum from tumor-bearing mice. C57Bl/6 mice were injected s.c. with E.G7 tumor cells, and sera were collected on day 35. Endogenous antibodies specifically bound to E.G7 cells were detected by flow cytometry in the serum of tumor-bearing mice but not of naïve mice (Fig. 1a). Kinetics studies revealed that tumor-specific antibodies were detectable as early as 7 days after tumor inoculation and gradually increased thereafter (Fig. 1b). Similar results were observed in the TC-1 and LLC tumor models (Supplemental Fig. 1a). Additional studies were then performed to assess the deposition of these antibodies at the tumor site. Sections stained with anti-mouse IgG antibody revealed a patchy distribution of IgG deposition at the tumor site on day 30 after tumor inoculation (Fig. 1c). Consistent with the finding that tumor-specific antibodies were not detected early, deposition of IgG at the tumor site was not observed on day 9. These findings suggest that tumor-specific antibodies are spontaneously elicited in tumor-bearing mice and are deposited at the tumor site.
Fig. 1.
Detection of tumor-specific antibodies. E.G7 tumor cells (1.0 × 106) were injected s.c. into C57Bl/6 mice. Sera were collected at day 35 (a) or the indicated day (b). E.G7 tumor cells were incubated with 0.3% serum for 30 min and then washed, stained with an Alexa-Fluor-647-conjugated mouse IgG-specific secondary antibody and analyzed by flow cytometry. a Representative example and b the fold change in the MFI of naïve mice (day 0) over time with SD (N = 8 mice per group). c Representative images of antibody deposition in tumor on days 9 and 30. A representative 20 × field of staining is presented. *P < 0.05, **P < 0.01 and ***P < 0.001 when compared with the naïve (day 0) group
The role of FcγRIIB in tumor growth
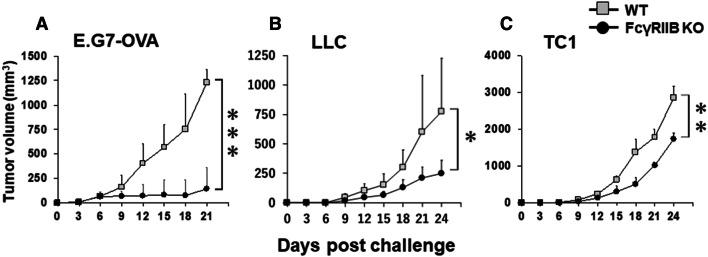
Theoretically, spontaneously elicited tumor-specific antibodies can bind tumor cells and can then be recognized by antigen-presenting cells through FcR binding. Various FcRs that perform these functions have been identified. To examine the role of inhibitory FcRs (FcγRIIB in this context), E.G7 cells were inoculated into FcγRIIB KO mice, and tumor growth was monitored and compared with WT (Fig. 2a). Of interest, the incidence and early growth (up to 9 days) in FcγRIIB KO mice were similar compared with WT, but the tumors in FcγRIIB KO mice were regressed or slowed. Consistent with this observation, the growth of LLC and TC-1 cells was significantly delayed in FcγRIIB KO mice (Fig. 2b, c). These findings led us to focus on analyzing tumor microenvironment in FcγRIIB KO mice.
Fig. 2.
Tumor growth in FcγRIIB KO mice. E.G7 tumor cells (106), LLC (105) or TC-1 (105) were injected s.c. into WT/FcγRIIB HT or FcγRIIB KO mice. Data represent the combined means with SD of 10–15 mice/group from two or three independent experiments (aN = 12; WT, N = 15 (8 mice cleared tumors in 15 mice and never relapsed); FcγRIIB KO, bN = 12; WT, N = 8; FcγRIIB KO, cN = 12; WT, N = 8; FcγRIIB KO). *P < 0.05, **P < 0.01 and ***P < 0.001 compared with WT/HT mice
Effect of FcγRIIB deficiency on tumor-specific cellular and humoral immunity
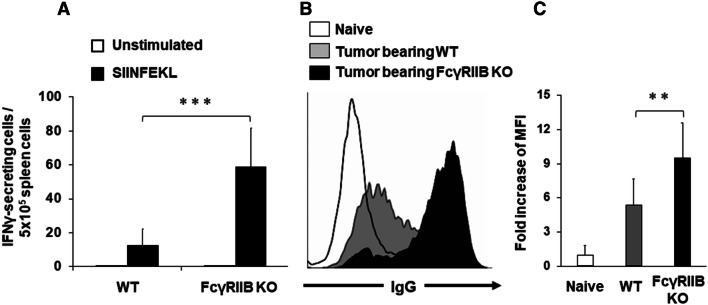
To clarify the mechanism by which tumor growth was reduced in FcγRIIB KO mice, single-cell suspensions were prepared from spleen cells of E.G7 tumor-bearing mice and these were then stimulated ex vivo with SIINFEKL peptide, which is a CD8-restricted epitope of OVA, and analyzed by ELISpot for IFNγ production. The number of IFNγ-producing cells in FcγRIIB KO mice was significantly higher than that in WT (Fig. 3a). Next, the induction of tumor-specific antibodies was evaluated and these were significantly elevated in FcγRIIB KO mice (Fig. 3b, c). These findings suggest that a deficiency of FcγRIIB enhances tumor-specific cellular and humoral immunity and results in delayed tumor growth.
Fig. 3.
Tumor-specific IFNγ and antibody production in FcγRIIB KO mice. a Mice were treated as described in Fig. 2. Spleen cells from E.G7 tumor-bearing mice were isolated on day 28, re-stimulated ex vivo with 0.1 μg/ml SIINFEKL peptide, and monitored for IFNγ secretion using an ELISpot assay. Results represent the mean with SD of five mice per group from two independent experiments. b, c Serum and cells were prepared as described in Fig. 1. b Representative results from one mouse per group and c the fold change in the MFI of naïve mice with SD (n = 5 mice per group). **P < 0.01 and ***P < 0.001
Effect of FcγRIIB deficiency on tumor-infiltrated immune cells and gene expressions
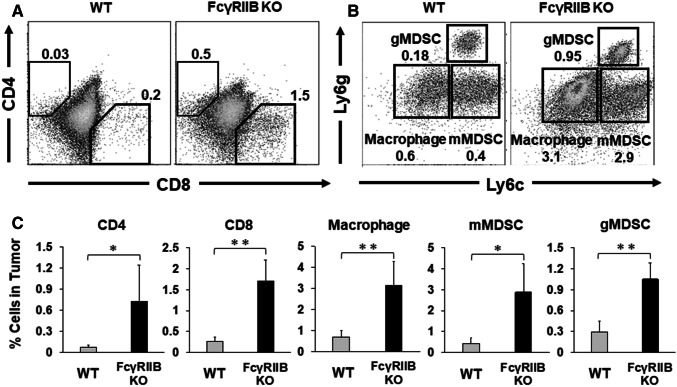
To identify the cellular mechanisms underlying regression or slowing of tumor growth in FcγRIIB KO mice, tumor-infiltrated immune cells were evaluated by flow cytometry. Consistent with the observed enhancement in antitumor immunity, the fraction of CD4 and CD8 T cells infiltrating tumors was significantly elevated in FcγRIIB KO mice (Fig. 4a, c). Furthermore, the frequencies of tumor-infiltrating myeloid cells, monocytic myeloid-derived suppressor cells (mMDSC; CD11b+, Ly6cHigh, Ly6g−), granulocytic MDSCs (gMDSC; CD11b+, Ly6cdim, Ly6g+) and macrophages (CD11b+, Ly6c−, Ly6g−) were also significantly elevated in FcγRIIB KO mice (Fig. 4b, c and Supplemental Fig. 1B). Thus, the total number of immune cells (CD45+) infiltrating the tumor also increased.
Fig. 4.
Tumor-infiltrating immune cells in FcγRIIB KO mice. Mice were treated as described in Fig. 2. E.G7 tumors were removed at day 21 and the number of tumor-infiltrating CD45 + CD4 cells, CD8 cells, mMDSC (CD11b+, Ly6c+, Ly6g−), gMDSCs (CD11b+, Ly6cint, Ly6g+) and macrophages (CD11b+, Ly6c−, Ly6g−) were determined by flow cytometry. a, b Representative results from one mouse per group. c Results were evaluated independently for each mouse, and the data represent the means with SD of 10 mice per group from two independent experiments. *P < 0.05 and **P < 0.01
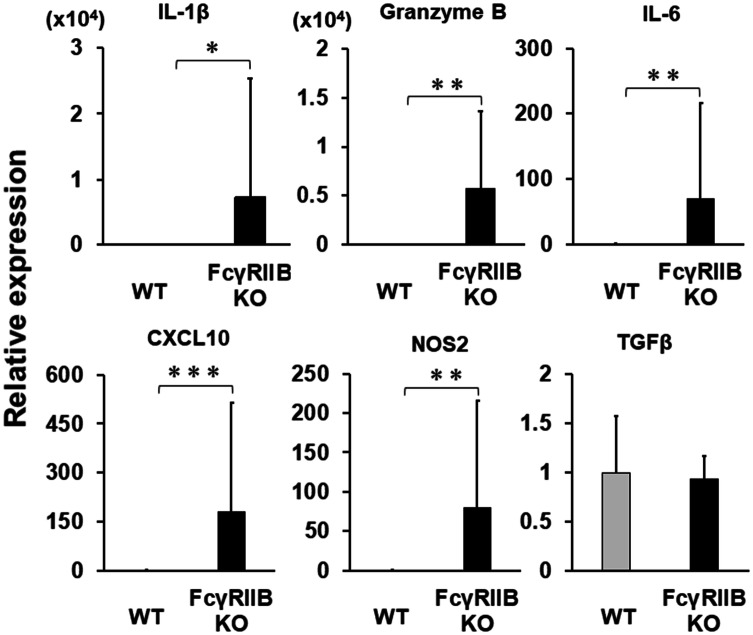
To evaluate the molecular mediators associated with immunological functions, the mRNA expression of cytokines or chemokines within the tumors was evaluated by qPCR. Consistent with the increase in the number of effector and CD8 T cells at the tumor site, the expression of granzyme B and IFN-inducible genes such as Cxcl10 and Nos2 increased significantly in FcγRIIB KO mice (Fig. 5), as did IL-1β and IL-6, suggesting that inflammation at tumor site was extensively activated. Similar results for tumor-infiltrated immune cells and gene expression were observed in the LLC model (Supplemental Figs. 1B and 2A). Initial studies demonstrated no difference in myeloid and lymphoid cell lineages in the organs of FcγRIIB KO naïve mice compared with WT naïve mice [17], and we confirmed that the baseline of inflammatory gene expressions in tumor-free KO mice were comparable to that in WT mice (Supplemental Fig. 2B).
Fig. 5.
Gene expression at tumor sites in FcγRIIB KO mice. Mice were treated as described in Fig. 2. E.G7 tumors were removed at day 28 and analyzed for the expression of the indicated mRNAs by qPCR. Data represent the means with SD of 10–12 mice per group from two independent experiments (N = 12; WT, N = 10; FcγRIIB KO). *P < 0.05, **P < 0.01 and ***P < 0.001
FcR binding induces the expression of cytokines by macrophages in vitro
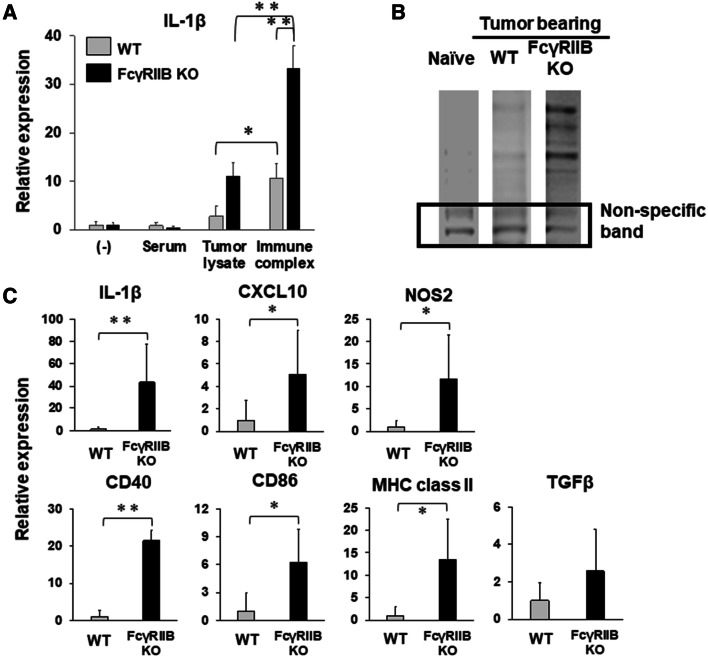
It is well established that the binding of immune complexes to FcRs on dendritic cells or macrophages induces their maturation and antigen presentation. To determine whether this was the case in this study, a mixture of tumor lysate and serum antibodies, which should contain immune complexes, was prepared and incubated with BMDMs. Consistent with previous studies, incubation with immune complexes significantly upregulated the expression of IL-1β in WT macrophages, whereas antibodies (serum) or antigen (tumor lysate) alone did not (Fig. 6a and [20]). Furthermore, IL-1β expression stimulated by immune complexes was strongly upregulated in FcγRIIB KO macrophages (Fig. 6a). An immunoblot of E.G7 lysate with sera from E.G7 tumor-bearing mice confirmed that the endogenous antibodies recognized numerous tumor antigens (Fig. 6b). These findings suggest that tumor antibody immune complexes induce the activation of macrophages and that the inhibitory receptor FcγRIIB regulates FcR-mediated activation.
Fig. 6.
Gene expression in macrophages from FcγRIIB KO mice. a BMDMs from WT or FcγRIIB KO were incubated with 1:300 dilution of serum and 10 µg/ml of tumor lysates for 16 h and analyzed for IL-1β mRNA levels by qPCR. Results represent the mean fold increase with SD from 3 to 5 independent experiments. b Immunoblot analysis of E.G7 tumor cell lysate using serum from naïve, tumor-bearing WT or tumor-bearing FcγRIIB KO mice using an anti-mouse IgG-specific secondary antibody. A non-specific band was used as a loading control. A representative example from two independent experiments is shown. c Mice were treated as described in Fig. 5. Tumor-infiltrated macrophages (CD45+, CD11b+, Ly6c−, Ly6g−) were purified by flow cytometry and analyzed for the indicated mRNAs by qPCR. Results represent the mean with SD of results from 10 to 12 independently sorted cell populations (cN = 12; WT, N = 10; FcγRIIB KO). *P < 0.05, **P < 0.01 and ***P < 0.001
Tumor-infiltrated macrophages are activated in FcγRIIB-deficient mice
We next examined whether FcγRIIB regulates the functional activity of macrophages in the tumor immune microenvironment. To evaluate whether macrophages from tumor-bearing mice are activated, tumor-infiltrated macrophages (CD11b+, Ly6c−, Ly6g−) were sorted by flow cytometry and analyzed for gene expression by real-time RT-PCR. Consistent with our in vitro study (Fig. 6a), the expressions of IL-1β were significantly elevated in tumor-infiltrated macrophages from FcγRIIB KO mice. Furthermore, the expression of IFN-inducible genes, such as Cxcl10, and Nos2, was significantly elevated, suggesting that the macrophages were activated. In addition, antigen presentation molecules, such as CD40, CD86, and MHC class II, were also significantly elevated, suggesting the macrophages had maturated. However, the expression of TGFβ was not elevated in the macrophages as well as at the tumor site (Figs. 5, 6c). These results suggest that tumor-infiltrated macrophages from FcγRIIB-deficient mice are activated and have matured at tumor site and so contribute to antitumor immunity.
Discussion
Pathogen or tumor cells bound to antibodies can be recognized by dendritic cells or macrophages through their FcRs. FcRs transduce signals that lead to either up- or downregulation of immune responses. We therefore examined the potential role of the inhibitory FcR, FcγRIIB in tumor immunity using animal models. Preliminary studies in multiple tumor models showed that tumor growth was regressed or was significantly inhibited in FcγRIIB KO mice compared with WT (Fig. 2). Based on these findings, we focused on the tumor microenvironment in FcγRIIB KO mice. Our results demonstrated that the deletion of FcγRIIB significantly improved both cellular and humoral antitumor immunity and enhanced inflammation at the tumor site in multiple tumor models (Figs. 2, 3, 4, 5). The induction of antitumor immunity and inflammation at the tumor site varied depending on the tumor cell line. These data are summarized in Supplemental Table 1. Our in vitro studies supported the concept that tumor antigen-related immune complexes enhanced the activation of macrophages in FcγRIIB KO mice (Fig. 6a); indeed, tumor-infiltrated macrophages from FcγRIIB KO mice were activated (Fig. 6c). These findings suggested that humoral immunity contributes to the immunosuppressive microenvironment that supports tumor growth.
Recent reports have demonstrated that spontaneous antibodies against tumors are present in tumor-bearing animals and in patients with cancer [23–25]. We previously demonstrated that IL-4 expression in Tfh cells and the number of B cells increased in the draining lymph nodes, suggesting an enhancement of antibody production in the tumor microenvironment [26]. Consistent with previous reports, our study showed that tumor-specific antibodies are spontaneously induced 7 days after tumor inoculation (Fig. 1b) [27]. Although these antibodies may regulate antitumor immune responses, how they affect the immunity against tumor remains unclear. These effector systems could be induced via FcRs. Indeed, spontaneous tumor-specific antibodies were deposited at tumor sites (Fig. 1c). Recent studies have examined whether passive transfer from serum containing tumor-specific antibodies affects tumor growth [27, 28]. Pearce et al. showed that serum transferred from tumor antigen Neu5Gc-immunized mice enhanced tumor growth at low concentrations but was inhibitory at higher concentrations in the MC38 tumor model. Moynihan et al. showed that combination therapy (AIPV)-induced antibodies were functional because the serum transferred from AIPV-treated mice protected naïve mice against intravenous B16F10 challenge. However, the mechanisms underlying these observations have not been elucidated.
Recent studies have demonstrated that the administration of tumor-reactive (therapeutic) antibodies, such as anti-HER2 or anti-CD20, can alter tumor progression [29]. Furthermore, FcγRIIB influences the efficacy of antibody therapy. Clynes et al. showed that FcγRIIB-deficient mice could inhibit tumor growth and prevent metastasis more effectively than WT mice when treated with a therapeutic monoclonal antibody, indicating that FcγRIIB expression on effector cells, i.e., macrophages, leads to the suppression of their phagocytic and cytotoxic potential in vivo [30–32]. However, the involvement of spontaneously induced tumor-specific antibodies and FcRs in antitumor immunity has not been addressed. The present results demonstrated that the deletion of FcγRIIB exacerbates inflammation within tumors and regresses or inhibits their growth (Figs. 2, 5), suggesting that spontaneous tumor-reactive antibodies create a suppressive immune environment at the tumor site through FcγRIIB. FcγRIIB is known to play an important role in inhibiting the activation of antigen-presenting cells after binding immune complexes [33]. The tumor-reactive antibodies do not target specific antigens. Under physiological conditions, this may contribute to the amelioration of excessive inflammation and prevention of autoimmunity or allergic reaction. Recent reports have demonstrated that mice that lack the gene encoding FcγRIIB exhibit abnormalities in various immune reactions and inflammatory responses involving antibodies and spontaneously develop autoimmune diseases, such as collagen-induced arthritis and immune complex-mediated alveolitis [13–18, 32].
The immune system contains various mechanisms to prevent autoimmunity, and these immune tolerance systems are hijacked by tumor cells to promote tumor progression. Dendritic cell subtypes, MDSCs, and regulatory T cells are involved in the development of an immunosuppressive microenvironment in tumors [2]. Conversely, immunotherapeutic targets to break immune tolerance are designed around immunosuppressive molecules such as PD-1 or CTLA4 [34, 35]. Current studies indicate that signaling through FcγRIIB contributes to the suppression of inflammation at tumor sites and, therefore, a blockade of FcγRIIB in tumors should be considered as a target for cancer immunotherapy. Indeed, some researchers have explored and achieved the generation of FcγRIIB Abs in mouse and human. Williams et al. had developed anti-FcγRIIB mouse antibodies, which promoted antibody-dependent cell-mediated phagocytosis [36]; however, their antibodies did not work well due to their rapid half-life in the circulation. Roghanian et al. also generated human FcγRIIB-specific antibodies and demonstrated the ability of FcγRIIB-blocking antibodies to enhance tumor cell depletion and block therapeutic antibody internalization without FcγRIIB signaling [31]. Further studies may hopefully develop the selective blockade of FcγRIIB and clinical testing of blockade as adjuncts to cancer immunotherapy.
FcγRIIB is widely expressed on B cells and many myeloid cells (including macrophages and dendritic cells) [37]. The present findings are consistent with the enhanced activation of macrophages and dendritic cells in FcγRIIB KO mice leading to an increase in their capacity to activate T cells. They are also consistent with previous in vitro studies showing that macrophage and dendritic cells from FcγRIIB KO mice enhance the activation and induction of T cells via immune complexes [33], since engagement of FcγRIIB by immune complexes results in the downregulation of the maturation and activation of dendritic cells and macrophages. The present study used FcγRIIB KO mice where only stimulation from activating FcRs was present and inhibition from FcγRIIB signaling was absent (Fig. 6). These results suggest that the physiological role of FcγRIIB is to downregulate inflammation in the tumor microenvironment. The affinity of FcRs for antibodies depends on the IgG subclass, which affords greater flexibility to the immune system in invoking only the appropriate immune mechanisms for distinct pathogens [14]. This study did not examine tumor-specific IgG subtypes, since it is technically difficult to separate the function of specific IgG subclasses. Recent studies have, however, demonstrated that FcγRIIB contributes varying levels of negative regulation depending on the specific IgG subclass involved [14, 38]. It might therefore be possible to induce antitumor immunity through FcRs without stimulating FcγRIIB by selecting the appropriate IgG subtype.
In summary, this works demonstrates that spontaneous antibodies against tumors create a suppressive tumor microenvironment through FcγRIIB signaling. These findings suggest that the expression of FcγRIIB serves as a potent mechanism for tumors to escape host immune responses and immunotherapy, and that a blockade of the interaction between FcγRIIB and immune complexes may provide an attractive therapeutic target for cancer immunotherapy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Dr. Toshiyuki Takai for kindly providing FcγRIIB KO mice and a critical review of this manuscript. The authors would like to thank Enago (http://www.enago.jp) for the English language review.
Abbreviations
- BMDMs
Bone marrow-derived macrophages
- FcRs
Fc receptors
- FcγRIIB
Fcγ receptor IIB
- gMDSCs
Granulocytic MDSCs
- HT
Heterozygous
- KO
Knockout
- LLC
Lewis lung carcinoma
- mMDSCs
Monocytic myeloid-derived suppressor cells
- OVA
Ovalbumin
- WT
Wild-type
Author contributions
HS and CI conceived the concept. HS and YK designed the experiments. YK and SU performed the in vitro and in vivo experiments. HS, YK and SU analyzed the data. HS and YK wrote the paper.
Funding
This work was supported by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) KAKENHI Grant nos. 25430103 and 16K07106, Japan.
Compliance with ethical standards
Ethical approval
The study was approved by the Institutional Committee for the Use and Care of Laboratory Animals of Tohoku University. Animal research approval number: 2017-171-1. All in vivo experiments were conducted in strict accordance with good animal practice and complied with ethics committee guidelines of Tohoku University.
Conflict of interest
The authors declare that they have no conflicts of interests.
Animal source
C57Bl/6 mice were obtained from Japan SLC (Hamamatsu, Japan). FcγRIIB KO mice were provided by Takai et al. (Department of Experimental Immunology, Tohoku University, Sendai, Japan).
Cell line authentication
The following cell lines were purchased from American Type Culture collection (Manassas, VA): E.G7, LLC, TC-1 and B16 melanoma. No cell line authentication was necessary.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006;6:715–727. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- 3.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science (New York, N.Y.) 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 4.van Kempen LC, Ruiter DJ, van Muijen GN, Coussens LM. The tumor microenvironment: a critical determinant of neoplastic evolution. Eur J Cell Biol. 2003;82:539–548. doi: 10.1078/0171-9335-00346. [DOI] [PubMed] [Google Scholar]
- 5.Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science (New York, N.Y.) 2015;348:74–80. doi: 10.1126/science.aaa6204. [DOI] [PubMed] [Google Scholar]
- 6.Qin Z, Schwartzkopff J, Pradera F, Kammertoens T, Seliger B, Pircher H, Blankenstein T. A critical requirement of interferon gamma-mediated angiostasis for tumor rejection by CD8+ T cells. Cancer Res. 2003;63:4095–4100. [PubMed] [Google Scholar]
- 7.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 9.Pincetic A, Bournazos S, DiLillo DJ, Maamary J, Wang TT, Dahan R, Fiebiger BM, Ravetch JV. Type I and type II Fc receptors regulate innate and adaptive immunity. Nat Immunol. 2014;15:707–716. doi: 10.1038/ni.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DiLillo DJ, Ravetch JV. Fc-receptor interactions regulate both cytotoxic and immunomodulatory therapeutic antibody effector functions. Cancer Immunol Res. 2015;3:704–713. doi: 10.1158/2326-6066.CIR-15-0120. [DOI] [PubMed] [Google Scholar]
- 11.Bournazos S, Wang TT, Dahan R, Maamary J, Ravetch JV. Signaling by antibodies: recent progress. Annu Rev Immunol. 2017;35:285–311. doi: 10.1146/annurev-immunol-051116-052433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nimmerjahn F, Ravetch JV. Fcgamma receptors: old friends and new family members. Immunity. 2006;24:19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Bolland S, Yim YS, Tus K, Wakeland EK, Ravetch JV. Genetic modifiers of systemic lupus erythematosus in Fc gamma RIIB(−/−) mice. J Exp Med. 2002;195:1167–1174. doi: 10.1084/jem.20020165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clynes R, Maizes JS, Guinamard R, Ono M, Takai T, Ravetch JV. Modulation of immune complex-induced inflammation in vivo by the coordinate expression of activation and inhibitory Fc receptors. J Exp Med. 1999;189:179–185. doi: 10.1084/jem.189.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang Y, Hirose S, Abe M, Sanokawa-Akakura R, Ohtsuji M, Mi X, Li N, Xiu Y, Zhang D, Shirai J, Hamano Y, Fujii H, Shirai T. Polymorphisms in IgG Fc receptor IIB regulatory regions associated with autoimmune susceptibility. Immunogenetics. 2000;51:429–435. doi: 10.1007/s002510050641. [DOI] [PubMed] [Google Scholar]
- 16.McGaha TL, Karlsson MC, Ravetch JV. FcgammaRIIB deficiency leads to autoimmunity and a defective response to apoptosis in Mrl–MpJ mice. J Immunol. 2008;180:5670–5679. doi: 10.4049/jimmunol.180.8.5670. [DOI] [PubMed] [Google Scholar]
- 17.Takai T, Ono M, Hikida M, Ohmori H, Ravetch JV. Augmented humoral and anaphylactic responses in Fc gamma RII-deficient mice. Nature. 1996;379:346–349. doi: 10.1038/379346a0. [DOI] [PubMed] [Google Scholar]
- 18.Yuasa T, Kubo S, Yoshino T, Ujike A, Matsumura K, Ono M, Ravetch JV, Takai T. Deletion of fc gamma receptor IIB renders H-2(b) mice susceptible to collagen-induced arthritis. J Exp Med. 1999;189:187–194. doi: 10.1084/jem.189.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobayashi N, Hong C, Klinman DM, Shirota H. Oligodeoxynucleotides expressing polyguanosine motifs promote antitumor activity through the upregulation of IL-2. J Immunol. 2013;190:1882–1889. doi: 10.4049/jimmunol.1201063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shirota Y, Shirota H, Klinman DM. Intratumoral injection of CpG oligonucleotides induces the differentiation and reduces the immunosuppressive activity of myeloid-derived suppressor cells. J Immunol. 2012;188:1592–1599. doi: 10.4049/jimmunol.1101304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito SE, Shirota H, Kasahara Y, Saijo K, Ishioka C. IL-4 blockade alters the tumor microenvironment and augments the response to cancer immunotherapy in a mouse model. Cancer Immunol Immunother CII. 2017;66:1485–1496. doi: 10.1007/s00262-017-2043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warren MK, Vogel SN. Bone marrow-derived macrophages: development and regulation of differentiation markers by colony-stimulating factor and interferons. J Immunol. 1985;134:982–989. [PubMed] [Google Scholar]
- 23.Benvenuto M, Mattera R, Masuelli L, Tresoldi I, Giganti MG, Frajese GV, Manzari V, Modesti A, Bei R. The crossroads between cancer immunity and autoimmunity: antibodies to self antigens. Front Biosci (Landmark Ed) 2017;22:1289–1329. doi: 10.2741/4545. [DOI] [PubMed] [Google Scholar]
- 24.Reuschenbach M, von Knebel Doeberitz M, Wentzensen N. A systematic review of humoral immune responses against tumor antigens. Cancer Immunol Immunother CII. 2009;58:1535–1544. doi: 10.1007/s00262-009-0733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaenker P, Gray ES, Ziman MR. Autoantibody production in cancer-the humoral immune response toward autologous antigens in cancer patients. Autoimmun Rev. 2016;15:477–483. doi: 10.1016/j.autrev.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 26.Shirota H, Klinman DM, Ito SE, Ito H, Kubo M, Ishioka C. IL4 from T follicular helper cells downregulates antitumor immunity. Cancer Immunol Res. 2017;5:61–71. doi: 10.1158/2326-6066.CIR-16-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moynihan KD, Opel CF, Szeto GL, Tzeng A, Zhu EF, Engreitz JM, Williams RT, Rakhra K, Zhang MH, Rothschilds AM, Kumari S, Kelly RL, Kwan BH, Abraham W, Hu K, Mehta NK, Kauke MJ, Suh H, Cochran JR, Lauffenburger DA, Wittrup KD, Irvine DJ. Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses. Nat Med. 2016;22:1402–1410. doi: 10.1038/nm.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pearce OM, Laubli H, Verhagen A, Secrest P, Zhang J, Varki NM, Crocker PR, Bui JD, Varki A. Inverse hormesis of cancer growth mediated by narrow ranges of tumor-directed antibodies. Proc Natl Acad Sci USA. 2014;111:5998–6003. doi: 10.1073/pnas.1209067111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gul N, van Egmond M. Antibody-dependent phagocytosis of tumor cells by macrophages: a potent effector mechanism of monoclonal antibody therapy of cancer. Cancer Res. 2015;75:5008–5013. doi: 10.1158/0008-5472.CAN-15-1330. [DOI] [PubMed] [Google Scholar]
- 30.Dahan R, Sega E, Engelhardt J, Selby M, Korman AJ, Ravetch JV. FcgammaRs modulate the anti-tumor activity of antibodies targeting the PD-1/PD-L1 axis. Cancer Cell. 2015;28:285–295. doi: 10.1016/j.ccell.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Roghanian A, Teige I, Martensson L, Cox KL, Kovacek M, Ljungars A, Mattson J, Sundberg A, Vaughan AT, Shah V, Smyth NR, Sheth B, Chan HT, Li ZC, Williams EL, Manfredi G, Oldham RJ, Mockridge CI, James SA, Dahal LN, Hussain K, Nilsson B, Verbeek JS, Juliusson G, Hansson M, Jerkeman M, Johnson PW, Davies A, Beers SA, Glennie MJ, Frendeus B, Cragg MS. Antagonistic human FcgammaRIIB (CD32B) antibodies have anti-tumor activity and overcome resistance to antibody therapy in vivo. Cancer Cell. 2015;27:473–488. doi: 10.1016/j.ccell.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 32.Takai T. Roles of Fc receptors in autoimmunity. Nat Rev Immunol. 2002;2:580–592. doi: 10.1038/nri856. [DOI] [PubMed] [Google Scholar]
- 33.van Montfoort N, t Hoen PA, Mangsbo SM, Camps MG, Boross P, Melief CJ, Ossendorp F, Verbeek JS. Fcgamma receptor IIB strongly regulates Fc gamma receptor-facilitated T cell activation by dendritic cells. J Immunol. 2012;189:92–101. doi: 10.4049/jimmunol.1103703. [DOI] [PubMed] [Google Scholar]
- 34.Muller AJ, Scherle PA. Targeting the mechanisms of tumoral immune tolerance with small-molecule inhibitors. Nat Rev Cancer. 2006;6:613–625. doi: 10.1038/nrc1929. [DOI] [PubMed] [Google Scholar]
- 35.Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8:1069–1086. doi: 10.1158/2159-8290.CD-18-0367. [DOI] [PubMed] [Google Scholar]
- 36.Williams EL, Tutt AL, Beers SA, French RR, Chan CH, Cox KL, Roghanian A, Penfold CA, Butts CL, Boross P, Verbeek JS, Cragg MS, Glennie MJ. Immunotherapy targeting inhibitory Fcγ receptor IIB (CD32b) in the mouse is limited by monoclonal antibody consumption and receptor internalization. J Immunol. 2013;19:4130–4140. doi: 10.4049/jimmunol.1301430. [DOI] [PubMed] [Google Scholar]
- 37.Daeron M, Latour S, Malbec O, Espinosa E, Pina P, Pasmans S, Fridman WH. The same tyrosine-based inhibition motif, in the intracytoplasmic domain of Fc gamma RIIB, regulates negatively BCR-, TCR-, and FcR-dependent cell activation. Immunity. 1995;3:635–646. doi: 10.1016/1074-7613(95)90134-5. [DOI] [PubMed] [Google Scholar]
- 38.Nimmerjahn F, Ravetch JV. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science (New York, N.Y.) 2005;310:1510–1512. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.