Abstract
Treatment strategies for patients with advanced solid tumors have traditionally been based on three different paradigms: surgery, cytotoxics (chemotherapy or radiation therapy) and targeted therapies. Immunotherapy has emerged as a novel treatment paradigm in our armamentarium. Unfortunately, most patients still do not benefit from immunotherapy. These patients often have “cold tumors” characterized by a paucity of effector T cells in the tumor microenvironment, low mutational load, low neoantigen burden and often an immunosuppressive tumor microenvironment. TIGIT is an immunoreceptor inhibitory checkpoint that has been implicated in tumor immunosurveillance. Expression of TIGIT has been demonstrated in both NK cells and T cells and plays a role in their activation and maturation. TIGIT competes with immunoactivator receptor CD226 (DNAM-1) for the same set of ligands: CD155 (PVR or poliovirus receptor) and CD112 (Nectin-2 or PVRL2). TIGIT’s role in tumor immunosurveillance is analogous to the PD-1/PD-L1 axis in tumor immunosuppression. Both TIGIT and PD-1 are upregulated in a variety of different cancers. Anti-TIGIT antibodies have demonstrated synergy with anti-PD-1/PD-L1 antibodies in pre-clinical models. Currently, there are multiple first-in-man phase I trials hoping to exploit this new pathway and improve response rates with existing immunotherapies.
Keywords: TIGIT, Immunotherapy, Immuno-oncology, Combination immunotherapy
Introduction
Treatment strategies for patients with advanced solid tumors have traditionally been based on three different paradigms: surgery, cytotoxics (chemotherapy or radiation therapy) and targeted therapies. Immunotherapy has emerged as a novel treatment paradigm in our armamentarium. At the Huntsman Cancer Institute, 38% of phase 1 trials are now immunotherapy studies. Following the initial success of checkpoint inhibitors in melanoma, indications for this class of drugs have expanded to different tumor types. At least six checkpoint inhibitors have been approved by the FDA for different indications including: melanoma, non-small cell lung cancer, squamous cell carcinoma of the head and neck, Hodgkin lymphoma, bladder cancer and Merkel cell carcinoma [1–6]. More recently, pembrolizumab became the first tissue-agnostic regulatory approval for patients with mismatch repair deficient advanced cancers that have exhausted standard of care [7]. Unfortunately, a vast majority of patients still do not benefit from immunotherapy. These patients often have “cold tumors” (MSI-low colorectal cancer, pancreatic cancer, sarcoma) characterized by a paucity of effector T cells in the tumor microenvironment, low mutational load, low neoantigen burden and often an immunosuppressive tumor microenvironment. Different strategies are undergoing clinical testing to expand indications of immunotherapy to patients with “cold” tumors. This includes a combination of checkpoint inhibitors with targeted therapies, vaccines, radiation or chemotherapy as well as T cell therapies. T cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domain (TIGIT) is an immunoreceptor inhibitory checkpoint that has been implicated in tumor immunosurveillance [8–11]. Anti-TIGIT antibodies have demonstrated synergy with anti-PD-1/PD-L1 antibodies in pre-clinical models [12–14]. Currently, there are multiple first-in-man phase I trials hoping to exploit this new pathway and improve response rates with existing immunotherapies. Here, we will review TIGIT pathway inhibitors undergoing clinical development as they represent an additional opportunity for combination therapy.
TIGIT pathway
TIGIT was first identified in 2009 as an immune checkpoint that inhibits activation of T cells and NK cells [11, 15, 16]. The TIGIT receptor has an IgV domain, a transmembrane domain and an immunoreceptor tyrosine-based domain [11]. TIGIT has been found to be upregulated in tumor antigen-specific CD8+ T-cells and CD8+ TILs involved in adaptive immune tumor surveillance [17]. TIGIT is also expressed on NK cells, binding of which inhibits NK cytotoxicity directly [16]. This downregulation of NK cell activity occurs through the phosphorylation of immunoreceptor tail tyrosine (ITT)-like domain of the cytoplasmic tail of TIGIT. The ITT-like domain is phosphorylated at Tyr225 and binds Grb2, recruiting SHIP1 to terminate PI3K and MAPK signaling in the NK cell [18]. NK cell maturation is dependent on the presence of the TIGIT receptor and essential for development of self-tolerance [19, 20].
TIGIT competes with immunoactivator receptor CD226 or DNAX accessory molecule-1 (DNAM-1) for the same set of ligands: CD155 (PVR or poliovirus receptor) and CD112 [Nectin-2 or poliovirus receptor-related 2 (PVRL2)] [8]. DNAM-1 is expressed on monocytes, T cells and NK cells [21]. In opposition to TIGIT’s immunosuppressive effects, DNAM-1 enhances cytotoxicity of T lymphocytes and NK cells [22]. DNAM-1’s interactions with PVR and Nectin-2 were found to enhance NK-mediated lysis of tumor cells. This was demonstrated by antibody mediated masking of receptors and its ligands [23]. Without the presence of PVR ligand, NK cells become hypo-responsive [19]. DNAM-1 binds PVR and likely provides positive costimulation to induce interferon-γ (IFN-γ) production [24]. Conversely, TIGIT binding of PVR suppresses IFN-γ production leading to downregulation of NK cells [25].
Multiple mechanisms have been demonstrated to explain TIGIT’s ability to suppress immune function. First described was TIGIT’s role in production of pro- and anti-inflammatory cytokines. Following TIGIT ligation, PVR is phosphorylated which modulates Erk activity and cytokine production via monocyte dendritic cells. This enhances production of IL-10 and diminishes release of IL12p40 [11, 24]. IL10 is an anti-inflammatory cytokine that contributes to T cell exhaustion and immunosuppression [11]. Knock down of TIGIT using RNA interference induced T cell proliferation and increased IFN-γ production [11, 24]. Blocking of TIGIT on cytokine-induced killer cells using an anti-TIGIT functional antibody increased proliferation and cytotoxic targeting of tumor cells expressing PVR and also increased IFN-γ, IL-6 and TNF-α production [8]. This was also demonstrated in siRNA knockdown of TIGIT on CD8+ T cells derived from AML patients which lead to a significant increase in TNF-α and IFN-γ production and decreased susceptibility to apoptosis of CD8+ T cells [26].
A second mechanism in which TIGIT inhibits immunosurveillance is through both competition and direct inhibition of DNAM-1 [13]. Before the discovery of TIGIT, it was known that DNAM-1 binds ligands PVR and Nectin [27, 28]. TIGIT was found to bind PVR with a higher affinity compared to DNAM-1; therefore, outcompeting with DNAM-1 for binding and inducing T-cell suppression [11]. Additionally, it was demonstrated that TIGIT directly interacts on the cell surface with DNAM-1, impairing its ability to homodimerize and thus activate immunosurveillance. TIGIT intrinsically may affect T-cell proliferation even without antigen and antigen presenting cells present. In mouse models, agonistic anti-TIGIT antibodies inhibited T cell proliferation in the absence of any other cell type [15].
TIGIT is expressed on a subset of Foxp3+ Treg cells in isolated human lymphocyte populations [11]. Foxp3 is essential for maturation of naïve T-cell development toward regulatory T cell phenotype [29]. It was demonstrated that TIGIT-deficient T-cells had lower Foxp3+ expression [30]. Additionally, TIGIT+ Tregs co-express an immunosuppressive gene signature including CTLA-4 and PD-1 [30]. Activation of TIGIT+ Treg cells through agonistic anti-TIGIT antibodies induces expression of fibrinogen-like protein 2 (FGL2). Neutralization of FGL2 lead to TIGIT+ Treg-cell suppressive abilities to similar levels as TIGIT− Treg cells. TIGIT- Treg cells, unlike TIGIT+ Treg cells, are able to suppress differentiation of Th2 cells; however, when FGL2 expression is lost; TIGIT+ Treg cells suppress differentiation of Th2 cells similar to TIGIT− Treg cells [30]. Therefore, TIGIT+ Treg cells shift the balance of activity away from proinflammatory Th1 and Th17 cells to Th2 cells via FGL2 allowing for specialized suppressive effects in inflammatory tissue. TIGIT activation in Tregs release a gene program to inactivate CD8+ T cells. In a murine model, mice with TIGIT deficient Tregs resulted in significantly delayed tumor growth compared to wild type Tregs, whereas mice with TIGIT deficient CD8+ T cells had similar tumor growth compared to wild type CD8+ T cells [9]. It was proposed that upregulation of IL-10 production by TIGIT+ Tregs and CD8+ T cells leads to the dysfunctional phenotype of CD8+ cytotoxic TILs [9].
CD96 is a member of the immunoglobulin gene superfamily and is an important receptor that allows adhesive interactions of NK and T cells in immune response [31, 32]. CD96 has been found to have similar immunosuppressant effects as TIGIT, binding to its ligand PVR with an affinity higher than DNAM-1 but weaker than TIGIT [11]. CD96’s effect on tumor surveillance was initially demonstrated in CD96−/− mice, which were found to have fewer lung metastases than DNAM-1 −/− mice injected with melanoma cell lines [33]. Furthermore, they demonstrated that TIGIT−/− knockout mice treated with anti-CD96 mAb also had reduced lung metastases compared to TIGIT−/− knockout alone. Like TIGIT, CD96 is found to be expressed on NK cells and T-cells in humans [32]. It was proposed that CD96/TIGIT/DNAM-1/PVR form a dynamic axis of inhibitory signals from TIGIT and CD96 opposing stimulatory signals from DNAM-1 [34].
TIGIT’s role in tumor biology
TIGIT and PD-1 were found to be upregulated in a 15-gene signature of multiple tumor-associated T cells [13]. Specifically, this was seen in colon, endometroid, breast and renal clear cell carcinoma. The correlation between TIGIT and PD-1 expression in tumor samples suggests both receptors are partners in inducing T cell exhaustion. Furthermore, upregulation of TIGIT and downregulation of DNAM-1 in CD8+ TILs was seen in advanced melanoma patients, and most of these cells co-expressed PD-1 [17]. PVR, a TIGIT ligand, was found to be strongly expressed on tumor cells in patients with cutaneous T cell lymphoma in parallel with TIGIT expression [35]. Additionally, it was found that DNAM-1 expression was decreased on NK cells and CD8+ cells in the peripheral blood of these patients suggesting the antagonistic nature of DNAM-1 and TIGIT in tumor suppression [35].
DNAM-1 appears to serve a role in immunosurveillance for cancer detection. Mice deficient with DNAM-1 had enhanced development of fibrosarcoma and papilloma when exposed to carcinogens [36]. PVR and Nectin-2 appear to serve as backup redundancy with each other as PVR-deficient mice demonstrated compensatory tumor surveillance using Nectin-2 binding [22]. Increased levels of soluble PVR were seen in serum samples from patients with lung, esophageal, gastric and pancreaticobiliary malignancies compared to healthy controls [37]. Counterintuitively, TIGIT was found to be the immunologic-related gene associated with the best relapse-free survival and overall survival in a retrospective study of triple-negative breast cancer [38]. Of note, they were unable to confirm this finding with other datasets.
TIGIT’s role in suppression of circulating T lymphocytes (CTL) was also demonstrated in melanoma. PVR was found to be constitutively expressed in melanoma cell lines. IFN-γ producing cells were found to have higher PD-1 and TIGIT expression and decreased DNAM-1 expression. Overexpression of PVR was found to significantly suppress CTL activation [10]. Furthermore, patients with metastatic melanoma expressed a higher TIGIT/CD226 ratio on Tregs in the tumor microenvironment compared to that seen in the peripheral blood [39]. Subgroup analysis of patients with the highest TIGIT/CD226 ratio that were treated with anti-PD1 and/or anti-CTLA-4 agents had a significantly worse PFS of 2 months versus 12 months (p = 0.039) [39]. Eleven AML patients who received allogenic stem cell transplants were found to have increased TIGIT expressed on CD8+ T cells compared to controls. A higher rate of primary refractory disease was seen in the high-TIGIT expressers [26].
Interestingly, TIGIT’s role in the tumor microenvironment may also be intertwined with the microbiome. Fusobacterium nucleatum, a bacterium indigenous to the oral cavity, was found to be inversely associated with CD3+ T-cell density in colorectal carcinoma tissue, suggesting a role in tumorigenesis [40]. F. nucleatum was found in humans to produce a protein, Fap2, that directly interacts with TIGIT, causing inhibition of NK cell cytotoxicity [41, 42]. Further establishing TIGIT’s role in colorectal cancer, PVR specifically has been found to be overexpressed in colorectal cancers [43].
Synergy of anti-TIGIT therapy with other immunotherapies
Despite the expanding indications for immunotherapy antibodies in the treatment of cancers, most patients still do not respond to these agents. Combination of immunotherapies using different mechanisms of actions are ongoing [44]. Most notably, in CheckMate-067, a randomized phase 3 study in patients with metastatic melanoma comparing nivolumab versus nivolumab plus ipilimumab versus ipilimumab, improved overall survival (OS) was seen in the combination arm compared to ipilimumab monotherapy [45]. However, improved progression-free or overall survival was not demonstrated between monotherapy nivolumab and combination nivolumab and ipilimumab arms (the study was not powered for statistical comparisons between these two arms). Early data from a variety of phase 1 studies testing IDO inhibitors in combination with checkpoint inhibitors suggest that the combination may increase response rates when compared to historical controls [46].
In preclinical trials, anti-TIGIT candidate drug OMP-313M32 demonstrated a statistically significant reduction of tumor volume in human melanoma PDX in humanized NOD scid gamma (NSG) mice [47]. The poliovirus receptor-related immunoglobulin (PVRIG) is a recently discovered inhibitory immune checkpoint expressed on the surface of T and NK cells [48]. In syngeneic tumor models, COM701, a PVRIG inhibitor, increased effector CD8 T cell activation and synergized with PD-1 and TIGIT inhibitor in an in vivo model leading to tumor growth inhibition and increased survival [12].
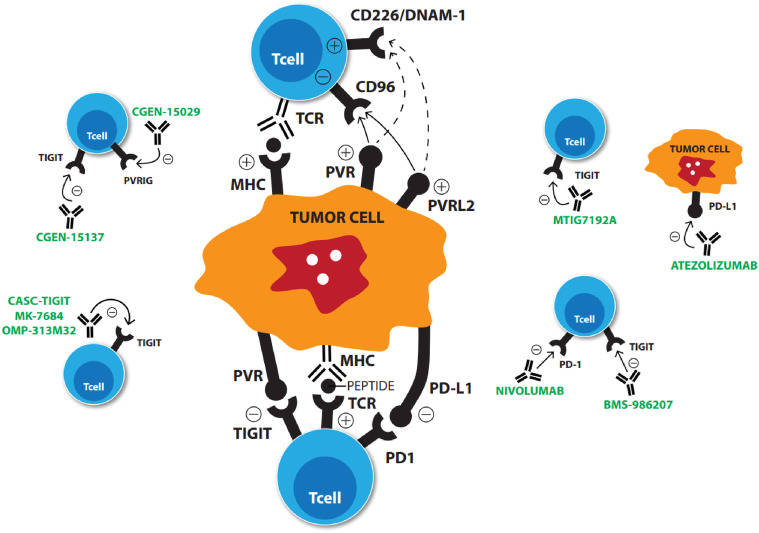
Inhibitors used in combination, as discussed above, may increase response rates with anti-TIGIT therapies (Fig. 1). Increased expression of TIGIT and PD-1 has been demonstrated in human non-small cell lung cancer and advanced melanoma-associated CD8+ T cells suggesting a role for synergy [13, 17]. In harvested human metastatic melanoma tumors, addition of anti-TIGIT and anti-PD-1 blocking antibodies resulted in increased proliferation of CD8+ TILs with increased degranulation of these cells as compared to those treated with anti-TIGIT or anti-PD1 antibodies alone [17]. Synergy of anti-TIGIT agents has also been demonstrated with anti-PD-L1 antibodies. Mice treated with CT26 cell lines, analogous to human aggressive, undifferentiated, refractory human colorectal carcinoma, showed response to combination anti-TIGIT and PD-L1 inhibitors. This response was not demonstrated with monotherapy of either agent [13]. In melanoma cell lines, TILs had slightly enhanced activation by addition of anti-TIGIT and anti-PD-L1 antibodies, also suggesting synergy [10]. OMP-313R12, a novel TIGIT antibody, induced tumor growth inhibition in a murine colon carcinoma model (CT26 WT) [47]. Combination OMP-313R12 and anti-PD-L1 had statistically significant improved overall survival in mice models as compared to controls [14]. First-in-man clinical trials with anti-TIGIT either alone or in combination with other immunotherapies are ongoing (Table 1).
Fig. 1.
Mechanism of action of ongoing pre-clinical and phase I trial drugs
Table 1.
Ongoing clinical trials in August 2018
| Trial sponsor | Agent | Clinicaltrials.gov identifier | Estimated enrollment | Type of trial | Combination immunotherapy |
|---|---|---|---|---|---|
| Genentech | Anti-TIGIT monoclonal antibody (MTIG7192A) | NCT02794571 | 300 | Phase I | PD-L1 (atezolizumab) |
| Bristol-Myers Squibb | Anti-TIGIT monoclonal antibody (BMS-986207) | NCT02913313 | 170 | Phase I/II | PD-1 (nivolumab) |
| OncoMed pharmaceuticals | Anti-TIGIT monoclonal antibody (OMP-313M32) | NCT03119428 | 30 | Phase I | N/A |
| Merck | Anti-TIGIT monoclonal antibody (MK-7684) | NCT02964013 | 240 | Phase 1 | N/A |
| Arcus biosciences | Anti-TIGIT monoclonal antibody (AB154) | NCT03628677 | 42 | Phase 1 | PD-1 (AB122) |
| Compugen [49] | Anti-TIGIT antibody (CGEN-15137) | N/A | N/A | Pre-clinical | Anti-PVRIG antibody (CGEN-15029) |
| Cascadian therapeutics [50] | Anti-TIGIT antibody (CASC-TIGIT) | N/A | N/A | Pre-clinical | N/A |
Anticipated adverse events
In pre-clinical models of TIGIT knock out 2e2 TCR transgenic mice, which are known to develop spontaneous autoimmune optic neuritis, all developed experimental autoimmune encephalomyelitis [15]. However, this contrasts with CTLA-4-deficient mice who universally develop fatal lymphoproliferative disease [51]. Preclinical toxicology studies across different TIGIT molecules have shown excellent safety profiles with no toxicities that can be directly attributed to the TIGIT antibody (unpublished data).
Translation of TIGIT to the clinic
Current clinical trials using TIGIT inhibitors alone or in combination with anti-PD-1/PD-L1 therapy are all phase I designed to determine dosing and safety (Table 1). Once we have phase I data of these agents, planning for phase II trials will need to target specific cancers that have the highest likelihood of response. Tumors with higher mutational burden and have subsequently demonstrated response to PD-1/PD-L1 inhibitors (i.e., melanoma, NSCLC, bladder) would be natural targets [52]. Increased expression of TIGIT and synergy between anti-TIGIT and anti-PD-1/PD-L1 antibodies has already been shown in preclinical models of melanoma, NSCLC and colon cancer [10, 13, 17]. As discussed above, colon cancer’s association with the bacterium F. nucleatum and its interaction with TIGIT make it a potential target for anti-TIGIT therapies as well [42, 43]. However, one study looking at human cancers found that some of the highest TIGIT expression was in tumors not associated with response to anti-PD-1/PD-L1 therapy including triple-negative breast cancer, T-cell lymphoma and cervical cancer suggesting we may need to consider a broader range of tumor targets [49].
Potential target populations for TIGIT therapy would be those who failed PD-1/PD-L1 monotherapy in which these agents are already approved. One may hypothesize that anti-TIGIT antibodies would be dependent on presence of significant levels of TILs; therefore, targeting MSI/MMR “hot” tumors that do not respond or lose response to PD-1/PD-L1 may be most efficacious. Approximately, one-third of patients with MMR-high mutations do not respond to anti-PD-1 therapy [53]. The hope would be for a synergistic effect like that seen in the CheckMate-067 trial combining anti-PD-1 and anti-CTLA-4 antibodies where improved response rates in advanced melanoma was demonstrated [45]. However, recent negative data from the ECHO-301/KEYNOTE-252 trial, a randomized phase 3 trial with nivolumab and epacadostat (IDO inhibitor) in metastatic melanoma suggests that perhaps a better understanding of immunotherapy combinations is needed before launching large randomized trials [54]. The role of TIGIT inhibition in “cold” tumors will depend on its ability to potentially overcome T cell exclusion.
Useful biomarkers for predicting response to therapy will be of paramount importance especially as we conduct trials with multiple immunotherapies where we anticipate increased immune-mediated adverse events. Current clinically available biomarkers in the case of checkpoint inhibitors have been unsatisfactory. The most commonly used biomarker, PD-L1 by IHC, has been inconsistent with tumor response to these therapies [55]. TIGIT expression or a potential gene signature predictive of TIGIT driven immunosupppresion could be useful marker of disease that is refractory to standard cytotoxic agents for targeting patients for anti-TIGIT therapies. In AML patients, TIGIT expression in circulating CD8+ T cells correlated with a higher rate of primary refractory disease to cytotoxic therapy [26].
Optimal biologic dosing of targeted therapies such as checkpoint inhibitors does not necessarily correlate with maximum-tolerated dosing (MTD) as it is often the case with cytotoxic agents [56]. Therefore, correlative biomarkers of biologic activity may be more useful than MTD for determining dosing of checkpoint inhibitors. A proposed mechanism of PD-1 inhibitor resistance is through mutations in interferon pathway genes such as JAK1 and JAK2 [57]. Ongoing clinical trials are evaluating an 18-gene signature of IFN-γ pathway activation as a predictor of response to PD-1 inhibition [58]. Given IFN-γ is an indirect marker of TIGIT and PVR binding, IFN-γ concentration could be a good marker of biologic activity of anti-TIGIT agents [25]. Additionally, murine models have shown that qRT-PCR analysis of known markers of CD4+/CD8+ T cell and NK cell activity (CD3e, CD8a, NCR1, IFN-γ, GZMA, CD226) in both the tumor and whole blood showed dose-dependent correlation with anti-TIGIT antibody and would be informative exploratory correlatives in human trials [49].
Whether anti-TIGIT monotherapy results in anti-tumor activity in humans is currently unknown. Pending results of ongoing phase I trials may help elucidate if monotherapy should be attempted in larger phase II trials. In pre-clinical murine models, anti-TIGIT monotherapy has shown independent activity [14, 47]. Furthermore, anti-TIGIT monotherapy has a dose-dependent association with decrease in tumor size and infiltration and activation of CD8+ and CD4+ T cells in the tumor microenvironment [49]. The safety profile of monotherapy compared to dual therapy with anti-PD-1/PD-L1 inhibitors from ongoing phase I trials will also determine if dual therapy should be evaluated in larger phase II trials.
Conclusion
Immunotherapy has rapidly revolutionized the cancer treatment landscape across different tumor types. Although some immune-related toxicities could be potentially life-threatening if not identified early, the toxicity profile of immunotherapy is generally better than the mainstay of cytotoxic agents. In addition, immunotherapy has offered unprecedented long-term responses in advanced and metastatic disease. However, even among sensitive tumor types, a large group of patients will not respond to these therapies. TIGIT has demonstrated great promise in preclinical models as a new target for cancer immunotherapy and potentially may work synergistically to expand the activity of approved checkpoint inhibitors. Synergy of anti-TIGIT and anti-PD1/PD-L1 antibodies has been demonstrated in in vitro and mice models. Ongoing first-in-man phase I trials will help answer if TIGIT has a place in the next generation of immunotherapy.
Acknowledgements
Jonathan Martinez provided the original illustration for this manuscript.
Abbreviations
- FGL2
Fibrinogen-like protein 2
- ITT
Immunoreceptor tail tyrosine
- MTD
Maximum-tolerated dosing
- PVR
Poliovirus receptor
- PVRL2
Poliovirus receptor-related 2
- TIGIT
T cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domain
Author contributions
BLS was primary author of the manuscript. IG-L aided with additional content and editing.
Funding
No relevant funding.
Compliance with ethical standards
Conflict of interest
The authors declare they have no conflicts of interest.
References
- 1.Eggermont AMM, Chiarion-Sileni V, Grob J-J, Dummer R, Wolchok JD, Schmidt H, Hamid O, Robert C, Ascierto PA, Richards JM, Lebbé C, Ferraresi V, Smylie M, Weber JS, Maio M, Bastholt L, Mortier L, Thomas L, Tahir S, Hauschild A, Hassel JC, Hodi FS, Taitt C, de Pril V, de Schaetzen G, Suciu S, Testori A. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016;375(19):1845–1855. doi: 10.1056/NEJMoa1611299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhäufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crinò L, Blumenschein GRJ, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferris RL, Blumenschein GJ, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, Worden F, Saba NF, Iglesias Docampo LC, Haddad R, Rordorf T, Kiyota N, Tahara M, Monga M, Lynch M, Geese WJ, Kopit J, Shaw JW, Gillison ML. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ, Rodig SJ, Chapuy B, Ligon AH, Zhu L, Grosso JF, Kim SY, Timmerman JM, Shipp MA, Armand P. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee J-L, Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK, Necchi A, Gerritsen W, Gurney H, Quinn DI, Culine S, Sternberg CN, Mai Y, Poehlein CH, Perini RF, Bajorin DF. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nghiem PT, Bhatia S, Lipson EJ, Kudchadkar RR, Miller NJ, Annamalai L, Berry S, Chartash EK, Daud A, Fling SP, Friedlander PA, Kluger HM, Kohrt HE, Lundgren L, Margolin K, Mitchell A, Olencki T, Pardoll DM, Reddy SA, Shantha EM, Sharfman WH, Sharon E, Shemanski LR, Shinohara MM, Sunshine JC, Taube JM, Thompson JA, Townson SM, Yearley JH, Topalian SL, Cheever MA. PD-1 blockade with pembrolizumab in advanced merkel-cell carcinoma. N Engl J Med. 2016;374(26):2542–2552. doi: 10.1056/NEJMoa1603702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drugs@FDA pembrolizumab. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125514s024lbl.pdf. Accessed 7 Oct 2017
- 8.Zhang B, Zhao W, Li H, Chen Y, Tian H, Li L, Zhang L, Gao C, Zheng J. Immunoreceptor TIGIT inhibits the cytotoxicity of human cytokine-induced killer cells by interacting with CD155. Cancer Immunol Immunother. 2016;65(3):305–314. doi: 10.1007/s00262-016-1799-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurtulus S, Sakuishi K, Ngiow S-F, Joller N, Tan DJ, Teng MWL, Smyth MJ, Kuchroo VK, Anderson AC. TIGIT predominantly regulates the immune response via regulatory T cells. J Clin Investig. 2015;125(11):4053–4062. doi: 10.1172/JCI81187. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Inozume T, Yaguchi T, Furuta J, Harada K, Kawakami Y, Shimada S. Melanoma cells control antimelanoma CTL responses via interaction between TIGIT and CD155 in the effector phase. J Investig Dermatol. 2016;136(1):255–263. doi: 10.1038/JID.2015.404. [DOI] [PubMed] [Google Scholar]
- 11.Yu X, Harden K, Gonzalez LC, Francesco M, Chiang E, Irving B, Tom I, Ivelja S, Refino CJ, Clark H, Eaton D, Grogan JL. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol. 2009;10(1):48–57. doi: 10.1038/ni.1674. [DOI] [PubMed] [Google Scholar]
- 12.Levy Ofer, Chan Chris, Cojocaru Gady, Liang Spencer, Ophir Eran, Ganguly Sudipto, Kotturi Maya, Friedman Tal, Murter Benjamin, Dassa Liat, Leung Ling, Greenwald Shirley, Azulay Meir, Kumar Sandeep, Alteber Zoya, Pan Xiaoyu, Drake Andy, Salomon Ran, Machlenkin Arthur, Hunter John, Levine Zurit, Pardoll Drew, White Mark. Abstract 581: Discovery and development of COM701, a therapeutic antibody targeting the novel immune checkpoint PVRIG. Cancer Research. 2017;77(13 Supplement):581–581. doi: 10.1158/1538-7445.AM2017-581. [DOI] [Google Scholar]
- 13.Johnston RJ, Comps-Agrar L, Hackney J, Yu X, Huseni M, Yang Y, Park S, Javinal V, Chiu H, Irving B, Eaton DL, Grogan JL. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell. 2014;26(6):923–937. doi: 10.1016/j.ccell.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 14.Srivastava Minu K., Yun Rui, Mayes Erin, Yu Janice, Jie Hyun-Bae, Axelrod Fumiko, Xie Ming-Hong, Monteon Jorge, Lam Andrew, Ji May, Liu Yuwang, Lewicki John, Hoey Tim, Gurney Austin, Park Angie Inkyung. Abstract 2612: Anti-Tigit induces T cell mediated anti-tumor immune response and combines with immune checkpoint inhibitors to enhance strong and long term anti-tumor immunity. Cancer Research. 2017;77(13 Supplement):2612–2612. doi: 10.1158/1538-7445.AM2017-2612. [DOI] [Google Scholar]
- 15.Joller N, Hafler JP, Brynedal B, Kassam N, Spoerl S, Levin SD, Sharpe AH, Kuchroo VK. Cutting edge: TIGIT has T cell-intrinsic inhibitory functions. J Immunol. 2011;186(3):1338–1342. doi: 10.4049/jimmunol.1003081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stanietsky N, Simic H, Arapovic J, Toporik A, Levy O, Novik A, Levine Z, Beiman M, Dassa L, Achdout H, Stern-Ginossar N, Tsukerman P, Jonjic S, Mandelboim O. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc Natl Acad Sci USA. 2009;106(42):17858–17863. doi: 10.1073/pnas.0903474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chauvin JM, Pagliano O, Fourcade J, Sun Z, Wang H, Sander C, Kirkwood JM, Chen TH, Maurer M, Korman AJ, Zarour HM. TIGIT and PD-1 impair tumor antigen-specific CD8(+) T cells in melanoma patients. J Clin Investig. 2015;125(5):2046–2058. doi: 10.1172/JCI80445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu S, Zhang H, Li M, Hu D, Li C, Ge B, Jin B, Fan Z. Recruitment of Grb2 and SHIP1 by the ITT-like motif of TIGIT suppresses granule polarization and cytotoxicity of NK cells. Cell Death Differ. 2013;20(3):456–464. doi: 10.1038/cdd.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He Y, Peng H, Sun R, Wei H, Ljunggren HG, Yokoyama WM, Tian Z. Contribution of inhibitory receptor TIGIT to NK cell education. J Autoimmun. 2017;81:1–12. doi: 10.1016/j.jaut.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Bi J, Zhang Q, Liang D, Xiong L, Wei H, Sun R, Tian Z. T-cell Ig and ITIM domain regulates natural killer cell activation in murine acute viral hepatitis. Hepatology. 2014;59(5):1715–1725. doi: 10.1002/hep.26968. [DOI] [PubMed] [Google Scholar]
- 21.Shibuya A, Campbell D, Hannum C, Yssel H, Franz-Bacon K, McClanahan T, Kitamura T, Nicholl J, Sutherland GR, Lanier LL, Phillips JH. DNAM-1, a novel adhesion molecule involved in the cytolytic function of T lymphocytes. Immunity. 1996;4(6):573–581. doi: 10.1016/S1074-7613(00)70060-4. [DOI] [PubMed] [Google Scholar]
- 22.Nagumo Y, Iguchi-Manaka A, Yamashita-Kanemaru Y, Abe F, Bernhardt G, Shibuya A, Shibuya K. Increased CD112 expression in methylcholanthrene-induced tumors in CD155-deficient mice. PLoS One. 2014;9(11):e112415. doi: 10.1371/journal.pone.0112415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pende D, Bottino C, Castriconi R, Cantoni C, Marcenaro S, Rivera P, Spaggiari GM, Dondero A, Carnemolla B, Reymond N, Mingari MC, Lopez M, Moretta L, Moretta A. PVR (CD155) and Nectin-2 (CD112) as ligands of the human DNAM-1 (CD226) activating receptor: involvement in tumor cell lysis. Mol Immunol. 2005;42(4):463–469. doi: 10.1016/j.molimm.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 24.Lozano E, Dominguez-Villar M, Kuchroo V, Hafler DA. The TIGIT/CD226 axis regulates human T cell function. J Immunol. 2012;188(8):3869–3875. doi: 10.4049/jimmunol.1103627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li M, Xia P, Du Y, Liu S, Huang G, Chen J, Zhang H, Hou N, Cheng X, Zhou L, Li P, Yang X, Fan Z. T-cell immunoglobulin and ITIM domain (TIGIT) receptor/poliovirus receptor (PVR) ligand engagement suppresses interferon-gamma production of natural killer cells via beta-arrestin 2-mediated negative signaling. J Biol Chem. 2014;289(25):17647–17657. doi: 10.1074/jbc.M114.572420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kong Y, Zhu L, Schell TD, Zhang J, Claxton DF, Ehmann WC, Rybka WB, George MR, Zeng H, Zheng H. T-cell immunoglobulin and ITIM domain (TIGIT) associates with CD8 + T-cell exhaustion and poor clinical outcome in AML patients. Clin Cancer Res. 2016;22(12):3057–3066. doi: 10.1158/1078-0432.CCR-15-2626. [DOI] [PubMed] [Google Scholar]
- 27.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 28.Joller N, Lozano E, Burkett Patrick R, Patel B, Xiao S, Zhu C, Xia J, Tan Tze G, Sefik E, Yajnik V, Sharpe Arlene H, Quintana Francisco J, Mathis D, Benoist C, Hafler David A, Kuchroo Vijay K. Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity. 2014;40(4):569–581. doi: 10.1016/j.immuni.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tahara-Hanaoka S, Shibuya K, Onoda Y, Zhang H, Yamazaki S, Miyamoto A, Honda S, Lanier LL, Shibuya A. Functional characterization of DNAM-1 (CD226) interaction with its ligands PVR (CD155) and nectin-2 (PRR-2/CD112) Int Immunol. 2004;16(4):533–538. doi: 10.1093/intimm/dxh059. [DOI] [PubMed] [Google Scholar]
- 30.Bottino C, Castriconi R, Pende D, Rivera P, Nanni M, Carnemolla B, Cantoni C, Grassi J, Marcenaro S, Reymond N, Vitale M, Moretta L, Lopez M, Moretta A. Identification of PVR (CD155) and Nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J Exp Med. 2003;198(4):557–567. doi: 10.1084/jem.20030788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fuchs A, Cella M, Giurisato E, Shaw AS, Colonna M. Cutting edge: CD96 (Tactile) promotes NK cell-target cell adhesion by interacting with the poliovirus receptor (CD155) J Immunol. 2004;172(7):3994–3998. doi: 10.4049/jimmunol.172.7.3994. [DOI] [PubMed] [Google Scholar]
- 32.Wang PL, O’Farrell S, Clayberger C, Krensky AM. Identification and molecular cloning of tactile. A novel human T cell activation antigen that is a member of the Ig gene superfamily. J Immunol. 1992;148(8):2600–2608. [PubMed] [Google Scholar]
- 33.Chan CJ, Martinet L, Gilfillan S, Souza-Fonseca-Guimaraes F, Chow MT, Town L, Ritchie DS, Colonna M, Andrews DM, Smyth MJ. The receptors CD96 and CD226 oppose each other in the regulation of natural killer cell functions. Nat Immunol. 2014;15:431. doi: 10.1038/ni.2850. [DOI] [PubMed] [Google Scholar]
- 34.Blake SJ, Dougall WC, Miles JJ, Teng MW, Smyth MJ. Molecular pathways: targeting CD96 and TIGIT for cancer immunotherapy. Clin Cancer Res. 2016;22(21):5183–5188. doi: 10.1158/1078-0432.CCR-16-0933. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi N, Sugaya M, Suga H, Oka T, Kawaguchi M, Miyagaki T, Fujita H, Inozume T, Sato S. Increased soluble CD226 in sera of patients with cutaneous T-cell lymphoma mediates cytotoxic activity against tumor cells via CD155. J Investig Dermatol. 2017;137(8):1766–1773. doi: 10.1016/j.jid.2017.03.025. [DOI] [PubMed] [Google Scholar]
- 36.Iguchi-Manaka A, Kai H, Yamashita Y, Shibata K, Tahara-Hanaoka S, Honda S, Yasui T, Kikutani H, Shibuya K, Shibuya A. Accelerated tumor growth in mice deficient in DNAM-1 receptor. J Exp Med. 2008;205(13):2959–2964. doi: 10.1084/jem.20081611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iguchi-Manaka A, Okumura G, Kojima H, Cho Y, Hirochika R, Bando H, Sato T, Yoshikawa H, Hara H, Shibuya A, Shibuya K. Increased soluble CD155 in the serum of cancer patients. PLoS One. 2016;11(4):e0152982. doi: 10.1371/journal.pone.0152982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinez-Canales S, Cifuentes F, Lopez De Rodas Gregorio M, Serrano-Oviedo L, Galan-Moya EM, Amir E, Pandiella A, Gyorffy B, Ocana A. Transcriptomic immunologic signature associated with favorable clinical outcome in basal-like breast tumors. PLoS One. 2017;12(5):e0175128. doi: 10.1371/journal.pone.0175128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fourcade J, Sun Z, Chauvin J-M, Ka M, Davar D, Pagliano O, Wang H, Saada S, Menna C, Amin R, Sander C, Kirkwood JM, Korman AJ, Zarour HM. CD226 opposes TIGIT to disrupt Tregs in melanoma. JCI Insight. 2018 doi: 10.1172/jci.insight.121157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mima K, Sukawa Y, Nishihara R, Qian ZR, Yamauchi M, Inamura K, Kim SA, Masuda A, Nowak JA, Nosho K, Kostic AD, Giannakis M, Watanabe H, Bullman S, Milner DA, Harris CC, Giovannucci E, Garraway LA, Freeman GJ, Dranoff G, Chan AT, Garrett WS, Huttenhower C, Fuchs CS, Ogino S. Fusobacterium nucleatum and T cells in colorectal carcinoma. JAMA Oncol. 2015;1(5):653–661. doi: 10.1001/jamaoncol.2015.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mima K, Cao Y, Chan AT, Qian ZR, Nowak JA, Masugi Y, Shi Y, Song M, da Silva A, Gu M, Li W, Hamada T, Kosumi K, Hanyuda A, Liu L, Kostic AD, Giannakis M, Bullman S, Brennan CA, Milner DA, Baba H, Garraway LA, Meyerhardt JA, Garrett WS, Huttenhower C, Meyerson M, Giovannucci EL, Fuchs CS, Nishihara R, Ogino S. Fusobacterium nucleatum in colorectal carcinoma tissue according to tumor location. Clin Transl Gastroenterol. 2016;7(11):e200. doi: 10.1038/ctg.2016.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gur C, Ibrahim Y, Isaacson B, Yamin R, Abed J, Gamliel M, Enk J, Bar-On Y, Stanietsky-Kaynan N, Coppenhagen-Glazer S, Shussman N, Almogy G, Cuapio A, Hofer E, Mevorach D, Tabib A, Ortenberg R, Markel G, Miklic K, Jonjic S, Brennan CA, Garrett WS, Bachrach G, Mandelboim O. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity. 2015;42(2):344–355. doi: 10.1016/j.immuni.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Masson D, Jarry A, Baury B, Blanchardie P, Laboisse C, Lustenberger P, Denis MG. Overexpression of the CD155 gene in human colorectal carcinoma. Gut. 2001;49(2):236–240. doi: 10.1136/gut.49.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168(4):707–723. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, Wolchok JD. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zakharia Yousef, McWilliams Robert, Shaheen Monaster, Grossman Kenneth, Drabick Joseph, Milhem Mohammed, Rixie Olivier, Khleif Samir, Lott Ryan, Kennedy Eugene, Munn David, Vahanian Nicholas, Link Charles. Abstract CT117: Interim analysis of the Phase 2 clinical trial of the IDO pathway inhibitor indoximod in combination with pembrolizumab for patients with advanced melanoma. Cancer Research. 2017;77(13 Supplement):CT117–CT117. doi: 10.1158/1538-7445.AM2017-CT117. [DOI] [Google Scholar]
- 47.Park Angie Inkyung, Srivastava Minu, Mayes Erin, Jie Hyun-Bae, Yun Rui, Murriel Christopher, Xie Ming-hong, Lam Andrew, Ji May, Axelrod Fumiko, Monteon Jorge, Lewicki John, Hoey Tim, Gurney Austin. Abstract 2003: Antibody against TIGIT (T cell immunoreceptor with Ig and ITIM domains) induces anti-tumor immune response and generates long-term immune memory. Cancer Research. 2017;77(13 Supplement):2003–2003. doi: 10.1158/1538-7445.AM2017-2003. [DOI] [Google Scholar]
- 48.Zhu Y, Paniccia A, Schulick AC, Chen W, Koenig MR, Byers JT, Yao S, Bevers S, Edil BH. Identification of CD112R as a novel checkpoint for human T cells. J Exp Med. 2016;213(2):167–176. doi: 10.1084/jem.20150785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cattaruzza Fiore, Yeung Pete, Wang Min, Brunner Alayne, Scolan Erwan Le, Cain Jennifer, Argast Gretechen, O'Young Gilbert, Liu YuWang, Cancilla Belinda, Gurney Austin, Hoey Tim, Lewicki John, Kapoun Ann. Abstract 599: Pharmacodynamic biomarkers for anti-TIGIT treatment and prevalence of TIGIT expression in multiple solid tumor types. Cancer Research. 2017;77(13 Supplement):599–599. doi: 10.1158/1538-7445.AM2017-599. [DOI] [Google Scholar]
- 50.Piasecki Julia C., Brasel Kenneth, Rosler Robert, Klucher Kevin M., Peterson Scott R. Abstract 578: Discovery and characterization of novel antagonistic antibodies that bind with high affinity to human, cynomolgus, and murine TIGIT, an immune checkpoint receptor. Cancer Research. 2017;77(13 Supplement):578–578. doi: 10.1158/1538-7445.AM2017-578. [DOI] [Google Scholar]
- 51.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3(5):541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 52.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SAJR, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A, Børresen-Dale A-L, Boyault S, Burkhardt B, Butler AP, Caldas C, Davies HR, Desmedt C, Eils R, Eyfjörd JE, Foekens JA, Greaves M, Hosoda F, Hutter B, Ilicic T, Imbeaud S, Imielinsk M, Jäger N, Jones DTW, Jones D, Knappskog S, Kool M, Lakhani SR, López-Otín C, Martin S, Munshi NC, Nakamura H, Northcott PA, Pajic M, Papaemmanuil E, Paradiso A, Pearson JV, Puente XS, Raine K, Ramakrishna M, Richardson AL, Richter J, Rosenstiel P, Schlesner M, Schumacher TN, Span PN, Teague JW, Totoki Y, Tutt ANJ, Valdés-Mas R, van Buuren MM, van ’Veer L, Vincent-Salomon A, Waddell N, Yates LR, Australian Pancreatic Cancer Genome I. Consortium IBC, Consortium IM-S, PedBrain I, Zucman-Rossi J, Futreal PA, McDermott U, Lichter P, Meyerson M, Grimmond SM, Siebert R, Campo E, Shibata T, Pfister SM, Campbell PJ, Stratton MR. Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LAJ. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hamid O, Gajewski TF, Frankel AE et al (2017) Epacadostat plus pembrolizumab in patients with advanced melanoma: phase 1 and 2 efficacy and safety results from ECHO-202/ KEYNOTE-037. In: Proceedings from the 2017 ESMO congress; September 8–12, Madrid, Spain. (Abstract 1214O)
- 55.Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016;17(12):e542–e551. doi: 10.1016/S1470-2045(16)30406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sachs JR, Mayawala K, Gadamsetty S, Kang SP, de Alwis DP. Optimal dosing for targeted therapies in oncology: drug development cases leading by example. Clin Cancer Res. 2016;22(6):1318–1324. doi: 10.1158/1078-0432.ccr-15-1295. [DOI] [PubMed] [Google Scholar]
- 57.Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, Torrejon DY, Abril-Rodriguez G, Sandoval S, Barthly L, Saco J, Homet Moreno B, Mezzadra R, Chmielowski B, Ruchalski K, Shintaku IP, Sanchez PJ, Puig-Saus C, Cherry G, Seja E, Kong X, Pang J, Berent-Maoz B, Comin-Anduix B, Graeber TG, Tumeh PC, Schumacher TNM, Lo RS, Ribas A. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med. 2016;375(9):819–829. doi: 10.1056/NEJMoa1604958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ayers M, Lunceford J, Nebozhyn M, Murphy E, Loboda A, Kaufman DR, Albright A, Cheng JD, Kang SP, Shankaran V, Piha-Paul SA, Yearley J, Seiwert TY, Ribas A, McClanahan TK. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Investig. 2017;127(8):2930–2940. doi: 10.1172/JCI91190. [DOI] [PMC free article] [PubMed] [Google Scholar]