Abstract
Since the first bone marrow transplantation, adoptive T cell therapy (ACT) has developed over the last 80 years to a highly efficient and specific therapy for infections and cancer. Genetic engineering of T cells with antigen-specific receptors now provides the possibility of generating highly defined and efficacious T cell products. The high sensitivity of engineered T cells towards their targets, however, also bears the risk of severe off-target toxicities. Therefore, different safety strategies for engineered T cells have been developed that enable removal of the transferred cells in case of adverse events, control of T cell activity or improvement of target selectivity. Receptor avidity is a crucial component in the balance between safety and efficacy of T cell products. In clinical trials, T cells equipped with high avidity T cell receptor (TCR)/chimeric antigen receptor (CAR) have been mostly used so far because of their faster and better response to antigen recognition. However, over-activation can trigger T cell exhaustion/death as well as side effects due to excessive cytokine production. Low avidity T cells, on the other hand, are less susceptible to over-activation and could possess better selectivity in case of tumor antigens shared with healthy tissues, but complete tumor eradication may not be guaranteed. In this review we describe how ‘optimal’ TCR/CAR affinity can increase the safety/efficacy balance of engineered T cells, and discuss simultaneous or sequential infusion of high and low avidity receptors as further options for efficacious but safe T cell therapy.
Keywords: CAR, TCR avidity, Safeguards, T cell engineering
Clinical advancement in adoptive cell therapy
Adoptive cell therapy (ACT) refers to the use of immune cells for the treatment of human diseases. Immune cells are retrieved from a patient or a healthy donor (autologous or allogeneic transplantation) and usually manipulated ex vivo for antigen-specific selection, expansion and/or introduction of a specific T cell receptor (TCR) or chimeric antigen receptor (CAR) before re-infusion into the patient. According to the extent of such manipulations, we can broadly classify the different ACT approaches that already reached the clinic into two categories, based on the use of naturally occurring or genetically engineered immune cells.
Naturally occurring, ‘physiological’ T cells
Hematopoietic stem cell transplantation (HSCT) is the first example of ACT, with the first bone marrow transfusion reported in 1939 [1]. Currently, 45,000–60,000 HSCTs are performed worldwide every year [2]. Human leukocyte antigen (HLA)-matched transplants occur only in 20–30% of cases and, despite a safe profile, a high probability of disease relapse has been reported [3]. The use of alternative stem cell sources in absence of perfect HLA-matched donors (HLA-mismatched, haploidentical and umbilical cord blood transplantation) dramatically increased the percentage of HSCT treated patients and intrinsically provided a good graft-versus-tumor (GvT) effect. However, donor alloreactive T cells triggering GvT are also mainly responsible for graft-versus-host disease (GvHD), representing a major threat for HSCT patients. To separate these two effects is an important challenge for future improvements [4, 5].
Besides GvHD, the onset of life-threatening infections is a second main safety issue in HSCT, especially when HSCT is combined with donor T cell depletion to reduce the risk of GvHD. The time needed for the transplanted stem cells to restore a functional immune system generates a temporal window during which the patient is strongly immunosuppressed. One promising option to provide immune protection during this time is the direct infusion of purified virus-specific T cells [6], for example isolated by peptide-major histocompatibility complex (pMHC) multimer staining [7]. Since conventional, non-reversible pMHC multimer staining can trigger TCR activation, we developed the StrepTamer technology for the isolation of minimally manipulated, pMHC multimer-specific T cells with complete preservation of the initial differentiation and functional status [8]. In a recent clinical trial, we demonstrated that transfer of cytomegalovirus (CMV)-specific T cells isolated from CMV seropositive donors via StrepTamer technology exerts protection from CMV infection in immunosuppressed patients following allogeneic HSCT [9]. Parallel clinical trials corroborated the safety and the clinical benefits of virus-specific T cell infusions also for Epstein–Barr-Virus and adenovirus [10–12]. However, the approach is still limited to the setting of HCST (when patients are usually lymphopenic anyway) and the availability of detectable antigen-specific T cell populations from antigen-experienced donors.
The use of naturally occurring antigen-specific T cells provided interesting clinical benefits also in cancer therapy. Infusion of lymphocytes from marrow donors induced complete remission in patients with hematological relapse after HSCT [13]. In metastatic melanoma patients, tumor infiltrating lymphocyte (TIL) therapy, pioneered by Rosenberg and colleagues in 1987 [14], showed astonishing objective response rates since the first clinical studies [15–17], with a protocol for short-term ex vivo expansion of TILs immediately applied worldwide [18–20]. Remarkably, despite differences in the pre-conditioning regimens of patients and in the TIL expansion protocols, these clinical trials reported consistent outcomes: a dropout rate and an objective response of approx. 38% and 40% of all treated patients, respectively, with approx. 9% of complete response on average [18, 19, 21]. Besides toxicity coming from non-myeloablative chemotherapy and high-dose bolus IL-2, treatment was usually well tolerated. In addition, TIL treatment did not induce any of the severe toxicities reported in melanoma patients infused with engineered T cells expressing a high-avidity TCR against melanoma antigens (i.e. Melan-A/MART1 or gp100) [22], despite the fact that CD8+ T cells reactive to these antigens were frequently found in the TIL fraction. These results invigorated further investigations of TIL treatment in other tumor settings. Objective responses were observed in metastatic ovarian and breast cancers but the results are still too preliminary to be conclusive [23, 24].
Engineered T cells
The strong protective activity demonstrated with naturally occurring antigen-specific T cells indicates high potency and efficacy of ACT. However, transfer of virus-specific T cells is limited by their low frequencies in seronegative donors and the availability of seropositive donors. In TIL therapy, the dropout rate is still high and applications in tumor subsets other than melanoma are at their infancy (tumor accessibility is also often limited); in addition, TILs are of unknown composition in terms of TCR specificity and functionality with often unpredictable clinical outcome. To make ACT broadly applicable, the use of engineered T cells offers two main advantages. First, generation of off-the-shelf products can be envisioned; second, TCRs/CARs can be pre-clinically well-characterized and T cell products with highly defined functionality can be produced. In this approach, (currently usually autologous) T cells are genetically modified ex vivo to express either a specific TCR or a CAR against a desired antigen, and are finally re-infused into the patient.
Since the conceptualization and first clinical success of CARs in 1989 and 2008 [25, 26], respectively, more than 1290 clinical trials with CAR-T cells have been conducted worldwide. Striking results were reported in patients with relapsing, non-responding CD19+ B cell malignancies for which CD19 CAR-T cell treatment showed up to 90% complete remission [27]. This culminated in the first US Food and Drug Administration approval of a genetically engineered cell product for the treatment of a human disease [28]. The success of CD19 CARs is also attributable to the great quality of the CD19 molecule as a target, since CD19 is widely expressed on tumor cells at high levels and is a B cell linage-specific marker. In line with this elitist expression pattern, B cell aplasia represents the main long-term on-target off-tumor side effect of CD19 CAR-T cell therapy, which can be managed by intravenous administration of immunoglobulins, similarly to B cell deficiencies due to CD19 mutations [29]. Cytokine-release syndrome (CRS) and CAR-related encephalopathy syndrome (CRES) are, instead, two acute side effects commonly observed in clinical trials with CD19 CARs and have already been extensively revised [30–32]. Briefly, CRS is a form of systemic inflammation which develops within a few days after cell infusion (1–6 days on average with > 95% of CRS events occurring within 12 days) and is driven by a storm of cytokines produced by activated CAR-T cells and host immune cells; CRES usually manifests within 1 month with brain tissue inflammation, edema and sometimes even necrosis [33]. In most cases early therapeutic intervention with corticosteroids and/or the anti-IL-6 receptor antibody Tocilizumab, according to the grade of side effects, can mitigate CRS and CRES [34]. Interestingly, in a dose-escalating clinical study at the Fred Hutchinson Cancer Research Center, six of seven patients with grade ≥ 4 CRES, with occurrence of lethal events, were treated with a dose of CAR-T cells later established to be higher than the maximum tolerated dose [33]. In the same study, anti-tumor responses were achieved with lower CAR-T cells doses, which led to the speculation that the most severe toxicities could be avoided in future applications by the use of appropriate T cell amounts.
CAR-T cell therapy was not that successful in solid tumors so far. First, the tumor microenvironment is often highly immunosuppressive and thereby limits T cell function. To overcome this issue, CAR-T cells have been engineered in an increasingly sophisticated way, e.g. via knock-out of the inhibitory receptor PD-1 [35] or by additional expression of anti-inflammatory cytokines or chemokines for T cell recruitment at the tumor site [36, 37]. Although these new generation CARs already showed better anti-tumor efficacy in pre-clinical models, these studies have not investigated the potential for long-term adverse effects. Loss-of-function of PD-1 in T cells, for example, increases the risk of lymphomagenesis [38], highlighting how carefully protein engineering should be carried on. Second, severe, sometimes fatal, toxicities followed ACT with CARs targeting human epidermal growth factor receptor 2 (HER2), carbonic anhydrase IX (CAIX) and carcinoembryonic antigen-related cell adhesion molecule 5 (CEACAM5) [39–41], commonly imputable to on-target off-tumor effects due to the high affinity of CARs. Whether therapeutic efficacy without toxicity can be achieved for target antigens with off-tumor expression is still an open question.
Similar results have been observed also in ACT with TCR-engineered T cells. Half of the patients treated with high-avidity MART-1 specific TCRs showed severe toxicity due to the disruption of Melan-1-expressing melanocytes in skin, ear and eye [22]. Similarly, Zhong and colleagues demonstrated how anti-tumor activity and ocular autoimmunity positively correlate and that both increase according to TCR avidity [42]. On the other hand, targeting the cancer-testis antigen NY-ESO-1 with T cells expressing an affinity-enhanced TCR showed objective clinical responses in melanoma and sarcoma patients without appreciable toxicity [43]. Some of these inconsistencies could be explained by differences in expression and distribution of self-antigens in tumor and normal tissues. However, unpredictable severe adverse events occurred as well, in particular with affinity-enhanced TCRs, due to cross-reactivity towards different epitopes. Fatal neurotoxicity and cardiotoxicity were reported in TCR-based therapies directed against the cancer-testis antigen MAGE-A3 due to unspecific recognition of MAGE-A12 and Titin-derived epitopes, respectively [44, 45].
Taken together, lessons from clinical trials are that a ‘therapeutic window’ for ACTs with engineered T cells exists, but the line between efficacy and toxicity can be very thin and often hard to predict for individual patients. Thus, engineered T cells need to be more controllable in temporal, spatial and functional dimensions and the development of safety strategies is at least as urgent as the development of more functional T cell products.
Safety strategies for ACT with engineered T cells
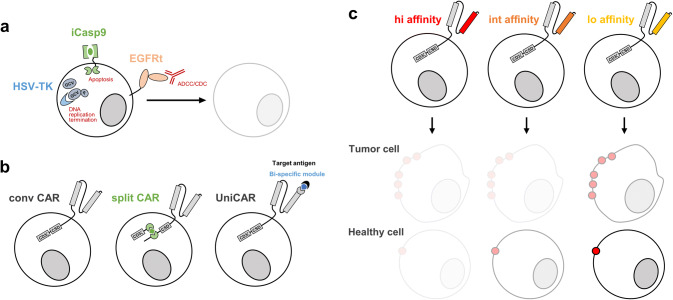
Immunotherapy with engineered T cells is often accompanied by mild to severe toxicities. First safety mechanisms aimed at the eradication of the transferred cells as well as the development of synthetic pathways for fine tuning T cell activation (Fig. 1a, b). A third, more recently introduced option is to use antigen-specific receptors with ‘optimal’ (rather than highest possible) avidity, meaning an avidity that represents the best compromise between efficacy and safety (Fig. 1c).
Fig. 1.
Safety strategies for ACT with engineered T cells. Safety strategies can be categorized into killing of transferred cells (a), tuning of engineered T cell activity (b) or improving selectivity of target recognition (c). a HSV-TK, iCas9 and EGFRt are three examples of safety switches leading to depletion of transferred cells by, respectively, DNA replication termination, apoptosis and ADCC/CDC. b Compared to conventional CARs, split CARs are activated only upon small molecule-induced dimerization of the antigen binding domain and intracellular signaling component, whereas UniCARs can be adapted to target any antigen since recognition is driven by bi-specific modules. c High avidity CARs can effectively target tumor cells, but may also recognize healthy cells with low target antigen expression; low avidity CARs may be less efficient in tumor cell killing, but spare healthy tissues
Selective depletion of adoptively transferred engineered cells
An efficient safety strategy to intervene in case of adverse effects is to specifically ‘kill’ the transferred T cells (Fig. 1a). This was first achieved by the introduction of a so-called ‘suicide gene’. The herpes simplex virus thymidine kinase (HSV-TK) was the first clinically tested suicide gene [46]. It efficiently ablates cycling cells by converting the non-toxic prodrug ganciclovir into a toxic antimetabolite that interferes with DNA replication [47]. However, due to immunogenicity of the viral TK, TK-expressing T cells can be prematurely rejected [48], greatly limiting its applications in the clinic [49, 50]. Inducible caspase 9 (iCasp9), on the other hand, is non-immunogenic [51]. iCasp9 is composed of the intracellular domain of the human pro-apoptotic Cas9 protein and a human FK506 binding protein which dimerize upon administration of the small molecule AP1903, leading to activation of the mitochondrial apoptotic pathway. A single dose of the dimerizing molecule in patients who developed GvHD after engineered T cells infusion was able to rapidly induce apoptosis of the transferred cells and thus terminate GvHD [51].
Antibody-dependent depleting mechanisms are based on the recognition of selective surface markers on engineered T cells by clinically approved monoclonal antibodies, with consequent initiation of antibody-dependent cellular cytotoxicity (ADCC) or complement-dependent cytotoxicity (CDC). The truncated epidermal growth factor receptor (EGFRt) is the most promising marker. It contains the EGFR extracellular and transmembrane domains, including the binding site for the monoclonal antibody Cetuximab, but lacks its intracellular domain in order to prevent any signaling activation upon antibody binding [52]. We recently demonstrated in syngeneic mouse models that Cetuximab administration is able to eradicate CD19 CAR-T cells and to rescue from B cell aplasia [53]. The clinical benefits of this suicide marker are now under investigation in several CAR-T cell clinical trials, targeting CD19 (NCT02028455), CD171 (NCT02311621) and CD123 (NCT02159495).
Cetuximab could, however, lead to off-target effects since the expression of non-truncated EGFR is ubiquitous and EGFR is upregulated in inflamed tissues. A novel approach could be the use of a second CAR-T cell (anti-CAR CAR-T cell) able to target therapeutically transferred CAR-T cells through the recognition of an additional tag introduced into the primary CAR construct. The approach seems worth to be investigated also in light of the potentially higher tissue infiltration of the anti-CAR CAR-T cells compared to an antibody. Preliminary data generated in our laboratory showed successful B cell rescue in mouse models treated with a murine, Strep-tagged CD19 CAR upon administration of a second anti-Strep-tag CAR without tumor relapse (unpublished data). For the above-mentioned strategies, reverting long-term B cell aplasia in patients with stable leukemia remission after CD19 CAR therapy could represent an immediate clinical application.
Tuning of engineered T cell activity
The efficient and permanent ablation of the infused ACT product in case of adverse events leads to non-reversible and often premature termination of the therapy. Positive regulation and fine-tuning of T cell activation represents an alternative strategy for avoiding this issue (Fig. 1b).
In the synthetic splitting receptor approach, engineered T cell activity is regulated by the administration of small molecules; thereby, the timing and power of T cell activity can be pharmacologically controlled. To achieve this, the antigen binding and intracellular signaling components are separated and each fused to an additional module, but heterodimerize upon small molecule administration. One example is the FKBP-FRB module, that assembles in the presence of the rapamycin analog AP21967. This small molecule could efficiently control the activity of CAR-T cells expressing the FKBP-FRB module in vivo and, more importantly, the magnitude of the response was dependent on the dosage of the heterodimerizing molecule [54].
The combination of CARs and bispecific target modules is an alternative design to regulate CAR-T cell activity. In this case, the CAR cannot bind directly to the cognate antigen and the engineered T cells are thus inactive. Only the presence of a bispecific molecule—specific for both the CAR and the target antigen—makes the CAR-T cell functional. The ‘universal’ CAR platform technology [55] is one example for the described approach. So-called UniCARs can be armed against different targets by changing the bispecific target module. Following this idea, engineered human T cells with a UniCAR were redirected against the acute myeloid leukemia (AML) antigens CD33 and CD123, showing how it is possible to target different antigens subsequently as well as simultaneously [55]. This can enhance the efficacy of the therapy and reduce the risk for development of antigen-loss tumor variants under treatment.
The activities of engineered T cells can also be controlled by inhibitory strategies. The Sadelain laboratory developed CTLA-4 and PD1-based inhibitory CARs (iCARs) to reduce off-target responses during immunotherapy [56]. When the iCAR recognizes its antigen expressed on normal cells, cytokine secretion, cytotoxicity and proliferation induced through the TCR or CAR of the T cell can be selectively limited. Thus, the iCAR can inhibit the activating TCRs or CARs in an antigen-restricted manner and allows with this for the discrimination between target and off-target cells. Furthermore, it was shown that this inhibition is only temporary and reversible, maintaining the function of most of the engineered T cells for therapy [56].
Finally, the introduction of the TCR/CAR transgene into the endogenous TCR locus [57, 58, 59] could be considered as a novel approach for the fine-tuning of T cell activation. TCR downregulation is a natural inhibitory feedback loop preventing overt T cell activation and still, T cells with a CAR or TCR placed in the endogenous locus have proven to be highly effective in pre-clinical tumor models [57, 58, 59]. It will be exciting to use these new technologies in order to delineate if natural receptor regulation may also prove beneficial in terms of safety.
Improve the specificity of target recognition
The safety of ACT can be improved by increasing the tumor selectivity of the engineered T cells. This is particularly important since most tumor antigens are not exclusively expressed on tumor cells but also shared by host cells, leading to on-target off-tumor toxicities (Fig. 1c).
An approach to render engineered T cells specific for a tumor even in the absence of truly tumor specific antigens is by combinatorial antigen recognition. T cells are engineered with two different tumor-specific CARs providing either the activation or the costimulatory signal. Thus, engineered T cells are fully activated only by dual-antigen expressing malignant cells but not by single-antigen expressing normal cells. This combinatorial antigen recognition was successfully tested in different mouse models. Kloss and colleagues engineered T cells with two CARs specific for the prostate stem cell antigen (PSCA) and the prostate specific membrane antigen (PSMA); T cells co-transduced with both CARs efficiently killed PC3 prostate tumor lines expressing both antigens sparing tumor cells expressing either antigen alone [60]. In a different model, T cells with two CARs directed against mesothelin and folate receptor showed only weak activity against single positive tumors [61].
In addition, Kim and colleagues recently provided a new vision of how to improve target selectivity, by converting the canonical myeloid CD33 marker into a leukemia specific antigen. With CD33 being expressed also on normal myeloid cells, AML patients treated with CD33 CARs experienced severe myelosuppression. The ablation of CD33 in the bone marrow transplants of the patients generated chimera of CD33+ and CD33− myeloid cells. CD33− stem cells resisted to CD33 CAR-T cell therapy and were able to regenerate a functional myeloid compartment [62].
Finally, modulation of TCR/CAR avidity also represents an approach to enhance targeting specificity (Fig. 1c). Target antigens are normally expressed at higher levels in tumor cells than in healthy tissues; in turn, TCRs/CARs with lower avidities should recognize and kill effectively tumor cells sparing low-antigen expressing normal cells. This could be even more crucial for CARs because of their extremely high affinities, with the soluble single-chain variable fragments (scFv) being derived from antibodies. Recently, an increasing number of studies using CARs with low affinity scFvs confirmed this hypothesis [63–66]. On the other hand, the use of a low-affinity Herceptin-based CAR showed a safer profile in sarcoma patients but also only modest clinical activity [67]. It will be challenging to define optimal avidity for each specific clinical setting.
How to manage acute toxicity
Among the different safety approaches, to our knowledge currently only iCas9 and EGFRt are under investigation in clinical settings, whereas the general number of clinical trials with CAR-T cells is increasing fast. In addition, at least for iCas9, complete clearance of the transferred cells upon suicide gene activation requires a few days. Especially for acute toxicities like CRS and CRES, it is not clear yet whether these in vivo depletion kinetics are sufficient to turn around life-threatening conditions mediated by engineered T cells.
CRS and CRES occurr particularly often in clinical trials with CD19 CAR-T cells. Retrospective analyses of data coming from these clinical trials identified risk factors associated to CRS/CRES onset [32] (i.e. tumor burden, CAR-T cell dose and pre-existing endothelial activation) and, more interestingly, a signature of clinical variables which enables to identify patients with high risk of developing severe toxicities [33]. A more informed and earlier intervention based on such risk profiles will likely help in increasing the safety profile of engineered T cell therapy. In addition, molecular mechanisms behind CRS/CRES were recently unraveled attributing monocyte/macrophage-expressed IL-6 and IL-1 the pivotal role in the onset of these side effects [68, 69]. This evidence is further supported by the clinical efficacy of the anti-IL-6 receptor antibody Tocilizumab. The two studies for the first time modeled CRS in preclinical mouse models [68, 69]. Remarkably, the group of Bondanza studied direct interactions between human CAR-T cells and human immune system in a novel humanized mouse model, with efficient human CD34+ hematopoietic stem cell engraftment and human immune system reconstitution [68, 70]. Further establishment of preclinical models for the study of ACT-related toxicities will be of high importance to the field.
T cell receptor avidity
For all of the aforementioned safety problems, the immune system has developed natural solutions to find the ‘right’ balance between robust protective immune responses on the one side, and lack of autoimmunity and immunopathology on the other side. Natural T cell responses are composed of polyclonal repertoires with the majority of TCRs having low avidity against their target antigen. Therefore, exploration of low avidity and/or polyclonal transgenic receptor cell therapies may contribute to finding the optimal balance between therapeutic efficacy and safety.
In natural T cells, the TCR has a profound impact on antigen-specificity, functionality, and even phenotypic and proliferative fate. Key determinant is the affinity of the TCR to its cognate pMHC. As presence of co-receptors and TCR expression levels can influence the quality of TCR-pMHC binding, the overall receptor-ligand interaction is best described by the term ‘structural avidity’. Therefore, we here speak of ‘CAR avidity’ when CAR-T cells are considered, although affinity of scFv can be precisely measured. Antigen sensitivity of T cells is often described as ‘functional avidity’ and can be determined by antigen concentration-dependent effector functions (e.g. cytokine release and/or target cell lysis). The structural avidity (i.e. kD = kon-rate/koff-rate of the antigen receptor–ligand interaction) can be accurately quantified by biophysical assays such as surface plasmon resonance, which is however time-consuming and laborious (sometimes even impossible for certain TCRs) as it makes recombinant expression of both TCR and pMHC necessary [71]. As particularly the koff-rate seems to determine the overall structural avidity of TRCs, assays have been developed that enable fast, but also precise and reproducible measurements of TCR-pMHC koff-rates [72, 73]. TCR koff-rates have proven to not only correlate with functional avidity, but also indicate the likelihood of in vivo protectivity [72, 74], even if the exact relationship of these parameters is still unclear due to the low number of individual TCRs that have so far been investigated side-by-side.
Upon recall infection, selective expansion of T cells with high avidity TCRs occurs [75, 76] and during primary infection, high avidity T cells dominate at the acute phase of the infection [77]. TCR avidity-dependent repertoire evolution is less well studied in tumor settings, but high avidity TCRs also seem to mediate particularly good anti-tumor protection [42]. Taken together, these findings could generally speak for the therapeutic usage of high avidity receptors—TCR or CAR—for adoptive immunotherapy.
However, vast evidence actually exists that the highest avidity receptors do not necessarily represent the best receptors in terms of functionality, and particularly—as introduced above—also in terms of safety. Kalergis and colleagues pointed out nearly 20 years ago that an ‘optimal’ (rather than ultra-long) dwell-time results in efficient T cell activation [78] and many other studies that followed also support a kinetic proof-reading model of T cell activation with limited signaling that predicts an optimal dissociation time for maximum T cell activation [79].
Unique characteristics of low avidity receptors
Low avidity TCRs have an important role in antigen-specific immune responses [80, 81]. Zehn and colleagues showed that T cells with low avidity TCRs even lead the very early phase (day 4 after infection) of the immune response while high avidity T cells are still being primed in the lymph node before they dominate the immune response shortly afterwards [77]. TCR avidity has also been shown to modulate CD4+ and CD8+ T cell differentiation [82] in a probabilistic manner [83], with low avidity preferentially leading to central memory precursor T cells (TCMp) generation at least in relative numbers [83, 84].
Two important facets of immune responses derived from T cells with TCRs of heterogenous avidities are therefore that, first, high and low avidity T cells may be of distinct importance during immune responses over time, and second, that differential TCR avidity leads to phenotypic diversity. Obviously, the phenotypic state of T cells in turn again determines their role during recall or ongoing chronic infections. Phenotypic imprinting through low avidity receptor-ligand interaction may have very beneficial consequences for therapeutic T cell products in certain clinical settings. During chronic antigen exposure, for example, low avidity T cells have been reported to be less susceptible to exhaustion and therefore showed improved maintenance of T cell responses [85, 86]. TCR avidity-dependent repertoire evolution during chronic antigen exposure is surprisingly poorly understood, and low avidity T cells may regain importance particularly when high avidity T cells are driven into exhaustion or replicative senescence [87].
Furthermore, an avidity increase to very high levels may actually deteriorate, rather than enhance, in vivo performance. It has been shown for TCRs with avidities beyond a certain threshold that T cell functionality at best plateaus or deteriorates again [42, 79, 88]. TCRs with an overly high avidity are usually affinity-enhanced, ‘supraphysiological’ TCRs [88]. To what extent high-avidity/low-functionality TCRs are present in physiological TCR repertoires remains to be investigated. For CAR-T cells already 14 years ago it was shown by the Abken group that very high scFv affinity does not improve T cell activation, but instead decreases selectivity against cells expressing the target HER2 at low levels [89]. Furthermore, CAR-T cells targeting CD20 with overly high avidity were later shown to proliferate less well than avidity-reduced CAR-T cells targeting the same antigen, at least in part due to more activation-induced cell death upon high avidity receptor-ligand interaction [90].
An important caveat is that many pre-clinical studies used (1) unphysiologically high numbers of T cells for in vivo experiments, (2) T cells from transgenic mice or virally transduced human PBMCs expressing TCRs or CARs constitutively from extrinsic promotors, and (3) usually only compare a very limited number of receptors (mostly two). In the future, a vast spectrum of receptors specific for the same antigen (best re-expressed in a physiological manner [57, 58]) needs to be investigated. Furthermore, sophisticated techniques to monitor avidity-dependent TCR repertoire evolution of endogenous populations ex vivo are urgently needed, and T cell re-transfer experiments will need to more truthfully recapitulate physiological TCR repertoires.
Importantly, low avidity receptors may render cell products safer. The aforementioned decreased selectivity of targets with increasing avidity becomes a serious problem when self-antigens are targeted, with on-target off-tumor toxicities being the main obstacle to clinical success of ACT. Preclinical studies support the concept that low-avidity TCR/CAR are highly selective in the killing of only tumor cells with higher antigen expression. Few clinical studies, enrolling an equally low number of patients, investigated the therapeutic efficacy of low-avidity engineered T cells so far, reporting on a safer profile but also a weak anti-tumor response [67]. These data further highlight the difficulty of finding ‘optimum avidity’ receptors. What exactly the ‘optimum’ avidity level is may thereby depend significantly on the type of antigen targeted (i.e. self or non-self antigen), and the antigen load.
‘Optimum’ avidity receptors for future cell therapies
If one antigen-specific receptor is being used, in terms of efficacy the avidity should be high enough for proper T cell activation and effector function, but not too high so that activation-induced cell death and an exhausted or too differentiated phenotype are circumvented. Avidity maturation through selection of high avidity TCRs e.g. upon recall infection can provide a hint which avidity level is most suitable for protection. Of note, this ‘optimum’ avidity should have stood the test of millions of years of co-evolution with pathogens. As the human immune system has co-evolved with pathogens rather than cancer, it is an intriguing question to what extent this natural selection of ‘optimum’ TCRs can be translated from the infection to the cancer setting. For CARs, even less guidance on which avidity will be best can be provided through natural selection mechanisms, as scFv affinity/CAR avidity will differ significantly from native antibody affinity/B cell avidity. As CAR-T cells also do not undergo somatic hypermutation, their in vivo population dynamics will be better represented by natural T cells that also do not change the TCR, but are selected in an evolutionary process within the antigen-specific response.
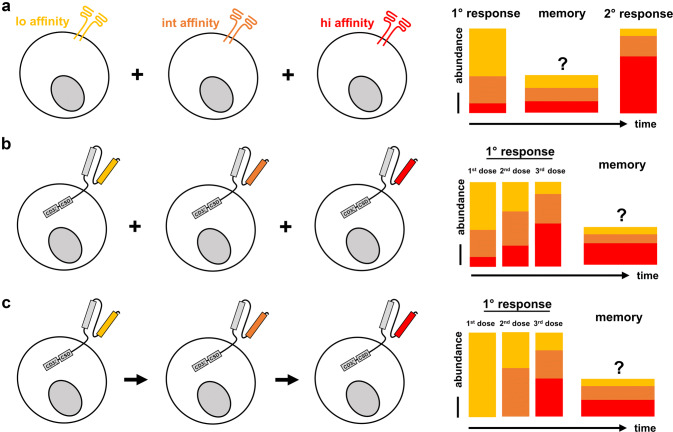
It is teleologically appealing that there should be a good reason why natural T cell responses are composed of heterogeneous TCR repertoires spanning a wide range of avidities for a given antigen (Fig. 2a). The use of multiple receptors with different avidities either sequentially or simultaneously may therefore also constitute an important consideration for the setting of ACT with transgenic receptors (Fig. 2b, c). Simultaneous transfer of CARs with different avidities, for example, could reduce initial antigen load through relative domination of low avidity receptors—but with every dose or after resting memory, high avidity receptors would be selectively expanded and ensure robust tumor control (Fig. 2b). In absolute numbers, a high avidity TCR-pMHC interaction is likely to lead to more TCMp cells due to the positive effect on the expansion of T cells in general, and re-call responses robustly enrich for high avidity T cells. Still, a low avidity TCR-pMHC interaction can induce functional memory [77]. Excitingly, this approach could kill two birds with one stone. It is attractive to speculate that with CD19 CARs particularly the high antigen load through healthy B cells is an important contributor to the high frequency of CRS. In this regard, low avidity CD19 CARs may be able to reduce the initial antigen load without induction of strong cytokine storms. This feature of low avidity CARs enhances safety through e.g. milder killing kinetics and should be differentiated from improved safety through better selectivity when antigens are targeted that are also present on healthy cells at low expression levels. ACT with receptors of different avidities could therefore constitute a balanced approach between protectivity and safety. Consistent with that is, as one example, the evidence that TIL therapy is based on the use of polyclonal T cell populations and was not associated with any of the severe and lethal toxicities reported in patients treated with single high-avidity melanoma antigen-specific engineered T cells.
Fig. 2.
Strategies of using antigen-specific receptors with different avidities for immunotherapy. a Natural T cell responses are polyclonal and mediated by TCRs of different avidities; while low avidity TCRs contribute to primary responses and generate T cell memory, selective expansion of high avidity TCRs leads to ‘avidity maturation’ of the population as a whole. High avidity TCRs, thus, dominate and ensure protection during recall responses. b Simultaneous application of CARs with different avidities could ‘re-build’ natural TCR repertoires. The infusion of a T cell product with higher doses of low avidity receptors may reduce the initial antigen load with less cytokine release, whereas enrichment of high avidity CARs over time would ensure robust protection and tumor clearance. c CARs with different avidities may be also applied sequentially with a similar effect, but may be easier to implement than the approach presented in (b)
Due to the powerful proliferative capacity of weakly differentiated T cells [91], it may prove difficult to titrate the amount of high avidity T cells, so that both engraftment of high avidity T cells and domination of low avidity T cells is ensured at the same time. The same problem may prevent that a ‘balanced’ tumor response (mild initial reaction, strict later control) could be copied by application of different dosages of the same avidity receptor. As an alternative to the simultaneous transfer, T cells equipped with transgenic receptors with different avidities may therefore also be applied sequentially (Fig. 2c). By this, initial antigen reduction could be provided by low avidity receptors only, and higher avidity receptors would be supplied afterwards to ensure initial remission and maintenance of tumor control.
Future perspectives
Clinical results from TIL and CD19 CAR-T cell therapies showed how powerful the immune system can be in fighting human disorders. The same power, however, can lead to dramatically severe toxicities. With engineered T cells, the equilibrium between efficacy and toxicity seems to mainly depend on the quality of the target and the TCR/CAR construct. Unfortunately, the number of disease-specific antigens, and in turn of antigen-specific receptors available for cell therapy, is still limited. Thus, first priority is the discovery of new targets, in particular in the promising field of neo-antigens. In parallel, increasing the safety profile of the already available TCR/CAR is crucial. We propose that TCRs/CARs with optimal avidity, and simultaneous or sequential use high- and low-avidity receptors, could provide the ideal balance between efficacy and safety.
Abbreviations
- ADCC
Antibody-dependent cellular cytotoxicity
- CDC
Complement-dependent cytotoxicity
- CRES
CAR-related encephalopathy syndrome
- CRS
Cytokine-release syndrome
- EGFRt
Truncated epidermal growth factor receptor
- GvT
Graft-versus-tumor
- HSV-TK
Herpes simplex virus thymidine kinase
- iCAR
Inhibitory chimeric antigen receptor
- iCasp9
Inducible caspase 9
- pMHC
Peptide-major histocompatibility complex
- TCMp
Central memory precursor T cells
Author contribution
All authors contributed to the writing and to the revisions of the manuscript. They all approved the final version.
Funding
This work was supported by the Deutsche Forschungsgemeinschaft (SFB1321/TP17)
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Osgoog Aplastic anemia treated with daily transfusion and intravenous marrow; case report. Ann Intern Med. 1939;13:357. doi: 10.7326/0003-4819-13-2-357. [DOI] [Google Scholar]
- 2.Passweg JR, Baldomero H, Bader P, et al. Hematopoietic stem cell transplantation in Europe 2014: more than 40,000 transplants annually. Bone Marrow Transplant. 2016 doi: 10.1038/bmt.2016.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrett AJ, Battiwalla M. Relapse after allogeneic stem cell transplantation. Hematol: Expert Rev; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi SW, Reddy P. Current and emerging strategies for the prevention of graft-versus-host disease. Nat Rev Clin Oncol. 2014;11:536–547. doi: 10.1038/nrclinonc.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Remberger M, Mattsson J, Hentschke P, et al. The graft-versus-leukaemia effect in haemotopoietic stem cell transplantation using unrelated donors. Bone Marrow Transplant. 2002;30:761–768. doi: 10.1038/sj.bmt.1703735. [DOI] [PubMed] [Google Scholar]
- 6.Riddell, Watanabe K, Goodrich J, et al. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science (80-) 1992;257:238–241. doi: 10.1126/science.1352912. [DOI] [PubMed] [Google Scholar]
- 7.Cobbold M, Khan N, Pourgheysari B, et al. Adoptive transfer of cytomegalovirus-specific CTL to stem cell transplant patients after selection by HLA–peptide tetramers. J Exp Med. 2005 doi: 10.1084/jem.20040613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knabel M, Franz TJ, Schiemann M, et al. Reversible MHC multimer staining for functional isolation of T-cell populations and effective adoptive transfer. Nat Med. 2002 doi: 10.1038/nm0602-631. [DOI] [PubMed] [Google Scholar]
- 9.Neuenhahn M, Albrecht J, Odendahl M, et al. Transfer of minimally manipulated CMV-specific T cells from stem cell or third-party donors to treat CMV infection after allo-HSCT. Leukemia. 2017 doi: 10.1038/leu.2017.16. [DOI] [PubMed] [Google Scholar]
- 10.Rooney CM, Ng CYC, Loftin S, et al. Use of gene-modified virus-specific T lymphocytes to control Epstein–Barr-virus-related lymphoproliferation. Lancet. 1995 doi: 10.1016/S0140-6736(95)91150-2. [DOI] [PubMed] [Google Scholar]
- 11.Doubrovina E, Oflaz-Sozmen B, Prockop SE, et al. Adoptive immunotherapy with unselected or EBV-specific T cells for biopsy-proven EBV + lymphomas after allogeneic hematopoietic cell transplantation. Blood. 2012;119:2644–2656. doi: 10.1182/blood-2011-08-371971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tzannou I, Leen AM. Preventing stem cell transplantation-associated viral infections using T-cell therapy. Immunotherapy. 2015;7:793–810. doi: 10.2217/imt.15.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolb HJ, Mittermüller J, Clemm C, et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood. 1990;76:2462–2465. doi: 10.1182/blood.V76.12.2462.2462. [DOI] [PubMed] [Google Scholar]
- 14.Spiess PJ, Yang JC, Rosenberg SA. In vivo antitumor activity of tumor-infiltrating lymphocytes expanded in recombinant interleukin-2. J Natl Cancer Inst. 1987;79:1067–1075. [PubMed] [Google Scholar]
- 15.Topalian SL, Solomon D, Avis FP, et al. Immunotherapy of patients with advanced cancer using tumor-infiltrating lymphocytes and recombinant interleukin-2: a pilot study. J Clin Oncol. 1988 doi: 10.1200/JCO.1988.6.5.839. [DOI] [PubMed] [Google Scholar]
- 16.Dudley ME, Wunderlich JR, Robbins PF, et al. Supplemental online materials to cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–855. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenberg SA, Yang JC, Sherry RM, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Besser MJ, Shapira-Frommer R, Treves AJ, et al. Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2010 doi: 10.1158/1078-0432.CCR-10-0041. [DOI] [PubMed] [Google Scholar]
- 19.Radvanyi LG, Bernatchez C, Zhang M, et al. Specific lymphocyte subsets predict response to adoptive cell therapy using expanded autologous tumor-infiltrating lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2012 doi: 10.1158/1078-0432.CCR-12-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andersen R, Donia M, Ellebaek E, et al. Long-lasting complete responses in patients with metastatic melanoma after adoptive cell therapy with tumor-infiltrating lymphocytes and an attenuated il2 regimen. Clin Cancer Res. 2016;22:3734–3745. doi: 10.1158/1078-0432.CCR-15-1879. [DOI] [PubMed] [Google Scholar]
- 21.Dudley ME, Gross CA, Langhan MM, et al. CD8 + enriched “Young” tumor infiltrating lymphocytes can mediate regression of metastatic melanoma. Clin Cancer Res. 2010 doi: 10.1158/1078-0432.CCR-10-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson LA, Morgan RA, Dudley ME, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009 doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujita K, Ikarashi H, Takakuwa K, et al. Prolonged disease-free period in patients with advanced epithelial ovarian cancer after adoptive transfer of tumor-infiltrating lymphocytes. Clin Cancer Res. 1995;1(5):501–507. [PubMed] [Google Scholar]
- 24.Zacharakis N, Chinnasamy H, Black M, et al. Immune recognition of somatic mutations leading to complete durable regression in metastatic breast cancer. Nat Med. 2018 doi: 10.1038/s41591-018-0040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci. 1989 doi: 10.1073/pnas.86.24.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pule MA, Savoldo B, Myers GD, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14:1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park JH, Geyer MB, Brentjens RJ. CD19-targeted CAR T-cell therapeutics for hematologic malignancies: interpreting clinical outcomes to date. Blood. 2016;127:3312–3320. doi: 10.1182/blood-2016-02-629063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with b-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Zelm MC, Reisli I, Van Der Burg M, et al. An antibody-deficiency syndrome due to mutations in the CD19 gene. N Engl J Med. 2006 doi: 10.1056/NEJMoa051568. [DOI] [PubMed] [Google Scholar]
- 30.Hay KA. Cytokine release syndrome and neurotoxicity after CD19 chimeric antigen receptor-modified (CAR-) T cell therapy. Br J Haematol. 2018;183:364–374. doi: 10.1111/bjh.15644. [DOI] [PubMed] [Google Scholar]
- 31.Gauthier J, Turtle CJ. Insights into cytokine release syndrome and neurotoxicity after CD19-specific CAR-T cell therapy. Curr Res Transl Med. 2018;66:50–52. doi: 10.1016/j.retram.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Z, Han W. Biomarkers of cytokine release syndrome and neurotoxicity related to CAR-T cell therapy. Biomark Res. 2018 doi: 10.1186/s40364-018-0116-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gust J, Hay KA, Hanafi LA, et al. Endothelial activation and blood–brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 2017 doi: 10.1158/2159-8290.CD-17-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy-assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15:47–62. doi: 10.1038/nrclinonc.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rupp LJ, Schumann K, Roybal KT, et al. CRISPR/Cas9-mediated PD-1 disruption enhances anti-Tumor efficacy of human chimeric antigen receptor T cells. Sci Rep. 2017 doi: 10.1038/s41598-017-00462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peng W, Ye Y, Rabinovich BA, et al. Transduction of tumor-specific T cells with CXCR36 chemokine receptor improves migration to tumor and antitumor immune responses. Clin Cancer Res. 2010 doi: 10.1158/1078-0432.CCR-10-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adachi K, Kano Y, Nagai T, et al. IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat Biotechnol. 2018 doi: 10.1038/nbt.4086. [DOI] [PubMed] [Google Scholar]
- 38.Wartewig T, Kurgyis Z, Keppler S, et al. PD-1 is a haploinsufficient suppressor of T cell lymphomagenesis. Nature. 2017;552:121–125. doi: 10.1038/nature24649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morgan RA, Yang JC, Kitano M, et al. Case report of a serious adverse event following the administration of t cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lamers CHJ, Sleijfer S, Van Steenbergen S, et al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol Ther. 2013 doi: 10.1038/mt.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thistlethwaite FC, Gilham DE, Guest RD, et al. The clinical efficacy of first-generation carcinoembryonic antigen (CEACAM5)-specific CAR T cells is limited by poor persistence and transient pre-conditioning-dependent respiratory toxicity. Cancer Immunol Immunother. 2017 doi: 10.1007/s00262-017-2034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhong S, Malecek K, Johnson LA, et al. T-cell receptor affinity and avidity defines antitumor response and autoimmunity in T-cell immunotherapy. Proc Natl Acad Sci. 2013;110:6973–6978. doi: 10.1073/pnas.1221609110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robbins PF, Morgan RA, Feldman SA, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol. 2011 doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morgan RA, Chinnasamy N, Abate-Daga D, et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother. 2013 doi: 10.1097/CJI.0b013e3182829903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cameron BJ, Dukes J, Harper JV, et al. Identification of a titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells. Sci Transl Med. 2013 doi: 10.1126/scitranslmed.3006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tiberghien P, Ferrand C, Lioure B, et al. Administration of herpes simplex-thymidine kinase-expressing donor T cells with a T-cell-depleted allogeneic marrow graft. Blood. 2001 doi: 10.1182/blood.V97.1.63. [DOI] [PubMed] [Google Scholar]
- 47.Ciceri F, Bonini C, Teresa M, et al. Infusion of suicide-gene-engineered donor lymphocytes after family haploidentical haemopoietic stem-cell transplantation for leukaemia (the TK007 trial): a non-randomised phase I-II study. Lancet Oncol. 2009;10:489–500. doi: 10.1016/S1470. [DOI] [PubMed] [Google Scholar]
- 48.Berger C, Flowers ME, Warren EH, Riddell SR. Analysis of transgene-specific immune responses that limit the in vivo persistence of adoptively transferred HSV-TK-modified donor T cells after allogeneic hematopoietic cell transplantation. Blood. 2006 doi: 10.1182/blood-2005-08-3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bonini C, Ferrari G, Verzeletti S, et al. HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science. 1997;276:1719–1724. doi: 10.1126/science.276.5319.1719. [DOI] [PubMed] [Google Scholar]
- 50.Lee WYW, Zhang T, Lau CPY, et al. Immortalized human fetal bone marrow-derived mesenchymal stromalcell expressing suicide gene for anti-tumor therapy in vitro andin vivo. Cytotherapy. 2013 doi: 10.1016/j.jcyt.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 51.Di Stasi A, Tey S-K, Dotti G, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med. 2011 doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang X, Chang WC, Wong CLW, et al. A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood. 2011 doi: 10.1182/blood-2011-02-337360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paszkiewicz PJ, Fräßle SP, Srivastava S, et al. Targeted antibody-mediated depletion of murine CD19 CAR T cells permanently reverses B cell aplasia. J Clin Invest. 2016;126:4262–4272. doi: 10.1172/jci84813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu C-Y, Roybal KT, Puchner EM, et al. Remote control of therapeutic T cells through a small molecule-gated chimeric receptor. Science (80-) 2015 doi: 10.1126/science.aab4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cartellieri M, Feldmann A, Koristka S, et al. Switching CAR T cells on and off: a novel modular platform for retargeting of T cells to AML blasts. Blood Cancer J. 2016;6:e458. doi: 10.1038/bcj.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fedorov VD, Themeli M, Sadelain M. PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci Transl Med. 2013;5:215ra172. doi: 10.1126/scitranslmed.3006597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eyquem J, Mansilla-Soto J, Giavridis T, et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature. 2017 doi: 10.1038/nature21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roth TL, Puig-Saus C, Yu R, et al. Reprogramming human T cell function and specificity with non-viral genome targeting. Nature. 2018 doi: 10.1038/s41586-018-0326-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schober K, Müller T, Gökmen F, et al. Orthotopic replacement of T-cell receptor α- and β-chains with preservation of near-physiological T-cell function. Nat Biomed Eng. 2019 doi: 10.1038/s41551-019-0409-0. [DOI] [PubMed] [Google Scholar]
- 60.Kloss CC, Condomines M, Cartellieri M, et al. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat Biotechnol. 2013;31:71–75. doi: 10.1038/nbt.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lanitis E, Poussin M, Klattenhoff AW, et al. Chimeric antigen receptor T cells with dissociated signaling domains exhibit focused antitumor activity with reduced potential for toxicity in vivo. Cancer Immunol Res. 2013 doi: 10.1158/2326-6066.CIR-13-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim MY, Yu KR, Kenderian SS, et al. Genetic inactivation of CD33 in hematopoietic stem cells to enable CAR T cell immunotherapy for acute myeloid leukemia. Cell. 2018 doi: 10.1016/j.cell.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu X, Jiang S, Fang C, et al. Affinity-tuned ErbB2 or EGFR chimeric antigen receptor T cells exhibit an increased therapeutic index against tumors in mice. Cancer Res. 2015 doi: 10.1158/0008-5472.CAN-15-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Caruso HG, Hurton LV, Najjar A, et al. Tuning sensitivity of CAR to EGFR density limits recognition of normal tissue while maintaining potent antitumor activity. Cancer Res. 2015 doi: 10.1158/0008-5472.CAN-15-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Park S, Shevlin E, Vedvyas Y, et al. Micromolar affinity CAR T cells to ICAM-1 achieves rapid tumor elimination while avoiding systemic toxicity. Sci Rep. 2017 doi: 10.1038/s41598-017-14749-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Drent E, Themeli M, Poels R, et al. A rational strategy for reducing on-target off-tumor effects of CD38-chimeric antigen receptors by affinity optimization. Mol Ther. 2017;25:1946–1958. doi: 10.1016/j.ymthe.2017.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ahmed N, Brawley VS, Hegde M, et al. Human epidermal growth factor receptor 2 (HER2)—specific chimeric antigen receptor—modified T cells for the immunotherapy of HER2-positive sarcoma. J Clin Oncol. 2015;33:1688–1696. doi: 10.1200/JCO.2014.58.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Norelli M, Camisa B, Barbiera G, et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med. 2018;24:739–748. doi: 10.1038/s41591-018-0036-4. [DOI] [PubMed] [Google Scholar]
- 69.Giavridis T, Van Der Stegen SJC, Eyquem J, et al. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade letter. Nat Med. 2018 doi: 10.1038/s41591-018-0041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wunderlich M, Chou FS, Link KA, et al. AML xenograft efficiency is significantly improved in NOD/SCID-IL2RG mice constitutively expressing human SCF, GM-CSF and IL-3. Leukemia. 2010;24:1785–1788. doi: 10.1038/leu.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Corr M, Slanetz AE, Boyd LF, et al. T cell receptor-MHC class I peptide interactions: affinity, kinetics, and specificity. Science. 1994;265:946–949. doi: 10.1126/science.8052850. [DOI] [PubMed] [Google Scholar]
- 72.Nauerth M, Weissbrich B, Knall R, et al. TCR-ligand koff rate correlates with the protective capacity of antigen-specific CD8 + T Cells for adoptive transfer. Sci Transl Med. 2013;5:192ra87. doi: 10.1126/scitranslmed.3005958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hebeisen M, Schmidt J, Guillaume P, et al. Identification of rare high-avidity, tumor-reactive CD8 + T cells by monomeric TCR-ligand off-rates measurements on living cells. Cancer Res. 2015;75:1983–1991. doi: 10.1158/0008-5472.CAN-14-3516. [DOI] [PubMed] [Google Scholar]
- 74.Allard M, Couturaud B, Carretero-Iglesia L, et al. TCR-ligand dissociation rate is a robust and stable biomarker of CD8 + T cell potency. JCI Insight. 2017 doi: 10.1172/jci.insight.92570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Busch DH, Pamer EG. T cell affinity maturation by selective expansion during infection. J Exp Med. 1999;189:701–710. doi: 10.1084/jem.189.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Savage PA, Boniface JJ, Davis MM. A Kinetic Basis For T Cell Receptor Repertoire Selection during an Immune Response primes a specific CD4 ϩ T helper response primarily di- rected toward an immunodominant epitope restricted by the I-E k MHC molecule (Schwartz, 1985) McHeyzer Immun. 1999;10:485–492. doi: 10.1016/S1074-7613(00)80048-5. [DOI] [PubMed] [Google Scholar]
- 77.Zehn D, Lee SY, Bevan MJ. Complete but curtailed T-cell response to very low-affinity antigen. Nature. 2009;458:211–214. doi: 10.1038/nature07657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kalergis AH, Boucheron N, Doucey MA, et al. Efficient T cell activation requires an optimal dwell-time of interaction between the TCR and the pMHC complex. Nat Immunol. 2001;2:229–234. doi: 10.1038/85286. [DOI] [PubMed] [Google Scholar]
- 79.Lever M, Maini PK, van der Merwe PA, Dushek O. Phenotypic models of T cell activation. Nat Rev Immunol. 2014;14:619–629. doi: 10.1038/nri3728. [DOI] [PubMed] [Google Scholar]
- 80.Sabatino JJ, Huang J, Zhu C, Evavold BD. High prevalence of low affinity peptide–MHC II tetramer–negative effectors during polyclonal CD4+ T cell responses. J Exp Med. 2011;208:81–90. doi: 10.1084/jem.20101574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martinez RJ, Evavold BD. Lower affinity T cells are critical components and active participants of the immune response. Front Immunol. 2015;6:1–10. doi: 10.3389/fimmu.2015.00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim C, Williams MA. Nature and nurture: t-cell receptor-dependent and T-cell receptor-independent differentiation cues in the selection of the memory T-cell pool. Immunology. 2010;131:310–317. doi: 10.1111/j.1365-2567.2010.03338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cho YL, Flossdorf M, Kretschmer L, et al. TCR signal quality modulates fate decisions of single CD4 + T cells in a probabilistic manner. Cell Rep. 2017;20:806–818. doi: 10.1016/j.celrep.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 84.Knudson KM, Goplen NP, Cunningham CA, et al. Low-affinity T cells are programmed to maintain normal primary responses but are impaired in their recall to low-affinity ligands. Cell Rep. 2013;4:554–565. doi: 10.1016/j.celrep.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 85.Caserta S, Kleczkowska J, Mondino A, Zamoyska R. Reduced functional avidity promotes central and effector memory CD4 T cell responses to tumor-associated antigens. J Immunol. 2010;185:6545–6554. doi: 10.4049/jimmunol.1001867. [DOI] [PubMed] [Google Scholar]
- 86.Utzschneider DT, Alfei F, Roelli P, et al. High antigen levels induce an exhausted phenotype in a chronic infection without impairing T cell expansion and survival. J Exp Med. 2016;213:1819–1834. doi: 10.1084/jem.20150598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schober K, Buchholz VR, Busch DH. TCR repertoire evolution during maintenance of CMV-specific T-cell populations. Immunol Rev. 2018;283:113–128. doi: 10.1111/imr.12654. [DOI] [PubMed] [Google Scholar]
- 88.Irving M, Zoete V, Hebeisen M, et al. Interplay between T cell receptor binding kinetics and the level of cognate peptide presented by major histocompatibility complexes governs CD8 + T cell responsiveness. J Biol Chem. 2012;287:23068–23078. doi: 10.1074/jbc.M112.357673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chmielewski M, Hombach A, Heuser C, et al. T cell activation by antibody-like immunoreceptors: increase in affinity of the single-chain fragment domain above threshold does not increase T cell activation against antigen-positive target cells but decreases selectivity. J Immunol. 2004;173:7647–7653. doi: 10.4049/jimmunol.173.12.7647. [DOI] [PubMed] [Google Scholar]
- 90.Watanabe K, Terakura S, Martens AC, et al. Target antigen density governs the efficacy of anti-CD20-CD28-CD3 ζ chimeric antigen receptor-modified effector CD8 + T cells. J Immunol. 2015;194:911–920. doi: 10.4049/jimmunol.1402346. [DOI] [PubMed] [Google Scholar]
- 91.Busch DH, Fräßle SP, Sommermeyer D, et al. Role of memory T cell subsets for adoptive immunotherapy. Semin Immunol. 2016;28:28–34. doi: 10.1016/j.smim.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]