Abstract
Background
An association between immune-related adverse events (irAEs) caused by immunotherapeutic agents and the clinical benefit of immunotherapy has been suggested. We retrospectively evaluated by means of 18F-FDG PET/CT lymphoid tissue changes in the mediastinal/hilar lymph nodes and the spleen in response to ipilimumab administration in metastatic melanoma.
Methods
A total of 41 patients with unresectable metastatic melanoma underwent 18F-FDG PET/CT before the start of ipilimumab (baseline PET/CT), after two cycles (interim PET/CT) and at the end of treatment (late PET/CT). Data analysis was focused on the mediastinal/hilar lymph nodes and the spleen. The patients’ best clinical response (BCR) was used as reference.
Results
According to the BCR reference, 31 patients showed disease control (DC) and 10 patients showed progressive disease (PD). Mediastinal/hilar lymph node evaluation revealed that in total 4 patients in the interim or late PET/CT (10%) demonstrated a ‘sarcoid-like lymphadenopathy’ as response to treatment (LN-positive). All LN-positive patients responded to ipilimumab with DC. On the other hand, no significant differences between the DC and PD groups regarding both semi-quantitative and quantitative 18F-FDG PET spleen-related parameters at baseline and as response to treatment were detected.
Conclusion
Based on our findings, 10% patients in the interim or late PET/CT showed ‘sarcoid-like lymphadenopathy’ as response to treatment. All these patients showed disease control, implying a relation between the appearance of sarcoid-like lymphadenopathy and the clinical benefit of anti-CTLA-4 therapy. On the other hand, quantitative 18F-FDG PET analysis of the spleen showed a poor performance in predicting clinical benefit to ipilimumab.
Keywords: Metastatic melanoma, Ipilimumab, ‘Sarcoid-like lymphadenopathy’, Spleen glucose metabolism, 18F-FDG PET/CT
Introduction
Metastatic melanoma has a highly aggressive clinical behavior and a very poor prognosis, historically associated with a median survival of approximately 7 months and a 5-year survival probability of less than 10% [1]. Nevertheless, the recent introduction and increasing application of immunotherapeutic agents in clinical practice has resulted in unprecedented improvements in patients’ survival. In particular, ipilimumab, a monoclonal antibody that inhibits the antigen CTLA-4, has shown a survival benefit in metastatic melanoma patients, achieving a median overall survival of 10.1 months, a progression-free survival of 4.4 months and a response rate of 11% [2, 3]. The response rate has been documented to rise up to 61% when ipilimumab is combined with an antibody against another antigen, the PD-1 inhibitor nivolumab [4].
Nevertheless, despite these undoubtedly promising results, the percentage of non-responders to immunotherapy remains high. In addition, due to their unique anti-cancer mechanism partly involving cytokine release by activated T cells, these agents are associated with cumulative, dose dependent, immune-related adverse events (irAEs) [5]. The early recognition of these irAEs is of utmost importance, since the discontinuation of immunotherapy and initiation of corticosteroid treatment leads to their successful management. Interestingly, an association between irAEs and the clinical benefit of immunotherapeutic agents has been suggested [1, 6, 7].
PET/CT is an imaging modality providing functional metabolic tissue assessment. 18F-FDG, the workhorse of PET/CT imaging, is a glucose analogue that is transported into cells by glucose transporters and phosphorylated by hexokinase enzyme to 18F-FDG-6 phosphate, which cannot be further metabolized and becomes trapped intracellularly. Apart from its well-documented affinity for tumor tissue, 18F-FDG also accumulates at sites of inflammation. This is explained by the fact that activated cells involved in inflammation, especially neutrophils and monocytes/macrophages, demonstrate increased expression of glucose transporters, mostly GLUT1 and GLUT3, as well as increased hexokinase activity [8–13]. Two 18F-FDG PET/CT patterns of inflammatory changes—particularly under anti-CTLA-4 administration—which are potentially representative of an ‘immune flare’ response, are the symmetric hilar and mediastinal nodal uptake in a pattern similar to sarcoidosis (‘sarcoid-like lymphadenopathy’) and the diffuse splenic uptake [14].
In the present retrospective study, we aimed to evaluate lymphoid tissue changes in response to the administration of immunotherapy in metastatic melanoma and in correlation with the patients’ best clinical response (BCR). For this reason the glucose metabolism in the mediastinal and hilar lymph nodes as well as in the spleen was studied by means of 18F-FDG PET/CT in a group of metastatic melanoma patients receiving ipilimumab. Our hypothesis was that monitoring glucose metabolism during immunotherapy by means of 18F-FDG PET/CT would provide information regarding activation of the immune system, the degree of which may be of prognostic significance regarding the outcome of this treatment.
Materials and methods
Patients
Forty-one patients (30 males, 11 females; mean age 57.6 years) with unresectable, stage IV metastatic melanoma (American Joint Committee on Cancer, 7th edition [15]) were studied with 18F-FDG PET/CT in order to evaluate lymphoid tissue glucose metabolism during ipilimumab administration. PET/CT scans were performed before the start of ipilimumab (baseline PET/CT), after two cycles of treatment (interim PET/CT) and after completion of the four-cycle treatment (late PET/CT). Regarding mediastinal and hilar lymph node analysis, all 41 patients were included in the study. In terms of spleen glucose metabolism analysis, ten patients were excluded: one patient had undergone splenectomy, 5 patients had spleen metastases, and in 4 patients the dynamic study in the area of the thorax and upper abdomen was not completed at least in one of the 3 scans; in total, 31 patients were identified and included in this analysis. Ipilimumab was administered intravenously in 4 doses at a dose of 3 mg/kg every 3 weeks. The patients of this cohort have already been evaluated in previous publications of our group, but with a different concept, approach and analysis than in the here presented study [16–18].
Data acquisition
All patients underwent 18F-FDG PET/CT scans during the course of ipilimumab treatment in a Biograph mCT PET/CT system (Siemens Co., Erlangen, Germany). For attenuation correction a low-dose attenuation CT (120 kV, 30 mA) was used. Iterative image reconstruction was based on the ordered subset expectation maximization (OSEM) algorithm. Data acquisition consisted of dynamic PET/CT over the thorax and upper abdomen (dPET/CT) for 60 min after intravenous administration of 18F-FDG using a 24-frame protocol and static, whole body PET/CT. Details about PET/CT acquisition methods have been previously described from our group [18].
Data analysis
Data analysis was focused on the mediastinal and hilar lymph nodes, and the spleen. The lymph nodes were evaluated visually/qualitatively and semi-quantitatively based on standardized uptake value (SUV) calculations. Spleen glucose metabolism was assessed by means of semi-quantitative and quantitative analysis of the dynamic 18F-FDG PET data.
Regarding the lymph node evaluation, patients demonstrating a newly emerging, increased, symmetric 18F-FDG uptake in the mediastinal and hilar lymph nodes (similar to the one observed in sarcoidosis) as response to treatment without evidence of infection nor being suspicious for metastasis were classified as LN-positive. All other patients were characterized as LN-negative. The mediastinal and hilar lymph nodes were assessed independently at individual time points. These evaluations were performed by two nuclear medicine physicians (Christos Sachpekidis, Antonia Dimitrakopoulou-Strauss). The readers were blinded to potential other existing imaging results as well as to each other’s reading of the scan.
Spleen and lymph node SUV (SUVmean and SUVmax) calculations were performed at the 60-min post-injection frames and were based on volumes of interest (VOIs) placed over all the slices of the spleen and the lymph nodes, drawn using the pseudo-snake algorithm of the Pmod software (http://www.pmod.com/files/download/v31/doc/pbas/4729.htm).
Quantitative evaluation of the dynamic 18F-FDG PET/CT data was performed in VOIs drawn all over the spleen. The analysis was performed using a dedicated software and based on a two-tissue compartment model with a blood component (VB), with methods already reported in literature and performed previously by our group [18–23]. Two-tissue compartment modeling leads to the extraction of the following kinetic parameters: K1, representing the transport of the radiopharmaceutical 18F-FDG from plasma to tissue, k2 reflecting its transport back from tissue to plasma, k3 reflecting its phosphorylation rate, and k4 representing its dephosphorylation rate. The global tracer influx (Ki) is also calculated according to the formula:
Furthermore, a non-compartment model was used to calculate the parameter of heterogeneity, fractal dimension (FD), which describes the more deterministic or chaotic distribution of the tracer activity via time in a local volume defined by a VOI [24].
Response evaluation
Patients’ best clinical response (BCR), based on clinical follow-up, serum tumor markers (LDH) and standard of care imaging (including brain MRI and whole body 18F-FDG PET/CT scans), was assessed by the dermato-oncologists (Jessica C. Hassel) at a median of 21.4 months (range: 6.3–41.9 months) and was used as reference. BCR was defined either as disease control (DC)—including stable disease (SD), partial response (PR) and complete response (CR)-, or as progressive disease (PD).
In search of statistically significant differences that could be predictive of BCR, we compared the DC and PD groups regarding the mediastinal/hilar lymph node as well as the spleen-related glucose metabolism changes.
Statistical analysis
For the parameters SUVmean, SUVmax, VB, K1, k3, influx (Ki) and FD, the following comparisons were made: for every PET measurement, parameters were compared between the DC and PD groups. Separately for both patient groups, it was tested whether a significant change in parameters between PET measurements could be observed and, finally, the change in parameters was compared between both patient groups. p values were corrected for multiplicity of testing by the Bonferroni–Holm method. The results were considered significant for p value less than 0.05 (p < 0.05).
Results
According to the reference of BCR, 31 patients showed DC and 10 patients showed PD. Qualitative evaluation of the mediastinal/hilar lymph nodes revealed that in total 4 patients demonstrated a sarcoid-like lymphadenopathy as response to treatment (LN-positive) either in the interim (Fig. 1a) or late (Fig. 1b) PET/CT (10%). Two of these patients demonstrated pulmonary metastatic disease. The lymph node SUVmean ranged from 2.4 to 5.1, and the SUVmax from 3.1 to 6.8. Due to the small number of LN-positive patients, no statistical analysis was performed. All patients classified as LN-positive responded to ipilimumab therapy with DC.
Fig. 1.
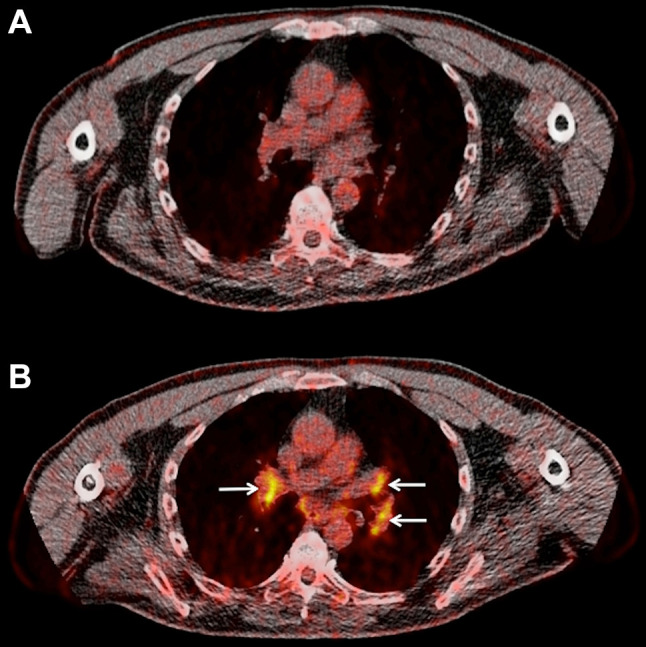
Sarcoid-like lymphadenopathy in a 55-year-old stage IV melanoma patient undergoing immunotherapy with ipilimumab. Transaxial 18F-FDG PET/CT images at the thoracic level of before onset of treatment (a), and after two cycles of ipilimumab treatment (b). Newly emerging, 18F-FDG avid mediastinal and hilar lymph nodes bilaterally (arrows) after the end of treatment with a SUVmean ranging from 3.3 to 3.8, and a SUVmax ranging from 4.5 to 6.0. Based on his clinical status the patient was characterized as SD thus, disease control (DC)
Thirty-one patients were included in the spleen glucose metabolism evaluations. According to the reference criteria of BCR, 24 patients were classified as showing DC and 7 of them as showing PD. Linear mixed model analysis revealed no statistically significant differences between the DC and PD groups regarding both semi-quantitative (SUVmean, SUVmax) and quantitative (VB, K1, k3, Ki, FD) baseline spleen-related parameters. We also studied the spleen-related parameters’ changes during the whole course of ipilimumab administration and found that no significant changes took place in each group as response to treatment. Moreover, there were no significant between-groups differences concerning the parameters’ changes. Similarly, in the subgroup of LN-positive patients no ‘homogenous’ pattern of response was observed regarding spleen parameters. The results of this analysis are presented in Table 1.
Table 1.
Descriptive statistics of mean (median) values for the 18F-FDG semi-quantitative and quantitative parameters of the spleen before, after two cycles and after four cycles of ipilimumab in the DC and PD groups
| Parameter | First PET/ CT (baseline) |
Second PET/CT (interim) |
Third PET/CT (late) |
|
|---|---|---|---|---|
| DC group | SUVmean | 2.0 (2.0) | 2.1 (2.1) | 2.1 (2.0) |
| SUVmax | 3.2 (3.1) | 3.2 (3.1) | 3.3 (3.3) | |
| K 1 | 0.50 (0.51) | 0.45 (0.43) | 0.48 (0.46) | |
| k 3 | 0.02 (0.01) | 0.01 (0.004) | 0.01 (0.01) | |
| Influx (Ki) | 0.01 (0.01) | 0.01 (0.003) | 0.01 (0.004) | |
| V B | 0.06 (0.06) | 0.07 (0.06) | 0.06 (0.05) | |
| FD | 1.09 (1.09) | 1.09 (1.09) | 1.09 (1.09) | |
| PD group | SUVmean | 2.1 (2.2) | 2.0 (2.1) | 2.0 (2.1) |
| SUVmax | 3.4 (3.2) | 3.3 (3.5) | 3.8 (3.4) | |
| K 1 | 0.44 (0.38) | 0.51 (0.58) | 0.37 (0.33) | |
| k 3 | 0.02 (0.01) | 0.02 (0.003) | 0.01 (0.01) | |
| Influx (Ki) | 0.01 (0.01) | 0.01 (0.002) | 0.01 (0.01) | |
| V B | 0.06 (0.04) | 0.06 (0.05) | 0.06 (0.07) | |
| FD | 1.11 (1.10) | 1.10 (1.10) | 1.10 (1.09) |
The units of parameters K1, k3, influx and VB are 1 /min. SUVmean, SUVmax and FD have no unit
Discussion
Immunotherapy has been associated with atypical-response patterns and occurrence of several irAEs, due to activation of the immune system accompanying the administration of these agents. In the context of such a generalized inflammatory response, an activation of the benign lymphoid tissue can be considered an expected finding. Given that both melanoma and immune infiltrates demonstrate increased 18F-FDG uptake, reliable assessment of response to immunotherapy by means of PET/CT represents a diagnostic challenge. In an attempt to deepen our understanding of the role of 18F-FDG PET/CT in immunotherapy response assessment of malignant melanoma we tried to determine whether metabolic changes in the non-melanoma-involved mediastinal and hilar lymph nodes as well as the spleen could predict response to therapy with the monoclonal anti-CTLA-4 antibody ipilimumab.
A ‘sarcoid-like’ lymph node distribution has been described as a radiographic finding in up to 5% of patients receiving immune checkpoint inhibitors [25–27]. To our knowledge, this is the largest study focusing exclusively on benign lymphoid tissue metabolic changes in the immunotherapeutic context by means of 18F-FDG PET/CT during and after anti-CTLA-4 administration. In accordance with the previously mentioned results, our findings showed that 10% of patients exhibited an increased, symmetrical 18F-FDG uptake as response to treatment either in the interim or late PET/CT. Interestingly, all the above-mentioned patients showed disease control according to the reference criteria of BCR. This finding is of significance and comes to support the suggested relationship between the appearance of this adverse event and tumor response: ‘sarcoid-like’ lymphadenopathy should not be evaluated as disease progression, since it may be associated with improved tumor response and disease control [25]. However, the non-appearance of this adverse event does not imply the opposite, since 27 patients with DC did not have this reaction.
The second organ studied in the present analysis was the spleen. The spleen is the largest lymphoid organ and blood-filtering unit in the body. It contains diverse populations of immune cells and provides an important defensive role against pathogens, including the development of T and B-lymphocytes in specific compartments. Being at the center of both innate and adaptive immune responses, the spleen plays, therefore, an essential role for immune homeostasis [28, 29]. The rationale for using 18F-FDG PET/CT to evaluate spleen metabolic changes during immunotherapy is the following: the blockade of CTLA-4 during ipilimumab administration results in an activation of the T cells and the immune system in general. In this context, the spleen will also exhibit a form of activation [30]. During this activation, the metabolism of the dendritic cells and T cells in the organ increases and the aerobic glycolysis pathway is used to cover their high-energy demands [31].
The existing PET data regarding the effect of immunotherapy on spleen metabolism are limited [32]. Ribas et al. [33] studied a group of 12 patients receiving the CTLA-4 blocking antibody, tremelimumab, with two different PET agents, 18F-FDG and 18F-FLT. Although no significant changes in splenic 18F-FDG uptake were observed within 3 months after initial dosing, a significant increase in spleen SUV was demonstrated in the follow-up 18F-FLT PET scans; this finding was considered consistent with lymphoid cell activation as response to the immunotherapeutic agent. A recently published case report documented an increased 18F-FDG uptake in the lymph nodes and spleen in a melanoma patient successfully treated with a combination of CTLA-4 and PD-1 blockade. The authors concluded that increased metabolic consumption in benign lymphoid tissue detected on PET/CT may be a surrogate marker of immune activation and treatment response in immunotherapy [34]. Most recently, in terms of a phase I clinical trial investigating safety of RNA-lipoplexes in advanced melanoma patients (Lipo-MERIT, NCT02410733), spleen 18F-FDG PET changes were studied as response to vaccination in two patients. The results were somewhat inconclusive with one patient showing unchanged SUV values during the course of vaccination and the second patient showing a more than twofold increased spleen uptake [35].
In the present study, we performed monitoring of the spleen glucose metabolism during ipilimumab administration by applying semi-quantitative and quantitative 18F-FDG PET evaluations. Semi-quantitative analysis based on SUV calculations revealed that the degree of 18F-FDG uptake in the spleen (SUVmean, SUVmax) had no differences between the groups DC and PD. Moreover, SUV values showed no significant change potentially correlating with the clinical response to ipilimumab in both groups and there were also no between-groups differences regarding the SUV changes. This is in agreement with the results of a recent study in a group of 18 advanced lung cancer patients receiving nivolumab, in which the spleen displayed no significant SUV differences pre-therapy and during therapy [36].
Although SUV calculation is the simplest and most widely used method for semi-quantitative evaluation of PET, requiring only static imaging when the tracer has reached equilibrium, the generally accepted method for a quantitative evaluation of 18F-FDG metabolism is full kinetic analysis after application of a two-tissue compartment model [37]. This model leads to extraction of specific tracer-related parameters. A pre-requisite for this is the performance of full dynamic PET studies for at least 60 min, as carried out in the present study. Similarly to SUV calculations, the results of quantitative glucose metabolism analysis showed no significant differences between the two BCR groups. In particular, the carrier-mediated transport of 18F-FDG from plasma to tissue (K1), the phosphorylation rate of the tracer (k3), its influx (Ki), the fractional blood volume (VB)—also known as vessel density—and the fractal dimension (FD) were not significantly different between DC and PD. Further, no single parameter showed a significant change as response to ipilimumab in both groups. The present results imply a limited utility of studying spleen 18F-FDG metabolism by means of SUV calculations and two-tissue compartment modeling in clinical response prediction of immunotherapy with the immune checkpoint inhibitor ipilimumab in melanoma, and are in line with previous findings of our group regarding application of quantitative 18F-FDG PET analysis in melanoma lesions [18]. In that study we had shown that quantitative analysis of 18F-FDG PET data of 25 metastatic melanoma patients could not provide additional information in treatment response evaluation of patients receiving ipilimumab. The main difference between the previous study and the present one is that the first focused on 18F-FDG changes melanoma lesions and not on benign lymphoid tissue.
Besides the small number of reported patients, a limitation of our analysis is the lack of histological validation of the symmetrically activated mediastinal/hilar lymph nodes (LN-positive), which is, however, usually not possible in clinical practice. In cases where the diagnostic dilemma of slow progression vs. pseudoprogression (sarcoid-like reaction) was present, the patients were continued on treatment and a short-term follow-up was made 4 weeks later. In one patient with suspicion of a sarcoid-like reaction, serum angiotensin-converting enzyme (ACE) and soluble interleukin-2 receptor (sIL2-R) were measured and demonstrated positive results, indicating that these PET findings were indeed attributed to sarcoid-like reaction and not tumor progression. Finally, the retrospective nature of the analysis does not allow for more robust conclusions to be made. Further prospective studies potentially including a larger number of patients and a larger spectrum of immune checkpoint inhibitors, like PD-1 inhibitors or their combination are necessary to validate these results.
Conclusion
Recent advancements in cancer immunotherapy can challenge ‘conventional’ radiologic treatment response evaluation through manifestation of atypical imaging responses and irAEs. We aimed to evaluate lymphoid tissue metabolic changes in response to the administration of a 4-cycle ipilimumab treatment in metastatic melanoma. For this reason, the glucose metabolism in the mediastinal and hilar lymph nodes as well as in the spleen was studied by means of 18F-FDG PET/CT. Based on our findings, 4/41 patients (10%) patients in the interim or late PET/CT showed an increased, symmetrical 18F-FDG uptake in the mediastinal and hilar lymph nodes as response to treatment. These 4 LN-positive patients were characterized as responders according to the BCR reference, suggesting that ‘sarcoid-like’ lymphadenopathy should not be evaluated as disease progression. On the other hand, the non-appearance of this adverse event does not imply the opposite, since 27 also responding patients did not show a sarcoid-like reaction. Finally, our results show a poor performance of quantitative 18F-FDG PET analysis of the spleen in predicting clinical benefit to ipilimumab.
Abbreviations
- 18F-FDG
2-Deoxy-2-(18F)fluoro-d-glucose
- BCR
Best clinical response
- CR
Complete response
- CT
Computed tomography
- DC
Disease control
- dPET/CT
Dynamic positron emission tomography/computed tomography
- FD
Fractal dimension
- irAEs
Immune-related adverse events
- LN-negative
Negative mediastinal/hilar lymph nodes
- LN-positive
Positive mediastinal/hilar lymph nodes
- PD
Progressive disease
- PET
Positron emission tomography
- PET/CT
Positron emission tomography/computed tomography
- PR
Partial response
- SD
Stable disease
- SUV
Standardized uptake value
- VOI
Volume of interest
Author contributions
CS performed the PET/CT studies, carried out the PET/CT data analysis, drafted and performed final editing of the manuscript. LL contributed to the conception of the study and co-drafted the manuscript. AK-S was responsible for the statistical analysis of the study. JCH was responsible for the selection of the patients who received the ipilimumab therapy and co-drafted the manuscript. AD-S was responsible for the PET-CT study design and the data evaluation and coordinated the project.
Funding
This study was supported in part by the German Cancer Aid under the project with the title ‘Therapy monitoring of ipilimumab based on the quantification of F-18-FDG kinetics with 4D PET/CT (dPET-CT) in patients with melanoma (stage 4)’. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding was received for this study.
Compliance with ethical standards
Conflict of interest
Jessica C. Hassel received honoraria for talks and travel expenses from Bristol-Myers Squibb (BMS), Merck, Sharp & Dohm (MSD), Roche, Novartis, Pfizer and is a member of an advisory board for MSD and Amgen. The other authors declare that they have no conflict of interest.
Ethical approval
The presented results are part of the study entitled “Quantification of 18F-FDG kinetics with 4D PET-CT in patients with melanoma stage IV”, which was approved by the Ethical Committee of the University of Heidelberg (Ethikvotum: S-107 /2012—Ethical Committee 1 of the University of Heidelberg) and the Federal Office for Radiation Protection (Bundesamt für Strahlenschutz; BfS: Z5- 22463 / 2 2012a-016). This study does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study. The patient presented in Fig. 1 agreed to the publication of this figure.
References
- 1.Downey SG, Klapper JA, Smith FO, et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13(22 Pt 1):6681–6688. doi: 10.1158/1078-0432.CCR-07-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691–2697. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- 6.Attia P, Phan GQ, Maker AV, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23(25):6043–6053. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaehler KC, Piel S, Livingstone E, Schilling B, Hauschild A, Schadendorf D. Update on immunologic therapy with anti-CTLA-4 antibodies in melanoma: identification of clinical and biological response patterns, immune-related adverse events, and their management. Semin Oncol. 2010;37(5):485–498. doi: 10.1053/j.seminoncol.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Kubota R, Yamada S, Kubota K, Ishiwata K, Tamahashi N, Ido T. Intratumoral distribution of fluorine-18-fluorodeoxyglucose in vivo: high accumulation in macrophages and granulation tissues studied by microautoradiography. J Nucl Med. 1992;33:1972–1980. [PubMed] [Google Scholar]
- 9.Gamelli RL, Liu H, He LK, Hofmann CA. Augmentations of glucose uptake and glucose transporter-1 in macrophages following thermal injury and sepsis in mice. J Leukoc Biol. 1996;59:639–647. doi: 10.1002/jlb.59.5.639. [DOI] [PubMed] [Google Scholar]
- 10.Mochizuki T, Tsukamoto E, Kuge Y, et al. FDG uptake and glucose transporter subtype expressions in experimental tumor and inflammation models. J Nucl Med. 2001;42:1551–1555. [PubMed] [Google Scholar]
- 11.Zhuang H, Alavi A. 18-fluorodeoxyglucose positron emission tomographic imaging in the detection and monitoring of infection and inflammation. Semin Nucl Med. 2002;32:47–59. doi: 10.1053/snuc.2002.29278. [DOI] [PubMed] [Google Scholar]
- 12.Paik JY, Lee KH, Choe YS, et al. Augmented 18F-FDG uptake in activated monocytes occurs during the priming process and involves tyrosine kinases and protein kinase C. J Nucl Med. 2004;45:124–128. [PubMed] [Google Scholar]
- 13.Jamar F, Buscombe J, Chiti A, et al. EANM/SNMMI guideline for 18F-FDG use in inflammation and infection. J Nucl Med. 2013;54(4):647–658. doi: 10.2967/jnumed.112.112524. [DOI] [PubMed] [Google Scholar]
- 14.Wong ANM, McArthur GA, Hofman MS, Hicks RJ. The advantages and challenges of using FDG PET/CT for response assessment in melanoma in the era of targeted agents and immunotherapy. Eur J Nucl Med Mol Imaging. 2017;44(Suppl 1):67–77. doi: 10.1007/s00259-017-3691-7. [DOI] [PubMed] [Google Scholar]
- 15.Balch CM, Gershenwald JE, Soong S-J, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anwar H, Sachpekidis C, Winkler J, et al. Absolute number of new lesions in 18F-FDG PET/CT is more predictive of clinical outcome than SUV changes in metastatic melanoma patients receiving ipilimumab. Eur J Nucl Med Mol Imaging. 2018;45(3):376–383. doi: 10.1007/s00259-017-3870-6. [DOI] [PubMed] [Google Scholar]
- 17.Sachpekidis C, Anwar H, Winkler J, et al. The role of interim 18F-FDG PET/CT in prediction of response to ipilimumab treatment in metastatic melanoma. Eur J Nucl Med Mol Imaging. 2018;45(8):1289–1296. doi: 10.1007/s00259-018-3972-9. [DOI] [PubMed] [Google Scholar]
- 18.Sachpekidis C, Anwar H, Winkler JK, et al. Longitudinal studies of the 18F-FDG kinetics after ipilimumab treatment in metastatic melanoma patients based on dynamic FDG PET/CT. Cancer Immunol Immunother. 2018;67:1261–1270. doi: 10.1007/s00262-018-2183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sokoloff L, Smith CB. Basic principles underlying radioisotopic methods for assay of biochemical processes in vivo. In: Greitz T, Ingvar DH, Widén L, editors. The metabolism of the human brain studied with positron emission tomography. New York: Raven Press; 1983. pp. 123–148. [Google Scholar]
- 20.Ohtake T, Kosaka N, Watanabe T, et al. Noninvasive method to obtain input function for measuring tissue glucose utilization of thoracic and abdominal organs. J Nucl Med. 1991;32:1432–1438. [PubMed] [Google Scholar]
- 21.Miyazawa H, Osmont A, Petit-Taboué MC, et al. Determination of 18F-fluoro-2-deoxy-d-glucose rate constants in the anesthetized baboon brain with dynamic positron tomography. J Neurosci Methods. 1993;50:263–272. doi: 10.1016/0165-0270(93)90033-N. [DOI] [PubMed] [Google Scholar]
- 22.Burger C, Buck A. Requirements and implementation of a flexible kinetic modeling tool. J Nucl Med. 1997;38:1818–1823. [PubMed] [Google Scholar]
- 23.Sachpekidis C, Thieke C, Askoxylakis V, et al. Combined use of (18)F-FDG and (18)F-FMISO in unresectable non-small cell lung cancer patients planned for radiotherapy: a dynamic PET/CT study. Am J Nucl Med Mol Imaging. 2015;5(2):127–142. [PMC free article] [PubMed] [Google Scholar]
- 24.Dimitrakopoulou-Strauss A, Strauss LG, Mikolajczyk K, Burger C, Lehnert T, Bernd L, Ewerbeck V. On the fractal nature of dynamic positron emission tomography (PET) studies. World J Nucl Med. 2003;2:306–313. [Google Scholar]
- 25.Bronstein Y, Ng CS, Hwu P, Hwu WJ. Radiologic manifestations of immune-related adverse events in patients with metastatic melanoma undergoing anti-CTLA-4 antibody therapy. Am J Roentgenol. 2011;197(6):W992–W1000. doi: 10.2214/AJR.10.6198. [DOI] [PubMed] [Google Scholar]
- 26.Kwak JJ, Tirumani SH, Van den Abbeele AD, Koo PJ, Jacene HA. Cancer immunotherapy: imaging assessment of novel treatment response patterns and immune-related adverse events. Radiographics. 2015;35(2):424–437. doi: 10.1148/rg.352140121. [DOI] [PubMed] [Google Scholar]
- 27.Howard SA, Krajewski KM, Jagannathan JP, et al. A new look at toxicity in the era of precision oncology: imaging findings, their relationship with tumor response, and effect on metastasectomy. Am J Roentgenol. 2016;207(1):4–14. doi: 10.2214/AJR.15.15480. [DOI] [PubMed] [Google Scholar]
- 28.MacDonald IC, Ragan DM, Schmidt EE, Groom AC. Kinetics of red blood cell passage through interendothelial slits into venous sinuses in rat spleen, analyzed by in vivo microscopy. Microvasc Res. 1987;33:118–134. doi: 10.1016/0026-2862(87)90011-2. [DOI] [PubMed] [Google Scholar]
- 29.Bratosin D, Mazurier J, Tissier JP, et al. Cellular and molecular mechanisms of senescent erythrocyte phagocytosis by macrophages. A review. Biochimie. 1998;80(2):173–195. doi: 10.1016/S0300-9084(98)80024-2. [DOI] [PubMed] [Google Scholar]
- 30.Yin Y, Choi SC, Xu Z, et al. Normalization of CD41 T cell metabolism reverses lupus. Sci Transl Med. 2015;7:274ra18. doi: 10.1126/scitranslmed.aaa0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahn SS, Hwang SH, Jung SM, et al. Evaluation of spleen glucose metabolism using 18F-FDG PET/CT in patients with febrile autoimmune disease. J Nucl Med. 2017;58(3):507–513. doi: 10.2967/jnumed.116.180729. [DOI] [PubMed] [Google Scholar]
- 32.Sachpekidis C, Larribere L, Kopp-Schneider A, Haberkorn U, Hassel J, Dimitrakopoulou-Strauss A. Benign lymphoid tissue changes as response to immunotherapy in metastatic melanoma patients: an 18F-FDG PET/CT study. Eur J Nucl Med Mol Imaging. 2018;45(Suppl 1):S517. [Google Scholar]
- 33.Ribas A, Benz MR, Allen-Auerbach MS, et al. Imaging of CTLA4 blockade-induced cell replication with (18)F-FLT PET in patients with advanced melanoma treated with tremelimumab. J Nucl Med. 2010;51:340–346. doi: 10.2967/jnumed.109.070946. [DOI] [PubMed] [Google Scholar]
- 34.Tsai KK, Pampaloni MH, Hope C, et al. Increased FDG avidity in lymphoid tissue associated with response to combined immune checkpoint blockade. J Immunother Cancer. 2016;4:58. doi: 10.1186/s40425-016-0162-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pektor S, Hilscher L, Walzer KC, et al. In vivo imaging of the immune response upon systemic RNA cancer vaccination by FDG-PET. EJNMMI Res. 2018;8(1):80. doi: 10.1186/s13550-018-0435-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eshghi N, Garland LL, Nia E, Betancourt R, Krupinski E, Kuo PH. 18F-FDG PET/CT can predict development of thyroiditis due to immunotherapy for lung cancer. J Nucl Med Technol. 2018;46:260–264. doi: 10.2967/jnmt.117.204933. [DOI] [PubMed] [Google Scholar]
- 37.Phelps ME, Huang SC, Hoffman EJ, et al. Tomographic measurement of local cerebral glucose metabolic rate in humans with (F-18)2-fluoro-2-deoxy-d-glucose: validation of method. Ann Neurol. 1979;6:371–388. doi: 10.1002/ana.410060502. [DOI] [PubMed] [Google Scholar]


