Abstract
Introduction
In this phase I study using a 3 + 3 dose escalation design, the safety, dose-limiting toxicity (DLT), immunogenicity and efficacy of intravenous Lipovaxin-MM—a multi-component dendritic cell-targeted liposomal vaccine against metastatic melanoma—was investigated.
Methods
Twelve subjects with metastatic cutaneous melanoma were recruited in three cohorts. Patients in Cohort A (n = 3) and Cohort B (n = 3) received three doses of 0.1 and 1 mL of Lipovaxin-MM, respectively, every 4 weeks. Patients in Cohort C (n = 6) received four doses of 3 mL vaccine weekly. Immunologic assessments of peripheral blood were made at regular intervals and included leukocyte subsets, cytokine levels, and Lipovaxin-MM-specific T-cell and antibody reactivities. Tumor responses were assessed by RECIST v1.0 at screening, then 8 weekly in Cohorts A and B and 6 weekly in Cohort C.
Results
Of a total of 94 adverse events (AEs) reported in ten subjects, 43 AEs in six subjects were considered to be possibly or probably vaccine-related. Most (95%) vaccine-related AEs were grade 1 or 2, two (5%) grade 3 vaccine-related AEs of anemia and lethargy were recorded, and higher grade AEs and DLTs were not observed. No consistent evidence of vaccine-specific humoral or cellular immune responses was found in post-immunization blood samples. One patient had a partial response, two patients had stable disease, and the remaining patients had progressive disease.
Conclusions
Lipovaxin-MM was well tolerated and without clinically significant toxicity. Immunogenicity of Lipovaxin-MM was not detected. Partial response and stable disease were observed in one and two patients, respectively.
Electronic supplementary material
The online version of this article (10.1007/s00262-018-2207-z) contains supplementary material, which is available to authorized users.
Keywords: Melanoma, Vaccine, Liposomes, Dendritic cells, DC-SIGN, Clinical trial
Introduction
Cutaneous melanoma accounts for 1.6% of all new cancer cases and 0.7% of cancer-related deaths worldwide, according to estimates made in 2012 [2]. Patients with distant metastatic disease have a 5-year survival rate of 10–15% [3]. Despite encouraging results from recently approved small-molecule inhibitors of the mitogen-activated protein kinase (MAPK) pathway and immune checkpoint inhibitory antibodies, the development of new therapeutic modalities may extend therapeutic benefits to more metastatic melanoma patients [4].
Dendritic cells (DCs) are unique antigen-presenting cells (APCs), which initiate and control immune responses and also play an important role in vaccine action. Immature DCs acquire and process antigens in the immunologic periphery before migrating to draining lymph nodes. During this process, DCs mature and can thus present the antigens to cognate T cells, which become activated, and result in the induction of potent cellular immune responses. Ex vivo techniques involved in DC-based vaccine preparation are usually cumbersome, labor intensive, and expensive. These methods may involve the isolation of monocytes or CD34+ cells from the patient, culturing these cells in vitro with cytokines to induce DC differentiation, loading the DCs with tumor antigens, and after further maturation with cytokines, injecting these antigen-primed DCs as vaccines [5, 6]. Furthermore, this approach has had poor efficacy partly because these DC preparations fail to migrate adequately to draining lymph nodes [7]. Hence, strategies for in vivo targeting of antigen payloads to DCs that might then directly activate immune responses are very attractive in tumor vaccine development [8, 9].
DCs capture antigens through different cell-surface receptors. C-type lectin receptors (CLRs) are DC cell surface receptors that recognize carbohydrate structures on antigens [10]. One particular CLR, dendritic cell-specific intercellular adhesion molecule 3-grabbing non-integrin (DC-SIGN; CD209), is specifically and abundantly expressed on immature human DCs, and is downregulated in mature DCs [11]. After ligand-specific antigen capture, DC-SIGN receptors rapidly internalize, leading to lysosome-based antigen processing with subsequent presentation to T cells of the antigen-derived peptides bound by both MHC Class I and Class II molecules [11, 12]. Consequently, anti-DC-SIGN antibodies can be used as targeting moieties to deliver antigens or molecular adjuvants to DCs in vivo to raise strong antigen-specific T-cell responses, as shown in murine models [13].
Liposomes are uni- or in some cases multi-lamellar membrane-bound nanoparticles, which can encapsulate immunomodulatory factors and tumor antigens and can then be targeted to cell-surface receptors in vivo via the incorporation of a specific antibody fragment in the liposomal surface. In this study, we used a multicomponent and multivalent DC−targeted liposomal allogeneic melanoma vaccine called Lipovaxin-MM. The details of each component of the vaccine and its specific function are outlined below. Tumor antigens including the melanocyte differentiation antigens, gp100, tyrosinase, and melanA/MART-1, are derived from plasma membrane vesicles (PMVs) prepared from the MM200 human melanoma cell line. MM200 crude tumor lysates have shown immunogenicity in the past [14]. The PMVs are modified using a liposomal mixture comprising the metal-chelating lipid, 3(nitrilotriacetic acid)-ditetradecylamine (3NTA-DTDA) together with the carrier lipid α−palmitoyl-ß−oleoyl-phosphatidylcholine (POPC), which enhances the insertion into PMVs of 3NTA-DTDA. The DC-maturing cytokine, interferon gamma (IFNγ), ισ ινχορπορατεδ ιντο τηε resulting liposome–PMV conjugate. The DC-targeting moiety is DMS-5000, which is a DC-SIGN-specific, VH domain antibody fragment. In the presence of nickel sulfate (NiSO4), DMS5000 is engrafted via its modified poly-histidine C-terminal tail and metal-chelating linkage to 3NTA-DTDA, which has been inserted into the membrane vesicles [15–17]. The preclinical utility of this in vivo DC-targeting vaccine method for the induction of effective antitumor T cells has been demonstrated previously in melanoma-bearing mice [17].
This study was aimed at determining the safety, immunogenicity, and efficacy of the DC-directed liposomal vaccine, Lipovaxin-MM. It was hypothesized that because this multi-component vaccine comprised melanoma PMVs modified to target DC-SIGN on DCs and to encapsulate the DC-activating cytokine, IFNγ, this construct would target melanoma antigens to DCs in vivo and would circumvent the need for ex vivo manipulation of DCs to produce a melanoma vaccine. Our results show that Lipovaxin-MM is safe but did not consistently induce specific immune responses in patients.
Methods
Study objectives
The primary objectives of this study were to determine the safety profile, dose-limiting toxicities (DLTs), and immunogenicity of escalating doses of Lipovaxin-MM, given as three doses separated by 4 weeks, or four doses separated by 1 week. The secondary objective of this study was to document any tumor responses as evidenced by partial or complete response or stable disease lasting longer than 6 weeks and confirmed at 12 weeks in any patient who received Lipovaxin-MM.
Study design
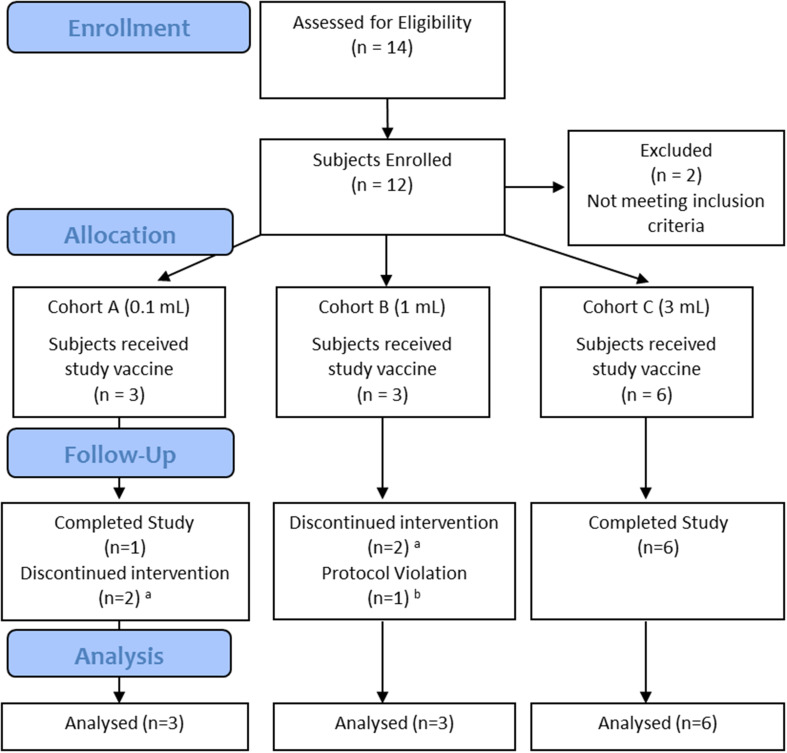
The Phase I clinical trial used a non-randomized, dose escalation design. Participants, those administering the intervention, and those assessing the outcome were not blinded. The trial employed an open label, 3 + 3 dose escalation design (three to six patients per cohort) to establish the maximum tolerated dose (MTD). Three intravenous vaccine doses (0.1, 1 and 3 mL) were tested among three patient cohorts (Fig. 1). Another 10 mL dose, although planned, was not tested because the technical challenges in preparing this large dose made it not feasible. In Cohorts A and B, three doses of vaccine were given at 4 weekly intervals, and in Cohort C, four doses were tested at weekly intervals. The study was conducted at a single site (Royal Adelaide Hospital, South Australia) over a 23-month period. The first participant was screened on 18 November 2009, and 20 October 2011 was the date on which the last participant was assessed. Patients were followed 21–28 days after the last dose of Lipovaxin-MM to evaluate the resolution of any treatment-emergent toxicity.
Fig. 1.
Flowchart of trial subjects. Of 14 screened subjects, 12 were enrolled into three Cohorts. The flowchart is based on CONSORT flow diagram template, and adapted for a non-randomized trial design. a subjects withdrawn early because of progressive disease. b protocol violation because of previous investigational immunotherapy
An adaptive design component was used for within cohort and between cohort dose escalations based on vaccine-specific immune responses. In Cohorts A (0.1 mL) and B (1 mL), Lipovaxin-MM-specific immune responses were sought 7 days after each of the first and second doses to detect priming and booster responses, respectively. On detection of a priming response, the subsequent two doses remained at the same level. In the absence of priming and booster responses, the third vaccine dose was escalated to the next higher level. On detection of a booster response, the third vaccine dose remained unchanged.
Before enrolling patients into Cohorts B and C, a review of the safety, immunogenicity, and efficacy data collected 28 days after the last dose for the three patients enrolled in the previous cohort was conducted. Dose escalation was allowed to proceed in the absence of study vaccine-related adverse events, which were grade 2 or higher. In Cohort C (3 mL), four doses of vaccine were given at weekly intervals in three patients. Cohort C was extended to enroll another three patients in the absence of any vaccine-specific immune response or ≥ grade 2 vaccine-related toxicity.
Study population
Eligible patients were aged ≥ 18, had histologically confirmed and incurable stage IV cutaneous melanoma (according to the AJCC Cancer Staging Manual, 2002) with no available standard therapy, or locoregionally recurrent melanoma with no therapeutic surgical option; Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) of ≤ 1; and life expectancy of ≥ 12 weeks. Key exclusion criteria were brain metastases or spinal cord compression; inadequate bone marrow, liver and renal function; evidence of severe or uncontrolled systemic diseases; unresolved toxicity ≥ CTC grade 2 from previous anti-cancer therapy except alopecia; participation in a trial of an investigational agent within the 30 days prior to study; pregnant or breast-feeding females; patients with an active seizure disorder; QTc interval of greater than 450 milliseconds (males) and 480 milliseconds (females); known HIV infection; immunosuppressive therapy including corticosteroids within 4 weeks of screening.
Safety assessments
Safety assessments included continuous adverse event data collection and the results of baseline and weekly vital sign measurements, electrocardiograms and traces, physical examinations and clinical laboratory tests including hematology, biochemistry, urinalysis, serum ferritin, and CRP and serum cytokine values. Adverse events were graded according to the NCI Common Terminology Criteria for Adverse Events (CTCAE v.3.0). In Cohorts A and B, DLT assessments were conducted on AEs occurring from post-dose on Day 0 to Day 56 and for Cohort C from post-dose on Day 0 until Day 49.
Delayed-type hypersensitivity assessment
Delayed-type hypersensitivity (DTH) skin tests were performed using 100–200 µL of the MM200 membrane-vesicle component of the vaccine (component LPVN [A]09/1), which was given by subcutaneous injection in the forearm of subjects. A positive reaction was defined as skin erythema and induration ≥ 5 mm and was measured 48 h after the injection. Sterile phosphate-buffered saline was used as a negative control. For Cohorts A and B, the DTH injection was given on Day 84 and for Cohort C, the injection was given at screening and on Day 42.
Primary immunologic data
Immune responses were measured for Cohorts A and B on Day 0, 7 days after each vaccination, and 28 days after the third dose. For Cohort C, immune responses were measured on Days 0, 28 and 42. Lipovaxin-MM-specific cell-mediated immune responses were evaluated using several different assays: (i) production of cytokines (IFNγ, TNF, IL-2, IL-4, IL-17, IL-10, and lymphotoxin) by cultured peripheral blood mononuclear cells (PBMCs) using the Cytometric Bead Array (CBA)(Becton, Dickinson and Company, Franklin Lakes, NJ); (ii) production of IFNγ by cultured PBMCs using intracellular cytokine staining and Enzyme-Linked ImmunoSpot (ELISpot) analysis; (iii) leukocyte subsets (CD20, CD3, CD4, CD8, CD11b, CD11c, CD16/CD56, CD19) and activation markers (CD69, CD25, CD44, CD45RA, CD45RO and CD62L) by flow cytometry; (iv) Lipovaxin-MM-specific antibodies by enzyme-linked immunosorbent assay (ELISA); and (v) cytokine levels in serum by ELISA. For assays (i) and (ii), autologous patient-derived monocytic dendritic cells were used to enable in vitro restimulation of patient PBMCs. Formalin-fixed paraffin-embedded (FFPE) melanoma tissues from patients were used in immunohistochemistry analysis for the expression of a key melanoma antigen melan-A/MART-1.
Efficacy measures
Tumor responses were determined for all patients with measurable lesions using RECIST criteria (v1.0). The assessments were made using computed tomographic (CT) scanning at screening, then every 8 weeks for Cohorts A and B, and every 6 weeks for Cohort C. Partial response status required confirmation at least 4 weeks after the scan demonstrating partial response. Stable disease status was only assigned if the tumor assessment occurred at a minimum interval of at least 8 weeks after the baseline CT scan.
Preparation of vaccine for injection
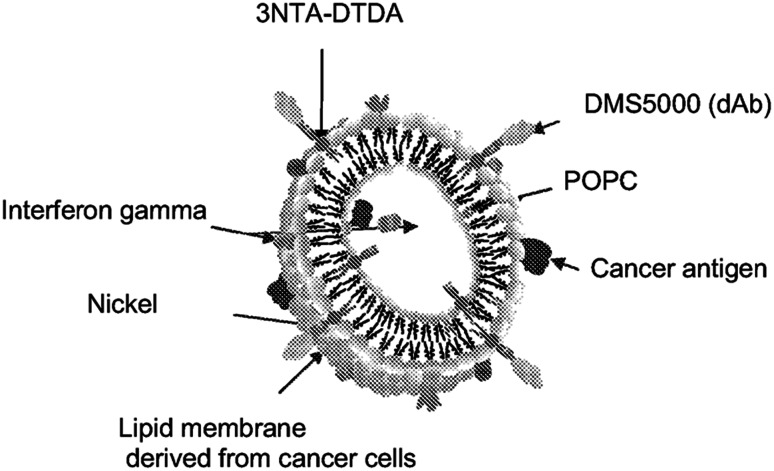
Each dose of Lipovaxin-MM was formulated at the Royal Adelaide Hospital pharmacy. If the final product could not be administered immediately to the patient, it was stored at 2–8 °C, but all products were administered to the patient within 6 h of product formulation. The vaccine was formulated from 4 pre-mix components: MM200 membrane vesicles (prepared at 107 cells/mL; component LPVN[A]09/1), lyophilized POPC/Ni-3NTA-DTDA liposomes (0.98 mM, 60µΜ, 20µM, respectively; component LPVN[B]09/1), IFNγ (40,000U/mL [equivalent to dose of 2 µg/mL] Imukin, Boehringer Ingelheim, Ingelheim am Rhein, Germany; component LPVN[C]09/1), and the DC-SIGN-specific domain Ab DMS5000 (26µM; component LPVN[D]09/1) (Fig. 2). Each dose of Lipovaxin-MM prepared was tested for DC-SIGN binding, IFN-γ activity, protein content and melanoma antigen profile (gp100, tyrosinase and melanA). For the vaccine doses of 0.1 and 1 mL, the appropriate dose of vaccine was administered in normal saline. The vaccine was given by slow intravenous injection into a peripheral arm vein at a rate no greater than 1 mL/min, by a chemotherapy certified nurse.
Fig. 2.
Diagrammatic representation of Lipovaxin-MM lipids derived from the MM200 membrane fraction, POPC and various cancer antigens are visible. The dendritic cell-targeting domain antibody DMS5000 is also depicted. Interferon gamma is depicted as being mainly associated with the outer surface of the vaccine
Results
This study was completed when neither BRAF inhibitors nor immune checkpoint inhibitors were approved or publically reimbursed in Australia. Out of 14 screened subjects, 12 were allocated to study treatment in three cohorts and were included in the intent to treat analysis (Fig. 1). Three subjects each were recruited in Cohorts A (0.1 mL) and B (1 mL), and six subjects were recruited in Cohort C (3 mL). Another 10 mL dose, although planned, was not tested because of the technical challenges associated with the preparation of the larger dose. No DLT was observed, and therefore, the MTD of the vaccine was not determined.
All subjects had a European ethnic origin, AJCC stage IV malignant melanoma, and ECOG PS ≤ 1. In both Cohorts A and B, two subjects were male and one subject was female. In Cohort C, three subjects were male and three subjects were female. The mean age of subjects in Cohorts A, B and C were 69.7, 53 and 61.5 years, respectively (Table 1).
Table 1.
Subject demographic data
| Patient | Age | Sex | Stage at start of treatment | ECOG performance status | Previous systemic therapies for melanoma | |
|---|---|---|---|---|---|---|
| AJCC | TNM | |||||
| 001001 | 63 | M | IV | M1a | 0 | Dacarbazine |
| 001002 | 76 | M | IV | M1c | 0 | Nab-paclitaxel |
| 001003 | 73 | F | IV | M1a | 0 | Dacarbazine |
| 001004 | 55 | M | IV | M1c | 1 | VMCL vaccinea |
| 001006 | 61 | M | IV | M1c | 1 | Dacarbazine |
| 001007 | 45 | F | IV | M1b | 0 | No prior systemic therapyb |
| 001008 | 28 | F | IV | M1c | 0 | BRAF inhibitor, ipilimumabc, dacarbazine |
| 001009 | 68 | M | IV | M1c | 0 | Nab-paclitaxel |
| 001010 | 78 | M | IV | M1c | 0 | No prior treatment |
| 001012 | 46 | F | IV | M1c | 0 | Nab-paclitaxel |
| 001013 | 70 | F | IV | M1c | 0 | Interferon therapy, dacarbazine |
| 001014 | 78 | M | IV | M1c | 0 | No prior systemic therapy |
AJCC American Joint Committee on Cancer staging (6th edition), TNM (Tumor Node Metastasis) stage, ECOG Eastern Cooperative Oncology Group
aPrevious immunotherapy (vaccine)—protocol violation
bPreviously received carboplatin, paclitaxel, 5-fluorouracil, methotrexate, cyclophosphamide and trastuzumab for ovarian and breast cancer
cRoyal Adelaide Hospital Human Research Ethics Committee granted a protocol waiver for previous immunotherapy (ipilimumab) because of the patient’s exceptional youth
All 12 subjects received at least one dose of the study vaccine and were included in the safety population. Four subjects were withdrawn from the study because of disease progression. Three subjects received two doses and one subject received one dose of vaccine before withdrawal. Subject 001 in Cohort A was escalated to a 1 mL dose for the third dose because a tumor response was observed after two doses. Six patients in Cohort C received four scheduled vaccine doses. A minor protocol violation was discovered after enrollment of subject 004 (Cohort B) who received three (1 mL) doses of the study vaccine but who had also had previous investigational immunotherapy. Specifically, the patient had received multiple intradermal injections of VCML (vaccinia virus-induced melanoma cell lysate), the last of which was administered approximately 19 months before enrollment into the study.
Adverse events
No DLTs were observed during the study. No subject was withdrawn from the study because of an AE. Grade 4 or 5 AEs were not observed during the protocol-specified study evaluation periods. Two serious adverse events (SAEs) were recorded after the hospitalization of two subjects during the study and were classified as grade 2 in severity: asymptomatic atrial fibrillation (possibly vaccine-related) and pneumonia (not vaccine-related).
A total of 94 adverse events (AEs) (83.3%) were reported in ten subjects across the three dose cohorts (Table 2). The majority of AEs (67%) were classified as grade 1, with 27% assessed as grade 2 and 7% as grade 3.
Table 2.
Adverse events according to NCI CTCAE (v3.0) grades
| Cohort A (n = 3) subject events (%) | Cohort B (n = 3) subject events (%) | Cohort C (n = 6) subject events (%) | |
|---|---|---|---|
| Subjects with at least one AE (n = 94) | 3 31 (33%) | 3 23 (24%) | 4 40 (43%) |
| Grade 1 AE (n = 63) | 3 26 (41%) | 3 14 (22%) | 3 23 (37%) |
| Grade 2 AE (n = 25) | 2 4 (16%) | 3 8 (32%) | 4 13 (52%) |
| Grade 3 AE (n = 6) | 1 1 (17%) | 1 1 (17%) | 2 4 (66%) |
AE adverse event
Forty-three AEs reported in six subjects out of a total of 94 events were considered possibly or probably vaccine related (Table 3), although no AE was definitely attributed to the vaccine. Of these, 41 were grade 1 or 2 AEs and included one AE of pruritus (in a Cohort A subject), which was considered to be probably vaccine-related. Two other AEs, anemia and lethargy (reported as grade 3 in one Cohort B subject), were also considered to be probably vaccine-related. Subject 001 reported the most frequent AEs, which included dizziness (four events) and musculoskeletal pain (eight events).
Table 3.
Summary of vaccine-related adverse events by system organ class
| System organ class | Grade 1 (n = 35) subject events, n (%) | Grade 2 (n = 6) subject events, n (%) | Grade 3 (n = 2) subject events, n (%) |
|---|---|---|---|
| Musculoskeletal and connective tissue disorders | |||
| Musculoskeletal pain | 1 8 (23%) | ||
| General disorders and administration site conditions | |||
| Pruritus | 1 1 (3%) | ||
| Fatigue | 2 2 (6%) | ||
| Thirst | 1 1 (3%) | ||
| Pyrexia | 1 4 (12%) | ||
| Nervous system disorders | |||
| Dysgeusia | 1 1 (17%) | ||
| Dizziness | 1 4 (12%) | ||
| Lethargy | 1 1 (3%) | 1 1 (50%) | |
| Sensory neuropathy | 1 1 (3%) | ||
| Infections and infestations | |||
| Cold symptoms | 1 2 (6%) | ||
| Flu symptoms | 1 2 (6%) | ||
| Gastrointestinal disorders | |||
| Nausea | 1 1 (3%) | ||
| Gastroesophageal reflux symptoms | 1 1 (3%) | ||
| Investigations | |||
| Weight gain | 1 1 (3%) | 1 1 (17%) | |
| Low serum iron | 1 1 (17%) | ||
| Increased basal temperature | 1 2 (6%) | ||
| Metabolism and nutrition disorders | |||
| Anorexia | 2 2 (6%) | 1 1 (17%) | |
| Skin and subcutaneous tissue disorders | |||
| Pruritus | 1 1 (3%) | ||
| Cold sweats | 1 1 (17%) | ||
| Respiratory disorders | |||
| Rhinorrhea | 1 1 (3%) | ||
| Blood and lymphatic system disorders | |||
| Anemia | 1 1 (50%) | ||
| Cardiac disorders | |||
| Atrial fibrillation | 1 1 (17%) | ||
Possibly or probably vaccine-related adverse events
Significant changes in health status, as determined by safety laboratory parameters (hematology, biochemistry, urinalysis, serum ferritin, CRP, serum cytokines), vital signs, ECGs, physical examination and use of concomitant medications, were not observed in any of the patients at any of the protocol-specified time points. No trends were observed between the dose cohorts with regard to the frequency, study vaccine relationship or severity of AEs.
Tumor response
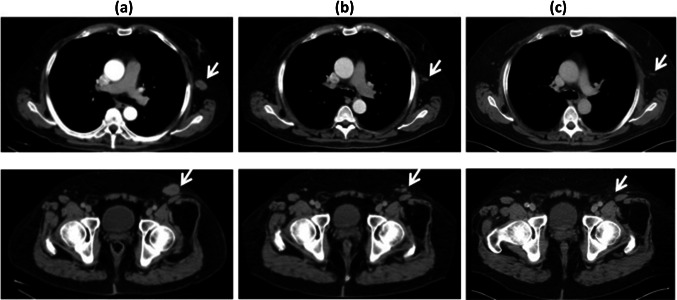
Tumor responses are summarized in Table 4. In Cohort A, one patient (001) had a partial response (PR) demonstrated at his restaging CT scan after receiving all three scheduled vaccine doses. He had a 55% reduction in the size of all three target lesions including a left lateral chest wall lesion and left inguinal lymphadenopathy, the latter lesion resolving completely (Fig. 3). Interestingly, this patient developed an itch at the left inguinal tumor site several days after the first Lipovaxin-MM infusion and before shrinkage of the lesion. The PR persisted from the end of the study (Day 112) until unequivocal progression of a 4-cm left flank, non-target, subcutaneous lesion (observed at baseline), which was documented on a CT scan 52 weeks after the first vaccine dose and which led to initiation of ipilimumab therapy two weeks later (Table 4).
Table 4.
Tumor responses and number of vaccine doses received
| Patient | Number of doses received | Best overall response | New lesions | Day of final study assessment | Duration of response | Study completion |
|---|---|---|---|---|---|---|
| 001001 | 3 | PR | No | Day 112 | 52 weeks | Completed study |
| 001002 | 2 | PD | Yes | Day 56 | NA | Withdrawnb |
| 001003 | 1 | PD | Yes | Day 26 | NA | Withdrawnb |
| 001004 | 3 | SD | Yes | Day 112 | 8 weeks | Protocol violationa |
| 001006 | 2 | PD | Yes | Day 56 | NA | Withdrawnb |
| 001007 | 2 | PD | No | Day 56 | NA | Withdrawnb |
| 001008 | 4 | PD | No | Day 42 | NA | Protocol waivera |
| 001009 | 4 | PD | No | Day 84 | NA | Completed study |
| 001010 | 4 | PD | No | Day 84 | NA | Completed study |
| 001012 | 4 | PD | No | Day 84 | NA | Completed study |
| 001013 | 4 | SD | No | Day 84 | 6 weeks | Completed study |
| 001014 | 4 | PD | No | Day 84 | NA | Completed study |
PR partial response, PD progressive disease, SD stable disease
aSubject had previous immunotherapy
bWithdrawn because progressive disease
Fig. 3.
Computed tomographic (CT) scans showing responses for two of three target lesions in Subject 001. a Lesions at baseline, b first CT response assessment after all three cycles of Lipovaxin-MM (week 8), c after completion of treatment (and off-study; week 25). Upper panels show CT scans of chest; lower panels show CT scans of abdomen/pelvis
Two Cohort A subjects, 002 who received two doses and 003 who received one dose, were withdrawn from the study before Day 56 because of progressive disease (PD). In Cohort B, two subjects, who each received two doses, were withdrawn from the study because of PD. Subject 004 in Cohort B, who received three doses, had RECIST-defined stable disease (SD) at Day 56 but had PD at Day 112. In Cohort C, all six patients received four vaccine doses. Five subjects had PD at the first tumor assessment on Day 42. The remaining subject (013) had RECIST-defined SD on Day 42 but subsequently had PD at the second assessment on Day 84.
Immunologic evaluation
At screening, all patients demonstrated evidence of peripheral blood lymphocyte (PBL) responsiveness in vitro by measurement of intracellular IFNγ production in CD4+ and CD8+ T cells after stimulation by the T-cell mitogen, phytohaemagglutinin (PHA). Targeting and maturation of DC by Lipovaxin-MM was tested in vitro for selected patients using a fluorescently labelled Lipovaxin-MM, flow cytometric analysis of activation markers and detection of IL-12 production by ELISA (Supplementary Fig. 1), and each dose of Lipovaxin-MM was confirmed to contain three key melanoma antigens (gp100, melanA/MART-1 and tyrosinase) by western blot (Supplementary Fig. 2).
After administration of Lipovaxin-MM, there were no consistent and meaningful patterns observed in the levels of circulating antibody to vaccine components, in the proportions of different PBL subsets, the expression of surface activation markers by these subsets, or in the levels of secreted cytokines as measured by CBA (Supplementary Fig. 3–7). T-cell responses were also measured by IFNγ ELISpot assay following ex vivo restimulation with vaccine components. For some patients T-cell responses were elevated significantly above baseline and negative control levels, suggesting that DCs had been successfully targeted in vivo. Responses to DMS500 antibody and dummy Lipovaxin vesicle (Lipovaxin-MM minus IFNγ) components of the vaccine were detected in 5/8 patients analyzed. Less frequently, IFNγ ELISpot responses to the MM200 plasma membrane vesicles containing melanoma antigens were observed in 2/8 patients analyzed (Subjects 008 and 013, Supplementary Fig. 8). MelanA/MART-1 expression was confirmed for 6/9 available patient tumor samples (Supplementary Fig. 2). Interestingly for Subject 001, in the post-vaccination specimen of the progressing subcutaneous non-target tumor, which had been resected from his left flank after ipilimumab and then dacarbazine chemotherapy, expression of melanA was not detected unlike in the pre-vaccination resected small bowel metastasis (Supplementary Fig. 2).
In summary, with the exception of rare T-cell responses to melanoma antigens contained within the liposomal vaccine as measured by ELISPOT, significant cellular and humoral immune responses were not detected in the blood of study subjects, including for Subject 001 where an objective tumor response was observed.
Delayed-type hypersensitivity assessment
Although positive DTH responses were not observed, two subjects in Cohort A (001 and 003) developed post-vaccination erythema (without induration) 48 h after injection of MM200 PMVs. No erythema or induration was observed for the remaining subjects in Cohorts B and C.
Discussion
In this phase I study, we evaluated the safety and immunogenicity of Lipovaxin-MM, a dendritic cell-targeted liposomal vaccine. The vaccine consists of liposome particles that are prepared to carry melanoma cell-derived membrane-associated antigens, multiple copies of an antibody fragment specific for the DC-SIGN receptor and a small dose of human IFNγ. The results showed that the vaccine was safe and well tolerated in metastatic melanoma patients. One confirmed objective tumor response was seen in the first enrolled subject. Two patients, one patient each in Cohorts B and C, had stable disease at the first evaluation done on Days 56 and 42, respectively. The remaining patients exhibited progressive disease.
None of the subjects experienced a DLT, and hence, a maximum tolerated dose was not determined. Moreover, clinically significant toxicities were not observed, and most AEs were grade 1 or 2. Only six AEs were grade 3 and two of these (anemia and lethargy) in one subject were deemed to be probably vaccine-related. High-grade AEs were not recorded.
Before this first-time-in-human clinical trial, a preclinical study used a vaccine composed of PMVs derived from the B16-OVA murine melanoma cell line fused with liposomes encapsulating IFNγ and carrying the 3NTA-DTDA metal chelator lipid and His-tagged recombinant antibody fragments specific for the murine DC surface receptors, CD11c and DEC205. In this study, the modified and DC-targeted PMVs were shown to target melanoma antigens to DCs in vivo following intravenous administration, with the consequent induction of potent tumor antigen-specific immune responses and marked anti-melanoma activity [17]. In another study, PMVs derived from P815 tumor cells, which were modified to encapsulate the cytokines, IL-2 and IL-12, and which were engrafted with the T-cell costimulatory molecules, CD80 and CD40, showed induction of potent antitumor immune responses and tumor regression when used as vaccines in syngeneic mice [16, 18]. Therefore, a preclinical rationale was provided for using DC-targeted liposomes as a simpler and less expensive way to manufacture a melanoma vaccine than previous preparations of ex vivo antigen-loaded DC vaccines.
In our study, we used DMS 5000, a domain antibody specific for DC-SIGN, which is a DC internalizing surface receptor [11] expressed in vivo by immature DC in peripheral tissues as well as on DC present in secondary lymphoid tissues such as lymph nodes, tonsils, and spleen [19]. It was hypothesized that intravenous administration of Lipovaxin-MM would target immature DC, and in the presence of the cytokine IFN-γ and melanoma antigens, DC maturation and presentation of tumor antigens to T-cells in lymphoid organs would be more effective.
DC targeting by Lipovaxin-MM was demonstrated in vitro, and the presence of key melanoma antigens including melanA was confirmed for all vaccine doses (Supplementary Figs. 1 and 2). The plasma membranes of the MM200 cell line in Lipovaxin-MM contain multiple tumor antigens. Given that metastatic melanoma is known to be highly heterogeneous [20], we assayed for the presence of a common, immunogenic melanoma differentiation antigen, melanA/MART-1, in pre-vaccination melanoma tumor tissues of the study patients with expression detected in 6/9 patient samples (Supplementary Fig. 2).
Although T-cell responses to vaccine components were detected by IFN-γ ELISpot following ex vivo restimulation, only 2/8 evaluable patient PBMC samples showed specific post-vaccination IFN-γ production after restimulation with MM200 PMVs (Supplementary Fig. 8). In addition, no other consistent post-vaccination immune responses were observed, even in the case of the single responding subject (Supplementary Fig. 3–7). We have therefore concluded there was minimal immunogenicity, with no apparent relationship to the clinical activity associated with the vaccine.
Despite our current findings, correlation between immunologic and clinical responses has been observed in a previous multiple peptide vaccine study conducted in the adjuvant setting after complete resection of melanoma [21]. However, the subjects in our study had advanced metastatic disease and also showed lower than expected responsiveness to the tetanus toxoid control antigen in the in vitro stimulation assays (Supplementary Fig. 3). These findings can indicate age-related immuno-senescence [22, 23], disease-related immune suppression [24, 25], or both, and can thus be associated with inhibited melanoma-specific and non-specific immune responses.
It has been acknowledged that active immunotherapy with melanoma antigens has been less than successful in inducing anti-melanoma activity [26]. More recently, the remarkable success of immune checkpoint blockade in metastatic melanoma treatment [27] has shown that endogenous repression of pre-existing anti-melanoma immunity is more common than lack of anti-melanoma immunity per se. Another reason for impaired anti-melanoma immunity is the reduced quantity and quality of intratumoral DCs, which are required to prime T cell-mediated immunity [28, 29]. Recent data indicate melanoma-intrinsic signaling impairs recruitment of key DC subpopulations responsible for cross-priming CD8+ T cells [30]. As all study subjects had advanced disease, systemic immune suppression may contribute to the observed lack of specific vaccine-induced immune responses. Hence, optimal active melanoma immunotherapy may require not only the induction of melanoma-specific responses but also the reversal of immune suppression [31]. Alternative routes of administration, such as subcutaneous, intradermal or intratumoral, may further enhance DC targeting and cell-mediated immune responses to melanoma antigens.
Given that Subject 001, whose partial tumor response was rapid, did not demonstrate vaccine-specific immunity, it is possible that he had an IFN-γ−ρεσπονσιϖε tumor that had reacted to a small amount of IFN-γ delivered at the tumor site by Lipovaxin-MM. Indeed, any such effect need not have resulted from DC-targeting because of the well-known phenomenon of enhanced tumor permeability and retention of liposomal particles [32]. A previous literature describes infrequent clinical anti-melanoma effects of systemic and intratumoral administration of IFN-γ [33–35]. Higher IFN-γ doses may increase the overall vaccine efficacy but would need to be explored in further studies.
As a final remark, Lipovaxin-MM is a complex multi-component allogeneic liposome-based vaccine, which uses the Lipovaxin metallo-chelating liposome platform. Although we exploited the Lipovaxin platform exclusively to decorate the surface of tumor-derived membrane vesicles with multiple copies of the DC-targeted molecule, DMS5000, the platform can easily be adapted as a delivery system for surface-attached synthetic peptides or recombinant protein antigens. The versatility of liposomes as delivery vehicles is well accepted and the physicochemical attributes of Lipovaxin liposomes could provide the means for active modification of the tumor microenvironment with resulting favorable antitumor effects [36].
Conclusions
This study successfully demonstrated that Lipovaxin-MM, a DC-targeted liposomal vaccine against melanoma, is safe and feasible to administer in further clinical studies. One partial response and two instances of stable disease were seen. PBL responsiveness, although seen in vitro at initial screening, did not result in any meaningful association between immune and clinical responses even when a clinical response was seen. Further exploitation of the Lipovaxin platform for immunotherapeutic applications is possible.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We acknowledge the technical assistance of Ms Kathlyn Carman at SA Pathology, and the work of the staff of the RAH Cancer Clinical Trials Unit. We dedicate this publication to the memory of Richard Perkins.
Abbreviations
- 3NTA-DTDA
3(nitrilotriacetic acid)-ditetradecylamine
- AE
Adverse event
- AJCC
American Joint Committee on Cancer staging (6th edition)
- BRAF
B-raf kinase protein
- CLR
C-type lectin receptor
- CR
Complete response
- CRP
C-reactive protein
- CT
Computed tomographic
- CTCAE
Common Terminology Criteria for Adverse Events
- DC-SIGN
Dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin
- DLT
Dose-limiting toxicity
- DMS-5000
a DC-SIGN-specific VH single domain antibody fragment
- DTH
Delayed-type hypersensitivity
- ECOG
Eastern Cooperative Oncology Group
- FFPE
Formalin-fixed paraffin embedded
- Lipovaxin-MM
Multi-component dendritic cell-targeted liposomal vaccine against metastatic melanoma
- LPVN
Lipovaxin component
- melanA/MART1
Melanoma antigen recognized by T cells 1
- MAPK
Mitogen activated protein kinase
- MM200
Human melanoma cell line MM200
- NCI
National Cancer Institute
- PD
Progressive disease
- PMV
Plasma membrane vesicles
- POPC
α−palmitoyl-ß−oleoyl-phosphatidylcholine
- PR
Partial response
- RAH
Royal Adelaide Hospital
- SD
Stable disease
- VH
Human immunoglobulin heavy chain variable domain
Author contributions
Trial conception and design: PR, IICA, JA, CRP; development of methodology: PR, JDP, KMG, AF, AR; acquisition of data: TG, PR, JDP, KMG, AF, AR, MPB; analysis and interpretation of data: TG, MPB, AR, AF. Writing, review, and/or revision of the manuscript: TG, MNA, CRP, PR, MPB; administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): TG, JDP, KMG, IICA, MNA, MPB; study supervision: MPB, IICA.
Funding
The clinical trial was funded by Lipotek Pty Ltd in a grant paid to Royal Adelaide Hospital.
Compliance with ethical standards
Conflict of interest
Jason D. Price, Katharine M. Gosling, and Ines I.C. Atmosukarto are employed by Lipotek Pty Ltd. Paul Rolan, Joseph Altin, and Christopher R. Parish are Lipotek shareholders. Paul Rolan and Ines I.C. Atmosukarto are Directors of Lipotek. All other authors declare that they have no conflict of interest.
Ethical approval and ethical standards
The Royal Adelaide Hospital Human Research Ethics Committee provided specific written approval of the Clinical Protocol and its subsequent amendments before participants were enrolled to the study (RAH Protocol No: 081124). This study was performed in accordance with the Therapeutic Goods Administration (TGA) Note for Guidance on Good Clinical Practice (2000), the ethical rules contained in the National Statement on Ethical Conduct in Human Research (2007) and the Declaration of Helsinki (1964). The trial was registered with the Australia and New Zealand Clinical Trials Registry (ACTRN12610000149066).
Informed consent
Informed written consent was obtained from all patients before enrollment.
Footnotes
Sections of this work have been previously published in the conference proceedings for the Clinical Oncology Society of Australia, COSA, 16th–19th November 2015, Hobart Tasmania, [1].
References
- 1.Abbas N, Rolan P, Price JD, Gosling KM, Atmosukarato IIC, Parish CR, Brown MP. Final report of a phase I trial of lipovaxin-MM, a novel dendritic cell-targeted liposomal vaccine for malignant melanoma. Asia-Pacific J Clin Oncol. 2015;11(S4):46–53. doi: 10.1007/s00262-018-2207-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S, Eggermont AM, Flaherty KT, Gimotty PA, Kirkwood JM, McMasters KM, Mihm MC, Morton DL, Ross MI, Sober AJ, Sondak VK. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keller HR, Zhang X, Li L, Schaider H, Wells JW. Overcoming resistance to targeted therapy with immunotherapy and combination therapy for metastatic melanoma. Oncotarget. 2017;8(43):75675–75686. doi: 10.18632/oncotarget.18523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12(4):265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Figdor CG, de Vries IJ, Lesterhuis WJ, Melief CJ. Dendritic cell immunotherapy: mapping the way. Nat Med. 2004;10(5):475–480. doi: 10.1038/nm1039. [DOI] [PubMed] [Google Scholar]
- 7.Anguille S, Smits EL, Bryant C, Van Acker HH, Goossens H, Lion E, Fromm PD, Hart DN, Van Tendeloo VF, Berneman ZN. Dendritic Cells as Pharmacological Tools for Cancer Immunotherapy. Pharmacol Rev. 2015;67(4):731–753. doi: 10.1124/pr.114.009456. [DOI] [PubMed] [Google Scholar]
- 8.Cohn L, Delamarre L. Dendritic cell-targeted vaccines. Front Immunol. 2014;5:255. doi: 10.3389/fimmu.2014.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bozzuto G, Molinari A. Liposomes as nanomedical devices. Int J Nanomedicine. 2015;10:975–999. doi: 10.2147/IJN.S68861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geijtenbeek TB, van Vliet SJ, Engering A, t Hart BA, van Kooyk Y. Self- and nonself-recognition by C-type lectins on dendritic cells. Annu Rev Immunol. 2004;22:33–54. doi: 10.1146/annurev.immunol.22.012703.104558. [DOI] [PubMed] [Google Scholar]
- 11.Engering A, Geijtenbeek TB, van Vliet SJ, Wijers M, van Liempt E, Demaurex N, Lanzavecchia A, Fransen J, Figdor CG, Piguet V, van Kooyk Y. The dendritic cell-specific adhesion receptor DC-SIGN internalizes antigen for presentation to T cells. J Immunol. 2002;168(5):2118–2126. doi: 10.4049/jimmunol.168.5.2118. [DOI] [PubMed] [Google Scholar]
- 12.Tacken PJ, de Vries IJ, Gijzen K, Joosten B, Wu D, Rother RP, Faas SJ, Punt CJ, Torensma R, Adema GJ, Figdor CG. Effective induction of naive and recall T-cell responses by targeting antigen to human dendritic cells via a humanized anti-DC-SIGN antibody. Blood. 2005;106(4):1278–1285. doi: 10.1182/blood-2005-01-0318. [DOI] [PubMed] [Google Scholar]
- 13.Kretz-Rommel A, Qin F, Dakappagari N, Torensma R, Faas S, Wu D, Bowdish KS. In vivo targeting of antigens to human dendritic cells through DC-SIGN elicits stimulatory immune responses and inhibits tumor growth in grafted mouse models. J Immunother. 2007;30(7):715–726. doi: 10.1097/CJI.0b013e318135472c. [DOI] [PubMed] [Google Scholar]
- 14.Hersey P, Edwards A, Coates A, Shaw H, McCarthy W, Milton G. Evidence that treatment with vaccinia melanoma cell lysates (VMCL) may improve survival of patients with stage II melanoma. Treatment of stage II melanoma with viral lysates. Cancer Immunol Immunother. 1987;25(3):257–265. doi: 10.1007/BF00199156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Altin JG, Parish CR. Liposomal vaccines–targeting the delivery of antigen. Methods. 2006;40(1):39–52. doi: 10.1016/j.ymeth.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 16.van Broekhoven CL, Altin JG. The novel chelator lipid 3(nitrilotriacetic acid)-ditetradecylamine (NTA(3)-DTDA) promotes stable binding of His-tagged proteins to liposomal membranes: potent anti-tumor responses induced by simultaneously targeting antigen, cytokine and costimulatory signals to T cells. Biochim Biophys Acta. 2005;1716(2):104–116. doi: 10.1016/j.bbamem.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 17.van Broekhoven CL, Parish CR, Demangel C, Britton WJ, Altin JG. Targeting dendritic cells with antigen-containing liposomes: a highly effective procedure for induction of antitumor immunity and for tumor immunotherapy. Cancer Res. 2004;64(12):4357–4365. doi: 10.1158/0008-5472.CAN-04-0138. [DOI] [PubMed] [Google Scholar]
- 18.Van Broekhoven CL, Altin JG. A novel system for convenient detection of low-affinity receptor-ligand interactions: chelator-lipid liposomes engrafted with recombinant CD4 bind to cells expressing MHC class II. Immunol Cell Biol. 2001;79(3):274–284. doi: 10.1046/j.1440-1711.2001.01010.x. [DOI] [PubMed] [Google Scholar]
- 19.Geijtenbeek TB, Torensma R, van Vliet SJ, van Duijnhoven GC, Adema GJ, van Kooyk Y, Figdor CG. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100(5):575–585. doi: 10.1016/S0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- 20.Harbst K, Lauss M, Cirenajwis H, Winter C, Howlin J, Torngren T, Kvist A, Nodin B, Olsson E, Hakkinen J, Jirstrom K, Staaf J, Lundgren L, Olsson H, Ingvar C, Gruvberger-Saal SK, Saal LH, Jonsson G. Molecular and genetic diversity in the metastatic process of melanoma. J Pathol. 2014;233(1):39–50. doi: 10.1002/path.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slingluff CL, Petroni GR, Chianese-Bullock KA, Smolkin ME, Hibbitts S, Murphy C, Johansen N, Grosh WW, Yamshchikov GV, Neese PY, Patterson JW, Fink R, Rehm PK. Immunologic and clinical outcomes of a randomized phase II trial of two multipeptide vaccines for melanoma in the adjuvant setting. Clin Cancer Res. 2007;13(21):6386–6395. doi: 10.1158/1078-0432.CCR-07-0486. [DOI] [PubMed] [Google Scholar]
- 22.Lang PO, Govind S, Bokum AT, Kenny N, Matas E, Pitts D, Aspinall R. Immune senescence and vaccination in the elderly. Curr Top Med Chem. 2013;13(20):2541–2550. doi: 10.2174/15680266113136660181. [DOI] [PubMed] [Google Scholar]
- 23.Weinberger B, Schirmer M, Matteucci Gothe R, Siebert U, Fuchs D, Grubeck-Loebenstein B. Recall responses to tetanus and diphtheria vaccination are frequently insufficient in elderly persons. PLoS One. 2013;8(12):e82967. doi: 10.1371/journal.pone.0082967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Gast GC, The TH, Schraffordt Koops H, Huiges HA, Oldhoff J, Nieweg HO. Humoral and cell-mediated immune response in patients with malignant melanoma. I. In vitro lymphocyte reactivity to PHA and antigens following immunization. Cancer. 1975;36(4):1289–1297. doi: 10.1002/1097-0142(197510)36:4<1289::AID-CNCR2820360415>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 25.Lizee G, Radvanyi LG, Overwijk WW, Hwu P. Immunosuppression in melanoma immunotherapy: potential opportunities for intervention. Clin Cancer Res. 2006;12(7 Pt 2):2359s–2365s. doi: 10.1158/1078-0432.CCR-05-2537. [DOI] [PubMed] [Google Scholar]
- 26.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10(9):909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33(17):1974–1982. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tucci M, Stucci S, Passarelli A, Giudice G, Dammacco F, Silvestris F. The immune escape in melanoma: role of the impaired dendritic cell function. Expert Rev Clin Immunol. 2014;10(10):1395–1404. doi: 10.1586/1744666X.2014.955851. [DOI] [PubMed] [Google Scholar]
- 29.Hargadon KM, Bishop JD, Brandt JP, Hand ZC, Ararso YT, Forrest OA. Melanoma-derived factors alter the maturation and activation of differentiated tissue-resident dendritic cells. Immunol Cell Biol. 2016;94(1):24–38. doi: 10.1038/icb.2015.58. [DOI] [PubMed] [Google Scholar]
- 30.Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature. 2015;523(7559):231–235. doi: 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- 31.Atkins M. Immunotherapy combinations with checkpoint inhibitors in metastatic melanoma: current approaches and future directions. Semin Oncol. 2015;42(Suppl 3):S12–S19. doi: 10.1053/j.seminoncol.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 32.Harrington KJ, Mohammadtaghi S, Uster PS, Glass D, Peters AM, Vile RG, Stewart JS. Effective targeting of solid tumors in patients with locally advanced cancers by radiolabeled pegylated liposomes. Clin Cancer Res. 2001;7(2):243–254. [PubMed] [Google Scholar]
- 33.Schiller JH, Pugh M, Kirkwood JM, Karp D, Larson M, Borden E. Eastern cooperative group trial of interferon gamma in metastatic melanoma: an innovative study design. Clin Cancer Res. 1996;2(1):29–36. [PubMed] [Google Scholar]
- 34.Ernstoff MS, Trautman T, Davis CA, Reich SD, Witman P, Balser J, Rudnick S, Kirkwood JM. A randomized phase I/II study of continuous versus intermittent intravenous interferon gamma in patients with metastatic melanoma. J Clin Oncol. 1987;5(11):1804–1810. doi: 10.1200/JCO.1987.5.11.1804. [DOI] [PubMed] [Google Scholar]
- 35.Nemunaitis J, Fong T, Burrows F, Bruce J, Peters G, Ognoskie N, Meyer W, Wynne D, Kerr R, Pippen J, Oldham F, Ando D. Phase I trial of interferon gamma retroviral vector administered intratumorally with multiple courses in patients with metastatic melanoma. Hum Gene Ther. 1999;10(8):1289–1298. doi: 10.1089/10430349950017978. [DOI] [PubMed] [Google Scholar]
- 36.Kobayashi H, Watanabe R, Choyke PL. Improving conventional enhanced permeability and retention (EPR) effects; what is the appropriate target? Theranostics. 2013;4(1):81–89. doi: 10.7150/thno.7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.