Abstract
Despite the significant progress in tumor prevention, early detection, diagnosis and treatment made over recent decades, cancer is still an enormous public health challenge all around the world, with the number of people affected increasing every year. A great deal of effort is therefore being devoted to the search for novel safe, effective and economically sustainable treatments for the growing population of neoplastic patients. One main obstacle to this process is the extremely low percentage of therapeutic approaches that, after successfully passing pre-clinical testing, actually demonstrate activity when finally tested in humans. This disappointing and expensive failure rate is partly due to the pre-clinical murine models used for in vivo testing, which cannot faithfully recapitulate the multifaceted nature and evolution of human malignancies. These features are better mirrored in natural disease models, i.e., companion animals affected by cancers. Herein, we discuss the relevance of spontaneous canine tumors for the evaluation of the safety and anti-tumor activity of novel therapeutic strategies before in-human trials, and present our experience in the development of a vaccine that targets chondroitin sulphate proteoglycan (CSPG)4 as an example of these comparative oncology studies.
Keywords: Comparative oncology, CSPG4, DNA vaccination, Melanoma, Osteosarcoma, PIVAC 18
Introduction
Since the concept of translational oncology officially emerged from the National Cancer Institute (NCI) of the United States in 1992, an increasing number of comprehensive mouse models have been developed and used to test new therapies before their clinical application, strongly consolidating the bridge between basic research and clinical practice [1]. This has greatly contributed to our knowledge of cancer biology and to the improved clinical outcomes observed for many types of cancer over recent decades. Nevertheless, the survival benefits achieved are relatively modest, often measurable in months, and the short- and long-term toxicities of therapies are quite significant and not predicted by pre-clinical testing in mice. Even though phylogenetic and physiological similarities between mice and humans do exist, experimental therapies tested in murine models have, all too often, elicited responses that only poorly predict the outcomes of that therapy being translated to a human setting [2]. Indeed, transplantable models, genetically engineered mice and patient-derived xenograft models have been shown to not accurately mimic the complexity of human cancer, limiting their reliability for subsequent translational applications [2–4]. One of the main criticisms raised is the limited life-span of mice, which does not allow several fundamental features of the nature of human cancers, i.e., growth over long periods of time, genomic instability and tumor heterogeneity, to be reproduced [4, 5]. Furthermore, the microenvironment of the tumors that are modeled in mice is quite different from that which characterizes human neoplastic lesions, resulting in a favorable predictive response to chemo- and radio-therapy [6]. Importantly, from the safety point of view, murine bone marrow is generally less sensitive to the toxicity induced by chemotherapy than human bone marrow, suggesting that mice are not suitable for use in the evaluation of the adverse effects of novel chemotherapies or combinatorial approaches with chemotherapeutic agents [7]. Similar considerations can also be made for the response to immunotherapy, which has now become the fourth pillar of cancer treatment. The discrepancies between the immune systems of mice and humans, in terms of both innate and adaptive immunity, highlight the concerns raised as to the use of mouse models for the rigorous evaluation of immunotherapeutic strategies [3, 8]. Overall, many of these limitations may be overcome by evaluating novel treatments in companion animals—particularly dogs—that are affected by naturally occurring malignancies, in accordance with another important concept promoted by the NCI, that of comparative oncology.
The rationale for evaluating therapeutics in domestic tumor-bearing dogs before carrying out in-human studies will be discussed in the following sections. In particular, the unique opportunity found in assessing, with high translational value, both the safety and anti-tumor activity of novel immunotherapies in canine patients will be uncovered, with a specific focus on the comparative oncology studies that we have performed in recent years.
Why dogs are humans’ best friends, even in disease
Tumor-bearing dogs capture the “essence” of the problem of cancer in a way that is not achievable with other animal models [4, 5]. This awareness comes from decades of investigations into canine oncology. In 1929, the Nobel laureate August Krogh was the first to propose the study of diseases that naturally occur in animals and not just those induced experimentally in laboratory animals [9]. However, it took more than 30 years for the first anti-cancer therapy to be evaluated in dogs [5]. From that moment on, the concept of comparative oncology has spread all over the scientific world.
Several different factors have contributed to the solid rationale for the use of naturally occurring cancer in pet dogs as a translational model for human malignancies. In fact, new dimensions in the comparative oncology field opened up with the decoding of the canine genome in 2005 [10]. Dog-genome sequencing revealed that all 19,000 identified genes are orthologous, or at least similar, to human genes [11]. In particular, comparative gene expression studies in canine and human tumors have revealed that there is close correspondence in terms of genetics and molecular markers [4, 12], thus supporting the overlap between canine and human cancer biology.
Cancer incidence in the pet animal population has increased in recent years, due to pets’ increased life expectancy [5]. It is estimated that 1 out of 3 people develop cancer; almost the same incidence is predicted in dogs. For certain tumor types, the incidence is higher in dogs than in humans, and this may be important for those low occurrence-rate human cancers whose treatment is still an unmet need. In this case, studying the same tumor in dogs could provide a larger patient population for the evaluation of new strategies, with rapid enrolment and faster study completion [13]. Moreover, canine cancers have shown some breed predispositions, providing us with an opportunity to understand the genetic links to different types of cancer [13].
Tumor initiation and progression processes in both human and dogs are influenced by the same factors, including age, nutrition, sex and environment [5]. Living in close proximity and sharing the same environment with their owners, dogs show the same pattern of cancer development, and could, therefore, be considered epidemiologic or etiologic sentinels of the disease [4, 13].
Pet tumors grow slowly in an intact immune system, allowing immune and cancer cells to interact for a long period of time, shaping one each other as well as showing the intratumor heterogeneity and genetic instability that is typical of human lesions [5]. Moreover, cancer development in companion animals resembles the natural step-wise evolution of human tumors, giving rise to spontaneous recurrences and metastasis. Overall, dog tumors reflect, better than any other animal model, the complex genetic, environmental, and physiological aspects present in human malignancies [2, 4, 5, 14]. An additional and fundamental point for translational research is the evidence that canine cancer patients often show the same clinical response to conventional treatments as those observed in human patients. Indeed, it has been demonstrated that several therapeutic protocols used in human clinics have a similar spectrum of activity in veterinary application [5]. Furthermore, drugs that have failed to give rise to significant effects in humans are also ineffective in dogs [5].
All these considerations mean that it is now widely accepted that cancer in canine patients faithfully reproduces fundamental aspects of the corresponding human malignancies. In fact, on one hand, we have growing scientific interest in exploiting naturally occurring cancers in dogs as an important predictive tool for human oncology, and, on the other, there are the owners who are increasingly willing to secure innovative experimental therapies for their pets [5]. The combination of these two considerations contributes to the “one medicine” concept, opening up possibilities to quite easily investigate innovative therapeutic approaches, with high translational power for human patients, in client-owned dogs. In this panorama, performing clinical trials in tumor-bearing companion animals could provide such considerable advantages over conventional pre-clinical mouse testing that a Comparative Oncology Trials Consortium (COTC) was established at the NCI to provide the infrastructure and resources needed to integrate veterinary oncology studies into the development pathways of new therapies for human cancers. More recently, not only veterinary teaching hospitals, but also several private veterinary hospitals are contributing to the “one medicine” practice by providing cutting-edge options and clinical trials for pet cancer patients.
While the patients entering human clinical trials generally have already been treated with standard-of-care therapies or have a disease in its advanced stages, in-dog trials, also newly diagnosed patients not yet been exposed to other treatment modalities can be enrolled, especially for those tumors for which standard-of-care is still inadequate.
As a result, clinical trials for pet patients can enhance and accelerate drug-development efforts by providing unique information that cannot be obtained from traditional pre-clinical models or trials performed directly on human patients. However, this does not mean that some limitations cannot be envisaged. Using pet as a model for studying human tumors and the potential of immunotherapeutic approaches entails possible high cost and long time to get the proper number of canine patients needed for a single veterinary study. Moreover, non-homogenous results can be obtained due to the influence of the owners when applying post-operative treatments and following up the study [15]. Moreover, a critical point could be related to the difficulties in the readout of results coming from veterinary immunotherapy trials, since the availability of tools for immune-monitoring is reduced as compared to those used in traditional inbreed mouse model experiments [16].
The importance of veterinary clinical trials for translation to human patients
Despite the unquestionable role that murine models have had and still hold for human cancer research, attrition rates for oncological therapies that move from the pre-clinical stage to human clinics are significantly higher than those in other therapeutic areas. Indeed, approximately 60% of anti-neoplastic drugs entering Phase III clinical trials fail, and only around 10% of anti-cancer treatments that proved successful in mice have been approved in human oncology [17]. This is even more dramatic if we consider that the development of a new cancer therapy from discovery to the marketplace is extremely time consuming and expensive. These disappointing results place the emphasis on the need of a “bridge” between murine models and human clinical trials, which could increase this success rate and improve our ability to select the safest and most promising therapeutics to be tested in humans. Because of this and all the previously mentioned considerations, we and others support the translational value of oncological canine patients [2, 4]. Interestingly, after the NCI established the Comparative Oncology Program and a European initiative launched the LUPA project to foster the use of naturally occurring cancer in dogs as a model for human tumors, several companies also introduced clinical trials in pet patients into their overall workflow, as was highlighted by the National Academies of Sciences back in 2015.
The use of canine models to evaluate innovative therapies has a long-standing history in other branches of medicine, with the first successful blood transfusion performed in dogs by Richard Lower in 1666; this technique was perfected much later, in the early 1900s, again in dogs [18]. The 1950s was the turn of surgical techniques for kidney transplantation and for the reduction of rejection risk, which were refined in dogs before becoming routine in humans [19]. Again, in the 1970s, one of the first clinical trials involving dogs assisted in the development of a regimen for bone marrow transplantation and then for the treatment of lymphoma canine patients with chemotherapy and myeloablative radiation [20, 21], leading to clinical protocols that were then used in human medical centers. These early examples of studies in pet dogs paved the way for important achievements in human clinics, and were a foretaste of how veterinary trials could strongly benefit both species.
Soon after, a number of studies performed in canine cancer patients collected proofs of clinical efficacy, dose definitions and toxicity assessments of anti-cancer drugs in a way that would be impossible to achieve in murine models. For example, two similar molecules, sunitinib and toceranib, which have been approved for the treatment of gastrointestinal tumors, renal cell carcinoma and pancreatic neuroendocrine tumors in human patients, and of mastocytoma in canine patients, were demonstrated to have similar toxicities in the two species, leading to lethargy, weakness and vomiting that could not be observed so easily in mice [22, 23]. Clinical trials on pet patients can therefore also allow graded and standardized toxicity assessments to be performed.
Other interesting examples include recent Phase I/II veterinary trials using ibrutinib [24], exportin-1, protein inhibitor KPT-335 [23] and the GS-9219 drug [25]; these trials were all helpful in demonstrating not only the anti-tumor activity of the drugs, but also in giving important clues regarding the toxicity profile and the re-definition of the dosing schedule prior to human clinical trials.
A particularly interesting case is that of Ibrutinib, a Bruton’s tyrosine kinase (BTK) inhibitor. This drug was proved to be effective in the treatment of lymphoma in vitro [8]. However, no appropriate in vivo murine models of lymphoma were available to confirm the efficacy of this inhibitor. The availability of pet dogs bearing naturally occurring lymphomas with sustained B cell receptor signaling was fundamental to the ability to demonstrate the drug’s clinical efficacy and to identify a useful biomarker for use as an endpoint in human clinical trials. Moreover, the regimen of Ibrutinib administration in human patients was re-defined thanks to the data, obtained in dogs, on the minimum tolerated and biologically effective dose [8]. Another remarkable story is that of GS-9291, an anti-proliferative nucleotide analog prodrug, which was found to be ineffective in murine models, while subsequent studies showed that the drug did have effects on canine lymphocytes [26]. When tested in canine patients with hematological malignancies, GS-9291 proved its clinical safety and efficacy [25], providing the basis for its evaluation in human patients. This molecule was entered into the process of regulatory approval for veterinary commercialization for the treatment of canine B cell lymphomas [27].
Another exciting pillar in the comparative oncology field is procaspase-activating compound-1 (PAC-1), a synthesized chemical product [28], which has now been granted Orphan Drug Designation by the Food and Drug Administration (FDA) for the treatment of advanced human cancers. PAC-1 is an outstanding paradigm because of the unique development path that has brought it to the human clinic, since it was first evaluated in pet dogs with spontaneous cancer to identify the best application for human clinical trials. Indeed, the safety, tolerability and anti-tumor potential of PAC-1, whether used as a single agent, or in combination with conventional drugs, was first demonstrated in canine patients [29], leading quite promptly to the approval of a first human trial (NCT02355535) for the treatment of advanced malignancies, such as breast cancer, lymphomas, melanomas and other solid tumors. Thanks to the veterinary trials, PAC-1 was also shown to be able to penetrate the blood–brain barrier, suggesting that this drug may be promising for the treatment of cancers of the central nervous system [30]. All these results drove the approval of an additional clinical trial for the combination of PAC-1 with temozolomide for the treatment of glioblastoma (NCT03332355).
Other fundamental achievements are found in the immune-oncology field. Indeed, as explained above, canine patients are of extraordinary relevance for the evaluation of immunotherapeutic strategies since tumors spontaneously develop in an immune-competent environment, and long-lasting and mutual relationships develop between host immune system and cancer cells.
In 2003, Bergman and collaborators started veterinary trials in dogs affected by advanced malignant melanoma (MM) to exploit the safety, immunogenicity and the anti-tumor potential of a xenogeneic DNA vaccine coding for the human tyrosinase [31–33]. The positive results obtained by these studies, led, in 2010, to the approval, by the United States Department of Agriculture (USDA), of the first anti-human tyrosinase DNA vaccine (ONCEPT, Merial) for the treatment of MM-bearing dogs and to a rapid translation of the proposed therapeutic approach to human clinical trials [34, 35]. Even though with the coming out of the most recent results from multiple veterinary and human trials, the therapeutic efficacy of ONCEPT has been questioned in both species [36–38], in-human trials demonstrated the safety and immunogenicity profile of the vaccine previously found in dogs. This is currently the only licensed anti-cancer DNA vaccine in any species and has driven several groups, including our own (see below), to investigate the translational efficacy of the immune-targeting of other antigens that are relevant for human and canine tumors [39, 40].
The study of another immunomodulatory agent, the liposomal muramyl tripeptide phosphatidyl ethanolamine (L-MTP-PE), corroborated the valuable potential of canine tumor models for the advancement of human treatments. L-MTP-PE has been studied because of its ability to activate macrophages and monocytes, which in turn can release proinflammatory cytokines with tumoricidal effects. The first evidence of L-MTP-PE’s potential efficacy in the treatment of osteosarcoma (OSA) came from veterinary studies in OSA-bearing dogs who showed higher survival when treated with this agent than controls that were treated with the placebo [41]. Considering the strong similarities between canine and human OSA (see below), the results of these veterinary assessments laid the foundation for L-MTP-PE’s evaluation in human clinical studies (NCT00631631, NCT02441309, NCT03643133) [42–44] and to its approval in Europe for the adjuvant treatment of patients with non-metastatic, resectable OSA [45]. Interestingly, strong anti-metastatic potential was shown when L-MTP-PE was tested in a mouse model of OSA. However, no increase in survival was observed, unlike findings that had previously been described in dogs, and this was most likely because OSA progression in mice was too rapid [46]. This confirms the idea that investigating immunotherapy in models that display the slow and step-wise progression of spontaneous metastatic disease may be of paramount importance for the identification of a survival benefit, which may be masked when using fast-progressing tumors in mice.
A vaccine named ADXS31-164, which is based on recombinant Listeria monocytogenes that express a chimeric human HER2/neu, has more recently been successfully investigated in canine OSA patients, resulting in a significant reduction in metastatic disease and increased overall survival [47]. Soon afterwards, this became the first Listeria-based vaccine to gain conditional approval for its clinical use in veterinary clinics, and a Phase I/II trial in human patients (NCT02386501) is ongoing. Many other immunotherapies and immunotherapeutic combination approaches are now under investigation in well-designed clinical trials using dogs with cancers, and thus provide increasing amounts of evidence to support the value of comparative oncology approaches to advance both canine and human oncological patient management (see below).
Melanoma and osteosarcoma on the comparative stage
As discussed above, canine oncological patients that spontaneously develop tumors in the same anatomic sites as humans are an interesting avatar for pre-clinical therapeutic studies endowed with a high translational value [4]. This is particularly true for MM and OSA, which are the two most challenging tumors “under the microscope” of comparative oncology nowadays.
MM is the most aggressive form of skin cancer in humans. It represents the sixth most common cancer worldwide and its incidence is increasingly rising [2, 48]. Several advantages for MM clinical outcome have undoubtedly been achieved [2] with the introduction of checkpoint inhibitors (CIs), i.e., monoclonal antibodies directed against the cytotoxic T lymphocyte antigen-4 (CTLA-4) and the programmed cell death receptor-1 (PD-1) or its ligands. However, CIs have been proven to work well in an, as yet, unsatisfactory percentage of patients, the vast majority of whom displayed a pre-existing T-cell-mediated immune response against the tumor [49]. A high proportion of MM patients, however, exhibit innate or acquired resistance to CIs and suffer from disease progression despite the treatment, and most display severe toxicity issues. Improvements and new therapies are therefore needed to increase the survival of patients. Although pre-clinical mouse models have contributed to our understanding of the molecular mechanisms of melanoma carcinogenesis, they are inadequate for the study of novel (immune) therapeutic approaches [2]. As a consequence, we, and others, have looked at spontaneous MM-bearing dogs as models because the canine malignancy shares many characteristics with human MM, including overlapping cytological, histopathological and architectural features [50]. Clinical behavior is another important aspect. Indeed, canine MM comes in a very aggressive form, as in humans, with a strong resistance to treatment [2, 4]. Furthermore, conventional therapies are quite effective in the early stages of the disease both in canine and human MM patients, but not very successful in the advanced stages, with one third of patients experiencing recurrence and metastasis [4]. Moreover, once the tumor has metastasized, the survival rate of canine MM patients after 1-year is only 30%, resembling the human-patient 5-year survival rate, which is only 15–20% [4, 48]. From the genetic point of view, several alterations and signaling-pathway abnormalities have been found in canine MM, including phosphorylated forms of AKT and ERK1/2, alterations in KIT and PTEN, which overlap with some of those widely described in specific human MM subtypes.
However, it must be noted that the well-known BRAFV600E mutation, which has been widely identified in almost 60% of human MM, is absent in canine MM, which are universally BRAF wild type (WT). Moreover, although MM in dogs can affect a range of anatomical sites, such as the lips, skin and digit/footpad, the oral MM subtype is the most prevalent clinically significant form affecting dogs. Therefore, canine MM can serve, in particular, to model human mucosal MM, an aggressive histological subtype that is predominantly BRAF, RAS and NF1 WT (triple wild type or TWT), with markedly poor survival. The possibility of deeply investigating this subtype in humans is limited by its very low prevalence, increasing the value of canine MM, which instead accounts for up to 100,000 diagnoses/year in the United States alone [51]. These characteristics mean that canine oral MM has been proposed as an invaluable pre-clinical model of mucosal, TWT MM and UV-independent melanomagenesis [52]. The consequent identification of novel effective therapies may be successful for both veterinary and human oncology fields.
Another urgent medical need is found in OSA, an aggressive malignancy with poor prognosis and that still has few therapeutic options [53]. OSA is one of the most common malignant bone tumors in both humans and dogs. Several investigations have brought to light the considerable similarities that exist in OSA biological behavior in human and canine patients, including an identical site of onset, histology and proclivity for metastasis [54]. Moreover, it has been demonstrated that genomic alterations that have been linked to OSA pathogenesis and progression are highly conserved in human and canine tumors [13]. In addition, a similar pattern of response to traditional treatments has been observed in both species. A combination of surgery and radio- or chemotherapy is the first-line treatment and has been shown to enhance the survival time for both human and dog OSA patients [55]. However, for those patients with the metastatic form of the disease, which is indeed the vast majority, the prognosis remains dismally poor, with a 2-year survival for canine patients [55] and a 5-year survival for human patients [53] of only about 20%. Therefore, the identification of novel and effective approaches to improve patient survival is urgently needed. In particular, the use of canine OSA as a surrogate for pediatric OSA could be of paramount impact. Indeed, there is still a lack of knowledge regarding the etiology of this tumor and a paucity of therapeutic targets involved in OSA initiation, progression and development. One of the major challenges to overcome when developing OSA clinical trials in the human setting is the young age and the low percentage of affected patients. In this condition, the high number of canine OSA patients diagnosed each year offers a tremendous opportunity that can accelerate advancements in the identification of the key initiating events that are involved in the etiopathogenesis and progression of OSA, thus improving the management of the disease for both humans and dogs.
Overall, spontaneously occurring canine MM and OSA are, in our opinion, attractive models for the identification and development of novel therapeutic strategies.
CSPG4: “all for one and one for all”
The power of comparative oncology studies obviously relies on the identification of shared tumor antigens that are significantly relevant for both human and canine cancers. This would allow unique therapeutic strategies, which can benefit both species, to be developed.
Of the numerous tumor antigens that have been identified so far, our attention has been focused on chondroitin sulphate proteoglycan (CSPG)4. CSPG4 is restrictedly present in normal healthy tissues, as it was widely stated [56–61] and recently supported by Rivera and colleagues [62] which performed an IHC analysis of an FDA Standard Frozen Tissue Array, including 30 different organs, demonstrating that no CSPG4 expression was found in healthy tissues. Indeed, in adults CSPG4 expression is mainly limited to stem cells and adult progenitor cells, while it is post-translationally down-regulated at terminal differentiation [63].
It is becoming increasingly clear from the literature that CSPG4 is implicated in several of the most aggressive and treatment-resistant forms of cancer, including MM, basal-like breast cancers, leukemia, mesothelioma, glioblastoma, soft-tissue sarcomas, pancreatic carcinoma and squamous-cell carcinoma of the head and neck, where it plays a key, and indispensable, oncogenic role [56, 64]. CSPG4 therefore meets all the requirements of the definition of “oncoantigen” [40, 65, 66], i.e., it is an ideal target for anti-tumor (immuno)therapy.
CSPG4 is endowed with multivalent functions, which make it a sort of master regulator of several cancer cell-associated pathways. A great deal of data have demonstrated that CSPG4 can be involved in the sustenance of tumor cell proliferation through its ability to sequester growth factors and concomitantly to associate with the corresponding receptors to form ternary complexes [56]. This has been demonstrated for platelet derived growth factor AA and several fibroblast growth factors [67]. CSPG4 perceive and capture these mitogens, while promoting ligand-binding and dimerization of the corresponding receptors. In this way, CSPG4 can potentiate the activation of the MAPK pathway, resulting in the selective growth of CSPG4-positive tumor cells and providing a survival advantage. Moreover, its extended extracellular arm means that CSPG4 can link different components of the extracellular matrix (ECM), such as tenascin-C, laminin, perlecan and collagens (types II, V and VI) [68]. Its strong interplay with ECM molecules suggests that CSPG4 is involved in optimal cancer cell adhesion and migration. Furthermore, CSPG4 has been demonstrated to interact with several integrins, and thus to cooperate in the activation of integrin-dependent cellular phenomena, such as cell proliferation, motility and survival. Filopodial CSPG4 can also sequester plasminogen and has consequently been implicated in the control of matrix degradation [69, 70]. All these data suggest that binding through the extracellular portion of CSPG4 to a huge variety of molecules in the extracellular space means that this unique proteoglycan may be involved in numerous steps in cancer progression, from sustained proliferation to migration and invasion. Indeed, the CSPG4 cytoplasmic tail is directly linked to a multitude of different signaling cascades, with the two major involved pathways being PI3K–AKT-1 and focal adhesion kinase (FAK) [71].
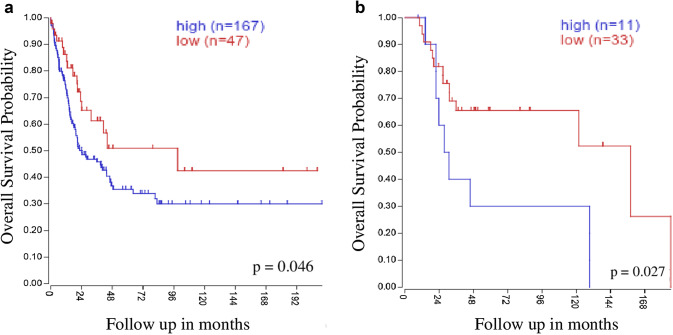
As mentioned above, the oncogenic role of CSPG4 in a number of tumor histotypes has recently been revealed. Nevertheless, the best-established implication is with MM, because of its widespread expression in the majority of human MM patients [72]. In this regard, we have evaluated two publicly available comprehensive microarray datasets that include gene expression data from 214 samples of primary MM [73] and 44 samples from MM metastatic lesions [74]. Interestingly, we observed, by querying the R2 Kaplan–Meier scanner (https://hgserver1.amc.nl/cgi-bin/r2/main.cgi) for prognostic studies, that CSPG4 over-expression in MM tumors showed a significant correlation with shorter overall survival (Fig. 1a). Furthermore, CSPG4 over-expression was associated with significantly reduced overall survival in a selected metastatic setting (Fig. 1b). These data corroborate the link between high CSPG4 expression and poor prognosis, supporting the idea of the potential direct implication of CSPG4 in melanoma progression [64, 72].
Fig. 1.
CSPG4 clinical impact on melanoma patient survival. The mRNA expression levels of CSPG4 in human MM samples were determined by querying the R2 Kaplan–Meier scanner (https://hgserver1.amc.nl/cgi-bin/r2/main.cgi) using previously deposited gene expression analysis datasets from a [73] (GSE65904, including 214 melanoma tumor samples) and b [74] (GSE19234, including 44 metastatic melanoma biopsies). For prognostic studies, R2 analysis software was used and patients were stratified according to CSPG4 expression. Kaplan–Meier curves depict overall survival probability, in years, for melanoma patients stratified by high (blue) or low (red) mRNA CSPG4 expression. In order to define the cutoff between high and low gene expression, all percentiles between the lower and upper quartiles were computed; the best performing threshold was used as a cutoff. Overall survival data were tested for significance using the log-rank test
These considerations and evidence that the amino-acid sequence of CSPG4 is highly evolutionarily conserved, showing over 82% homology with its canine counterpart, led us to evaluate the potential relevance of CSPG4 for comparative oncology in MM. We were the first to investigate, by means of immunohistochemical (IHC) analysis, CSPG4 expression in canine MM. After evaluating a cohort of 65 canine MM samples, collected between 2000 and 2010 at the Diagnostic Laboratory of the Department of Animal Pathology at the University of Turin (Italy), we demonstrated the over-expression of CSPG4 antigen in almost 60% of canine MM, in which the staining was mostly restricted to the tumor cell membrane [75]. Moreover, positive staining was more frequent, albeit not significantly so, in amelanotic rather than in melanotic tumors, and this correlation with a more aggressive phenotype was also suggested by the Kaplan–Meier curve, which indicate lower survival in cases of higher CSPG4 expression levels [40]. In addition to the well-known role of CSPG4 in human MM, this molecule therefore also constitutes a potential IHC marker and a promising targetable antigen in canine MM. These results laid the foundation for the evaluation of CSPG4 as a prototype oncoantigen for translational immunotherapy studies against MM [66].
What makes anti-CSPG4 directed therapies an even more attractive approach is the recently recognized widespread expression of this oncoantigen in a huge variety of other aggressive tumors [66]. We have recently expanded our focus of research to another challenging malignancy with very poor prognosis and few treatments available; OSA. We demonstrated that CSPG4 is over-expressed in both human and canine OSA biopsies and that an evident correlation exists between CSPG4 over-expression and a shorter survival for both OSA-affected humans and dogs [76]. This study indicates that CSPG4 may possibly be clinically implicated in OSA progression, highlighting that CSPG4 is also an interesting therapeutic target in the comparative oncology field of OSA.
Finally, it has been emerging in recent decades that a minority of cells inside a tumor, named cancer stem cells (CSC), are endowed with more resistant behavior to conventional therapies, i.e., chemo- and radio-therapy, than more differentiated cancer cells [77, 78]. This implies that CSC are the cells that are principally responsible for treatment failure and local or distant recurrences/metastases. Considering that conventional anti-cancer therapies are predominantly directed against the bulk of differentiated tumor cells, the CSC model has important clinical implications, and suggests that there is a need for innovative approaches that can also impact upon the CSC compartment. Against this background, the potential of immunotherapies against CSC has recently become an appealing field of research that may yet succeed where conventional therapies have failed.
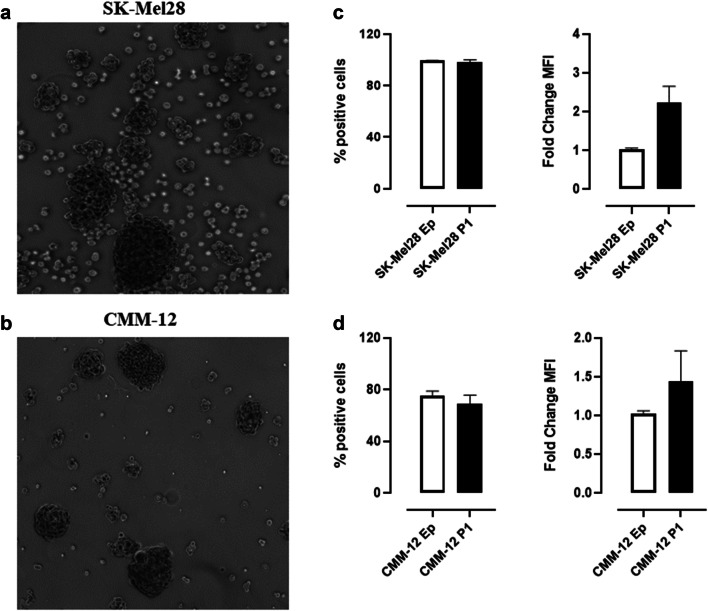
Considering its significant oncogenic role, it is not surprising that CSPG4 over-expression has been identified in CSC subsets in several tumor histotypes [56, 79]. We have also confirmed the overexpression of CSPG4 in human (Fig. 2a) and canine (Fig. 2b) MM- and OSA-derived CSC [76], thanks to the generation of “melanospheres” and “osteospheres” [80]. These findings make CSPG4 an even more interesting target for the design of approaches to target both differentiated cells and CSC.
Fig. 2.
CSPG4 expression in melanospheres. Representative images of human SK-Mel28- (a) and canine CMM-12- (b) derived melanospheres. Both human and canine melanospheres were generated according to the protocol described in [80]. Flow cytometry analysis of CSPG4 expression on Ep and P1-derived human SK-Mel28 (c) and canine CMM-12 (d) cells. Flow cytometry was performed using a FACS Verse (BD Biosciences) and the results were analyzed using BDFacs Suite software. Results are expressed as percentage (%) of CSPG4-positive cells (left panels) and as P1/Ep fold-change of CSPG4 mean fluorescence intensity (MFI, right panels)
In conclusion, the development of effective anti-CSPG4 therapies may represent a “crosswise bullet” that can simultaneously strike a wide range of tumors, and impair a number of oncogenic features in tumor cells. We consider the possibility of investigating anti-CSPG4 targeting in spontaneous canine tumors that express CSPG4 to be a priceless opportunity for the development of advancements in the veterinary field that can be successfully and rapidly translated into treatment in human clinics.
Testing anti-CSPG4 DNA vaccines
Once an appealing tumor antigen has been identified, as in the case of CSPG4, the rational design of immunotherapeutic strategies becomes a precious opportunity in the fight against cancer.
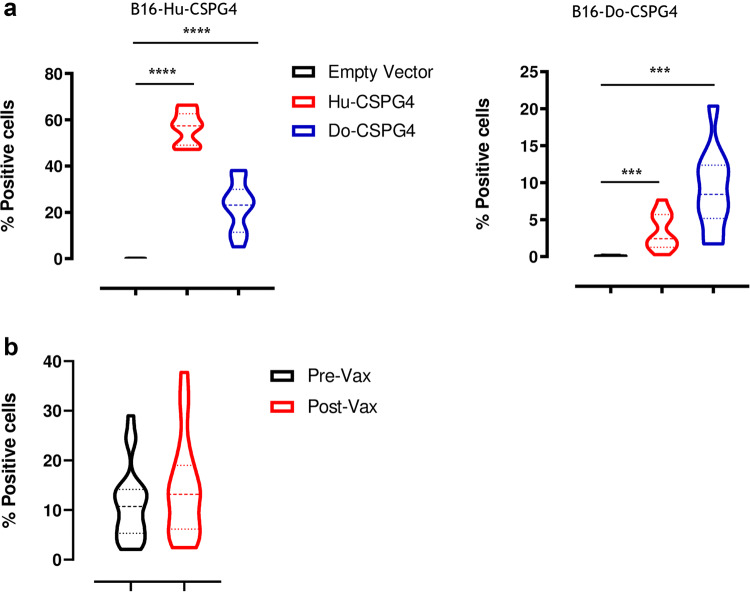
The potential of targeting CSPG4 by means of passive and active immunotherapeutic strategies has been well documented in recent decades. For this reason, monoclonal antibody (mAb)-based anti-tumor approaches [79, 81], and genetically engineered T cells with chimeric antigen receptors (CAR-T) [82] that are reactive against CSPG4 have been developed. These techniques have demonstrated the efficacy of anti-CSPG4 immune-targeting in impairing cancer cell proliferation, migration and invasion in a number of cancer types and in various experimental settings. Furthermore, active immunization approaches, such as anti-idiotypic antibodies or mimotopes [83, 84] have been investigated. These approaches never reached clinics because of the difficulties in the standardization and the induction of a frequent and efficient immune response. Nevertheless, they have shown evidence of immunogenicity and clinical effectiveness, without collateral effects. This has provided a strong rationale for the development of innovative and more effective strategies of immunization against CSPG4. DNA vaccination may well represent an easy and versatile strategy with which to achieve this aim [85]. DNA vaccination offers many advantages over other immunotherapies, as DNA plasmids are relatively simple and inexpensive to design and produce on large scales, as well as being well tolerated and safe [40]. Indeed, it has been demonstrated in pre-clinical models and by many clinical trials that the risk for plasmid genomic integration is very low, and no evidence of anti-DNA immune response following vaccination have been reported so far, which allows multiple administrations to be carried out. We therefore investigated the immunogenic potential of two plasmids, one carrying the human (Hu; Gene ID_1464) and one the dog (Do; Gene ID_487658) sequence of CSPG4, initially in a murine model, where both Hu- and Do-CSPG4 are xenogeneic antigens. Specifically, we vaccinated C57BL/6 mice twice, at 2-week intervals, with either the Hu- or Do-CSPG4 plasmids. DNA vaccination was performed by plasmid intramuscular injection followed by electroporation, one of the most effective methods for securing safe and efficient DNA immunization [86]. Sera of vaccinated mice were collected 2 weeks after the last vaccination and tested, by flow cytometry, for their ability to stain B16 murine melanoma cells that had been stably transfected with either the Hu- or Do-CSPG4. No staining was found on the B16 WT cells with all the tested sera. However, as shown in Fig. 3, sera from Hu-CSPG4 DNA vaccinated mice were effective in binding the B16-Hu-CSPG4 (Fig. 3a, left panel) and to a lesser extent the B16-Do-CSPG4 (Fig. 3a, right panel) cell lines, indicating the presence of anti-CSPG4 antibodies. Similarly, Do-CSPG4 DNA vaccination was effective in inducing a significant antibody response that could bind B16-Do-CSPG4 cells (Fig. 3a, right panel), but the induced antibodies had a very low ability to bind B16-Hu-CSPG4 cells (Fig. 3a, left panel). The empty plasmid did not induce antibodies that were able to bind any of the two cell lines tested. Overall, these results demonstrate that both Hu-CSPG4 and Do-CSPG4 DNA vaccines can be immunogenic in a xenogeneic host. However, one of the major limitations in anti-cancer vaccination is host immune tolerance to the self-target antigen. Indeed, the homologous sequence used as an immunogen frequently fails to induce an effective immune response. To overcome this issue, we decided to test the Hu-CSPG4 vaccine in dogs in order to circumvent immune tolerance and induce a proper immunogenic response.
Fig. 3.
Anti-CSPG4 vaccine-induced antibody response. a Anesthetized C57BL/6 mice were vaccinated as previously described in [94] and sera collected 2 weeks after vaccination were tested for their ability to stain murine B16 melanoma cells stably transfected with either the human (left panel) or canine (right panel) CSPG4 antigen. Results are expressed as percentage (%) of CSPG4 positive cells. Student’s t test ***P < 0.0006; ****P < 0.0001. b Canine MM patients were vaccinated with the Hu-CSPG4 DNA plasmid, as previously described in [39, 40], and sera collected before the first immunization (Pre-Vax) and after the fourth vaccination (Post-Vax) were selected for further analysis. Sera were tested for their ability to stain the canine CSPG4 antigen on the canine CSPG4+ MM cell line (CMM-12). The IgA-specific binding was revealed using a goat anti-dog IgA secondary antibody. Results are expressed as percentage (%) of CSPG4 positive cells. Flow cytometry was performed using a FACS Verse (BD Biosciences) and the results were analyzed using BDFacs Suite software
To this aim, we conducted a non-randomized prospective veterinary clinical trial of adjuvant vaccination with the xenogeneic Hu-CSPG4 DNA plasmid in client-owned dogs with en bloc surgically resected CSPG4-positive oral MM [39, 40]. This trial included, after written informed consent signed by the owners, dogs without concurrent life-threatening diseases and with histologically confirmed oral stage II and III surgically resected MM and a minimum follow-up of 6 months. Basically, after primary MM resection, canine patients included in the vaccination group were injected intramuscularly with the Hu-CSPG4 DNA plasmid, and then in vivo electroporation was performed [40]. The purpose of this adjuvant vaccination was to eliminate the tumor cells that may remain after surgery, hampering the development of recurrences and metastasis, which are actually the main causes of MM-related death.
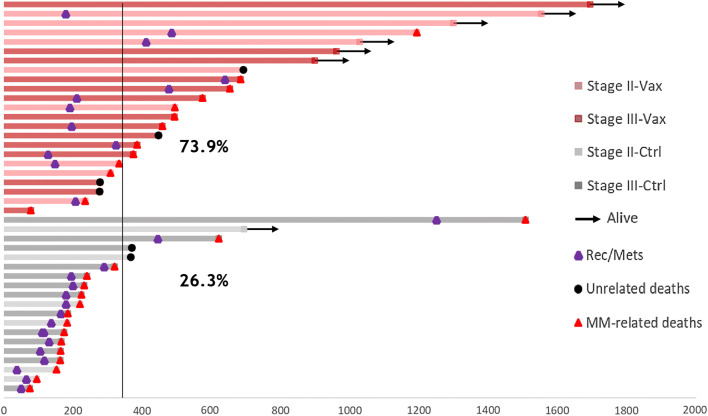
The trial demonstrated the safety, immunogenicity and clinical efficacy of the vaccine. No evidence of acute or late (up to 3 years for some of the vaccines), local or systemic side effects were observed. Moreover, the vaccine was able to induce, in the sera of all the vaccinated dogs, an IgG antibody response that was able to bind not only the Hu-CSPG4, but also the Do-CSPG4 antigen [39, 40]. In addition, recent preliminary data suggest that the vaccine also induces IgA antibodies (Fig. 3b). This could be of paramount relevance for mucosal protection and consequently for blocking recurrences in the oral cavity. A deeper analysis of the immunoglobulin repertoire, not only in the sera, but also in the saliva of vaccinated dogs, may provide interesting insights to better explain the clinical-protection mechanisms observed in the vaccines. Indeed, the most important result from this veterinary trial is the significant prolongation of the overall and the disease-free survival of vaccinated dogs compared to dogs treated with conventional therapies alone ([39, 40] and Fig. 4).
Fig. 4.
Clinical efficacy of the Hu-CSPG4 vaccine in canine MM patients. Swimmer plot depicting the overall survival of canine MM patients enrolled in the veterinary trials [39, 40]. Briefly, the survival (in days) of dogs with surgically resected CSPG4-positive MM, either vaccinated (Vax) or non-vaccinated (Ctrl), is reported. Arrows indicate that the patients were still alive at the time of publication [39]. The purple dots indicate, for each patient, the day of recurrence or metastasis (Rec/Mets) detection, if any. Black dots indicate patients who died because of unrelated reasons, while red dots indicate patients who died because of MM. Percentage of canine patients, vaccinated or treated with conventional therapies alone, that are still alive at 1 year after the diagnosis, is indicated in the plot
The polyvalent role that CSPG4 plays in regulating numerous pathways of the “life” of cancer cells means that there may be many different mechanisms of action by which anti-CSPG4 antibodies exert their therapeutic effects. We demonstrated that sera derived from both vaccinated mice and dogs were able to interfere with MM cell proliferation [40], by inducing CSPG4 down-regulation [39] and CSPG4 internalization (data not shown). Moreover, after demonstrating the over-expression and clinical relevance of CSPG4 in human and canine OSA (see above), we explored whether CSPG4 immune-targeting by monoclonal antibodies, or sera derived from vaccinated dogs, were able to inhibit both human and canine OSA cell proliferation and osteosphere viability [76]. The results show that this is indeed the case, suggesting that anti-CSPG4 DNA vaccination also exerts a potential therapeutic effect in the treatment of OSA. It is highly likely that other mechanisms, such as the ability of vaccine-induced antibodies to interfere with cancer cell migration and/or adhesion, may impact upon the clinical efficacy of Hu-CSPG4 DNA vaccination, and these are currently under investigation.
Overall, the results reported in these studies [39, 40, 76] have endorsed DNA vaccination against CSPG4 as a valid adjuvant option for the treatment of strongly aggressive diseases, such as MM and OSA, while also indicating that it has the potential to be extended to the treatment of a wide range of CSPG4-expressing tumors. To this end, we are now testing a second-generation anti-CSPG4 DNA vaccine that codes for a chimeric human/dog protein. The chimeric CSPG4 protein provides xenogeneic epitopes to both human and dog patients, granting a tolerance brake in both species.
Conclusions
As discussed, companion animals naturally develop tumors in a chronologically relevant time and in an immunocompetent environment, realistically reproducing most of the fundamental processes involved in human tumor development, which are major clinical hurdles in the treatment of human patients. For these reasons, tumors arising in companion dogs are becoming an increasingly recognized tool with which to study the therapeutic potential of anti-cancer treatments. This is particularly true for some types of tumors, for which physiological, anatomical, biological and clinical features are shared by the canine and human diseases, as has been clearly demonstrated.
In recent years, several groups have performed veterinary studies in order to test their innovative strategies in a high translational setting, against a wide range of comparative tumors, such as lymphomas [87–90], melanoma [91–93], osteosarcomas [47] and many others (http://vetcancersociety.org/pet-owners/clinical-trials/; https://ebusiness.avma.org/aahsd/study_search.aspx).
We focused our research on CSPG4, demonstrating that it is expressed by both human and canine MM and OSA, and that its targeting with antibodies can reduce tumor proliferation in vitro. Moreover, our DNA vaccine coding for Hu-CSPG4 was safe and immunogenic in dogs with surgically resected MM and significantly increased their survival [39, 40]. Interestingly, our results also demonstrate that CSPG4 is over-expressed by human and canine melano- and osteospheres, suggesting that the use of immunotherapeutic strategies against CSPG4 might not only be effective against the tumor bulk population, but also against CSC [76]. This could be of paramount importance for the ability to target cells with more aggressive and stem features, in order to more efficiently counteract the onset of recurrences and metastatic lesions. In conclusion, our observations (1) support the idea that comparative oncology may have a significant impact on the development of effective new anti-cancer therapies; and (2) underline the relevance of anti-CSPG4 vaccination for the treatment of the wide range of CSPG4-expressing tumors, starting from MM and OSA.
As a final, more general consideration, we believe that, on one hand, new therapies that are developed in dogs can be quickly translated for the management of human patients. On the other hand, it may be also true that human therapies that have already been approved (e.g., CIs) could be used to treat canine tumors, making the investigation of combinatorial approaches that can be added to clinical protocols easier. This mutual benefit for the veterinary and the human clinical worlds is also starting to capture the attention of industry and financial markets, leading to the hope that there will be a time reduction in the jump from pre-clinic to in-human clinical trials and a consequent acceleration in the drug-development process.
Acknowledgements
Monoclonal antibodies directed towards different epitopes of the CSPG4 antigen (225.2, TP32, TP49 and VF20-VT87.41) used to perform flow cytometry analysis were kindly provided by Prof. Soldano Ferrone (Department of Surgery, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA). We thank Dr. Dale Lawson for his revision and editing of the manuscript.
Abbreviations
- BTK
Bruton’s tyrosine kinase
- CAR-T
Genetically engineered T cells with chimeric antigen receptors
- CSC
Cancer stem cells
- CIs
Checkpoint inhibitors
- CSPG4
Chondroitin sulphate proteoglycan 4
- COTC
Comparative Oncology Trials Consortium
- CTLA-4
Cytotoxic T lymphocyte antigen-4
- ECM
Extracellular matrix
- FAK
Focal adhesion kinase
- FDA
Food and Drug Administration
- IHC
Immunohistochemical
- L-MTP-PE
Liposomal muramyl tripeptide phosphatidyl ethanolamine
- MM
Malignant melanoma
- NCI
National Cancer Institute
- OSA
Osteosarcoma
- PDX
Patients-derived xenograft
- PAC-1
Procaspase-activating compound-1
- PD-1
Programmed cell death receptor-1
- TWT
Triple wild type
- USDA
United States Department of Agriculture
- WT
Wild type
Authors’ contribution
LT, GB, SI, DG and FR produced the results discussed in this review. FR, LT and GB performed mouse experiments and flow cytometry analysis, supervised by FC. SI and DG, under the supervision of PB, collaborated to produce the results in canine patients. FC, FR and LT provided major contributions in writing and discussing the manuscript. FC and EQ critically revised the manuscript. All authors read and approved the final version of the manuscript.
Funding
This work was supported by Grants from Fondazione Ricerca Molinette Onlus, the University of Turin (ex 60% 2018, intramural funds) and the Italian Ministry of Health (Progetti ordinari di Ricerca Finalizzata, RF-2013-02359216). FR was supported by a fellowship from Fondazione Italiana per la Ricerca sul Cancro (FIRC).
Compliance with ethical standards
Conflict of interest
The authors declare that no potential conflicts of interest exist.
Ethical approval and ethical standards
All the in vivo experiments were approved by the Italian Ministry of Health, authorization numbers 0006939-P-18/03/2015 (164/2015-PR) and 0004230-20/02/2018-DGSAF-MDS-P.
Animal source
Mice used for the vaccination experiments reported in this paper were purchased from Charles River Laboratories or bred at the Molecular Biotechnology Center, University of Turin, where all mice were maintained and treated in accordance with University Ethical Committee and European Union guidelines under Directive 2010/63. The canine patients that were enrolled in veterinary trials were client-owned dogs, whose institutes of reference were the Veterinary Teaching Hospital of the University of Turin and the Veterinary clinics of South Rome, Italy. Dogs were treated according to the Good Clinical Practice guidelines for animal clinical studies, and rules imposed by the Ethical Committee of the University of Turin (Italy).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dragani TA, Castells A, Kulasingam V, Diamandis EP, Earl H, Iams WT, Lovly CM, Sedelaar JP, Schalken JA. Major milestones in translational oncology. BMC Med. 2016;14:110. doi: 10.1186/s12916-016-0654-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barutello G, Rolih V, Arigoni M, Tarone L, Conti L, Quaglino E, Buracco P, Cavallo F, Riccardo F. Strengths and weaknesses of pre-clinical models for human melanoma treatment: dawn of dogs’ revolution for immunotherapy. Int J Mol Sci. 2018 doi: 10.3390/ijms19030799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mak IW, Evaniew N, Ghert M. Lost in translation: animal models and clinical trials in cancer treatment. Am J Transl Res. 2014;6:114–118. [PMC free article] [PubMed] [Google Scholar]
- 4.Riccardo F, Aurisicchio L, Impellizeri JA, Cavallo F. The importance of comparative oncology in translational medicine. Cancer Immunol Immunother CII. 2015;64:137–148. doi: 10.1007/s00262-014-1645-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paoloni M, Khanna C. Translation of new cancer treatments from pet dogs to humans. Nat Rev Cancer. 2008;8:147–156. doi: 10.1038/nrc2273. [DOI] [PubMed] [Google Scholar]
- 6.Talmadge JE, Singh RK, Fidler IJ, Raz A. Murine models to evaluate novel and conventional therapeutic strategies for cancer. Am J Pathol. 2007;170:793–804. doi: 10.2353/ajpath.2007.060929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon MY, Blackett NM. The sensitivities of human and murine hemopoietic cells exposed to cytotoxic drugs in an in vivo culture. Cancer Res. 1976;36:2822–2826. [PubMed] [Google Scholar]
- 8.Honigberg LA, Smith AM, Sirisawad M, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci U S A. 2010;107:13075–13080. doi: 10.1073/pnas.1004594107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krogh A. The progress of physiology. Science. 1929;70:200–204. doi: 10.1126/science.70.1809.200. [DOI] [PubMed] [Google Scholar]
- 10.Lindblad-Toh K, Wade CM, Mikkelsen TS, et al. Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature. 2005;438:803–819. doi: 10.1038/nature04338. [DOI] [PubMed] [Google Scholar]
- 11.Olson PN. Using the canine genome to cure cancer and other diseases. Theriogenology. 2007;68:378–381. doi: 10.1016/j.theriogenology.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 12.Mueller F, Fuchs B, Kaser-Hotz B. Comparative biology of human and canine osteosarcoma. Anticancer Res. 2007;27:155–164. [PubMed] [Google Scholar]
- 13.Gardner HL, Fenger JM, London CA. Dogs as a model for cancer. Annu Rev Anim Biosci. 2016;4:199–222. doi: 10.1146/annurev-animal-022114-110911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Medicine Io, National Academies of Sciences E, Medicine . The role of clinical studies for pets with naturally occurring tumors in translational cancer research: workshop summary. Washington, DC: The National Academies Press; 2015. [PubMed] [Google Scholar]
- 15.Abdelmegeed SM, Mohammed S. Canine mammary tumors as a model for human disease. Oncol Lett. 2018;15:8195–8205. doi: 10.3892/ol.2018.8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan TM, Selting KA. Exploring the potential utility of pet dogs with cancer for studying radiation-induced immunogenic cell death strategies. Front Oncol. 2018;8:680. doi: 10.3389/fonc.2018.00680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hay M, Thomas DW, Craighead JL, Economides C, Rosenthal J. Clinical development success rates for investigational drugs. Nat Biotechnol. 2014;32:40–51. doi: 10.1038/nbt.2786. [DOI] [PubMed] [Google Scholar]
- 18.Barsoum N, Kleeman C. Now and then, the history of parenteral fluid administration. Am J Nephrol. 2002;22:284–289. doi: 10.1159/000063775. [DOI] [PubMed] [Google Scholar]
- 19.Murray JE, Sheil AG, Moseley R, Knight P, McGavic JD, Dammin GJ. Analysis of mechanism of immunosuppressive drugs in renal homotransplantation. Ann Surg. 1964;160:449–473. doi: 10.1097/00000658-196409000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiden PL, Storb R, Lerner KG, Kao GF, Graham TC, Thomas ED. Treatment of canine malignancies by 1200 R total body irradiation and autologous marrow grafts. Exp Hematol. 1975;3:124–134. [PubMed] [Google Scholar]
- 21.Storb R, Tsoi MS, Weiden PL, Graham TC, Thomas ED. Studies on the mechanism of stable graft-host tolerance in canine and human radiation chimeras. Transplant Proc. 1976;8:561–564. [PubMed] [Google Scholar]
- 22.Faivre S, Delbaldo C, Vera K, et al. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol. 2006;24:25–35. doi: 10.1200/JCO.2005.02.2194. [DOI] [PubMed] [Google Scholar]
- 23.London CA, Bernabe LF, Barnard S, et al. Preclinical evaluation of the novel, orally bioavailable selective inhibitor of nuclear export (SINE) KPT-335 in spontaneous canine cancer: results of a phase I study. PLoS ONE. 2014;9:e87585. doi: 10.1371/journal.pone.0087585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burger JA, Keating MJ, Wierda WG, et al. Safety and activity of ibrutinib plus rituximab for patients with high-risk chronic lymphocytic leukaemia: a single-arm, phase 2 study. Lancet Oncol. 2014;15:1090–1099. doi: 10.1016/s1470-2045(14)70335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vail DM, Thamm DH, Reiser H, et al. Assessment of GS-9219 in a pet dog model of non-Hodgkin’s lymphoma. Clin Cancer Res. 2009;15:3503–3510. doi: 10.1158/1078-0432.CCR-08-3113. [DOI] [PubMed] [Google Scholar]
- 26.Reiser H, Wang J, Chong L, et al. GS-9219–a novel acyclic nucleotide analogue with potent antineoplastic activity in dogs with spontaneous non-Hodgkin’s lymphoma. Clin Cancer Res. 2008;14:2824–2832. doi: 10.1158/1078-0432.CCR-07-2061. [DOI] [PubMed] [Google Scholar]
- 27.De Clercq E. Tanovea(R) for the treatment of lymphoma in dogs. Biochem Pharmacol. 2018;154:265–269. doi: 10.1016/j.bcp.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 28.West DC, Qin Y, Peterson QP, et al. Differential effects of procaspase-3 activating compounds in the induction of cancer cell death. Mol Pharm. 2012;9:1425–1434. doi: 10.1021/mp200673n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peterson QP, Hsu DC, Novotny CJ, West DC, Kim D, Schmit JM, Dirikolu L, Hergenrother PJ, Fan TM. Discovery and canine preclinical assessment of a nontoxic procaspase-3-activating compound. Cancer Res. 2010;70:7232–7241. doi: 10.1158/0008-5472.CAN-10-0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joshi AD, Botham RC, Schlein LJ, et al. Synergistic and targeted therapy with a procaspase-3 activator and temozolomide extends survival in glioma rodent models and is feasible for the treatment of canine malignant glioma patients. Oncotarget. 2017 doi: 10.18632/oncotarget.19085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bergman PJ, Camps-Palau MA, McKnight JA, et al. Development of a xenogeneic DNA vaccine program for canine malignant melanoma at the animal medical center. Vaccine. 2006;24:4582–4585. doi: 10.1016/j.vaccine.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 32.Liao JC, Gregor P, Wolchok JD, Orlandi F, Craft D, Leung C, Houghton AN, Bergmann PJ. Vaccination with human tyrosinase DNA induces antibody responses in dogs with advanced melanoma. Cancer Immun. 2006;6:21. [PMC free article] [PubMed] [Google Scholar]
- 33.Grosenbaugh DA, Leard AT, Bergman PJ, et al. Safety and efficacy of a xenogeneic DNA vaccine encoding for human tyrosinase as adjunctive treatment for oral malignant melanoma in dogs following surgical excision of the primary tumor. Am J Vet Res. 2011 doi: 10.2460/ajvr.72.12.1631. [DOI] [PubMed] [Google Scholar]
- 34.Wolchok JD, Yuan J, Houghton AN, et al. Safety and immunogenicity of tyrosinase DNA vaccines in patients with melanoma. Molecular Ther J Am Soc Gene Ther. 2007;15:2044–2050. doi: 10.1038/sj.mt.6300290. [DOI] [PubMed] [Google Scholar]
- 35.Yuan J, Ku GY, Adamow M, et al. Immunologic responses to xenogeneic tyrosinase DNA vaccine administered by electroporation in patients with malignant melanoma. J Immunother Cancer. 2013 doi: 10.1186/2051-1426-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ottnod JM, Smedley RC, Walshaw R, Hauptman JG, Kiupel M, Obradovich JE. A retrospective analysis of the efficacy of Oncept vaccine for the adjunct treatment of canine oral malignant melanoma. Vet Comp Oncol. 2013;11:219–229. doi: 10.1111/vco.12057. [DOI] [PubMed] [Google Scholar]
- 37.Treggiari E, Grant JP, North SM. A retrospective review of outcome and survival following surgery and adjuvant xenogeneic DNA vaccination in 32 dogs with oral malignant melanoma. J Vet Med Sci. 2016;78:845–850. doi: 10.1292/jvms.15-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verganti S, Berlato D, Blackwood L, Amores-Fuster I, Polton GA, Elders R, Doyle R, Taylor A, Murphy S. Use of Oncept melanoma vaccine in 69 canine oral malignant melanomas in the UK. J Small Anim Pract. 2017;58:10–16. doi: 10.1111/jsap.12613. [DOI] [PubMed] [Google Scholar]
- 39.Piras LA, Riccardo F, Iussich S, et al. Prolongation of survival of dogs with oral malignant melanoma treated by en bloc surgical resection and adjuvant CSPG4-antigen electrovaccination. Vet Comp Oncol. 2017;15:996–1013. doi: 10.1111/vco.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riccardo F, Iussich S, Maniscalco L, et al. CSPG4-specific immunity and survival prolongation in dogs with oral malignant melanoma immunized with human CSPG4 DNA. Clin Cancer Res Off J Am Assoc Cancer Res. 2014;20:3753–3762. doi: 10.1158/1078-0432.CCR-13-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kurzman ID, MacEwen EG, Rosenthal RC, et al. Adjuvant therapy for osteosarcoma in dogs—results of randomized clinical trials using combined liposome-encapsulated muramyl tripeptide and cisplatin. Clin Cancer Res. 1995;1:1595–1601. [PubMed] [Google Scholar]
- 42.Kleinerman ES, Gano JB, Johnston DA, Benjamin RS, Jaffe N. Efficacy of liposomal muramyl tripeptide (CGP 19835A) in the treatment of relapsed osteosarcoma. Am J Clin Oncol. 1995;18:93–99. doi: 10.1097/00000421-199504000-00001. [DOI] [PubMed] [Google Scholar]
- 43.Kleinerman ES, Jia SF, Griffin J, Seibel NL, Benjamin RS, Jaffe N. Phase II study of liposomal muramyl tripeptide in osteosarcoma: the cytokine cascade and monocyte activation following administration. J Clin Oncol. 1992;10:1310–1316. doi: 10.1200/JCO.1992.10.8.1310. [DOI] [PubMed] [Google Scholar]
- 44.Creaven PJ, Cowens JW, Brenner DE, et al. Initial clinical trial of the macrophage activator MTP-PE encapsulated in liposomes in patients with advanced cancer. J Biol Resp Modifier. 1990;9:492–498. [PubMed] [Google Scholar]
- 45.Pa M. Muramyl tripeptide (mifamurtide) for the treatment of osteosarcoma. Expert Rev Anticancer Ther. 2009 doi: 10.1586/era.09.69. [DOI] [PubMed] [Google Scholar]
- 46.Biteau K, Guiho R, Chatelais M, Taurelle J, Chesneau J, Corradini N, Heymann D, Redini F. L-MTP-PE and zoledronic acid combination in osteosarcoma- preclinical evidence of positive therapeutic combination for clinical transfer. Am J Cancer Res. 2016;6:677. [PMC free article] [PubMed] [Google Scholar]
- 47.Mason NJ, Gnanandarajah JS, Engiles JB, Gray F, Laughlin D, Gaurnier-Hausser A, Wallecha A, Huebner M, Paterson Y. Immunotherapy with a HER2-Targeting Listeria Induces HER2-Specific Immunity and Demonstrates Potential Therapeutic Effects in a Phase I Trial in Canine Osteosarcoma. Clin Cancer Res Off J Am Assoc Cancer Res. 2016;22:4380–4390. doi: 10.1158/1078-0432.CCR-16-0088. [DOI] [PubMed] [Google Scholar]
- 48.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 49.Jenkins RW, Barbie DA, Flaherty KT. Mechanisms of resistance to immune checkpoint inhibitors. Br J Cancer. 2018;118:9–16. doi: 10.1038/bjc.2017.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nishiya AT, Massoco CO, Felizzola CR, et al. Comparative Aspects of Canine Melanoma. Vet Sci. 2016 doi: 10.3390/vetsci3010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bosenberg M, Arnheiter H, Kelsh R. Melanoma in mankind’s best friend. Pigment Cell Melanoma Res. 2014;27:1. doi: 10.1111/pcmr.12196. [DOI] [PubMed] [Google Scholar]
- 52.Mochizuki H, Kennedy K, Shapiro SG, Breen M. BRAF mutations in canine cancers. PLoS ONE. 2015;10:e0129534. doi: 10.1371/journal.pone.0129534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taran SJ, Taran R, Malipatil NB. Pediatric osteosarcoma: an updated review. Indian J Med Paediatr Oncol. 2017;38:33–43. doi: 10.4103/0971-5851.203513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Varshney J, Scott MC, Largaespada DA, Subramanian S. Understanding the osteosarcoma pathobiology: a comparative oncology approach. Vet Sci. 2016 doi: 10.3390/vetsci3010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fenger JM, London CA, Kisseberth WC. Canine osteosarcoma: a naturally occurring disease to inform pediatric oncology. ILAR J. 2014;55:69–85. doi: 10.1093/ilar/ilu009. [DOI] [PubMed] [Google Scholar]
- 56.Nicolosi PA, Dallatomasina A, Perris R. Theranostic impact of NG2/CSPG4 proteoglycan in cancer. Theranostics. 2015;5:530–544. doi: 10.7150/thno.10824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Benassi MS, Pazzaglia L, Chiechi A, Alberghini M, Conti A, Cattaruzza S, Wassermann B, Picci P, Perris R. NG2 expression predicts the metastasis formation in soft-tissue sarcoma patients. J Orthop Res Off Publ Orthop Res Soc. 2009;27:135–140. doi: 10.1002/jor.20694. [DOI] [PubMed] [Google Scholar]
- 58.Wang X, Katayama A, Wang Y, et al. Functional characterization of an scFv-Fc antibody that immunotherapeutically targets the common cancer cell surface proteoglycan CSPG4. Cancer Res. 2011;71:7410–7422. doi: 10.1158/0008-5472.CAN-10-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garusi E, Rossi S, Perris R. Antithetic roles of proteoglycans in cancer. Cell Mol Life Sci CMLS. 2012;69:553–579. doi: 10.1007/s00018-011-0816-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beard RE, Abate-Daga D, Rosati SF, Zheng Z, Wunderlich JR, Rosenberg SA, Morgan RA. Gene expression profiling using nanostring digital RNA counting to identify potential target antigens for melanoma immunotherapy. Clinical Cancer Res Off J Am Assoc Cancer Res. 2013;19:4941–4950. doi: 10.1158/1078-0432.CCR-13-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ziai MR, Imberti L, Nicotra MR, Badaracco G, Segatto O, Natali PG, Ferrone S. Analysis with monoclonal antibodies of the molecular and cellular heterogeneity of human high molecular weight melanoma associated antigen. Cancer Res. 1987;47:2474–2480. [PubMed] [Google Scholar]
- 62.Rivera Z, Ferrone S, Wang X, Jube S, Yang H, Pass HI, Kanodia S, Gaudino G, Carbone M. CSPG4 as a target of antibody-based immunotherapy for malignant mesothelioma. Clin Cancer Res Off J Am Assoc Cancer Res. 2012;18:5352–5363. doi: 10.1158/1078-0432.CCR-12-0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kozanoglu I, Boga C, Ozdogu H, Sozer O, Maytalman E, Yazici AC, Sahin FI. Human bone marrow mesenchymal cells express NG2: possible increase in discriminative ability of flow cytometry during mesenchymal stromal cell identification. Cytotherapy. 2009;11:527–533. doi: 10.1080/14653240902923153. [DOI] [PubMed] [Google Scholar]
- 64.Campoli M, Ferrone S, Wang X. Functional and clinical relevance of chondroitin sulfate proteoglycan 4. Adv Cancer Res. 2010;109:73–121. doi: 10.1016/B978-0-12-380890-5.00003-X. [DOI] [PubMed] [Google Scholar]
- 65.Cavallo F, De Giovanni C, Nanni P, Forni G, Lollini PL. 2011: the immune hallmarks of cancer. Cancer Immunol Immunother. 2011;60:319–326. doi: 10.1007/s00262-010-0968-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rolih V, Barutello G, Iussich S, De Maria R, Quaglino E, Buracco P, Cavallo F, Riccardo F. CSPG4: a prototype oncoantigen for translational immunotherapy studies. J Transl Med. 2017;15:151. doi: 10.1186/s12967-017-1250-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cattaruzza S, Ozerdem U, Denzel M, et al. Multivalent proteoglycan modulation of FGF mitogenic responses in perivascular cells. Angiogenesis. 2013;16:309–327. doi: 10.1007/s10456-012-9316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Price MA, Colvin Wanshura LE, Yang J, et al. CSPG4, a potential therapeutic target, facilitates malignant progression of melanoma. Pigment Cell Melanoma Res. 2011;24:1148–1157. doi: 10.1111/j.1755-148X.2011.00929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goretzki L, Lombardo CR, Stallcup WB. Binding of the NG2 proteoglycan to kringle domains modulates the functional properties of angiostatin and plasmin(ogen) J Biol Chem. 2000;275:28625–28633. doi: 10.1074/jbc.M002290200. [DOI] [PubMed] [Google Scholar]
- 70.Tamburini E, Dallatomasina A, Quartararo J, Cortelazzi B, Mangieri D, Lazzaretti M, Perris R. Structural deciphering of the NG2/CSPG4 proteoglycan multifunctionality. FASEB J Off Publ Fed Am Soc Exp Biol. 2019;33:3112–3128. doi: 10.1096/fj.201801670R. [DOI] [PubMed] [Google Scholar]
- 71.Tamburini Elisa, Dallatomasina Alice, Quartararo Jade, Cortelazzi Barbara, Mangieri Domenica, Lazzaretti Mirca, Perris Roberto. Structural deciphering of the NG2/CSPG4 proteoglycan multifunctionality. The FASEB Journal. 2019;33(3):3112–3128. doi: 10.1096/fj.201801670R. [DOI] [PubMed] [Google Scholar]
- 72.Campoli MR, Chang CC, Kageshita T, Wang X, McCarthy JB, Ferrone S. Human high molecular weight-melanoma-associated antigen (HMW-MAA): a melanoma cell surface chondroitin sulfate proteoglycan (MSCP) with biological and clinical significance. Crit Rev Immunol. 2004;24:267–296. doi: 10.1615/CritRevImmunol.v24.i4.40. [DOI] [PubMed] [Google Scholar]
- 73.Cirenajwis H, Ekedahl H, Lauss M, et al. Molecular stratification of metastatic melanoma using gene expression profiling: prediction of survival outcome and benefit from molecular targeted therapy. Oncotarget. 2015;6:12297–12309. doi: 10.18632/oncotarget.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bogunovic D, O’Neill DW, Belitskaya-Levy I, et al. Immune profile and mitotic index of metastatic melanoma lesions enhance clinical staging in predicting patient survival. Proc Natl Acad Sci U S A. 2009;106:20429–20434. doi: 10.1073/pnas.0905139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mayayo SL, Prestigio S, Maniscalco L, et al. Chondroitin sulfate proteoglycan-4: a biomarker and a potential immunotherapeutic target for canine malignant melanoma. Vet J. 2011;190:e26–e30. doi: 10.1016/j.tvjl.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 76.Riccardo F, Tarone L, Iussich S, Giacobino D, Arigoni M, Sammartano F, Morello E, Martano M, Gattino F, De Maria R, Ferrone S, Buracco P, Cavallo F. Identification of CSPG4 as a promising target for translational combinatorial approaches in osteosarcoma. Ther Adv Med Oncol. 2019 doi: 10.1177/1758835919855491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ruiu R, Rolih V, Bolli E, et al. Fighting breast cancer stem cells through the immune-targeting of the xCT cystine-glutamate antiporter. Cancer Immunol Immunother CII. 2019;68:131–141. doi: 10.1007/s00262-018-2185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Koren A, Rijavec M, Kern I, Sodja E, Korosec P, Cufer T. BMI1, ALDH1A1, and CD133 transcripts connect epithelial-mesenchymal transition to cancer stem cells in lung carcinoma. Stem Cells Int. 2016;2016:9714315. doi: 10.1155/2016/9714315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rivera Z, Ferrone S, Wang X, Jube S, Yang H, Pass HI, Kanodia S, Gaudino G, Carbone M. CSPG4 as a target of antibody-based immunotherapy for malignant mesothelioma. Clin Cancer Res. 2012;18:5352–5363. doi: 10.1158/1078-0432.Ccr-12-0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Conti L, Lanzardo S, Arigoni M, Antonazzo R, Radaelli E, Cantarella D, Calogero RA, Cavallo F. The noninflammatory role of high mobility group box 1/Toll-like receptor 2 axis in the self-renewal of mammary cancer stem cells. FASEB J Off Publ Fed Am Soc Exp Biol. 2013;27:4731–4744. doi: 10.1096/fj.13-230201. [DOI] [PubMed] [Google Scholar]
- 81.Wang X, Osada T, Wang Y, et al. CSPG4 protein as a new target for the antibody-based immunotherapy of triple-negative breast cancer. J Natl Cancer Inst. 2010;102:1496–1512. doi: 10.1093/jnci/djq343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang Y, Geldres C, Ferrone S, Dotti G. Chondroitin sulfate proteoglycan 4 as a target for chimeric antigen receptor-based T-cell immunotherapy of solid tumors. Expert Opin Ther Targets. 2015;19:1339–1350. doi: 10.1517/14728222.2015.1068759. [DOI] [PubMed] [Google Scholar]
- 83.Mittelman A, Chen GZ, Wong GY, Liu C, Hirai S, Ferrone S. Human high molecular weight-melanoma associated antigen mimicry by mouse anti-idiotypic monoclonal antibody MK2-23: modulation of the immunogenicity in patients with malignant melanoma. Clinical Cancer Res Off J Am Assoc Cancer Res. 1995;1:705–713. [PubMed] [Google Scholar]
- 84.Wang X, Ko EC, Peng L, Gillies SD, Ferrone S. Human high molecular weight melanoma-associated antigen mimicry by mouse anti-idiotypic monoclonal antibody MK2-23: enhancement of immunogenicity of anti-idiotypic monoclonal antibody MK2-23 by fusion with interleukin 2. Cancer Res. 2005;65:6976–6983. doi: 10.1158/0008-5472.CAN-04-2328. [DOI] [PubMed] [Google Scholar]
- 85.Quaglino E, Riccardo F, Macagno M, Bandini S, Cojoca R, Ercole E, Amici A, Cavallo F. Chimeric DNA vaccines against ErbB2 + carcinomas: from mice to humans. Cancers. 2011;3:3225–3241. doi: 10.3390/cancers3033225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aurisicchio L, Mancini R, Ciliberto G. Cancer vaccination by electro-gene-transfer. Expert Rev Vaccines. 2013;12:1127–1137. doi: 10.1586/14760584.2013.836903. [DOI] [PubMed] [Google Scholar]
- 87.Impellizeri JA, Gavazza A, Greissworth E, Crispo A, Montella M, Ciliberto G, Lubas G, Aurisicchio L. Tel-eVax: a genetic vaccine targeting telomerase for treatment of canine lymphoma. J Transl Med. 2018;16:349. doi: 10.1186/s12967-018-1738-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gavazza A, Lubas G, Fridman A, et al. Safety and efficacy of a genetic vaccine targeting telomerase plus chemotherapy for the therapy of canine B-cell lymphoma. Human Gene Ther. 2013;24:728–738. doi: 10.1089/hum.2013.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Peruzzi D, Gavazza A, Mesiti G, et al. A vaccine targeting telomerase enhances survival of dogs affected by B-cell lymphoma. Mol Ther J Am Soc Gene Ther. 2010;18:1559–1567. doi: 10.1038/mt.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marconato L, Stefanello D, Sabattini S, et al. Enhanced therapeutic effect of APAVAC immunotherapy in combination with dose-intense chemotherapy in dogs with advanced indolent B-cell lymphoma. Vaccine. 2015;33:5080–5086. doi: 10.1016/j.vaccine.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 91.Milevoj N, Tratar UL, Nemec A, Brozic A, Znidar K, Sersa G, Cemazar M, Tozon N. A combination of electrochemotherapy, gene electrotransfer of plasmid encoding canine IL-12 and cytoreductive surgery in the treatment of canine oral malignant melanoma. Res Vet Sci. 2019;122:40–49. doi: 10.1016/j.rvsc.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 92.Kurupati RK, Zhou X, Xiang Z, Keller LH, Ertl HCJ. Safety and immunogenicity of a potential checkpoint blockade vaccine for canine melanoma. Cancer Immunol Immunother CII. 2018;67:1533–1544. doi: 10.1007/s00262-018-2201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Finocchiaro LM, Fondello C, Gil-Cardeza ML, Rossi UA, Villaverde MS, Riveros MD, Glikin GC. Cytokine-enhanced vaccine and interferon-beta plus suicide gene therapy as surgery adjuvant treatments for spontaneous canine melanoma. Human Gene Ther. 2015;26:367–376. doi: 10.1089/hum.2014.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Riccardo F, Bolli E, Macagno M, Arigoni M, Cavallo F, Quaglino E. Chimeric DNA vaccines: an effective way to overcome immune tolerance. Curr Top Microbiol Immunol. 2017;405:99–122. doi: 10.1007/82_2014_426. [DOI] [PubMed] [Google Scholar]