Abstract
Adoptive cell therapy with T-cell receptor (TCR)-engineered T cells represents a powerful method to redirect the immune system against tumours. However, although TCR recognition is restricted to a specific peptide–MHC (pMHC) complex, increasing numbers of reports have shown cross-reactivity and off-target effects with severe consequences for the patients. This demands further development of strategies to validate TCR safety prior to clinical use. We reasoned that the desired TCR signalling depends on correct pMHC recognition on the outside and a restricted clustering on the inside of the cell. Since the majority of the adverse events are due to TCR recognition of the wrong target, we tested if blocking the signalling would affect the binding. By over-expressing the c-SRC kinase (CSK), a negative regulator of LCK, in redirected T cells, we showed that peripheral blood T cells inhibited anti-CD3/anti-CD28-induced phosphorylation of ERK, whereas TCR proximal signalling was not affected. Similarly, overexpression of CSK together with a therapeutic TCR prevented pMHC-induced ERK phosphorylation. Downstream effector functions were also almost completely blocked, including pMHC-induced IL-2 release, degranulation and, most importantly, target cell killing. The lack of effector functions contrasted with the unaffected TCR expression, pMHC recognition, and membrane exchange activity (trogocytosis). Therefore, co-expression of CSK with a therapeutic TCR did not compromise target recognition and binding, but rendered T cells incapable of executing their effector functions. Consequently, we named these redirected T cells “dummy T cells” and propose to use them for safety validation of new TCRs prior to therapy.
Keywords: T-cell receptor, TCR, TCR signalling, CSK, Immunotherapy
Introduction
When a T cell binds its target through its TCR to the pMHC on the target cell, a series of intracellular reactions take place leading to effector functions. The amplitude and duration of TCR–pMHC binding and hence intracellular signalling are critical for the functional outcome and effector functions such as proliferation, production of cytokines, and cytotoxic activity [1, 2]. TCR activation by binding to MHC–peptide results in recruitment of LCK to the vicinity of the CD3–TCR complex, and activated LCK then phosphorylates the immunoreceptor tyrosine-based activation motifs (ITAMs) in CD3zeta chain. These domains, once phosphorylated, become anchoring points for the recruitment of the TCR-signalling machinery. This includes zap70, which together with LCK phosphorylate the adaptor proteins SLP76 and LAT, which create a signalling hub, a signalosome, leading to phosphorylation and activation of multiple downstream effectors [3, 4].
TCR signalling is under strict control and the balance between negative and positive regulators will determine the degree of activation [5]. A powerful negative regulator of TCR signal is the c-terminal SCR kinase (CSK) [6]. The activity of this kinase is restricted to the phosphorylation of the inhibitory tyrosine of SCR family kinases [7], but it can also regulate LYP, a tyrosine phosphatase, by direct binding [8]. CSK is believed to tune down TCR activation by phosphorylating LCK on Tyr505, which recruits phosphatases to negatively regulate the activity of LCK, even at steady state. It was elegantly demonstrated that selective inhibition of CSK was sufficient to turn on the TCR intracellular signalling cascade, without extracellular stimulation [9]. In addition, the same group showed that CSK played a pivotal role in the TCR orientation at the immune synapse by regulating actin remodelling [10].
The modulation of TCR signalling has been exploited in adoptive cell therapy [11]. While much focus has been on how to increase TCR stimulation and hence improve the cytotoxic effect [12–17], the possibility of non-specific binding or cross reactivity still represents a severe complication [18]. Various safeguard methods have been proposed to control and block the unexpected side effects [19, 20], but novel methods for pre-clinical or early clinical validation of TCR selectivity and specificity still represent an important innovation, since safeguard kinetics might be too slow to rescue a patient if T-cell activation occurs against an unpredicted target or at an unpredicted site.
It was shown more than a decade ago that inhibition of CSK could increase TCR signalling [21], but this feature has never been exploited in a therapeutic setting. We tested the effect of CSK overexpression on TCR-redirected T cells, and demonstrated that while these T cells have retained their TCR expression and TCR–pMHC binding strength, TCR distal signalling was suppressed as well as effector functions. Hence, these T cells had become “dummy T cells”; despite strong binding to their target, they were not able to mediate killing of target cells. We propose the use of these dummy T cells as a clinical in vivo tracer validation strategy to test the specificity of a therapeutic TCR.
Materials and methods
Cell lines, media, chemicals, and peptides
J76 [22] and SupT1 (kind gifts from M. Heemskerk, Leiden University Medical Center, and M. Pulé, University College London, UK, respectively) were maintained in RPMI (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% FCS (HyClone, Logan, UT, USA). T2 cells were maintained in the same medium. The packaging cells were the modified Human Embryonic Kidney cells-293, Hek-Phoenix (Hek-P) and they were grown in DMEM (SIGMA, St Louis, MO, USA) with 10% FCS. HeLa cells were grown in the same medium. K562:HLA-A2 was created using K562 cell line stably expressing an HLA-A2-GFP construct described in [23]. T cells were grown in CellGro DC medium (CellGenix, Freiburg, Germany) supplemented with 5% heat-inactivated human serum (HS) (Trina Bioreactives AG, Nänikon, Switzerland), 10 mM N-acetylcysteine (Mucomyst 200 mg/mL, AstraZeneca AS, London, UK), 0.01 M HEPES (Life Technologies, Oslo, Norway), and gentamycin 0.05 mg/mL (Thermo Fisher Scientific). All cell lines are routinely checked for mycoplasma infection using VenorGeM Mycoplasma kit (Minerva Biolabs, Berlin, Germany) and MycoAlert kit (Lonza, Basel, Switzerland). The transforming growth factor β II (TGFβR2) frameshift peptide131–139 RLSSCVPVA was provided by Norsk Hydro ASA (Porsgrunn, Norway). The MART-1 peptide26–35 EAAGIGILTV was manufactured by ProImmune Ltd (Oxford, UK) and MART-1 dextramer was from Immudex (Copenhagen, Denmark).
DNA constructs
CSK cloning was performed by amplifying cDNA from PBMC isolated from a healthy donor using the following primers: 5′-CACCATGTCAGCAATACAGGC-3′ (full length construct) or 3′-CACCATGGGCTGTGGCTGCAGCTCACACCCCGAAGATGCAATACAGGCCG-5′ (membrane targeted CSK with LCK myristoylation domain) and 5′-CTCTCTTGGCTCTCAGGTGCAGCTCGTG-3′. The amplicon was subsequently cloned into pENTR vector (Thermo Fisher Scientific) and sequence verified (Eurofins MWG Operon, Ebersberg, Germany). It was then transferred into pMP71 (retroviral vector) or pCIpA102 (mRNA synthesis construct) as described in [24].
TCR expression constructs were prepared by amplifying TCR-α and β chains separately with specific primers and followed by a second PCR to fuse the TCR chains as a TCR-2A construct, as described in [24, 25] for DMF5 and Radium-1, respectively.
HLA-A2-peptide single-chain trimer (SCT) expression plasmids were constructed, as described in [26]. Peptide-coding sequence exchange to create a TGFβR2 frameshift peptide SCT (SCT–TGF) was performed by site direct mutagenesis using these peptides: 5′-GGCCTCGAGGCTCGTCTGTCATCATGCGTTCCTGTGGCTGGCTGTGGCAGC-3′ and 5′-GCTGCCACAGCCAGCCACAGGAACGCATGATGACAGACGAGCCTCGAGGCC-3′.
In vitro mRNA transcription
The in vitro mRNA synthesis was performed essentially as previously described [27]. Anti-Reverse Cap Analog (Trilink Biotechnologies Inc., San Diego, CA, USA) was used to cap the RNA. The mRNA was assessed by agarose gel electrophoresis and Nanodrop (Thermo Fisher Scientific).
In vitro expansion, electroporation, and retroviral transduction of human T cells
T cells from healthy donors were expanded using a protocol adapted for GMP production of T cells employing Dynabeads CD3/CD28, as described in [27]. In brief, PBMCs were isolated from buffy coats by density gradient centrifugation and cultured with Dynabeads (Dynabeads® ClinExVivo™ CD3/CD28, Thermo Fischer Scientific) at a 3:1 ratio in complete CellGro DC Medium with 100 U/mL recombinant human IL-2 (Novartis, Emeryville, CA, USA) for 10 days. The cells were frozen and aliquots were thawed and rested in complete medium before transfection.
Expanded T cells were washed twice and resuspended in CellGro DC medium to 70 × 106 cells/mL. The mRNA was mixed with the cell suspension at 100 μg/mL, and electroporated in a 4-mm gap cuvette at 500 V and 2 ms using a BTX 830 Square Wave Electroporator (BTX Technologies Inc., Hawthorne, NY, USA). Immediately after transfection, T cells were transferred to complete culture medium at 37 °C in 5% CO2 overnight.
Viral particles produced as described in [24] were used to transduce cells (cell line or activated T cells) as follows: spinoculation was performed with 1 Volume of retroviral supernatant in a 12-well or a 24-well culture non-treated plate (Nunc A/S, Roskilde, Denmark) pre-coated with 20 µg/mL retronectin (Takara Bio. Inc., Shiga, Japan). T cells were spinoculated twice, whereas all the other cell lines only once. Cells were then harvested with PBS–EDTA (0.5 mM) and grown in their medium or in CellGro DC, 5% HS, 100U/mL IL-2 for T cells. Cells were used for experiments after 3–7 days.
Functional assay, trogocytosis measurements, and flow cytometry
Functional assays: T cells were stimulated for 5 h with antigen presenting cells (APCs), loaded or not with the indicated peptide, at a T-cell to target ratio of 2:1 and in the presence of BD GolgiPlug and BD Golgistop (BD Biosciences, Chicago, IL, USA) at a 1/1000 dilution.
Measurement of trogocytosis was performed as follows: TCR/CSK_2_GFP or GFP expressing J76 cells were incubated with T2 cells that had been loaded O/N with the indicated peptide at saturating concentration (5 µM) at 1:1 ratio for 4 h. Cells were then extensively washed and stained for flow analysis (see below) using specific antibodies. Trogocytosis was measured by determining the increase of signal of the transferred markers.
The following antibodies were used: Vβ3-FITC (Beckman Coulter-Immunotech SAS, Marseille, France) and CD107a-PE-Cy5 (BD Biosciences). The following antibodies were from eBiosciences (Thermo Fisher Scientific): CD3-eFluor450, CD4-eFluor 450, CD4-PE-Cy7, CD8-APC, and CD8-PE-Cy7. Cells were washed in flow buffer [FB; phosphate buffered saline (PBS) with 2% human bovine serum albumin (BSA) and 0.5 µM EDTA]. For dextramer and antibody staining, cells were incubated for 30 min at room temperature (RT) with the recommended dilution in FB. If fixed, cells were incubated in FB containing 1% paraformaldehyd (PFA). Cells were acquired on a BD LSR II flow cytometer and the data analyzed using the FlowJo software (Treestar Inc., Ashland, OR, USA).
Killing assay
Non-radioactive Europium TDA (EuTDA) cytotoxicity assay based on DELFIA technology was performed. The EuTDA assay uses time-resolved fluorometry (TRF) and the measured fluorescence signal correlates directly with the amount of lysed cells (PerkinElmer Inc, Boston, MA, USA). T cells were stimulated for 2 h with target cells (K562:HLA-A2 cells) loaded or not with 100 nM of the indicated peptide and incubated at the indicated effector to target ratio (E:T). Measurement of the fluorescent signal was performed on a VICTOR X4™ plate reader (PerkinElmer Inc) and the data analyzed using the GraphPad prism software.
Phospho-specific flow cytometry
Prior to activation, T cells were incubated at 37 °C O/N at 1 million/mL in RPMI 1640, supplemented with 10% FCS, and then redistributed at 400 µL/tube and given another 20 min rest placed in water bath at 37 °C. TCR activation reagents were from eBioscience, and TCR signalling was initiated by anti-CD3-biotin (0.4, 2.0 or 10 µg/mL) and anti-CD28-biotin (5.0 µg/mL, Thermo Fisher Scientific) for 2 min, before crosslinking with avidin (50 µg/mL, Thermo Fisher Scientific) for 2 min. PFA at a final concentration of 1.6% was added to stop signalling and incubated for 5 min at RT, followed by permeabilization in > 90% freezer-cold methanol. At this point, the samples could be stored at − 80 °C, before further processing. In experiments using APCs loaded with peptide (relevant or irrelevant) to activate TCR, APCs and T cells were mixed at 1:1 ratio and spun down at 1 min for 1000 rpm, before placed in water bath (37 °C) for 5 or 15 min before PFA fixation and permeabilization as described. After rehydrating the cells by centrifugation 2 times in PBS, the cells were fluorescent barcoded, using 4 different levels of Pacific Blue as previously described [28], before combining all stimulation conditions per sample type into 1 tube and then proceed with antibody staining: CSK, CD5, CD4, CD20, p-CD3ς, p-SLP-76, p-Zap70/p-SYK and pSFK/p-LCK were all from BD Biosciences, whereas p-ERK was from Cell Signalling Technologies. Stained samples were collected on a LSR II flow cytometer (BD Biosciences). Data were analyzed using the Cytobank Software (http://www.Cytobank.org), and phosphorylation levels were calculated relative to unstimulated T cells, using arcsinh transformed data [23].
T-cell binding assay and microscopy
Visualization of T-cell binding to cognate APC was performed as follows: 0.15 × 106 HeLa cells were seeded into Glass Bottom 6-Well Plates (MatTek Corp., Boston, MA, USA); after 12 h, they were transfected with SCT constructs (1 µg DNA/well) and left another 24 h. Then, 0.6 × 106 T cells were added, left for 35 min, and cells were washed once or not with RPMI before confocal microscopy. Images were taken on an Olympus Fluoview 1000 IX-81 inverted confocal laser scanning microscope using 40 × objective lens. HeLa cells were identified by their morphological characteristics or anti-HLA-A2 antibody staining whereas T cells were traced by GFP expression. Ten images per condition were acquired; Image J software (https://imagej.nih.gov/ij/) was used to analyze images. The number of cells per frame was counted; plotting and statistical analyses were performed using the GraphPad prism software.
Results
Overexpression of CSK blocks TCR distal signalling
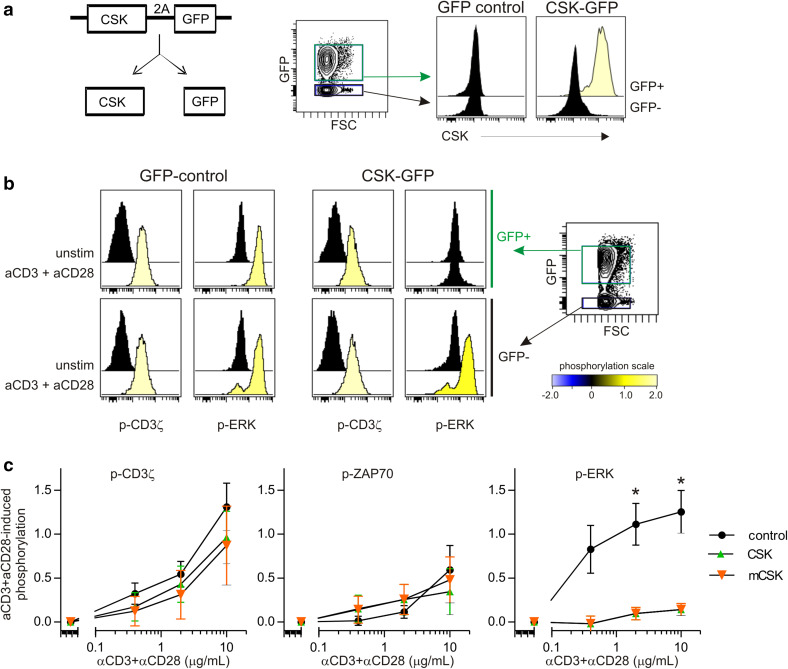
To test if TCR signalling could be blocked by modulating negative regulation, we created retroviral vectors by fusing the sequence encoding for CSK or membrane-bound LCK myristoylation domain-CSK to GFP via a 2A ribosome skipping sequence [29], termed CSK–GFP and mCSK–GFP (Fig. 1a). This design enabled the CSK variants and GFP to be translated as separate proteins, yet CSK expression was directly correlated with GFP expression in human T cells transduced with the CSK_GFP vector (data not shown). Hence, GFP could be used to track cells that over-expressed CSK. To test the impact of CSK overexpression on TCR signalling, GFP, CSK_GFP, or mCSK_GFP-transduced T cells were activated with varying concentrations of anti-CD3/anti-CD28 for 2 min, followed by detection of phosphorylated kinases using phospho-specific flow cytometry. TCR-induced phosphorylation (p) of CD3ς and ZAP70 was similar between T cells over-expressing CSK or mCSK (GFP+ T cells) and the corresponding GFP− T cells within the same sample (Fig. 1b, c). In contrast, TCR-induced p-ERK was almost completely blocked in the presence of CSK or mCSK (Fig. 1b, c). No difference was observed between GFP+ and GFP− T cells for cells transduced with control vector (without CSK) (Fig. 1b), confirming that our system was not biased by GFP presence.
Fig. 1.
Overexpression of CSK potently suppresses TCR-induced phosphorylation of ERK. a Design of the retroviral vector used for overexpression of CSK. CSK_2A_GFP construct (referred to as CSK–GFP) consists of a fusion of the CSK coding sequence with GFP via picornavirus 2A sequence. One mRNA is transcribed into two distinct proteins and CSK expression follows GFP as shown in the histogram overlays. b PBMC-derived T cells were transduced with GFP (control) or CSK–GFP. Incubation with anti-CD3/CD28-biotin, followed by avidin crosslinking for 2 min, was used to activate TCR and signalling was detected by phospho-specific flow cytometry. Histogram overlays from one representative experiment. The colour code shows induced phosphorylation level relative to unstimulated cells, using arcsinh transformation of the median fluorescence intensity. c Anti-CD3/CD28-induced phosphorylation of CD3z, ZAP70, and ERK, relative to unstimulated cells. Mean ± SEM, n = 4 (control, CSK–GFP) or n = 3 (mCSK–GFP) of arcsinh transformed data. *P < 0.05, paired t test
Next, we studied TCR signalling in a more physiological setting utilizing pMHC presenting APCs to activate signalling. T cells were first stably transduced with a TGFβR2-frameshift specific TCR referred to as Radium-1 [25], followed by transfection of GFP, CSK_GFP, or mCSK_GFP as specified and incubated with or without APC loaded with relevant (TGFβR2p) or irrelevant (M1p) peptide. The presence of relevant APCs induced phosphorylation of ERK in a fraction of CD8+ and CD8− T cells, whereas the presence of irrelevant APCs did not (Fig. 2a, b). Of the measured TCR-signalling nodes (CD3z, Zap70, SLP76 and ERK), ERK became phosphorylated in the highest fraction of T cells with 40 and 22% positive CD8 T cells and 5 and 15 min post APC stimulation, respectively (Fig. 2b). When the T cells also over-expressed mCSK, the APC-induced phosphorylation of ERK was almost completely blocked. In contrast to the potent suppressive effect of mCSK, overexpression of CSK had limited effect (Fig. 2b). Together, these data show that mCSK had the ability to block MHC–peptide induced TCR signalling in T cells engineered to express a therapeutic TCR.
Fig. 2.
mCSK is more potent than CSK in suppressing specific TCR-induced signalling. PBMC-derived T cells were transduced with Radium-1 TCR, expanded, and transiently transfected with GFP, CSK–GFP, or mCSK–GFP mRNA. APCs were loaded with relevant (APC1) or irrelevant (APC2) peptide and mixed with the T cells to induce TCR signalling. a One representative experiment (n = 2). b TCR-induced phosphorylation of SLP76 and ERK, relative to unstimulated cells. Mean ± SEM, n = 2 of arcsinh transformed data
Overexpression of CSK blocks TCR-dependent functions
We next tested if CSK overexpression was sufficient to block TCR-induced IL-2 release. The TCR-negative cell line J76 [22] was transduced to constitutively express a MART1-specific TCR, DMF5 [30]. These cells were super-infected with CSK–GFP and GFP+ CD3+ positive cells were sorted to obtain a pure population. J76 cells expressing CSK–GFP only or DMF5 only were also prepared (Fig. 3a). To analyze IL-2 release from the different J76 cells, these were incubated with SupT1 cells expressing Single-chain trimer (SCT) [31] fused to the MART1 peptide (M1p) or an irrelevant peptide. Whereas M1p efficiently induced IL-2 release in DMF5 transduced cells, the presence of CSK co-expression completely blocked it (Fig. 3b).
Fig. 3.
Overexpression of mCSK suppresses TCR-induced effector functions. a J76 cells were stably transduced with DMF5 TCR, CSK–GFP, or both constructs. The J76 variants were stained with anti-CD3 for 10 min and analyzed by flow cytometry. Representative plots are shown. b Cells from a were FACS sorted to obtain highly purified populations and then incubated at 1:1 ratio with SupT1 cells expressing melanoma antigen recognized by T cells 1 (MART-)1 SCT (SCT-M1p) or a control SCT (SCT-irr). Supernatants were harvested after 12 h and analyzed for the presence of IL-2 by ELISA, Mean ± SD, n = 2. c PBMC-derived T cells were first transduced with Radium-1 TCR and then with GFP, CSK–GFP, or mCSK–GFP. T cells were then incubated with APC loaded with or without peptide or left alone for 5 h and CD107a expression was quantified, Mean ± SD, n = 2. d PBMC-derived T cells were electroporated with the indicated constructs. Eight hours later, they were incubated with target cells pre-loaded with Europium at an effector to target ratio (E:T) of 50:1 and killing was measured 2 h later. Percent target cell lysis was calculated from triplicates, Mean ± SD. The figure is representative of two separate experiments. *P < 0.05, **P < 0.01 paired t test
We then tested T cells transduced with Radium-1 TCR using the same strategy. We super-infected the T cells with GFP, CSK_GFP, or mCSK_GFP constructs and incubated them with APC loaded or not with specific peptides. Similar to the TCR-signalling experiments (Fig. 2), the presence of CSK decreased the TCR-induced degranulation as measured by CD107a, whereas mCSK completely blocked it (Fig. 3c). Finally, the potency of CSK and mCSK to suppress TCR-induced killing was tested. Mock-electroporated T cells were used as negative control and peptide-loaded APC (K562:HLA-A2) as target cells. Again, whereas CSK showed some suppressive effect by reducing TCR-induced killing of APCs, mCSK was able to completely block TCR-induced killing (Fig. 3d). These results suggest that overexpression of CSK is sufficient to block TCR-induced activation of J76 cells, whereas membrane targeting of CSK is necessary to block TCR-induced functional effects in PBMC-derived T cells redirected with therapeutic TCR.
pMHC–TCR binding is improved by CSK
The lack of TCR-induced signalling and effector functions could be due to disturbed plasma membrane trafficking of the TCR, but the staining shown in Fig. 3a suggests that the TCR–CD3 complex was indeed located at the membrane. Another hypothesis could be that CSK affects TCR binding to pMHC. To test this, J76 cells expressing DMF5 TCR were transduced with either GFP or the CSK–GFP constructs and stained with MART1 pMHC multimers. In this case, the cells were not sorted to enable comparison of CSK over-expressing cells (GFP+) vs. non-transduced cells (GFP−) in the same test tube (Fig. 4a). As expected, GFP only (negative control) had no impact on the staining intensity of the TCR by MART1p-MHC–multimer when compared to the GFP negative population (Fig. 4b). However, both CSK and mCSK expression lead to an increased staining intensity, suggesting that the presence of CSK proteins improved TCR binding to pMHC, probably through kinase inhibition as recently shown in a similar assay using the tyrosine kinase inhibitor dasatinib [32].
Fig. 4.
CSK overexpression increases TCR membrane localization (a, b). J76 cells transduced with DMF5 TCR and the indicated constructs were stained using MART-1 multimer. The mean fluorescence intensity (MFI) was plotted and each cell population was split into CSK–GFP positive and negative (dark green and light green, respectively) to compare cells from the same tube. a GFP vs. MART-1 multimer staining in CSK–GFP-transduced cells; and b one experiment representative of two separate experiments. c Sorted J76 cells expressing DMF5 TCR together with the indicated construct were stained with CD3 antibody and the MFI was plotted. This is representative of two separate experiments
We then asked if increased pMHC–multimer-binding intensity was due to an increased TCR expression level. Since J76 cells have no endogenous TCR expressed at the membrane, they are CD3 negative. However, upon TCR overexpression, they became CD3 positive, and the CD3 signal intensity in the double positive population (GFP+ CD3+) was increased in the presence of CSK (Fig. 4c). Taken together, these data support that CSK over-expressing TCR-redirected T cells have kept their TCR-binding specificity and have higher TCR expression, but with very limited effector functions.
CSK over-expressing T cells are able to perform trogocytosis with antigen presenting cells
Membrane exchange between T cell and APC, termed trogocytosis, seems to play a crucial role in target recognition [33]. As trogocytosis might be TCR signalling dependent [34–36], we tested if CSK expressing cells were still able to perform trogocytosis. To test this, J76 TCR (Radium-1) cells expressing either GFP, CSK, or mCSK constructs were incubated together with CD3 negative T2 target cells loaded or not with relevant or irrelevant peptides, and the presence of transferred CD3 molecules was analyzed by flow cytometry. As shown, CD3 was transferred from J76 cells to T2 cells only when the latter were incubated with the relevant peptide, confirming that trogocytosis was TCR-specific (Fig. 5a). The presence of CSK or mCSK did not affect trogocytosis. Furthermore, J76 (Radium-1) cells, which are HLA-A2 negative, became HLA-A2 positive only when the specific peptide was presented (Fig. 5b). Although the HLA-A2 signal on T2 cells increased with peptide loading (Fig. 5c), the irrelevant peptide (M1p) which induces HLA-A2 recruitment was not able to trigger trogocytosis. Interestingly, CSK or mCSK expressing cells had statistically significant improved membrane exchange (Fig. 5b, P < 0.05). Taken together, these data suggest that introducing CSK to suppress TCR signalling did not negatively affect trogocytosis, but rather enhanced it.
Fig. 5.
CSK overexpression does not affect trogocytosis, but increases TCR-dependent target cell attachment. a T2 cells were loaded or not with a relevant (TGFβR2p) or an irrelevant peptide (M1p) O/N prior to co-incubation with J76 cells constitutively expressing Radium-1 TCR in combination with GFP (control), CSK–GFP or mCSK–GFP. The presence of surface CD3 was then monitored on T2 cells and the geometric mean plotted. b Same as in a but GFP+ cells (J76) were gated and the presence of HLA-A2 detected. Mean ± SD, n = 2. *P < 0.05, paired t test. c T2 cells used for a and b were stained with anti-HLA-A2 antibodies and MFI was plotted. Mean ± SD, n = 2. d T cells were electroporated with mRNA encoding for Radium-1 TCR and either GFP or e the mCSK–GFP. SCT expressing HeLa cells were used as APCs and were co-incubated with T cells for 15 min prior to live cell imaging. A total of 20 pictures per co-culture were taken; 10 pictures before washing (data not shown) and 10 pictures after washing the plate. GFP+ T cells bound to APCs were counted per frame. The dots represent the number of counts per frame after washing. Mean ± SD, n = 10. **P < 0.02, paired t test
Dummy T cells trace antigen presenting cells
To analyze T-cell binding to their targets, we set up a microscopy-based assay, where T cells are incubated with adherent target cells and bound T cells are counted after washing out non-bound T cells. Here, T cells electroporated with Radium-1 TCR mRNA in combination with mCSK–GFP or GFP mRNA were incubated with the adherent HLA-A2- cell line, HeLa cells, expressing SCT exposing a relevant antigenic peptide (TGFβR2, SCT–TGF), or an irrelevant one (MART-1p, SCT-M1). Thirty minutes later, cells were washed and the number of T cells per frame was counted. Although a tendency to stronger binding to cognate target was observed when T cells did not express mCSK, no statistical significance was reached (Fig. 5d). However, when mCSK was expressed, T-cell specific binding became significantly increased compared to non-specific peptide (Fig. 5e, P = 0.0029, n = 10). In agreement with our previous data, these results suggest that blocking distal TCR signalling did not negatively affect binding but rather improved it.
Discussion
Methods have been proposed to control and block unexpected side effects of adoptive T-cell therapy [19, 20]. Here, we demonstrated that the presence of mCSK in redirected T cells could completely block distal TCR signalling, cytokine production, degranulation, and cytotoxicity, while the TCR expression and its ability to specifically recognize and bind its cognate pMHC was maintained or even increased. Based on this, we propose that introducing mCSK into redirected T cells with therapeutic TCR could potentially be used as a safeguard system to evaluate target specificity in vivo.
We have shown that by exploiting a picornavirus 2A-based sequence construct and link CSK to GFP, we could specifically trace CSK-transduced cells and study their function in vitro. This is very important for kinetics analysis of signalling, because the test and the control are present in the same tube, thereby reducing variation contrary to the conventional techniques used to study signalling [37]. Phosphoflow cytometry enabled us to detect the specific signalling of a TCR when stimulated by APCs presenting the cognate pMHC, which was previously shown by only few labs [38]. This pMHC-restricted stimulation system is far more physiologically relevant than the commonly used CD3/CD28 antibody-based activation. Indeed, CD3/CD28 stimulation overrides TCR specificity and, therefore, represents an over-saturated stimulation condition. The specific pMHC stimulation allowed precise analysis of signalling components in the presence of CSK, confirming the central role of ERK for downstream effector functions [39, 40]. In addition, the early signalling, which is LCK dependent, was not affected. This important point is in agreement with the standby model proposed by [41] stating that a pool of the kinase is kept constitutively active to respond faster to TCR clustering.
Modification of signalling components within redirected T cells is becoming increasingly attractive; indeed, a recent report demonstrated that T-cell efficacy could be improved when intracellular signalling was modulated [12]. We here showed that the opposite is possible by introducing CSK, a negative regulator of TCR signalling, and thereby creating a tool for a safe in vivo investigation of possible cross reactivity or on-target/off-tumour toxicity prior to TCR therapy. This is in agreement with a report, where controlled regulation of CD4 T-cell cytokine release could be used to prevent adverse effects such as cytokine release syndrome. Here, an inducible system controlled the expression of bacterial signalling components that in turn affected TCR response [42]. Due to the risk of creating an immune response against bacterial components, it might be favourable to use human proteins. Adoptive TCR transfer has shown that precision in targeting is a crucial step. In a recent clinical trial using TCR targeting (Melanoma-associated antigen 3) MAGE-A3, an unexpected cross-reactive target was found to be expressed in cardiomyocytes [43, 44], leading to the death of patients from heart failure. Thus, the current prediction methods do not seem to fulfil all the necessary requirements. Although antigenic peptides are screened against databases covering the entire proteome, allelic variations between individuals, splice variants and secondary modifications such as phosphorylations [45] are difficult to detect. Furthermore, the protein expression pattern is complicated to analyze in all tissues and under variable conditions. Finally, the inherent alloreactivity of a given TCR for another MHC allele is almost impossible to predict [46, 47]. The combination of all the above aspects which is difficult to foresee has implications for the safety of TCR-based therapies, with inappropriate targeting carrying the risk of fatal outcome [18].
Safeguard systems such as inducible suicide genes in which the redirected T cells can be deleted before fatal events happen have been proposed [11]. However, the time of response might still represent a challenge. It is, therefore, becoming obvious that computer-based predictions and in vitro testing of off-target toxicity for a therapeutic TCR are not sufficient for accurate safety evaluation. One could imagine alternative methods to test a TCR in vivo: the use of soluble TCR combined with a tracer is an attractive one, but the lack of all TCR companion proteins such as co-receptors (CD4, CD8, CD28, etc.) might hinder proper detection and may simply not be complex enough to simulate an immune synapse. Systems such as mRNA electroporation are attracting more and more attention in the CAR therapy field [48, 49] due to the transient nature of the mRNA which might limit side effects.
We herein postulate that an efficient alternative to validate therapeutic TCRs would be to employ modified effector cells unable to be activated, directly in the patient (Fig. 6). These cells are termed “dummy”, because they are similar to the therapeutic ones in their binding to the target cell, but unable to mount effector functions such as killing or cytokine release. We, therefore, propose to transiently co-transfect CSK with TCRs into T cells in combination with cell tracking systems [50] to detect off-target/off-tumour sites at the body level. In conclusion, our data demonstrate that CSK is an ideal candidate to make dummy T cells. This tool could help clinicians to evaluate the safety of a TCR before therapeutic use.
Fig. 6.
Dummy T cells in a clinical setting. Design of the proposed procedure: patient T cells are isolated by leukapheresis and transfected with the therapeutic TCR and mCSK, preferably as mRNA. These cells are subsequently labelled with a bio-detectable agent and infused back into the patient. Accumulation of the signal will finally be monitored and exclusive homing to the tumour site or homing to additional sites will give a go/no go information to validate further use of the TCR for ACT
Acknowledgements
The authors would like to thank the members of the Smeland lab and of the Department of Cellular Therapy for their positive input and support. We are also thankful to Dr. Pierre Dillard for commenting on this manuscript.
Abbreviations
- APC
Antigen presenting cells
- CSK
c-SRC kinase
- EuTDA
Europium TDA
- FB
Flow buffer
- FCS
Fetal calf serum
- GFP
Green fluorescent protein
- GMP
Good manufacturing practice
- HS
Human serum
- ITAMs
Immunoreceptor tyrosine-based activation motifs
- MAGE-A3
Melanoma-associated antigen 3
- MART-1
Melanoma antigen recognized by T cells 1
- MFI
Mean fluorescence intensity
- PBMC
Peripheral blood mononuclear cells
- PFA
Paraformaldehyde
- pMHC
Peptide–MHC
- RT
Room temperature
- SCT
Single-chain trimer
- TCR
T-cell receptor
- TGFβR2
Transforming growth factor β II
Author contributions
EMI, SW, GES, LEF, and JHM conceived and designed the experiments. EMI, NM, MPO, AF, GS, CP, and JHM performed the experiments. SW, OB, GK, and JHM interpreted the data. SW, EMI, NM, and JHM wrote the manuscript and all authors edited the manuscript.
Funding
This work was supported by the Gene Therapy program of the Radium Hospital to Sébastien Wälchli and Anne Fåne, The Norwegian Research Council to Else Marit Inderberg (#244388), and partly supported by an Innovation Grant from Southern and Eastern Norway Regional Health Authority to Nadia Mensali (#13/00367-88).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the Regional Committee for Medical and Health Research Ethics (REC South-East, Norway) (Approval no. 2013/624).
Informed consent
Informed consent from healthy donors was given.
References
- 1.Comrie WA, Burkhardt JK. Action and traction: cytoskeletal control of receptor triggering at the immunological synapse. Front Immunol. 2016;7:68. doi: 10.3389/fimmu.2016.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Werlen G, Palmer E. The T-cell receptor signalosome: a dynamic structure with expanding complexity. Curr Opin Immunol. 2002;14(3):299–305. doi: 10.1016/S0952-7915(02)00339-4. [DOI] [PubMed] [Google Scholar]
- 3.Malissen B, Bongrand P. Early T cell activation: integrating biochemical, structural, and biophysical cues. Annu Rev Immunol. 2015;33:539–561. doi: 10.1146/annurev-immunol-032414-112158. [DOI] [PubMed] [Google Scholar]
- 4.Chakraborty AK, Weiss A. Insights into the initiation of TCR signaling. Nat Immunol. 2014;15(9):798–807. doi: 10.1038/ni.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13(4):227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chow LM, Fournel M, Davidson D, Veillette A. Negative regulation of T-cell receptor signalling by tyrosine protein kinase p50csk. Nature. 1993;365(6442):156–160. doi: 10.1038/365156a0. [DOI] [PubMed] [Google Scholar]
- 7.Okada M. Regulation of the SRC family kinases by Csk. Int J Biol Sci. 2012;8(10):1385–1397. doi: 10.7150/ijbs.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vang T, Liu WH, Delacroix L, Wu S, Vasile S, Dahl R, Yang L, Musumeci L, Francis D, Landskron J, Tasken K, Tremblay ML, Lie BA, Page R, Mustelin T, Rahmouni S, Rickert RC, Tautz L. LYP inhibits T-cell activation when dissociated from CSK. Nat Chem Biol. 2012;8(5):437–446. doi: 10.1038/nchembio.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schoenborn JR, Tan YX, Zhang C, Shokat KM, Weiss A. Feedback circuits monitor and adjust basal Lck-dependent events in T cell receptor signaling. Sci Signal. 2011;4(190):ra59. doi: 10.1126/scisignal.2001893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan YX, Manz BN, Freedman TS, Zhang C, Shokat KM, Weiss A. Inhibition of the kinase Csk in thymocytes reveals a requirement for actin remodeling in the initiation of full TCR signaling. Nat Immunol. 2014;15(2):186–194. doi: 10.1038/ni.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu CY, Rupp LJ, Roybal KT, Lim WA. Synthetic biology approaches to engineer T cells. Curr Opin Immunol. 2015;35:123–130. doi: 10.1016/j.coi.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palmer DC, Guittard GC, Franco Z, Crompton JG, Eil RL, Patel SJ, Ji Y, Van Panhuys N, Klebanoff CA, Sukumar M, Clever D, Chichura A, Roychoudhuri R, Varma R, Wang E, Gattinoni L, Marincola FM, Balagopalan L, Samelson LE, Restifo NP. Cish actively silences TCR signaling in CD8+ T cells to maintain tumor tolerance. J Exp Med. 2015;212(12):2095–2113. doi: 10.1084/jem.20150304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daniel-Meshulam I, Horovitz-Fried M, Cohen CJ. Enhanced antitumor activity mediated by human 4-1BB-engineered T cells. Int J Cancer. 2013;133(12):2903–2913. doi: 10.1002/ijc.28320. [DOI] [PubMed] [Google Scholar]
- 14.Weichsel R, Dix C, Wooldridge L, Clement M, Fenton-May A, Sewell AK, Zezula J, Greiner E, Gostick E, Price DA, Einsele H, Seggewiss R. Profound inhibition of antigen-specific T-cell effector functions by dasatinib. Clin Cancer Res. 2008;14(8):2484–2491. doi: 10.1158/1078-0432.CCR-07-4393. [DOI] [PubMed] [Google Scholar]
- 15.Okoye I, Wang L, Pallmer K, Richter K, Ichimura T, Haas R, Crouse J, Choi O, Heathcote D, Lovo E, Mauro C, Abdi R, Oxenius A, Rutschmann S, Ashton-Rickardt PG. T cell metabolism. The protein LEM promotes CD8(+) T cell immunity through effects on mitochondrial respiration. Science. 2015;348(6238):995–1001. doi: 10.1126/science.aaa7516. [DOI] [PubMed] [Google Scholar]
- 16.Ebert PJR, Cheung J, Yang Y, McNamara E, Hong R, Moskalenko M, Gould SE, Maecker H, Irving BA, Kim JM, Belvin M, Mellman I. MAP kinase inhibition promotes T cell and anti-tumor activity in combination with PD-L1 checkpoint blockade. Immunity. 2016;44(3):609–621. doi: 10.1016/j.immuni.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 17.Eil R, Vodnala SK, Clever D, Klebanoff CA, Sukumar M, Pan JH, Palmer DC, Gros A, Yamamoto TN, Patel SJ, Guittard GC, Yu Z, Carbonaro V, Okkenhaug K, Schrump DS, Linehan WM, Roychoudhuri R, Restifo NP. Ionic immune suppression within the tumour microenvironment limits T cell effector function. Nature. 2016;537(7621):539–543. doi: 10.1038/nature19364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hinrichs CS, Restifo NP. Reassessing target antigens for adoptive T-cell therapy. Nat Biotechnol. 2013;31(11):999–1008. doi: 10.1038/nbt.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones BS, Lamb LS, Goldman F, Di Stasi A. Improving the safety of cell therapy products by suicide gene transfer. Front Pharmacol. 2014;5:254. doi: 10.3389/fphar.2014.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roybal KT, Rupp LJ, Morsut L, Walker WJ, McNally KA, Park JS, Lim WA. Precision tumor recognition by T cells with combinatorial antigen-sensing circuits. Cell. 2016;164(4):770–779. doi: 10.1016/j.cell.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vang T, Abrahamsen H, Myklebust S, Enserink J, Prydz H, Mustelin T, Amarzguioui M, Tasken K. Knockdown of C-terminal Src kinase by siRNA-mediated RNA interference augments T cell receptor signaling in mature T cells. Eur J Immunol. 2004;34(8):2191–2199. doi: 10.1002/eji.200425036. [DOI] [PubMed] [Google Scholar]
- 22.Heemskerk MH, Hoogeboom M, de Paus RA, Kester MG, van der Hoorn MA, Goulmy E, Willemze R, Falkenburg JH. Redirection of antileukemic reactivity of peripheral T lymphocytes using gene transfer of minor histocompatibility antigen HA-2-specific T-cell receptor complexes expressing a conserved alpha joining region. Blood. 2003;102(10):3530–3540. doi: 10.1182/blood-2003-05-1524. [DOI] [PubMed] [Google Scholar]
- 23.Walchli S, Kumari S, Fallang LE, Sand KM, Yang W, Landsverk OJ, Bakke O, Olweus J, Gregers TF. Invariant chain as a vehicle to load antigenic peptides on human MHC class I for cytotoxic T-cell activation. Eur J Immunol. 2014;44(3):774–784. doi: 10.1002/eji.201343671. [DOI] [PubMed] [Google Scholar]
- 24.Walchli S, Loset GA, Kumari S, Johansen JN, Yang W, Sandlie I, Olweus J. A practical approach to T-cell receptor cloning and expression. PLoS One. 2011;6(11):e27930. doi: 10.1371/journal.pone.0027930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inderberg EM, Walchli S, Myhre MR, Trachsel S, Almasbak H, Kvalheim G, Gaudernack G. T cell therapy targeting a public neoantigen in microsatellite instable colon cancer reduces in vivo tumor growth. Oncoimmunology. 2017;6(4):e1302631. doi: 10.1080/2162402X.2017.1302631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walseng E, Walchli S, Fallang LE, Yang W, Vefferstad A, Areffard A, Olweus J. Soluble T-cell receptors produced in human cells for targeted delivery. PLoS One. 2015;10(4):e0119559. doi: 10.1371/journal.pone.0119559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Almasbak H, Rian E, Hoel HJ, Pule M, Walchli S, Kvalheim G, Gaudernack G, Rasmussen AM. Transiently redirected T cells for adoptive transfer. Cytotherapy. 2011;13(5):629–640. doi: 10.3109/14653249.2010.542461. [DOI] [PubMed] [Google Scholar]
- 28.Myklebust JH, Irish JM, Brody J, Czerwinski DK, Houot R, Kohrt HE, Timmerman J, Said J, Green MR, Delabie J, Kolstad A, Alizadeh AA, Levy R. High PD-1 expression and suppressed cytokine signaling distinguish T cells infiltrating follicular lymphoma tumors from peripheral T cells. Blood. 2013;121(8):1367–1376. doi: 10.1182/blood-2012-04-421826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Felipe P, Luke GA, Hughes LE, Gani D, Halpin C, Ryan MD. E unum pluribus: multiple proteins from a self-processing polyprotein. Trends Biotechnol. 2006;24(2):68–75. doi: 10.1016/j.tibtech.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Johnson LA, Heemskerk B, Powell DJ, Jr, Cohen CJ, Morgan RA, Dudley ME, Robbins PF, Rosenberg SA. Gene transfer of tumor-reactive TCR confers both high avidity and tumor reactivity to nonreactive peripheral blood mononuclear cells and tumor-infiltrating lymphocytes. J Immunol. 2006;177(9):6548–6559. doi: 10.4049/jimmunol.177.9.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu YY, Netuschil N, Lybarger L, Connolly JM, Hansen TH. Cutting edge: single-chain trimers of MHC class I molecules form stable structures that potently stimulate antigen-specific T cells and B cells. J Immunol. 2002;168(7):3145–3149. doi: 10.4049/jimmunol.168.7.3145. [DOI] [PubMed] [Google Scholar]
- 32.Lissina A, Ladell K, Skowera A, Clement M, Edwards E, Seggewiss R, van den Berg HA, Gostick E, Gallagher K, Jones E, Melenhorst JJ, Godkin AJ, Peakman M, Price DA, Sewell AK, Wooldridge L. Protein kinase inhibitors substantially improve the physical detection of T-cells with peptide-MHC tetramers. J Immunol Methods. 2009;340(1):11–24. doi: 10.1016/j.jim.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis DM. Intercellular transfer of cell-surface proteins is common and can affect many stages of an immune response. Nat Rev Immunol. 2007;7(3):238–243. doi: 10.1038/nri2020. [DOI] [PubMed] [Google Scholar]
- 34.Hudrisier D, Riond J, Mazarguil H, Gairin JE, Joly E. Cutting edge: CTLs rapidly capture membrane fragments from target cells in a TCR signaling-dependent manner. J Immunol. 2001;166(6):3645–3649. doi: 10.4049/jimmunol.166.6.3645. [DOI] [PubMed] [Google Scholar]
- 35.Osborne DG, Wetzel SA. Trogocytosis results in sustained intracellular signaling in CD4(+) T cells. J Immunol. 2012;189(10):4728–4739. doi: 10.4049/jimmunol.1201507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aucher A, Magdeleine E, Joly E, Hudrisier D. Capture of plasma membrane fragments from target cells by trogocytosis requires signaling in T cells but not in B cells. Blood. 2008;111(12):5621–5628. doi: 10.1182/blood-2008-01-134155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Irish JM, Kotecha N, Nolan GP. Mapping normal and cancer cell signalling networks: towards single-cell proteomics. Nat Rev Cancer. 2006;6(2):146–155. doi: 10.1038/nrc1804. [DOI] [PubMed] [Google Scholar]
- 38.Wolchinsky R, Hod-Marco M, Oved K, Shen-Orr SS, Bendall SC, Nolan GP, Reiter Y. Antigen-dependent integration of opposing proximal TCR-signaling cascades determines the functional fate of T lymphocytes. J Immunol. 2014;192(5):2109–2119. doi: 10.4049/jimmunol.1301142. [DOI] [PubMed] [Google Scholar]
- 39.Adams CL, Grierson AM, Mowat AM, Harnett MM, Garside P. Differences in the kinetics, amplitude, and localization of ERK activation in anergy and priming revealed at the level of individual primary T cells by laser scanning cytometry. J Immunol. 2004;173(3):1579–1586. doi: 10.4049/jimmunol.173.3.1579. [DOI] [PubMed] [Google Scholar]
- 40.Schmid DA, Irving MB, Posevitz V, Hebeisen M, Posevitz-Fejfar A, Sarria JC, Gomez-Eerland R, Thome M, Schumacher TN, Romero P, Speiser DE, Zoete V, Michielin O, Rufer N Evidence for a TCR affinity threshold delimiting maximal CD8 T cell function. J Immunol 184(9):4936–4946 [DOI] [PubMed]
- 41.Nika K, Soldani C, Salek M, Paster W, Gray A, Etzensperger R, Fugger L, Polzella P, Cerundolo V, Dushek O, Hofer T, Viola A, Acuto O. Constitutively active Lck kinase in T cells drives antigen receptor signal transduction. Immunity. 2010;32(6):766–777. doi: 10.1016/j.immuni.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei P, Wong WW, Park JS, Corcoran EE, Peisajovich SG, Onuffer JJ, Weiss A, Lim WA. Bacterial virulence proteins as tools to rewire kinase pathways in yeast and immune cells. Nature. 2012;488(7411):384–388. doi: 10.1038/nature11259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Linette GP, Stadtmauer EA, Maus MV, Rapoport AP, Levine BL, Emery L, Litzky L, Bagg A, Carreno BM, Cimino PJ, Binder-Scholl GK, Smethurst DP, Gerry AB, Pumphrey NJ, Bennett AD, Brewer JE, Dukes J, Harper J, Tayton-Martin HK, Jakobsen BK, Hassan NJ, Kalos M, June CH. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood. 2013;122(6):863–871. doi: 10.1182/blood-2013-03-490565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cameron BJ, Gerry AB, Dukes J, Harper JV, Kannan V, Bianchi FC, Grand F, Brewer JE, Gupta M, Plesa G, Bossi G, Vuidepot A, Powlesland AS, Legg A, Adams KJ, Bennett AD, Pumphrey NJ, Williams DD, Binder-Scholl G, Kulikovskaya I, Levine BL, Riley JL, Varela-Rohena A, Stadtmauer EA, Rapoport AP, Linette GP, June CH, Hassan NJ, Kalos M, Jakobsen BK. Identification of a Titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells. Sci Transl Med. 2013;5(197):197ra103. doi: 10.1126/scitranslmed.3006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Depontieu FR, Qian J, Zarling AL, McMiller TL, Salay TM, Norris A, English AM, Shabanowitz J, Engelhard VH, Hunt DF, Topalian SL. Identification of tumor-associated, MHC class II-restricted phosphopeptides as targets for immunotherapy. Proc Natl Acad Sci USA. 2009;106(29):12073–12078. doi: 10.1073/pnas.0903852106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Piepenbrink KH, Blevins SJ, Scott DR, Baker BM. The basis for limited specificity and MHC restriction in a T cell receptor interface. Nat Commun. 2013;4:1948. doi: 10.1038/ncomms2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sewell AK. Why must T cells be cross-reactive? Nat Rev Immunol. 2012;12(9):669–677. doi: 10.1038/nri3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Almasbak H, Lundby M, Rasmussen AM. Non-MHC-dependent redirected T cells against tumor cells. Methods Mol Biol. 2010;629:453–493. doi: 10.1007/978-1-60761-657-3_28. [DOI] [PubMed] [Google Scholar]
- 49.Kenderian SS, Ruella M, Shestova O, Klichinsky M, Aikawa V, Morrissette JJ, Scholler J, Song D, Porter DL, Carroll M, June CH, Gill S. CD33-specific chimeric antigen receptor T cells exhibit potent preclinical activity against human acute myeloid leukemia. Leukemia. 2015;29(8):1637–1647. doi: 10.1038/leu.2015.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Z, Li Z. Molecular imaging in tracking tumor-specific cytotoxic T lymphocytes (CTLs) Theranostics. 2014;4(10):990–1001. doi: 10.7150/thno.9268. [DOI] [PMC free article] [PubMed] [Google Scholar]