Abstract
Adoptive transfer of T cells genetically modified by TCRs or CARs represents a highly attractive novel therapeutic strategy to treat malignant diseases. Various approaches for the development of such gene therapy medicinal products (GTMPs) have been initiated by scientists in recent years. To date, however, the number of clinical trials commenced in Germany and Europe is still low. Several hurdles may contribute to the delay in clinical translation of these therapeutic innovations including the significant complexity of manufacture and non-clinical testing of these novel medicinal products, the limited knowledge about the intricate regulatory requirements of the academic developers as well as limitations of funds for clinical testing. A suitable good manufacturing practice (GMP) environment is a key prerequisite and platform for the development, validation, and manufacture of such cell-based therapies, but may also represent a bottleneck for clinical translation. The German Cancer Consortium (DKTK) and the Paul-Ehrlich-Institut (PEI) have initiated joint efforts of researchers and regulators to facilitate and advance early phase, academia-driven clinical trials. Starting with a workshop held in 2016, stakeholders from academia and regulatory authorities in Germany have entered into continuing discussions on a diversity of scientific, manufacturing, and regulatory aspects, as well as the benefits and risks of clinical application of CAR/TCR-based cell therapies. This review summarizes the current state of discussions of this cooperative approach providing a basis for further policy-making and suitable modification of processes.
Keywords: CAR/TCR-transgenic T cells, Cellular therapy, Regulatory aspects, Clinical translation
Introduction
Cellular therapy has been traditionally applied in the context of allogeneic BMT or SCT in patients with high-risk leukemia, lymphoma and myeloma [1, 2]. T cells and other immune cells present in the stem cell product or transferred after SCT as donor lymphocyte infusion (DLI) may efficiently eliminate residual leukemia cells but are also associated with GvHD [3–5]. The high treatment-related toxicity associated with GvHD has been accepted in these high-risk patients as their prognosis without this treatment option is particularly poor, and the therapeutic outcome has the potential to result in long-term response and survival [3] based on the memory potential of adaptive anti-tumor immune responses [6, 7].
Over the past decades, a series of approaches were put forward to target tumor cells in a more specific manner via, e.g., adoptive transfer of isolated and expanded tumor-specific T cells, development of bi- or multi-specific antibodies, or genetic modification of immune effector cells. Thereby, cellular therapies using genetically modified T cells experienced a major advance by the introduction of either a CAR or TCR that target defined antigens presented by tumor cells [8, 9]. Clinical trials using CAR-T cells have demonstrated substantial efficacy especially in CD19-positive advanced B-cell malignancies including acute lymphoblastic leukemia, chronic lymphocytic leukemia and aggressive B-cell lymphomas [10–12]. Recently, the first CD19-CAR-T-cell products from Novartis and Kite Pharma received approval in the US for the treatment of childhood and adolescent acute lymphoblastic leukemia and large B-cell lymphoma in adults, respectively. TCR-transgenic T cells are also evaluated in clinical trials, and anticancer activity has been reported in hematopoietic and non-hematopoietic malignancies [13–16].
Although genetic modification of T cells has been shown to be therapeutically effective in a variety of malignancies, a careful clinical translation is needed given the potential risks associated with these therapeutic approaches. Previous clinical trials have demonstrated the occurrence of severe and life-threatening toxicities as well as some fatalities after therapeutic administration of CAR- and TCR-engineered T cells [17, 18]. The complexity of the risk profile is high and related to diverse but interconnected characteristics of the investigational medicinal product (IMP) as well as the individual medical condition and disease state of the recipient. Furthermore, the prediction of toxicities from non-clinical studies is hampered as suitable animal models are often missing. Therefore, careful considerations are needed with regard to the design of first-in-human trials with such products and a robust infrastructure should be in place for identification and mitigation of toxicities. A joint effort of scientists, clinicians and regulators is required to enable successful translation of these complex products to the benefit of the patients.
Apart from these challenges, genetically engineered T cells are considered as advanced therapy medicinal products (ATMPs) which need to be manufactured in compliance with good manufacturing practice (GMP). The GMP requirements for these highly complex products render these therapeutic approaches quite cost-intensive, further slowing down clinical translation.
The Deutsches Konsortium für Translationale Krebsforschung—DKTK (German Cancer Consortium) is one of the Deutsche Zentren der Gesundheitsforschung—DZG (German Health Research Centers), established around the Deutsches Krebsforschungszentrum—DKFZ (German Cancer Research Center) in Heidelberg. Together with universities, university hospitals and academic research centers at the partner sites in Berlin, Dresden, Essen/Düsseldorf, Frankfurt/Mainz, Freiburg, Heidelberg, Munich and Tübingen, this institution aims to focus, improve and enhance clinically oriented innovative cancer research. DKTK researchers set out to systematically address issues associated with clinical translation of advanced cellular therapies, specifically the academic, societal and socio-economic challenges. DKTK, therefore, fosters close collaboration between translational research and regulatory authorities on specific research topics. DKTK workshops organized together with the regulators of the Paul-Ehrlich-Institut (PEI), the German Federal Authority for Vaccines and Biomedicines, provide a new format to discuss the regulatory challenges related to the quality, non-clinical and clinical aspects of certain biomedicines. A DKTK/PEI workshop held in 2016 initiated the problem-oriented discussion about novel therapies based on CAR- and TCR-modified T cells within this group, as a starting point for a continuing dialog between research scientists, clinicians and regulators towards more efficient translational processes in Germany and Europe. The following chapters provide the present state of discussions within the cooperative DKTK/PEI approach.
Legal framework in Germany and the European Union
In the EU, T cells genetically modified by TCRs or CARs are classified as ATMPs. The corresponding regulation (European Commission (EC), No 1394/2007) applies since 2008 and provides a common regulatory framework for gene therapies, somatic cell therapies and tissue engineered products. Article 2(5) of the ATMP regulation specifies that a product which may fall within the definition of a somatic cell therapy medicinal product and a gene therapy medicinal product (GTMP) shall be considered as a GTMP. Since this scenario applies for genetically engineered T cells, CAR- and TCR-modified T cells are classified as GTMPs [19]. However, in addition to the regulatory classification, in scientific terms CAR- and TCR-modified T cells are also considered as cancer immunotherapies, and, more specifically, as adoptive cellular immunotherapies in accordance with their mechanism of action [20].
In general, the same standards regarding manufacture, non-clinical characterization and clinical trial design apply for clinical trials with CAR- and TCR-modified T cells as for other medicinal products independent of the phase of the clinical trial. This does especially include the overarching principle of clinical trials approval which consists of weighing benefits and risks. Thus, a clinical trial may only be undertaken if the foreseeable risks and inconveniences have been considered against the anticipated benefit for the individual trial subject and other present and future patients as stipulated in Directive 2001/20/EU.
In the EU, the approval of clinical trials is in the remit of the member states. In Germany, clinical trial applications for GTMPs are authorized by the PEI. Multinational clinical trial applications can be submitted based on the Voluntary Harmonization Procedure, a prototype of the new procedure laid down in Regulation 536/2014/EU, which is coordinated by the Clinical Trials Facilitation Group office, located at the PEI. The Voluntary Harmonization Procedure aims to provide a coordinated assessment of a clinical trial application between the participating member states, despite the fact that some critical issues are currently treated in a different manner between EU member states [21]. In Germany, as an additional prerequisite for approval of a clinical trial, a positive opinion from independent (and in the future: registered) Ethics Committees has to be obtained. A manufacturing license is granted by the respective local competent authority (Landesbehörde) in accordance with Sect. 13 of the German Medicinal Products Act (see below). Since genetically engineered T cells fall under the definition of a genetically modified organism (GMO), an environmental risk assessment has to be performed by the sponsor for each clinical trial. Appropriateness of the environmental risk assessment is evaluated by the PEI in consultation with the Federal Office for Consumer Protection and Food Safety (Bundesamt für Verbraucherschutz und Lebensmittelsicherheit), which is the German competent authority for the deliberate release approval of a GMO into the environment.
There is a significant concern in the cell therapy community in Germany and the EU about a delayed and ineffective translation of concepts for innovative therapies into clinical applications. It is a common understanding of both, researchers and regulators, that a balance has to be created between requirements of GMP that need to be considered, a coherent and integrative risk assessment that includes the clinical scenario of a severely ill patient population deprived of therapeutic alternatives, and novel approaches to respond to safety issues that are difficult to address such as target specificity, uncontrolled expansion of the genetically modified cells within the recipient and insertion of the CAR/TCR constructs into the genome of the modified cells.
The recently released Guidelines on Good Manufacturing Practice specific to Advanced Therapy Medicinal Products (https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-4/2017_11_22_guidelines_gmp_for_atmps.pdf) have been adopted from an extensive consultation process that stakeholders were invited to contribute to. The document is understood to develop an understanding of the variability in manufacturing inherent to the nature of biological products, and to enable flexibility in the development of ATMPs, where the acceptable quality is related to the stage of development and a concept of responsibility in the assessment and acceptance of risks. Similarly, industry has shown awareness of the need to respond to challenges in ATMP development and manufacture. In many instances, industry stakeholders have committed themselves to interact with academic institutions as the major source of ATMP development [22]. The multifold interactions show the willingness of the different stakeholders to communicate with each other and to find solutions. However, the highly different perspectives as well as the complexity of the matter currently impede options for reconcilement.
Already early on, when the DZG were established to promote efficient translation of basic research findings into new treatment approaches for patients, the PEI joined such efforts by offering regulatory guidance. Regulatory support for ATMPs and other innovative products is also needed in view of certain specificities in the German regulatory environment. The regulatory situation in Germany is different from other EU member states: (a) the regulatory status of certain cellular blood products including peripheral blood stem cells is higher than in the other member states, as these are regulated as medicinal products under the German Medicinal Products Act; (b) due to the federal structure, local competent authorities are in charge of GMP oversight and grant manufacturing licenses. Hence, from the academic developers’ perspective, the establishment of focused “Academic Competence Centers for ATMPs” with a defined mission appears to be highly desirable (Table 1). In addition, harmonization of the regulatory framework on national, EU and in best case global level is seen as highly important to handle comparable requirements when preparing and conducting early and advanced phase clinical cellular therapy trials.
Table 1.
A proposal of the academic developers group for the establishment of Academic Competence Centers for ATMPs and their mission
| Mission of Academic Competence Centers for ATMPs |
|---|
| Provide a defined quality assurance and infrastructure that will allow (in total or in part) |
| The manufacture, quality control, storage, release and delivery of ATMPs |
| The design and execution of early phase clinical trials with ATMPs |
| The interdisciplinary management of toxicities |
| The active follow-up of patients treated with ATMPs, |
| Interact on both national and EU level |
| Should be accredited in terms of process and structure quality by the local competent authority as the basis for a national accreditation |
| May apply for the coverage of costs based on their national accreditation at health insurance companies for certain products |
| Should be obliged to establish and maintain a network of exchange with other accredited centers to avoid redundancy and excessive consumption of resources |
Especially in academic centers, the speed of technological development will allow to make use of technology platforms and medical devices that challenge the conventional borders between processes and products. Highly standardized, semiautomatic systems will support the collaboration and mutual support between academic manufacturing sites, and the comparability of products that emerge from the standardized manufacturing process.
From a regulatory perspective, it is proposed to align structures and efforts to shape an interactive network of academic institutions that will allow for faster access to innovative therapies in Germany. For academic institutions, the challenge lies in the development of a common, risk-based pathway for the manufacture and quality control in compliance with standards of GMP. This calls for high-level proposals to the competent authorities to involve them in a collaborative effort to shape new models of accreditation of academic centers, process-oriented dossiers, generic tools, and fast-track development strategies for innovative medicines.
Critical aspects of engineered T cells
Many aspects substantially define the active pharmaceutical ingredient of genetically modified cells, and therefore, efficacy and safety of the therapeutic approach. These include the type of cells to be modified, the means of genetic transfer, the CAR/TCR construct itself including its specificity, as well as the transfer of additional genes that aim at either improving efficacy or safety of the therapeutic approach. All these aspects interrelate and incorporate many sub-aspects resulting in a high complexity of the medicinal product.
Immune effector cell type
In case of transfer of CAR or TCR coding genes, diverse hematopoietic cells such as T cells, NK cells, hematopoietic stem cells or subsets thereof have been investigated in preclinical and clinical studies [10, 23–25]. With respect to toxicity there are a number of cell-related risk factors. For example, longevity of T cells is considered to be of high importance for durable responses [26, 27] but may represent a particular problem in case of serious adverse reactions. In contrast to T cells, NK cells lack long-term survival which may reduce the risk for long-term toxicity. Stem cell characteristics of effector cells may provide the advantage of long-term efficacy [28] but concurrently may be associated with an enhanced potential for malignant transformation. Furthermore, many aspects of defined cell populations have not been studied in detail, making a risk assessment challenging. The plasticity of hematopoietic stem cells in general and the potential to differentiate [29] may add to the general risks of defined cell populations and challenges the benefit of meticulous characterization of cellular components. Thus, detailed definition of a cellular product and subsequent correlation with clinical outcome will be an important step to further clarify these issues.
Mode of gene transfer
Redirection of cells to target cancer cells can be accomplished by a diversity of techniques. With respect to efficacy, high and long-term transgene expression is an important requirement for effective tumor rejection which can only be achieved by genomic integration of the transgene encoding the CAR or TCR. Currently, retroviral and lentiviral vectors are mainly used for this purpose [30], with induction of insertional oncogenesis as observed in early gene therapy clinical trials initially being a concern [31, 32]. However, despite the high proliferative capacity of T cells, cases of insertional oncogenesis have not been observed so far when using genetically modified T cells. This indicates a certain resistance of T cells to malignant transformation and suggests that retroviral and lentiviral vectors may be safe for genetic modification of T cells [33]. Nevertheless, developments such as non-viral genetic transfer by RNA [34], the use of transposons [35–37], or of novel gene-editing technologies [38] may further reduce risks associated with viral gene transfer. In any case, the mode of gene transfer represents one option to define a common standardized master approach which subsequently may be applied for different constructs.
Chimeric antigen receptors
CARs typically recognize the target antigen through a single chain fragment variable (scFv) domain of an antibody fused to a spacer/hinge domain of variable length which is usually derived from CD8-alpha chain or immunoglobulin G molecules. Over the past years, several generations of CAR constructs have been developed mainly differing with respect to their intracellular signaling domains. The signaling domains play a crucial role in determining efficacy as well as adverse reactions of CAR T cells [39–42], requiring an individual risk assessment for each construct. Consequently, to minimize unexpected serious adverse reactions it is advisable to base as far as possible future generations of CARs on elements already tested successfully in clinical trials. With respect to antigen recognition by defined CARs, preclinical in vivo activity and safety may be investigated in selected animal models in case the target antigen provides a high homology to the target structure in humans, although minor differences in reactivity may result in unforeseen major toxicities as previously shown for other types of immunotherapy [43]. Thus, residual risks may persist when conducting a clinical trial, and an adequate benefit-risk analysis based on a clinical study design including an appropriately adapted starting dose needs to be established.
T-cell receptors
With respect to TCRs, the mode of action is more complex than that of CARs due to several special features of T-cell mediated target recognition. As conventional TCRs consist of two chains [44], introduction of a transgenic TCR into T cells may result in mispairing between endogenous and the transgenic TCR chains potentially resulting in lethal autoimmunity as seen in mouse models [45]. However, in humans this has not been observed so far. Moreover, a number of approaches have been developed to reduce the risk for mispairing, including the introduction of additional cysteine bonds, murinization, also encompassing potentially less immunogenic minimal murine sequences of constant regions, and the construction of single-chain TCRs by fusing the introduced alpha- and beta-chains [46–50]. Another special feature of TCRs is the binding affinity which is much lower as compared to antibodies [51]. In the autologous system, TCRs with enhanced peptide-independent MHC binding are either deleted, or differentiate into selected T-cell subpopulations during thymic development [52]. As soon as the TCR derives from an allogeneic or xenogeneic environment or is affinity-maturated in vitro, off-target reactivity is more likely, even if the respective HLA molecule presenting the target peptide ligand is matched. Due to the large peptide ligandome presented by MHC molecules, preclinical exclusion of any cross-reactivity is currently not feasible. Since cross-reactivity can usually not be addressed sufficiently in animal models, alternative testing strategies are important to potentially detect cross-reactivity before clinical application. As for CARs, also for TCRs an adequate benefit-risk analysis based on an appropriate study design is essential to balance the remaining risk.
Introduction of additional genetic modifications
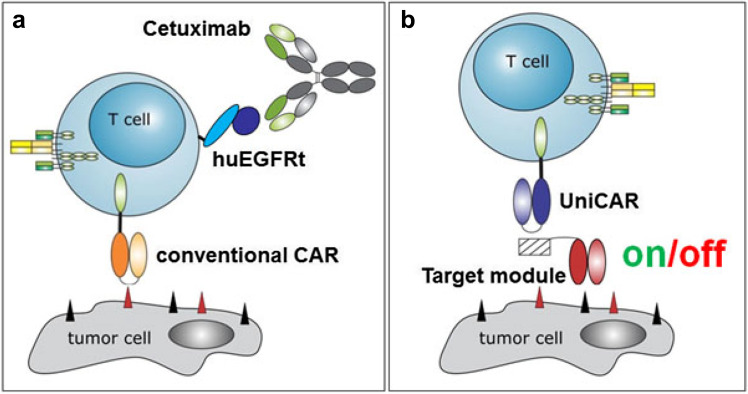
Other genetic modifications are currently applied in addition to the redirecting construct, with the aim to enhance efficacy, modify function or reduce toxicity [53–56]. These modifications may have an impact on the benefit-risk analysis of the primary construct which needs to be considered. For instance, the inclusion of immunostimulatory cytokines or checkpoint-modulating constructs may enhance effector cell activity but also increase the risk for toxicity. In contrast, inclusion of a suicide gene that allows removal of the genetically modified cells may reduce the severity of potential adverse reactions and should be considered accordingly in the benefit-risk analysis. There are various approaches to deplete potential autoreactive T cells by introduction of additional genes as, e.g., the HSV thymidine kinase suicide gene with ganciclovir prodrug [57], the transgene encoding an inducible caspase-9 [55], and a truncated version of EGFR as target for antibody-mediated cell killing (Fig. 1a) [58]. The risk of serious adverse reactions may alternatively be reduced by controlled activation of the modified T cells using a modular system, for example, the UniCAR system (Fig. 1b) [59]. Irrespective of the chosen strategy, the functionality of such systems needs to be investigated in non-clinical studies.
Fig. 1.
Technologies to overcome unwanted side effects of genetically modified (CAR) T cells. a In case severe side effects on healthy tissue occur upon adoptive transfer of genetically modified T cells, these cells can be eliminated by application of antibodies targeting genetically modified T cells; e.g., CAR T cells expressing a truncated EGFR domain can be eliminated via the clinically used antibody cetuximab. b T cells modified with the UniCAR system are inert (‘off’ status). Only in the presence of a target module, UniCAR T cells can recognize target cells and get activated (‘on’). As the respective target modules can reversibly and rapidly associate and dissociate with the UniCAR domain in a concentration-dependent manner, UniCAR T cells can be turned off in case severe side effects occur, simply by stopping infusion of the target module, and switched on again if necessary [60–62]
While the non-clinical program for a GTMP before its first clinical administration is expected to be performed according to the European Medicines Agency (EMA) guideline on ‘Non-clinical studies required before first clinical use of gene therapy medicinal products’ [63], several compromises have to be made for CAR- and TCR-modified T cells. These are necessary, since relevant animal models expected to provide reliable and translatable data for humans are often not available. Therefore, an adapted non-clinical program which is tailored to the specificities of the engineered T cells is usually performed.
Pharmacology studies and their limitations
Antigen-specific activation, expansion, and cytotoxic activity against tumor cells as well as engraftment and persistence of the CAR- and TCR-modified T cells are considered essential parameters for achieving clinical efficacy. However, some of these parameters are not exclusively defined by the product-specific characteristics, but also influenced by patient-specific conditions such as the tumor load and the preconditioning regimen [64]. For example, non-clinical dose-finding studies are hampered in several ways. First, the administered number of genetically modified cells is expected to increase in vivo due to the activation and subsequent expansion of these cells. However, the extent of T-cell expansion in vivo largely depends on diverse and as yet insufficiently defined determinants which can hardly be mimicked in animal studies. Second, if genetically modified human cells are administered to immunocompromised animals, the endogenous TCR may be activated through a xenogeneic immune reaction which could induce cell expansion that is not expected to occur in patients in an autologous setting. In contrast, the survival of the human cells may be compromised in the animal models in the absence of human cytokines and other species-specific factors. Thus, even if administered to tumor-bearing animals, the expansion rate of the genetically modified human T cells may not be predictive for the expansion of the cells when administered to a patient. Therefore, it is generally accepted that the starting dose in humans is not based on the actual non-clinical testing of the CAR- or TCR-modified T cells, but instead derived from clinical experiences with other CAR- and TCR-modified T cells. As a consequence, it is in most cases not necessary to perform non-clinical dose-finding studies. However, other parameters can and should be tested in non-clinical studies. These include the expression of the transgenic CAR or TCR, the specificity of the modified T cells for the target antigen and their biological activity upon target recognition. These parameters can be investigated to some extent in vitro. In addition, animal pharmacology studies are generally expected to demonstrate tumor control by the genetically modified T cells in vivo. Usually, such studies are performed using human cells in an appropriate tumor-bearing immunocompromised mouse model. However, the decision whether or not to include such studies should be made on an individual basis and in consultation with the responsible competent authority. Apart from that, non-clinical pharmacokinetic studies addressing the biodistribution of the genetically modified cells are usually not requested due to the difficulties of translating such data to the patient’s situation. Nevertheless, from a regulatory point of view it is advisable to evaluate the expansion and persistence of the genetically modified T cells concomitant to their anti-tumor activity in animal models, provided that such models are included in the pharmacology program.
Safety studies and their limitations
Some adverse events, such as tumor lysis syndrome, CRS, hemophagocytic lymphohistiocytosis, CAR-T cell-related encephalopathy syndrome (CREST) and macrophage activation syndrome, have been repeatedly observed in clinical studies with genetically modified T cells [10, 17, 39, 65, 66]. These side effects are dependent on many different factors that are difficult to model in non-clinical experiments. Hence, it is generally accepted that these potential toxicities are not investigated in non-clinical studies. Instead, they have to be expected when administering genetically modified T cells, with the severity of these adverse events assumed to largely depend on the tumor load of the patients and the cell type composition of the product. Consequently, appropriate risk mitigation measures including a close monitoring of such adverse events and appropriate treatment options must be in place at the study site. A CAR-T-Cell-associated toxicity (CARTOX) group has been formed in the USA, working on recommendations for monitoring, grading and managing acute toxicities associated with this therapeutic approach [39].
Other potential toxicities relate more directly to the antigen specificity of the CAR or TCR and include potential on- and off-target effects, which should be addressed to the extent possible before administering the genetically modified T cells to patients. Since the possibilities and limitations of non-clinical analyses for identifying potential on- and off-target toxicities differ between CARs and TCRs, they are considered separately.
For CARs, a key question is whether or not the scFv antibody domain exclusively recognizes the human antigen. If binding is restricted to the human antigen, a relevant animal model is most likely not available. Even the use of a fully homologous animal model as previously described for CD19-CAR T cells has clear limitations for predicting on- and off-target toxicities [67]. Thus, it is rather recommended to address potential on-target toxicity of the CAR by a detailed investigation of the expression pattern of the target antigen in human cells, tissues and organs, while potential off-target toxicities might be addressed at least to some extent in in vitro studies using human cells and tissues. Clinical experiences with other CARs targeting the same antigen or with otherwise related or informative medicinal products might also be valuable for evaluating the potential risks of a given CAR approach before its translation to the human setting. For scFv antibodies which cross-react with the target antigen in another species, the situation is different, as safety studies could in principle be performed in that species. In such case, it should be carefully evaluated what the limitations of the potential animal model are and how informative the obtained safety data will be for the translation to humans. As these considerations are essential with regard to the strategy and extent of the non-clinical safety testing, it is advisable to discuss this early on with the competent higher federal authority.
For TCRs it is generally accepted that on-target and off-target toxicity cannot be adequately addressed in animal models mainly due to the HLA-restriction of the TCR. Even in case that a transgenic animal model expressing the respective HLA molecule should exist, it is still possible that the relevant peptides including the target peptide as well as potential cross-reactive peptides are not adequately presented by defined cells of the animal. Therefore, a combination of in silico analyses and in vitro studies is usually performed to address the potential on-target and off-target toxicities of a TCR, while safety studies in animals are not performed. Such an alternative safety program ideally includes the following: (a) a detailed investigation of the expression pattern of the target antigen in human cells, tissues and organs; (b) a thorough characterization of the core peptide binding motif of the TCR, in particular if the TCR has been derived from a xenogeneic origin or has been affinity-maturated in vitro; (c) an in silico analysis with the core peptide binding motif for identification of potentially cross-reactive peptides; (d) an appropriate in vitro testing for actual evaluation of cross-reactivity; and (e) an extensive alloreactivity screen. Such a dataset is expected to provide sufficient information on potential on-target and off-target toxicities, and to enable a well-balanced benefit-risk analysis.
Challenges of personalized therapies
Personalized therapies, e.g., when targeting somatic mutations by neoantigen-specific TCRs [68, 69], represent a major challenge due to several reasons: (a) individual vector constructs need to be manufactured, resulting in currently unreasonably high costs. This is even more demanding as usage of multiple TCRs targeting diverse neoantigens would be favorable [70, 71]; (b) the time period currently required for regulatory processes will often extend beyond the lifespan of the diseased patient. These hurdles may be best addressed in focused round table discussions, involving all stakeholders and including representatives from the PEI and the competent authorities of the German federal states. Production of redirecting constructs using pre-established master processes may represent a conceivable development. In case of usage of such a TCR in an autologous setting, extensive toxicity screens may not be needed. Nevertheless, the use of such TCRs in an allogeneic or xenogeneic setting would still require preclinical toxicity screens for the TCRs themselves as outlined above.
Modular concepts and off-the-shelf products for a broader application
Modular concepts and off-the-shelf products represent an interesting strategy with potential advantages concerning the safety, flexibility, and costs of genetically modified T cells. This can be effective by the development of new CAR constructs from earlier-generation CARs using the same vector backbone to which additional costimulatory molecules, hinge regions, and spacer elements may be added [72]. Another approach combines a universal CAR with modular adapter proteins for tumor cell recognition (e.g., UniCARs [60]).
Off-the-shelf products are based on allogeneic cells. However, in general, allogeneic T cells have a high risk of inducing GvHD due to the endogenous TCR of unknown specificity. While attempts are being made to eliminate the endogenous TCR using gene editing, allogeneic NK cells may provide a suitable alternative due to their considerable lower risk of inducing GvHD. Nevertheless, the development of CAR-engineered primary NK cells is still in its early stages. More progress has been made with genetic modification of the clinically usable NK cell line NK-92 [73], where a single center phase I clinical trial with ErbB2-specific CAR NK-92 cells in patients with recurrent ErbB2-positive glioblastoma is ongoing (NCT03383978; clinicaltrials.gov) [74].
Aspects of first-in-human clinical trials with CARs/TCRs
Adoptive transfer of genetically modified cells has shown high potential [75]. However, patient populations for phase I clinical trials need to be carefully selected considering type and stage of disease, the target antigen, and lack of alternative treatment options. In addressing the potential risks it is useful to take into account the published experience with other CARs/TCRs, and to implement appropriate risk mitigation in the trial design. Important risk mitigation strategies include the selection of a safe starting dose, staggered enrollment of trial subjects [76] and close patient monitoring, well trained clinical staff and availability of intensive care facilities. Different views have been expressed regarding the appropriateness of dose-escalation studies of CAR T cells. The administered cell dose may be less relevant, as CAR T-cell toxicity is related to the cell expansion in vivo, and the individual disease burden of the patients is expected to modulate the extent of CAR T-cell activation and proliferation [77], resulting in a high subject-to-subject variability. On the other hand, dose-escalation studies have demonstrated higher toxicity with higher doses [78], thus arguing in favor of including dose escalation in the clinical study design. From a regulatory point of view, a rationale for the dosing regimen is needed. As outlined above, non-clinical studies are less informative for dose-finding, and it is generally accepted that the starting dose in humans is derived from clinical experiences with other CAR- and TCR-modified T cells. Product-specific factors like transduction efficiency, proliferation capacity, and disease-specific criteria like antigen expression and tumor load should also be taken into account. Algorithms for the treatment of most prominent toxicities should be in place [39, 79, 80] and high alertness for other toxicities including on-target/off-tumor and off-target reactions, anaphylaxis, insertional mutagenesis, GvHD, as well as novel so far non described toxicities should be given. It is advisable to implement an independent safety monitoring board for decisions related to dose escalation, if appropriate, and adherence to stopping rules (http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2017/07/WC500232186.pdf). Feasibility to produce and apply the medicinal product is an important aspect in case of personalized therapies. With respect to GMP production and product validation, product stability as well as the patient condition needs to be taken into account. Therefore, failure to treat may be an important trial endpoint.
Future challenges, tasks and potential solutions
Clinical translation of complex novel therapeutics such as CAR- and TCR-engineered T cells is a high promise but also an enormous hurdle for academia with regard to the associated costs. The fact that usually autologous cells are used for CAR- and TCR-based therapies, which need to be manufactured and controlled for each patient separately, and the risk-mitigation measures needed during the clinical trial, increase the costs even further. However, as for most other ATMPs, the development of new CAR- and TCR-based therapies is for the most part driven in academic environments, while pharmaceutical companies usually step in at later stages of clinical development. This is expected to limit clinical translation of novel and innovative approaches, since the funding of academia for bringing new therapies into clinical trials is highly limited. Approaches chosen in the USA, with major investments channeled into a few institutions jointly funded by academia, industry and charity, seem to be promising for overcoming some limitations. Further analysis of the reasons for the hurdles that delay clinical translation, and discussion of potential solutions should take into account the perspectives of research scientists, regulators and clinicians. Hence, regular round table discussions on the national but also on the European level appear mandatory. With a better understanding of the clinical efficacy and adverse reactions, it might also be possible to adapt the regulatory requirements accordingly. Both, additional characterizations of the genetically modified cells before administration to the patients, and analyses of the genetically modified cells in patient samples after treatment might help to correlate, e.g., a certain design of the CAR, a minimal transduction efficiency of the cells, and a specific cellular composition of CAR- and TCR-modified cells, respectively, with a certain clinical outcome. Inclusion of independent expert opinions with respect to the complex characteristics of the novel medicinal product is highly important for the development of such therapies towards clinical use. Likewise, discussing the ethical aspects of a benefit-risk analysis as well as a differentiated evaluation of costs for safety measurements is urgently needed within the society, including not only research scientists, regulators and clinicians, but also patients, health care providers, and politicians. Chances and limitations of the current legal framework need to be evaluated, and modifications discussed and followed under consideration of the state of the art. Master manufacturing processes and generic manufacturing licenses may help to reduce the efforts for novel highly personalized therapeutic agents. Communication of the outcome of discussions within the scientific community and among regulators is of high value to accelerate processes and harmonize procedures. In addition, public disclosure of product development proceedings between academia and the regulatory authorities may be a probate measure to push the whole field. This may be of particular importance to broadly drive improvements of the clinical translation process.
Conclusion
There is an urgent need for the structured promotion of clinical application of CAR/TCR T-cell therapies within Germany and the EU. Towards this aim, a cooperative initiative has been formed by scientists and clinicians within the DKTK, and representatives of the regulatory authorities. Based on their combined technological, regulatory and clinical expertise, this initiative aims to overcome existing hurdles for translation of academic research innovations into clinical studies. Key challenges and potential solutions have been formulated by respective partners including the vision of researchers towards the establishment of Academic Competence Centers for ATMPs as well as the structured exchange between all stakeholders to discuss the different aspects for clinical translation and first-in-human studies. These need to focus on patients’ risks and benefits as well as societal and socio-economic issues to make CAR/TCR therapy more rapidly available for patients with hematological and solid malignancies who are desperately awaiting novel treatments for their life-threatening disease.
Disclaimer statement
The views expressed in this article are the personal views of the authors and may not be understood or quoted as being made on behalf of or reflecting the official position of the Paul-Ehrlich-Institut, or the European Medicines Agency or one of its Committees or Working Parties.
Abbreviations
- ATMP
Advanced therapy medicinal product
- CARTOX
CAR-T-Cell-associated toxicity
- CREST
chimeric antigen receptor (CAR)-T cell related encephalopathy syndrome
- CRS
cytokine release syndrome
- DKFZ
Deutsches Krebsforschungszentrum (German Cancer Research Center)
- DKTK
Deutsches Konsortium für Translationale Krebsforschung (German Cancer Consortium)
- DLI
donor lymphocyte infusion
- DZG
Deutsche Zentren der Gesundheitsforschung
- EGFR
epidermal growth factor receptor
- ErbB2, HER2/neu
human epidermal growth factor receptor 2
- GMO
genetically modified organism
- GMP
good manufacturing practice
- GTMP
gene therapy medicinal product
- HZDR
Helmholtz Zentrum Dresden Rossendorf
- IMP
investigational medicinal product
- NCT
Nationales Centrum für Tumorerkrankungen
- PEI
Paul-Ehrlich-Institut
- TUM
Technische Universität München
Author Contributions
All authors participated in writing and editing of the manuscript.
Funding
Support for this publication was provided by the DKTK.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Thomas ED. Bone marrow transplantation: prospects for leukemia and other conditions. Proc Inst Med Chic. 1975;30:256–258. [PubMed] [Google Scholar]
- 2.Gyurkocza B, Rezvani A, Storb RF. Allogeneic hematopoietic cell transplantation: the state of the art. Expert Rev Hematol. 2010;3:285–299. doi: 10.1586/ehm.10.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood. 2008;112:4371–4383. doi: 10.1182/blood-2008-03-077974. [DOI] [PubMed] [Google Scholar]
- 4.Billingham RE. The biology of graft-versus-host reactions. Harvey Lect. 1966;62:21–78. [PubMed] [Google Scholar]
- 5.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373:1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schadendorf D, Hodi FS, Robert C, et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015;33:1889–1894. doi: 10.1200/JCO.2014.56.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Busch DH, Frassle SP, Sommermeyer D, et al. Role of memory T cell subsets for adoptive immunotherapy. Semin Immunol. 2016;28:28–34. doi: 10.1016/j.smim.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Couzin-Frankel J. Breakthrough of the year 2013. Cancer Immunother Sci. 2013;342:1432–1433. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- 9.Maus MV, Fraietta JA, Levine BL, et al. Adoptive immunotherapy for cancer or viruses. Annu Rev Immunol. 2014;32:189–225. doi: 10.1146/annurev-immunol-032713-120136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, Popplewell LL, Wagner JR, et al. Phase 1 studies of central memory-derived CD19 CAR T-cell therapy following autologous HSCT in patients with B-cell NHL. Blood. 2016;127:2980–2990. doi: 10.1182/blood-2015-12-686725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porter DL, Levine BL, Kalos M et al. (2011) Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 365: 725 – 33 [DOI] [PMC free article] [PubMed]
- 13.Johnson LA, Morgan RA, Dudley ME, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535–546. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robbins PF, Morgan RA, Feldman SA, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol. 2011;29:917–924. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rapoport AP, Stadtmauer EA, Binder-Scholl GK, et al. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat Med. 2015;21:914–921. doi: 10.1038/nm.3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgan RA, Yang JC, Kitano M, et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linette GP, Stadtmauer EA, Maus MV, et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood. 2013;122:863–871. doi: 10.1182/blood-2013-03-490565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.European Medicines Agency (2015) Reflection paper on classification of advanced therapy medicinal products. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/06/WC500187744.pdf. Accessed 21 May 2015
- 20.Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348:62–68. doi: 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartmann J, Schussler-Lenz M, Bondanza A, Buchholz CJ. Clinical development of CAR T cells-challenges and opportunities in translating innovative treatment concepts. EMBO Mol Med. 2017;9:1183–1197. doi: 10.15252/emmm.201607485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pearce KF, Hildebrandt M, Greinix H, et al. Regulation of advanced therapy medicinal products in Europe and the role of academia. Cytotherapy. 2014;16:289–297. doi: 10.1016/j.jcyt.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Gschweng E, De Oliveira S, Kohn DB. Hematopoietic stem cells for cancer immunotherapy. Immunol Rev. 2014;257:237–249. doi: 10.1111/imr.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robbins PF, Kassim SH, Tran TL, et al. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T cell receptor: long term follow up and correlates with response. Clin Cancer Res. 2014;21:1019–1027. doi: 10.1158/1078-0432.CCR-14-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glienke W, Esser R, Priesner C, et al. Advantages and applications of CAR-expressing natural killer cells. Front Pharmacol. 2015;6:21. doi: 10.3389/fphar.2015.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gattinoni L, Klebanoff CA, Palmer DC, et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8 + T cells. J Clin Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berger C, Jensen MC, Lansdorp PM, et al. Adoptive transfer of effector CD8 + T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008;118:294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gattinoni L, Restifo NP. Moving T memory stem cells to the clinic. Blood. 2013;121:567–568. doi: 10.1182/blood-2012-11-468660. [DOI] [PubMed] [Google Scholar]
- 29.Riemke P, Czeh M, Fischer J, et al. Myeloid leukemia with transdifferentiation plasticity developing from T-cell progenitors. EMBO J. 2016;35:2399–2416. doi: 10.15252/embj.201693927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Reilly M, Shipp A, Rosenthal E, et al. NIH oversight of human gene transfer research involving retroviral, lentiviral, and adeno-associated virus vectors and the role of the NIH recombinant DNA advisory committee. Methods Enzymol. 2012;507:313–335. doi: 10.1016/B978-0-12-386509-0.00016-8. [DOI] [PubMed] [Google Scholar]
- 31.Hacein-Bey-Abina S, Von Kalle C, Schmidt M, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 32.Braun CJ, Boztug K, Paruzynski A, et al. Gene therapy for Wiskott–Aldrich syndrome–long-term efficacy and genotoxicity. Sci Transl Med. 2014;6:227ra33. doi: 10.1126/scitranslmed.3007280. [DOI] [PubMed] [Google Scholar]
- 33.Heemskerk MH. T-cell receptor gene transfer for the treatment of leukemia and other tumors. Haematologica. 2010;95:15–19. doi: 10.3324/haematol.2009.016022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riet T, Holzinger A, Dorrie J, et al. Nonviral RNA transfection to transiently modify T cells with chimeric antigen receptors for adoptive therapy. Methods Mol Biol. 2013;969:187–201. doi: 10.1007/978-1-62703-260-5_12. [DOI] [PubMed] [Google Scholar]
- 35.Maiti SN, Huls H, Singh H, et al. Sleeping beauty system to redirect T-cell specificity for human applications. J Immunother. 2013;36:112–123. doi: 10.1097/CJI.0b013e3182811ce9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kebriaei P, Singh H, Huls MH, et al. Phase I trials using sleeping beauty to generate CD19-specific CAR T cells. J Clin Invest. 2016;126:3363–3376. doi: 10.1172/JCI86721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monjezi R, Miskey C, Gogishvili T, et al. Enhanced CAR T-cell engineering using non-viral Sleeping Beauty transposition from minicircle vectors. Leukemia. 2016;31:186–194. doi: 10.1038/leu.2016.180. [DOI] [PubMed] [Google Scholar]
- 38.Eyquem J, Mansilla-Soto J, Giavridis T, et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature. 2017;543:113–117. doi: 10.1038/nature21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy—assessment and management of toxicities. Nat Rev Clin Oncol. 2017;15:47–62. doi: 10.1038/nrclinonc.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahmed N, Brawley VS, Hegde M, et al. Human epidermal growth factor receptor 2 (HER2) -specific chimeric antigen receptor-modified T cells for the immunotherapy of HER2-positive sarcoma. J Clin Oncol. 2015;33:1688–1696. doi: 10.1200/JCO.2014.58.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6:224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5:177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suntharalingam G, Perry MR, Ward S, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 44.Bentley GA, Mariuzza RA. The structure of the T cell antigen receptor. Annu Rev Immunol. 1996;14:563–590. doi: 10.1146/annurev.immunol.14.1.563. [DOI] [PubMed] [Google Scholar]
- 45.Bendle GM, Linnemann C, Hooijkaas AI, et al. Lethal graft-versus-host disease in mouse models of T cell receptor gene therapy. Nat Med. 2010;16:565–570. doi: 10.1038/nm.2128. [DOI] [PubMed] [Google Scholar]
- 46.Aggen DH, Chervin AS, Schmitt TM, et al. Single-chain ValphaVbeta T-cell receptors function without mispairing with endogenous TCR chains. Gene Ther. 2012;19:365–374. doi: 10.1038/gt.2011.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cohen CJ, Li YF, El-Gamil M, Robbins PF, Rosenberg SA, Morgan RA. Enhanced antitumor activity of T cells engineered to express T-cell receptors with a second disulfide bond. Cancer Res. 2007;67:3898–3903. doi: 10.1158/0008-5472.CAN-06-3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Provasi E, Genovese P, Lombardo A, et al. Editing T cell specificity towards leukemia by zinc finger nucleases and lentiviral gene transfer. Nat Med. 2012;18:807–815. doi: 10.1038/nm.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sommermeyer D, Uckert W. Minimal amino acid exchange in human TCR constant regions fosters improved function of TCR gene-modified T cells. J Immunol. 2010;184:6223–6231. doi: 10.4049/jimmunol.0902055. [DOI] [PubMed] [Google Scholar]
- 50.Knies D, Klobuch S, Xue SA, et al. An optimized single chain TCR scaffold relying on the assembly with the native CD3-complex prevents residual mispairing with endogenous TCRs in human T-cells. Oncotarget. 2016;7:21199–21221. doi: 10.18632/oncotarget.8385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsui K, Boniface JJ, Steffner P, et al. Kinetics of T-cell receptor binding to peptide/I-Ek complexes: correlation of the dissociation rate with T-cell responsiveness. Proc Natl Acad Sci USA. 1994;91:12862–12866. doi: 10.1073/pnas.91.26.12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gascoigne NR, Rybakin V, et al. TCR signal strength and T cell development. Annu Rev Cell Dev Biol. 2016;32:327–348. doi: 10.1146/annurev-cellbio-111315-125324. [DOI] [PubMed] [Google Scholar]
- 53.Cherkassky L, Morello A, Villena-Vargas J, et al. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Invest. 2016;126:3130–3144. doi: 10.1172/JCI83092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guedan S, Chen X, Madar A, et al. ICOS-based chimeric antigen receptors program bipolar TH17/TH1 cells. Blood. 2014;124:1070–1080. doi: 10.1182/blood-2013-10-535245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou X, Di Stasi A, Brenner MK. iCaspase 9 suicide gene system. Methods Mol Biol. 2015;1317:87–105. doi: 10.1007/978-1-4939-2727-2_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singh H, Figliola MJ, Dawson MJ, et al. Reprogramming CD19-specific T cells with IL-21 signaling can improve adoptive immunotherapy of B-lineage malignancies. Cancer Res. 2011;71:3516–3527. doi: 10.1158/0008-5472.CAN-10-3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ciceri F, Bonini C, Marktel S, et al. Antitumor effects of HSV-TK-engineered donor lymphocytes after allogeneic stem-cell transplantation. Blood. 2007;109:4698–4707. doi: 10.1182/blood-2006-05-023416. [DOI] [PubMed] [Google Scholar]
- 58.Paszkiewicz PJ, Frassle SP, Srivastava S, et al. Targeted antibody-mediated depletion of murine CD19 CAR T cells permanently reverses B cell aplasia. J Clin Invest. 2016;126:4262–4272. doi: 10.1172/JCI84813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cartellieri M, Bachmann M, Feldmann A, et al. Chimeric antigen receptor-engineered T cells for immunotherapy of cancer. J Biomed Biotechnol. 2010;2010:956304. doi: 10.1155/2010/956304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cartellieri M, Feldmann A, Koristka S, et al. Switching CAR T cells on and off: a novel modular platform for retargeting of T cells to AML blasts. Blood Cancer J. 2016;6:e458. doi: 10.1038/bcj.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Albert S, Arndt C, Feldmann A, et al. A novel nanobody-based target module for retargeting of T lymphocytes to EGFR-expressing cancer cells via the modular UniCAR platform. Oncoimmunology. 2017;6:e1287246. doi: 10.1080/2162402X.2017.1287246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Feldmann A, Arndt C, Bergmann R, et al. Retargeting of T lymphocytes to PSCA- or PSMA positive prostate cancer cells using the novel modular chimeric antigen receptor platform technology “UniCAR”. Oncotarget. 2017;8:31368–31385. doi: 10.18632/oncotarget.15572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.European Medicines Agency (2008) Guideline on the non-clinical studies required before first clinical use of gene therapy medicinal products. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003942.pdf. Accessed 30 May 2008
- 64.Turtle CJ, Hanafi LA, Berger C, et al. CD19 CAR-T cells of defined CD4+:CD8 + composition in adult B cell ALL patients. J Clin Invest. 2016;126:2123–2138. doi: 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maude SL, Barrett D, Teachey DT, Grupp SA. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J. 2014;20:119–122. doi: 10.1097/PPO.0000000000000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brentjens R, Yeh R, Bernal Y, et al. Treatment of chronic lymphocytic leukemia with genetically targeted autologous T cells: case report of an unforeseen adverse event in a phase I clinical trial. Mol Ther. 2010;18:666–668. doi: 10.1038/mt.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kochenderfer JN, Yu Z, Frasheri D, et al. Adoptive transfer of syngeneic T cells transduced with a chimeric antigen receptor that recognizes murine CD19 can eradicate lymphoma and normal B cells. Blood. 2010;116:3875–3886. doi: 10.1182/blood-2010-01-265041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bassani-Sternberg M, Braunlein E, Klar R, et al. Direct identification of clinically relevant neoepitopes presented on native human melanoma tissue by mass spectrometry. Nat Commun. 2016;7:13404. doi: 10.1038/ncomms13404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stronen E, Toebes M, Kelderman S, et al. Targeting of cancer neoantigens with donor-derived T cell receptor repertoires. Science. 2016;352:1337–1341. doi: 10.1126/science.aaf2288. [DOI] [PubMed] [Google Scholar]
- 70.Verdegaal EM, de Miranda NF, Visser M, et al. Neoantigen landscape dynamics during human melanoma-T cell interactions. Nature. 2016;536:91–95. doi: 10.1038/nature18945. [DOI] [PubMed] [Google Scholar]
- 71.Tran E, Robbins PF, Lu YC, et al. T-cell transfer therapy targeting mutant KRAS in cancer. N Engl J Med. 2016;375:2255–2262. doi: 10.1056/NEJMoa1609279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schubert ML, Hückelhoven A, Hoffmann JM, et al. Chimeric antigen receptor T cell therapy targeting CD19-positive leukemia and lymphoma in the context of stem cell transplantation. Hum Gene Ther. 2016;27:758–771. doi: 10.1089/hum.2016.097. [DOI] [PubMed] [Google Scholar]
- 73.Schonfeld K, Sahm C, Zhang C, et al. Selective inhibition of tumor growth by clonal NK cells expressing an ErbB2/HER2-specific chimeric antigen receptor. Mol Ther. 2015;23:330–338. doi: 10.1038/mt.2014.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang C, Burger MC, Jennewein L, et al. ErbB2/HER2-Specific NK Cells for Targeted Therapy of Glioblastoma. J Natl Cancer Inst. 2016;108:djv375. doi: 10.1093/jnci/djv375. [DOI] [PubMed] [Google Scholar]
- 75.Kalos M, June CH. Adoptive T cell transfer for cancer immunotherapy in the era of synthetic biology. Immunity. 2013;39:49–60. doi: 10.1016/j.immuni.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.European Medicines Agency (2016) Guideline on strategies to identify and mitigate risks for first-in-human and early clinical trials with investigational medicinal products. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2016/11/WC500216158.pdf. Accessed 10 November 2016 [DOI] [PMC free article] [PubMed]
- 77.Davila ML, Brentjens R. Chimeric antigen receptor therapy for chronic lymphocytic leukemia: what are the challenges? Hematol Oncol Clin North Am. 2013;27:341–353. doi: 10.1016/j.hoc.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bonifant CL, Jackson HJ, Brentjens RJ, Curran KJ. Toxicity and management in CAR T-cell therapy. Mol Ther Oncolytics. 2016;3:16011. doi: 10.1038/mto.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]