Abstract
Fusobacterium nucleatum (Fn) has been shown to promote colorectal cancer (CRC) development by inhibiting host anti-tumour immunity. However, the impact of Fn infection on macrophage polarization and subsequent intestinal tumour formation as well as the underlying molecular pathways has not been investigated. We investigated the impact of Fn infection on macrophage polarization in human CRCs and cultured macrophages as well as the effects on macrophage phenotype and intestinal tumour formation in ApcMin/+ mice. We also examined whether macrophage-polarized activation challenged by Fn infection via a TLR4-dependent mechanism involved the IL-6/STAT3/c-MYC signalling cascade. Our data showed that macrophages are a major tumour-infiltrating immune cell type in human CRCs with Fn infection (P < 0.001). Fn infection increased M2 polarization of macrophages in vitro and in vivo, leading to intestinal tumour growth in ApcMin/+ mice. Moreover, Fn infection induced high expression of TLR4, IL-6, STAT3, p-STAT3, and c-MYC in cultured macrophages challenged with Fn, which was blocked by TAK-242 pre-treatment (P < 0.05). Interestingly, c-MYC protein was mainly co-localized with CD206+ M2 macrophages with Fn infection. In conclusion, we show that Fn infection increased M2 polarization of macrophages in vitro and in vivo. Furthermore, Fn infection enhanced colorectal tumour growth in a TLR4-dependent manner involving activation of the IL-6/p-STAT3/c-MYC signalling pathway. For the first time, our results indicate an immunosuppressive effect of Fn by promoting M2 polarization of macrophages through a TLR4-dependent mechanism, which may serve as a promising target for immunotherapy of Fn-related CRC.
Electronic supplementary material
The online version of this article (10.1007/s00262-018-2233-x) contains supplementary material, which is available to authorized users.
Keywords: Fusobacterium nucleatum, Macrophages, Colorectal cancer, Immune, Toll-like receptor 4
Introduction
Macrophages are immune cells that participate in host defence, tissue repair, angiogenesis, and inflammation [1, 2]. Tumour associated macrophages (TAM) play an important role in the development and progression of colorectal cancer (CRC) [3, 4]. Macrophage-polarized activation has significant effects on immune responses in CRC [3]. Macrophages are characterized by their heterogeneity, which can be shaped by the microenvironment [5]. In response to diverse signals within the tumour environment, macrophages can differentiate into distinct functional phenotypes that range from a pro-inflammatory M1-phenotype to a tumour-promoting M2-phenotype [6, 7]. Moreover, the molecular mechanisms that determine the activation state of TAMs within CRC are still not completely understood. Understanding the mechanisms that direct macrophage differentiation in response to diverse environmental signals will provide a basis for novel cancer therapeutic strategies.
In recent years, increasing evidence has indicated that Fusobacterium nucleatum (Fn) may promote CRC development by inhibiting the anti-tumour adaptive immunity [8–10]. However, the presence and activation state of TAMs within CRCs with Fn infection remains to be elucidated. To target the immunity of Fn-related CRC prevention and therapy, a better understanding of the molecular mechanisms that determine the activation state of TAMs within the tumour microenvironment of Fn-related CRC is clearly warranted. However, information on the underlying molecular pathways in macrophage-polarized activation in Fn-related CRC remains largely unknown.
In our previous study, it has been shown that Fn infection can increase c-MYC expression in colon cancer cells via a TLR4-dependent pathway [11]. IL-6 trans-signalling via STAT3 is a key modulator of TLR4-driven inflammatory responses [12]. The activation of the IL-6/STAT3 signalling pathway has been implicated in the pathogenesis of CRC [13]. IL-6-mediated STAT3 activation blocks the functional maturation of dendritic cells, leading to a suppression of anti-tumour immunity in cancers [14]. Furthermore, tumour-induced IL-6 can suppress intratumoural immunity by impairing the ketogenic response to reduced caloric intake [15]. Moreover, IL-6 can enhance c-MYC protein expression and translocation in multiple myeloma [16]. Recent studies have suggested that c-MYC is a key player in alternative macrophage activation [17, 18] and IL-6 can promote M2 polarization of adipose tissue macrophages via upregulation of the IL-4 receptor α [19]. Therefore, it would be interesting to investigate whether Fn infections can determine macrophage phenotypes in a TLR4-dependent manner involving the IL-6/STAT3/c-MYC cascade.
In the current study, we investigated the impact of Fn infection on TAM polarization in human CRCs and cultured macrophages along with the phenotype of TAMs and consequences on intestinal tumour formation in Apcmin/+ mice. We also examined whether macrophage-polarized activation challenged by Fn infection is TLR4-dependent via the IL-6/STAT3/c-MYC signalling pathway.
Methods
Sample collection
Formalin-fixed, paraffin-embedded CRC tissues (n = 16) were obtained from the pathology department archives of the Affiliated Hospital of Southwest Medical University (Sichuan, China) between February 2016 and December 2016.
Animal experiments
Fifteen male C57BL/6-ApcMin/+ mice were randomly and equally divided into three groups: Fn, Fn + TAK-242, and control. Mice in the control group were given fastidious anaerobe broth (FAB), and mice in the other two groups were fed Fn at 109 colony forming units suspended with 0.25 ml FAB per day. The period of bacterial feeding experiments was performed for 8 weeks to ensure tumour formation. Tumours in the colon and small intestine were counted and tumour volume was calculated as described previously [20]. Colon and small intestine tissues were resected and fixed in 4% paraformaldehyde and embedded in paraffin for histological analysis.
Cell culture and bacterial infection
The mouse macrophage cell line RAW 264.7 was graciously supplied by Dr. Jing Ren of Medical Laboratory Centre of the Southwest Medical University. RAW 264.7 macrophages were cultured in high glucose DMEM supplemented with 10% fetal bovine serum (FBS) (Pan Biotech, Germany), 100 U/mL penicillin, and 0.1 mg/mL streptomycin (Beyotime, china) in a 5% CO2, 95% air incubator at 37 °C. RAW 264.7 cells were seeded in 6-well plates and incubated for 6 h (until 60% confluency) at 37 °C. The cells were then infected with bacteria (MOI 1:100) for 2 and 6 h. The plates were shaken gently and incubated at 37 °C in an incubator with controlled 5% CO2.
TAK-242 treatment of mice and macrophages
TAK-242 (10 mg) was dissolved in 1 ml DMSO and further diluted in 24 ml sterile endotoxin-free water. The final concentration of TAK-242 and DMSO was 0.4 mg/ml and 4%, respectively. TAK-242 was injected intraperitoneally into mice at a dose of 4 mg/kg with an injection volume of 0.1 ml/10 g body weight before bacterial feeding every 2 days for 8 weeks [21]. For intervention of cultured macrophages, a stock solution of TAK-242 was prepared in DMSO and further diluted with DMEM to yield a final concentration of 1 µM. TLR4 was inhibited with 1 µM TAK-242 for 1 h prior to Fn stimulation.
Fn and Escherichia coli (E. coli) culture
Fn (F01) strains were isolated and confirmed as previously described [11]. Fn (ATCC10953) was kindly supplied by Dr. Junqiang Jiang of the Affiliated Dental Hospital of Southwest Medical University and non-pathogenic E. coli (ATCC25922) was kindly supplied by Dr. Yinshun Zhou of the Microbiology Department of Southwest Medical University. Fn was incubated for 4–5 days in FAB supplemented with vancomycin (4 µg/mL) and neomycin sulphate (30 µg/mL) under anaerobic conditions at 37 °C. E. coli was cultured for 24 h in Luria–Bertani broth at 37 °C using an orbital shaker incubator.
Microbial fluorescence in situ hybridization (FISH) analysis
Sections (5 µm-thick) were prepared and hybridized as previously described [22]. Five random 200× magnification fields per sample were evaluated by an observer blind to sample status and the number of bacteria per field was calculated. We defined negative, weak, and positive Fn as those cases with < 5, between 5 and 20, and > 20 visualized FUS664 probes per field on average, respectively.
IHC
Indirect immunohistochemistry was performed with formalin-fixed, paraffin-embedded tissue sections as previously described [23]. The primary antibodies used in the study were as follows: IL-6 (1:100; Bioworld), STAT3 (1:100; Cell Signaling Technology).
Western blot analysis
Total cellular protein was isolated from cultured cells with a protein extraction solution (Beyotime, China). Proteins were subjected to 10% SDS–PAGE and transferred to polyvinylidene fluoride membranes. Following this, membranes were blocked with 5% skimmed milk in PBST for 2 h at room temperature and incubated overnight at 4 °C with the following primary antibodies: TLR4 (1:500; Santa Cruz), IL-6 (1:1000; Bioworld), STAT3 (1:1000; Cell Signaling Technology), phosphorylated-STAT3 (1:1000; Cell Signaling Technology), c-MYC (1:100; Bioworld), and GAPDH (1:10,000; Bioworld, USA). Membranes were then incubated with the appropriate secondary antibodies for 1 h at room temperature. Blots were quantified by densitometry using Quantity One 4.5.0 software (Bio-Rad Laboratories, CA).
Immunofluorescence
Immunofluorescence staining was performed as previously described [24]. Tissue immunofluorescence studies were performed on paraffin-embedded 5 µm sections. The immunofluorescence staining of RAW 264.7 macrophages was performed on glass coverslips and sections were incubated with the following primary antibodies: CD3-ε (1:100; Santa Cruz), CD4 (1:100; Santa Cruz), CD8-α (1:100; Santa Cruz), CD83 (1:100; Santa Cruz), CD68 (1:100; Santa Cruz), Neutrophil Elastase (NE) (1:100; Beijing Biosynthesis), CD86 (1:100; Santa Cruz), CD206 (1:100; Santa Cruz), and c-MYC (1:100; Bioworld) for 16 h at 4 °C. Slides were then incubated with appropriate secondary antibodies for 1 h at 37 °C in the dark; DAPI (Beyotime, China) was used as a nuclear counterstain. Immunofluorescence staining was analysed using a microscope (BX53F; Olympus, Tokyo, Japan).
Quantification of immune cells and M1/M2-polarized macrophages
Immunofluorescence for eight immune cell markers (CD3, CD4, CD8, CD83, NE, CD68, CD86, and CD206) was counted manually from captured images. As described previously, the cell density of M1 and M2 macrophages was determined by CD86+ or CD206+ staining, respectively [25]. Additionally, CD68 antibody was used as a pan-macrophage marker. Five random high-power fields of vision at 400× magnification were counted. Immune cell density was calculated and presented as the average number of positive cells per square millimetre [24, 26].
Quantitative real-time PCR (qPCR)
The extraction of total RNA from treated RAW 264.7 macrophages was accomplished using a RNA extract kit (TIANGEN, China) according to the manufacturer’s instructions. Reverse transcription was done using a Reverse Transcription kit (TOYOBO, Japan) to obtain cDNA. The qPCR process was carried out using a QuantiNova™ SYBR Green PCR Kit (Qiagen, Germany) on an Applied Biosystems StepOnePlus Real-Time PCR system [27]. Primer sequences used in this study are presented in Supplementary Table 1. All experiments were performed in triplicate.
Statistical analysis
Data are presented as the mean and standard deviation for continuous variables and as proportions for categorical variables. Data were analysed using one-way ANOVA followed by Bonferroni test for multiple comparisons. Differences were considered significant if P < 0.05. All significance tests were two-tailed. All statistical tests were performed using SPSS software Version 13.0 (SPSS Inc., Chicago, IL, USA).
Results
Macrophages were the predominant infiltrating immune cell population in human CRC with Fn infection
Since tumour infiltrating immune cells play a role in anti-tumour immune responses to CRC, we analysed the densities of CD3+, CD4+, and CD8+ T cells, dendritic cells (CD83+), neutrophils (NE), and macrophages (CD68+) by immunofluorescence in CRC tissues with and without Fn infection. The densities of CD3+, CD4+, and CD8+ T cells in Fn-negative CRCs were not significantly different from those in Fn-positive CRCs (Fig. 1a). We observed significantly higher number of dendritic cells (P = 0.031), neutrophils (P = 0.048), and macrophages (P < 0.001) in Fn-positive CRCs compared to Fn-negative CRCs. It is worth noting that CD68+ macrophages were the most predominantly increased immune cell populations in Fn-positive CRCs. These findings suggest that macrophages in the CRC microenvironment play an important role in the immune response to Fn infection.
Fig. 1.
Infiltrating immune cell populations detected by immunofluorescence in human colorectal cancer. a The average number of CD3+, CD4+, and CD8+ T cells was not significantly different between Fn-negative (n = 8) and Fn-positive CRCs (n = 8). The densities of CD83+ cells, neutrophils, and CD68+ cells were significantly increased in Fn-positive CRCs compared to Fn-negative CRCs. Fn was detected by FISH. b CD206+ M2-phenotype macrophages were significantly higher than CD86+ M1 macrophages in Fn-positive CRCs, while no different in Fn-negative CRCs. c Representative images of CD206+ and CD86+ macrophages in Fn-positive CRCs compared to Fn-negative CRCs. NE neutrophil elastase. *, P < 0.05; **, P < 0.001
Increased M2-phenotype macrophage populations in the human CRC microenvironment with Fn infection
Since the phenotype of macrophages determines the immune response to bacterium infection and cancers, CD86+ M1-phenotype macrophages and CD206+ M2-phenotype macrophages were examined by immunofluorescence in human CRC tissues with and without Fn infection. In Fn-negative CRCs, the densities of M1 and M2 macrophages were not significantly different (P > 0.05). In Fn-positive CRCs, the densities of M2 macrophages were significantly higher than those of M1 macrophages (P = 0.001). Moreover, the densities of M2 macrophages in Fn-positive CRCs were significantly higher than those in Fn-negative CRCs (P < 0.001) (Fig. 1b, c). These results suggest that macrophage polarization into the tumour promoting M2-phenotype in CRCs is enhanced in the presence of Fn.
Fn infection favoured M2 polarization in cultured macrophages
To determine the effect of Fn infection on macrophage phenotypes in vitro, M1 and M2 markers were measured by immunofluorescence on a murine macrophage cell line (RAW 264.7). CD206+ M2 macrophages were significantly increased in Fn (ATCC10953) and Fn (F01) challenged macrophages compared to E. coli challenged macrophages at both 2 and 6 h (all P values < 0.05). CD86+ M1 macrophages were significantly decreased in Fn (ATCC10953) and Fn (F01) challenged macrophages compared to E. coli challenged macrophages at both 2 and 6 h (all P values < 0.05) (Fig. 2).
Fig. 2.
Fn infection favours M2 polarization in cultured macrophages. a CD86+ M1 macrophages and its markers (IL-12 and TNF-α) were significantly decreased in Fn (ATCC10953) and Fn (F01) challenged macrophages compared to E. coli challenged macrophages at 6 h. The mRNA levels of cytokines were determined by quantitative real-time PCR. b CD206+ M2 macrophages were significantly increased in Fn (ATCC10953) and Fn (F01) challenged macrophages compared to E. coli challenged macrophages. IL-10 and TGF-β were expressed at a significantly higher level in Fn (F01) challenged macrophages than in Fn (ATCC10953) and E. coli challenged macrophages. TAK-242 pre-treatment before Fn (F01) challenge significantly decreased CD206+ M2 macrophages compared to Fn (F01) treatment. c Representative immunofluorescence images of CD206+ and CD86+ macrophages after E. coli, Fn (ATCC10953), Fn (F01) and TAK-242 pre-treatment. *P < 0.05 compared to E. coli group; **P < 0.001 compared to E. coli group; #P < 0.05 compared to Fn (F01) group; ##P < 0.001 compared to Fn (F01) group; ‡P < 0.05 compared to Fn (ATCC10953) group
Moreover, the mRNA levels of M1 markers (IL-12 and TNF-α) and M2 markers (IL-10 and TGF-β) were determined by qPCR. IL-12 and TNF-α were expressed at a significantly lower level in Fn (ATCC10953) and Fn (F01) challenged macrophages compared to E. coli challenged macrophages at 6 h (all P values < 0.05) (Fig. 2). Importantly, IL-10 was expressed at a significantly higher level in Fn (F01) challenged macrophages than in Fn (ATCC10953) as well as E. coli challenged macrophages at 2 and 6 h (all P values < 0.05). Moreover, TGF-β was expressed at a much higher level in Fn (F01) challenged macrophages compared to Fn (ATCC10953) and E. coli challenged macrophages at 6 h (P = 0.003, P = 0.005, respectively). These results demonstrate that Fn (F01) infection promotes the accumulation as well as differentiation of M2 macrophages.
The host can recognize microbial structures and components via pattern-recognition receptors such as TLR4, which is expressed by a variety of immune cells. Therefore, we asked whether macrophage polarization is affected by blocking TLR4 signalling. M1 and M2 markers were measured on Fn (F01) challenged macrophages following pre-treatment with the TLR4 inhibitor TAK-242. Notably, CD206+ M2 macrophages were significantly decreased in macrophages pre-treated with TAK-242 compared to Fn (F01) challenged macrophages (P < 0.001). Moreover, mRNA levels of M2 markers (IL-10 and TGF-β) were dramatically reduced in the TAK-242 pre-treatment group compared to the Fn (F01) group (P < 0.05) (Fig. 2). These in vitro results suggest that TLR4 signalling is required for macrophage M2 activation in response to Fn challenge.
Fn infection increased M2 polarization and intestinal tumour growth in ApcMin/+ mice and was TLR4-dependent
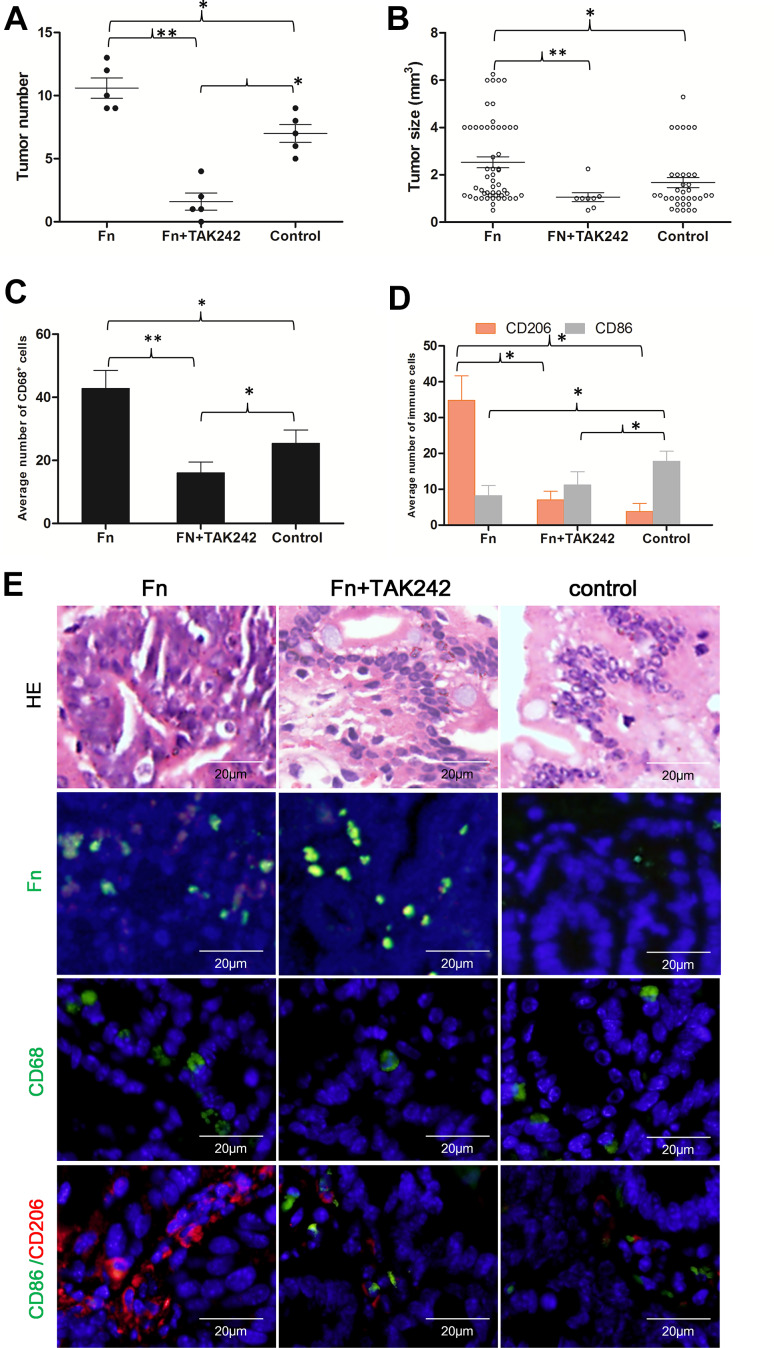
To determine whether Fn could promote the formation of intestinal tumours, we fed C57BL/6-ApcMin/+ mice with Fn (F01). All mice were killed after 8 weeks of treatment. Fn-fed mice had higher number of tumours (P = 0.029) as well as larger average intestinal tumour volume (P = 0.027) compared to the control group (Fig. 3A-B), suggesting that Fn potentiates intestinal tumourigenesis in ApcMin/+ mice.
Fig. 3.
Fn infection increased M2 polarization of macrophages and intestinal tumour growth in ApcMin/+ mice. a, b Fn (F01) fed mice had higher number and larger volume of intestinal tumours compared to the control group. TAK-242 pre-treatment significantly decreased intestinal tumour number and size compared to the Fn-treated group. c Fn infection significantly increased the densities of macrophages (CD68+) compared to the control group. TAK-242 pre-treatment significantly decreased the densities of macrophages. d CD206+ M2 macrophages were significantly higher in the intestinal tumours of Fn-fed mice than in the control group. CD86+ M1 macrophages were significantly lower in Fn-fed mice than in the control group. TAK-242 pre-treatment significantly reduced M2 polarization of macrophages compared to the Fn treatment group. e Representative immunofluorescence images of CD206+ and CD86+ macrophages in the intestinal tissue microenvironment of ApcMin/+ mice in the Fn (F01) treatment, TAK-242 pre-treatment, and control group. *P < 0.05, **P < 0.001
To further test the hypothesis that Fn infection polarizes macrophages towards the tumour promoting M2-phenotype in vivo, M1 and M2 markers were measured by immunofluorescence on macrophages in the Apcmin/+ mice intestinal tumour microenvironment. The density of CD68+ macrophages in the intestinal tumour microenvironment was significantly higher in Fn-fed mice compared to the control group (P = 0.002). CD206+ M2 macrophages were significantly higher in the intestinal tumour microenvironment of Fn-fed mice compared to the control group (P < 0.05). Moreover, CD86+ M1 macrophages were significantly lower in Fn-fed mice compared to the control group (P < 0.05) (Fig. 3c–e). These findings offer direct support that Fn infection favours the M2 polarization of macrophages and contributes to the tumourigenesis of intestinal tumours.
To confirm that TLR4 signalling polarizes macrophages towards the M2-phenotype in vivo, ApcMin/+ mice were pre-treated with TAK-242. Compared to the Fn-treated group, TAK-242 pre-treatment significantly reduced the M2 polarization of macrophages within the tumour microenvironment (P < 0.05). Moreover, the average number of intestinal tumours in the TAK-242 pre-treated group was significantly lower compared to the Fn-treated group (P < 0.001) and the control group (P = 0.002). The average size of intestinal tumours in the TAK-242 pre-treated group was significantly smaller compared to the Fn-treated group (P < 0.001) (Fig. 3). Collectively, these results suggest that TLR4 signalling is required for M2 activation of TAMs in response to Fn challenge in vivo.
Macrophage phenotype was associated with increased IL-6/STAT3/c-MYC signalling in human CRCs with Fn infection
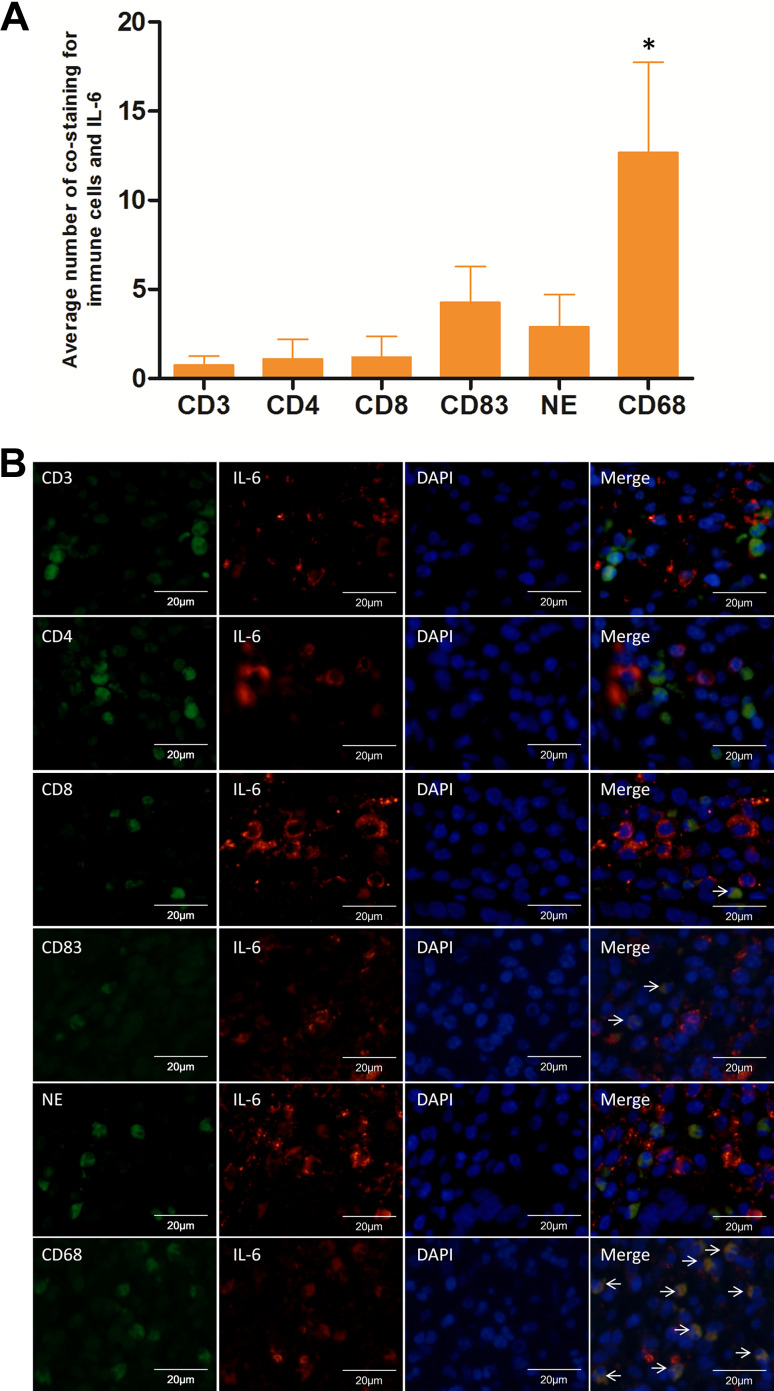
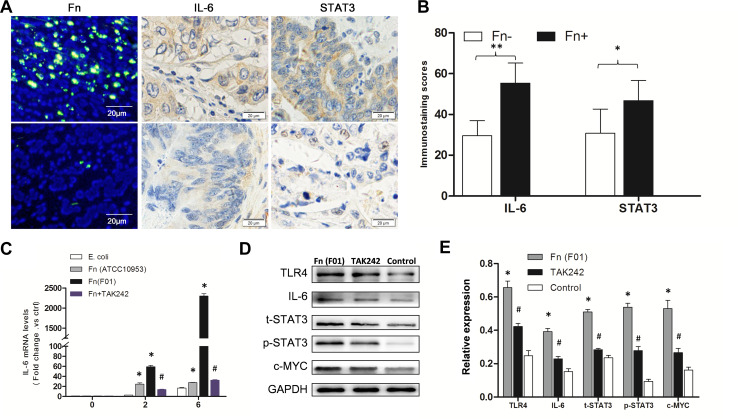
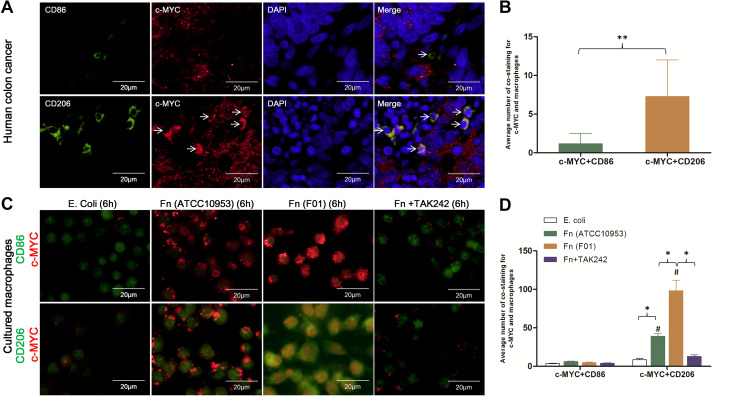
Using immunofluorescence co-staining for immune cells (CD3+, CD4+, CD8+, NE, CD83+, and CD68+ cells) and IL-6 in 8 human CRC tissues with Fn infection, IL-6 was found mainly co-localized with CD68+ macrophages (P < 0.05) (Fig. 4). These results indicate that increased IL-6 expression in human CRCs with Fn infection was mainly associated with CD68+ macrophages. Furthermore, IL-6 and STAT3 protein abundance was compared in 8 Fn-positive and 8 Fn-negative CRCs by IHC. The abundance of IL-6 and STAT3 protein was significantly higher in Fn-positive CRCs compared to Fn-negative CRCs (P < 0.001, P = 0.004, respectively) (Fig. 5A-B). To investigate whether macrophage polarization is associated with c-MYC expression, immunofluorescence co-staining for macrophages (CD86+ and CD206+ cells) and c-MYC protein was performed in human CRCs with Fn infection. In human CRCs with Fn infection, the frequency of co-staining for CD206+ macrophages and c-MYC protein was significantly higher than that for CD86+ macrophages and c-MYC protein (P < 0.001) (Fig. 6a, b). These findings suggest that M2 polarization of macrophages in human CRCs with Fn infection may be associated with the activation of the IL-6/STAT3/c-MYC signalling pathway.
Fig. 4.
Immunofluorescence co-staining for immune cells and IL-6 in human CRC with Fn infection. a The frequency of immunofluorescence co-staining for macrophages (CD68+) and IL-6 was significantly higher than that for other immune cells (CD3+, CD4+, CD8+, NE+, and CD83+) and IL-6 in Fn-positive CRCs (n = 8). b Representative images of immunofluorescence co-staining for immune cells (CD3+, CD4+, CD8+, NE, CD83+, and CD68+ cells) and IL-6. NE neutrophil elastase. *P < 0.05
Fig. 5.
Fn infection induced the activation of the IL-6/STAT3/c-MYC signalling in a TLR4-dependent manner in vivo and in vitro. a Examples of high expression of IL-6 and STAT3 in Fn-positive CRC compared to low expression of IL-6 and STAT3 in Fn-negative CRC using immunocytochemistry. b Expression of IL-6 and STAT3 was significantly higher in Fn-positive CRCs (n = 8) than that in Fn-negative CRCs (n = 8). *P < 0.05; **P < 0.001. c Using quantitative real-time PCR, IL-6 mRNA was expressed at a significantly higher level in Fn (F01) challenged macrophages compared to Fn (ATCC10953) and E. coli challenged macrophages at 2 and 6 h (*P < 0.05). Pre-treatment with TAK-242 led to a significant decrease in IL-6 mRNA compared to the Fn (F01) treatment group (#P < 0.05). d, e Western blots showing significantly increased levels of TLR4, IL-6, t-STAT3, p-STAT3, and c-MYC protein in cultured macrophages after Fn (F01) infection compared to control group, while TLR4, IL-6, t-STAT3, p-STAT3, and c-MYC protein levels were significantly decreased in the TAK-242 pre-treatment group compared to the Fn (F01) treatment group. Bar diagrams represent the results obtained after densitometric scanning from three different experiments. Bars represent the mean ± SD. *P < 0.05 compared to control group; #P < 0.05 compared to Fn (F01) group
Fig. 6.
High frequency of co-staining for CD206+ macrophages and c-MYC protein after exposure to Fn infection. a Examples of immunofluorescence co-staining for c-MYC and M1-polarized cells (CD86+) or M2 cells (CD206+) in human colon cancer with Fn infection. b The frequency of co-staining for CD206+ macrophages and c-MYC protein was significantly higher than that for CD86+ macrophages and c-MYC. c Examples of immunofluorescence co-staining for c-MYC and M1-polarized cells (CD86+) or M2-polarized cells (CD206+) in cultured macrophages after exposure to E. coli, Fn (ATCC10953), Fn (F01), and TAK-242 pre-treatment with Fn (F01). d The frequency of co-staining for CD206+ macrophages and c-MYC in Fn (ATCC10953) and Fn (F01) challenged cells was significantly higher than that for CD86+ macrophages and c-MYC (#P < 0.05). The frequency of co-staining for CD206+ macrophages and c-MYC in Fn (F01) challenged cells was significantly higher than that in Fn (ATCC10953) and E. coli challenged cells. TAK-242 pre-treatment significantly decreased the frequency of co-staining for CD206+ macrophages and c-MYC compared to Fn (F01) challenged cells. *P < 0.05
Fn promoted M2 polarization of macrophages via a TLR4-dependent mechanism involving the IL-6/p-STAT3/c-MYC cascade
To delineate the cellular mechanisms by which Fn infection increased M2 macrophage polarization within the CRC microenvironment, the expression of TLR4, IL-6, STAT3, p-STAT3, and c-MYC was further assessed by qPCR or Western blot in cultured macrophages challenged with Fn (F01). The mRNA of IL-6 was expressed at a significantly higher level in Fn (F01) challenged macrophages compared to Fn (ATCC10953) and E. coli challenged macrophages at 2 and 6 h (P < 0.05). The expression of TLR4, IL-6, STAT3, p-STAT3, and c-MYC protein was significantly increased in cultured macrophages after Fn (F01) infection compared to the control group (all P values < 0.05) (Fig. 5c–e).
The frequency of co-staining of CD206+ macrophages and c-MYC in Fn (F01) challenged cells was significantly higher than that for CD86+ macrophages and c-MYC (P < 0.05). Furthermore, it was significantly higher than that for CD206+ macrophages and c-MYC in Fn (ATCC10953) and E. coli challenged cells (P < 0.05, P < 0.05, respectively). Moreover, TAK-242 pre-treatment before Fn (F01) challenge significantly decreased the frequency of co-staining of CD206+ macrophages and c-MYC compared to Fn (F01) challenged cells (P < 0.05) (Fig. 6c, d). These findings indicate that c-MYC is a specific signature of M2-phenotype macrophage activation.
Furthermore, we examined whether the inhibition of TLR4 affects IL-6/p-STAT3/c-MYC expression in cultured macrophages. Pre-treatment with TAK-242 prior to challenge with Fn (F01) led to a significant decrease of IL-6 mRNA abundance compared to the Fn (F01) treatment group (P < 0.05) (Fig. 5c). Western blot analysis found that the expression of TLR4, IL-6, STAT3, p-STAT3, and c-MYC was significantly decreased in the TAK-242 pre-treatment group compared to the Fn (F01) treatment group (P < 0.05) (Fig. 5d, e).
Discussion
The type, density, and location of immune cells within human CRC can influence the behaviour of tumours, predict clinical outcome, and potentially identify appropriate immunotherapies [28, 29]. We have made the discovery that macrophages are a major tumour-infiltrating immune cell type in a small sample of CRCs with Fn infection. This finding is consistent with a previous study, which revealed that Fn abundance was significantly associated with macrophage infiltration [30]. Macrophages in the microenvironment of cancers can differentiate into either an M1-phenotype, which impedes tumour progression or an M2-phenotype, which can enhance tumour cell proliferation, migration, angiogenesis, and metastasis [6, 7]. M2 macrophages are generally associated with tumour progression in various cancers [31]. Another important finding in this study was that M2 macrophages were the main population compared to M1 population in the microenvironment of human CRCs with Fn infection. Moreover, as demonstrated by the immunofluorescence data and qPCR results, Fn infection increased M2 polarization of macrophages in vitro and in vivo in intestinal tumours in ApcMin/+ mice. Our results suggested that TAMs in the microenvironment of Fn-positive CRCs mostly possess an M2-phenotype. These findings can explain the impaired immune response and poor clinical prognosis of CRC patients with Fn infection.
In the ApcMin/+ mouse model, we found that Fn infection increased M2 polarization of macrophages within the tumour microenvironment and intestinal tumour growth. Inhibition of TLR4 by TAK-242 significantly decreased M2-polarized macrophages and the formation of intestinal tumours. LPS-TLR4 signalling is classically involved in M1 macrophages’ polarization [32]. However, our results suggest that TLR4 signalling is required for tumour-associated M2 macrophage activation in response to Fn challenge in vivo. This is similar to a recent report in which M2 macrophage polarization was induced in a TLR4-dependent manner [33]. These findings indicate a novel mechanism by which Fn in the CRC microenvironment may contribute to tumour progression by promoting M2 macrophage polarization. However, the mechanism behind this altered response to Fn infection by macrophages is yet to be fully understood.
Previous studies have reported that stimulation of TLR4 through the MyD88-dependent signalling pathway leads to the secretion of IL-6 [34, 35]. Our data demonstrated that inhibition of TLR4 reduced IL-6 expression in response to Fn infection. Our findings are consistent with previous studies, which reported that TAK-242 inhibited TLR4-induced secretion of IL-6 in a dose-dependent manner [34, 36]. IL-6 has been reported to promote the M2 polarization of macrophages [19, 37]. These findings further support a role for TLR4 in promoting IL-6 gene transcription to promote M2 polarization of macrophages.
IL-6/STAT3 signalling has been shown to play an important role in the pathogenesis of CRC [13, 38, 39]. We found high abundance of IL-6 and STAT3 in CRCs with Fn infection as well as high expression of IL-6, STAT3, p-STAT3, and c-MYC in cultured macrophages challenged with Fn. A recent study reported that cancer-associated fibroblasts can promote endometrial cancer growth via activation of the IL-6/STAT3/c-MYC pathway [40]. In the present study, TAK-242 pre-treatment significantly decreased the expression of IL-6, STAT3, p-STAT3, and c-MYC in cultured macrophages. Moreover, TAK-242 pre-treatment significantly decreased the frequency of co-staining for c-MYC and CD206+ macrophages challenged with Fn. These preliminary findings suggest that Fn infection may activate the IL-6/p-STAT3/c-MYC signalling pathway in macrophages in a TLR4-dependent manner.
The biological functions of c-MYC have been investigated mostly in cancer cells and its effects on immune cells have not been well characterized. Interestingly, our study found that c-MYC was mainly co-localized with CD206+ M2 macrophages in human CRCs with Fn infection as well as in cultured macrophages challenged by Fn. Our results were consistent with previous studies which suggested that c-MYC is a key player in macrophage M2 activation [18, 41]. Taken together, these findings demonstrate that the IL-6/p-STAT3/c-MYC signalling pathway in macrophages is key in the control of macrophage differentiation and suggests that interfering with this pathway should be taken into account when targeting TAM activation.
As a heterogeneous group of organisms, Fn is a common inhabitant of both the oral cavity and the human gut [42]. Some oral bacteria were detected abundant in the stool of CRC patients, indicating an oral-gut translocation route [43]. In our study, both a gut-derived Fn (F01) strain from a CRC patient and an Fn (ATCC10953) strain originating from the oral cavity induced M2 polarization of macrophages. It is worth to noting that compared to Fn (ATCC10953), Fn (F01) strain induced significantly higher levels of M2 macrophages, suggesting some oral Fn may have evolved towards higher levels of virulence, and subsequently play a role in the CRC tumourigenesis. Further studies are needed to investigate the mechanism for the evolved virulence of CRC-related Fn.
In conclusion, our results showed that Fn infection increased M2 macrophage polarization in vitro and in vivo, enhanced colorectal tumour growth, which is likely TLR4-dependent involving the IL-6/p-STAT3/c-MYC signalling pathway. Our study has provided additional targets for CRC therapeutic strategies, which could be used in combination with other targeted approaches. The use of TLR4 antagonists can potentially be another therapeutic approach to suppress tumour-promoting M2 macrophage polarization. We propose for the first time, a novel mechanism of action, which may explain the immunosuppressive effect of Fn by promoting M2 polarization of macrophages via a TLR4-dependent mechanism. This may serve as a promising target for immunotherapy of Fn-related CRC.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- CRC
Colorectal cancer
- E. coli
Escherichia coli
- FAB
Fastidious anaerobe broth
- FISH
Fluorescence in situ hybridization
- Fn
Fusobacterium nucleatum
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- NE
Neutrophil elastase
- TAM
Tumour associated macrophages
Author contributions
TC, QL, JW, YW, WP, and HL conducted the experiments. JW and XT analyzed the data. XF wrote the paper. YP and XF conceived and designed the study.
Funding
This work was supported by the Youth Program of National Science Fund under Grant no. 81602163.
Compliance with ethical standards
Conflict of interest
All authors declare no financial disclosures and no conflict of interest.
Ethical approval
The Ethical Committee on Animal Care and Animal Experiment of the Southwest Medical University gave approval to the use of mice for the animal research described in this paper (approval number: No. 201702004). The animals were maintained in accordance with the guidelines approved by this committee.
Animal source
All mice were purchased from the Nanjing Biomedical Research Institute of Nanjing University and maintained in specific pathogen-free conditions at the Animal Experimental Centre of Southwest Medical University.
Informed consent
Informed consent was obtained from all participants and the project was approved by the review board of Southwest Medical University (No. K2017027).
Footnotes
Ting Chen and Qing Li contributed equally to this work.
References
- 1.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11(11):723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Odegaard JI, Chawla A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science. 2013;339(6116):172–177. doi: 10.1126/science.1230721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Isidro RA, Appleyard CB. Colonic macrophage polarization in homeostasis, inflammation, and cancer. Am J Physiol Gastrointest Liver Physiol. 2016;31(1)1):G59–G73. doi: 10.1152/ajpgi.00123.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yin Y, Yao S, Hu Y, Feng Y, Li M, Bian Z, Zhang J, Qin Y, Qi X, Zhou L, Fei B, Zou J, Hua D, Huang Z. The Immune-microenvironment confers chemoresistance of colorectal cancer through macrophage-derived IL6. Clin Cancer Res. 2017;23(23):7375–7387. doi: 10.1158/1078-0432.CCR-17-1283. [DOI] [PubMed] [Google Scholar]
- 5.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 6.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122(3):787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang X, Mo C, Wang Y, Wei D, Xiao H. Anti-tumour strategies aiming to target tumour-associated macrophages. Immunology. 2013;138(2):93–104. doi: 10.1111/imm.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaplan CW, Ma X, Paranjpe A, Jewett A, Lux R, Kinder-Haake S, Shi W. Fusobacterium nucleatum outer membrane proteins Fap2 and RadD induce cell death in human lymphocytes. Infect Immun. 2010;78(11):4773–4778. doi: 10.1128/IAI.00567-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gur C, Ibrahim Y, Isaacson B, Yamin R, Abed J, Gamliel M, Enk J, Bar-On Y, Stanietsky-Kaynan N, Coppenhagen-Glazer S, Shussman N, Almogy G, Cuapio A, Hofer E, Mevorach D, Tabib A, Ortenberg R, Markel G, Miklic K, Jonjic S, Brennan CA, Garrett WS, Bachrach G, Mandelboim O. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity. 2015;42(2):344–355. doi: 10.1016/j.immuni.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, Clancy TE, Chung DC, Lochhead P, Hold GL, El-Omar EM, Brenner D, Fuchs CS, Meyerson M, Garrett WS. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013;14(2):207–215. doi: 10.1016/j.chom.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y, Peng Y, Yu J, Chen T, Wu Y, Shi L, Li Q, Wu J, Fu X. Invasive Fusobacterium nucleatum activates beta-catenin signaling in colorectal cancer via a TLR4/P-PAK1 cascade. Oncotarget. 2017;8(19):31802–31814. doi: 10.18632/oncotarget.15992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenhill CJ, Rose-John S, Lissilaa R, Ferlin W, Ernst M, Hertzog PJ, Mansell A, Jenkins BJ. IL-6 trans-signaling modulates TLR4-dependent inflammatory responses via STAT3. J Immunol. 2011;186(2):1199–1208. doi: 10.4049/jimmunol.1002971. [DOI] [PubMed] [Google Scholar]
- 13.Waldner MJ, Neurath MF. Master regulator of intestinal disease: IL-6 in chronic inflammation and cancer development. Semin Immunol. 2014;26(1):75–79. doi: 10.1016/j.smim.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Kitamura H, Ohno Y, Toyoshima Y, Ohtake J, Homma S, Kawamura H, Takahashi N, Taketomi A. Interleukin-6/STAT3 signaling as a promising target to improve the efficacy of cancer immunotherapy. Cancer Sci. 2017;108(10):1947–1952. doi: 10.1111/cas.13332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flint TR, Janowitz T, Connell CM, Roberts EW, Denton AE, Coll AP, Jodrell DI, Fearon DT. Tumor-induced IL-6 reprograms host metabolism to suppress anti-tumor immunity. Cell Metab. 2016;24(5):672–684. doi: 10.1016/j.cmet.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi Y, Frost PJ, Hoang BQ, Benavides A, Sharma S, Gera JF, Lichtenstein AK. IL-6-induced stimulation of c-Myc translation in multiple myeloma cells is mediated by myc internal ribosome entry site function and the RNA-binding protein, hnRNP A1. Cancer Res. 2008;68(24):10215–10222. doi: 10.1158/0008-5472.CAN-08-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pello OM. Macrophages and c-Myc cross paths. Oncoimmunology. 2016;5(6):e1151991. doi: 10.1080/2162402X.2016.1151991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pello OM, De Pizzol M, Mirolo M, Soucek L, Zammataro L, Amabile A, Doni A, Nebuloni M, Swigart LB, Evan GI, Mantovani A, Locati M. Role of c-MYC in alternative activation of human macrophages and tumor-associated macrophage biology. Blood. 2012;119(2):411–421. doi: 10.1182/blood-2011-02-339911. [DOI] [PubMed] [Google Scholar]
- 19.Braune J, Weyer U, Hobusch C, Mauer J, Bruning JC, Bechmann I, Gericke M. IL-6 regulates M2 polarization and local proliferation of adipose tissue macrophages in obesity. J Immunol. 2017;198(7):2927–2934. doi: 10.4049/jimmunol.1600476. [DOI] [PubMed] [Google Scholar]
- 20.Naito S, von Eschenbach AC, Giavazzi R, Fidler IJ. Growth and metastasis of tumor cells isolated from a human renal cell carcinoma implanted into different organs of nude mice. Cancer Res. 1986;46(8):4109–4115. [PubMed] [Google Scholar]
- 21.Farzi A, Halicka J, Mayerhofer R, Frohlich EE, Tatzl E, Holzer P. Toll-like receptor 4 contributes to the inhibitory effect of morphine on colonic motility in vitro and in vivo. Sci Rep. 2015;5:9499. doi: 10.1038/srep09499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiahui Yu YC, Xiangsheng Fu X, Zhou Y, Peng L, Shi T, Chen Y. Invasive Fusobacterium nucleatum may play a role in the carcinogenesis of proximal colon cancer through the serrated neoplasia pathway. Int J Cancer. 2016;139(6):1318–1326. doi: 10.1002/ijc.30168. [DOI] [PubMed] [Google Scholar]
- 23.Li L, Fu X, Zhang W, Xiao L, Qiu Y, Peng Y, Shi L, Chen X, Zhou X, Deng M. Wnt signaling pathway is activated in right colon serrated polyps correlating to specific molecular form of β-catenin. Hum Pathol. 2013;44(6):1079–1088. doi: 10.1016/j.humpath.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 24.Chew V, Lai L, Pan L, Lim CJ, Li J, Ong R, Chua C, Leong JY, Lim KH, Toh HC, Lee SY, Chan CY, Goh BKP, Chung A, Chow PKH, Albani S. Delineation of an immunosuppressive gradient in hepatocellular carcinoma using high-dimensional proteomic and transcriptomic analyses. Proc Natl Acad Sci USA. 2017;114(29):E5900–E5909. doi: 10.1073/pnas.1706559114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klar AS, Michalak-Micka K, Biedermann T, Simmen-Meuli C, Reichmann E, Meuli M. Characterization of M1 and M2 polarization of macrophages in vascularized human dermo-epidermal skin substitutes in vivo. Pediatr Surg Int. 2018;34(2):129–135. doi: 10.1007/s00383-017-4179-z. [DOI] [PubMed] [Google Scholar]
- 26.Mima K, Sukawa Y, Nishihara R, Qian ZR, Yamauchi M, Inamura K, Kim SA, Masuda A, Nowak JA, Nosho K, Kostic AD, Giannakis M, Watanabe H, Bullman S, Milner DA, Harris CC, Giovannucci E, Garraway LA, Freeman GJ, Dranoff G, Chan AT, Garrett WS, Huttenhower C, Fuchs CS, Ogino S. Fusobacterium nucleatum and T Cells in colorectal carcinoma. JAMA Oncol. 2015;1(5):653–661. doi: 10.1001/jamaoncol.2015.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nei Y, Obata-Ninomiya K, Tsutsui H, Ishiwata K, Miyasaka M, Matsumoto K, Nakae S, Kanuka H, Inase N, Karasuyama H. GATA-1 regulates the generation and function of basophils. Proc Natl Acad Sci USA. 2013;110(46):18620–18625. doi: 10.1073/pnas.1311668110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Becht E, de Reynies A, Giraldo NA, Pilati C, Buttard B, Lacroix L, Selves J, Sautes-Fridman C, Laurent-Puig P, Fridman WH. Immune and stromal classification of colorectal cancer is associated with molecular subtypes and relevant for precision immunotherapy. Clin Cancer Res. 2016;22(16):4057–4066. doi: 10.1158/1078-0432.CCR-15-2879. [DOI] [PubMed] [Google Scholar]
- 29.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 30.Park HE, Kim JH, Cho NY, Lee HS, Kang GH. Intratumoral Fusobacterium nucleatum abundance correlates with macrophage infiltration and CDKN2A methylation in microsatellite-unstable colorectal carcinoma. Virchows Arch. 2017;471(3):329–336. doi: 10.1007/s00428-017-2171-6. [DOI] [PubMed] [Google Scholar]
- 31.Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer. 2006;42(6):717–727. doi: 10.1016/j.ejca.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 32.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paynich ML, Jones-Burrage SE, Knight KL. Exopolysaccharide from Bacillus subtilis induces anti-inflammatory M2 macrophages that prevent T cell-mediated disease. J Immunol. 2017;198(7):2689–2698. doi: 10.4049/jimmunol.1601641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park S, Shin HJ, Shah M, Cho HY, Anwar MA, Achek A, Kwon HK, Lee B, Yoo TH, Choi S. TLR4/MD2 specific peptides stalled in vivo LPS-induced immune exacerbation. Biomaterials. 2017;126:49–60. doi: 10.1016/j.biomaterials.2017.02.023. [DOI] [PubMed] [Google Scholar]
- 35.O’Neill LA, Golenbock D, Bowie AG. The history of Toll-like receptors—redefining innate immunity. Nat Rev Immunol. 2013;13(6):453–460. doi: 10.1038/nri3446. [DOI] [PubMed] [Google Scholar]
- 36.Ii M, Matsunaga N, Hazeki K, Nakamura K, Takashima K, Seya T, Hazeki O, Kitazaki T, Iizawa Y. A novel cyclohexene derivative, ethyl (6R)-6-[N-(2-Chloro-4-fluorophenyl)sulfamoyl]cyclohex-1-ene-1-carboxylate (TAK-242), selectively inhibits toll-like receptor 4-mediated cytokine production through suppression of intracellular signaling. Mol Pharmacol. 2006;69(4):1288–1295. doi: 10.1124/mol.105.019695. [DOI] [PubMed] [Google Scholar]
- 37.Sanmarco LM, Ponce NE, Visconti LM, Eberhardt N, Theumer MG, Minguez AR, Aoki MP. IL-6 promotes M2 macrophage polarization by modulating purinergic signaling and regulates the lethal release of nitric oxide during Trypanosoma cruzi infection. Biochim Biophys Acta. 2017;1863(4):857–869. doi: 10.1016/j.bbadis.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 38.Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, Karin M. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer cell. 2009;15(2):103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.West NR, McCuaig S, Franchini F, Powrie F. Emerging cytokine networks in colorectal cancer. Nat Rev Immunol. 2015;15(10):615–629. doi: 10.1038/nri3896. [DOI] [PubMed] [Google Scholar]
- 40.Subramaniam KS, Omar IS, Kwong SC, Mohamed Z, Woo YL, Mat Adenan NA, Chung I. Cancer-associated fibroblasts promote endometrial cancer growth via activation of interleukin-6/STAT-3/c-Myc pathway. Am J Cancer Res. 2016;6(2):200–213. [PMC free article] [PubMed] [Google Scholar]
- 41.Martinez FO, Helming L, Milde R, Varin A, Melgert BN, Draijer C, Thomas B, Fabbri M, Crawshaw A, Ho LP, Ten Hacken NH, Cobos Jimenez V, Kootstra NA, Hamann J, Greaves DR, Locati M, Mantovani A, Gordon S. Genetic programs expressed in resting and IL-4 alternatively activated mouse and human macrophages: similarities and differences. Blood. 2013;121(9):e57–e69. doi: 10.1182/blood-2012-06-436212. [DOI] [PubMed] [Google Scholar]
- 42.Strauss J, Kaplan GG, Beck PL, Rioux K, Panaccione R, Devinney R, Lynch T, Allen-Vercoe E. Invasive potential of gut mucosa-derived Fusobacterium nucleatum positively correlates with IBD status of the host. Inflamm Bowel Dis. 2011;17(9):1971–1978. doi: 10.1002/ibd.21606. [DOI] [PubMed] [Google Scholar]
- 43.Ding T, Schloss PD. Dynamics and associations of microbial community types across the human body. Nature. 2014;509(7500):357–360. doi: 10.1038/nature13178. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.