Abstract
The goal of this study is to evaluate PD-L1 prevalence and its association with major clinical characteristics in Chinese non-small cell lung cancer (NSCLC) patients to inform the clinical development of anti-PD1/PD-L1 agents in this population. We used phosphatase and tensin homolog (PTEN) expression through IHC as a surrogate tissue quality marker to screen surgical NSCLC samples in tissue microarray (TMA; 172 cases) or whole-section (268 cases) format. The samples were then analyzed with a clinically validated PD-L1 IHC assay. The results were correlated with baseline characteristics and clinical outcomes. PTEN IHC showed that 108 TMA samples and 105 whole-section samples qualified for PD-L1 IHC. With a clinically relevant cutoff, 41.7% of the TMA samples were PD-L1 positive. PD-L1 level was much lower in EGFR-mutant patients and seemed to be a favorable prognostic factor for both overall survival (OS) and recurrence-free survival (RFS). These findings were confirmed in the whole-section samples except that their survival data were not mature enough for correlation analysis. In summary, PD-L1 expression was detected in approximately 40% of PTEN-qualified Chinese NSCLC samples, negatively correlated with EGFR mutation and seemed to be a favorable prognostic factor for both OS and RFS. Notably, the different results from PTEN-qualified and PTEN-disqualified samples underscore the importance of tissue quality control prior to biomarker testing.
Electronic supplementary material
The online version of this article (10.1007/s00262-017-2098-4) contains supplementary material, which is available to authorized users.
Keywords: NSCLC, PD-L1, EGFR, PTEN IHC, Tissue quality screening, Favorable prognostic factor
Introduction
Lung cancer has been the most common cancer in the world for several decades. Worldwide, there were approximately 1.8 million new cases and 1.6 million deaths in 2012 [2]. Recent data from national cancer statistics showed that in 2015, there would be 221,200 new cases and 158,040 deaths in the United States [3] and 733,300 new cases and 610,200 deaths in China [4].
Non-small cell lung cancer (NSCLC) is one of the two major types of lung cancer, accounting for approximately 85% of all lung cancer cases [5]. The two predominant histologic types of NSCLC are adenocarcinoma, which accounts for more than half of cases, and squamous cell carcinoma, which accounts for approximately 25% of cases [6, 7]. The overall 5-year survival rate for stage 3b/4 NSCLC is 2–4% [8]. More than half of the patients with NSCLC present with distant metastatic disease at the time of initial diagnosis, which directly contributes to poor survival prospects.
Management of patients with advanced NSCLC is individualized based upon molecular and histologic features of the tumor. The presence of somatic activating mutations in the EGFR gene or re-arrangements in the anaplastic lymphoma kinase gene are strongly predictive of sensitivity to EGFR tyrosine kinase inhibitors or to anaplastic lymphoma kinase inhibitors, respectively [9, 10]. For patients who do not harbor these activating genetic abnormalities, platinum-based regimens remain the standard first-line option. Virtually, all patients progress following first-line chemotherapies, with median progression-free survival ranging from 3.1 to 6.5 months [11, 12]. As a result, most NSCLC patients will continue to need more effective second-line treatment options. Patients with a good performance status in second-line studies have a median survival duration of approximately 8–10 months.
Encouraging clinical data emerging in the field of cancer immunotherapy have demonstrated that such therapies can result in significant survival benefits in patients with advanced malignancies, including NSCLC [13–16]. The programed death 1 receptor (PD-1; also known as CD279) is a negative costimulatory receptor expressed mainly on activated T cells [17]. The binding of PD-1 to its ligands, PD-L1 and PD-L2, down-regulates excessive immune responses by inhibiting effector T-cell function [18]. Over-expression of PD-L1 in several tumor types, including NSCLC, leads to the suppression of anti-tumor immune response and is believed to be a major mechanism of immune surveillance evasion [19]. Clinical studies of anti-PD-1 antibodies (e.g., Nivolumab or Pembrolizumab) have established the therapeutic value of targeting the PD-1/PD-L1 pathway in NSCLC in both second-line [20, 21] and first-line [22] settings. Atezolizumab (MPDL3280A; F Hoffmann-La Roche/Genentech) is an engineered, humanized IgG1 monoclonal anti-PD-L1 antibody that blocks the binding between PD-L1 and its receptors, PD-1 and B7.1 (also known as CD80) [23, 24]. In addition to demonstrating clinical utilities similar to those of the PD-1 antibodies in second-line treatment of NSCLC [14, 25], Atezolizumab might inhibit down-regulation of immune responses by additionally blocking PD-L1/B7.1 binding on T cells and antigen-presenting cells [26, 27].
The clinical utility of Atezolizumab and other PD1/PD-L1 inhibitors in Chinese NSCLC patients remains to be verified in large-scale clinical trials. Rational design of such trials requires reliable data on the prevalence of PD-L1 expression in Chinese NSCLC patients as well as its correlation with important clinical parameters such as age, gender, tumor stage, tumor molecular sub-type, and prognosis. The objective of this study was to collect such biomarker data from Chinese NSCLC samples, whose tissue quality was assessed with a method that first determined the expression of phosphatase and tensin homolog (PTEN) on non-tumoral cells as an internal control and a surrogate for tissue quality, thereby ensuring the reliability of the subsequently obtained PD-L1 expression data.
Materials and methods
Patient populations
Patients included in this study were those who underwent surgical resection at Guangdong General Hospital in Guangzhou, China between 2006 and 2014, had histologically confirmed NSCLC with sufficient tissue for PTEN IHC and PD-L1 IHC staining and also had complete clinical records.
The tissue microarray (TMA) and the whole-section cohorts contained 268 and 172 formalin-fixed paraffin-embedded samples, respectively. Clinical parameters including age, gender, tumor histology, smoking status, tumor stage, treatment type (“Surgery only” or “Surgery plus others”, where “others” include neo/adjuvant chemotherapy and radiation therapy), tumor differentiation level, and EGFR mutation status were collected from the hospital’s medical records. Pathologic tumor stage was defined using the American Joint Committee on Cancer Staging Manual, seventh edition. Stage was assigned retrospectively for patients, whose tumors were staged before publication of the seventh edition. Smoking status was defined as never (< 100 lifetime cigarettes), former (≥ 100 lifetime cigarettes and quit ≥ 1 year before diagnosis), or current (≥ 100 lifetime cigarettes and quit < 1 year before diagnosis).
PTEN IHC for tissue quality assessment
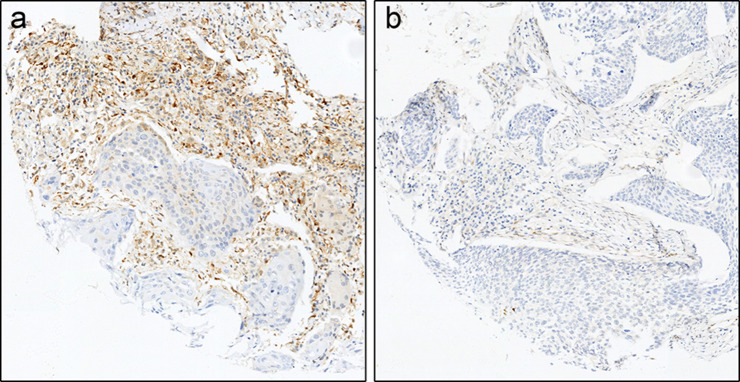
All samples were provided as 3–4 μm formalin-fixed paraffin-embedded sections. To assess tissue quality, one section from each sample was stained for PTEN expression for which the primary antibody was clone 138G6 from Cell Signaling Technology (Massachusetts, USA). As a tumor suppressor gene, PTEN is usually expressed at least moderately on stromal cells (e.g., stromal and epithelial cells) in various tissues [28], making it a surrogate biomarker for tissue quality: if most stromal cells demonstrate at least moderate PTEN staining intensity, the sample is considered to have acceptable tissue quality; if there are no or only a few stromal cells demonstrating at least moderate PTEN staining intensity, the sample is considered of poor tissue quality (presumably caused by pre-analytical factors and/or storage conditions) [29] and should thus be excluded from the subsequent PD-L1 IHC assessment (Fig. 1).
Fig. 1.
Examples of PTEN staining observed in patient samples analyzed in this study. a PTEN-qualified sample. b PTEN-disqualified sample. PTEN staining is evident in a from the presence of the chromogen (brown), but is absent in b. The counterstain is hematoxylin (blue)
PD-L1 IHC
PD-L1 expression on tumor cells (TC) and tumor-infiltrating immune cells (IC) was assessed with the Ventana SP142 PD-L1 IHC assay (Ventana Medical Systems, Tucson, AZ, USA). PD-L1 expression was as previously described [30]:
For TCs, the score is the percentage of TCs showing any discernible membrane PD-L1 staining of any intensity among the total number of TCs. The cutoffs are TC3: ≥ 50%, TC2: ≥ 5 and < 50%, TC1: ≥ 1 and < 5%, and TC0: < 1%.
For ICs, the score is the proportion of tumor area covered with ICs with any PD-L1 staining of any intensity. The cutoffs are IC3: ≥ 10%, IC2: ≥ 5 and < 10%, IC1: ≥ 1 and < 5%, and IC0: < 1%.
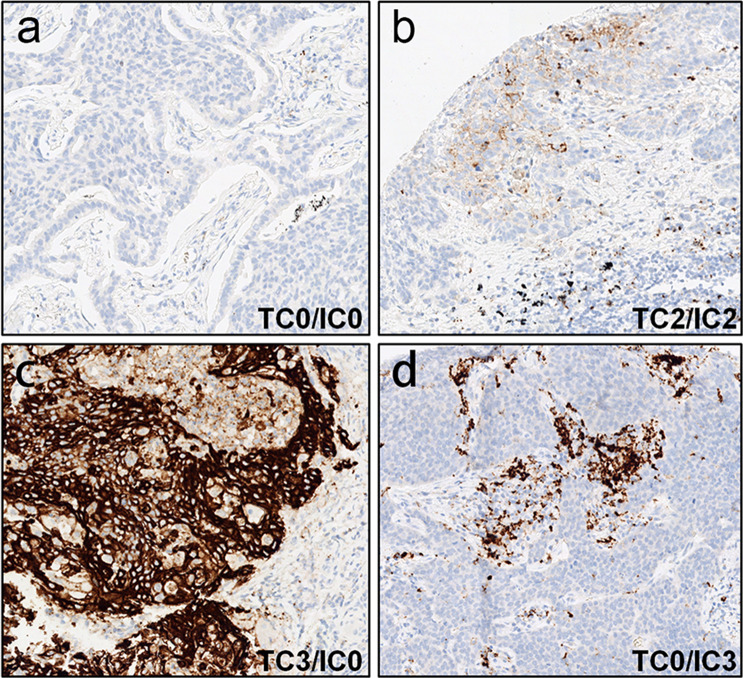
Typical PD-L1 staining and scoring results with this assay are shown in Fig. 2. It should be noted that according to two recent reports, staining intensity with this Ventana SP142 assay is relatively weaker than with four other PD-L1 IHC assays including 22C3 (approved by FDA), 28-8 (approved by FDA), SP263 (investigational use only), and E1L3N (laboratory-developed test) [31, 32]. Therefore, results from this study were compared only to those from the previous studies that used the same SP142 assay.
Fig. 2.
Examples of the range of PD-L1 staining observed in patient samples analyzed in this study. a TC0/IC0 sample. b TC2/IC2 sample. c TC3/IC0 sample. d TC0/IC3 sample. PD-L1 staining is shown by the presence of the chromogen (brown). The counterstain is hematoxylin (blue)
EGFR mutation
Patients’ EGFR mutation status was obtained from Guangdong General Hospital’s patient record database. At the time of the test, genomic DNA was extracted from patient samples using DNeasy Blood and Tissue Kit (No. 69504; Qiagen, Valencia, CA, USA), and exons 18–21 were amplified with four pairs of primers. Polymerase chain reaction (PCR) products were purified and labeled using BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA), followed by sequencing in an ABI 3100 Genetic Analyzer (Applied Biosystems).
Some patient samples were also tested for EGFR mutations using ARMS, according to the protocol of the DxS EGFR mutation test kit (DxS). A total of 29 mutations in the EGFR gene can be detected using this kit. All quantitative PCR reactions were performed using the LightCycler 480 instrument (Roche Applied Science, Indianapolis, IN, USA).
Patients whose tumor samples were not analyzed for EGFR mutation status were categorized as “Unknown” for this clinical characteristic.
Statistical analysis
The statistical analyses were conducted by cohort. The prevalence of PD-L1 was estimated as a proportion of patients who are PD-L1-positive or PD-L1-negative for the pre-specified cutoffs. To assess the correlation between PD-L1 level and patient characteristics, descriptive statistics were provided for each PD-L1 category. The corresponding p values were calculated using Fisher’s exact test. To assess the prognostic effect of PD-L1 level with OS and RFS, Cox regression model was performed to estimate the hazard ratio (HR) and 95% confidence interval (CI). The corresponding p values were calculated using log-rank test. The p values reported are for descriptive purpose, and no multiple testing adjustments have been done.
Results
Patient characteristics and tissue quality
After tissue quality assessment with PTEN IHC, 108 out of 268 TMA samples (40.3%) and 105 out of 172 whole-section samples (61.0%) were found qualified for PD-L1 IHC. Patient characteristics for these samples are shown in Table 1. For the TMA cohort and the whole-section cohort, respectively: the median age of the patients was 59 years and 64.8 years; male patients accounted for 73.1 and 61.0%; majority of the patients were non-squamous (64.8 and 73.3%); the combined fractions of current and previous smokers were 57.0 and 34.7%; 94.4 and 89.5% patients had 1/2/3a stage NSCLC; and 55.6 and 33.3% patients had the other types of treatment besides surgery. In terms of EGFR mutation, mutant patients accounted for 14.8 and 35.2% in the TMA cohort and the whole-section cohort, respectively. The relatively lower value in the TMA cohort is likely due to the fact that some of those samples were collected before EGFR mutation test became part of the routine clinical diagnosis of NSCLC in China. As a result, there is a higher fraction of patients with “Unknown” EGFR mutation status in the TMA cohort than in whole-section cohort (38.9 vs. 18.1%).
Table 1.
Patient characteristics
| TMA cohort | Whole-section cohort | |
|---|---|---|
| Gender | ||
| n | 108 | 105 |
| Female | 29 (26.9%) | 41 (39%) |
| Male | 79 (73.1%) | 64 (61%) |
| Tumor histology | ||
| n | 108 | 105 |
| Non-squamous | 70 (64.8%) | 77 (73.3%) |
| Squamous | 38 (35.2%) | 28 (26.7%) |
| Smoking status | ||
| n | 100 | 104 |
| Current | 40 (40%) | 35 (33.7%) |
| Never | 43 (43%) | 68 (65.4%) |
| Previous | 17 (17%) | 1 (1%) |
| Tumor stage | ||
| n | 108 | 105 |
| 1, 2, 3a | 102 (94.4%) | 94 (89.5%) |
| 3b, 4 | 6 (5.6%) | 11 (10.5%) |
| Treatment type | ||
| n | 108 | 105 |
| Surgery only | 48 (44.4%) | 70 (66.7%) |
| Surgery + others | 60 (55.6%) | 35 (33.3%) |
| Differentiation level | ||
| n | 93 | 98 |
| Moderately differentiated | 56 (60.2%) | 57 (58.2%) |
| Poorly differentiated | 29 (31.2%) | 39 (39.8%) |
| Well differentiated | 8 (8.6%) | 2 (2%) |
| EGFR | ||
| n | 108 | 105 |
| Mutation | 16 (14.8%) | 37 (35.2%) |
| Unknown | 42 (38.9%) | 19 (18.1%) |
| Wild type | 50 (46.3%) | 49 (46.7%) |
PD-L1 prevalence
Our results showed that PD-L1 prevalence in the PTEN-qualified samples of the TMA cohort was 58.3, 41.7, 18.5, and 10.2% for the “TC0 and IC0”, “TC1/2/3 or IC1/2/3”, “TC2/3 or IC2/3”, and “TC3/IC3” categories, respectively (Table 2). Because we have noticed before that for some types of cancer, the physical size of tumor tissue sections used for PD-L1 IHC seemed to affect the subsequent readout (data not published), and we carried out the same analysis in a separate cohort of whole-section samples. The results from the whole-section cohort are fairly comparable to those from the TMA cohort, with 60.0, 40.0, 19.0, and 4.8% for the corresponding categories, respectively (Table 2).
Table 2.
PD-L1 prevalence
| PD-L1 score | Cohort | PTEN-qualified | PTEN-disqualified | All |
|---|---|---|---|---|
| TC3 or IC3 | TMA | 11/108 (10.2%) | 7/160 (4.4%) | 18/268 (6.7%) |
| Whole-section | 5/105 (4.8%) | 0/67 (0%) | 5/172 (2.9%) | |
| TC2/3 or IC2/3 | TMA | 20/108 (18.5%) | 11/160 (6.9%) | 31/268 (11.6%) |
| Whole-section | 20/105 (19%) | 2/67 (3%) | 22/172 (12.8%) | |
| TC1/2/3 or IC1/2/3 | TMA | 45/108 (41.7%) | 20/160 (12.5%) | 65/268 (24.3%) |
| Whole-section | 42/105 (40%) | 3/67 (4.5%) | 45/172 (26.2%) | |
| TC0 or IC0 | TMA | 63/108 (58.3%) | 140/160 (87.5%) | 203/268 (75.7%) |
| Whole-section | 63/105 (60%) | 64/67 (95.5%) | 127/172 (73.8%) |
The PTEN-disqualified samples in both cohorts showed lower PD-L1 prevalence than the PTEN-qualified ones (Table 2). For the “TC1/2/3 or IC1/2/3” category in particular, the value was 12.5 vs. 41.7 and 4.5 vs. 40.0% for the TMA cohort and the whole-section cohort, respectively. Similar differences were observed for the “TC2/3 or IC2/3” and “TC3/IC3” categories. As a result, the fraction of “TC0 and IC0” samples was significantly higher for the PTEN-disqualified samples in both cohorts (87.5 vs. 58.3% and 95.5 vs. 60.0%, respectively; Table 2).
Association between PD-L1 expression and patient characteristics
With TC1/IC1 as the cutoff for PD-L1 positivity, we then examined the association between PD-L1 positivity and patient characteristics in both cohorts, but found none with gender, tumor histology, smoking status, tumor stage, treatment type, or tumor differentiation level in the TMA cohort (Supplementary Table 1). In the whole-section cohort, however, patients who were male, had squamous histology, were current or previous smokers, or had poorly differentiated tumors appeared to show higher PD-L1 positivity rates (Supplementary Table 2).
Interestingly, we found in both cohorts that EGFR-mutant patients had lower fractions of PD-L1 positivity than EGFR wild-type patients (Table 3). With the TC1/IC1 cutoff, the proportions of PD-L1 positivity are 18.8 vs. 44.0% in the EGFR-mutant (n = 16) and wild-type (n = 50) sub-groups, respectively, in the TMA cohort (p = 0.083) and 18.9% (n = 37) vs. 51.0% (n = 49) in the whole-section cohort (p = 0.003). This trend persisted with more stringent cutoffs for PD-L1 expression. In the PTEN-disqualified samples of the TMA cohort, the fraction of TC0/IC0 samples was higher for both EGFR-mutant (95.0 vs. 81.2%) and EGFR wild-type (83.8 vs. 56.0%) sub-groups, thus resulting in a less pronounced correlation between EGFR mutation status and PD-L1 expression level. Similar results were seen in the whole-section cohort.
Table 3.
PD-L1 expression and EGFR mutation status
| Samples | PTEN-qualified | PTEN-disqualified | All | |||
|---|---|---|---|---|---|---|
| EGFR status | Mutation | Wild type | Mutation | Wild type | Mutation | Wild type |
| The TMA cohort | ||||||
| n | 16 | 50 | 40 | 68 | 56 | 118 |
| TC1/IC1 as cutoff | p = 0.083 | p = 0.126 | p = 0.005 | |||
| TC0 and IC0 | 13 (81.2%) | 28 (56%) | 38 (95%) | 57 (83.8%) | 51 (91.1%) | 85 (72%) |
| TC1/2/3 or IC1/2/3 | 3 (18.8%) | 22 (44%) | 2 (5%) | 11 (16.2%) | 5 (8.9%) | 33 (28%) |
| TC2/IC2 as cutoff | p = 0.271 | p = 0.025 | p = 0.004 | |||
| TC0/1 and IC0/1 | 15 (93.8%) | 40 (80%) | 40 (100%) | 59 (86.8%) | 55 (98.2%) | 99 (83.9%) |
| TC2/3 or IC2/3 | 1 (6.2%) | 10 (20%) | 0 (0%) | 9 (13.2%) | 1 (1.8%) | 19 (16.1%) |
| TC3/IC3 as cutoff | p = 0.323 | p = 0.083 | p = 0.01 | |||
| TC0/1/2 and IC0/1/2 | 16 (100%) | 44 (88%) | 40 (100%) | 62 (91.2%) | 56 (100%) | 106 (89.8%) |
| TC3 or IC3 | 0 (0%) | 6 (12%) | 0 (0%) | 6 (8.8%) | 0 (0%) | 12 (10.2%) |
| The whole-section cohort | ||||||
| n | 37 | 49 | 28 | 26 | 65 | 75 |
| TC1/IC1 as cutoff | p = 0.003 | p = 0.604 | p = 0.002 | |||
| TC0 and IC0 | 30 (81.1%) | 24 (49%) | 27 (96.4%) | 24 (92.3%) | 57 (87.7%) | 48 (64%) |
| TC1/2/3 or IC1/2/3 | 7 (18.9%) | 25 (51%) | 1 (3.6%) | 2 (7.7%) | 8 (12.3%) | 27 (36%) |
| TC2/IC2 as cutoff | p = 0.083 | p = 0.227 | p = 0.017 | |||
| TC0/1 and IC0/1 | 34 (91.9%) | 37 (75.5%) | 28 (100%) | 24 (92.3%) | 62 (95.4%) | 61 (81.3%) |
| TC2/3 or IC2/3 | 3 (8.1%) | 12 (24.5%) | 0 (0%) | 2 (7.7%) | 3 (4.6%) | 14 (18.7%) |
| TC3/IC3 as cutoff | p = 0.131 | – | p = 0.123 | |||
| TC0/1/2 and IC0/1/2 | 37 (100%) | 45 (91.8%) | 28 (100%) | 26 (100%) | 65 (100%) | 71 (94.7%) |
| TC3 or IC3 | 0 (0%) | 4 (8.2%) | 0 (0%) | 0 (0%) | 0 (0%) | 4 (5.3%) |
Association between PD-L1 expression and prognosis
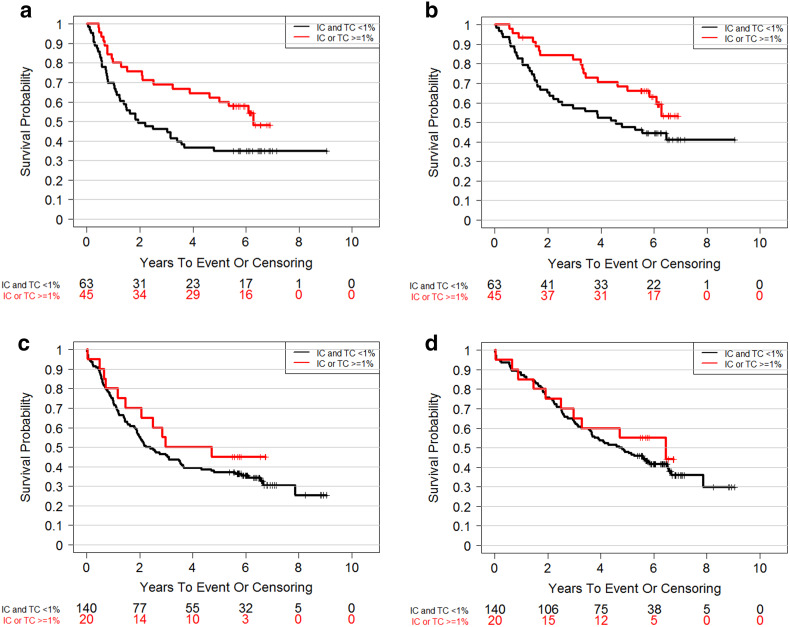
In the PTEN-qualified sub-group of the TMA cohort, out of 108 patients, 54 deaths (50.0%) and 62 RFS events (57.4%) occurred during a median follow-up of 6.24 years after resection. We found that RFS appeared to be longer in the PD-L1-positive sub-group than in the PD-L1-negative sub-group (HR 0.55, 95% CI [0.32, 0.93], p = 0.024; Fig. 3a). A similar trend was observed on the association between PD-L1 expression and OS (HR 0.58, 95% CI [0.33, 1.03], p = 0.056; Fig. 3b). In the PTEN-disqualified sub-group of the TMA cohort, the difference between PD-L1-positive and PD-L1-negative sub-groups was significantly reduced (HR 0.75, 95% CI [0.4, 1.4], p = 0.36 for RFS and HR 0.81, 95% CI [0.42, 1.56], p = 0.53 for OS; Fig. 3c, d).
Fig. 3.
Prognostic effect of PD-L1 level in the TMA cohort and the impact of tissue quality. a RFS in PTEN-qualified samples, HR 0.55, 95% CI [0.32, 0.93], p = 0.024. b OS in PTEN-qualified samples, HR 0.58, 95% CI [0.33, 1.02], p = 0.056. c RFS in PTEN-disqualified samples, HR 0.75, 95% CI [0.4, 1.4], p = 0.36. d OS in PTEN-disqualified samples, HR 0.81, 95% CI [0.42, 1.56], p = 0.53
In the PTEN-qualified sub-group of the whole-section cohort, out of 105 patients, 18 OS events (17.1%) and 25 RFS events (23.8%) had been observed during a median follow-up of 2.6 years after resection. Such low event rates did not allow for deriving the association between PD-L1 expression and patient prognosis.
Discussion
The objective of this study is to understand the prevalence of PD-L1 expression in Chinese NSCLC patients and its association with major clinical characteristics such as age, gender, tumor stage, tumor molecular sub-type, and prognosis.
The patient population in this study, which included a TMA cohort and a whole-section cohort, was fairly representative of the general Chinese NSCLC population except for a lower proportion of female patients (26.9%, Table 1) and EGFR-mutant patients (14.8%, Table 1) in the TMA cohort. For example, smoking is believed to induce higher neo-antigen burden in NSCLC [33], which may require a higher level of PD-L1 expression by the tumor cells to maintain immune surveillance evasion. The fraction of male current smokers in the TMA cohort is very close to that in the general patient population (53.3 vs. 53% [34, 35]).
To exclude the potential influence of tissue quality on our results, we employed PTEN IHC as a tissue quality assessment method. Samples whose internal positive control cells did not exhibit at least moderate staining intensity were considered disqualified for the PD-L1 IHC. We found that 40.3% of the TMA samples and 61.0% of the surgical samples were qualified. The reason for the higher fraction of disqualified samples in the TMA cohort needs further investigation. We suspect that it could be due to the longer time that the TMA samples have been in storage than the whole-section samples.
PD-L1 prevalence results vary with the method used to evaluate it, including the antibody clone, the staining procedure, and the scoring algorithm. Until recently, many methods that have not been validated in prospective clinical trials have been used in this field, which may have contributed to the very different results that have been reported in the literature (Supplementary Table 3). We used a method that has been validated in recent phase II and phase III clinical trials with the anti-PD-L1 drug Atezolizumab [14, 25]. The PD-L1 prevalence from this study is lower than what has been reported in an Atezolizumab phase II clinical trial with second-line NSCLC patients who were mostly Caucasian [14] (TC1/2/3 or IC1/2/3: 40.0 and 41.7 vs. 68%; TC2/3 or IC2/3: 18.5 and 19.0 vs. 37%; TC3 or IC3: 10.2 and 4.8 vs. 16%). A recent study conducted with a cohort of 1,070 surgically resected NSCLC samples from South Korean reported PD-L1 prevalence of 44% (strongly positive + weakly positive) [36], but the authors used a different clinically validated IHC assay (clone 22C3, Merck), so it is difficult to make a direct comparison to our data. Nonetheless, these results may reflect potential ethnic difference in PD-L1 expression and certainly warrant further investigation.
PD-L1 expression in the PTEN-disqualified sub-groups of both cohorts was much lower than that from the PTEN-qualified sub-groups (Table 2), which clearly underscores the importance of tissue quality control in similar PD-L1 IHC assays.
The association between PD-L1 expression and various clinical characteristics has been very controversial. For example, Pan et al. did not find any significant correlation between gender, smoking status, or histological type with PD-L1 expression [37]. However, two other studies found that higher PD-L1 expression was associated with poorer tumor differentiation [37, 38]. D’Incecco et al. reported that PD-1 expression was significantly associated with current smoking status [39]. Sun et al. found a significantly higher prevalence of PD-L1 in older, male, smoker, squamous or later-stage Korean NSCLC patients [36]. Cooper et al. reported a higher, though statistically insignificant, PD-L1 expression in squamous cell carcinomas and large cell carcinomas than in adenocarcinomas [40]. Azuma et al. found that PD-L1 expression was significantly higher for women than for men, for never smokers than for smokers, and for patients with adenocarcinoma than for those with squamous cell carcinoma. These and other relevant reports are summarized in Supplementary Table 3. As mentioned earlier, the different IHC methods used in different studies as well as various patient characteristics may have contributed to such varying results.
While we saw a lack of correlation between PD-L1 expression and any clinical characteristics except EGFR mutation status in the TMA cohort, the whole-section cohort results indicated that patients who were males or smokers or had squamous or poorly differentiated tumors tended to demonstrate higher levels of PD-L1 expression. The fact that these two cohorts were from the same hospital and were evaluated with the same assays/methods clearly shows the complexity of the correlation between PD-L1 expression and patients’ clinical characteristics. Future studies with much large sample sizes are needed to verify our results.
In terms of EGFR mutation, several previous studies have reported either a lack of correlation [40] or a positive correlation [39, 41–44] with PD-L1 expression, which is contradictory to the negative correlation observed in this study. Though verification in future studies is obviously needed, our finding may help explain the more inferior efficacy of Atezolizumab in EGFR-mutant vs. wild-type NSCLC patients in a recent phase III trial on second-line NSCLC (OAK study; HR 1.24 [85 patients] vs. 0.69 [628 patients]; p value was not available) [25]. For the PTEN-disqualified samples, many of them have false-negative results for PD-L1 expression due to poor tissue quality, which likely led to a significantly diminished correlation between PD-L1 expression and EGFR mutation status.
The prognostic value of PD-L1 expression in NSCLC has been equally controversial. Two previous meta-analyses, each with more than 1,000 patients from multiple studies, found that PD-L1 expression was associated with poorer OS (pooled HR 1.75, 95% CI [1.40, 2.20], p < 0.001 [38] and pooled HR 1.47, 95% CI [1.19, 1.83], p = 0.0004 [37]). This has been shown in many individual studies in the last 3 years [37, 38, 41, 44–52]. However, a more recent study with 1070 Korean NSCLC samples reported that that correlation only existed for patients without post-operative therapies or with adenocarcinoma sub-type [36]. Another study showed that PD-L1 is not a strong prognostic marker in advanced NSCLC patients treated with chemotherapies [53]. However, several other studies found PD-L1 expression to be a favorable prognostic factor in defined sub-groups of NSCLC patients [39, 40, 44, 54–60]. More details on these reports are summarized in Supplementary Table 3.
In the TMA cohort, we found PD-L1 expression to be a favorable factor for both RFS and OS (Fig. 3). This trend seemed to persist when the patients were divided into different sub-groups according to different clinical parameters (Supplementary Table 4). Interestingly, the Atezolizumab phase III trial in second-line NSCLC (OAK study) showed that for patients treated with docetaxel, those with TC1/2/3 or IC1/2/3 had a longer OS than those with TC0/IC0 (median OS: 10.3 months in 222 patients, 95% CI [8.8, 12.0] vs. 8.9 months in 199 patients, 95% CI [7.7, 11.5]; p value was not available) [25].
In conclusion, we used a clinically validated companion/complementary diagnostic assay to evaluate PD-L1 prevalence in Chinese NSCLC patients treated in the same hospital and then analyzed its correlation with various clinical characteristics and treatment outcome. Importantly, we employed PTEN IHC as a means to exclude samples with poor tissue quality, so that their influence on the results could be excluded. We found that in PTEN-qualified samples, PD-L1 prevalence is lower than what has been reported for Caucasian patients, but is comparable to that from other Asian patients. Moreover, we found that EGFR-mutant patients had lower PD-L1 expression compared with wild-type patients and that PD-L1-positive patients had longer RFS and OS compared with PD-L1-negative patients. In PTEN-disqualified samples, PD-L1 level was lower and the aforementioned correlations were less pronounced or absent, potentially due to a much higher likelihood of false-negative PD-L1 expression data from such samples.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Robert Clarke (Oncology Biomarker Development, Genentech Inc., Basel, Switzerland) for his project management support to this study and Ventana Medical Systems, Inc. (Tucson, AZ, USA) for making the PD-L1 IHC assay available for this study.
Abbreviations
- CI
Confidence interval
- HR
Hazard ratio
- IC
Immune cell
- NSCLC
Non-small cell lung cancer
- PTEN
Phosphatase and tensin homolog
- RFS
Recurrence-free survival
- TC
Tumor cell
- TMA
Tissue microarray
Author contributions
Conception and design of study: YW, GC, XZ, JH, and DS. Acquisition of data: XC, CS, ZX, JG, JY, XY, and HK. Analysis and/or interpretation of data: GC, XZ, XC, CS, HD, SL, XX, YZ, and MC. Drafting the manuscript: GC, XZ, XC, JG, and CS. Revising the manuscript critically for important intellectual content: ZX, JY, XY, HD, SL, XX, YZ, MC, HK, JH, AK, DS, and YW. Approval of the version of the manuscript to be published (the names of all authors listed): XZ, XC, CS, ZX, JG, JY, XY, HD, SL, XX, YZ, MC, HK, JH, AK, DS, GC, and YW.
Compliance with ethical standards
Funding
This study was supported by Genentech Inc., a member of the Roche Group. This work was also supported by Special Fund of Public Interest from National Health and Family Control Committee (Grant No. 201402031), Key Lab System Project of Guangdong S&T Department (Grant No. 2012A061400006), The Guangdong Provincial Applied S&T Research and Development Program (Grant No. 2016B020237006), General Research Project of Guangzhou Science and Technology Bureau (Grant No. 201607010391), The National Key Research and Development Program of China (Grant No. 2016YFC1303800), and Public Welfare and Capacity Establishment Program of Guangdong Science and Technology Department (Grant No. 2014A020212225).
Conflict of interest
Chun Sun, Hang-jun Dai, Su-chun Li, and Yun-xia Zuo are employees of F. Hoffmann-La Roche/Genentech Inc. Xu Cao, Jian-jun Guo, Xin-ran Xu, Meng Chen, Hartmut Koeppen, Jing He, Astrid Kiermaier, David Shames, and Gang Cheng are employees of F. Hoffmann-La Roche/Genentech Inc. and hold stocks of F. Hoffmann-La Roche. All the other authors declare no conflict of interest.
Ethical approval and ethical standards
This study was approved by the institutional review board of Guangdong General Hospital in Guangzhou, China according to the Helsinki declaration.
Informed consent
The acquisition of written informed consent was waived by the institutional review board of Guangdong General Hospital in Guangzhou, China with the condition that all samples must be anonymized during the study.
Footnotes
Results in this paper have been published before as a poster titled “Characterization of PD-L1 expression in Chinese non-small cell lung cancer patients with PTEN IHC as a means for sample quality screening” in ESMO Asia 2016, December 16–19, 2016, Singapore [1].
Xu-chao Zhang, Xu Cao, Gang Cheng, and Yi-long Wu contributed equally to this work.
Contributor Information
Gang Cheng, Phone: +86 185 0166 0591, Email: gcheng00@hotmail.com.
Yi-long Wu, Phone: +86 138 0977 5415, Email: syylwu@live.cn.
References
- 1.Zhang X-c, Cao X, Sun C, Xie Z, Guo J-j, Yang J-j et al. Characterization of PD-L1 expression in Chinese non-small cell lung cancer patients with PTEN IHC as a means for sample quality screening. In ESMO Asia. 2016. Singapore. Ann Oncol 27(suppl_9):ix9–ix18. 10.1093/annonc/mdw574
- 2.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C et al. Cancer incidence and mortality worldwide: IARC CancerBase No. 11. GLOBOCAN 2012 v1.0. 2012. http://globocan.iarc.fr. Accessed 31 Mar 2017
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 4.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 5.Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584–594. doi: 10.1016/S0025-6196(11)60735-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langer CJ, Besse B, Gualberto A, Brambilla E, Soria JC. The evolving role of histology in the management of advanced non-small-cell lung cancer. J Clin Oncol. 2010;28(36):5311–5320. doi: 10.1200/JCO.2010.28.8126. [DOI] [PubMed] [Google Scholar]
- 7.Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, et al. International association for the study of lung cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6(2):244–285. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cetin K, Ettinger DS, Hei YJ, O’Malley CD. Survival by histologic subtype in stage IV nonsmall cell lung cancer based on data from the Surveillance, Epidemiology and End Results Program. Clin Epidemiol. 2011;3:139–148. doi: 10.2147/CLEP.S17191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janku F, Stewart DJ, Kurzrock R. Targeted therapy in non-small-cell lung cancer—is it becoming a reality? Nat Rev Clin Oncol. 2010;7(7):401–414. doi: 10.1038/nrclinonc.2010.64. [DOI] [PubMed] [Google Scholar]
- 10.Mok TS. Personalized medicine in lung cancer: what we need to know. Nat Rev Clin Oncol. 2011;8(11):661–668. doi: 10.1038/nrclinonc.2011.126. [DOI] [PubMed] [Google Scholar]
- 11.Bareschino MA, Schettino C, Rossi A, Maione P, Sacco PC, Zeppa R, et al. Treatment of advanced non small cell lung cancer. J Thorac Dis. 2011;3(2):122–133. doi: 10.3978/j.issn.2072-1439.2010.12.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayashi H, Okamoto I, Morita S, Taguri M, Nakagawa K. Postprogression survival for first-line chemotherapy of patients with advanced non-small-cell lung cancer. Ann Oncol. 2012;23(6):1537–1541. doi: 10.1093/annonc/mdr487. [DOI] [PubMed] [Google Scholar]
- 13.Chen DS, Irving BA, Hodi FS. Molecular pathways: next-generation immunotherapy—inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res. 2012;18(24):6580–6587. doi: 10.1158/1078-0432.CCR-12-1362. [DOI] [PubMed] [Google Scholar]
- 14.Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837–1846. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 15.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 17.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1 (PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24(2):207–212. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 21.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 23.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 24.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8(6):467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 25.Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7–1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27(1):111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang J, Riella LV, Chock S, Liu T, Zhao X, Yuan X, et al. The novel costimulatory programmed death ligand 1/B7.1 pathway is functional in inhibiting alloimmune responses in vivo. J Immunol. 2011;187(3):1113–1119. doi: 10.4049/jimmunol.1100056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15(4):356–362. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- 29.Sakr RA, Barbashina V, Morrogh M, Chandarlapaty S, Andrade VP, Arroyo CD, et al. Protocol for PTEN expression by immunohistochemistry in formalin-fixed paraffin-embedded human breast carcinoma. Appl Immunohistochem Mol Morphol. 2010;18(4):371–374. doi: 10.1097/PAI.0b013e3181d50bd5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirsch FR, McElhinny A, Stanforth D, Ranger-Moore J, Jansson M, Kulangara K, et al. PD-L1 immunohistochemistry assays for lung cancer: results from phase 1 of the blueprint PD-L1 IHC assay comparison project. J Thorac Oncol. 2017;12(2):208–222. doi: 10.1016/j.jtho.2016.11.2228. [DOI] [PubMed] [Google Scholar]
- 32.Rimm DL, Han G, Taube JM, Yi ES, Bridge JA, Flieder DB, et al. A prospective multi-institutional, pathologist-based assessment of 4 immunohistochemistry assays for PD-L1 expression in non-small cell lung cancer. JAMA Oncol. 2017;3(8):1051–1058. doi: 10.1001/jamaoncol.2017.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J, Ou JX, Bai CX. Tobacco smoking in China: prevalence, disease burden, challenges and future strategies. Respirology. 2011;16(8):1165–1172. doi: 10.1111/j.1440-1843.2011.02062.x. [DOI] [PubMed] [Google Scholar]
- 35.Zheng R, Zeng H, Zuo T, Zhang S, Qiao Y, Zhou Q, et al. Lung cancer incidence and mortality in China, 2011. Thorac Cancer. 2016;7(1):94–99. doi: 10.1111/1759-7714.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun JM, Zhou W, Choi YL, Choi SJ, Kim SE, Wang Z, et al. Prognostic significance of PD-L1 in patients with non-small cell lung cancer: a large cohort study of surgically resected cases. J Thorac Oncol. 2016;11(7):1003–1011. doi: 10.1016/j.jtho.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 37.Pan ZK, Ye F, Wu X, An HX, Wu JX. Clinicopathological and prognostic significance of programmed cell death ligand1 (PD-L1) expression in patients with non-small cell lung cancer: a meta-analysis. J Thorac Dis. 2015;7(3):462–470. doi: 10.3978/j.issn.2072-1439.2015.02.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang A, Wang HY, Liu Y, Zhao MC, Zhang HJ, Lu ZY, et al. The prognostic value of PD-L1 expression for non-small cell lung cancer patients: a meta-analysis. Eur J Surg Oncol. 2015;41(4):450–456. doi: 10.1016/j.ejso.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 39.D’Incecco A, Andreozzi M, Ludovini V, Rossi E, Capodanno A, Landi L, et al. PD-1 and PD-L1 expression in molecularly selected non-small-cell lung cancer patients. Br J Cancer. 2015;112(1):95–102. doi: 10.1038/bjc.2014.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cooper WA, Tran T, Vilain RE, Madore J, Selinger CI, Kohonen-Corish M, et al. PD-L1 expression is a favorable prognostic factor in early stage non-small cell carcinoma. Lung Cancer. 2015;89(2):181–188. doi: 10.1016/j.lungcan.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 41.Azuma K, Ota K, Kawahara A, Hattori S, Iwama E, Harada T, et al. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol. 2014;25(10):1935–1940. doi: 10.1093/annonc/mdu242. [DOI] [PubMed] [Google Scholar]
- 42.Lin K, Cheng J, Yang T, Li Y, Zhu B. EGFR-TKI down-regulates PD-L1 in EGFR mutant NSCLC through inhibiting NF-kappaB. Biochem Biophys Res Commun. 2015;463(1–2):95–101. doi: 10.1016/j.bbrc.2015.05.030. [DOI] [PubMed] [Google Scholar]
- 43.Song Z, Yu X, Cheng G, Zhang Y. Programmed death-ligand 1 expression associated with molecular characteristics in surgically resected lung adenocarcinoma. J Transl Med. 2016;14(1):188. doi: 10.1186/s12967-016-0943-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang Y, Fang W, Zhang Y, Hong S, Kang S, Yan Y, et al. The association between PD-L1 and EGFR status and the prognostic value of PD-L1 in advanced non-small cell lung cancer patients treated with EGFR-TKIs. Oncotarget. 2015;6(16):14209–14219. doi: 10.18632/oncotarget.3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huynh TG, Morales-Oyarvide V, Campo MJ, Gainor JF, Bozkurtlar E, Uruga H, et al. Programmed cell death ligand 1 expression in resected lung adenocarcinomas: association with Immune Microenvironment. J Thorac Oncol. 2016;11(11):1869–1878. doi: 10.1016/j.jtho.2016.08.134. [DOI] [PubMed] [Google Scholar]
- 46.Inamura K, Yokouchi Y, Sakakibara R, Kobayashi M, Subat S, Ninomiya H, et al. Relationship of tumor PD-L1 expression with EGFR wild-type status and poor prognosis in lung adenocarcinoma. Jpn J Clin Oncol. 2016;46(10):935–941. doi: 10.1093/jjco/hyw087. [DOI] [PubMed] [Google Scholar]
- 47.Ji M, Liu Y, Li Q, Li X, Ning Z, Zhao W, et al. PD-1/PD-L1 expression in non-small-cell lung cancer and its correlation with EGFR/KRAS mutations. Cancer Biol Ther. 2016;17(4):407–413. doi: 10.1080/15384047.2016.1156256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koh J, Go H, Keam B, Kim MY, Nam SJ, Kim TM, et al. Clinicopathologic analysis of programmed cell death-1 and programmed cell death-ligand 1 and 2 expressions in pulmonary adenocarcinoma: comparison with histology and driver oncogenic alteration status. Mod Pathol. 2015;28(9):1154–1166. doi: 10.1038/modpathol.2015.63. [DOI] [PubMed] [Google Scholar]
- 49.Meniawy TM, Lake RA, McDonnell AM, Millward MJ, Nowak AK. PD-L1 on peripheral blood T lymphocytes is prognostic in patients with non-small cell lung cancer (NSCLC) treated with EGFR inhibitors. Lung Cancer. 2016;93:9–16. doi: 10.1016/j.lungcan.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 50.Takada K, Okamoto T, Shoji F, Shimokawa M, Akamine T, Takamori S, et al. Clinical significance of PD-L1 protein expression in surgically resected primary lung adenocarcinoma. J Thorac Oncol. 2016;11(11):1879–1890. doi: 10.1016/j.jtho.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y, Wang L, Li Y, Pan Y, Wang R, Hu H, et al. Protein expression of programmed death 1 ligand 1 and ligand 2 independently predict poor prognosis in surgically resected lung adenocarcinoma. Onco Targets Ther. 2014;7:567–573. doi: 10.2147/OTT.S59959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou ZJ, Zhan P, Song Y. PD-L1 over-expression and survival in patients with non-small cell lung cancer: a meta-analysis. Transl Lung Cancer Res. 2015;4(2):203–208. doi: 10.1016/j.lungcan.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sorensen SF, Zhou W, Dolled-Filhart M, Georgsen JB, Wang Z, Emancipator K, et al. PD-L1 expression and survival among patients with advanced non-small cell lung cancer treated with chemotherapy. Transl Oncol. 2016;9(1):64–69. doi: 10.1016/j.tranon.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ameratunga M, Asadi K, Lin X, Walkiewicz M, Murone C, Knight S, et al. PD-L1 and tumor infiltrating lymphocytes as prognostic markers in resected NSCLC. PLoS One. 2016;11(4):e0153954. doi: 10.1371/journal.pone.0153954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ishii H, Azuma K, Kawahara A, Yamada K, Imamura Y, Tokito T, et al. Significance of programmed cell death-ligand 1 expression and its association with survival in patients with small cell lung cancer. J Thorac Oncol. 2015;10(3):426–430. doi: 10.1097/JTO.0000000000000414. [DOI] [PubMed] [Google Scholar]
- 56.Lin C, Chen X, Li M, Liu J, Qi X, Yang W, et al. Programmed death-ligand 1 expression predicts tyrosine kinase inhibitor response and better prognosis in a cohort of patients with epidermal growth factor receptor mutation-positive lung adenocarcinoma. Clin Lung Cancer. 2015;16(5):e25–e35. doi: 10.1016/j.cllc.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 57.Miao L, Lu Y, Xu Y, Zhang G, Huang Z, Gong L, et al. PD-L1 and c-MET expression and survival in patients with small cell lung cancer. Oncotarget. 2017;8(33):53978–53988. doi: 10.18632/oncotarget.9765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmidt LH, Kummel A, Gorlich D, Mohr M, Brockling S, Mikesch JH, et al. PD-1 and PD-L1 expression in NSCLC indicate a favorable prognosis in defined subgroups. PLoS One. 2015;10(8):e0136023. doi: 10.1371/journal.pone.0136023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Toyokawa G, Takada K, Haratake N, Takamori S, Akamine T, Katsura M, et al. Favorable disease-free survival associated with programmed death ligand 1 expression in patients with surgically resected small-cell lung cancer. Anticancer Res. 2016;36(8):4329–4336. [PubMed] [Google Scholar]
- 60.Yang CY, Lin MW, Chang YL, Wu CT, Yang PC. Programmed cell death-ligand 1 expression is associated with a favourable immune microenvironment and better overall survival in stage I pulmonary squamous cell carcinoma. Eur J Cancer. 2016;57:91–103. doi: 10.1016/j.ejca.2015.12.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.