Abstract
Regulatory T cells (Treg) and myeloid-derived suppressor cells (MDSC) are the two important and interactive immunosuppressive components of the tumor microenvironment that hamper anti-tumor immune responses. Therefore, targeting these two populations together might be beneficial for overcoming immune suppression in the tumor microenvironment. We have recently shown that prophylactic Foxp3 DNA/recombinant protein vaccine (Foxp3 vaccine) promotes immunity against Treg in tumor-free conditions. In the present study, we investigated the immune modulatory effects of a prophylactic regimen of the redesigned Foxp3 vaccine in the B16F10 melanoma model. Our results indicate that Foxp3 vaccination continuously reduces Treg population in both the tumor site and the spleen. Surprisingly, Treg reduction was associated with a significant decrease in the frequency of MDSC, both in the spleen and in the tumor environment. Furthermore, Foxp3 vaccination resulted in a significant reduction of arginase-1(Arg-1)-induced nitric oxide synthase (iNOS), reactive oxygen species (ROS) and suppressed MDSC activity. Moreover, this concurrent depletion restored production of inflammatory cytokine IFN-γ and enhanced tumor-specific CTL response, which subsequently resulted in the reduction of tumor growth and the improved survival rate of vaccinated mice. In conclusion, our results revealed that Foxp3 vaccine promotes an immune response against tumor by targeting both Treg and MDSC, which could be exploited as a potential immunotherapy approach.
Keywords: Regulatory T cells, Myeloid-derived suppressor cells, Foxp3, Melanoma
Introduction
A growing body of evidence suggests that infiltrated immunosuppressive cells in the tumor microenvironment, such as regulatory T cells (Treg) and myeloid-derived suppressor cells (MDSC), hinder anti-tumor immune responses. In healthy individuals, Treg modulate many aspects of immune response, such as tolerance to self-antigen and prevention of autoimmune diseases [1]. These cells are a highly immunosuppressive subset of CD4+ T cells characterized by expression of the master regulatory transcription factor Foxp3 and high expression of IL-2 receptor α chain (CD25) [2]. In mice, according to the origin and suppressive function, Treg are classified into different subsets [3]. The pivotal role of Treg in the inhibition of anti-tumor immune responses is widely investigated. Treg are expanded at the tumor sites in various experimental models, and their presence is associated with higher tumor burden and poor prognosis, but their depletion promotes anti-tumor immune responses [4–6].
In addition to Treg, the failure of anti-tumor T-cell response also depends on the presence of MDSC, which increase in virtually all types of animal tumor models and human cancers. In mouse models of tumors, MDSC are characterized by the expression of granulocyte differentiation marker 1 (Gr-1) and CD11b. According to the expression of Gr-1 subsets (Ly6G and Ly6C), MDSC are classified into granulocytic (Ly6G+Ly6C−) and monocytic (Ly6Glow/–Ly6C+) subpopulations; however, these phenotypic markers are not completely able to define these cells, but are functionally associated with immunosuppressive mechanisms, such as reactive oxygen species (ROS), inducible nitric oxide synthase (iNOS), and arginase-1 (Arg-1) [7, 8].
Treg, together with MDSC, are the essential cells that contribute to the immunosuppressive network in the tumor site, and the close interactions between these cells profoundly enhance tumor progression and metastasis [9]. The previous studies have determined that MDSC can induce Foxp3+Treg [10]. On the other hand, Treg have the ability to promote the suppressive activity of MDSC, which represents the impact of cross interactions in tumor growth [11]. Therefore, targeting both MDSC and Treg could be a promising approach to ablate the immunosuppressive network in the tumor microenvironment and provide enhanced anti-tumor immune responses.
Focusing on surface markers, numerous attempts have been employed to reduce the number of Treg [12–14]; however, these therapeutic strategies have been demonstrated to be only marginally successful [15]. An alternative approach involves targeting transcription factor Foxp3 due to its essential role in Treg suppressive function. The previous studies have reported that dendritic cells transfected with Foxp3 mRNA can induce CTL response against Foxp3 in melanoma-bearing mice [16]. Although this attractive strategy is robust and effective in depleting Foxp3+Treg, elicitation of active and more comprehensive therapeutic approaches that target Treg through Foxp3 may provide more effective protection.
Over the past two decades, DNA vaccination has become a powerful immunotherapy to induce humoral and cellular immune responses against encoded antigens in tumor models [17, 18]. In addition, DNA vaccination has advantages over other cancer therapeutic approaches; it can induce the immune system against itself and weakly immunologic antigens such as transcription factors by facilitating their MHC class I presentation [19, 20]. Therefore, we produced a DNA vaccine against Foxp3 that includes a plasmid-encoding truncated Foxp3 gene and Foxp3 recombinant protein in a prime-boost regimen. We have recently shown that our novel Foxp3 DNA/recombinant protein vaccine has the ability to reduce the accumulation of CD4+Foxp3+Treg through the induction of CTL. This vaccine also potently induces humoral immune responses [21, 22]. In this regard, here, we reproduced our DNA vaccine, which is tested in the B16F10 melanoma model to evaluate not only the accumulation of Treg but also to determine the effect of Treg depletion on the frequency and functions of MDSC and anti-tumor T-cell response.
Materials and methods
Construction of plasmids
The plasmid pSP73-Spf/Foxp3/A64 encoding Foxp3 mRNA without nuclear localization signal sequence was kindly provided by E. Gilboa (Miami, USA). The plasmid pEGFP-N1 under control of cytomegalovirus promoter and the prokaryotic expression vector pET24a+ carrying (poly)-histidine-tagged (His-tag) sequence for protein detection and purification (purchased from National Gene Bank, Pasteur Institute of Iran), both containing kanamycin resistance gene, were used as the backbone for the DNA vaccine. According to distinct plasmids for DNA and recombinant protein fractions, two pairs of primers were designed to obtain the sequence-encoding truncated Foxp3 from pSP73-Spf/Foxp3/A64 plasmid using PCR. The PCR products using the Kozak/HindIII 5′-TAT AAGCTT GCCACCATGGCTCCTTCCTTGGCCCT-3′ (forward) and KpnI 5′-CTTGGTACC AGGGCGAACATGCGAGTAAAC-3′ (reverse) primers were inserted into the pEGFP-N1 plasmid using T4 DNA ligase to construct the Fox-GFP vector. Subsequently, the products using the EcoRI 5′-TATGAATTCGCTCCTTCCTTGGCCC-3′ (forward) and XhoI 5′-CTTCTCGAG GGCGAACATGCGAG-3′ (reverse) primers were inserted into pET24a+ and ligated to generate Fox-pET expression plasmid. The Fox-GFP and Fox-pET plasmids were transformed and amplified in DH5α and BL21 (DE3) competent Escherichia coli (E. coli), respectively, and then confirmed through kanamycin resistance, colony PCR, and digestion with restriction enzymes. Finally, constructs were purified by Plasmid Mini Kit (Qiagen, Germany) and verified through DNA sequencing.
EndoFree plasmid purification for immunization
The constructed plasmid Fox-GFP and the plasmid pEGFP-N1, as a control vector that was cloned into DH5α E. coli, were purified with EndoFree Plasmid Purification Giga Kit according to the manufacturer’s instructions (Qiagen). DNA concentration was determined using a Nanodrop analyzer (Thermo Fisher, USA).
Expression and purification of Foxp3 recombinant protein
The procedure for expression and purification of Foxp3 recombinant protein in BL21-transformed Fox-pET cells was previously explained in detail [21]. To confirm Foxp3 recombinant protein expression, SDS-PAGE was performed on BL21 E. coli transformed with the Fox-pET plasmid. The Foxp3-His-tag recombinant protein was purified using nickel–nitrilotriacetic acid (Ni–NTA) affinity chromatography according to the manufacturer’s instructions (Qiagen, Germany). The samples of different steps of purification were analyzed using SDS-PAGE. Purified protein was dialyzed using Slide-A-Lyzer dialysis cassette (10 K, Thermo Fisher, USA) for 48 h in PBS (pH 7.4), to prevent protein aggregation, and Western blot analysis was performed on dialyzed Foxp3-His-tag protein using anti-His-tag HRP-conjugated antibody (Sigma, USA). The protein concentration was measured using Bicinchoninic Acid (BCA) Protein Assay Kit (Thermo Fisher).
Animals and tumor cell line
Five-week-old C57BL/6 female mice were purchased from Pasteur Institute (Tehran, Iran) and were housed under standard laboratory conditions in a temperature controlled and light-cycled facility.
Mouse melanoma cell line B16F10 was purchased from Cell Bank of Iran (Pasteur Institute, Tehran, Iran) and maintained in RPMI 1640 medium (R10, Gibco, USA) supplemented with 10% FBS (Gibco), 2 mM l-glutamine (Gibco), 100 U/ml penicillin and 100 μg/ml streptomycin (Gibco) at 37 °C under a humidified atmosphere of 5% CO2.
The B16F10 lysate at 107 tumor cells/ml was prepared by repeated rounds of freezing in liquid nitrogen and thawing in a 37 °C water bath. The concentration of cell line lysate was measured using a BCA protein assay.
Prophylactic immunization
Mice were randomly assigned to Foxp3-vaccinated and control groups (n = 11 per group). For prophylactic vaccination, the vaccinated group mice were injected i.m. with 100 μg of Fox-GFP plasmid suspended in 100 μl of sterile PBS into the upper leg muscle on day 38 before tumor challenge. The control group mice were injected with 100 µg of pEGFP-N1, as the control vector. On day − 24, the vaccinated mice were injected i.d. into the loose skin over the neck with 20 µg of recombinant Foxp3 protein emulsified in incomplete Freund’s adjuvant (IFA, Sigma), as the primary injection. Two weeks later, these mice were given a boost injection with 20 µg of recombinant Foxp3 protein emulsified in IFA. The control group was given 20 µg of molecular grade BSA emulsified in IFA at each indicated timepoint. Three mice from each group were killed 1 week after the last immunization to assess the effect of the vaccine on the frequency of CD4+Foxp3+ T cells.
Tumor challenge
Ten days following the last immunization, both Foxp3-vaccinated and control groups were s.c. inoculated with 5 × 105 B16F10 melanoma cell line (in 200 µl of RPMI medium) in the right flank. When the tumor was palpable, tumor area (mm2) was measured every other day using digital calipers. The survival of tumor-bearing mice was monitored daily. Thirteen days after tumor challenge, three mice from each group were sacrificed and spleen and tumor tissues were used for further analysis.
Spleen and tumor tissue cell isolation
The cells from spleen and tumor tissues were isolated, as previously described [23]. Briefly, mechanically dissociated spleens were treated with RBC lysis buffer (0.15 mM NH4Cl, 10 mM KHCO3, 0.1 mM disodium EDTA, pH 7.2) and splenocytes were suspended in R10 media. Tumor tissues were minced and then digested with 1 mg/ml of collagenase IV and 500 µg/ml of DNase (Roche, Germany) at 37 °C for 30 min followed by passing through 50 μm nylon mesh. Dead cells were removed by centrifugation over Ficoll–Hypaque gradients (Lymphodex, InnoTrain, Germany).
Immunophenotyping by flow cytometry
The following antibodies and their relevant isotype controls were purchased from BioLegend (USA): Gr-1-APC (clone RB6-8C5), CD11b-PE (clone M1/70), CD4-APC (clone GK 1.5), and Foxp3-Alexa Fluor 488 (clone MF-14). For cell surface staining, splenocytes and tumor cell suspensions were first incubated with FcR blocker (Miltenyi Biotec, Germany) and then with the above-mentioned antibodies. For intracellular staining, cells were fixed and permeabilized using Foxp3 fix/perm buffer (BioLegend) following cell surface staining. Cells were then incubated with anti-Foxp3 antibody and analyzed using FACS Calibur flow cytometry (BD Biosciences).
Intracellular IFN-γ assay
Splenic cells (106/200 µl R10) were stimulated with or without PMA (20 ng/ml, Sigma, USA) and ionomycin (2 µg/ml, Sigma, USA) in the presence of brefeldin (2 µl/ml, BioLegend) and/or monensin (2 µl/ml, BioLegend) for 6 h at 37 °C. Cells were then harvested and stained for CD8-FITC surface marker followed by IFN-γ-APC intracellular staining after fixation with fix/perm solution (BioLegend). The expression levels of IFN-γ in CD8+ T cells were assessed using FACS Calibur flow cytometry.
CD107a cytotoxicity assay
Splenocytes (106/200 µl R10) were stimulated with 200 µg/ml of B16F10 cell line lysate in the presence of CD107a-APC antibody (BioLegend) and monensin (BioLegend) in 96-well round bottom culture plates at 37 °C for 6 h. Harvested cells were then surface stained with CD8-FITC antibody to detect the production of CD107a in CTL using FACS Calibur flow cytometry.
MDSC and ROS
The expression level of ROS in Gr-1+CD11b+ cells was measured, as previously described [23]. Briefly, tumor-infiltrated immune cells and splenocytes suspended in PBS were incubated with 5 μM 2′, 7′-dichlorodihydrofluorescein diacetate (DCFDA, Sigma, USA) at 37 °C for 30 min. Cells were then labeled with Gr-1-APC and CD11b-PE, and the level of ROS in Gr-1+CD11b+ cells was detected using FACS Calibur flow cytometry.
Suppression assay
Gr-1+ cells were purified from splenocytes following the manufacturer’s instructions (Miltenyi Biotec). The suppression assay was performed, as previously described, with slight modification [23]. Briefly, nylon wool-purified naïve T cells (105 cells/well) from a healthy adult C57BL/6 mouse were labeled with carboxy fluorescein succinimidyl ester (CFSE) and co-cultured with Gr-1+ cells (T cells/Gr-1+ cells = 2:1) (5 × 104 cells/well) from Foxp3-vaccinated or control mice in the presence of soluble anti-CD3 (1 μg/ml) and anti-CD28 (5 μg/ml, Biolegend). Unstimulated and stimulated naïve T cells served as negative and positive controls (Co− and Co+), respectively. After 3 days, the supernatants were collected and the cells were harvested and stained with CD3-APC to analyze CFSE dilution using FACS Calibur flow cytometry. To assess the percentage of MDSC suppression, the proliferation index (PI) of samples was calculated by the following formula:
Nitrite production assay
The nitrite concentration in the co-culture supernatant was assessed by standard Griess reaction, as previously described [24].
RNA extraction and gene expression by real-time PCR
Total RNA was extracted from splenocytes and tumor tissues using RNXplus (Sinaclon, Iran), according to the manufacturer’s instructions. Then, cDNA was synthesized using two-step RT-PCR Kit (Vivantis, Malaysia). Real-time PCR was performed on Applied Biosystems Plus1 using Solis BioDyne 5 × HOT FIREPOL EvaGreen qPCR Mix Plus (ROX, Estonia), according to the manufacturer’s instructions using the following conditions: 95 °C for 15 min, and 95 °C for 15 s, 60 °C for 20 s, and 72 °C for 20 s for 40 cycles followed by 72 °C for 10 min. The sequences of primers were as follows: Arg-1 (forward) 5′-AAGAAAAGGCCGATTCACCT-3′; 5′-CACCTCCTCTGCTGTCTTCC-3′ (reverse); iNOS (forward) 5′-AGTGGTGTTCTTTGCTTC-3′; 5′-GCTTGCCTTATACTGGTC-3′ (reverse); Foxp3 (forward) 5′-GCAGGGCAGCTAGGTATCTGTAG-3′ 5′-TCGGAGATCCCCTTTGTCTTATC-3′ (reverse); β-actin (forward) 5′TGGGCTTACCTCACTGCTTTC 3′, 5′TCATCGCTAATCACGACGCT 3′ (reverse). The level of mRNA in each sample was quantified using the 2−ΔΔct method.
Statistical analysis
Statistical analyses were performed using the GraphPad Prism 6 software. Nonparametric means comparisons between two groups were conducted using Mann–Whitney U test. Survival data were analyzed by log-rank test. All flow cytometry data analyses were performed using the FlowJo software.
Results
Foxp3 DNA and recombinant protein generation
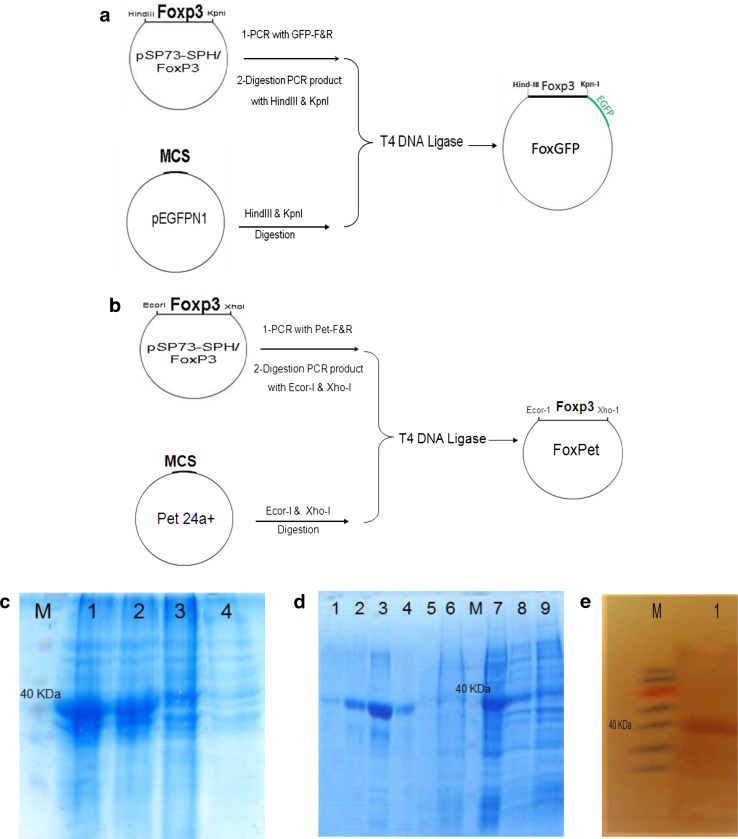
The Fox-GFP and Fox-pET vectors encoding function-inactivated Foxp3 were constructed, as schematically shown in Fig. 1a, b, respectively. Colony PCR assays and restriction enzyme digestion followed by sequence analysis confirmed the construction of the vectors (data not shown).
Fig. 1.
Construction and generation of Foxp3 plasmids and recombinant protein. a, b Schematic representation of the construction of Fox-GFP and Fox-pET plasmids is shown, respectively. c SDS-PAGE analysis of an Foxp3 recombinant protein expressed in BL21 E. coli. M, protein marker; lanes 1 and 2, Fox-pET-transfected BL21 E. coli cells 3 h after IPTG induction; lane 3, Fox-pET-transfected BL21 E. coli cells before induction; lane 4, non-transfected BL21 E. coli cells. d SDS-PAGE analysis of various steps of Foxp3 recombinant protein purification using Ni–NTA resin. Lanes 1–4, final fraction elution of the column (pH 4.5) consists of Foxp3 recombinant protein; lane 5, flow through of the column at pH 6.3; lane 6, flow through of the column at pH 8; M, protein marker; lane 7, Fox-Pet-transfected BL21 E. coli lysate before Ni–NTA resin mixing; lane 8, transfected BL21 E. coli after IPTG induction; and lane 9, transfected BL21 E. coli before IPTG induction. e Western blot analysis of the pooled fraction 2 and 3 elution (pH 4.5) from the Ni–NTA column (lane 1) and protein marker (M) represents the band at the molecular weight of approximately 40 kDa. IPTG isopropyl β d-thiogalactoside
To evaluate the expression of recombinant protein Foxp3, SDS-PAGE was performed using Fox-pET-transformed BL21 E. coli cell lysates. The expression of the Foxp3 protein was detected as approximately 42 kDa (Fig. 1c). To confirm Foxp3 recombinant protein purification, SDS-PAGE was performed using eluted fractions from different steps of Ni–NTA affinity chromatography (Fig. 1d). Finally, Western blot analysis was done on dialyzed fractions of 2 and 3 purified from Ni–NTA using anti-His-tag antibody, where a clear band corresponding to the molecular mass of Foxp3 recombinant protein (42 kDa) was detected (Fig. 1e).
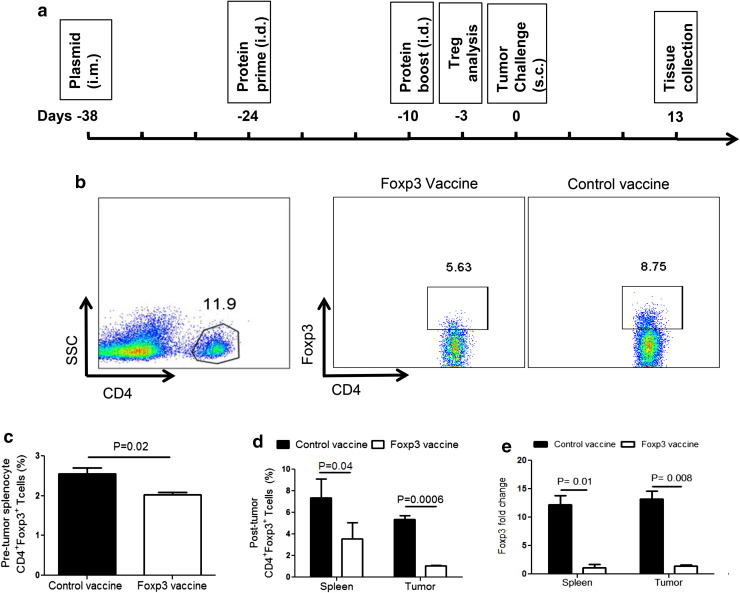
Foxp3 vaccine consistently reduces CD4+Foxp3+ Treg in B16F10 tumor
Transcription factor Foxp3 is the master regulator for suppressive activities of Treg. Thus, targeting Foxp3 could be an alternative approach to mitigate the adverse effects of Treg in tumors. We have recently shown that prophylactic Foxp3 vaccine reduces Treg in splenocytes of tumor-free mice [21]. In this regard, we investigated whether this vaccine could also reduce the number of Treg in melanoma-bearing mice. To address this question, C57BL/6 mice were immunized with Foxp3 DNA and recombinant protein in a protocol schematically indicated in Fig. 2a. We initially reassessed the impact of the Foxp3 vaccine on Treg before tumor challenge. Treg frequency was determined by CD4+Foxp3+ lymphocytes in splenocytes and tumor samples using flow cytometry, as depicted in Fig. 2b. As expected, the Foxp3 vaccine reduced the frequency of Treg in splenocytes of vaccinated mice 3 days before tumor challenge (Fig. 2c). We then investigated the impact of the Foxp3 vaccine on Treg in tumor conditions and revealed a continuous reduction in the number of Treg 13 days after tumor challenge in comparison with control mice. Surprisingly, this reduction was more significant in tumor-infiltrated immune cells than splenocytes (Fig. 2d). We also investigated the expression levels of Foxp3 gene by real-time PCR. In agreement with the flow cytometry data, we found a significant reduction in Foxp3 gene expression in both the tumor site and splenocytes (Fig. 2e). Of note, we did not observe any adverse autoimmune reactions such as vitiligo following vaccination. These data indicate that Foxp3 vaccination results in a consistent decrease in the frequency of CD4+Foxp3+ Treg even in tumor-bearing mice.
Fig. 2.
Foxp3 vaccine reduces CD4+Foxp3+ Treg. a Schematic representation of vaccination and tumor challenge timeline. C57BL/6 mice (n = 11 per group) were immunized i.m. with pEGFP-N1 or Fox-GFP plasmids on day − 38. Mice were then injected i.d. with molecular grade bovine serum albumin or Foxp3 recombinant protein emulsified in IFA on days − 24 and − 10, as a prime-boost regimen. A week after the boost injection, mice (n = 3 per group) was sacrificed and examined for the frequency of CD4+Foxp3+ T cells in splenocytes before tumor challenge. On day 0, B16F10 tumor cell line was injected s.c., and on day 13, the final analyses were assessed on spleens and tumors of mice (n = 3 mice per group). b Representative dot plots showing the frequency of CD4+Foxp3+ T cells in splenocytes and cells isolated from tumor tissues. c Percentages of CD4+Foxp3+ T cells in splenocytes before tumor challenge. d Percentages of CD4+Foxp3+ T cells in spleen and tumor tissue obtained from Foxp3 vaccinated versus control mice 13 day post-tumor challenge. e Foxp3 gene expression in splenocytes and tumor tissues of Foxp3 vaccinated versus control mice 13 day post-tumor challenge (β-actin was used as housekeeping gene). Data represent mean ± one standard error of two independent experiments
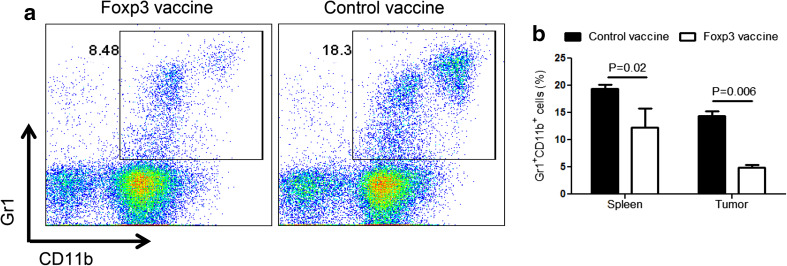
Foxp3 vaccine reduces MDSC in melanoma-bearing mice
The previous reports have revealed a close interaction between MDSC and Treg, thereby leading to the expansion of Treg [10]. To investigate whether targeting Treg with the Foxp3 vaccine could impact the MDSC populations, the frequency of Gr1+CD11b+ cells in splenocytes and tumor tissues 13 days after tumor challenge was measured. Remarkably, compared with control mice, we found a significant decrease in the proportion of Gr-1+CD11b+ cells in the splenocytes of Foxp3-vaccinated mice (Fig. 3a). More importantly, this reduction was more significant in the tumor site (Fig. 3b). Therefore, targeting Treg, using Foxp3 vaccine, results in the simultaneous reduction of MDSC in the spleen and the tumor site of melanoma-bearing mice.
Fig. 3.
Foxp3 vaccine reduces MDSC. The splenocytes and tumor cells from Foxp3-vaccinated or control mice 13 day post-tumor challenge were analyzed for Gr-1 and CD11b using flow cytometry (n = 6 mice per group). a Representative dot plots indicating Gr1+CD11b+ cells in Foxp3 vaccinated versus control mice. b Percentages of Gr1+CD11b+ cells in splenocytes versus tumor cells. Data represent mean ± one standard error of two independent experiments
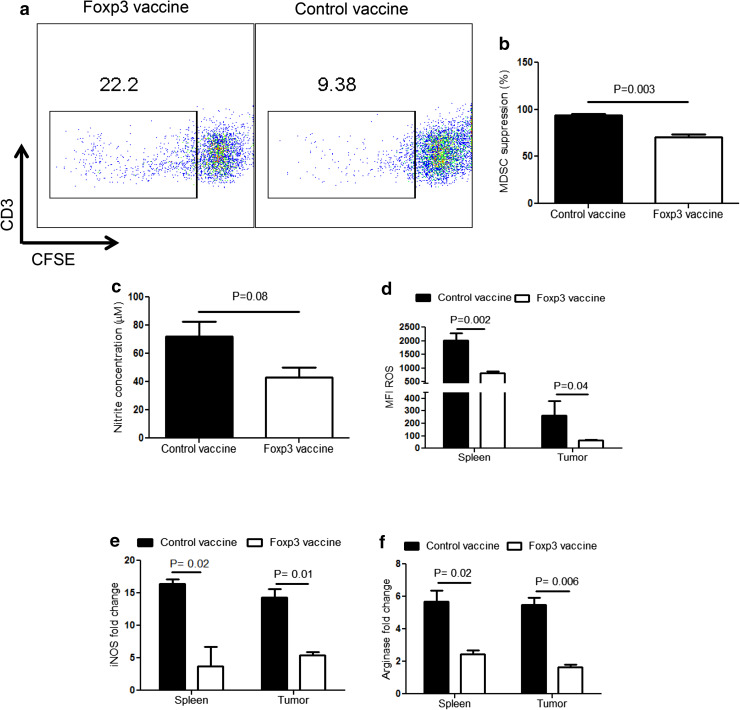
Foxp3 vaccine mitigates the suppressive activities of MDSC
We further evaluated the modulatory effects of the Foxp3 vaccine on the suppressive properties of MDSC. To this end, the in vitro suppressing capacity of MDSC isolated from Foxp3 and control-vaccinated mice was assessed on stimulated naïve T cells. As depicted in Fig. 4a, b, MDSC isolated from Foxp3-vaccinated mice exhibited significantly less suppressive effects on stimulated T cells than control-vaccinated mice. This reduction was also confirmed by the lower level of nitrite in the co-culture supernatants of the Foxp3-vaccinated group; however, the difference was not significant (Fig. 4c). Moreover, we observed that Foxp3 vaccine reduced the production of ROS by MDSC obtained from either splenocytes or tumor tissues (Fig. 4d). Finally, we found that the gene expression levels of both iNOS (Fig. 4e) and Arg-1 (Fig. 4f) were significantly reduced in splenocytes and tumor tissues. Collectively, these data illustrate the ability of Foxp3 vaccination to modulate the suppressive functions of MDSC in melanoma conditions.
Fig. 4.
Foxp3 vaccine strongly modulates the suppressive mechanisms of MDSC. Gr1+ cells isolated from splenocytes of Foxp3-vaccinated or control mice 13 day post-tumor challenge and CFSE-labeled naïve T cells isolated from splenocytes of healthy C57BL/6 mice were co-cultured, as described in the “Materials and methods” (n = 6 mice per group). a Representative dot plots showing percent proliferation of CFSE-labeled T cells in the presence of MDSC isolated from Foxp3-vaccinated versus control mice. b Percentages of splenocyte MDSC mediated suppression from Foxp3-vaccinated versus control mice. c Nitrite levels recovered from the supernatant of MDSC-T-cell co-culture. d MFI for ROS among Gr1+CD11b+ cells in splenocytes and tumor-infiltrated cells obtained from Foxp3-vaccinated and control mice. e, f Expression of iNOS and Arg-1 genes in the splenocytes and tumor tissue of Foxp3-vaccinated versus control mice. β-actin was used as housekeeping gene. Data represent mean ± one standard error of two independent experiments. MFI mean fluorescence intensity
Foxp3 vaccine induces anti-tumor T-cell responses
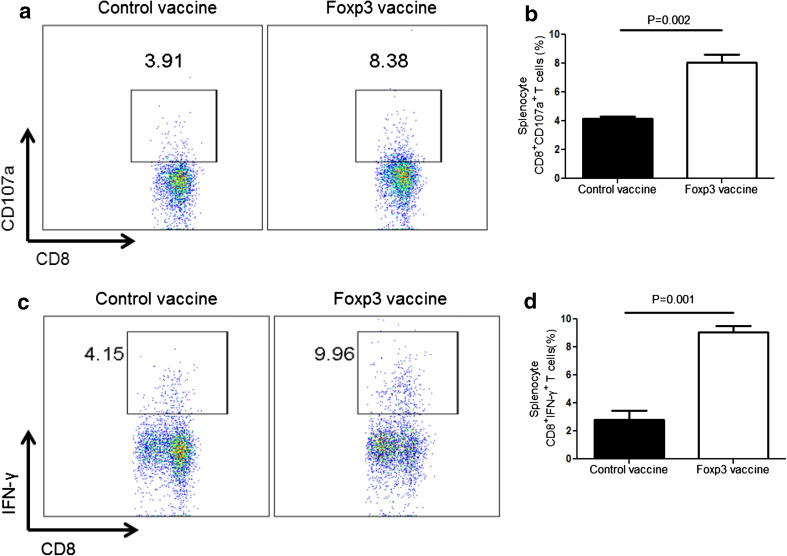
We next investigated whether the Foxp3 vaccine could enhance anti-tumor T-cell response following depletion of these immunosuppressive cells. Therefore, the production levels of CD107a and intracellular expression of IFN-γ in CD8+ T cells were measured in splenocytes. CD107a is the marker of lysosomal membrane and presented on the surface of cytotoxic T cells following perforin and granzyme secretion. We observed that compared to the controls, CD8+ T cells from Foxp3-vaccinated mice express higher levels of CD107a (Fig. 5a, b). Furthermore, we analyzed the anti-tumor activity of CTLs by IFN-γ production. We found that compared to the control group, depletion of Treg, following Foxp3 vaccination, results in enhanced IFN-γ production by CD8+ T cells from the vaccinated group (Fig. 5c, d). Taken together, these data demonstrate that Foxp3 vaccine improves CTL responses in the melanoma model.
Fig. 5.
Foxp3 vaccine promotes CTL responses. Splenocytes from Foxp3-vaccinated and control mice were analyzed for CD107a and IFN-γ expression in CD8+ T cells, as described in the “Materials and methods”. a Representative dot plots showing percent CD8+CD107a+ T cells from the Foxp3-vaccinated and control mice. b Percentages of CD8+CD107a+ T cells in Foxp3-vaccinated versus control mice. c Representative dot plots showing percentages of CD8+ IFN-γ+ T cells from the Foxp3-vaccinated and control mice. d Percentages of CD8+ IFN-γ+ T cells in Foxp3-vaccinated versus control mice (n = 6 mice per group). Data represent mean ± one standard error of two independent experiments
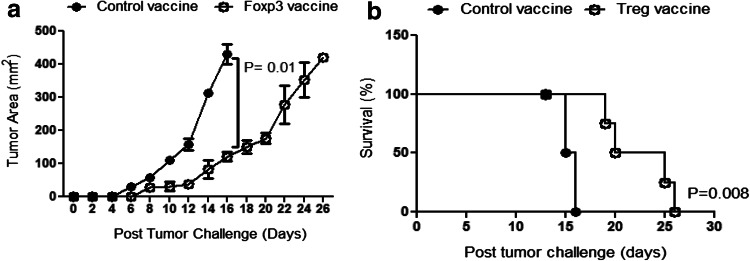
Vaccination against Foxp3 delays tumor growth and increases animal survival
Based on the enhanced CTL response, following Foxp3 vaccination, we aimed to determine the effects of this vaccine regimen on tumor growth and survival rate. Interestingly, compared to controls at day 16 after tumor challenge, we found a significant reduction in the tumor size in the Foxp3-vaccinated group (Fig. 6a). Although all the mice finally died, the survival of Foxp3-vaccinated mice was significantly increased compared to the controls (Fig. 6b). These data indicate that Foxp3 vaccination modulates the aggressive growth of melanoma in the animal model.
Fig. 6.
Foxp3 vaccine reduces the rate of tumor growth and enhances mice survival. a Rate of tumor growth reported in mm2 is shown. b Kaplan–Meier curve represents the percentage of mice survival in each group (n = 5 mice per group). Data represent mean ± one standard error of two independent experiments
Discussion
Tumor stroma cells produce various mediators that recruit versatile immunosuppressive cells into the tumor microenvironment. Treg and MDSC are recognized as the main tumor-infiltrating immunosuppressive cells and their constitutive interactions generate an immunosuppressive network, thereby enhancing the tumor progression in a protected site [8, 25]. Therefore, identification of a feasible approach to target these cells may promote anti-tumor immune responses. Various groups have already demonstrated that targeting key Treg markers could overcome tumor immunosuppression [13, 26, 27]. Among these markers, Foxp3 is the most important marker expressed in the thymus during central tolerance and plays an essential role in detection and function of Treg in the periphery [2, 28]. Moreover, it has been shown that Foxp3 can also be processed and presented as an endogenous antigen by MHC class I molecules, which finally leads to the induction of CTL [29]. It has been reported that some tumor cell lines also present this protein on their surfaces [30].
DNA vaccines have been introduced as one of the effective ways to enhance anti-tumor immune responses through cross presentation [18]. Our prophylactic DNA vaccine includes a plasmid-encoding truncated Foxp3 gene and Foxp3 recombinant protein emulsified in IFA in a prime-boost regimen. This vaccine was supposedly processed via MHC class I and class II pathways to induce CTL via cross presentation.
Our group has recently reported the modulatory effects of the Foxp3 vaccine on the frequency of Treg in tumor-free mice [21]. In the present study, we demonstrated that the prophylactic Foxp3 vaccine reduces accumulation of CD4+Foxp3+Treg in both the periphery and the tumor microenvironment. Notably, we found that Foxp3 vaccine significantly influenced tumor recruiting than circulating Treg. Depleting the tumor-infiltrated Treg has shown to be an effective strategy to eradicate tumors and Foxp3 is a specific marker for tumor-infiltrating Treg [31, 32]. The previous studies using transgenic mice, in which the expression of diphtheria toxin receptor is under the control of Foxp3 gene, have determined that targeting Foxp3 leads to depletion of tumor-infiltrating Treg [33]. Furthermore, Larsen et al. described CTL in cutaneous T-cell lymphoma patients who were enhanced against Foxp3 antigen. Notably, most malignant T cells in this type of cancer are Treg, which express Foxp3 in the context of MHC class I and could be detected and killed by CTL [34]. Recently, it was reported that compared with wild-type B16F10 cell line, reduction of Foxp3 expression on B16F10 murine melanoma cell line using siRNA resulted in reduced CD4+CD25+Foxp3+ Treg and improved tumor burden [35, 36]. Nair et al. have also shown that transfection of mice dendritic cells with Foxp3 mRNA in melanoma model induces CD8+ T cells against Foxp3 [16]. This group did not note any autoimmune disease, which is consistent with our observations. Thus, our results suggest that Foxp3 vaccine depletes mostly tumor-infiltrating rather than naturally occurring Treg, which play an essential role in immune hemostasis. However, the mechanism(s) by which Foxp3 vaccine differentiates these two Treg populations are unknown and merits further investigation.
Apart from Treg, MDSC represent another major cell population involved in tumor progression. Induction of Treg has been recognized as one of the main immunosuppressive mechanisms mediated by MDSC [7, 10, 37]. On the other hand, recent studies have determined that Treg promote the suppressive activity of MDSC [11]. These studies demonstrated a tight interaction between MDSC and Treg, thereby creating a network for tumor progression. The previous reports have shown a reduction in Treg following depletion of MDSC, which emphasizes the crosstalk between these immunosuppressive cells [37]. Therefore, we investigated the effects of the Foxp3 vaccine on MDSC and found, for the first time, that our Foxp3 vaccine reduces the accumulation of both MDSC and Treg in both the spleen and the tumor site. Moreover, this reduction was more prominent in the tumor site than the periphery. These results coupled with the greater reduction of Treg in the tumor site provide compelling evidence that the main target of our vaccine is the microenvironment of the tumor where the immunoregulatory network exists.
MDSC have emerged as potentially important regulators of immune response with several immunosuppressive properties. MDSC can be differentiated from mature granulocyte and/or monocyte cells based on these immunosuppressive capabilities, which mediate anti-tumor immune response [38–40]. Although we were unable to investigate different subtypes of MDSC in this study, we have shown that our novel Foxp3 vaccine dampens the expression of ROS, Arg-1 and iNOS as the most important suppressive functions of MDSC. Therefore, we speculate that Foxp3 vaccination results in reduced immunosuppressive activity of MDSC. It has been shown that MDSC have a function, as antigen-presenting cells, at an early timepoint of the tumor challenge [41]. Therefore, there is a possibility that the Foxp3 vaccine might temporarily impact the suppressive capacity of MDSC by interaction with other antigen-presenting cells; however, the cellular and molecular mechanisms underlying these effects remain to be determined.
The immunosuppressive Treg and MDSC mostly target CTL, as the essential players in anti-tumor immune response [40, 42]. We have previously shown the enhancement of Foxp3-specific cytotoxicity in vivo and in vitro in tumor-free mice [21]. Therefore, we assessed if depletion of Treg and MDSC could restore the cytotoxic function of tumor-specific CD8+ T cells in a melanoma model. The expression of the glycoprotein CD107a was analyzed on splenocyte CD8+ T cells to evaluate the cytotoxic activity, since this marker is expressed on the membrane of vesicles after perforin and granzyme exocytosis [43]. Our investigation revealed that when compared to the control vaccine, the cytotoxic activity of melanoma-specific CD8+ T lymphocytes markedly enhanced in response to the Foxp3 vaccine. Furthermore, compared to controls, we found higher levels of IFN-γ in CD8+ T lymphocytes from the Foxp3 vaccine-treated mice, which further confirmed the activation of CTL after depletion of Treg and MDSC. This finding is in agreement with the previous studies that have shown that targeting tumor-infiltrating Treg causes improved systemic CTL response and IFN-γ production in cancer models [32]. Regardless of some limitations in our studies, we demonstrated that the Foxp3 vaccine not only promotes Foxp3-specific CTL, as determined in our previous study, but also indirectly enhances tumor-specific CTL responses through reduction of both Treg and MDSC in the tumor microenvironment.
Depleting tumor-infiltrated Treg and MDSC along with increasing the cytotoxicity of tumor-specific T cells is likely to be essential in tumor immunotherapy. Here, we showed that although our Foxp3 vaccine did not induce complete eradication of the tumor, when compared to control animals, it drastically attenuated the tumor growth in accordance with longer survival of the Foxp3-vaccinated mice. Melanoma is one of the fastest growing tumors with a potent immunosuppressive microenvironment that is resistant to various conventional immunotherapies [44, 45]. There is a possibility that overcoming suppressive network using the Foxp3 vaccine promotes the other suppressive mechanisms that potently support the melanoma growth. Although our data are encouraging, the therapeutic effects of our vaccine in other types of tumor models need to be evaluated. These studies will enable us to better understand the differences in the microenvironment of tumors, which could be the main obstacle for controlling complete tumor growth by the Foxp3 vaccine.
In conclusion, we have provided critical evidence that Foxp3 DNA/recombinant protein vaccine can enhance the immune response in an animal model of melanoma by targeting both Treg and MDSC. We suggest that this vaccine in combination with other immunotherapy approaches, such as a dendritic cell cancer vaccine, has the potential to inhibit tumor growth. Future studies are required to evaluate the capacity of the Foxp3 vaccine and validate targeting Foxp3 for cancer treatment.
Abbreviations
- Arg-1
Arginase-1
- BCA
Bicinchoninic acid
- CFSE
Carboxy fluorescein succinimidyl ester
- DCFDA
2′,7′-Dichlorodihydrofluorescein diacetate
- E. coli
Escherichia coli
- Gr-1
Granulocyte differentiation marker 1
- His-tag
(Poly)-histidine-tagged
- IFA
Incomplete Freund’s adjuvant
- iNOS
Induced nitric oxide synthase
- MDSC
Myeloid-derived suppressor cell(s)
- Ni–NTA
Nickel nitrilotriacetic acid
- ROS
Reactive oxygen species
- Treg
Regulatory T cell(s)
Author contributions
AN performed plasmid and protein preparation, animal studies, flow cytometry analysis, real-time PCR, suppression assay, and nitrite assay, analyzed the data, and wrote the manuscript. RM contributed in flow cytometry analysis and animal studies. AM advised in plasmid design. RB performed intracellular cytokine staining. MS assisted in plasmid and protein preparation. HRM performed Treg staining and CD107a cytotoxic assay. HF performed RNA extraction. MZ performed MDSC staining. KA provided the main construct and co-supervised the designing of plasmids. SE contributed to writing and editing the article. FN advised on plasmid design and data analysis. AR co-supervised the study. JH supervised the study.
Compliance with ethical standards
Funding
The main part of this study was supported by the Iran National Science Foundation (INSF, Grant Number 90007957) and partially by the Isfahan University of Medical Sciences (Grant Number 391146).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Mouse experiments were approved by the animal ethics committee of Tehran University of Medical Sciences (Tehran, Iran).
References
- 1.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155(3):1151–1164. [PubMed] [Google Scholar]
- 2.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6(4):345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 4.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 5.Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9(2):606–612. [PubMed] [Google Scholar]
- 6.Viehl CT, Moore TT, Liyanage UK, Frey DM, Ehlers JP, Eberlein TJ, et al. Depletion of CD4+CD25+ regulatory T cells promotes a tumor-specific immune response in pancreas cancer-bearing mice. Ann Surg Oncol. 2006;13(9):1252–1258. doi: 10.1245/s10434-006-9015-y. [DOI] [PubMed] [Google Scholar]
- 7.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12(4):253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindau D, Gielen P, Kroesen M, Wesseling P, Adema GJ. The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology. 2013;138(2):105–115. doi: 10.1111/imm.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66(2):1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 11.Fujimura T, Ring S, Umansky V, Mahnke K, Enk AH. Regulatory T cells stimulate B7-H1 expression in myeloid-derived suppressor cells in ret melanomas. J Investig Dermatol. 2012;132(4):1239–1246. doi: 10.1038/jid.2011.416. [DOI] [PubMed] [Google Scholar]
- 12.Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, Sabzevari H. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105(7):2862–2868. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- 13.Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. 1999;59(13):3128–3133. [PubMed] [Google Scholar]
- 14.Foss FM. DAB(389)IL-2 (ONTAK): a novel fusion toxin therapy for lymphoma. Clin Lymphoma. 2000;1(2):110–116. doi: 10.3816/CLM.2000.n.009. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H, Chua KS, Guimond M, Kapoor V, Brown MV, Fleisher TA, et al. Lymphopenia and interleukin-2 therapy alter homeostasis of CD4+CD25+ regulatory T cells. Nat Med. 2005;11(11):1238–1243. doi: 10.1038/nm1312. [DOI] [PubMed] [Google Scholar]
- 16.Nair S, Boczkowski D, Fassnacht M, Pisetsky D, Gilboa E. Vaccination against the forkhead family transcription factor Foxp3 enhances tumor immunity. Cancer Res. 2007;67(1):371–380. doi: 10.1158/0008-5472.CAN-06-2903. [DOI] [PubMed] [Google Scholar]
- 17.Stevenson FK. DNA vaccines against cancer: from genes to therapy. Ann Oncol. 1999;10(12):1413–1418. doi: 10.1023/A:1008395012716. [DOI] [PubMed] [Google Scholar]
- 18.Stevenson FK, Ottensmeier CH, Rice J. DNA vaccines against cancer come of age. Curr Opin Immunol. 2010;22(2):264–270. doi: 10.1016/j.coi.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 19.Kyutoku M, Nakagami H, Koriyama H, Tomioka H, Nakagami F, Shimamura M, et al. Development of novel DNA vaccine for VEGF in murine cancer model. Sci Rep. 2013;3:3380. doi: 10.1038/srep03380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu S, Shi D, Wang HC, Yu YZ, Xu Q, Sun ZW. Co-immunization with DNA and protein mixture: a safe and efficacious immunotherapeutic strategy for Alzheimer’s disease in PDAPP mice. Sci Rep. 2015;5:7771. doi: 10.1038/srep07771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mousavi Niri N, Memarnejadian A, Pilehvar-Soltanahmadi Y, Agha Sadeghi M, Mahdavi M, Kheshtchin N, et al. Improved anti-Treg vaccination targeting Foxp3 efficiently decreases regulatory T cells in mice. J Immunother. 2016;39(7):269–275. doi: 10.1097/CJI.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 22.Niri NM, Hadjati J, Sadat M, Memarnejadian A, Aghasadeghi M, Akbarzadeh A, et al. Inducing humoral immune responses against regulatory T cells by Foxp3-Fc(IgG) fusion protein. Monoclon Antib Immunodiagn Immunother. 2015;34(6):381–385. doi: 10.1089/mab.2015.0048. [DOI] [PubMed] [Google Scholar]
- 23.Namdar A, Mirzaei HR, Jadidi-Niaragh F, Ashourpour M, Ajami M, Hadjati J, et al. Multiple low doses of 5-fluorouracil diminishes immunosuppression by myeloid derived suppressor cells in murine melanoma model. Iran J Immunol. 2015;12(3):176–187. [PubMed] [Google Scholar]
- 24.Liu Y, Van Ginderachter JA, Brys L, De Baetselier P, Raes G, Geldhof AB. Nitric oxide-independent CTL suppression during tumor progression: association with arginase-producing (M2) myeloid cells. J Immunol. 2003;170(10):5064–5074. doi: 10.4049/jimmunol.170.10.5064. [DOI] [PubMed] [Google Scholar]
- 25.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5(4):263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 26.Sutmuller RP, van Duivenvoorde LM, van Elsas A, Schumacher TN, Wildenberg ME, Allison JP, et al. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194(6):823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coe D, Begom S, Addey C, White M, Dyson J, Chai JG. Depletion of regulatory T cells by anti-GITR mAb as a novel mechanism for cancer immunotherapy. Cancer Immunol Immunother. 2010;59(9):1367–1377. doi: 10.1007/s00262-010-0866-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hori S, Sakaguchi S. Foxp3: a critical regulator of the development and function of regulatory T cells. Microbes Infect. 2004;6(8):745–751. doi: 10.1016/j.micinf.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 29.Andersen MH. FOXP3-specific immunity. Oncoimmunology. 2013;2(10):e26247. doi: 10.4161/onci.26247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ebert LM, Tan BS, Browning J, Svobodova S, Russell SE, Kirkpatrick N, et al. The regulatory T cell-associated transcription factor FoxP3 is expressed by tumor cells. Cancer Res. 2008;68(8):3001–3009. doi: 10.1158/0008-5472.CAN-07-5664. [DOI] [PubMed] [Google Scholar]
- 31.Ahmadzadeh M, Felipe-Silva A, Heemskerk B, Powell DJ, Jr, Wunderlich JR, Merino MJ, et al. FOXP3 expression accurately defines the population of intratumoral regulatory T cells that selectively accumulate in metastatic melanoma lesions. Blood. 2008;112(13):4953–4960. doi: 10.1182/blood-2008-06-163048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marabelle A, Kohrt H, Sagiv-Barfi I, Ajami B, Axtell RC, Zhou G, et al. Depleting tumor-specific Tregs at a single site eradicates disseminated tumors. J Clin Investig. 2013;123(6):2447–2463. doi: 10.1172/JCI64859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klages K, Mayer CT, Lahl K, Loddenkemper C, Teng MW, Ngiow SF, et al. Selective depletion of Foxp3+ regulatory T cells improves effective therapeutic vaccination against established melanoma. Cancer Res. 2010;70(20):7788–7799. doi: 10.1158/0008-5472.CAN-10-1736. [DOI] [PubMed] [Google Scholar]
- 34.Larsen SK, Munir S, Woetmann A, Frosig TM, Odum N, Svane IM, et al. Functional characterization of Foxp3-specific spontaneous immune responses. Leukemia. 2013;27(12):2332–2340. doi: 10.1038/leu.2013.196. [DOI] [PubMed] [Google Scholar]
- 35.Franco-Molina MA, Miranda-Hernandez DF, Mendoza-Gamboa E, Zapata-Benavides P, Coronado-Cerda EE, Sierra-Rivera CA, et al. Silencing of Foxp3 delays the growth of murine melanomas and modifies the tumor immunosuppressive environment. Onco Targets Ther. 2016;9:243–253. doi: 10.2147/OTT.S90476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miguel A, Sendra L, Noe V, Ciudad CJ, Dasi F, Hervas D, et al. Silencing of Foxp3 enhances the antitumor efficacy of GM-CSF genetically modified tumor cell vaccine against B16 melanoma. Onco Targets Ther. 2017;10:503–514. doi: 10.2147/OTT.S104393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 2008;68(13):5439–5449. doi: 10.1158/0008-5472.CAN-07-6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dolcetti L, Marigo I, Mantelli B, Peranzoni E, Zanovello P, Bronte V. Myeloid-derived suppressor cell role in tumor-related inflammation. Cancer Lett. 2008;267(2):216–225. doi: 10.1016/j.canlet.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 39.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182(8):4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ostrand-Rosenberg S. Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer Immunol Immunother. 2010;59(10):1593–1600. doi: 10.1007/s00262-010-0855-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ko HJ, Lee JM, Kim YJ, Kim YS, Lee KA, Kang CY. Immunosuppressive myeloid-derived suppressor cells can be converted into immunogenic APCs with the help of activated NKT cells: an alternative cell-based antitumor vaccine. J Immunol. 2009;182(4):1818–1828. doi: 10.4049/jimmunol.0802430. [DOI] [PubMed] [Google Scholar]
- 42.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6(4):295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 43.Betts MR, Koup RA. Detection of T-cell degranulation: CD107a and b. Methods Cell Biol. 2004;75:497–512. doi: 10.1016/S0091-679X(04)75020-7. [DOI] [PubMed] [Google Scholar]
- 44.MacKie RM, Hauschild A, Eggermont AM. Epidemiology of invasive cutaneous melanoma. Ann Oncol. 2009;20(Suppl 6):vi1–vi7. doi: 10.1093/annonc/mdp252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garbe C, Peris K, Hauschild A, Saiag P, Middleton M, Spatz A, et al. Diagnosis and treatment of melanoma: European consensus-based interdisciplinary guideline. Eur J Cancer. 2010;46(2):270–283. doi: 10.1016/j.ejca.2009.10.032. [DOI] [PubMed] [Google Scholar]