Abstract
Immune checkpoint inhibition suggests promising progress for the treatment of advanced hepatocellular carcinoma (HCC). However, the underlying cellular mechanisms remain unclear because liver cancer cells apparently do not upregulate inhibitory checkpoint molecules. Here, we analysed whether regulatory T cells (Tregs) can alternatively trigger checkpoint inhibition pathways in HCC. Using flow cytometry we analysed expression of checkpoint molecules (PD-1, PD-L1, CTLA-4, GITR, Tim-3) on peripheral CD4+CD25+Foxp3+ Tregs and their secretion of inhibitory mediators (IL-10, IL-35, TGF-beta, galectin-9) in 116 individuals (50 patients with HCC, 41 non-tumour bearing liver disease controls, 25 healthy controls). Functional activity of Tregs on T effector cells (IFN-gamma production, cytotoxicity) was characterized in vitro using a lectin-dependent cellular cytotoxicity (LDCC) assay against checkpoint inhibitor-negative P815 target cells. Unlike liver patients without malignancy and healthy controls, the frequency of checkpoint inhibitor-positive Tregs inversely correlated to age of patients with HCC (PD-L1, p = 0.0080; CTLA-4, p = 0.0029) and corresponded to enhanced numbers of Tregs producing IL-10 and IL-35 (p < 0.05 each). Tregs inhibited IFN-gamma secretion and cytotoxicity of CD8+ T cells when added to LDCC against P815 cells. Treg-induced inhibition of IFN-gamma secretion could be partially blocked by neutralizing PD-1 and PD-L1 antibodies specifically in HCC patients. In HCC peripheral Tregs upregulate checkpoint inhibitors and contribute to systemic immune dysfunction and antitumoural activity by several inhibitory pathways, presumably facilitating tumour development at young age. Blocking PD-L1/PD-1 interactions in vitro selectively interfered with inhibitory Treg -T effector cell interactions in the patients with HCC and resulted in improved antitumoural activity also against checkpoint inhibitor-negative tumour cells.
Electronic supplementary material
The online version of this article (10.1007/s00262-019-02427-4) contains supplementary material, which is available to authorized users.
Keywords: Hepatocellular carcinoma, CD4+ regulatory T cells, PD-L1/PD-1 checkpoint blockade, IFN-gamma production, T cell degranulation
Introduction
Hepatocellular carcinoma (HCC) has become the fifth most prevalent malignancy worldwide and holds the second rank concerning cancer-related mortality owing due to its limited treatment options [2–4]. Mostly as a result of viral hepatitis C, alcohol abuse and non-alcoholic steatohepatitis (NASH) the incidence of HCC is increasing also in many industrialized countries [5]. The discovery of the so-called immune checkpoint inhibitors has resulted in the unprecedented success of cancer therapy in several other forms of cancer, e.g., malignant melanoma and has prompted high expectations to also control advanced HCC [6–8].
Immune checkpoints involve membrane expression of distinct molecules which fine-tune immune responses [9]. Most immune checkpoint molecules display immunosuppressive activity, presumably preventing over-activation of T cells and limiting collateral tissue damage after immune activation [10]. Cancer cells may exploit this mechanism by upregulating ligands for inhibitory checkpoint molecules to escape immune surveillance [11]. Novel oncological immune therapies attempt to interrupt aberrant checkpoint inhibitor interactions between tumour cells and T lymphocytes with specific blocking antibodies [12, 13].
In HCC, expression of programmed death 1 receptor (PD-1) is increased on CD8+ T effectors cells and interaction with its ligands PD-L1 (programmed death ligand 1) and PD-L2 (programmed death ligand 2) on tumour cells blocked signalling, proliferation, and cytokine secretion of T cells [14, 15]. Several reports have shown an association between the infiltration of CD8+ T cells in HCC and patient survival [16, 17] suggesting that interferon (IFN)-gamma secretion by CD8+ cells has antitumour activity [18].
PD-1 inhibitors are supposed to interrupt this inhibitory interaction between PD-1 on activated CD4+ and CD8+ T effector cells and its ligands on tumour cells [19]. Indeed, the recent open-label phase1/2 dose escalation and expansion trial studying the PD-1 antibody nivolumab (CheckMate 040 trial) observed substantial tumour reductions with up to 20% objective response rates in patients with advanced HCC [20]. Another non-randomized, open-label phase 2 trial (KEYNOTE-224) with the PD-1 inhibitor pembrolizumab confirmed durable clinical efficacy in patients who had progressed after treatment with sorafenib or were intolerant to the drug [21]. However, expression of PD-1 ligands was unexpectedly low on tumour tissue in both studies and did not correlate with response rates suggesting that other pathways of checkpoint inhibitory interactions might be involved.
To explain the mechanism of immune checkpoint inhibition, it has been proposed that PD-1 ligand signalling might be provided by other immune cells surrounding the HCC microenvironment [22–24]. In particular, regulatory T cells (Tregs) stand out among the immunosuppressive candidate cells in and around the tumour niche [25, 26]. Tregs are a subset of CD4+CD127− T lymphocytes which constitutively express the transcription factor Foxp3 (forkhead box p3) and the interleukin-2 (IL-2) receptor α chain CD25 [27]. Activated Tregs inhibit different subsets of immune cells via contact-dependent interactions between checkpoint molecules and their ligands involving PD-1, PD-L1, CTLA-4 (cytolytic T lymphocyte-associated antigen), GITR (glucocorticoid-induced TNFR family-related gene), Tim-3 (T-cell immunoglobulin and mucin-domain containing-3), and galectin-9. Furthermore, Tregs can contribute to a strong immunosuppressive tumour environment by releasing inhibitory cytokines such as transforming growth factor (TGF)-beta, IL-10 and IL-35 [28, 29].
Since the effect of immune checkpoint inhibitors on Tregs is poorly understood in liver cancer, in this study we analysed the expression of checkpoint molecules on Tregs as well as release of inhibitory cytokines in patients with HCC compared to those in non-tumour bearing patients with chronic liver disease and in healthy controls. Furthermore, we analysed the impact of Tregs through the interaction with immune checkpoints on effector functions of CD8+ T cells in an in vitro model against tumour cells not expressing checkpoint inhibitory molecules.
Patients and methods
Study group
In this study, we prospectively recruited 50 patients with HCC, 41 patients with non-tumour bearing chronic liver disease and 25 healthy controls. All patients were recruited from the Department I of Internal Medicine and the Center for Integrated Oncology (CIO) at the Bonn University Hospitals between January 2016 and February 2019. Control blood samples were obtained from healthy volunteers via the Bonn university blood banking service. The clinical features of all study subjects are summarized in Table 1. The diagnosis of liver cancer was established either by histology and/or contrast-enhanced MRI (magnetic resonance imaging) and/or computer tomography (CT) scans according to internationally accepted radiological criteria [30]. Due to limitations in sample volume and cell yield, not all experiments could be performed in every subject.
Table 1.
Characteristics and demographics of subjects
| Healthy controls | Liver disease without HCC | HCC | |
|---|---|---|---|
| Numbers | 25 | 41 | 50 |
| Median age (years) | 42 (24–86) | 56 (27–76) | 68 (52–89)* |
| Sex | |||
| Male | 14 (56%) | 28 (68%) | 38 (76%) |
| Female | 11 (44%) | 13 (32%) | 12 (24%) |
| Etiology | |||
| HCV | NA | 32 (78%) | 22 (44%) |
| HBV | NA | 0 (0%) | 3 (6%) |
| NASH | NA | 4 (10%) | 15 (30%) |
| ASH | NA | 5 (12%) | 10 (20%) |
| ALT median (U/ml) | NA | 69 (25–753) | 53 (15–397) |
| Cirrhosis | NA | 10 (24%) | 48 (96%)** |
| Child–Pugh Score A (%) | NA | 6 (15%) | 31 (62%) |
| Child–Pugh Score B (%) | NA | 4 (10%) | 14 (28%) |
| Child–Pugh Score C (%) | NA | 0 (0%) | 3 (6%) |
| Alpha-fetoprotein | |||
| AFP median (ng/ml) | NA | 6.0 (1.9–35.9) | 32.5 (2.0–50,000)** |
| > 400 ng/ml (%) | NA | NA | 11 (22%) |
| Treatment prior to study inclusion and blood collection | |||
| Treatment Naive | NA | NA | 28 (56%) |
| Local therapy | |||
| Resection | NA | NA | 7 (14%) |
| Radiofrequency ablation (RFA) | NA | NA | 1 (2%) |
| Transarterial chemoembolization (TACE) | NA | NA | 12 (24%) |
| Selective internal radiation therapy (SIRT) | NA | NA | 5 (10%) |
| Systemic therapy | |||
| Sorafenib | NA | NA | 3 (6%) |
| More than 2 treatment options | NA | NA | 6 (12%) |
| Tumour stage at diagnosis of HCC | |||
| BCLB 0 | NA | NA | 6 (12%) |
| BCLB A | NA | NA | 15 (30%) |
| BCLB B | NA | NA | 14 (28%) |
| BCLB C | NA | NA | 13 (26%) |
| BCLB D | 2 (4%) | ||
| Tumour size, cm (< 5/> 5) | NA | NA | 34/16 |
| Tumour number (single/multiple) | NA | NA | 40/10 |
| Vascular invasion (present/absent) | NA | NA | 40/10 |
| Extrahepatic manifestation (present/absent) | NA | NA | 48/2 |
Other results were not significantly different
NA not applicable
*p < 0.001; HCC compared with disease controls and healthy controls
**p < 0.001; HCC compared with disease controls
Materials
Immunohistochemistry of HCC specimens
Immunostaining was specifically performed on whole sections (2 µm) from formalin-fixed, paraffin-embedded tumour tissue. All slides were processed on a BenchMark Ultra automated immunostainer (Ventana Medical Systems, Tucson, AZ, USA) according to the manufacturer’s instructions. The following primary antibodies were used: anti-PD-1 (Clone NAT105, Fa. Abcam, Cambridge, UK), anti-PD-L1 (Clone 22C3, Fa. Agilent, Santa Clara, CA, USA), anti-CD8 (Clone C8/144B, Fa. Agilent, Santa Clara, Ca, USA) and anti-perforin (Clone MRQ-23, Fa. Cell Marque, Rocklin, USA). Sections were counter-stained with haematoxylin and image acquisition performed with a Leica-microscope. Immune cells in the tumours were analysed using serially stained sections. All histopathology specimens were reviewed by an expert pathologist (Marieta Toma).
Isolation and immunostaining of peripheral blood mononuclear cells (PBMC)
PBMC were isolated from venous blood by Ficoll-Paque (PAA Laboratories; Cölbe, Germany) density gradient centrifugation, washed with phosphate-buffered saline (PBS), and cryopreserved in liquid nitrogen until further analysis.
For fluorescence-activated cell sorting (FACS) analysis, frozen PBMC were thawed and cultured in RPMI 1640 medium (PAA Laboratories). After 1 h, brefeldin A (BFA, 0.5 µg/ml Sigma-Aldrich, St. Louis, Missouri, USA) was added, and the cells were cultured at 37 °C and 5% CO2. After overnight culture, PBMC were stained with Zombie Aqua™ (Biolegend; Eching, Germany) to discriminate dead cells. Then, cell surface staining was performed using anti-CD3 (PE-Cy7-labelled), anti-CD4 (APC-Cy7-labelled), anti-CD8 and anti-CD25 (both PerCP-labelled), anti-PD-L1, anti-CTLA-4, and anti-GITR (all APC-labelled), anti-PD-1 and anti-Tim-3 (both Brilliant Violet 421-labelled). For intracellular staining cells were fixed and permeabilized using the Cytofix/Cytoperm Kit (BD Pharmingen, Heidelberg, Germany) and the Foxp3 staining Kit (eBioscience, Frankfurt, Germany), respectively. Next, cells were stained with intracellular antibodies (anti-Foxp3 [FITC-labelled], anti-TGF-beta, anti-IL-35 [both PE-labelled], anti-IL-10 and anti-galectin-9 [both APC-labelled]). Finally, samples were analysed on a FACSCanto II (BD Biosciences) with the FlowJo V10 software (TreeStar Inc., Ashland, OR, USA). Fluorescence minus one (FMO) controls and isotype controls were performed for all antibody panels to confirm proper compensation and define positive signals.
In vitro suppression of CD8+ T cells by autologous Tregs
To circumvent variation resulting from differential tumour epitope recognition and HLA-restriction we decided to study cytotoxic activity of CD8+ T cells in vitro using a lectin-dependent cellular cytotoxicity (LDCC) assay [31, 32]. In this assay, IFN-gamma secretion and CD107a degranulation of CD8+ T cells against P815 target cells were studied in the presence and absence of autologous Tregs. P815 cells are a natural killer cell (NK cell) resistant mouse mastocytoma cell line. Unlike human hepatoma cell lines P815 cells do not upregulate checkpoint inhibitory molecules in tissue culture such as PD-L1, PD-L2, PD-1, GITR, CTLA-4, Tim-3 nor galectin-9.
Pan T cell medium from PanBiotec (Aidenbach, Germany) was used for culture of T cells. Human recombinant proteins IL-2, OKT-3 (human anti-CD3) were obtained from R&D Systems (Wiesbaden, Germany), anti-CD28 from BD Biosciences. The following LEAF™ purified blocking antibodies were used: anti-PD-1 (EH12.2H7), anti-PD-L1 (clone 29E.2A3), anti-CTLA-4 (clone L3D10), anti-GITR (clone 621), and control antibodies (mouse IgG isotype control). All antibodies were purchased from Biolegend.
Untouched T cells and Tregs were isolated using the Human Pan T Cell Isolation Kit and the Human CD4+ CD25+ CD127dim/negative Regulatory T Cell Isolation Kit II (both Miltenyi, Bergisch Gladbach, Germany). Purity of isolated cell populations was > 95%. Then, untouched T cells (= Teff) were cultured with IL-2 (100 U/ml) in the presence or absence of autologous Tregs (Treg to Teff ratio 1:2). The next day, concanavalin A-loaded P815 cells (Con A; 5 µg/ml for 20 min) were added to the co-cultures (ratio 1:5) together with PE-conjugated CD107a mAb (Biolegend). After 1 h Golgi Stop (BD Biosciences) and monensin (Biolegend) were added for additional 3 h. In the co-culture, T cells were differentiated with the following antibodies: anti-CD8 (PE-Cy7-labelled), anti-CD3 (APC-Cy7-labelled), anti-CD56 (APC-labelled), anti-CD25 (PerCP-labelled), anti-IFN-gamma (FITC-labelled), anti-Foxp3 (FITC-labelled) (all BioLegend). Viability was checked with Zombie Aqua™ (Biolegend). Finally, intracellular accumulation of IFN-gamma and surface expression of CD107a was measured in CD8+ T effector cells via flow cytometry. IFN-gamma and CD107a expression in CD8+ T effector cells were determined at baseline and upon stimulation with Con A-loaded P815 cells. Suppression by Tregs is reported as frequencies of IFN-gamma+ and CD107a+CD8+ T effector cells that remain detectable when Tregs had been added and is given in percent relative to the number of cells measured in the absence of Tregs (= residual frequencies).
Adding cells to functional experiments in vitro could result in reduced numbers of cells per unit volume rather than true inhibition. To exclude this type of bias we also repeated the co-culture experiments in the presence of irradiated autologous PBMC (3000 rad) as proposed by Mayer et al. [33] (Supplementary Fig. 1a).
Statistics
Differences between groups were analysed using an unpaired non-parametric Mann–Whitney U test, Wilcoxon matched-pairs signed-rank test and paired Student t test as appropriate. Correlations were compared by linear regression analysis. p values below 0.05 were considered to indicate statistically significant differences. Calculations and graphs were obtained with the SPSS statistics software (version 24) and GraphPad Prism 8.0 software package (GraphPad Prism, San Diego California, USA), respectively.
Results
Clinical and histological features of patients
Patients with chronic liver disease—both with and without HCC—were mostly male and mainly suffered from hepatitis C, alcoholic and non-alcoholic fatty liver disease (Table 1). Patients with HCC were significantly older (p < 0.001) and had more frequently cirrhosis (47/50, 94%; p < 0.001). Histology was available in 25 patients with HCC (Fig. 1a). None of the tumour cells were positive for PD-1, and we only found PD-L1 positive tumour cells in 2 of the 25 HCC patients (8%). In addition, there was a variable number of CD8+ T cells infiltrating the tumours [4–250 cells/high power field (HPF)]. On average between 0.3 and 9 T cells/HPF were perforin positive. Interestingly, we found a linear relationship between the numbers of perforin positive cells in the tissue and serum alanine aminotransferase (ALT) levels (r2 = 0.522; p < 0.0001). Finally, we detected PD-1 and PD-L1 expression on immune cells in 6/25 and 8/25 of our HCC patients (PD1: 0.5–20%, PD-L1: 0.5–30% of mononuclear cells), respectively (Supplementary Table 1).
Fig. 1.
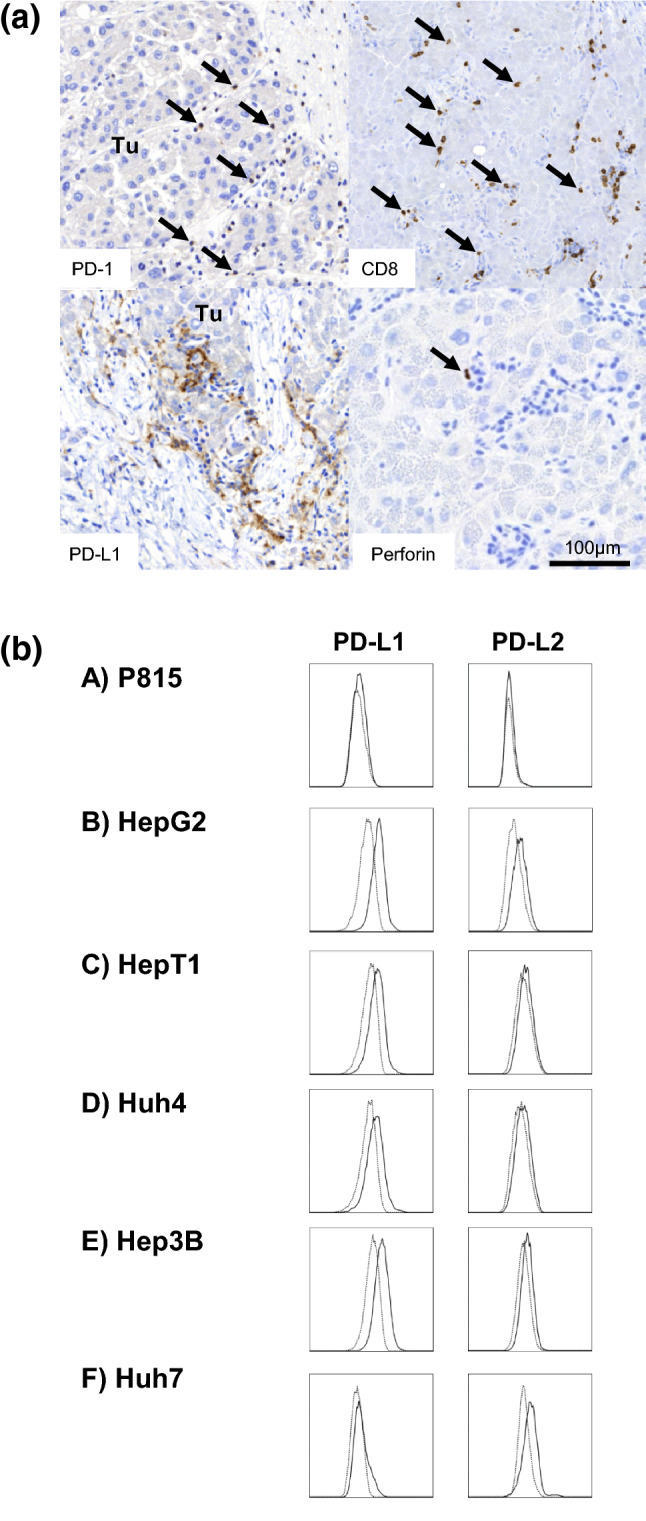
Expression of PD-1/PD-L1 on HCC tumour tissues and tumour cell lines. a Representative images of immunohistochemistry sections in the tumour tissue of HCC patient #20. Positive cells are stained brown by immunoperoxidase. Arrows mark PD-1-positive mononuclear cells (upper left), CD8+ T cells (upper right) and perforin-positive cells (lower right), respectively. PD-L1 staining shown in the lower-left panel indicates a greater number of positive mononuclear cells (macrophages and CD8+ T cells). Of note, HCC tumour cells did not stain positive for PD-1 and PD-L1. Tu tumour. b The flow cytometric analysis of PD-L1/L2 expression on P815 mouse mastocytoma cells in contrast to various hepatoma cell lines (HepG2, HepT1, Huh4, Hep3B, Huh7). Unlike hepatoma cells, which up-regulated PD-L1 and PD-L2, P815 cells remained PD-L1 and PD-L2 negative in cell culture. Thus, we used P815 cells as target cells in lectin-dependent cellular cytotoxicity (LDCC) assays to study the functional role of Tregs on T cell functions in a model of PD-L1/L2-negative tumour cells
Flow cytometric analysis of various hepatoma cell lines showed that all tested cells up-regulated PD-L1 and PD-L2 expression in tissue culture (Fig. 1b). In contrast, P815 mouse mastocytoma cells remained PD-L1 and PD-L2 negative in tissue culture and did also not express any of the other checkpoint molecules (PD-1, GITR, CTLA-4, Tim-3, galectin-9) (Fig. 1b and data not shown). Thus, P815 were chosen as target cells to study the role of Tregs on functional T cell capacity against checkpoint inhibitor-negative tumour cells using a lectin-dependent cellular cytotoxicity (LDCC) assay.
Phenotypic characterization of peripheral T cell subsets
Unlike overall frequency of T effector cell subsets and expression of CTLA-4, GITR and Tim-3 on CD4+ and CD8+ T cells (Supplementary Table 2a), the frequency of PD-1+ cells among CD4+ and PD-1+ cells among CD8+ T cells was significantly enhanced in the blood of patients with HCC (Fig. 2a, b; Supplementary Fig. 2a, b).
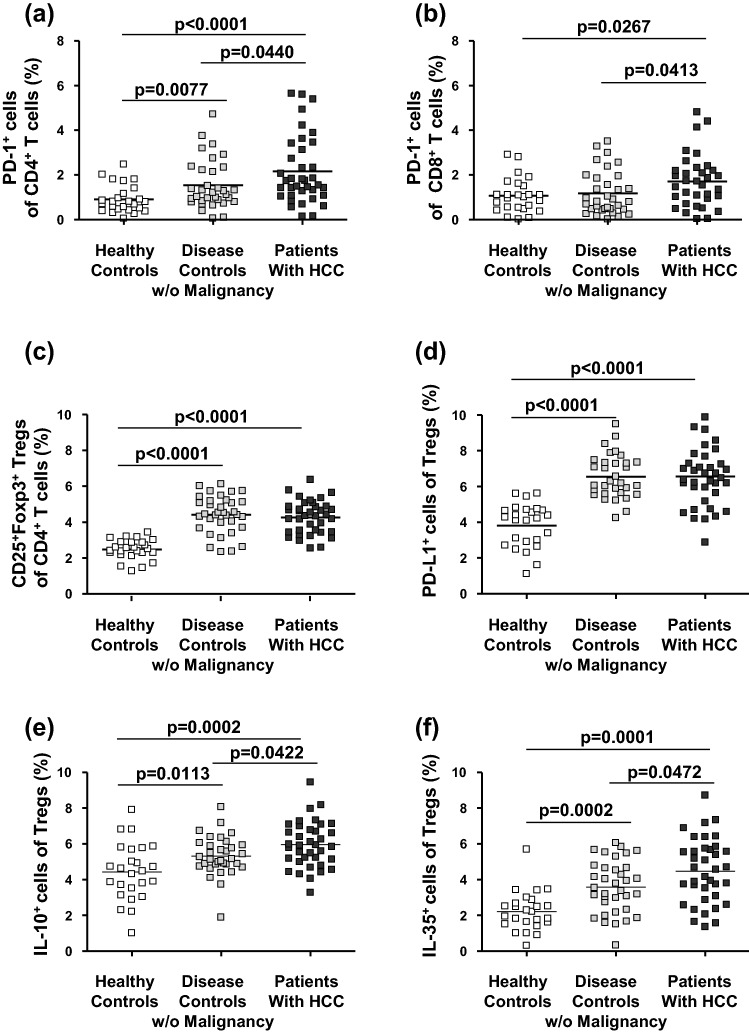
Fig. 2.
Frequency and phenotype of T cell subsets in the blood of patients with HCC. Frequencies of PD-1+ cells among CD4+ (a) and PD-1+ cells among CD8+ T effector cells (b) were significantly increased in the blood of patients with HCC. In addition, although increased over healthy controls, the frequencies of CD4+CD25+Foxp3+ Tregs (c) and PD-L1+ cells among Tregs (d) in HCC patients did not differ from those of the other study groups. Of note, frequencies of CD4+CD25+Foxp3+ Tregs staining positive for immunosuppressive cytokines IL-10 (e) and IL-35 (f) were higher in patients with HCC than in the other study groups. P-values refer to significances obtained by unpaired non-parametric Mann–Whitney U test for the differences marked by bars
Next, we analysed several Foxp3+CD25+CD4+ Treg subsets: Overall frequencies of Tregs and frequencies of PD-L1-positive cells among Tregs were increased in the blood of both patients with non-tumour-bearing liver disease without HCC and patients with HCC compared to healthy controls but did not differ between patients with HCC and patients with chronic liver diseases (Fig. 2c, d; Supplementary Fig. 2c, d). When we analysed cytokine production in Tregs at the single-cell level by intracellular cytokine staining, both IL-10- and IL-35-positive Treg subsets were more frequent in the patients with HCC than in the patients with chronic liver diseases without detectable malignancy (Fig. 2e, f; Supplementary Fig. 2e, f). To exclude that differences in IL-10- and IL-35-positive Tregs were due to the imbalance in the distribution of cirrhotic patients between the disease controls and patients with HCC, we analysed their frequency in cirrhotic and non-cirrhotic disease controls without malignancy and found identical numbers in the two subgroups (Supplementary Fig. 3). In addition, CTLA-4+, GITR+, PD-1+, and PD-L1+ Tregs apparently defined a closely related T cell subset, because the numbers of positively stained cells were significantly related to each other (Supplementary Fig. 4). Concerning expression of CTLA-4, GITR, PD-L1, and Tim-3 as well as production of galectin-9 and TGF-beta, Treg subsets did not differ significantly between the patients with and without HCC (Supplementary Table 2b).
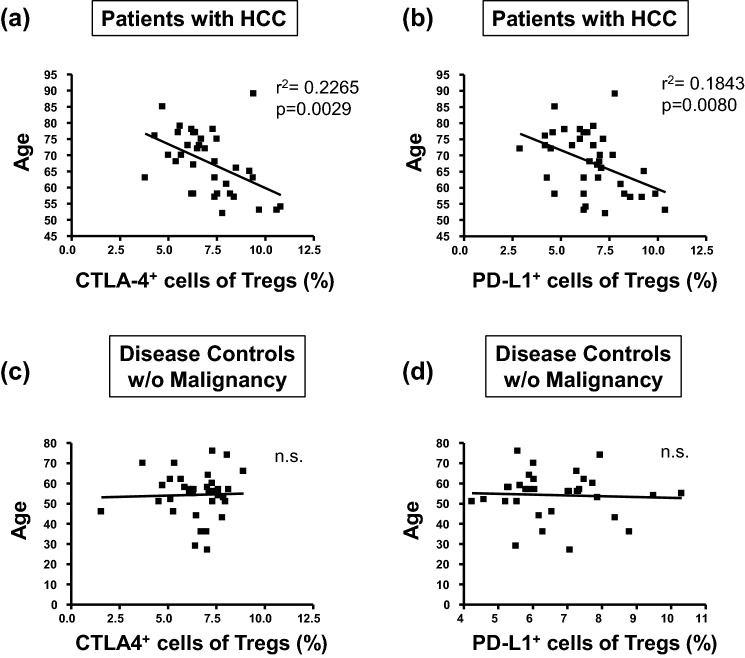
Of note, we observed inverse relationships between age of HCC patients and the frequency of the CTLA-4+ and PD-L1+ Treg subsets specifically in the patients with HCC (Fig. 3a, b). In contrast, analogous correlations were not detected in patients without HCC nor the healthy controls (Fig. 3c, d).
Fig. 3.
Correlation between the age of HCC patients and frequency of CD4+CD25+Foxp3+ Treg subsets. These figures illustrate inverse relationships between age of HCC patients and frequency of CD4+CD25+Foxp3+ Treg subsets expressing CTLA-4 (a) and PD-L1 (b). In contrast, such correlations were not detected between age und Treg subset size in the patients without malignancy (c, d), nor healthy controls (data not shown). Statistical parameters refer to correlation coefficients and levels of significances obtained by linear regression analysis. n.s. not significant
Functional in vitro analysis of Tregs
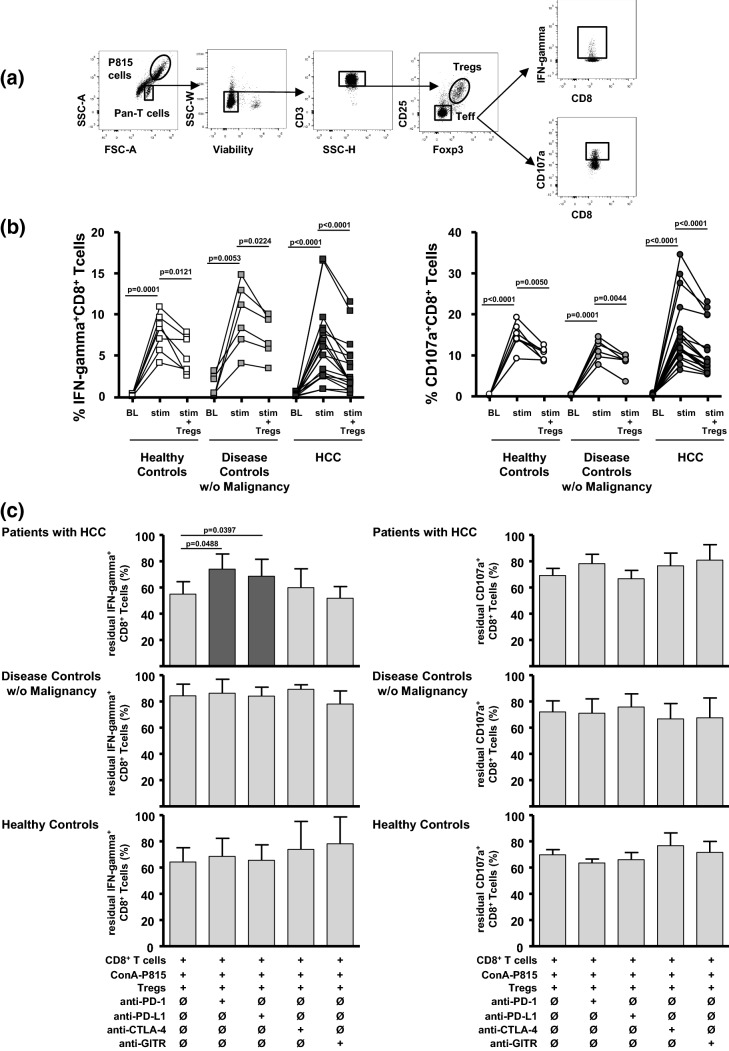
To study the regulation of T effector cell functions against PD-L1/L2-negative tumour cells by Tregs, we analysed lectin-dependent cellular cytotoxicity (LDCC) against Con A-loaded P815 cells (Fig. 4). Exposure to Con A-loaded P815 cells induced both IFN-gamma secretion and degranulation in CD8+ T effector cells (Fig. 4a). Peak induction of IFN-gamma production and degranulation did not differ significantly between the study groups (%IFN-gamma+CD8+ T cells (mean ± SD): 7.8 ± 2.4 in healthy controls; 9.7 ± 4.0 in disease controls; 6.2 ± 4.6 in HCC; %CD107a+CD8+ T cells (mean ± SD): 14.7 ± 3.1 in healthy controls; 11.5 ± 2.5 in disease controls; 15.4 ± 7.9 in HCC; Fig. 4b). Finally, adding autologous Tregs to the P815-stimulated CD8+ T cells in the LDCC assays resulted in both reduced IFN-gamma production and CD8+ T cell degranulation in each patient group (Fig. 4b).
Fig. 4.
IFN-gamma production and degranulation by CD8+ T cells in lectin-dependent cellular cytotoxicity (LDCC) assays and effects of checkpoint inhibition. a The gating strategy to analyse IFN-gamma production and T cell degranulation in CD8+ T effector cells by flow cytometry in co-cultures with Con A-loaded P815 cells and Tregs (Treg to Teff ratio: 1:2). b IFN-gamma production (left) and CD107a degranulation (right) of CD8+ T effector cells in LDCC assays before (BL), with exposure to Con A-loaded P815 target cells (stim = peak secretion) and upon adding Tregs (stim + Tregs). Adding autologous Tregs reduced P815-induced peak IFN-gamma secretion and degranulation of CD8+ T cells in all study groups. p values refer to significances obtained by paired Student t test marked by bars. c Provides the summary statistics concerning the differences of IFN-gamma-production (left) and CD107a degranulation (right) by CD8+ T cells after sequential addition of Con A-loaded P815 target cells and autologous Tregs (Treg to Teff ratio: 1:2), as well as in the presence of added neutralizing anti-PD-1, anti-PD-L1, and anti-CTLA-4, and anti-GITR (10 µg/ml each). Columns represent the mean ± SD of 6–18 different donors. Anti-PD-1 and anti-PD-L1—but not anti-CTLA-4 nor anti-GITR—partially reversed Treg-associated inhibition of IFN-gamma secretion in CD8+ T cells from patients with HCC. Treg-mediated inhibition of T cell degranulation was not reversed by any of the antibodies. p values refer to significances obtained by paired Student t test marked by bars
Analysis of checkpoint inhibition on Treg/T effector cell interactions
Tregs can inhibit T effector cell functions by several contact-dependent mechanisms. To clarify whether checkpoint inhibitory molecules can recover the functionality of CD8+ T cells by blocking the interaction between Tregs and T effector cells, we repeated the LDCC experiments and added neutralizing antibodies against PD-1, PD-L1, CTLA-4, and GITR to the Tregs in the co-culture (Fig. 4c). While Treg-mediated inhibition of T cell degranulation was not reversed by any of the antibodies, anti-PD-1 and anti-PD-L1—but not anti-CTLA-4 nor anti-GITR—partially reversed Treg-associated inhibition of IFN-gamma secretion in CD8+ T cells from the patients with HCC (Fig. 4c). This reversal was not observed in CD8+ T cells from the non-tumour-bearing patients with chronic liver disease nor the healthy controls. Furthermore, control experiments with anti-PD-1 and anti-PD-L1 in the LDCC assay without Tregs did not show that IFN-gamma production or degranulation of T effector cells were affected by inhibitory checkpoint antibodies (Supplementary Fig. 1b).
Discussion
Currently, monoclonal antibodies against immune checkpoint pathways are evaluated as new potential antitumour agents across a broad variety of different tumour types also comprising HCC [34–36]. This strategy is based on the idea that the monoclonal antibodies interrupt inhibitory interactions between checkpoint molecules on tumour cells and T effector cells. However, in HCC this mode of action seems implausible because the liver tumour cells themselves express only negligible amounts of checkpoint inhibitor ligands. Furthermore, expression of checkpoint molecules on tumour cells did apparently not correlate with response rates to checkpoint inhibitor antibodies in the first clinical trials in HCC [20, 21, 37, 38]. To account for this discrepancy, it has been suggested that other cells in the HCC microenvironment, which can also express checkpoint molecules, can inhibit antitumour responses. Several studies proposed that macrophages, myeloid-derived suppressor cells [22–24], and especially Tregs might substitute for the low expression of checkpoint molecules on liver tumour cells [25, 26]. Thus, these cell types are potential candidates to explain poor immune responsiveness to checkpoint inhibitor blockade in liver cancer. However, this hypothesis has not been checked thus far.
Since our studies confirmed negligible in situ expression of PD-1 and PD-L1 on liver tumour cells and up-regulated expression of PD-1 on immune cells in line with previous reports on hepatoma and various other solid cancers [14, 37, 39], we studied Foxp3+CD25+CD4+ Tregs in patients with liver cancer compared to patients with corresponding liver diseases without malignancy and healthy controls.
First, the study focused on the expression of inhibitory checkpoint molecules on Tregs (PD-1, PD-L1, CTLA-4, GITR, Tim-3), and their secretion of immunomodulatory factors (IL-10, IL-35, TGF-beta, galectin-9). In our ex vivo studies, we detected higher frequencies of Foxp3+CD25+CD4+ Treg subsets in the blood of patients with HCC than in either control group, which also secreted inhibitory cytokines IL-10 and IL-35. This difference appeared to be particularly linked to the presence of malignancy because cirrhotic and non-cirrhotic liver patients without malignancy did not differ concerning IL-10 and IL-35 secreting Tregs.
Next, we studied in vitro interactions between Tregs and T effector cells in a tumour model not expressing checkpoint inhibitory molecules on the tumour cells by analysing lectin-dependent cellular cytotoxicity (LDCC) of patient T cells against Con A-loaded P815 tumour cells. LDCC is a well characterized immune assay [31, 32] where Con A-loaded target cells cross-link T cell receptors and trigger calcium influx in T effector cells mimicking T cell activation by antigen. Thus, our LDCC assay offers several advantages: we could assess T cell functional capacity independently from antigen specificity and—with the choice of P815 cells—could analyse antitumoural activity against checkpoint inhibitor-negative tumour cells. Stimulation with Con A-loaded P815 cells activates CD8+ T effector cells independently from antigen recognition and thus assesses their functional capacity. Finding on average rather similar peak results for IFN-gamma production and T cell degranulation excludes intrinsic cell activation defects of T effector cells.
Inhibition by Foxp3+CD25+CD4+ Tregs may involve several molecular pathways. In previous studies, we had demonstrated that Tregs inhibit proliferation and cytokine secretion of T effector cells via IL-10 and IL-35, which is reversed by adding neutralizing antibodies [40, 41]. To clarify the role of additional contact-dependent pathways, we performed blocking experiments in our LDCC assays using anti-PD-1, anti-PD-L1, anti-CTLA-4, and anti-GITR, respectively. Here, IFN-gamma production was partially reversed in the patients with HCC when the interaction between PD-L1 on Tregs and its receptor PD-1 on T effector cells was blocked by either adding anti-PD-L1 or PD-1 antibodies. This novel finding suggests that Tregs contribute to suppression of antitumoural immunity in HCC via the PD-L1/PD-1 pathway, even when expression of inhibitory checkpoint molecules is negligible on the tumour cells. Immunosuppressive effects were apparently rather moderate, but it has to be taken into account that Con A provides a much stronger T cell activation signal than conventional antigen. On the other hand, inhibition was not fully reversed by adding PD-L1 and PD-1 antibodies, indicating that our study did not address all inhibitory mechanisms. For example, tumour-associated macrophages and myeloid-derived suppressor cells are further candidate cell types in the tumour microenvironment potentially contributing to T effector cell inhibition [23, 24]. Finally, perforin and granzyme release as well as triggering of death receptors by their cognate ligands—not analysed in this study—may also contribute to the control of tumour growth by cytotoxic T effector cells.
Our study had been designed to gain functional insights on Tregs at the cellular level rather than to find prognostic markers in patients with HCC. Interestingly, however, we observed an inverse correlation between the expansion of PD-L1- and CTLA-4-positive Tregs and the age of diagnosis in our patients with HCC. Increased frequencies of Tregs can be associated with various liver diseases even without malignancy [40, 41], however, the absence of a relationship between Treg frequencies and age both in healthy and disease controls without HCC largely is evidence against a general age-related change in Treg subset frequency. Thus, the inverse relationship between age and Treg frequencies selectively in patients with HCC may be a hint that patients with liver disease and pronounced expansions of CTLA-4/PD-L1-positive Tregs carry a particularly high risk to develop HCC and thus become diagnosed at an early age, in line with other reports claiming a substantial role of Tregs for tumour progression and survival [42–44].
In summary, our studies extend previous findings reported for Tregs in breast cancer and malignant melanoma to liver cancer [45, 46] and provide experimental evidence that Tregs contribute to immune dysfunction in HCC via several pathways. These pathways also involve Tregs and their interaction with T effector cells via the inhibitory checkpoint molecules PD-L1 and PD-1. Thus, further studies should also take into account circulating Tregs as a potential therapeutic target and predictive biomarker in HCC immunotherapy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Ingrid Braunschweiger, Eva-Maria Schumacher and Susanne Steiner for their perfect technical assistance.
Abbreviations
- ALT
Alanine aminotransferase
- BFA
Brefeldin A
- Con A
Concanavalin A
- CT
Computer tomography
- CTLA-4
Cytolytic T lymphocyte-associated antigen
- FACS
Fluorescence-activated cell sorting
- FMO
Fluorescence minus one
- Foxp3
Forkhead box p3
- GITR
Glucocorticoid-induced TNFR family-related gene
- HCC
Hepatocellular carcinoma
- HPF
High power field
- IFN
Interferon
- IL
Interleukin
- LDCC
Lectin-dependent cellular cytotoxicity
- MRI
Magnetic resonance imaging
- NASH
Non-alcoholic steatohepatitis
- NK cell
Natural killer cell
- PBMC
Peripheral blood mononuclear cells
- PBS
Phosphate-buffered saline
- PD-1
Programmed death 1 receptor
- PD-L1
Programmed death ligand 1
- PD-L2
Programmed death ligand 2
- TGF
Transforming growth factor
- Tim-3
T cell immunoglobulin and mucin-domain containing-3
- Treg
Regulatory T cell
Author contributions
BL, LD, PL, RM, AV, JN, CPS, MAGC and US were involved in the study concept and design as well as in patient acquisition. BL, HDN, BK, AV, MT, AMEH, MAGC and US were involved in the acquisition, analysis, and interpretation of the data. BL, LD, MAGC and US drafted the manuscript, and all authors were involved in critical revision of the manuscript.
Funding
This work was supported by the German Center for Infection Research [to B. Langhans, L. Dold and U. Spengler (TTU 05.808)], the German Research Foundation (SFB/TRR57 to U. Spengler and J. Nattermann), and the Deutsche Krebshilfe [to U. Spengler and H.D. Nischalke (70112169), to M.A. Gonzalez-Carmona (109255)]. The study sponsors had no role in the study design, collection, analysis, and interpretation of data.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval and ethical standards
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Institutional Review Board of the Bonn University Ethics Committee (Approval #150/15).
Informed consent
All patients and healthy blood donors gave written informed consent prior to blood sample collection for the use of biomaterials and clinical data for scientific purposes.
Cell line authentication
The mouse mastocytoma cell line P815 and the various hepatoma cell lines HepG2, Hep3B, and Huh7 were purchased from the American Type Culture Collection (ATCC®) TIB64™. Cell lines Huh4 and HepT1 were a kind gift of W. Caselmann and T. Pietsch, respectively. Cell line authentication for human HepG2, Hep3B, Huh7, Huh4, and HepT1 was not necessary, because these cell lines were excluded from our in vitro studies due to up-regulation of PD-L1/L2 in cell culture. Cell line authentication of murine P815 cells was performed by the Swiss DNA company Microsynth (Supplementary Table 3).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Langhans B, Nischalke HD, Krämer B, et al. Role of regulatory T cells and checkpoint inhibition in hepatocellular carcinoma. J Hepatol. 2019;70(Suppl):e377. doi: 10.1007/s00262-019-02427-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 3.Wallace MC, Preen D, Jeffrey GP, et al. The evolving epidemiology of hepatocellular carcinoma: a global perspective. Expert Rev Gastroenterol Hepatol. 2015;9:765–779. doi: 10.1586/17474124.2015.1028363. [DOI] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Zucman-Rossi J, Pikarsky E, et al. Hepatocellular carcinoma. Nat Rev Dis Primer. 2016;2:16018. doi: 10.1038/nrdp.2016.18. [DOI] [PubMed] [Google Scholar]
- 6.Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2015;12:681–700. doi: 10.1038/nrgastro.2015.173. [DOI] [PubMed] [Google Scholar]
- 7.Elsegood CL, Tirnitz-Parker JE, Olynyk JK, et al. Immune checkpoint inhibition: prospects for prevention and therapy of hepatocellular carcinoma. Clin Transl Immunol. 2017;6:e161. doi: 10.1038/cti.2017.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishida N, Kudo M. Immune checkpoint blockade for the treatment of human hepatocellular carcinoma. Hepatol Res Off J Jpn Soc Hepatol. 2018;48:622–634. doi: 10.1111/hepr.13191. [DOI] [PubMed] [Google Scholar]
- 9.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwai Y, Ishida M, Tanaka Y, et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Popovic A, Jaffee EM, Zaidi N. Emerging strategies for combination checkpoint modulators in cancer immunotherapy. J Clin Investig. 2018;128:3209–3218. doi: 10.1172/JCI120775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol. 2018;62:29–39. doi: 10.1016/j.intimp.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Shi F, Shi M, Zeng Z, et al. PD-1 and PD-L1 upregulation promotes CD8(+) T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int J Cancer. 2011;128:887–896. doi: 10.1002/ijc.25397. [DOI] [PubMed] [Google Scholar]
- 15.Kim H-D, Song G-W, Park S, et al. Association between expression level of PD1 by tumor-infiltrating CD8+ T cells and features of hepatocellular carcinoma. Gastroenterology. 2018;155(6):1936–1950.e17. doi: 10.1053/j.gastro.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 16.Unitt E, Marshall A, Gelson W, et al. Tumour lymphocytic infiltrate and recurrence of hepatocellular carcinoma following liver transplantation. J Hepatol. 2006;45:246–253. doi: 10.1016/j.jhep.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 17.Budhu A, Forgues M, Ye Q-H, et al. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell. 2006;10:99–111. doi: 10.1016/j.ccr.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 18.Brandacher G, Winkler C, Schroecksnadel K, et al. Antitumoral activity of interferon-gamma involved in impaired immune function in cancer patients. Curr Drug Metab. 2006;7:599–612. doi: 10.2174/138920006778017768. [DOI] [PubMed] [Google Scholar]
- 19.Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007;19:813–824. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 20.El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet Lond Engl. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940–952. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 22.Kalathil S, Lugade AA, Miller A, et al. Higher frequencies of GARP(+)CTLA-4(+)Foxp3(+) T regulatory cells and myeloid-derived suppressor cells in hepatocellular carcinoma patients are associated with impaired T-cell functionality. Cancer Res. 2013;73:2435–2444. doi: 10.1158/0008-5472.CAN-12-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeung OWH, Lo C-M, Ling C-C, et al. Alternatively activated (M2) macrophages promote tumour growth and invasiveness in hepatocellular carcinoma. J Hepatol. 2015;62:607–616. doi: 10.1016/j.jhep.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 24.Iwata T, Kondo Y, Kimura O, et al. PD-L1 + MDSCs are increased in HCC patients and induced by soluble factor in the tumor microenvironment. Sci Rep. 2016;6:39296. doi: 10.1038/srep39296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greten TF, Sangro B. Targets for immunotherapy of liver cancer. J Hepatol. 2017;18:1–20. doi: 10.1016/j.jhep.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shitara K, Nishikawa H. Regulatory T cells: a potential target in cancer immunotherapy. Ann N Y Acad Sci. 2018;1417:104–115. doi: 10.1111/nyas.13625. [DOI] [PubMed] [Google Scholar]
- 27.Shevach EM, Thornton AM. tTregs, pTregs, and iTregs: similarities and differences. Immunol Rev. 2014;259:88–102. doi: 10.1111/imr.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang F, Wan L, Zhang C, et al. Tim-3-Galectin-9 pathway involves the suppression induced by CD4 + CD25 + regulatory T cells. Immunobiology. 2009;214:342–349. doi: 10.1016/j.imbio.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Sakaguchi S, Miyara M, Costantino CM, et al. FOXP3 + regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 30.Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatol Baltim Md. 2018;67:358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 31.Perl A, González-Cabello R, Onody K, et al. Independence of depressed lectin-dependent cell-mediated cytotoxicity from interleukin 2 production in patients with systemic lupus erythematosus. Clin Exp Immunol. 1986;65:286–292. [PMC free article] [PubMed] [Google Scholar]
- 32.Tsukishiro T, Shimizu Y, Higuchi K, et al. Effect of branched-chain amino acids on the composition and cytolytic activity of liver-associated lymphocytes in rats. J Gastroenterol Hepatol. 2000;15:849–859. doi: 10.1046/j.1440-1746.2000.02220.x. [DOI] [PubMed] [Google Scholar]
- 33.Mayer E, Bannert C, Gruber S, et al. Cord blood derived CD4 + CD25(high) T cells become functional regulatory T cells upon antigen encounter. PLoS One. 2012;7:e29355. doi: 10.1371/journal.pone.0029355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Llovet JM, Montal R, Sia D, et al. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol. 2018;15(10):599–616. doi: 10.1038/s41571-018-0073-4. [DOI] [PubMed] [Google Scholar]
- 35.Shrestha R, Prithviraj P, Anaka M, et al. Monitoring immune checkpoint regulators as predictive biomarkers in hepatocellular carcinoma. Front Oncol. 2018;8:269. doi: 10.3389/fonc.2018.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iñarrairaegui M, Melero I, Sangro B. Immunotherapy of hepatocellular carcinoma: facts and hopes. Clin Cancer Res Off J Am Assoc Cancer Res. 2018;24:1518–1524. doi: 10.1158/1078-0432.CCR-17-0289. [DOI] [PubMed] [Google Scholar]
- 37.Semaan A, Dietrich D, Bergheim D, et al. CXCL12 expression and PD-L1 expression serve as prognostic biomarkers in HCC and are induced by hypoxia. Virchows Arch Int J Pathol. 2017;470:185–196. doi: 10.1007/s00428-016-2051-5. [DOI] [PubMed] [Google Scholar]
- 38.Zhou G, Sprengers D, Boor PPC, et al. Antibodies against immune checkpoint molecules restore functions of tumor-infiltrating T cells in hepatocellular carcinomas. Gastroenterology. 2017;153(1107–1119):e10. doi: 10.1053/j.gastro.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 39.Calderaro J, Rousseau B, Amaddeo G, et al. Programmed death ligand 1 expression in hepatocellular carcinoma: relationship with clinical and pathological features. Hepatol Baltim Md. 2016;64:2038–2046. doi: 10.1002/hep.28710. [DOI] [PubMed] [Google Scholar]
- 40.Langhans B, Braunschweiger I, Arndt S, et al. Core-specific adaptive regulatory T-cells in different outcomes of hepatitis C. Clin Sci Lond Engl. 2010;119:97–109. doi: 10.1042/CS20090661. [DOI] [PubMed] [Google Scholar]
- 41.Langhans B, Krämer B, Louis M, et al. Intrahepatic IL-8 producing Foxp3+CD4+ regulatory T cells and fibrogenesis in chronic hepatitis C. J Hepatol. 2013;59:229–235. doi: 10.1016/j.jhep.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 42.Kobayashi N, Hiraoka N, Yamagami W, et al. FOXP3 + regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res. 2007;13:902–911. doi: 10.1158/1078-0432.CCR-06-2363. [DOI] [PubMed] [Google Scholar]
- 43.Lin SZ, Chen KJ, Xu ZY, et al. Prediction of recurrence and survival in hepatocellular carcinoma based on two Cox models mainly determined by FoxP3 + regulatory T cells. Cancer Prev Res Phila. 2013;6:594–602. doi: 10.1158/1940-6207.CAPR-12-0379. [DOI] [PubMed] [Google Scholar]
- 44.Zhang A-B, Qian Y-G, Zheng S-S. Prognostic significance of regulatory T lymphocytes in patients with hepatocellular carcinoma. J Zhejiang Univ Sci B. 2016;17:984–991. doi: 10.1631/jzus.B1600264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ribas A, Shin DS, Zaretsky J, et al. PD-1 blockade expands intratumoral memory T cells. Cancer Immunol Res. 2016;4:194–203. doi: 10.1158/2326-6066.CIR-15-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Toor SM, Syed Khaja AS, Alkurd I, et al. In-vitro effect of pembrolizumab on different T regulatory cell subsets. Clin Exp Immunol. 2018;191:189–197. doi: 10.1111/cei.13060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.