Abstract
The MYCN oncogene is a strong genetic marker associated with poor prognosis in neuroblastoma (NB). Therefore, MYCN gene amplification and subsequent overexpression provide a possible target for new treatment approaches in NB. We first identified an inverse correlation of MYCN expression with CD45 mRNA in 101 NB tumor samples. KEGG mapping further revealed that MYCN expression was associated with immune-suppressive pathways characterized by a down-regulation of T cell activation and up-regulation of T cell inhibitory gene transcripts. We then aimed to investigate whether DNA vaccination against MYCN is effective to induce an antigen-specific and T cell-mediated immune response. For this purpose, we generated a MYCN-expressing syngeneic mouse model by MYCN gene transfer to NXS2 cells. MYCN-DNA vaccines were engineered based on the pCMV-F3Ub plasmid backbone to drive ubiquitinated full-length MYCN-cDNA and minigene expression. Vaccines were delivered orally with attenuated S. typhimurium strain SL7207 as a carrier. Immunization with both MYCN-DNA vaccines significantly reduced primary tumor growth of MYCN-expressing NB cells in contrast to negative controls. The immune response was mediated by tumor-infiltrating T cells in vivo, which revealed MYCN-specific and MHC class I-restricted lysis of inducible MYCN-expressing NB target cells in vitro. Finally, these antigen-specific T cells also killed MYCN-negative mammary carcinoma cells pulsed with MYCN peptides in contrast to controls. In summary, we demonstrate proof of concept that MYCN can be targeted by DNA vaccination, which may provide an approach to overcoming MYCN immune-suppressive activities in patients with MYCN-amplified disease.
Keywords: Pediatric oncology, Neuroblastoma, MYCN, DNA vaccination, Immunotherapy
Introduction
The amplification of the NB-derived myelocytomatosis viral-related oncogene (MYCN) is the most significant prognostic genetic marker in children with advanced-stage neuroblastoma (NB) and diagnosed in about 40 % of high-risk patients. High expression of MYCN is associated with rapid tumor progression and poor clinical outcome due to the development of metastases resistant even to the most intensive treatment regimen available today [1].
MYCN is overexpressed in 20 % of all NB and is restricted to early stages of embryonic development [2, 3], which are excellent characteristics of a target for novel approaches. However, MYCN is a transcription factor with a complex regulation of gene expression, and therefore it is not easily “druggable” in contrast to other oncogenes. Most strategies pursued so far involved indirect targeting of downstream MYCN-regulated proteins such as Aurora kinase A or extraterminal bromodomain Barone (BET) [4].
MYCN-specific cytotoxic T cells (CTLs) were found in NB patients with MYCN amplification (MNA), indicating a natural immune response against this self-antigen [5] and suggesting the accessibility of MYCN as a tumor-associated antigen (TAA). A promising and simple approach to further activating the immune system is the application of DNA vaccines [6]. In this context, it is important to designate a TAA as harmful [7] by co-administration of adjuvants such as CpG or lipopolysaccharide (LPS), both Toll-like receptor agonists [8, 9]. We have generated several DNA vaccines encoding for NB TAAs, i.e., tyrosine hydroxylase (TH), survivin and GD2 peptide surrogates and demonstrated their efficacy to induce an effective anti-NB immune response in an immunocompetent mouse model [10–12]. The coding sequence of a TAA within a DNA vaccine is usually comprised of the full-length cDNA. However, we could previously show that the identification of target epitope peptides of a TAA with high binding affinity to MHC class I molecules using algorithms like syfpheiti [13] allows the design of minigenes. This provides the advantage of avoiding transfer of a potentially harmful gene sequence to a mammalian host [14–16]. Efficacy of such minigenes was demonstrated to be similar to the corresponding full-length cDNA in a variety of model systems [11, 17–19].
One important prerequisite for successful DNA vaccination is an effective delivery system. In this respect, the use of attenuated S. typhimurium (SL7207) (AroA-) proved to be advantageous compared to other DNA delivery methods [20]. Oral application of Salmonella activates the mucosa-associated immune system, and lipopolysaccharides provide additional stimulation via Toll-like receptors [21].
Based on these considerations, we first investigated the role of MYCN expression for the immune environment in neuroblastoma tumors and designed and characterized MYCN-DNA vaccines encoding full-length MYCN-cDNA and a minigene, consisting of high-affinity MYCN epitopes.
Materials and methods
Gene expression and tissue microarray analysis
Data from Affymetrix HuEx1.0 arrays (GEO accession number GSE32664) were reanalyzed for MYCN and CD45 expression in patients with known MYCN status. Exon-level expression was recovered using Affymetrix power tools 1.10.0 and visualized using the R2 platform (http://hgserver1.amc.nl/cgi-bin/r2/main.cgi) as described [22]. In total, expression data from 101 primary NB tumors from the German and European clinical NB trials were included. Kyoto encyclopedia of gene and genome (KEGG) pathway mapping for the transcriptomic data set was performed using KEGG mapper software. Tissue microarrays (TMAs) from paraffin-embedded tissue of 78 primary NB tumors were stained using a monoclonal antibody against CD45 (1:1000, Abcam, Cambridge, UK) as previously described [23].
Tissue culture and flow cytometry
NXS2, NXS2-MYCN, C1300-MYCN and CHO cells were grown in high-glucose DMEM, SCK murine mammary carcinoma cells in RPMI 1640, all supplemented with 10 % FCS and 100 mg/ml penicillin/streptomycin (P/S) (PAA Laboratories, Pasching, Austria). Expression of MHC class I antigen H2-Kk on wild-type (wt) NXS2 and NXS2-MYCN was determined as described previously [10] and analyzed using a FACS Canto II (Becton–Dickinson, Heidelberg, Germany) and FlowJo (Tree Star, Inc, Ashland, USA). MHC class I restriction was investigated following blockade of H2-Kk with 1 µg/ml H2-Kk antibody (1 h) (H2-Kk clone 36-7-5; Biolegend, Fell, Germany).
Construction of the MYCN-expressing NXS2-MYCN cell line
Murine MYCN-cDNA was linked to an HA tag by PCR and ligated into the expression vector pcDNA 4 (T-REx™ Expression-System; Invitrogen, Darmstadt, Germany), followed by stable transfection of NXS2 cells. NXS2-MYCN cells were established following two rounds of limited dilution (0.3 cells/well, 96-well plates) in high-glucose DMEM (10 % FCS tetracycline negative, 100 mg/ml P/S, 5–10 µg/ml blasticidin (Sigma-Aldrich, Steinheim, Germany) and 10–20 µg/ml Zeocin™ (Invitrogen, Darmstadt, Germany)).
Stable integration of murine MYCN was demonstrated in genomic DNA (gDNA) (DNA isolation kit, Macherey–Nagel, Düren, Germany) by PCR. Transgene MYCN expression was induced by addition of 4 µg/ml doxycycline (Sigma-Aldrich) and determined by quantitative real-time PCR (q-PCR). Total RNA was isolated (NucleoSpin® RNA II kit, Macherey–Nagel), and equal amounts of total RNA were reverse-transcribed using hexanucleotide primers (Roche, Penzberg, Germany) and SuperScript III reverse transcriptase (Invitrogen). q-PCR for MYCN was performed using the Fast q-PCR Master Mix (Applied Biosystems, Darmstadt, Germany) and a StepOnePlus Cycler (Applied Biosystems). Signals were detected using a TaqMan sample set (MYCN, TIB MOLBIOL). MYCN expression was calculated relative to 18S rRNA and GAPDH of the same sample using the comparative threshold (ΔΔCt) method.
Cell lysates for MYCN Western blot were prepared by incubation of cells with RIPA buffer including protease inhibitor (Invitrogen) (4 °C, 10 min) and centrifugation (10,000×g, 4 °C, 30 min). Western blot was conducted as previously described [24]. Following antibodies were used: anti-HA (C29F4; Cell Signaling, Frankfurt am Main, Germany), MYCN-specific antibody B8.4.8 (Santa Cruz, Heidelberg, Germany), HRP-labeled anti-rabbit IgG (HA), anti-mouse IgG (MYCN) antibody (Bio-Rad; 1:3000, PBS, pH 7.4) and anti-actin antibody (clone AC15, Sigma-Aldrich). Signals were visualized using ECL™ Detection Reagents (Fisher Scientific, Schwerte, Germany) with a VersaDoc aperture (Bio-Rad). Image lab software (Bio-Rad) was utilized to estimate relative protein expression according to density of protein lanes.
Design and construction of full-length MYCN-DNA and minigene vaccines
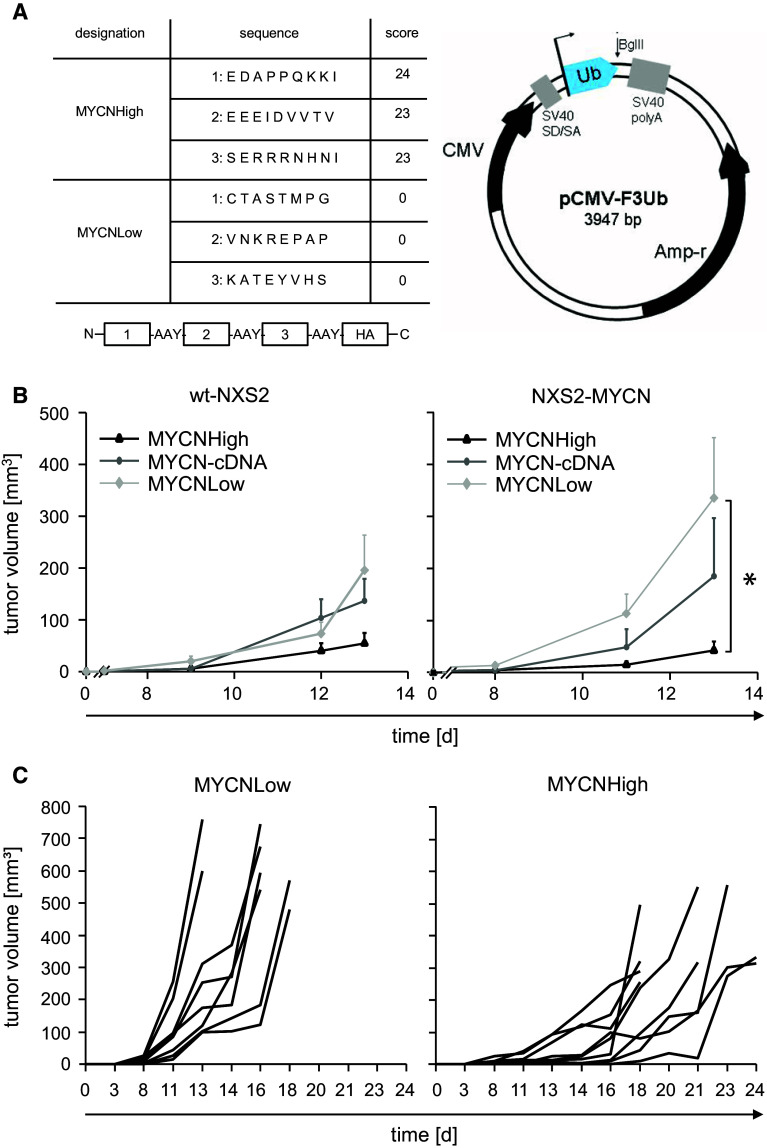
The sequence of the murine MYCN protein (accession number AAH49783) was analyzed for octamers and nonamers with high and low binding scores to the murine MHC class I molecule H2-Kk using the epitope prediction algorithm syfpeithi [13]. The predicted epitopes were not found in any other protein sequence. Two minigenes containing peptides of either high (MYCNHigh) or low (MYCNLow) binding affinity to MHC class I H2-Kk (Fig. 3a) separated by an AAY spacer for proteasomal degradation [25] were generated by overlapping PCR and ligated into pCMV-F3Ub [26, 27]. The full-length DNA vaccine (MYCN-cDNA) consisting of the murine MYCN-cDNA was inserted into the same expression vector. Sequences of all generated vaccines were verified by sequencing and will be provided upon request.
Fig. 3.
Vaccine design and efficacy of a MYCN-DNA vaccination. a The sequences of MYCNHigh and MYCNLow minigene epitopes identified by syfpeithi and their respective scores are shown. Epitopes were separated by an AAY spacer sequence, which represents a preferential proteasomal cleavage site (left panel). An HA tag was included to allow detection. Schematic of the expression vector pCMV-F3Ub with the BglII restriction site for insertion of minigenes (right panel). b Primary tumor growth of wt-NXS2 cells (n = 6, left panel) or NXS2-MYCN (n = 9, right panel) was analyzed after DNA vaccination with attenuated Salmonella typhimurium SL7207 as described in materials and methods. Results are presented as mean tumor volume in mm3 ± SE. The MYCNHigh-vaccinated group: black triangles, MYCN-cDNA: dark gray circles, MYCNLow: light gray diamonds. Differences in the NXS2-MYCN model growth were statistically significant (*p = 0.0019) in contrast to the wt-NXS2 tumor model (p = 0.0887). c Individual tumor growth in the NXS2-MYCN model immunized with MYCNLow (left panel) and MYCNHigh (right panel). One mouse died in the MYCNLow experimental group during vaccination. The time period between tumor challenge and tumor removal (size > 800 mm3/necrosis) was significantly extended in MYCNHigh-vaccinated mice (median 21 days) compared to MYCNLow-vaccinated mice (median 16 days) (*p < 0.05)
Preparation of DNA vaccines for oral delivery
The attenuated Salmonella typhimurium strain SL7207 (AroA-) was used as vehicle for oral application as previously described [11, 12].
Syngeneic neuroblastoma models
Syngeneic female A/J mice (8–10 weeks old) were purchased from Harlan-Laboratories (Mannheim, Germany) and housed according to the German guidelines for the care and use of laboratory animals, i.e., Tierschutzgesetz. Analysis of anti-tumor response in the wt-NXS2 (n = 6) and the NXS2-MYCN (n = 9) model was performed separately. Mice were vaccinated three times with MYCNHigh, MYCN-cDNA or MYCNLow in weekly intervals by oral gavage of 3 × 108 SL7207 as vaccine carrier. Seven days after the last immunization, mice were challenged by s.c. injection of 3 × 106 NB cells followed by analysis of primary tumor growth. Tumor growth was determined as previously described [28]. Primary tumors were surgically removed, when tumors reached a volume of 800 mm3 or showed signs of necrosis. Time of wound healing after surgery as well as inflammation, tissue fragility and bleeding were analyzed at necropsy.
Chromium release assay
Splenocytes of treated and control mice were harvested and cultured in RPMI 1640 (10 % FCS, 100 mg/ml P/S, 50 µmol/l β-mercaptoethanol (Sigma-Aldrich, Steinheim, Germany), with 100 IU/ml interleukin-2 for 5 days. NXS2-MYCN, wt-NXS2 and SCK cells pulsed with the three MYCN peptides (Fig. 3a) were used as MYCN-presenting target cells and compared to untreated SCK cells (negative control). Pulsing of SCK cells and chromium release assays was performed as previously described [29].
Analysis of IFN-γ
IFN-γ release was determined by ELISA (Mouse IFN-γ ELISA MAX™ Standard; Biolegend, San Diego, USA). Irradiated NXS2-MYCN or wt-NXS2 target cells (1 × 104) were cultured with splenocytes (E/T 100:1) from vaccinated or control mice in RPMI 1640 supplemented with 10 % FCS, 100 μg/ml P/S, 50 μmol/l β-mercaptoethanol and 100 IU/ml IL-2 (Sigma-Aldrich, Steinheim, Germany). Supernatants (50 µl) were collected over time and analyzed.
Immunohistochemistry
Cryo-sections of primary tumors (NXS2-MYCN) were analyzed for infiltrating T cells using rat anti-mouse CD8 and CD4 antibodies [both 1:25 (1 µg)] (anti-CD8 clone 53–6.7, rat (LOU) IgG2a, κ; anti-CD4 clone RM4-5, rat (DA) IgG2a, κ; BD Pharmingen, Heidelberg, Germany) for 2 h and polyclonal goat anti-rat IgG–biotin secondary antibody (anti-rat IgG clone G28-5; BD Pharmingen) for 1 h in Tris-buffered saline (TBS, pH 7.5) (1:125) and 2.5 % goat serum (Vector Laboratories, Peterborough, UK). Staining was accomplished as previously described [24]. To determine the average number of infiltrating T cells per µm2 of tumor tissue, 102 of 0.25 µm2 on the slides were analyzed under the microscope at a magnification of 400×.
Statistics
Mann–Whitney U test and log-rank test were used to determine statistical significance for nonparametric data. For parametric data, unpaired t test was applied. Differences were considered significant at *p < 0.05.
Results
MYCN and leukocyte infiltration in neuroblastoma
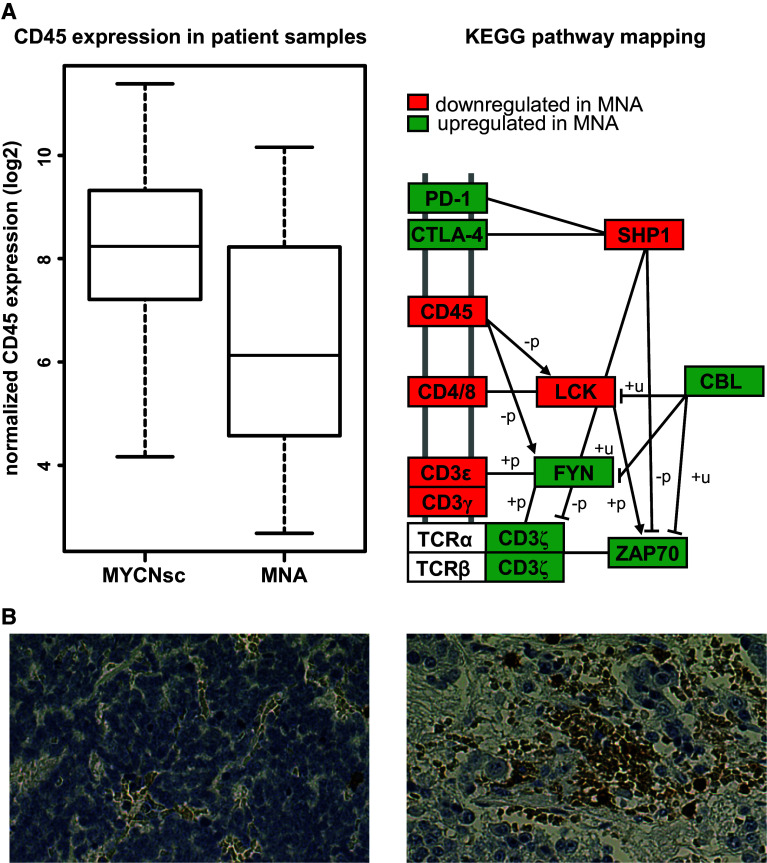
We investigated the correlation of MNA and leukocyte infiltration of 101 neuroblastoma tumor tissue samples by mRNA profiling and tissue microarray analyses (Fig. 1a). The CD45 mRNA expression is significantly lower in MNA patients (N = 19) compared to single copy MYCN controls (N = 82, p = 0.005). This finding was corroborated by immunohistochemistry, as all five MNA cases available for analysis revealed low or absent leukocyte infiltrates in contrast to MYCN single copy cases (Fig. 1b). Further analysis of the immune environment in MNA tumors was performed by KEGG pathway mapping, which indicated an up-regulation of inhibitors of T cell activation including CTLA-4 and PD1. Additionally, down-regulation of CD4/CD8 gene transcripts was identified in MNA tumors, further underlining immune-suppressive activity of MYCN. These findings provide an important rationale to target MYCN by means of DNA vaccination.
Fig. 1.
MYCN and leukocyte infiltration in neuroblastoma tissue. a MYCN and CD45 mRNA expression was analyzed by Affymetrix HuEx1.0 arrays in 101 NB patients (left panel). The difference of CD45 mRNA expression in MYCN-amplified patients (MNA; n = 19) compared to MYCN single copy cases (MYCNsc; n = 82) was statistically significant (p = 0.005). KEGG pathway mapping of corresponding transcriptomes shows up (green)- and down (red)-regulated genes involved in T cell signaling, and “+p” and “−p” depict phosphorylation and dephosphorylation, respectively, while “+u” denotes ubiquitination (right panel). b CD45 staining of representative samples from MNA (left) and MYCN single copy (right) tumor tissue (×40 magnification)
Generation and characterization of the NXS2-MYCN cell line
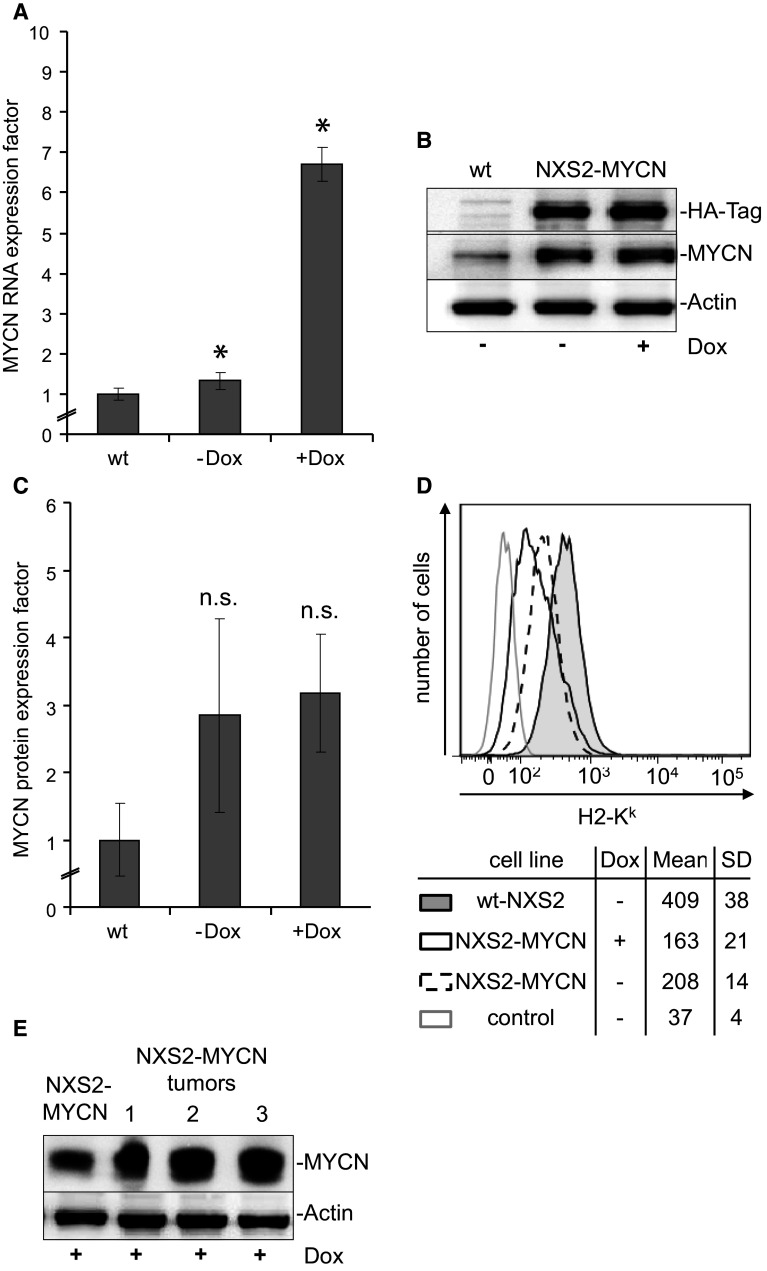
To establish a MYCN overexpression in NXS2 cells, wt-NXS2 cells were transfected with the doxycycline-inducible expression vector system T-REx™ encoding for an HA-tagged MYCN. After selection with Zeocin™ and blasticidin, stable transfection in single clones was verified by PCR, TaqMan q-PCR and Western blot analysis. One distinct clone was selected (NXS2-MYCN) and used for further studies. Typical characteristics of MYCN-amplified cells were demonstrated including an accelerated proliferation and sensitization for drug-induced apoptosis (data not shown).
MYCN-mRNA (Fig. 2a) and MYCN protein (Fig. 2b, c) expression increased by factors 7 (RNA) and 3 (protein) in NXS2-MYCN cells (4 µg/ml doxycycline, 6 h) compared to the wt-NXS2 cells. In these analyses, a weak endogenous MYCN-mRNA and protein expression was also demonstrated in wt-NXS2 cells (Fig. 2a, c). Interestingly, the doxycycline-induced difference in MYCN-mRNA expression (Fig. 2a) in NXS2-MYCN cells was not reflected by the MYCN protein expression (Fig. 2b, c). A posttranscriptional effect or “leakiness” of the NXS2-MYCN cell line could lead to the observed accumulation of the protein in the transfected cells [30]. However, the HA-tagged MYCN protein was only found in transfected cells (Fig. 2b). MYCN antigen expression was also analyzed in primary tumors induced by s.c. injection of NXS2-MYCN cells in A/J mice. To provide for highest MYCN transgene expression, mice constantly received doxycycline. In these tumors, MYCN protein expression was stable over time as demonstrated by Western blot analysis (Fig. 2e).
Fig. 2.
Generation and characterization of NXS2-MYCN cells. a MYCN-mRNA expression was determined by TaqMan q-PCR and analyzed following the comparative threshold (ΔΔCt) method. Bars represent MYCN RNA expression factors calculated by MYCN expression levels (2−ΔΔct) of NXS2-MYCN-transfected cells (±Dox) divided by that of wt-NXS2 cells (wt). Results represent mean values ± SD, n = 3 (*p < 0.05). b MYCN protein and MYCN-HA transgene expression were analyzed by Western blot before and 6 h after doxycycline treatment. c MYCN protein expression levels were related to the level of actin expression in each experiment (n = 3). Bars represent MYCN protein expression factors calculated from ratios of NXS2-MYCN-transfected cells (±Dox) divided by that of wt-NXS2 cells (wt) (mean values ± SD, n = 3). Increased protein expression in NXS2-MYCN-transfected (±Dox) cells compared to wt-NXS2 cells was not statistically significant (p > 0.1). The difference in NXS2-MYCN-transfected cells with doxycycline (+Dox) compared to NXS2-MYCN-transfected cells without doxycycline (−Dox) was not significant (p = 0.126). d Expression of H2-Kk on the cell surface of wt-NXS2 (filled black histogram) and untreated (dashed black histogram) as well as doxycycline-treated NXS2-MYCN (black histogram) was analyzed using flow cytometry and compared to isotype control used with wt-NXS2 cells (gray histogram). Results in the table below the histograms are presented as mean of geometric means of three experiments ± SD. H2-Kk expression on the cell surface of NXS2-MYCN is reduced but still detectable (*p < 0.05). e Western blot analysis of MYCN protein expression in NXS2-MYCN cells and three NXS2-MYCN primary tumors (1–3) from mice constantly treated with doxycycline
For vaccination experiments, sufficient expression of the MHC class I molecule H2-Kk is an important prerequisite. MYCN expression was described to have an impact on MHC class I expression [31]. H2-Kk was analyzed by flow cytometry (Fig. 2d). Increased MYCN expression after stable transfection of NXS2 resulted in significantly reduced but detectable H2-Kk expression on the cell surface of NXS2-MYCN, which was considered to be sufficient for further vaccination experiments.
Application of DNA vaccines encoding for MYCN-cDNA or its MHC class I epitopes reduced primary tumor growth without induction of autoimmunity
Mice received three oral applications of S. typhimurium (SL7207) carrying the respective MYCN-DNA vaccines (Fig. 3a) in weekly intervals starting 3 weeks prior to tumor challenge. The efficacy of the vaccines was analyzed in syngeneic tumor models established with wt-NXS2 and NXS2-MYCN cells (Fig. 3b) representing low and high MYCN target expression, respectively.
In the wt-NXS2 model (n = 6), we observed a reduction in primary tumor growth (70 %, day 13) in mice treated with the MYCNHigh minigene vaccine, in contrast to the MYCNLow control group (Fig. 3b, left panel). The full-length MYCN-cDNA vaccine was less effective compared to the MYCNLow control (30 %, day 13).
In the MYCN-NXS2 model (n = 9), mice immunized with the MYCNHigh minigene vaccine showed a significant 80 % reduction in primary tumor growth in contrast to controls (MYCNLow *p = 0.0019) (Fig. 3b, right panel). Interestingly, vaccination of mice with the minigene vaccine (MYCNHigh) resulted in a superior anti-tumor effect compared to the full-length MYCN-cDNA vaccine that reduced tumor growth by 45 % (day 13) compared to controls.
We also analyzed time to tumor surgery of individual mice of the MYCN-NXS2 tumor mice (Fig. 3c). Primary tumors of NXS2-MYCN cells had to be removed according to our institutional requirements when they reached a size of 800 mm3 or when tumors developed signs of necrosis. Results show that primary tumor growth in MYCNHigh-immunized mice is significantly delayed by an average of 5 days compared to MYCNLow-immunized controls (p = 0.0399, log-rank test). Notably, some tumors within the MYCNHigh-vaccinated group showed an early necrosis indicating an anti-tumor immune response and had to be removed before they reached the size of 800 mm3 (Fig. 3c, right panel).
Importantly, no vaccination-induced autoimmune response and potential toxic side effects were observed in our in vivo studies. The duration of wound healing (10 days) after removal of primary tumors was identical for all experimental groups, indicating unaffected cell survival and proliferation. Macroscopic analysis of organs of all treated groups did not reveal any signs of severe autoimmune reactions, such as bleeding, hyperemia, tissue fragility or inflammation (data not shown).
MYCN-DNA vaccination was associated with increased presence of CD4+ and CD8+ T cells at the primary tumor site
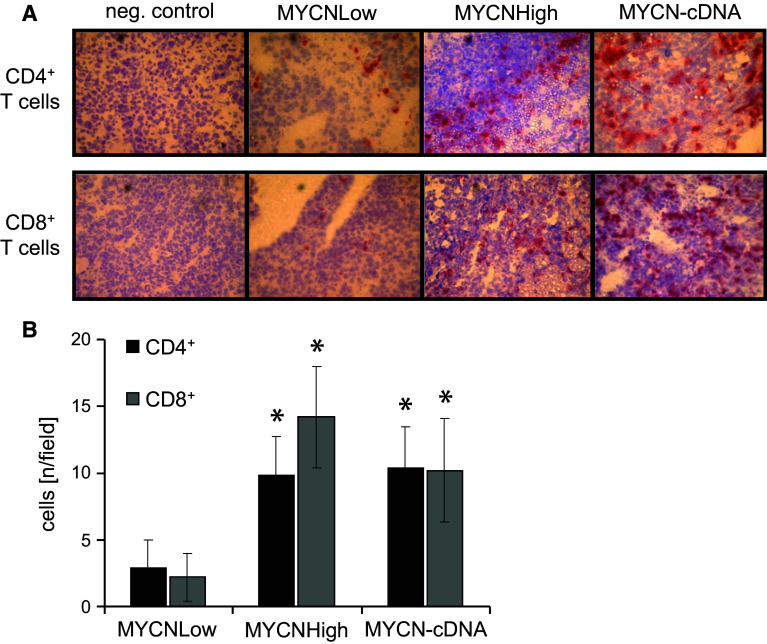
To further characterize the immune response induced by MYCN-DNA vaccination, primary tumors of immunized and control mice were analyzed by immunohistochemistry (Fig. 4). Increased infiltration of tumors with both CD4+ and CD8+ T cells was clearly found in mice immunized with MYCNHigh and MYCN-cDNA to a similar extent (Fig. 4a). Analysis of heart, kidneys, ovaries, lungs and adrenal medulla of MYCNHigh- or MYCN-cDNA-immunized mice showed no increased infiltration with CD4+ or CD8+ T cells compared to untreated and MYCNLow-treated mice (data not shown). Tumors of mice vaccinated with MYCNHigh or MYCN-cDNA showed an up to sevenfold increase in infiltrating CD4+ and CD8+ T cells compared to the MYCNLow group (Fig. 4b), suggesting the induction of MYCN-specific T cells by the vaccine.
Fig. 4.
Analysis of tumor-infiltrating CD4+ and CD8+ lymphocytes. a Tumors of each immunized mouse were stained. Red staining indicates infiltration with CD4+ T cells (upper row) and CD8+ T cells (lower row), respectively. b Numbers of CD8+ T cells (gray bars) and CD4+ T cells (black bars) per field were determined by light microscopy (ten fields, ×40 magnification, mean value ± SD) (*p < 0.05)
MYCN-DNA vaccination induced a cytotoxic MYCN-specific anti-NB immune response involving IFN-γ and increased target cell lysis
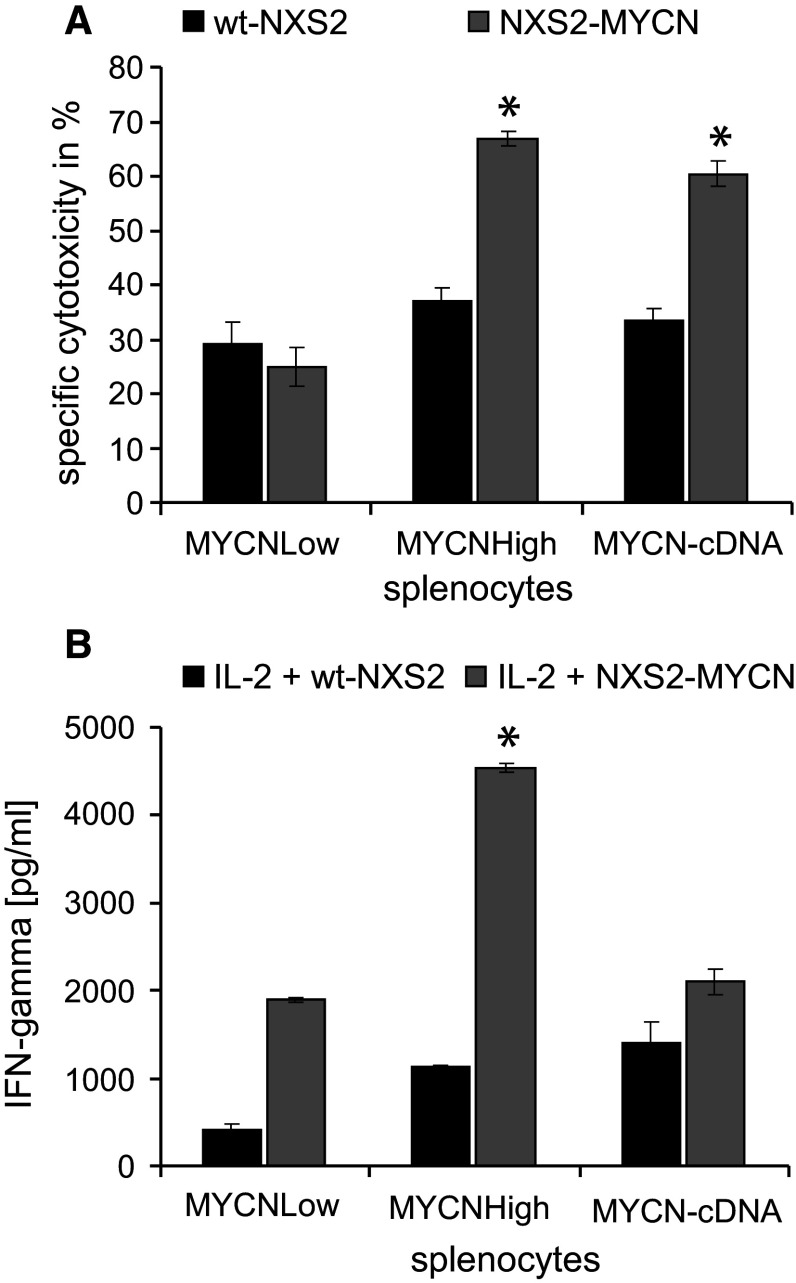
MYCN specificity of the response following MYCN-DNA vaccination was analyzed in cytotoxicity assays using splenocytes isolated from vaccinated groups of wt-NXS2 and MYCN-NXS2 in vivo models and target cells with differential MYCN antigen expression at an E/T ratio of 100:1 (Fig. 5a). Effector cells isolated from MYCNHigh-vaccinated NXS2-MYCN mice showed a 50 % (2.3-fold) higher cytotoxicity toward MYCN-expressing NXS2-MYCN target cells compared to splenocytes from MYCNLow-treated mice. In contrast, specific cytotoxicity of splenocytes from MYCNHigh-vaccinated mice toward wt-NXS2 target cells was significantly lower than toward NXS2-MYCN target cells and comparable to that of splenocytes isolated from MYCNLow-vaccinated mice. Interestingly, in vitro cytotoxicity of splenocytes from MYCN-cDNA-treated animals toward wt-NXS2 and NXS2-MYCN was comparable to that of splenocytes from the MYCNHigh-vaccinated group (Fig. 5a). These results indicate that the immune response is MYCN specific. This was further confirmed by quantification of IFN-γ release, an indicator for activated T cells in isolated splenocytes. IFN-γ was measured in response to co-incubation of effector cells with IL-2 and wt-NXS2 or NXS2-MYCN target cells (Fig. 5b). Only splenocytes from MYCNHigh-vaccinated wt-NXS2 mice showed a significant 2.5-fold elevated secretion of the TH1 cytokine IFN-γ after co-culture with irradiated NXS2-MYCN cells and IL-2 compared to splenocytes from the MYCNLow and also MYCN-cDNA group (Fig. 5b). Interestingly, splenocytes from all groups of mice showed a reduced IFN-γ release when wt-NXS2 instead of NXS2-MYCN cells were used in co-culture.
Fig. 5.
Cytotoxicity and IFN-γ release of splenocytes isolated from MYCN-vaccinated mice. a Cytotoxicity of splenocytes harvested in the NXS2-MYCN model toward wt-NXS2 (black bars) and MYCN-NXS2 (gray bars) as target cells was analyzed by 51Cr release assays (E/T ratio 100:1). Results are presented as mean cytotoxicity of three independent experiments ± SD. Differences between MYCNHigh- and MYCN-cDNA-vaccinated groups toward NXS2-MYCN target cells were significantly higher than toward wt-NXS2 (*p < 0.005). b CTL activation was determined by release of IFN-γ. Splenocytes were co-incubated (5 days) with irradiated wt-NXS2 (black bars) or NXS2-MYCN (gray bars) cells (E/T ratio 100:1). Results are presented as mean cytotoxicity of three independent experiments ± SD (*p < 0.005)
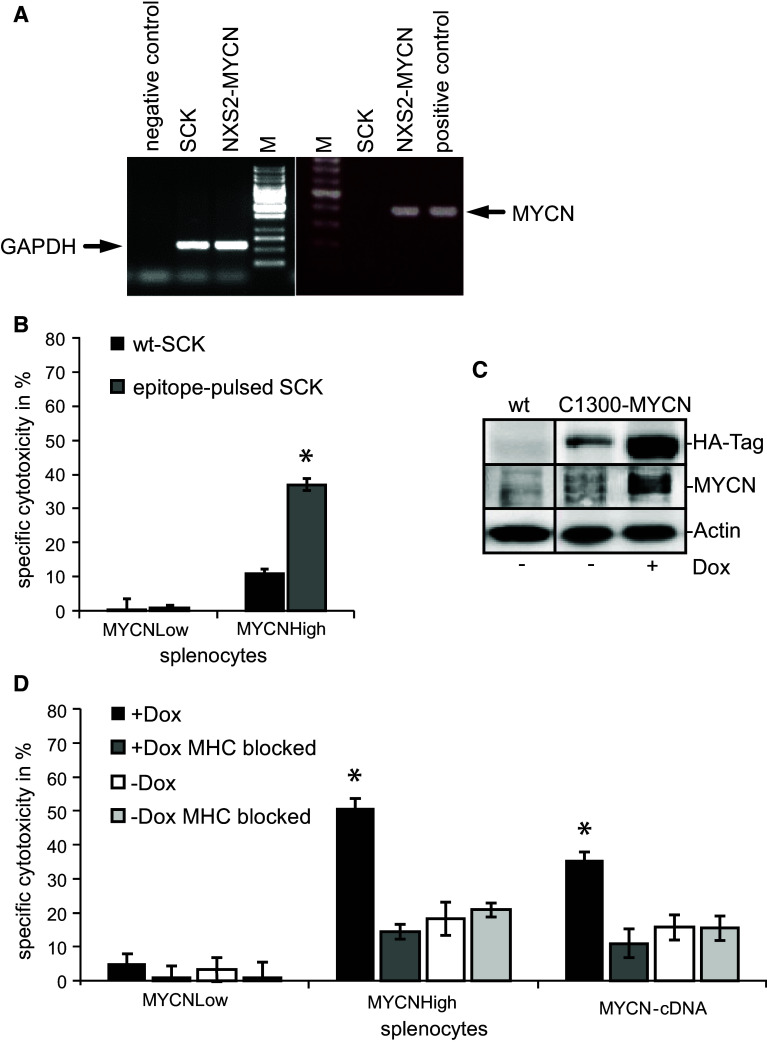
In order to confirm MYCN epitope specificity, the MYCN-negative mamma carcinoma cell line SCK (syngeneic to A/J mice, MHC class I H2-Kk positive, MYCN negative (Fig. 6a) was pulsed with the three MYCN peptides (EDAPPQKKI, EEEIDVVTV and SERRRNHNI).
Fig. 6.
Analysis of the mediated immune response and the MYCN specificity. a Differential MYCN expression in NXS2-MYCN and SCK mammary carcinoma cells. mRNA from both cells was isolated followed by reverse transcription and MYCN-specific PCR. GAPDH served as control. b MYCN epitope specificity was analyzed with splenocytes of immunized and control mice stimulated with IL-2 for 5 days. Cytotoxic activity against wt-SCK cells (black bars) or SCK cells pulsed with the three specific MYCN epitopes (gray bars) was analyzed in 51Cr release assays (E/T ratio 60:1) (mean in % ± SD, n = 3, *p < 0.005). c Western blot analysis of C1300-MYCN cells demonstrates a high transgene expression while treated with Dox (6 h) and a low expression in the absence of Dox. d MHC class I antigen molecule H2-Kk expressed on C1300-MYCN target cells was blocked by addition of anti-H2-Kk antibodies in cytotoxicity assays with (E/T ratio 50:1) (dark and light gray bars) while incubated in the absence (white and light gray bars) or presence (black and dark gray bars) of doxycycline. Black bars indicate unblocked cells. As effector cells, splenocytes from MYCNHigh-, MYCN-cDNA- and MYCNLow-treated NXS2-MYCN mice were isolated and stimulated for 5 days with IL-2. Differences in specific cytotoxicity (in % ± SD, n = 3) were statistically significant (*p < 0.05)
Effector cells of NXS2-MYCN mice immunized with the MYCNHigh vaccine mediated a significant cytotoxic effect against MYCN peptide-pulsed SCK target cells in contrast to unpulsed wt-SCK target cells (Fig. 6b). Splenocytes from the MYCNLow-vaccinated group were ineffective in mediating cytotoxicity toward MYCN peptide-pulsed SCK cells. These results indicate that the immune response induced by the MYCNHigh minigene vaccine is MYCN epitope specific.
MYCN specificity was also demonstrated using C1300-MYCN target cells with a doxycycline-inducible MYCN transgene at an E/T ratio of 50:1. This cell line is also syngeneic to A/J mice and expresses the MHC class I molecule H2-Kk on the cell surface. It shows no endogenous and only a weak transgene MYCN expression without doxycycline, but a high MYCN transgene expression in the presence of doxycycline (Fig. 6c). Doxycycline treatment of C1300-MYCN and subsequent MYCN induction resulted in significantly increased lysis mediated by splenocytes from MYCNHigh-vaccinated NXS2-MYCN mice, compared to untreated C1300-MYCN (50 % compared to 20 % lysis, respectively) (Fig. 6d). In order to show that the immune response is mainly mediated by CD8+ cytotoxic T cells, we demonstrated MHC class I restriction of the cytolytic response by blocking the MHC class I molecule H2-Kk. In fact, addition of an anti-H2-Kk mAb resulted in a 3.6-fold reduction in the MYCNHigh effector cell-mediated lysis of doxycycline-treated C1300-MYCN target cells. Consequently, addition of anti-H2-Kk mAb to antigen-negative C1300-MYCN cells that were not pre-treated with doxycycline had no impact on MYCNHigh lymphocyte-mediated lysis (Fig. 6d). Interestingly, lysis mediated by splenocytes isolated from MYCN-cDNA-vaccinated mice was comparable to that of splenocytes from MYCNHigh-vaccinated mice. Here, the addition of anti-H2-Kk mAb resulted in a 3.4-fold reduction in cell-mediated lysis of doxycycline-treated C1300-MYCN target cells.
In summary, we demonstrated in vivo activity of MYCN-DNA vaccination mediated by the induction of a MYCN-specific and MHC class I-restricted immune response, which is a typical feature of CD8+ T cells.
Discussion
MNA is the most significant marker for poor prognosis in NB and associated with rapid tumor progression and therapy-resistant relapses. Here we provide evidence that the immune-suppressive activity of MYCN (down-regulation of T cell activation and up-regulation of T cell inhibitory pathways, Fig. 1; down-regulation of MHC class I expression, Fig. 2d) could contribute to this clinical phenomenon. These findings are underlined by comparable observations with different malignant cells in other studies [22, 32–34].
Due to its high expression in MNA tumors and absent expression in healthy tissue after birth [2, 3], it is a promising target for new treatment approaches. Small-molecule inhibitors of the MYCN transcription factor are not easy to identify. Indirect inhibitors of MYCN-regulated gene products, e.g., BET—bromodomain, or Aurora-A inhibitors [4], are currently under investigation. We explored an immunotherapeutic approach to directly targeting MYCN. The strategy to immunize against MYCN is further supported by the fact that MYCN-specific CTLs were identified in NB patients with genomic MNA, indicating that MYCN can induce T cell-mediated immune responses [5]. Based on these considerations, we analyzed the potential of MYCN-DNA vaccines to induce MYCN-specific CTLs capable of mediating an effective immune response NB.
To avoid the expression of a potentially hazardous protein, we also generated a minigene DNA vaccine encoding for MYCN-derived peptides (Fig. 3a). These nona- and octamer peptides are MHC class I-restricted CTL epitopes identified by the syfpeithi epitope prediction algorithm [13]. Interestingly, studies suggest that epitopes encoded by minigenes could be more immunogenic than identical sequences encoded in full-length proteins [35].
In order to test our MYCN-DNA vaccination approach in vivo, we adapted the immunocompetent A/J mouse model, which is a suitable system to analyze active immunotherapeutic strategies against NB by generating NXS2 cells with high MYCN antigen expression (Fig. 2a–c) [11, 12, 28]. Characterization of these cells revealed sufficient expression of the MHC class I molecules for vaccination studies (Fig. 2d).
Here, we showed for the first time a strong induction of an anti-NB immune response (Fig. 3b, c). This immune response was characterized by elevated cellular cytotoxicity against NXS2 and NXS2-MYCN and increased secretion of IFN-γ by splenocytes isolated form vaccinated mice (Fig. 5). IFN-γ is known to up-regulate MHC class I molecules, resulting in increased T cell stimulation and T cell migration into the tumor site to eradicate cancer cells. Indeed, we found increased presence of CD4+ as well as CD8+ T cells at the primary tumor site (Fig. 4). CD4+ T cells are necessary for the initial process of CD8+ T effector cell activation and the induction of a CD8+ T cell memory [36–38]. The induction of tumor-infiltrating CTLs was further indicated by cytotoxicity assays and IFN-γ ELISAs and resulted in reduced tumor growth (Figs. 5, 6).
Importantly, observed anti-tumor responses were MYCN specific and MHC class I restricted as shown in Figs. 4, 5 and 6. In our experiments, the anti-tumor effect, specific lysis and IFN-γ secretion were significantly higher using NXS2-MYCN as target cells compared to wt-NXS2 cells as controls, demonstrating MYCN specificity of the induced immune response (Figs. 4, 5, 6).
Interestingly, the MYCNHigh minigene was more effective in vaccination experiments against high and low MYCN-expressing NB compared to the cDNA vaccine encoding for the entire range of MYCN MHC class I epitopes within the MYCN protein sequence (Figs. 3, 5). This finding suggests that a selection of high-affinity MHC class I epitope sequences provides an advantage over a wide range of MYCN epitopes derived from the entire MYCN sequence that also contains peptides with lower affinity. Importantly, minigene-based DNA vaccination is advantageous compared to the application of a full-length DNA vaccine with respect to safety of the therapeutic approach. Our results support the concept of rationally designed DNA vaccines by epitope prediction and demonstrate that the affinity of the selected epitopes as well as an optimal antigen processing is of key importance in inducing an effective immune response [27, 39].
Therefore, customized minigene DNA vaccine design for patients is interesting and applicable even if the tumor-associated antigen of choice is potentially hazardous [11]. Supporting this, we were able to demonstrate that MYCN vaccination was not associated with self-reactive CTLs in other tissues, suppression of wound healing or induction of autoimmunity despite abundant expression of the MYC homolog in healthy tissues. This is most likely related to the absence of an inflammatory microenvironment in healthy tissue in contrast to tumors [40].
Addressing the problem of down-regulated MHC class I expression in neuroblastoma, it has previously been shown that specific lysis by high-affinity CTLs can be induced by only a small number (<10–100) of ligands per cell [41–43]. Importantly, our vaccination strategy using attenuated Salmonella as a vehicle for DNA vaccines provides for the stimulation of the innate immune system and for a “danger signal” associated with the production of cytokines (interleukin-12, IFN-α and IFN-γ). These cytokines are known to induce up-regulation of MHC class I expression in and other genes encoding necessary subunits of the immunoproteasome to improve antigen presentation in neuroblastoma cells [44, 45].
In summary, we report the generation of MYCN-based minigene and full-length DNA vaccines and demonstrate their efficacy in a MYCN-expressing NB model using Salmonella typhimurium SL7207 as a vaccine carrier without signs of autoimmunity. These results indicate that MYCN-expressing NB cells are accessible for MHC class I-restricted immune responses induced by DNA vaccination.
Currently, immunotherapeutic approaches based on chimeric antigen receptor (CAR)-expressing effector cells or the application of antibodies are intensively studied in a variety of malignancies including neuroblastoma [46]. Although CAR technology offers the advantage of direct application of specific immune cells, generation of these effector cells for each individual patient requires substantial resources, in contrast to minigene DNA vaccination as a cost-effective and easy-to-handle alternative [47]. Minigene DNA vaccination also compares favorably to CAR technology or the application of antibodies due to the induction of immunological memory potentially resulting in a long-lasting anti-tumor immune response. Nevertheless, all of these immunotherapeutic approaches are promising alternatives to further improve the efficacy of current therapeutic protocols, and the choice of one approach over the other probably depends on the nature of the antigen and the immune status of the patient in each individual case. Based on the relevance of the intracellular antigen MYCN for NB patients and our results, we provide proof of concept that DNA vaccination targeting MYCN could provide an important new additional strategy to treat this malignant phenotype of NB.
Acknowledgments
This work was supported by NGFNplus (Bundesministerium für Bildung und Forschung, ENGINE).
Conflict of interest
We have no conflict of interest to disclose.
Ethical approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Abbreviations
- BET
Extraterminal bromodomain Barone
- CAR
Chimeric antigen receptors
- CD
Cluster of differentiation
- cDNA
Copy DNA
- CHO
Chinese hamster ovary
- CTLs
Cytotoxic T cells
- DOX
Doxycycline
- ELISA
Enzyme-linked immunosorbent assay
- FACS
Fluorescence-activated cell sorting
- FCS
Fetal calf serum
- gDNA
Genomic DNA
- H
Hour
- IFN
Interferon
- KEGG
Kyoto encyclopedia of genes and genomes
- LPS
Lipopolysaccharide
- MHC
Major histocompatibility complex
- MYCN
Neuroblastoma-derived myelocytomatosis viral-related oncogene
- MNA
MYCN amplification
- NB
Neuroblastoma
- PCR
Polymerase chain reaction
- RT
Room temperature
- SD
Standard deviation
- TAA
Tumor-associated antigen
- TBS
Tris-buffered saline
- TH
Tyrosine hydroxylase
- TMA
Tissue microarray
- wt
Wild type
References
- 1.Maris JM. Recent advances in neuroblastoma. N Engl J Med. 2010;362:2202–2211. doi: 10.1056/NEJMra0804577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamada S. Expression of c-myc and N-myc in mouse embryos during craniofacial development. Kokubyo Gakkai Zasshi. 1990;57:83–105. doi: 10.5357/koubyou.57.83. [DOI] [PubMed] [Google Scholar]
- 3.Zimmerman K, Alt FW. Expression and function of myc family genes. Crit Rev Oncog. 1990;2:75–95. [PubMed] [Google Scholar]
- 4.Barone G, Anderson J, Pearson AD, Petrie K, Chesler L. New strategies in neuroblastoma: therapeutic targeting of MYCN and ALK. Clin Cancer Res. 2013;19:5814–5821. doi: 10.1158/1078-0432.CCR-13-0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarkar AK, Nuchtern JG. Lysis of MYCN-amplified neuroblastoma cells by MYCN peptide-specific cytotoxic T lymphocytes. Cancer Res. 2000;60:1908–1913. [PubMed] [Google Scholar]
- 6.Donnelly JJ, Ulmer JB, Shiver JW, Liu MA. DNA vaccines. Annu Rev Immunol. 1997;15:617–648. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- 7.Matzinger P. An innate sense of danger. Ann N Y Acad Sci. 2002;961:341–342. doi: 10.1111/j.1749-6632.2002.tb03118.x. [DOI] [PubMed] [Google Scholar]
- 8.Krieg AM. The role of CpG motifs in innate immunity. Curr Opin Immunol. 2000;12:35–43. doi: 10.1016/S0952-7915(99)00048-5. [DOI] [PubMed] [Google Scholar]
- 9.Sizemore DR, Branstrom AA, Sadoff JC. Attenuated Shigella as a DNA delivery vehicle for DNA-mediated immunization. Science. 1995;270:299–302. doi: 10.1126/science.270.5234.299. [DOI] [PubMed] [Google Scholar]
- 10.Fest S, Huebener N, Weixler S, Bleeke M, Zeng Y, Strandsby A, Volkmer-Engert R, Landgraf C, Gaedicke G, Riemer AB, Michalsky E, Jaeger IS, Preissner R, Forster-Wald E, Jensen-Jarolim E, Lode HN. Characterization of GD2 peptide mimotope DNA vaccines effective against spontaneous neuroblastoma metastases. Cancer Res. 2006;66:10567–10575. doi: 10.1158/0008-5472.CAN-06-1158. [DOI] [PubMed] [Google Scholar]
- 11.Fest S, Huebener N, Bleeke M, Durmus T, Stermann A, Woehler A, Baykan B, Zenclussen AC, Michalsky E, Jaeger IS, Preissner R, Hohn O, Weixler S, Gaedicke G, Lode HN. Survivin minigene DNA vaccination is effective against neuroblastoma. Int J Cancer. 2009;125:104–114. doi: 10.1002/ijc.24291. [DOI] [PubMed] [Google Scholar]
- 12.Huebener N, Fest S, Strandsby A, Michalsky E, Preissner R, Zeng Y, Gaedicke G, Lode HN. A rationally designed tyrosine hydroxylase DNA vaccine induces specific antineuroblastoma immunity. Mol Cancer Ther. 2008;7:2241–2251. doi: 10.1158/1535-7163.MCT-08-0109. [DOI] [PubMed] [Google Scholar]
- 13.Rammensee H, Bachmann J, Emmerich NP, Bachor OA, Stevanovic S. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics. 1999;50:213–219. doi: 10.1007/s002510050595. [DOI] [PubMed] [Google Scholar]
- 14.Parker KC, Bednarek MA, Coligan JE. Scheme for ranking potential HLA-A2 binding peptides based on independent binding of individual peptide side-chains. J Immunol. 1994;152:163–175. [PubMed] [Google Scholar]
- 15.Whitton JL, Oldstone MB. Class I MHC can present an endogenous peptide to cytotoxic T lymphocytes. J Exp Med. 1989;170:1033–1038. doi: 10.1084/jem.170.3.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitton JL. Induction of protective immunity using minigenes. Ann N Y Acad Sci. 1994;730:107–117. doi: 10.1111/j.1749-6632.1994.tb44243.x. [DOI] [PubMed] [Google Scholar]
- 17.Huebener N, Lange B, Lemmel C, Rammensee HG, Strandsby A, Wenkel J, Jikai J, Zeng Y, Gaedicke G, Lode HN. Vaccination with minigenes encoding for novel ‘self’ antigens are effective in DNA-vaccination against neuroblastoma. Cancer Lett. 2003;197:211–217. doi: 10.1016/S0304-3835(03)00102-2. [DOI] [PubMed] [Google Scholar]
- 18.Lewen S, Zhou H, Hu H, Cheng T, Markowitz D, Reisfeld R, Xiang R, Luo Y. A Legumain-based minigene vaccine targets the tumor stroma and suppresses breast cancer growth and angiogenesis. Cancer Immunol Immunother. 2008;57:507–515. doi: 10.1007/s00262-007-0389-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lode HN, Huebener N, Zeng Y, Fest S, Weixler S, Gaedicke G. DNA minigene vaccination for adjuvant neuroblastoma therapy. Ann N Y Acad Sci. 2004;1028:113–121. doi: 10.1196/annals.1322.012. [DOI] [PubMed] [Google Scholar]
- 20.Berger E, Soldati R, Huebener N, Hohn O, Stermann A, Durmus T, Lobitz S, Zenclussen AC, Christiansen H, Lode HN, Fest S. Salmonella SL7207 application is the most effective DNA vaccine delivery method for successful tumor eradication in a murine model for neuroblastoma. Cancer Lett. 2013;331(2):167–173. doi: 10.1016/j.canlet.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 21.Levine MM, Galen J, Barry E, Noriega F, Chatfield S, Sztein M, Dougan G, Tacket C. Attenuated Salmonella as live oral vaccines against typhoid fever and as live vectors. J Biotechnol. 1996;44:193–196. doi: 10.1016/0168-1656(95)00094-1. [DOI] [PubMed] [Google Scholar]
- 22.Schramm A, Schowe B, Fielitz K, Heilmann M, Martin M, Marschall T, Koster J, Vandesompele J, Vermeulen J, De Preter K, Koster J, Versteeg R, Noguera R, Speleman F, Rahmann S, Eggert A, Morik K, Schulte JH. Exon-level expression analyses identify MYCN and NTRK1 as major determinants of alternative exon usage and robustly predict primary neuroblastoma outcome. Br J Cancer. 2012;107:1409–1417. doi: 10.1038/bjc.2012.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pajtler KW, Rebmann V, Lindemann M, Schulte JH, Schulte S, Stauder M, Leuschner I, Schmid KW, Kohl U, Schramm A, Eggert A. Expression of NTRK1/TrkA affects immunogenicity of neuroblastoma cells. Int J Cancer. 2013;133:908–919. doi: 10.1002/ijc.28096. [DOI] [PubMed] [Google Scholar]
- 24.Zeng Y, Huebener N, Fest S, Weixler S, Schroeder U, Gaedicke G, Xiang R, Schramm A, Eggert A, Reisfeld RA, Lode HN. Fractalkine (CX3CL1)- and interleukin-2-enriched neuroblastoma microenvironment induces eradication of metastases mediated by T cells and natural killer cells. Cancer Res. 2007;67:2331–2338. doi: 10.1158/0008-5472.CAN-06-3041. [DOI] [PubMed] [Google Scholar]
- 25.Velders MP, Weijzen S, Eiben GL, Elmishad AG, Kloetzel PM, Higgins T, Ciccarelli RB, Evans M, Man S, Smith L, Kast WM. Defined flanking spacers and enhanced proteolysis is essential for eradication of established tumors by an epitope string DNA vaccine. J Immunol. 2001;166:5366–5373. doi: 10.4049/jimmunol.166.9.5366. [DOI] [PubMed] [Google Scholar]
- 26.Michalek MT, Grant EP, Gramm C, Goldberg AL, Rock KL. A role for the ubiquitin-dependent proteolytic pathway in MHC class I-restricted antigen presentation. Nature. 1993;363:552–554. doi: 10.1038/363552a0. [DOI] [PubMed] [Google Scholar]
- 27.Sijts A, Zaiss D, Kloetzel PM. The role of the ubiquitin-proteasome pathway in MHC class I antigen processing: implications for vaccine design. Curr Mol Med. 2001;1:665–676. doi: 10.2174/1566524013363230. [DOI] [PubMed] [Google Scholar]
- 28.Lode HN, Reisfeld RA. Targeted cytokines for cancer immunotherapy. Immunol Res. 2000;21:279–288. doi: 10.1385/IR:21:2-3:279. [DOI] [PubMed] [Google Scholar]
- 29.Huebener N, Fest S, Hilt K, Schramm A, Eggert A, Durmus T, Woehler A, Stermann A, Bleeke M, Baykan B, Weixler S, Gaedicke G, Lode HN. Xenogeneic immunization with human tyrosine hydroxylase DNA vaccines suppresses growth of established neuroblastoma. Mol Cancer Ther. 2009;8:2392–2401. doi: 10.1158/1535-7163.MCT-09-0107. [DOI] [PubMed] [Google Scholar]
- 30.Rennel E, Gerwins P. How to make tetracycline-regulated transgene expression go on and off. Anal Biochem. 2002;309:79–84. doi: 10.1016/S0003-2697(02)00250-6. [DOI] [PubMed] [Google Scholar]
- 31.Forloni M, Albini S, Limongi MZ, Cifaldi L, Boldrini R, Nicotra MR, Giannini G, Natali PG, Giacomini P, Fruci D. NF-kappaB, and not MYCN, regulates MHC class I and endoplasmic reticulum aminopeptidases in human neuroblastoma cells. Cancer Res. 2010;70:916–924. doi: 10.1158/0008-5472.CAN-09-2582. [DOI] [PubMed] [Google Scholar]
- 32.Facchetti P, Prigione I, Ghiotto F, Tasso P, Garaventa A, Pistoia V. Functional and molecular characterization of tumour-infiltrating lymphocytes and clones thereof from a major-histocompatibility-complex-negative human tumour: neuroblastoma. Cancer Immunol Immunother. 1996;42:170–178. doi: 10.1007/s002620050267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin RF, Beckwith JB. Lymphoid infiltrates in neuroblastomas: their occurrence and prognostic significance. J Pediatr Surg. 1968;3:161–164. doi: 10.1016/0022-3468(68)91005-1. [DOI] [PubMed] [Google Scholar]
- 34.Pellat-Deceunynck C, Bataille R. Normal and malignant human plasma cells: proliferation, differentiation, and expansions in relation to CD45 expression. Blood Cells Mol Dis. 2004;32:293–301. doi: 10.1016/j.bcmd.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez F, An LL, Harkins S, Zhang J, Yokoyama M, Widera G, Fuller JT, Kincaid C, Campbell IL, Whitton JL. DNA immunization with minigenes: low frequency of memory cytotoxic T lymphocytes and inefficient antiviral protection are rectified by ubiquitination. J Virol. 1998;72:5174–5181. doi: 10.1128/jvi.72.6.5174-5181.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gerloni M, Zanetti M. CD4 T cells in tumor immunity. Springer Semin Immunopathol. 2005;27:37–48. doi: 10.1007/s00281-004-0193-z. [DOI] [PubMed] [Google Scholar]
- 37.Groux H. Type 1 T-regulatory cells: their role in the control of immune responses. Transplantation. 2003;75:8S–12S. doi: 10.1097/01.TP.0000067944.90241.BD. [DOI] [PubMed] [Google Scholar]
- 38.Tudor D, Dubuquoy C, Gaboriau V, Lefevre F, Charley B, Riffault S. TLR9 pathway is involved in adjuvant effects of plasmid DNA-based vaccines. Vaccine. 2005;23:1258–1264. doi: 10.1016/j.vaccine.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 39.Fruci D, Niedermann G, Butler RH, van Endert PM. Efficient MHC class I-independent amino-terminal trimming of epitope precursor peptides in the endoplasmic reticulum. Immunity. 2001;15:467–476. doi: 10.1016/S1074-7613(01)00203-5. [DOI] [PubMed] [Google Scholar]
- 40.Xiang R, Luo Y, Niethammer AG, Reisfeld RA. Oral DNA vaccines target the tumor vasculature and microenvironment and suppress tumor growth and metastasis. Immunol Rev. 2008;222:117–128. doi: 10.1111/j.1600-065X.2008.00613.x. [DOI] [PubMed] [Google Scholar]
- 41.Spierings DC, Agsteribbe E, Wilschut J, Huckriede A. Characterization of antigen-presenting properties of tumour cells using virus-specific cytotoxic T lymphocytes. Br J Cancer. 2000;82:1474–1479. doi: 10.1054/bjoc.1999.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sykulev Y, Joo M, Vturina I, Tsomides TJ, Eisen HN. Evidence that a single peptide–MHC complex on a target cell can elicit a cytolytic T cell response. Immunity. 1996;4:565–571. doi: 10.1016/S1074-7613(00)80483-5. [DOI] [PubMed] [Google Scholar]
- 43.Christinck ER, Luscher MA, Barber BH, Williams David B. Peptide binding to class I MHC on living cells and quantitation of complexes required for CTL lysis. Nature. 1991;352:67–70. doi: 10.1038/352067a0. [DOI] [PubMed] [Google Scholar]
- 44.Strehl B, Seifert U, Kruger E, Heink S, Kuckelkorn U, Kloetzel PM. Interferon-gamma, the functional plasticity of the ubiquitin-proteasome system, and MHC class I antigen processing. Immunol Rev. 2005;207:19–30. doi: 10.1111/j.0105-2896.2005.00308.x. [DOI] [PubMed] [Google Scholar]
- 45.Whitby FG, Masters EI, Kramer L, Knowlton JR, Yao Y, Wang CC, Hill CP. Structural basis for the activation of 20S proteasomes by 11S regulators. Nature. 2000;408:115–120. doi: 10.1038/35040607. [DOI] [PubMed] [Google Scholar]
- 46.Matthay KK, George RE, Yu AL. Promising therapeutic targets in neuroblastoma. Clin Cancer Res. 2012;18:2740–2753. doi: 10.1158/1078-0432.CCR-11-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heczey A, Louis CU. Advances in chimeric antigen receptor immunotherapy for neuroblastoma. Discov Med. 2013;16:287–294. [PMC free article] [PubMed] [Google Scholar]