Abstract
CD40 is a member of the TNF family of receptors that has been shown to play a crucial role in enhancing dendritic cell activity and fostering anti-tumor immune responses. In this study, we demonstrate the in vitro properties and in vivo efficacious activity of the CD40 agonist antibody, CP-870,893. CP-870,893 is a fully human, IgG2 antibody that selectively interacts with CD40 at a site distinct from its ligand-binding region with a KD of 0.4 nM. It enhances the expression of MHC class II, CD54, CD86, and CD23 on human B cells in vitro. CP-870,893 also enhances dendritic cell activity as evidenced by cytokine secretion (IL-12, IL-23, IL-8), the upregulation of CD86 and CD83, and the ability to prime T cells to secrete IFNγ. In SCID-beige mice, a single parenteral injection of CP-870,893 was therapeutically effective against several CD40pos human tumors (B-cell lymphoma, breast, colon, and prostate) indicating direct effects on tumor cell survival and/or growth. When mice were co-implanted with human T cells and dendritic cells, the activity of CP-870,893 against CD40pos tumors increased, and efficacy was also observed against CD40neg and CD40low tumors demonstrating the ability of CP-870,893 to enhance anti-tumor immune function in vivo. These studies suggest that CP-870,893 has the potential to be efficacious against a wide range of tumor types through both direct and immune-mediated effects.
Keywords: CD40, Immunotherapy, Dendritic cells, SCID-hu, IL-12
Introduction
The generation of an effective immune response against cancer is normally hampered by tolerance to cancer antigens, tumor suppressive factors that act to downregulate immune function, age related immune senescence, as well as the lack of danger or initiation signals in the tumor microenvironment that facilitate cell infiltration [1, 2]. Although, there has been clear evidence of tumor-associated antigens and the presence of antigen-specific T cells in cancer patients, the immune response is either insufficient or locally downregulated such that tumors are able to evade immune surveillance (3, 4). As such, agents that can break tolerance, enhance anti-tumor immune responses, and foster local cytokine responses at the tumor site should be beneficial to cancer patients.
Immunotherapy is in its early stages, both in our understanding of which cells to target as well as developing better techniques to monitor this response such that treatment regimens and combination therapies can be rationally designed. Although early approaches have primarily targeted T cell activity with agents such as IL-2 and more recently anti-CTLA4 (5, 6), an alternative and potentially synergistic approach is to target antigen presenting cells, particularly dendritic cells. Dendritic cells play a central role in both innate and adaptive immunity that may help to facilitate inflammatory responses by releasing key cytokines and chemotactic factors to increase immune cell migration and survival, as well as enhancing antigen presentation and T cell responses [3].
An important regulatory receptor on dendritic cells is CD40, a 50 kDa cell surface glycoprotein in the TNF-R family that is expressed on all antigen presenting cells including dendritic cells, monocytes, and B cells. Ligation of CD40 on the surface of these cells has been shown to increase costimulatory molecule expression, a requisite for the activation of T cells (8, 9). In fact, antigen presentation in the absence of CD40 can lead to tolerance, whereas CD40 activation can convert otherwise tolerogenic dendritic cells into competent antigen presenters. CD40 activation will also enhance cytokine secretion and, as such, CD40 agonists have been shown to replace the need for helper T cells in the generation of an effective CD8+ T cell response [4]. CD40 ligation on antigen presenting cells can also enhance the cytolytic function of NK and NKT cells via cytokine induction [5]. The importance of dendritic cells in the activity of CD40 agonists has been illustrated by the T cell response generated when antigen-pulsed CD40 stimulated dendritic cells are injected into mice [6].
The essential role of CD40 in anti-tumor immunity has been demonstrated both clinically and preclinically. In mice, studies have shown that CD40-/- mice have a diminished response to tumor vaccines as compared to their WT controls [7]. In addition, several groups have demonstrated the effectiveness of CD40 activation for anti-tumor immune responses using either mouse anti-CD40 activating antibodies, soluble CD40 ligand fusion proteins, or an adenovirus expressing CD40 ligand [8–16]. In fact, early clinical studies with a CD40 agonist, a CD40 ligand trimer fusion protein, demonstrated responses in cancer patents but was discontinued due to liver toxicity suggesting the clinical potential for a safe CD40 activating agent [17].
In addition to its role on enhancing immune function, CD40 is also expressed on many tumors (70% of solid tumors and 100% of B-cell malignancies [18]) where engagement of CD40 has been shown to induce cell cycle arrest, apoptosis, and increase cell surface expression of MHC and costimulatory molecules that can enhance tumor cell recognition [19–22]. Depending on the tumor type, CD40 has also been shown to increase the ability of the tumor to act as an antigen presenting cell and foster T cell responses [23]. As such, CD40-activating agents may provide a duel and inherently synergistic therapy for cancer by facilitating the release of tumor antigens, increasing tumor recognition by immune cells, and enhancing antigen presenting cell activity (25).
In this study, we describe the properties of the CD40 agonist antibody CP-870,893, its ability to activate B-cell and dendritic cell function in vitro, and its in vivo efficacious activity against human tumors in SCID-beige mice co-implanted with human T cells and dendritic cells.
Materials and methods
Generation of the IgG2 CD40 agonist CP-870,893
CP-870,893 was generated by immunizing 8 to 10-week-old XenoMice™ (Abgenix) with 300-19 cells transfected to express human CD40. Spleen and lymph node lymphocytes from immunized mice were fused with a myeloma cell line, and hybridomas were screened for their ability to differentially bind CD40 transfected versus non-transfected 300-19 cells, bind recombinant CD40-Ig fusion protein, and activate the CD40 receptor resulting in cytokine secretion.
Isolation of human T cells and monocyte derived dendritic cells
Peripheral blood was collected from normal human volunteers. Mononuclear cells were isolated using Sigma Accuspin tubes (St. Louis, MO), washed with RPMI media (Gibco/Invitrogen, Carlsbad, CA), and placed into tissue culture flasks at 5 × 106 cells/ml in complete RPMI medium (containing 100 μ/ml penicillin/streptomycin, 10 mM HEPES buffer, 2 mM glutamine, 0.1 mM non-essential amino acids; all from Gibco/Invitrogen). After a 3 h incubation at 37°C (5% CO2), non-adherent cells were removed and the T cells were isolated using selection columns (R&D Systems, Minneapolis, MN). In some cases, CD45RO+ cells were isolated using R&D columns.
The adherent cells from the same donor were washed with complete RPMI medium containing 10% fetal calf serum (Hyclone, Logan, Utah) and incubated for 7 days in complete RPMI medium supplemented with 10 ng/ml IL-4 (R&D systems) and 100 ng/ml GM-CSF (R&D systems). The non-adherent cells were then isolated, washed, confirmed to express CD11c by FACS, and utilized as monocyte derived dendritic cells (mDC) for all experiments. In some cases, T cells and mDC’s were frozen in liquid nitrogen prior to use.
Selectivity determinations
Human CD40-Ig (Alexis, Farmingdale, NY), TNFR1-Ig, TNFR2-Ig, CD137-Ig, and RANK-Ig (R&D Systems) were each plated onto Maxisorb ELISA plates (Ninc, VWR) in bicarbonate buffer at a concentration of 2.0 μg/ml and incubated overnight at 4°C. Plates were sealed and stored frozen at −20°C for up to 3 months. Prior to use, plates were thawed, washed once, and then blocked with 0.5% BSA in wash buffer (PBS with 0.05% Tween-20). Various concentrations of CP-870,893 or an anti-KLH control antibody (0.015–100 μg/ml) were then added, and the samples incubated for 1 h at room temperature, washed 3 times, and incubated with a 1:7,000 dilution of biotinylated anti-human IgG2 (Southern Biotech, Birmingham, AL) in blocking buffer for 1 h. Streptavidin-HRP (Jackson ImmunoResearch, West Grove, PA) was then added with TMB substrate (Thermo Scientific, Rockford, IL) and the optical density was read on a Spectramax plate reader at 650 nm. Selectivity was calculated as the ratio of the net signal against CD40 versus other targets.
BIAcore kinetic measurement experiments for estimation of affinity and epitope binning
Kinetic and epitope binning experiments were performed on a BIAcore 2000 (General Electric Health Care Division) using immobilized CD40-Ig. Epitope mapping was assessed against commercially available anti-human CD40 antibodies EA5 (Ancell), 5C3 (PharMingen), and LOB7/8 (Serotec) as well the CD40 ligand (CD154, Ancell).
Flow cytometry
Human CD40-transfected 300-19 cells, various tumor cell lines, isolated cells, and whole blood from various species (human, monkey, mouse, rat, rabbit, and dog) were assessed for their ability to bind CP-870,893. Cells were incubated with human anti-KLH IgG2 or CP-870,893 at various concentrations for 30 min at 4°C. In some cases, saturation binding was done by extending the incubation time for 3 h in the presence of 0.5 μg/ml of cytochalasin B at 25°C (Sigma, St. Louis, MO). Goat F(ab′)2 anti-human IgG Fc PE (Jackson ImmunoResearch, West Grove, PA) was used as the detecting antibody. Data were acquired using a Becton–Dickinson FACSCalibur and analyzed with CellQuest software.
Analysis of cell surface molecule upregulation on B cells and mDC’s
The following antibodies were used to assess cell surface marker expression: mouse IgG1 FITC, mouse IgG1 PE (Caltag/Invitrogen, Carlsbad, CA), mouse IgG2a, CD19 APC (Becton–Dickinson, San Jose, CA), CD54 FITC (Beckman Coulter, Fullerton, CA), the CD40 antibodies EA-5 (Alexis), LOB7/6 (Serotec, Oxford, UK), and 5C3 (Becton–Dickinson), HLA-DR PerCP, CD71 FITC, CD3 PerCP, CD20 APC, CD23 PE, CD83 PE, CD1a APC, CD11c, (Becton–Dickinson), CD86 PE (PharMingen), and goat F(ab’)2 anti-human IgG Fc specific PE (Caltag).
Human mDC, isolated lymphocytes, or heparinized whole blood diluted 1:1 with RPMI medium were incubated for 24 h with various concentrations of CP-870,893 or the control anti-KLH antibody. Cells were stained for 30 min (on ice, in the dark) with antibodies detecting HLA-DR, CD54, CD83, CD86, CD19/CD20, CD23, and CD71. The cells were then analyzed on a FACS Caliber (Becton–Dickinson). B cells were identified by gating on CD19 or CD20 positive cells.
Effects on mDC cytokine production
Human mDC cells were prepared as described above. The cells were then washed and incubated in the presence of various concentrations of CP-870,893 (0.01–10.0 μg/ml) alone or in the presence of LPS, IFNγ, or isolated T cells from a different donor to establish an allogeneic response. The supernatants were analyzed 24 h later for IFNγ, IL-8, TNFα, IL-6, RANTES, IL-15, IL-7, MDC, IL-12p40, IL-12p70, and IL-10 using commercially available ELISA kits obtained from R&D systems, Minneapolis, MN. IL-23 was determined by bioassay by adding serial dilutions of the supernatants along with 0.25 μg/ml IL-2 to CD45 RO+ T cells and measuring the production of IFNγ as an indicator [24]. The presence of IL-23 was deduced by the ability of anti-p40 (R&D systems) but not anti-p35 (R&D systems) to neutralize the activity.
Anti-tumor activity and immune enhancement experiments in SCID-beige mice
SCID-beige mice were obtained from Taconic (Washington, DC) and acclimated 1 week prior to use. All experimentation was done in accordance with the institutional guidelines. The plasma levels of CP-870,893 were determined by ELISA at various times after a single i.p. injection. Tumor cells were injected s.c. at a concentration of 1 × 107 cells/animal unless otherwise indicated. In some cases, T cells (1 × 106) and mDCs (5 × 105) from the same human donor were co-injected along with the tumor cells. CP-870,893 or an isotype matched control anti-KLH antibody were injected i.p. immediately prior to tumor injection or after tumors reached a size of 100 mm2. Tumor growth was measured using calipers at various times. Unless otherwise indicated, the results are presented in terms of the tumor size in mm2. Tumor cell lines utilized included: Daudi (ATCC, Manassas, VA; CCL 213), K562 (ATCC CCL 243), JIYOYE (ATCC CCL 87), Raji (ATCC CCL-86), BT474 (ATCC HTB-20), and PC-3 (ATCC CRL-1435).
Statistical analysis
Data were analyzed by the Student’s t-test. P values of <0.05 were considered significant.
Results
Characterization of CP-870,893
CP-870,893 was shown to bind 300-19 cells transfected with the human CD40 receptor with an EC50 of 0.15 ± 0.048 μg/ml. No binding was observed to parenteral 300-19 cells. Whole blood binding studies also confirmed the ability of CP-870,893 to bind CD40 on primary human B cells. However, CP-870,893 was not able to bind B cells in whole blood from mouse, rat, rabbit, or dog (data not shown). In addition, selectivity (as assessed by ELISA) using the extracellular domains of several other TNF family members indicated that CP-870,893 selectively binds to CD40 with greater than 100-fold selectivity versus RNFR1, TNFR2, RANK, and 4-1BB.
Biacore analysis using the extracellular domain of CD40 (CD40-Ig) indicated that CP-870,893 had a KD of 0.4 nM with a Ka of 4.42 × 104 M 1/Ms and a Kd of 9.11 × 10−6 M 1/s. Epitope analysis indicated that CP-870,893 binds to a site distinct from the ligand binding site, partially overlapping the binding with two commercially available CD40 antibodies, EA5 and 5C3. No overlap was observed with another commercially available CD40 antibody, LOB7/6.
In vitro effects of CP-870,893 on cell surface marker expression on B cells and dendritic cells
As shown in Fig. 1a, CP-870,893 induced the upregulation of cell surface marker expression on B cells. The highest expression was observed for class II, CD54, and CD23, however, CD71 and CD86 were also elevated. The concentration of CP-870,893 required to upregulate cell surface expression varied depending on the marker (EC50 range = 3.8–18.9 ng/ml). In addition to its effects on B cells, CP-870,893 also upregulated cell surface molecule expression on mDCs as shown in Fig. 1b. On dendritic cells, CP-870,893 had the greatest effect on CD83 and CD86 (up to 30-fold increased expression), however, class II and CD54 were also increased. As with B cells, the concentration of CP-870,893 required to upregulate surface molecule expression varied depending on the marker (EC50 range = 0.07–0.25 μg/ml). In addition to observing these effects on B-cell surface marker expression from healthy human donors, similar effects were also observed by adding CP-870,893 to blood obtained from patients with various cancers (data not shown). These results demonstrate that CP-870,893 is a potent and selective CD40 agonist antibody with the ability to upregulate costimulatory molecules on both B cells and dendritic cells.
Fig. 1.
Effects of CP-870,893 on cell surface marker upregulation on human CD19+ B cells and human mDC. a CP-870,893 or anti-KLH control was added to heparinized human whole blood for 24 h, and cell surface upregulation of various markers was determined using flow cytometry by gating on CD19+ B cells. Each data point is the mean of >6 experiments using different human donors. b mDCs were prepared as described in the materials and methods and incubated with various concentrations of CP-870,893 or an anti-KLH control antibody for 24 h. The upregulation of cell surface markers was determined by flow cytometry. Each data point is the mean of at least 6 studies
Effect of CP-870,893 on human dendritic cell cytokine production
Stimulation of human mDCs with CP-870,893 resulted in the significant production of IL-12p40, with an EC50 of 0.21 μg/ml. In addition to IL-12p40, IL-8 was also produced at high levels (Table 1). No induction of IFNγ, TNF, RANTES, or IL-10 was observed. IL-15, MDC, and IL-6 were also induced but levels were in the low pg/mL range. In addition, only low levels of IL-12p70 were induced. However, when cells were stimulated with both CP-870,893 and LPS, IL-12p70 was produced at higher levels as was IL-12p40. Similar results were obtained when IFNγ was used as a co-stimulus instead of LPS (data not shown). In addition to IL-12, IL-23 was also induced by the combination of CP-870,893 and LPS as indicated by the ability of IL-2 stimulated CD45RO+ T cells to secrete IFNγ in the presence of an anti-p35 antibody that will neutralize IL-12p70 (data not shown). Further, in an allogeneic culture where human T cells and dendritic cells from different donors were mixed and stimulated with CP-870,893, an increase in IFNγ production was observed (data not shown) demonstrating the ability of CP-870,893 to not only directly enhance dendritic cell function but also secondarily act on T cells either via cytokines (e.g. IL-12) and/or contact dependent co-stimulation. IFNγ was not observed by incubating either T cells or dendritic cells separately with CP-870,893 (data not shown). These results illustrate the ability of CP-870,893 to activate dendritic cells, enhance T cell function, and induce cytokines important for both cell recruitment and activation.
Table 1.
CP-870,893 Enhances the production of cytokines by dendritic cells alone and by costimulation with LPS
| IL-12p40 | IL-12p70 | IL-8 | |
|---|---|---|---|
| Anti-KLH | 44 ± 18 | 0 | 254 ± 53 |
| CP-870,893 | 4,685 ± 784* | 84 ± 14* | 4328 ± 1,630* |
| Anti-KLH + LPS | 15,756 ± 1,441 | 23 ± 18 | |
| CP-870,893 + LPS | 35,252 ± 1,515 | 1,214 ± 281* | >10,000* |
All values are in pg/ml
Data points represent the mean ± the SE for >3 experiments
CP-870,893 or anti-KLH was added at 1 μg/ml and LPS at 100 ng/ml where indicated
* P < 0.01 versus the anti-KLH control
Anti-tumor efficacy of CP-870,893 in SCID-beige mice
Since CP-870,893 did not interact with mouse CD40, we next examined its effects in SCID-beige mice against human tumors alone and when animals were co-injected with human T cells and mDCs. Plasma levels of CP-870,893 persisted in these animals for an extended period of time. After a single injection, the Cmax was 10 μg/ml with a 1.0 mg/kg dose and 2.0 μg/ml following a 0.1 mg/kg dose. Levels of CP-870,893 above 100 ng/ml were observed for greater than 50 days at both dose levels in these animals. The T1/2 was about 7 days.
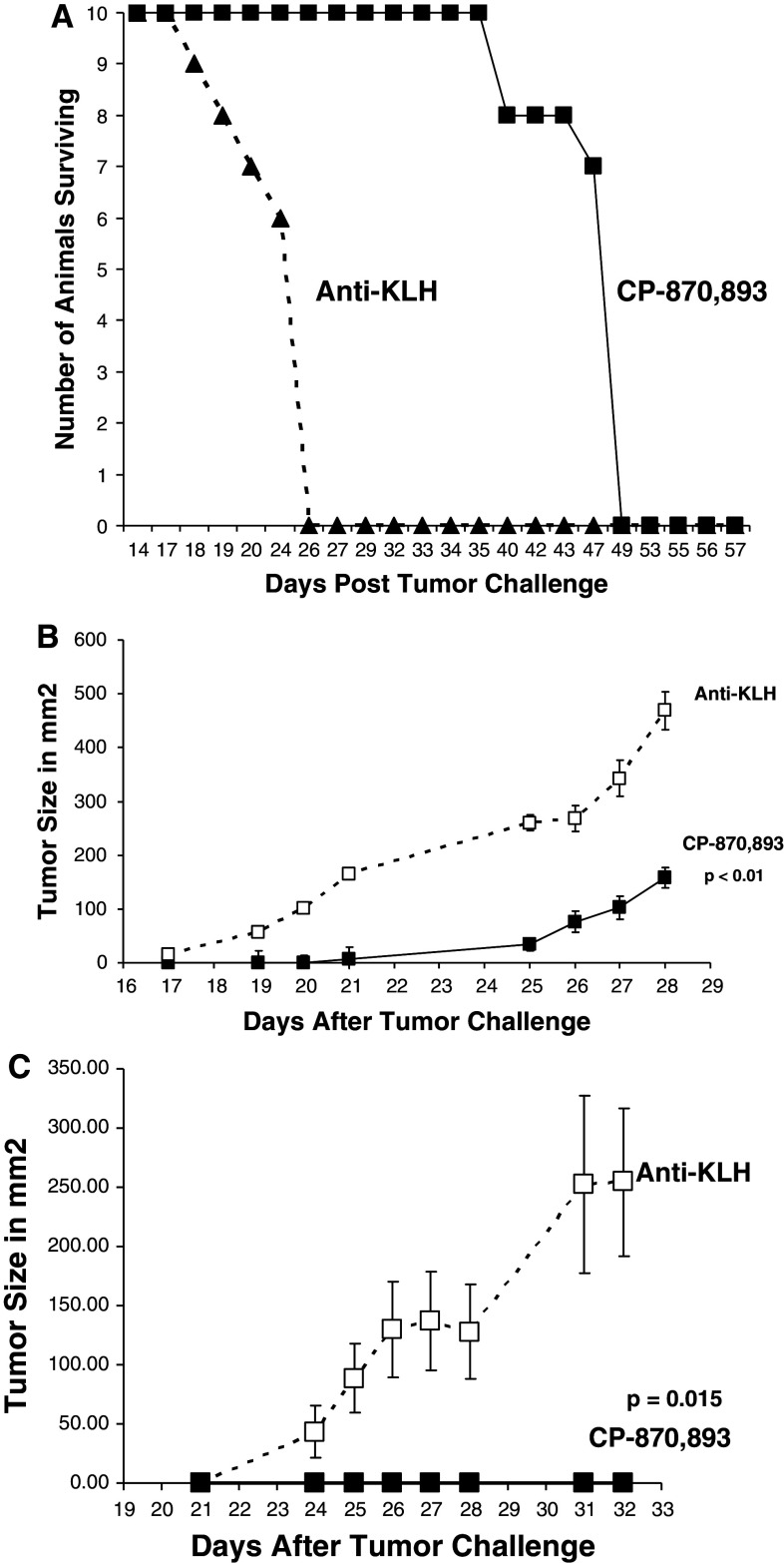
When animals were injected IV with the CD40pos B-cell lymphoma Daudi, a single i.p. injection of CP-870,893 administered at the time of tumor challenge delayed mortality for greater than 23 days versus controls (MST 48 days vs. 25 days for the control-antibody-injected animals) illustrating the ability of CP-870,893 to provide protection against a systemic hematological tumor (Fig. 2a). A single i.p. injection of CP-870,893 also delayed (Fig. 2b) or in some animals prevented (Fig. 2c) subcutaneous solid tumor growth of the CD40pos B-cell lymphomas Daudi and Jiyoye. These studies illustrate that CP-870,893 has direct anti-tumor effects on CD40pos tumors since SCID-beige mice lack lymphocytes and NK cells, and CP-870,893 does not interact with mouse CD40. Similar results were observed with BT474, a CD40pos breast tumor, and PC-3, a CD40pos prostate tumor (data not shown). The CD40, class I, and II expression status of the cell lines are listed in Table 2.
Fig. 2.
CP-870,893 prevents mortality induced by several B-cell lymphomas. a CP-870,893 was administered i.p. followed by the injection of 5 × 106 Daudi cells (IV). Mortality was monitored for 50 days. The MST was 25 days for the anti-KLH group and 48 days for the animals treated with CP-870,893. The group size was 10 animals per group and the study is representative of 5 separate studies. b CP-870,893 was administered i.p. at the time of s.c. tumor challenge with the B-cell lymphoma Daudi. Tumor size is represented in mm2. Each data point is the mean ± the SE for 5 animals per group and is representative of 3 separate experiments. c CP-870,893 was administered i.p. at the time of s.c. tumor challenge with the B-cell lymphoma Jijoye. Each data point refers to the mean tumor size in mm2 ± the SE for 5 animals per group. The study is representative of 3 separate studies
Table 2.
Cell surface expression analysis of CD40, MHC class I, and II on various tumor cell lines
| Detecting antibody | Daudi | K562 | JiJoye | Raji | BT474 | PC3 | Lovo |
|---|---|---|---|---|---|---|---|
| Isotype control | 10 | 10 | 11 | 11 | 13 | 12 | 16 |
| CD40 | 514 | 13 | 475 | 575 | 170 | 114 | 25 |
| MHC class I | 2,226 | 14 | ND | 1,625 | 11 | 8 | 30 |
| MHC class II | 2,594 | 11 | ND | 3,133 | 5 | 7 | 10 |
Values represent the average geometric mean from several experiments
ND Not done
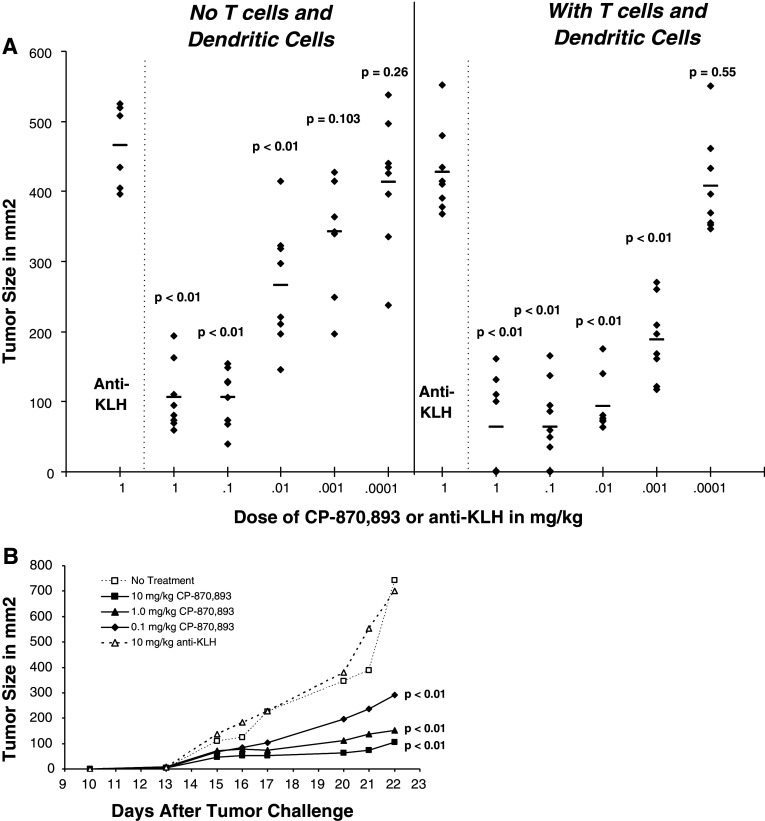
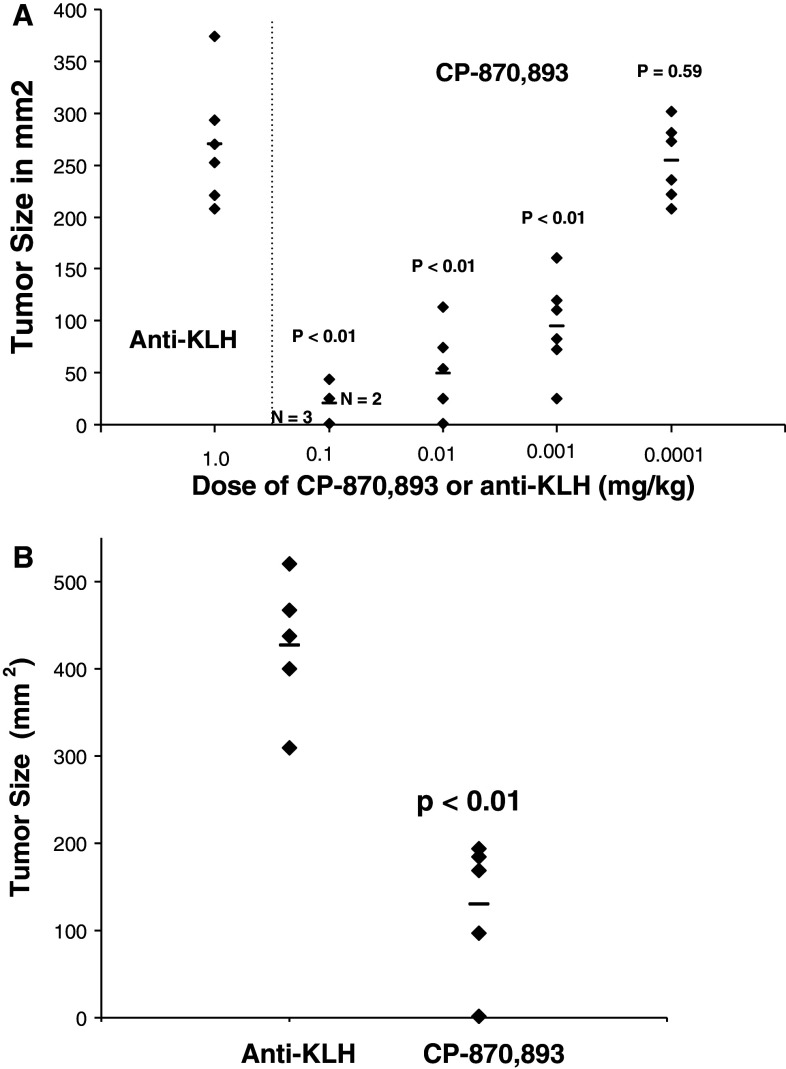
Although the direct anti-tumor effects of CP-870,893 can easily be assessed in SCID-beige mice, the potential in vivo immune-mediated activity for CP-870,893 is difficult to assess in mice given the lack of cross-reactivity on the mouse CD40 receptor. In order to examine this, we injected naive human T cells and mDCs into SCID-beige mice along with the tumor subcutaneously, and then administered CP-870,893 i.p. Using the CD40pos tumor Raji, CP-870,893 had the ability to decrease tumor growth with an ED50 of 0.016 mg/kg in the absence of T cells and mDCs. When human naïve T cells and mDCs were co-injected with the tumor, the dose level of CP-870,893 necessary to see activity decreased 20-fold to 0.0008 mg/kg suggesting a combined effect of direct anti-tumor and immune-enhancing activity (Fig. 3a). To further demonstrate the impact of CP-870,893 on human immune function, we also examined the effects of CP-870,893 against a CD40neg tumor K562 (Fig. 3b). In this case, CP-870,893 had no effect on the growth of this tumor in the absence of both human T cells and dendritic cells (data not shown). However, in the presence of human T cells and mDCs (but neither alone), a significant inhibition of tumor growth was observed. No inhibition of tumor growth was observed with T cells, and mDCs were injected with the tumor either alone or in animals injected with anti-KLH. Similar effects were observed with a CD40low colon carcinoma, Lovo (data not shown).
Fig. 3.
Effect of human T cells and mDCs on the in vivo activity of CP-870,893 in SCID-beige mice. a Dose-dependant inhibition of the CD40pos tumor Raji by CP-870,893 in the presence or absence of human T cells and mDC. CP-870,893 was administered i.p. at the time of s.c. tumor challenge. The data points represent the tumor size (mm2) for each individual animal taken on day 21 after tumor challenge. The mean tumor size for each treatment group (N = 10) is indicated by the horizontal line. The study is representative of at least 3 separate experiments. Significance is indicated as compared to the anti-KLH control. Significance in comparing groups with and without T cells and mDC are as follows: 0.1 mg/kg group P < 0.01; 0.01 mg/kg group P < 0.01; 0.001 mg/kg group P = 0.0103; 0.0001 mg/kg group P = 0.26. b Dose-dependant inhibition of the CD40neg tumor K562 by CP-870,893 in the presence human T cells and mDC. Animals received a single injection (IP) of various dose levels of CP-870,893 or 10 mg/kg anti-KLH at the time of s.c. tumor challenge. The data are representative of 3 experiments
In addition to B-cell lymphomas, CP-870,893 was also efficacious against other tumor types. CP-870,893 delayed the growth of the subcutaneous breast tumor BT474, both when the antibody was administered at the time of tumor injection (Fig. 4a) as well as when treatment was delayed until tumors reached a size of 100 mm2 (Fig. 4b) where in some cases, tumors regressed in size. When administered at the time of tumor challenge, the ED50 was 0.005 mg/kg and the ED90 0.06 mg/kg. This effect on tumor regression following therapeutic dosing was even more pronounced when a suboptimal level of cisplatinum was also administered to animals (data not shown). Therapeutic effects were also demonstrated with the CD40pos prostate tumor PC-3 (data not shown).
Fig. 4.
CP-870,893 prevents the growth of the human breast tumor cell line, BT 474 in the presence of human T cells and mDCs. BT474 tumor cells were administered s.c. along with human T cells and mDCs. a CP-870,893 was administered i.p. at the time of tumor challenge. Each data point refers to the tumor size of an individual animal measured on day 53 after challenge, a time when control animals reach a tumor size where they were euthanized. The horizontal lines represent the mean tumor size. b CP-870,893 was administered at a dose of 1.0 mg/kg once tumors reached a size of 100 mm2. Each data point refers to the tumor size of an individual animal measured on day 52 after challenge, a time when control animals reach a tumor size where they were euthanized. The horizontal lines represent the mean tumor size for all animals in that group
Discussion
In this study, we describe the identification and characterization of the CD40 agonist antibody, CP-870,893. CP-870,893 enhances mDC cytokine production, upregulates MHC and co-stimulatory molecules on both B cells and mDC, and is efficacious against both CD40pos and CD40neg tumors in SCID mice through both direct and immune-mediated mechanisms. These attributes may help to overcome some of the limitations of current therapies by providing a multi-pronged approach within a single agent to both directly attack the tumor while also enhancing anti-tumor immune responses. As such, CP-870,893 has the potential to be a robust anti-cancer therapeutic agent.
A key cytokine induced by CP-870,893 is IL-12. IL-12 has been shown to have anti-tumor efficacy in several preclinical models [25], and as such may be contributing to at least some of the immune-mediated efficacious effects observed CP-870,893. Since IL-12 can enhance both CTL and NK cell function, as well as macrophage antitumor activity [26], it provides a mechanism to kill both MHC class I positive and negative tumors, an important attribute as tumors often down-regulate class I expression to evade immune responses. This was supported by the ability of CP-870,893 to have efficacy against both class I positive and class I negative tumors in SCID mice.
The ability of CD40 agonists to induce cytokines from dendritic cells has been previously shown to replace the need for CD4+ helper T cells during an immune response [10]. A key attribute of CP-870,893 was its ability to not only induce cytokines from dendritic cells but to work with other agonist signals to increase this response. In fact, bioactive IL-12p70 and IL-23 were only produced at appreciable levels when a second stimulus was present. This property of CP-870,893 may help to localize cytokine production to sites of immune reactivity, thus, avoiding the issues encountered by excessive cytokine release [27].
Although the specific cellular mechanisms responsible for anti-tumor efficacy are difficult to dissect, eradication of MHC class I negative, CD40neg K562 cells required the presence of both T cells and mDC indicating that both cell types cooperated to decrease tumor growth. In addition, both T cells and mDC were necessary in order to see enhanced activity against CD40pos tumors. Although, one mechanism may be to enhance antigen-specific T cell killing of the tumor, we cannot exclude the possibility that the T cell preparations contained some NK cells that may also contribute to some of the anti-tumor activity observed in the SCID-hu model.
The ability of CP-870,893 to upregulate costimulatory molecules such as CD83 and CD86 on mDC is an important attribute to enhance T cell activation. These molecules have been shown to be essential to promote antigen-specific T cell responses and may also have the ability to facilitate breaking tolerance (32, 33). Tolerance to tumor antigens is likely as most tumor antigens originate from self and as such are not seen by the immune system. In fact, breaking tolerance has been reported for CD40 agonists (34). The upregulation of these co-stimulatory molecules, together with cytokine induction, should work together to promote T cell responses and possibly provide a local danger signal at the tumor site to facilitate cell infiltration.
The direct effects observed on CD40pos tumors indicate that CP-870,893 will not only have immune-based effects but also directly kill tumors. This is in line with reported effects of CD40 stimulation on inducing the apoptosis and cell cycle arrest on tumor cells (25–27, 35), and also consistent with the ability of CP-870,893 to promote ICAM-1 upregulation and homotypic aggregation on some B-cell lymphomas (in house data). Although there have been some reports that CD40 stimulation can enhance tumor growth, [28], our studies with CP-870,893 do not support this possibility. Our results do, however, indicate that an agonist signal delivered through CD40 on tumor cells results in decreased tumor growth in vivo in the absence of ADCC, substantiated by the lack of Fc interactions observed with the CP-870,893 (in house data) and the efficacy observed in SCID-beige mice against CD40pos tumors that lack NK cells, important effectors of any ADCC response.
The direct effects on CD40pos tumors will likely work together with the immune-enhancing effects of CP-870,893 to enhance efficacy. This is supported by the shift in the dose response observed for CP-870,893 in the presence versus absence of immune cells against CD40pos tumors. The rationale for this effect may be by enhancing the accessibility and/or release of tumor-associated antigens necessary to enhance an antigen-specific cell response, inflammatory signals established at the tumor site by tumor cell cytolysis that facilitate immune cell entry, and the combined effect of killing the tumor by two distinct mechanisms. In fact in some cases, tumor cells themselves can be stimulated by CD40 to act as an antigen-presenting cell that may be an added benefit [23]. Direct effects on CD40pos tumors could include: increased immunogenicity, enhanced apoptosis, increased radiosensitivity, and possibly tumor cell cytokine secretion, all of which may help break tolerance and enhance immune responses.
CP-870,893 represents a novel therapy for the treatment of cancer that has the potential to act directly on tumor cells, enhance immune responses, and synergize with direct anticancer therapies. Tumor model studies in SCID-beige mice demonstrated that CP-870,893 has a broad spectrum anti-tumor activity against B-cell lymphomas as well as breast, colon, and prostate tumors. Early studies with this agent in cancer patient have suggested both tolerability, the upregulation of cell surface markers on B cells, and signs of efficacy in melanoma patients [29]. However, further studies will be necessary to assess the full potential for this agent in cancer patients.
References
- 1.Chaudhuri D, Suriano R, Mittelman A, Tiwari RK. Targeting the immune system in cancer. Curr Pharm Biotechnol. 2009;10:166–184. doi: 10.2174/138920109787315114. [DOI] [PubMed] [Google Scholar]
- 2.Lustgarten J. Cancer, aging and immunotherapy: lessons learned from animal models. Cancer Immunol Immunother. 2009;58:1979–1989. doi: 10.1007/s00262-009-0677-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. 2009;229:152–172. doi: 10.1111/j.1600-065X.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diehl L, Den Boer AT, van der Voort EI, Melief CJ, Offringa R, Toes RE. The role of CD40 in peripheral T cell tolerance and immunity. J Mol Med. 2000;78:363–371. doi: 10.1007/s001090000126. [DOI] [PubMed] [Google Scholar]
- 5.Sartorius R, D’Apice L, Barba P, et al. Induction of human NK cell-mediated cytotoxicity by CD40 triggering on antigen presenting cells. Cell Immunol. 2003;221:81–88. doi: 10.1016/S0008-8749(03)00060-1. [DOI] [PubMed] [Google Scholar]
- 6.Davis ID, Chen Q, Morris L, et al. Blood dendritic cells generated with Flt3 ligand and CD40 ligand prime CD8+ T cells efficiently in cancer patients. J Immunother. 2006;29:499–511. doi: 10.1097/01.cji.0000211299.29632.8c. [DOI] [PubMed] [Google Scholar]
- 7.Mackey MF, Gunn JR, Ting PP, et al. Protective immunity induced by tumor vaccines requires interaction between CD40 and its ligand, CD154. Cancer Res. 1997;57:2569–2574. [PubMed] [Google Scholar]
- 8.Hanyu K, Iida T, Shiba H, Ohashi T, Eto Y, Yanaga K. Immunogene therapy by adenovirus vector expressing CD40 ligand for metastatic liver cancer in rats. Anticancer Res. 2008;28:2785–2789. [PubMed] [Google Scholar]
- 9.Dzojic H, Loskog A, Totterman TH, Essand M. Adenovirus-mediated CD40 ligand therapy induces tumor cell apoptosis and systemic immunity in the TRAMP-C2 mouse prostate cancer model. Prostate. 2006;66:831–838. doi: 10.1002/pros.20344. [DOI] [PubMed] [Google Scholar]
- 10.French RR, Chan HT, Tutt AL, Glennie MJ. CD40 antibody evokes a cytotoxic T cell response that eradicates lymphoma and bypasses T cell help. Nat Med. 1999;5:548–553. doi: 10.1038/5505. [DOI] [PubMed] [Google Scholar]
- 11.French RR, Taraban VY, Crowther GR, et al. Eradication of lymphoma by CD8 T cells following anti-CD40 monoclonal antibody therapy is critically dependent on CD27 costimulation. Blood. 2007;109:4810–4815. doi: 10.1182/blood-2006-11-057216. [DOI] [PubMed] [Google Scholar]
- 12.Honeychurch J, Glennie MJ, Johnson PW, Illidge TM. Anti-CD40 monoclonal antibody therapy in combination with irradiation results in a CD8 T cell-dependent immunity–B cell lymphoma. Blood. 2003;102:1449–1457. doi: 10.1182/blood-2002-12-3717. [DOI] [PubMed] [Google Scholar]
- 13.Tutt AL, O’Brien L, Hussain A, Crowther GR, French RR, Glennie MJ. T cell immunity to lymphoma following treatment with anti-CD40 monoclonal antibody. J Immunol. 2002;168:2720–2728. doi: 10.4049/jimmunol.168.6.2720. [DOI] [PubMed] [Google Scholar]
- 14.Todryk SM, Tutt AL, Green MH, et al. CD40 ligation for immunotherapy of solid tumours. J Immunol Methods. 2001;248:139–147. doi: 10.1016/S0022-1759(00)00349-5. [DOI] [PubMed] [Google Scholar]
- 15.van Mierlo GJ, den Boer AT, Medema JP, et al. CD40 stimulation leads to effective therapy of CD40(−) tumors through induction of strong systemic cytotoxic T lymphocyte immunity. Proc Natl Acad Sci USA. 2002;99:5561–5566. doi: 10.1073/pnas.082107699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diehl L, den Boer AT, Schoenberger SP, et al. CD40 activation in vivo overcomes peptide-induced peripheral cytotoxic T lymphocyte tolerance and augments anti-tumor vaccine efficacy. Nat Med. 1999;5:774–779. doi: 10.1038/10495. [DOI] [PubMed] [Google Scholar]
- 17.Vonderheide RH, Dutcher JP, Anderson JE, et al. Phase I study of recombinant human CD40 ligand in cancer patients. J Clin Oncol. 2001;19:3280–3287. doi: 10.1200/JCO.2001.19.13.3280. [DOI] [PubMed] [Google Scholar]
- 18.Toes RE, Schoenberger SP, van der Voort EI, Offringa R, Melief CJ. CD40-CD40 ligand interactions and their role in cytotoxic T lymphocyte priming and anti-tumor immunity. Semin Immunol. 1998;10:443–448. doi: 10.1006/smim.1998.0147. [DOI] [PubMed] [Google Scholar]
- 19.Hirano A, Longo DL, Taub DD, et al. Inhibition of human breast carcinoma growth by a soluble recombinant human CD40 ligand. Blood. 1999;93:2999–3007. [PubMed] [Google Scholar]
- 20.Hill SC, Youde SJ, Man S, et al. Activation of CD40 in cervical carcinoma cells facilitates CTL responses and augments chemotherapy-induced apoptosis. J Immunol. 2005;174:41–50. doi: 10.4049/jimmunol.174.1.41. [DOI] [PubMed] [Google Scholar]
- 21.Gomes EM, Rodrigues MS, Phadke AP, et al. Antitumor activity of an oncolytic adenoviral-CD40 ligand (CD154) transgene construct in human breast cancer cells. Clin Cancer Res. 2009;15:1317–1325. doi: 10.1158/1078-0432.CCR-08-1360. [DOI] [PubMed] [Google Scholar]
- 22.Georgopoulos NT, Merrick A, Scott N, Selby PJ, Melcher A, Trejdosiewicz LK. CD40-mediated death and cytokine secretion in colorectal cancer: a potential target for inflammatory tumour cell killing. Int J Cancer. 2007;121:1373–1381. doi: 10.1002/ijc.22846. [DOI] [PubMed] [Google Scholar]
- 23.Vonderheide RH, Butler MO, Liu JF, et al. CD40 activation of carcinoma cells increases expression of adhesion and major histocompatibility molecules but fails to induce either CD80/86 expression or T cell alloreactivity. Int J Oncol. 2001;19:791–798. doi: 10.3892/ijo.19.4.791. [DOI] [PubMed] [Google Scholar]
- 24.Frucht DM. IL-23: a cytokine that acts on memory T cells. Sci STKE. 2002;114:1–3. doi: 10.1126/stke.2002.114.pe1. [DOI] [PubMed] [Google Scholar]
- 25.Tahara H, Lotze MT. Antitumor effects of interleukin-12 (IL-12): applications for the immunotherapy and gene therapy of cancer. Gene Ther. 1995;2:96–106. [PubMed] [Google Scholar]
- 26.Tsung K, Meko JB, Peplinski GR, Tsung YL, Norton JA. IL-12 induces T helper 1-directed antitumor response. J Immunol. 1997;158:3359–3365. [PubMed] [Google Scholar]
- 27.Weerasekera N, Hudson I. Lessons from TGN-1412: the impact on phase I clinical trials. IDrugs. 2007;10:230–232. [PubMed] [Google Scholar]
- 28.Biancone L, Cantaluppi V, Boccellino M, et al. Activation of CD40 favors the growth and vascularization of Kaposi’s sarcoma. J Immunol. 1999;163:6201–6208. [PubMed] [Google Scholar]
- 29.Vonderheide RH, Flaherty KT, Khalil M, et al. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J Clin Oncol. 2007;25:876–883. doi: 10.1200/JCO.2006.08.3311. [DOI] [PubMed] [Google Scholar]