Abstract
Background
IMO-2055 is a Toll-like receptor 9 (TLR9) agonist that potentially enhances the efficacy of antitumor agents through immune stimulation. The objective of this phase Ib dose-escalation trial (3 + 3 design) was to determine the recommended phase II dose (RP2D) of IMO-2055 when combined with erlotinib and bevacizumab in patients with advanced non-small cell lung cancer (NSCLC).
Methods
Patients with stage 3/4 NSCLC and progressive disease (PD) following chemotherapy received IMO-2055 0.08, 0.16, 0.32, or 0.48 mg/kg once weekly plus erlotinib 150 mg daily and bevacizumab 15 mg/kg every 3 weeks. Patients could receive treatment until PD or unacceptable toxicity.
Results
Thirty-six patients were enrolled; 35 received at least one treatment dose. Two dose-limiting toxicities were observed across the dose range (Grade 3 dehydration and fatigue) with neither suggestive of a consistent toxicity pattern. IMO-2055 0.32 mg/kg was adopted as RP2D based on clinical and pharmacodynamic data. The most common treatment-emergent adverse events (TEAEs) were diarrhea (74 %), nausea (51 %), fatigue (51 %), rash (51 %), and injection-site reactions (49 %). Four patients experienced serious TEAEs considered to be study drug related. Five patients died, all due to PD. High-grade neutropenia and electrolyte disturbances previously reported with TLR9 agonists combined with platinum-based therapy were not observed in this study. Five of 33 patients evaluable for response (15 %) achieved partial response; another 20 (61 %) had stable disease, including 13 with stable disease ≥4 months.
Conclusions
IMO-2055 demonstrated good tolerability and possible antitumor activity in combination with erlotinib and bevacizumab in heavily pretreated patients with advanced NSCLC.
Keywords: Advanced/metastatic NSCLC, IMO-2055 (EMD 1201081), TLR9 agonist, Phase I, Recommended phase II dose
Introduction
Non-small cell lung cancer (NSCLC) accounts for more than 85 % of all lung cancer diagnoses and is the major cause of cancer deaths in the USA [1, 2]. The majority of NSCLC patients present with inoperable, advanced-stage disease at diagnosis [3], and only about 30–40 % of initially treated patients are eligible for subsequent therapy [4]. Docetaxel, pemetrexed, and erlotinib, an epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor, are the only currently approved therapies in the USA for second-line treatment of NSCLC [5–7]. These drugs are indicated regardless of the patient’s EGFR mutation status [4]. In Europe and Asian countries, gefitinib, another EGFR tyrosine kinase inhibitor, is approved for treatment of NSCLC patients with locally advanced or metastatic disease and activating mutations of the EGFR tyrosine kinase across all lines of therapy [8]. Nevertheless, for patients with advanced NSCLC survival remains limited, with a median survival of 9 months in patients with good performance status [9].
One approach to improve the survival of these patients is to simultaneously target the vascular endothelial growth factor (VEGF) and the EGFR pathways [10]. Both are interrelated and play a role in tumorigenesis and tumor growth in NSCLC [10, 11]. In phase I/II studies, the combination of the anti-VEGF recombinant humanized monoclonal antibody bevacizumab with erlotinib resulted in prolonged survival compared with chemotherapy alone in patients with advanced NSCLC [12, 13]. However, in a recent phase III trial of patients with advanced NSCLC for whom first-line treatment had failed, overall survival (OS) did not differ between patients treated with erlotinib plus bevacizumab (median 9.3 months) compared with erlotinib plus placebo (median 9.2 months; p = 0.7583), despite a trend toward increased progression-free survival (PFS) in the erlotinib and bevacizumab group [14]. Therefore, novel strategies to improve clinical outcomes with currently approved treatment options are needed.
Immunostimulating Toll-like receptor 9 (TLR9) agonists are associated with antitumor, antiproliferative, and antiangiogenic effects that are at least in part related to inhibition of EGFR-related signaling [15]. When combined with small-molecule EGFR tyrosine kinase inhibitors or anti-EGFR antibodies, TLR9 stimulation has been shown to produce a synergistic antitumor effect in vitro [15]. Furthermore, synthetic agonists of TLR9 have also been shown to act via EGFR-independent mechanisms to synergize with bevacizumab in both sensitive and cetuximab-resistant colon cancer xenografts [16].
IMO-2055 (EMD 1201081) is a TLR9 agonist with the potential to enhance the antitumor efficacy of biologics and small molecules through immune stimulation. In NSCLC xenograft models, it has been shown to enhance the antitumor effects of erlotinib and bevacizumab, and a synergistic effect of the triple combination was reported [17]. The objective of the present phase Ib trial was to determine the recommended phase II dose (RP2D) of IMO-2055 when combined with bevacizumab and erlotinib in patients with advanced or metastatic NSCLC whose disease was not amenable to curative therapy or for whom standard chemotherapy was not an option. Additional objectives were to evaluate safety, potential drug–drug interactions, and antitumor activity of the three-drug combination regimen.
Materials and methods
Study design and objectives
This was a phase Ib, open-label, dose-escalation trial of IMO-2055 combined with erlotinib and bevacizumab in patients with advanced or metastatic NSCLC who progressed during or after first-line therapy (clinicaltrials.gov no. NCT00633529). The primary objective was to determine the RP2D of IMO-2055 when combined with erlotinib and bevacizumab. Secondary objectives were to evaluate the safety profile, pharmacokinetics (PK), and antitumor activity of the triple-drug combination, as well as potential markers of IMO-2055 immune activation.
Dose escalation was performed using a standard 3 + 3 design [18]. IMO-2055 dose levels were escalated from 0.08 to 0.16, 0.32, and 0.48 mg/kg/week. All patients had to have the first 3-week treatment cycle 1 completed before a higher dose level could be initiated. Doses were escalated until the first IMO-2055–related dose-limiting toxicity (DLT) was observed. If one of three patients experienced an IMO-2055–related DLT, a total of six patients (3 + 3) had to be enrolled at that dose level. If only one of the six patients experienced an IMO-2055–related DLT, the next dose cohort could be initiated. If IMO-2055–related DLTs were seen in more than one of six patients, de-escalation to the previous dose level and expansion of this cohort to six patients was to occur. Once the RP2D was established, another 15 patients were planned to be enrolled at this dose level in order to further assess the secondary objectives.
The study received institutional review board approval at each participating center. The study was conducted in accordance with International Conference on Harmonization guidelines for Good Clinical Practice, as well as the principles of the Declaration of Helsinki.
Patient population
Patients ≥18 years old with histologically proven stage 3 or 4 NSCLC according to American Joint Committee on Cancer Classification (AJCC 6th ed) whose disease was not amenable to curative therapy and for whom erlotinib and bevacizumab therapy was appropriate were eligible. Patients were also required to have received at least one standard platinum-containing chemotherapy regimen prior to enrollment, a radiologic assessment within 21 days prior to inclusion if measurable disease was present, an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, and adequate bone marrow, liver, and renal function. Main exclusion criteria were as follows: presence of squamous cell carcinoma, except for patients with no or well-controlled intrathoracic disease or small peripheral lesions only and no cavitating lesions; known metastases of the central nervous system; <4 weeks between registration and last receipt of NSCLC-related treatment; or concurrent or planned use of hormonal agents. EGFR mutation status of tumors was not determined; at the time of study initiation, routine testing was neither common nor required according to erlotinib’s label. Patients were enrolled between November 2007 and November 2009. All patients provided their written informed consent prior to the start of any study-specific procedures. The study was completed in March 2011.
Treatment
IMO-2055 was administered subcutaneously on days 1, 8, and 15 of each 3-week treatment cycle. Each patient additionally received erlotinib 150 mg per os on days 1–21 (on an empty stomach) and bevacizumab 15 mg/kg intravenously on day 1 of each 3-week cycle. On day 1 of cycle 1, bevacizumab was administered simultaneously with erlotinib, followed by IMO-2055 administered 90 min later. On day 1 of cycle 2 and all subsequent cycles, all three drugs could be administered simultaneously. On days 8 and 15 of cycle 1 and all subsequent cycles, IMO-2055 and erlotinib could be given simultaneously.
Patients were eligible to continue treatment beyond the dose-escalation phase until progressive disease (PD), unacceptable treatment-related toxicity, or withdrawal of consent. Once treatment was discontinued, patients were followed until death or 1 year after the last study drug administration, with follow-up visits at 1 month post-treatment and every 3 months thereafter.
Outcome measures
Safety was assessed through adverse event (AE) monitoring, physical examinations, vital signs, ECOG performance status, electrocardiograms, and laboratory studies. AEs were classified according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 3.0 and coded using the Medical Dictionary for Regulatory Activities (MedDRA) version 10.1. DLTs were assessed during cycle 1 only and were defined as follows: Grade 4 neutropenia, anemia, or thrombocytopenia lasting 5 or more days; Grade 3 or higher nausea, diarrhea, or vomiting despite maximal support; any clinically significant Grade 3 or higher non-hematologic toxicity; treatment delay of more than 14 days due to prolonged recovery from a Grade 2 or higher drug-related toxicity; or toxicity leading to interruption of erlotinib treatment for more than 5 days.
Plasma samples for the evaluation of PK interactions of the three drugs were collected before study drug administration and at various times post-dosing during cycles 1 and 2 for the planned 15 patients treated with IMO-2055 at the RP2D once this had been established.
Tumor response and progression were evaluated after every second treatment cycle using Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0. Changes in tumor measurements were confirmed by repeat measurements no less than 4 weeks apart to determine partial response (PR) or complete response (CR).
Statistical methods
The planned sample size of 27–36 patients was based on three to six patients per dose cohort with an additional 15 patients enrolled once the RP2D level was established, for a total of 18 or more patients treated at the RP2D level. Baseline characteristics, safety, and preliminary antitumor activity outcomes were evaluated using summary data. DLTs were described according to dose level. Response outcomes were calculated as the percentage of patients with measurable disease at baseline. PFS and OS were evaluated using the Kaplan–Meier method [19]. PFS was calculated from first trial drug administration to documented PD or death from any cause. OS was calculated from first trial drug administration to death from any cause. PK parameters were calculated using non-compartmental methods with Kinetica 4.4.1 software. PK parameters obtained with single and multiple doses of erlotinib in combination with bevacizumab and IMO-2055 were compared with historical single-agent erlotinib PK results to assess any drug–drug interactions.
Results
Patient disposition and baseline characteristics
Thirty-six patients were enrolled in this study [intention to treat (ITT) population] and 35 of these received at least one dose of the study drugs (safety population). Thirty-three patients had measureable disease at baseline (antitumor activity evaluable population). Nineteen patients were enrolled during the dose-escalation phase. Four, six, six, and three patients were treated at dose level 0.08, 0.16, 0.32, and 0.48 mg/kg, respectively, including one patient assigned to the 0.08-mg/kg IMO-2055 dose cohort who discontinued the trial before completing the first treatment cycle. This patient was subsequently replaced. Demographic and baseline disease characteristics were comparable between dose cohorts (Table 1). Patients had a median age of 64 years (range 42–81 years), and were predominantly male (58 %) and Caucasian (81 %), and most patients (81 %) had an ECOG performance status score of 1 at baseline. Adenocarcinoma (69 % of patients) was the most common histopathology. Overall, patients had a median disease duration of 17.1 months (range 3.9–79.9 months; calculated from initial diagnosis to study entry). Forty-four percent of patients had 2 or more prior therapies for their cancer. Overall, 89 % of patients had a history of smoking.
Table 1.
Patient baseline and demographic characteristics (ITT population)
| Characteristic | IMO-2055, mg/kg/week | Overall (N = 36) | |||
|---|---|---|---|---|---|
| 0.08 (N = 4) | 0.16 (N = 6) | 0.32 (N = 23)a | 0.48 (N = 3) | ||
| Median age [years (range)] | 61.5 (52–81) | 67.5 (54–80) | 64.0 (42–79) | 65.0 (61–75) | 64.0 (42–81) |
| Male [n (%)] | 1 (25) | 3 (50) | 14 (61) | 3 (100) | 21 (58) |
| Ethnicity [n (%)] | |||||
| Caucasian | 4 (100) | 5 (83) | 18 (78) | 2 (67) | 29 (81) |
| Black | 0 | 1 (17) | 3 (13) | 1 (33) | 5 (14) |
| Asian | 0 | 0 | 1 (4) | 0 | 1 (3) |
| Other | 0 | 0 | 1 (4) | 0 | 1 (3) |
| ECOG performance status [n (%)] | |||||
| 0 | 1 (25) | 1 (17) | 4 (17) | 1 (33) | 7 (19) |
| 1 | 3 (75) | 5 (83) | 19 (83) | 2 (67) | 29 (81) |
| Histopathology [n (%)] | |||||
| Adenocarcinoma, NOS | 3 (75) | 4 (67) | 16 (70) | 2 (67) | 25 (69) |
| Large cell carcinoma, NOS | 1 (25) | 0 | 4 (17) | 0 | 5 (14) |
| Non-small cell carcinoma | 0 | 0 | 3 (13) | 1 (33) | 4 (11) |
| Squamous cell carcinoma, NOS | 0 | 2 (33) | 0 | 0 | 2 (6) |
| Median disease duration [months (range)] | 15.5 (5.7–22.5) | 23.3 (5.1–48.9) | 17.9 (3.9–79.9) | 13.6 (7.5–16.5) | 17.1 (3.9–79.9) |
| Prior cancer therapies [n (%)] | |||||
| 1 | 4 (100) | 3 (50) | 12 (52) | 1 (33) | 20 (56) |
| 2 | 0 | 3 (50) | 3 (13) | 2 (67) | 8 (22) |
| ≥3 | 0 | 0 | 8 (35) | 0 | 8 (22) |
ECOG Eastern Cooperative Oncology Group, ITT intention to treat, NOS not otherwise specified, RP2D recommended phase II dose
aIncludes 17 patients who were enrolled after the dose-escalation phase was completed and the RP2D of IMO-2055 (i.e., 0.32 mg/kg) was determined; 16 of these received ≥1 treatment dose
Dose-limiting toxicities and selection of the recommended dose
Dose-limiting toxicities were assessed during the first cycle of treatment and were reported in two patients; one patient in the 0.16-mg/kg IMO-2055 cohort experienced Grade 3 dehydration and one patient in the 0.48-mg/kg cohort experienced Grade 3 fatigue. The observed DLTs did not suggest a consistent dose-related toxicity pattern for the study treatment. Injection-site reactions, although not dose limiting, were more frequent and severe in patients receiving 0.48 mg/kg. Prior pharmacodynamic studies suggested that IMO-2055 immune stimulation as evidenced by cytokine release and cell activation was not consistently enhanced at increasing doses: IMO-2055 0.32 mg/kg yielded maximal responses in circulating IL-12, IL-6, and IFN-alpha in a previous phase I trial in patients with refractory solid tumors (data on file). In addition, a maximal effect on circulating lymphocytes was observed at IMO-2055 0.32 mg/kg (data on file). Based on these results and a better tolerability compared with IMO-2055 0.48 mg/kg seen in this trial, the 0.32 mg/kg IMO-2055 dose was selected as the RP2D for an expanded cohort of 16 additional patients.
Safety
All 35 patients in the safety population experienced at least one treatment-emergent AE (TEAE) during the overall treatment period. The most commonly reported TEAEs were diarrhea (74 %), nausea (51 %), fatigue (51 %), rash (51 %), and injection-site reactions (49 %). Overall, 25 patients (71 %) experienced Grade 3 or higher TEAEs, including dyspnea (17 % of patients), diarrhea (11 %), and acneiform dermatitis (11 %). Fifteen patients (43 %) experienced at least one serious TEAE of which the most common were PD and deep vein thrombosis, each reported in 9 % of patients.
Overall, 34 patients (97 %) reported at least one TEAE considered related to study drug (IMO-2055, erlotinib, or bevacizumab). There was no indication of cumulative toxicity. Drug-related TEAEs occurring in ≥20 % of patients are summarized in Table 2. Twelve patients (34 %) had one or more Grade 3 or higher drug-related TEAE of which the most commonly reported were fatigue (9 %) and diarrhea (9 %) (Table 2). Four patients (11 %) experienced serious TEAEs that were considered to be possibly related to the study medication. These serious TEAEs were as follows: in the 0.32-mg/kg cohort, one patient with a temporal lobe encephalomalacia at baseline experienced a Grade 3 cerebral hemorrhage. Another patient in this cohort had a Grade 2 enlarged uvula, and a third patient experienced Grade 4 blood creatinine increase, dyspnea, fatigue, hypotension, and metabolic acidosis. In the 0.48-mg/kg cohort, a single patient experienced Grade 1 pyrexia lasting for 7 days.
Table 2.
Study drug (IMO-2055, erlotinib, or bevacizumab)-related TEAEs (safety evaluable population)
| Preferred terma [n (%)] | IMO-2055, mg/kg/week | Overall (N = 35) | |||
|---|---|---|---|---|---|
| 0.08 (N = 4) | 0.16 (N = 6) | 0.32 (N = 22) | 0.48 (N = 3) | ||
| Any grade, occurring in ≥20 % of patients | |||||
| Injection-site reaction | 2 (50) | 3 (50) | 9 (41) | 3 (100) | 17 (49) |
| Diarrhea | 3 (75) | 2 (33) | 9 (41) | 3 (100) | 17 (49) |
| Fatigue | 2 (50) | 2 (33) | 8 (36) | 3 (100) | 15 (43) |
| Nausea | 1 (25) | 1 (17) | 6 (27) | 2 (67) | 10 (29) |
| Injection-site pain | 0 | 3 (50) | 5 (23) | 1 (33) | 9 (26) |
| Chills | 2 (50) | 1 (17) | 4 (18) | 1 (33) | 8 (23) |
| Anorexia | 2 (50) | 1 (17) | 4 (18) | 1 (33) | 8 (23) |
| Muscle spasms | 1 (25) | 0 | 5 (23) | 1 (33) | 7 (20) |
| Weight decreased | 3 (75) | 1 (17) | 3 (14) | 0 | 7 (20) |
| Grade ≥3, occurring in ≥5 % of patients | |||||
| Fatigue | 0 | 0 | 2 (9) | 1 (33) | 3 (9) |
| Diarrhea | 1 (25) | 0 | 2 (9) | 0 | 3 (9) |
| Anemia | 1 (25) | 0 | 1 (5) | 0 | 2 (6) |
| Dyspnea | 0 | 0 | 1 (5) | 1 (33) | 2 (6) |
TEAEs listed were considered to be “possibly related,” “probably related,” or “definitely related” to any of the three study drugs
TEAE treatment-emergent adverse event
aAccording to Medical Dictionary for Regulatory Activities (MedDRA) version 10.1
Sixteen patients (46 %) overall discontinued the study due to TEAEs, including two patients (50 %) in the 0.08-mg/kg cohort, three patients (50 %) in the 0.16-mg/kg cohort, eight patients (36 %) in the 0.32-mg/kg cohort, and three patients (100 %) in the 0.48-mg/kg cohort. The most common TEAEs leading to study discontinuation were chills (one patient each in the 0.08- and 0.16-mg/kg cohorts), injection-site reaction (two patients in the 0.16-mg/kg cohort), back pain (one patient each in the 0.08- and 0.32-mg/kg cohorts), fatigue (one patient each in the 0.32- and 0.48-mg/kg cohorts, and hypoxia (one patient each in the 0.08- and 0.48-mg/kg cohorts). Five patients (14 %) died either during the study or within 1 month post-treatment. All deaths were due to PD, with one patient also experiencing septic shock. None of these deaths was considered to be related to study medication. Beyond 1 month post-treatment, another 14 patients died, all due to PD.
Pharmacokinetics
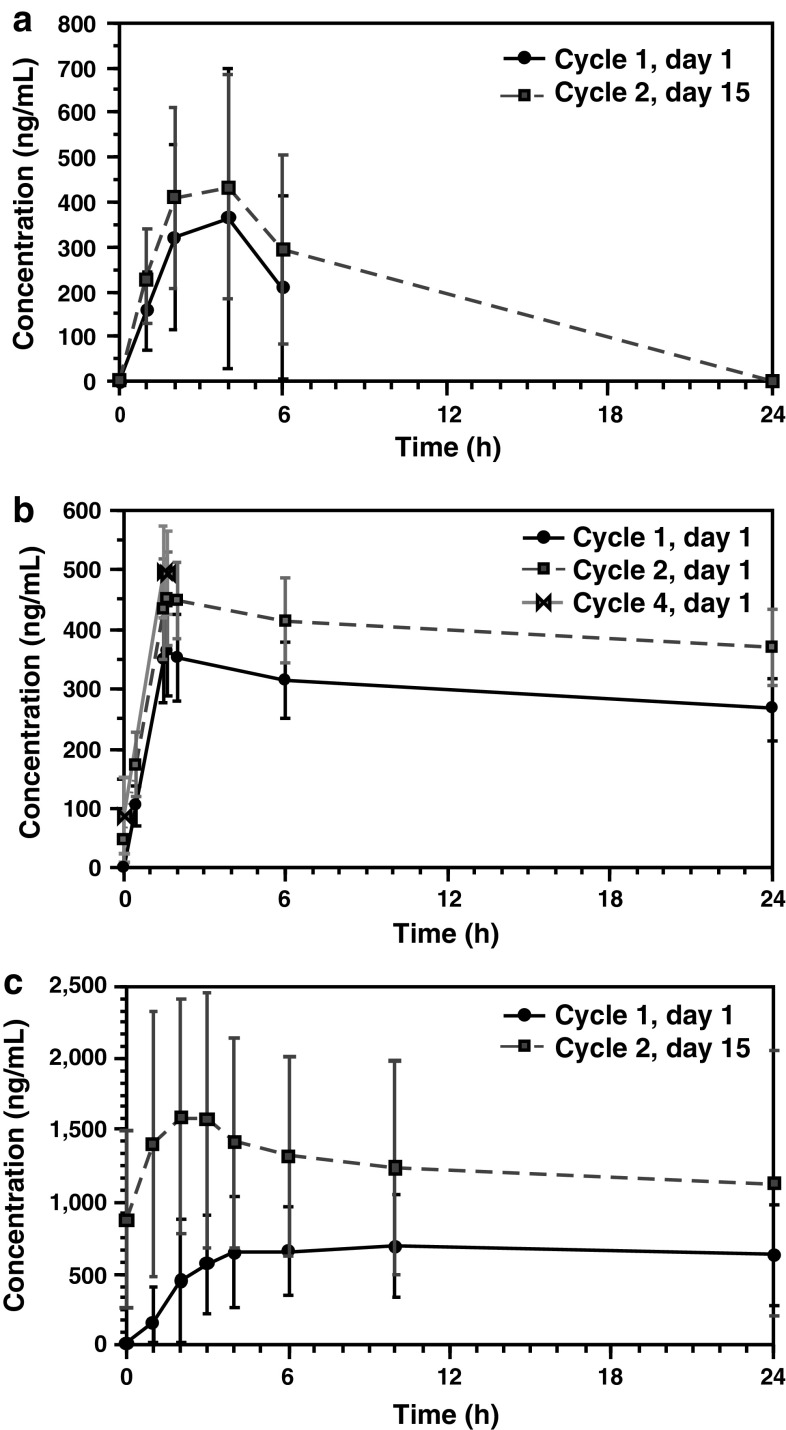
Pharmacokinetic evaluations included the determination of maximum plasma concentration (C max), time to C max (t max), and area under the plasma concentration–time curve from time zero to time t (AUC0–t) from plasma samples of patients treated with the IMO-2055 RP2D (i.e., 0.32 mg/kg) after the dose-escalation phase was completed. Results for IMO-2055, bevacizumab, and erlotinib, as obtained after the first administration during cycle 1 and again during cycle 2, are summarized in Table 3 and concentration–time profiles by treatment cycle and day are shown in Fig. 1. Variability of the main PK parameters was moderate to high for IMO-2055 and erlotinib, and low for bevacizumab. No apparent differences in PK parameters were observed between the two cycles and administration days for IMO-2055. However, as expected from the reported half-life of bevacizumab of ~20 days [12, 20], bevacizumab serum concentrations in cycle 2 were slightly higher than in the first cycle, with correspondingly higher C max and AUC0–t. Similarly, erlotinib, with a reported half-life of approximately 1.25 days [12], was associated with a moderate accumulation after multiple dosing resulting in a twofold higher C max and AUC0–t compared with cycle 1. Overall, the PK results for IMO-2055, bevacizumab, and erlotinib were within the expected ranges.
Table 3.
Pharmacokinetic parameters of IMO-2055, bevacizumab, and erlotinib
| Administration | Dose | N a | C max (ng/mL) | t max (h) | AUC0–t (ng/mL h) |
|---|---|---|---|---|---|
| IMO-2055 | |||||
| Cycle 1, day 1 | 0.32 mg/kg | 15 | 306.19 (69.70–1300.00) | 3.4 (1.8–4.0) | 1190.5 (289.1–4931.1) |
| Cycle 2, day 15 | 0.32 mg/kg | 12 | 418.05 (161.00–1130.00) | 4.0 (2.0–6.0) | 1732.8 (786.5–4810.6) |
| Bevacizumab | |||||
| Cycle 1, day 1 | 15 mg/kg | 15 | 370.19 (276.00–493.00) | 1.7 (1.5–6.0) | 6298.9 (2074.4–9443.2) |
| Cycle 2, day 1 | 15 mg/kg | 11 | 465.09 (357.00–580.00) | 1.7 (1.5–2.2) | 9247.4 (7187.1–10968.7) |
| Erlotinib | |||||
| Cycle 1, day 1 | 150 mg | 15 | 831.45 (348.00–1580.00) | 6.0 (2.0–24.0) | 12618.9 (6137.7–22898.9) |
| Cycle 2, day 15b | 150 mg | 12 | 1651.90 (704.00–3580.00) | 2.0 (1.0–24.0) | 21870.1 (5249.0–59983.1) |
Values for C max and AUC0–t are given as geometric mean (range); values for t max are given as median (range)
AUC 0-t area under the concentration–time curve from time zero to time t, C max maximum plasma concentration, t max time to Cmax
aNumber of patients analyzed
bTwo patients received 100 mg erlotinib in cycle 2
Fig. 1.
Mean plasma concentration–time profiles of IMO-2055 0.32 mg/kg (a), bevacizumab 15 mg/kg (b), and erlotinib 150 mg (c). Profiles are depicted in linear scale
Definitive analysis of drug–drug interactions was not performed because the sampling interval in this trial did not allow the evaluation of all PK parameters of the three drugs due to the long half-life of bevacizumab. Specifically, because there were no data from the elimination phase, the terminal phase could not be precisely estimated and thus half-life, total AUC, total clearance of the drug from plasma, and volume of distribution were not calculated. As a consequence, potential drug–drug interactions of IMO-2055 could not be determined.
Antitumor activity
Of the 33 patients with measurable disease at baseline, four patients had no post-baseline antitumor activity assessments while on treatment. Five patients (15 %) achieved a PR (confirmed according to RECIST), of which one patient previously failed to respond to bevacizumab-containing treatment (combination of bevacizumab plus paclitaxel/carboplatin). Another 20 patients (61 %) had stable disease (SD) as a best response, with 13 patients having SD for at least 4 months (Table 4). No patients achieved a CR. Four patients (12 %) in the 0.08-, 0.16- and 0.32-mg/kg cohorts had PD at cycle 2. Changes in tumor size per patient from baseline are shown in Fig. 2. In the ITT population, the median PFS was 5.6 months [95 % confidence interval (CI): 3.9–7.2 months] with a 1-year PFS rate of 24.2 %. The median OS was 16.0 months (95 % CI: 7.5–17.9 months), with 55.1 % of patients alive at 1 year after treatment start. One patient still showed no evidence of disease at the last confirmation visit 3.75 years after ending IMO-2055 treatment [CT scan and normal carcinoembryonic antigen (CEA) levels]. This patient was treated with IMO-2055 0.32 mg/kg for 21 weeks and is still alive without additional therapy ~4 years after IMO-2055 treatment was stopped (Boyd T, personal communication; Oct 2013).
Table 4.
Summary of patient responses to treatment (antitumor activity evaluable population)
| Best overall response (RECIST)a | IMO-2055, mg/kg/week | Overallb (N = 33) | |||
|---|---|---|---|---|---|
| 0.08 (N = 4) | 0.16 (N = 5) | 0.32 (N = 21) | 0.48 (N = 3) | ||
| PR [n (%)] | 2 (50) | 0 | 3 (14) | 0 | 5 (15) |
| SD [n (%)] | 1 (25) | 3 (60) | 13 (62) | 3 (100) | 20 (61) |
| PD [n (%)] | 0 | 2 (40) | 2 (10) | 0 | 4 (12) |
| Not evaluated [n (%)]c | 1 (25) | 0 | 3 (14) | 0 | 4 (12) |
PD progressive disease, PR partial response, RECIST Response Evaluation Criteria In Solid Tumors, SD stable disease
aNone of the patients achieved a complete response
bTwo patients (one patient each in the 0.16- and 0.32-mg/kg cohorts) of the treated 35 patients had no measurable disease at baseline and were not part of the antitumor activity evaluable population
cPatients had no post-baseline response assessment
Fig. 2.
Waterfall plot of best response overall per patient [changes in tumor size from baseline (%); antitumor activity evaluable population]. Four of 33 patients with measurable disease at baseline had no post-baseline tumor assessments at cycle 2
Discussion
This trial was launched to explore the promising preclinical data suggesting that IMO-2055 combined with erlotinib and bevacizumab would enhance the antitumor activity of the latter two drugs [15–17]. The success of other immune-stimulating agents has increased the interest in this approach to cancer drug development [21, 22]. This phase Ib trial established the RP2D of subcutaneously administered IMO-2055 as 0.32 mg/kg/week when combined with oral erlotinib 150 mg daily and intravenous bevacizumab 15 mg/kg once every 3 weeks. There were just two DLTs across the dose range and neither was suggestive of a consistent toxicity pattern. The decision to adopt the 0.32 mg/kg dose for subsequent phase II studies was based on pharmacodynamic and clinical data.
Subcutaneous weekly injections of IMO-2055 in combination with erlotinib and bevacizumab were generally well tolerated at all doses, including the expanded 0.32-mg/kg dose cohort. IMO-2055 did not appear to cause hematologic toxicity in this limited number of patients. The most commonly reported TEAEs of diarrhea, nausea, fatigue, and rash were consistent with previously reported trials of erlotinib combined with bevacizumab in a range of tumor types, including NSCLC [12, 23–25]. In addition, the frequency of observed injection-site reactions and chills was consistent with what was expected with the administration of an immunostimulatory agent; these TEAEs have been observed also in other clinical settings with IMO-2055 [26]. Two patients each withdrew from the study due to these two TEAEs (injection-site reactions: two patients in the 0.16-mg/kg cohort; chills: one patient each in the 0.08- and 0.16-mg/kg cohorts) despite prophylactic and symptomatic therapy.
There have been previous reports that the combination of TLR9 agonists with platinum-based therapy may exacerbate AEs in patients with advanced solid tumors [27–30]. Two recent phase III studies investigating the combination of platinum-based chemotherapy with the TLR9 agonist CPG 7909 (PF-3512676) as first-line treatment of advanced NSCLC were terminated at the first interim analysis, as the treatment group with CPG 7909 had increased rates of Grade 3 neutropenia but failed to show improved OS, PFS, or higher objective response rates compared with chemotherapy alone [28, 30]. Similarly, hematologic AEs were dose-limiting in two recent phase I trials of IMO-2055 combined with platinum-based chemotherapy in patients with advanced solid tumors [27, 29]. In addition, high rates of Grade 3 hypokalemia and hypomagnesemia were observed in these patients [27]. Myelosuppression and electrolyte disturbances are well-known side effects associated with platinum-based treatment [31, 32]. The precise role that TLR9 agonists played in these side effects when combined with platinum-based treatment is unclear; but these results show the difficulty of investigating increasingly complex regimens.
The combination of IMO-2055 with bevacizumab and erlotinib did not result in increased rates of myelosuppression or electrolyte imbalances in the limited number of patients treated in the present trial. The addition of IMO-2055 to erlotinib and bevacizumab was well tolerated and it is feasible to further explore IMO-2055 combined with these agents for the treatment of advanced NSCLC. This study is of interest because some of these heavily pretreated patients had prolonged responses, with one patient who received IMO-2055 0.32 mg/kg remaining in unmaintained remission for at least 3.75 years after study treatment was stopped. As a small uncontrolled trial, responses seen in this study are anecdotal and presented in a descriptive manner. However, the disease control rate of 76 % together with median PFS and OS of 5.6 and 16 months, respectively, are well within the range of historical data for the combination of erlotinib plus bevacizumab in this setting [12–14]. The disease control rates observed in these previously reported studies were 45–51 %, and the medians of PFS and OS ranged from 3.4–6.2 to 9.2–13.7 months. This suggests that IMO-2055 may have stimulated an antitumor response in these patients.
Any conclusions about the study must be tempered by the small number of patients enrolled. Further investigations in a larger patient population would be needed to establish the antitumor activity of IMO-2055 in combination with erlotinib and bevacizumab. The role of TLR9 agonists in the future of cancer drug development has not been established [33]. Current efforts are focused on their use as adjuvants in combination with other immune therapies (e.g., TLR2-neutralizing antibodies) and on evaluating direct application to a patient’s tumor in combination with irradiation [34–37]. As immune therapies become more widespread in the treatment of malignancies, the data presented here will be useful in planning the next combination of a TLR9 agonist with targeted therapies for use in both solid and hematologic tumors. Further investigation is required to see if the prolonged disease stabilization seen in the patients in this study can be replicated.
Acknowledgments
The trial was sponsored by EMD Serono Inc., Rockland, MA, USA. The authors would like to thank the patients and their families without whom this study would not have been possible. Editorial and medical writing assistance in the preparation of this manuscript was provided by Marianne Jenal-Eyholzer, Ph.D., CMPP, TRM Oncology, The Hague, The Netherlands, funded by Merck KGaA, Darmstadt, Germany.
Conflict of interest
Thomas E. Boyd: Consultant to Celgene; research funding by Genentech, Celgene, GSK, Onyx and Pharmacyclics. Guillaume de La Bourdonnaye: Former employee of Merck KGaA (2008–2012). David Wages: Former employee of EMD Serono. Alice S. Bexon: Previously employee of Idera Pharmaceuticals, now paid consultant to Idera Pharmaceuticals. David A. Smith, Paul Conkling, Donald A. Richards, John J. Nemunaitis, and Alain C. Mita: None.
Abbreviations
- AE
Adverse event
- AUC0–t
Area under the concentration–time curve from time zero to time t
- CI
Confidence interval
- Cmax
Maximum plasma concentration
- CR
Complete response
- DLT
Dose-limiting toxicity
- ECOG
Eastern Cooperative Oncology Group
- EGFR
Epidermal growth factor receptor
- ITT
Intention to treat
- MedDRA
Medical Dictionary for Regulatory Activities
- NCI CTCAE
National Cancer Institute Common Terminology Criteria for Adverse Events
- NOS
Not otherwise specified
- NSCLC
Non-small cell lung cancer
- OS
Overall survival
- PD
Progressive disease
- PFS
Progression-free survival
- PK
Pharmacokinetic
- PR
Partial response
- RECIST
Response Evaluation Criteria In Solid Tumors
- RP2D
Recommended phase II dose
- SD
Stable disease
- TEAE
Treatment-emergent adverse event
- TLR9
Toll-like receptor 9
- tmax
Time to C max
- VEGF
Vascular endothelial growth factor
Footnotes
The results of this study have been published in parts in abstract form at the ECCO-ESMO 2009 multidisciplinary congress [Smith et al. (2009) Eur J Cancer 7(Suppl): abstract 9148] and at the ASCO 2012 annual meeting [Smith et al. (2012) J Clin Oncol 30(Suppl): abstract e18047].
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network (NCCN) (2012) NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Non-small cell lung cancer version 3. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed 4 Sep 2013
- 3.Bareschino MA, Schettino C, Rossi A, Maione P, Sacco PC, Zeppa R, et al. Treatment of advanced non small cell lung cancer. J Thorac Dis. 2011;3:122–133. doi: 10.3978/j.issn.2072-1439.2010.12.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ho C, Davies AM, Lara PN, Jr, Gandara DR. Second-line treatment for advanced-stage non-small-cell lung cancer: current and future options. Clin Lung Cancer. 2006;7(Suppl 4):S118–S125. doi: 10.3816/CLC.2006.s.003. [DOI] [PubMed] [Google Scholar]
- 5.Taxotere [product monograph] (2013) Revised Aug 2013. Bridgewater, NJ: sanofi-aventis U.S. LLC. http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020449s059lbl.pdf. Accessed 16 Sep 2013
- 6.Alimta [prescribing information] (2013) Revised May 2013. Indianapolis, IN: Eli Lilly and Company. http://pi.lilly.com/us/alimta-pi.pdf. Accessed 16 Sep 2013
- 7.Tarceva [product information] (2013) Revised May 2013. South San Francisco, CA: Genentech, Inc. http://www.gene.com/download/pdf/tarceva_prescribing.pdf. Accessed 16 Sep 2013
- 8.Iressa [summary of product characteristics] (2011) Cheshire, UK: AstraZeneca UK Limited. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001016/WC500036358.pdf. Accessed 16 Sep 2013
- 9.Stinchcombe TE, Socinski MA. Considerations for second-line therapy of non-small cell lung cancer. Oncologist. 2008;13(Suppl 1):28–36. doi: 10.1634/theoncologist.13-S1-28. [DOI] [PubMed] [Google Scholar]
- 10.Byers LA, Heymach JV. Dual targeting of the vascular endothelial growth factor and epidermal growth factor receptor pathways: rationale and clinical applications for non-small-cell lung cancer. Clin Lung Cancer. 2007;8(Suppl 2):S79–S85. doi: 10.3816/CLC.2007.s.006. [DOI] [PubMed] [Google Scholar]
- 11.Reck M, Crinò L. Advances in anti-VEGF and anti-EGFR therapy for advanced non-small cell lung cancer. Lung Cancer. 2009;63:1–9. doi: 10.1016/j.lungcan.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 12.Herbst RS, Johnson DH, Mininberg E, Carbone DP, Henderson T, Kim ES, et al. Phase I/II trial evaluating the anti-vascular endothelial growth factor monoclonal antibody bevacizumab in combination with the HER-1/epidermal growth factor receptor tyrosine kinase inhibitor erlotinib for patients with recurrent non-small-cell lung cancer. J Clin Oncol. 2005;23:2544–2555. doi: 10.1200/JCO.2005.02.477. [DOI] [PubMed] [Google Scholar]
- 13.Herbst RS, O’Neill VJ, Fehrenbacher L, Belani CP, Bonomi PD, Hart L, et al. Phase II study of efficacy and safety of bevacizumab in combination with chemotherapy or erlotinib compared with chemotherapy alone for treatment of recurrent or refractory non small-cell lung cancer. J Clin Oncol. 2007;25:4743–4750. doi: 10.1200/JCO.2007.12.3026. [DOI] [PubMed] [Google Scholar]
- 14.Herbst RS, Ansari R, Bustin F, Flynn P, Hart L, Otterson GA, et al. Efficacy of bevacizumab plus erlotinib versus erlotinib alone in advanced non-small-cell lung cancer after failure of standard first-line chemotherapy (BeTa): a double-blind, placebo-controlled, phase 3 trial. Lancet. 2011;377:1846–1854. doi: 10.1016/S0140-6736(11)60545-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Damiano V, Caputo R, Bianco R, D’Armiento FP, Leonardi A, De Placido S, et al. Novel toll-like receptor 9 agonist induces epidermal growth factor receptor (EGFR) inhibition and synergistic antitumor activity with EGFR inhibitors. Clin Cancer Res. 2006;12:577–583. doi: 10.1158/1078-0432.CCR-05-1943. [DOI] [PubMed] [Google Scholar]
- 16.Damiano V, Caputo R, Garofalo S, Bianco R, Rosa R, Merola G, et al. TLR9 agonist acts by different mechanisms synergizing with bevacizumab in sensitive and cetuximab-resistant colon cancer xenografts. Proc Natl Acad Sci USA. 2007;104:12468–12473. doi: 10.1073/pnas.0705226104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang D, Kandimalla ER, Yu D, Karr R, Agrawal S (2008) Antitumor activity of IMO-2055, an agonist of TLR9, in combination with erlotinib and bevacizumab in non-small cell lung cancer xenografts in mice. AACR Meeting Abstracts 2008:abstr 2078
- 18.Storer BE. Design and analysis of phase I clinical trials. Biometrics. 1989;45:925–937. doi: 10.2307/2531693. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. doi: 10.1080/01621459.1958.10501452. [DOI] [Google Scholar]
- 20.Avastin [prescribing information] (2011) Revised September 2011. South San Francisco, CA: Genentech, Inc. http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/125085s225lbl.pdf. Accessed 16 Sep 2013
- 21.Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 22.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 23.Cohen EE, Davis DW, Karrison TG, Seiwert TY, Wong SJ, Nattam S, et al. Erlotinib and bevacizumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck: a phase I/II study. Lancet Oncol. 2009;10:247–257. doi: 10.1016/S1470-2045(09)70002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ko AH, Venook AP, Bergsland EK, Kelley RK, Korn WM, Dito E, et al. A phase II study of bevacizumab plus erlotinib for gemcitabine-refractory metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2010;66:1051–1057. doi: 10.1007/s00280-010-1257-5. [DOI] [PubMed] [Google Scholar]
- 25.Yau T, Wong H, Chan P, Yao TJ, Pang R, Cheung TT, et al. Phase II study of bevacizumab and erlotinib in the treatment of advanced hepatocellular carcinoma patients with sorafenib-refractory disease. Invest New Drugs. 2012;30:2384–2390. doi: 10.1007/s10637-012-9808-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore DJ, Hwang J, McGreivy J, Park S, Malik S, Martin RR et al (2005) Phase I trial of escalating doses of the TLR9 agonist HYB2055 in patients with advanced solid tumors. J Clin Oncol 23(16S): abstract 2503
- 27.Machiels JP, Kaminsky MC, Keller U, Brümmendorf TH, Goddemeier T, Forssman U, et al. Phase Ib trial of the Toll-like receptor 9 agonist IMO-2055 in combination with 5-fluorouracil, cisplatin, and cetuximab as first-line palliative treatment in patients with recurrent/metastatic squamous cell carcinoma of the head and neck. Invest New Drugs. 2013;31:1207–1216. doi: 10.1007/s10637-013-9933-z. [DOI] [PubMed] [Google Scholar]
- 28.Manegold C, van Zandwijk N, Szczesna A, Zatloukal P, Au JS, Blasinska-Morawiec M, et al. A phase III randomized study of gemcitabine and cisplatin with or without PF-3512676 (TLR9 agonist) as first-line treatment of advanced non-small-cell lung cancer. Ann Oncol. 2012;23:72–77. doi: 10.1093/annonc/mdr030. [DOI] [PubMed] [Google Scholar]
- 29.Malik S, Hwang J, Cotarla I, Sullivan T, Kerr R, Marshall J et al (2007) Initial phase 1 results of gemcitabine, carboplatin and IMO-2055, a toll like receptor 9 (TLR9) agonist, in patients (pts) with advanced solid tumors. J Thorac Oncol 2(8 Suppl 4):S726–S727, abstract P3-112
- 30.Hirsh V, Paz-Ares L, Boyer M, Rosell R, Middleton G, Eberhardt WE, et al. Randomized phase III trial of paclitaxel/carboplatin with or without PF-3512676 (Toll-like receptor 9 agonist) as first-line treatment for advanced non-small-cell lung cancer. J Clin Oncol. 2011;29:2667–2674. doi: 10.1200/JCO.2010.32.8971. [DOI] [PubMed] [Google Scholar]
- 31.Sánchez-González PD, López-Hernández FJ, López-Novoa JM, Morales AI. An integrative view of the pathophysiological events leading to cisplatin nephrotoxicity. Crit Rev Toxicol. 2011;41:803–821. doi: 10.3109/10408444.2011.602662. [DOI] [PubMed] [Google Scholar]
- 32.Yao X, Panichpisal K, Kurtzman N, Nugent K. Cisplatin nephrotoxicity: a review. Am J Med Sci. 2007;334:115–124. doi: 10.1097/MAJ.0b013e31812dfe1e. [DOI] [PubMed] [Google Scholar]
- 33.Guha M. Anticancer TLR agonists on the ropes. Nat Rev Drug Discov. 2012;11:503–505. doi: 10.1038/nrd3775. [DOI] [PubMed] [Google Scholar]
- 34.Kim YH, Gratzinger D, Harrison C, Brody JD, Czerwinski DK, Ai WZ, et al. In situ vaccination against mycosis fungoides by intratumoral injection of a TLR9 agonist combined with radiation: a phase 1/2 study. Blood. 2012;119:355–363. doi: 10.1182/blood-2011-05-355222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brody JD, Ai WZ, Czerwinski DK, Torchia JA, Levy M, Advani RH, et al. In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a phase I/II study. J Clin Oncol. 2010;28:4324–4332. doi: 10.1200/JCO.2010.28.9793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stevenson FK, Johnson PW. Harnessing innate immunity to suppress lymphoma. J Clin Oncol. 2010;28:4295–4296. doi: 10.1200/JCO.2010.30.4212. [DOI] [PubMed] [Google Scholar]
- 37.Yan J, Hua F, Liu HZ, Yang HZ, Hu ZW. Simultaneous TLR2 inhibition and TLR9 activation synergistically suppress tumor metastasis in mice. Acta Pharmocol Sin. 2012;33:503–512. doi: 10.1038/aps.2011.193. [DOI] [PMC free article] [PubMed] [Google Scholar]