Abstract
Due to its immunogenicity and overexpression concomitant with leukemia progression, Wilms tumor protein 1 (WT1) is of particular interest for immunotherapy of AML relapse after allogeneic hematopoietic stem cell transplantation (allo-HSCT). So far, WT1-specific T-cell responses have mainly been induced by vaccination with peptides presented by certain HLA alleles. However, this approach is still not widely applicable in clinical practice due to common limitations of HLA restriction. Dendritic cell (DC) vaccines electroporated with mRNA encoding full-length protein have also been tested for generating WT1-derived peptides for presentation to T-cells. Alternatively, an efficient and broad WT1 peptide presentation could be elicited by triggering receptor-mediated protein endocytosis of DCs. Therefore, we developed antibody fusion proteins consisting of an antibody specific for the DEC205 endocytic receptor on human DCs and various fragments of WT1 as DC-targeting recombinant WT1 vaccines (anti-hDEC205-WT1). Of all anti-hDEC205-WT1 fusion proteins designed for overcoming insufficient expression, anti-hDEC205-WT110–35, anti-hDEC205-WT191–138, anti-hDEC205-WT1223–273, and anti-hDEC205-WT1324–371 were identified in good yields. The anti-hDEC205-WT191–138 was capable of directly inducing ex vivo T-cell responses by co-incubation of the fusion protein-loaded monocyte-derived mature DCs and autologous T-cells of either healthy or HSCT individuals. Furthermore, the DC-targeted WT191–138-induced specific T-cells showed a strong cytotoxic activity by lysing WT1-overexpressing THP-1 leukemia cells in vitro while sparing WT1-negative hematopoietic cells. In conclusion, our approach identifies four WT1 peptide-antibody fusion proteins with sufficient production and introduces an alternative vaccine that could be easily translated into clinical practice to improve WT1-directed antileukemia immune responses after allo-HSCT.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-016-1938-y) contains supplementary material, which is available to authorized users.
Keywords: Wilms tumor protein 1, Tumor-associated antigen, Immunotherapy against high-risk AML, Anti-hDEC205-WT1 antibody fusion protein, Cytotoxic T-cells, Tumor vaccine
Introduction
Currently, patients with high-risk acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS) are treated with allogeneic hematopoietic stem cell transplantation (allo-HSCT) to benefit from a graft-versus-leukemia (GvL) effect and potential cure. However, a relapse rate of 20–70% after allo-HSCT [1] and an even poorer prognosis of patients who are not eligible for allo-HSCT urge alternative therapeutic approaches including antileukemic vaccination strategies [2].
In vaccination strategies for eradication of residual leukemic cells, tumor-associated antigens (TAAs) and dendritic cells (DCs) have been thoroughly investigated. Wilms tumor protein 1 (WT1) is an attractive vaccine candidate due to its therapeutic impact, tissue restricted expression, and leukemogenic characteristics [3]. Therefore, WT1-derived peptide vaccines are extensively tested for antileukemia vaccination. However, therapeutic use of single peptides is generally limited by human leukocyte antigen (HLA) restriction of the peptides, need for prior identification of immunogenic epitopes [4], and short-lived T-cell responses [5]. To overcome these limitations, several strategies including transfection of dendritic cells (DCs) with WT1 DNA [6] or full mRNA [7] as well as treatment with long WT1 peptides [8] and polyvalent peptide [9, 10] vaccines have been tested. Due to differences of the WT1 gene transcription and translation, at least 36 isoforms of WT1 protein are theoretically expressed from the gene [11]. Shuttling between the cytoplasm and nucleus [12], they bind to nucleotide sequences [13] as well as interact with posttranscriptional regulators [14]. Thus, recombinant expression of WT1 as a full-length protein is assumed to be difficult [15], and so far full-length human WT1 or fragments of it have not yet been expressed for protein vaccine approaches.
DCs initiate specific T-cell immunity and harmonize innate and adaptive immune responses [16, 17]. They are unique to cross-present extracellular antigens escaped from endolysosomal processing via MHC class I molecules and/or intracellular antigens engulfed by autophagy mechanisms via MHC class II molecules [18]. In addition, a significant association between lower DC count and higher incidence of relapse in allo-HSCT patients [19] supports the key role of DCs in induction of leukemia-specific immunity in the setting of transplantation. Therefore, effective allocation of tumor antigens to DCs is a promising option to evoke a sustained T-cell response. The DEC205 endocytic receptor on the surface of DCs is thought to be the appropriate receptor to translate this option. Indeed, MAGE-derived peptide [20], NY-ESO1 [21], or HER2/neu [22] protein fused to DEC205-specific antibodies improved immune responses against tumors expressing the aforementioned antigens in vitro and in vivo models.
The most crucial prerequisites for a more effective and sustained vaccination-induced T-cell response are diversity and immunogenicity of peptides as well as co-stimulatory signals, provision of cytokines and direct cell contact by the peptide presenting DCs. Thus, we have developed anti-hDEC205-WT1 antibody fusion proteins as DC-targeting recombinant WT1 vaccines and explored their T-cell stimulatory capacity by ex vivo and in vitro assays. By triggering DEC205 receptor-mediated endocytosis using anti-hDEC205-WT1 antibody fusion protein, monocyte-derived DCs (moDCs) were strengthened to uptake the WT1 protein fragments. Intracellular processing of the directly delivered WT1 fragments by moDCs resulted in HLA class I- and II-mediated presentation of a great diversity of WT1-derived peptides. Our approach may allow improved WT1-specific T-cell responses in vivo and give a translational opportunity for WT1-based immunotherapy to the broad patient population with high-risk AML irrespective of their HLA type.
Materials and methods
Molecular cloning, production, and purification of anti-hDEC205-WT1 antibody fusion proteins
A synthetic gene encoding scFv:hDEC205 was designed from an anti-hDEC205 antibody sequence (MG38-3 clone; Prof. R. Steinman, Rockefeller University, New-York, NY) published elsewhere [20]. Using various pCR3-based (Invitrogen) expression vectors encoding the constant domains of human IgG1 heavy (accession number P01857), kappa light chain (accession number AAA59000), or Gaussia princeps luciferase (GpL), FLAG-tagged scFv:hDEC205-GpL, FLAG-tagged full-length heavy and FLAG-tagged light chain of the anti-hDEC205 antibody expression constructs were cloned. cDNAs encoding diverse fragments of WT1_D, the longest isoform (accession number P19544-7), were obtained from THP-1, ML-2, and NALM-6 leukemia cell lines by RNA extraction (Qiagen) and RT-PCR using the primers indicated in Supplementary Table 1. Each WT1_D cDNA was inserted into cloning sites of the corresponding expression vectors to obtain anti-hDEC205-WT1_D antibody fusion constructs. The expression vectors were transiently transfected into HEK293 cells by electroporation (40 μg DNA, 40 × 106 cells, 250 V, 1800 μF, maximum resistance, Easyject Plus, PeqLab). After transfection, cells were recovered in RPMI 1640 culture medium containing 10% FCS overnight, followed by 6–7 days of culturing in serum-reduced medium (2% FCS). The various antibody fusion proteins were purified from collected culture supernatants by column affinity chromatography using anti-FLAG M2 affinity resin (Sigma–Aldrich) according to the manufacturer’s recommendations. Antibody fusion proteins were evaluated for their secretion, concentration, integrity, and purity by SDS-PAGE western blot and silver staining.
Binding studies
Parental and hDEC205-stably expressing CHO cells (CHO-hDEC205, kindly provided by Prof. D. Dudziak, Erlangen, Germany) were seeded into wells of a 24-well plate at a density of 2 × 105 cells per well and cultured overnight at 37 °C. For equilibrium binding studies, supernatants of parental CHO and CHO-hDEC205 cells were pairwise replaced by a medium containing increasing concentrations of the scFv:hDEC205-GpL and incubated at 37 °C for 1 h. Unbound scFv:hDEC205-GpL molecules were removed by washing the cells ten times with ice-cold PBS. Cells with bound GpL fusion protein molecules were transferred to a 96-well black plates in 50 μL medium (RPMI/0.5%FCS) and cell-bound GpL activity was measured using the Gaussia Luciferase assay kit (NEB) and a Lucy2 Luminometer according to manufacturers’ instructions. Specific binding values of scFv:hDEC205-GpL to hDEC205 were obtained by subtracting parental CHO-derived values (nonspecific binding) from the corresponding values derived of the CHO-hDEC205 cells (total binding). In another series of equilibrium binding studies, CHO-hDEC205 cells were blocked with a constant concentration (2 μg/mL) of the various anti-hDEC205-WT1 antibody fusion proteins at 37 °C for 1 h prior to incubation with increasing concentrations of scFv:hDEC205-GpL for an additional hour to obtain total and nonspecific binding to calculate the K d values. For the heterologous competition binding assay, CHO-hDEC205 cells were incubated with mixtures of a constant concentration [50 ng/mL (660 pM, C GpL)] of the scFv:hDEC205-GpL and increasing concentrations of the conventional anti-hDEC205 IgG1 antibody at 37 °C for 1.5 h. The K i (K d) of the antibody was calculated with the help of the K d-value of scFv:hDEC205-GpL and IC50 concentration of the anti-hDEC205 antibody using the formula: K i = IC50/(1 + C GpL/K d).
Patient and healthy donor samples and cell isolations
Peripheral blood samples from patients and buffy coats from healthy donors were collected after written informed consent approved by the Institutional Review Board of the University of Würzburg. All patients received allogeneic HSCT due to AML (n = 8) or MDS (n = 2). Peripheral blood mononuclear cells (PBMCs) were freshly isolated with Ficoll density gradient centrifugation. DCs were generated from fresh monocytes isolated with the CD14+ cell magnetic selection kit (Miltenyi Biotec) [23]. Briefly, monocytes were suspended in culture medium (RPMI1640 GlutaMax with 10% FCS and 50 μg/mL gentamycin) supplemented with 100 ng/mL GM-CSF, 20 ng/mL IL-4 and seeded into a 6-well culture plate at 3 × 106 cells per well. On day two and four, one third of the media was changed with fresh media supplemented with GM-CSF and IL-4. Maturation of monocyte-derived DCs (moDCs) was triggered by adding 10 ng/mL TNF-α twice. On day six, prior to cytokine supplementation, semi-mature moDCs dedicated for loading with the antibody fusion proteins were harvested and seeded into 96- or 48-well plates. Fresh CD3+ T-cells were negatively selected from the flow through fraction of CD14+ cell separation using the pan-T-cell selection kit (Miltenyi Biotec). CD3+ T-cells and remaining semi-mature moDCs were directly cryopreserved.
Expansion of WT1-specific T-cells
On day six, 7.5 × 105 semi-mature moDCs were incubated with 2 µg/mL of the anti-hDEC205-WT191–138 antibody fusion protein at 37 °C for 1.5 h to allow internalization. Then, GM-CSF, IL-4 and TNF-α were added and cells were further incubated for 20–22 h. On day seven, the loaded, fully matured moDCs were washed twice with 2 mL RPMI1640 GlutaMax and resuspended in 1 mL media. Parallel to this, autologous CD3+ T-cells were resuspended at a density of 3 × 106/mL in media. Then, moDCs and T-cells were mixed in one well of a 24-well culture plate and co-cultured at 37 °C. After two days, co-cultures were supplemented with 5 ng/mL IL-7 and IL-15 (Peprotech). Cytokines were then added every 2–3 days when half of the media was replenished. On day 12–13, cells were restimulated with 2 μg/mL of the antibody fusion protein for 24 h. The activated T-cells were selected using the CD137 selection kit in accordance with the manufacturer’s protocol (Miltenyi Biotec). The CD137+ T-cells and allogeneic irradiated feeder cells were seeded into a 48-well plate at a ratio of 1:10 and supplemented with 1 µg/mL PHA-L and 50 IU/mL IL-2. Specific T-cells were expanded further for 14–83 days by supplementation of IL-2, IL-7, and IL-15 every 2–3 days, together with a half media change, and restimulation of autologous moDCs loaded with the antibody fusion protein every 14 days.
Intracellular cytokine staining (ICS) and CD107a degranulation assay
The ICS assay was performed as described previously [24] with modifications. Briefly, 1 × 105 semi-mature moDCs per well of a 96-well plate were left unstimulated or loaded with 2 µg/mL anti-hDEC205 antibody (negative control), 2 µg/mL anti-hDEC205-WT1small antibody fusion proteins, 5 µg/mL WT1 peptide pool, or 1 µg/mL CMV pp65 recombinant protein (positive control) for 1.5 h prior to supplementation with GM-CSF, IL-4, and TNF-α. After 20 h of incubation at 37 °C, cells were washed twice with 200 µL RPMI1640 and resuspended in 100 µL medium. Then, 4 × 105 autologous CD3+ T-cells were added to the corresponding wells. The mixtures of moDCs and T-cells were incubated at 37 °C for 1 h, before addition of 10 µg/mL Brefeldin A (Sigma–Aldrich). For CD107a degranulation assay, 1 μL monensin and 2 μL anti-CD107a-APC (both BD Biosciences) were added. After 16 h of co-incubation, cells were harvested and stained with the following antibodies: anti-CD3-FITC or -PerCP, anti-CD8-FITC or -PerCP or -APC, anti-CD4-APC or -PerCP (BD Biosciences). After surface staining, cells were permeabilized with FACS Permeabilizing Solution 2 (BD Biosciences) and stained with anti-IFN-γ-PE (Beckman Coulter). Cells were analyzed with the FACS Calibur and CellQuest software (both from BD Biosciences).
ELISPOT assay
Semi-mature moDCs (5 × 104 per well) unloaded or loaded with 2 μg/mL anti-hDEC205-WT191–138 or control anti-hDEC205 or 5 μg/mL WT1 peptide pool (Miltenyi Biotec) were matured and co-incubated with PBMCs (2 × 105 per well) in a 96-well ELISPOT plate (Millipore) precoated with anti-IFN-γ capture antibody (BD Biosciences) for 16 h. After co-incubation, cells were removed from the wells, and the biotinylated anti-IFN-γ detection antibody (BD Biosciences) was added prior to visualization with Streptavidin-AP (Southern BioTech) and NBT/BCIP liquid substrate system (Sigma–Aldrich). Spots were determined on Immunospot S5 ELISPOT reader (C.T.L).
Vital-FR assay
VITAL-FarRed assays were performed as described elsewhere [25] with modifications. Briefly, 5 × 106 THP-1 cells were labeled with 5 μM CFSE and the same number of DG-75 cells was labeled with 5 μM FarRed (Invitrogen) for 5 min at 37 °C. Both cell lines are HLA-A*02 positive. Specific T-cells derived from HLA-A*02 positive patients were serially diluted, and each dilution of T-cells was mixed with 5 × 104 CFSE+ target and 5 × 104 FarRed+ control cells, resulting in graded effector: target (E:T) ratios. After 22 h of incubation at 37 °C in 5% CO2, cytotoxicity was assessed by flow cytometry by comparing specific lysis of target cells to that of control cells using a previously described method [25].
Statistics
Data are shown as median with IQR, mean ± SEM, or mean ± SD. Statistical significance was analyzed with GraphPad Prism 5.0 (GraphPad Software Inc.) using unpaired t test or one-and two-way analysis of variance (ANOVA) followed by Tukey’s and Bonferroni’s post hoc tests as indicated. p ≤ 0.05 was regarded to be significant.
Results
Construction of anti-hDEC205-WT1 antibody fusion proteins
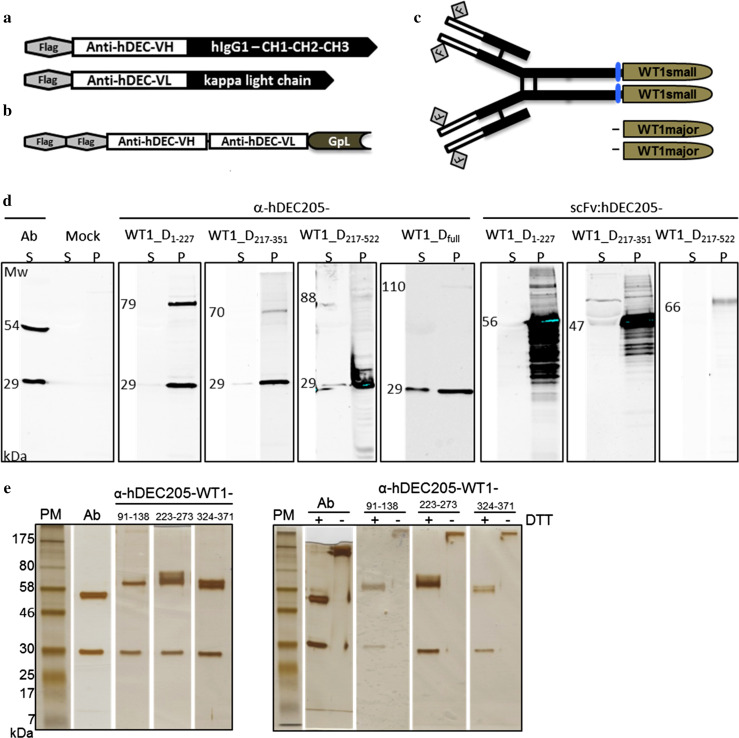
As a basis of the anti-hDEC205-WT1 antibody fusion proteins, expression constructs encoding the heavy and light chain of the anti-hDEC205 antibody were cloned. In order to quantify antibody binding to the DEC205 receptor, a GpL fusion protein of the corresponding DEC205-specific scFv (scFv:hDEC205-GpL) was also constructed (Fig. 1a, b).
Fig. 1.
Construction and production of anti-hDEC205-WT1 antibody fusion proteins. a Scheme of the anti-hDEC205 antibody. Heavy and light chains of the antibody were constructed by ligating the respective variable heavy and light chains to the N-terminus of the corresponding constant domains of human IgG1 isotype’s heavy chain (hIgG1–CH1–CH2–CH3) and kappa light chain. Each chain of the antibody was N-terminally FLAG-tagged. b Scheme of the scFv:hDEC205-GpL. Single chain variable fragment of anti-hDEC205 consisting of the variable heavy and light chains (anti-hDEC-VH and anti-hDEC-VL) was C-terminally fused to Gaussia princeps luciferase and tagged N-terminally by double FLAG. c Scheme of anti-hDEC205-WT1 antibody fusion proteins. The anti-hDEC205-WT1major and -WT1small fusion proteins were constructed by C-terminal linking of the respective WT1 fragments to the anti-hDEC205 heavy chain. Small fragments of WT1 were fused through a flexible spacer of four amino acids (little cylinders). d Production and secretion characteristics of anti-hDEC205-WT1_Dmajor antibody fusion proteins by SDS-PAGE and Western blot analyses. Proteins were detected with mouse anti-Flag M2 IgG (primary antibody) and goat anti-mouse IgG-IRDye 800CW (secondary antibody). S culture supernatant collected from transfected HEK293 cells, P lysate of pelleted HEK293 cells transfected with mock plasmids or encoding different anti-hDEC205-WT1_D antibody fusion proteins. e Purity and integrity of anti-hDEC205-WT1small fusion proteins by SDS-PAGE and silver staining. PM protein marker, molecular weights in kDa, + reducing, − non-reducing condition, DTT dithiothreitol
Due to the already known complexities in expression and purification of intracellular WT1, we generated a collection of cDNAs encoding fragments of WT1 isoform D (WT1_D) of different sizes. A full-length WT1_D cDNA was obtained by ligation of WT1_D1–227 to WT1_D217–522 fragment (Sup. Table 1). Subsequently, each cDNA was genetically fused to the C-terminus of the heavy chain of the anti-hDEC205 antibody (Fig. 1c), and antibody fusion proteins were expressed by transient transfection of HEK293 cells.
Production, secretion and purification of anti-hDEC205-WT1 antibody fusion proteins
To evaluate the productivity and secretion efficiency of intracellular WT1 fused to anti-hDEC205 antibody, we analyzed cell culture supernatants and cell lysates of transfected cells by Western blot. We identified four different anti-hDEC205 antibody fusion proteins with small WT1 peptides (anti-hDEC205-WT1small) yielding 2–4.4 μg/mL in culture supernatants (Table 1). Compared to the anti-hDEC205-WT1small antibody fusion proteins, all anti-hDEC205 antibody fusion proteins with large WT1 peptides (anti-hDEC205-WT1_Dmajor) and an anti-hDEC205 fusion protein with complete WT1 (anti-hDEC205-WT1_Dfull) were retained in the cells indicating that these large WT1 peptides interfere with efficient secretion (Fig. 1d).
Table 1.
Protein sequence of four secreted anti-hDEC205-WT1 antibody fusion proteins containing small fragments of WT1 protein
| Antibody fusion protein | Protein sequence of the WT1 cloned to the C-terminus of the α-hDEC205 | Sequence of known epitopes |
|---|---|---|
| Anti-hDEC205-WT110–35 | ALLPAVPSLGGGGGCALPVSGAAQWA | GGCALPVSGA |
| Anti-hDEC205-WT191–138 | AFTVHFSGQFTGTAGACRYGPFGPPPPSQASSGQARMFPNAPYLPSCL | RMFPNAPYL |
| QARMFPNAPYLPSCL | ||
| Anti-hDEC205-WT1223–273 | SDNLYQMTSQLECMTWNQMNLGATLKGVAAGSSSSVKWTEGQSNHSTGYES | WNQMNLGAT CMTWNQMNLGATLKG |
| KGVAAGSSSSVKWTE | ||
| Anti-hDEC205-WT1324–371 | MCAYPGCNKRYFKLSHLQMHSRKHTGEKPYQCDFKDCERRFSRSDQLK | DFKDCERRFa |
| KRYFKLSHLQMHSRKH |
aNot published
Three of the secreted antibody fusion proteins, anti-hDEC205-WT191–138, anti-hDEC205-WT1223–273, and anti-hDEC205-WT1324–371 that cover most of the previously defined immunogenic epitopes were purified via their FLAG tags at concentrations of 40–400 μg/mL with high purity and good integrity (Fig. 1e). The intracellularly trapped anti-hDEC205-WT1_D1–227 was also purified after Triton-100 extraction of cell lysates. However, due to the poor solubility of the antibody fusion protein, the obtained quantities were not sufficient for further translational efforts (Sup. Fig. 1).
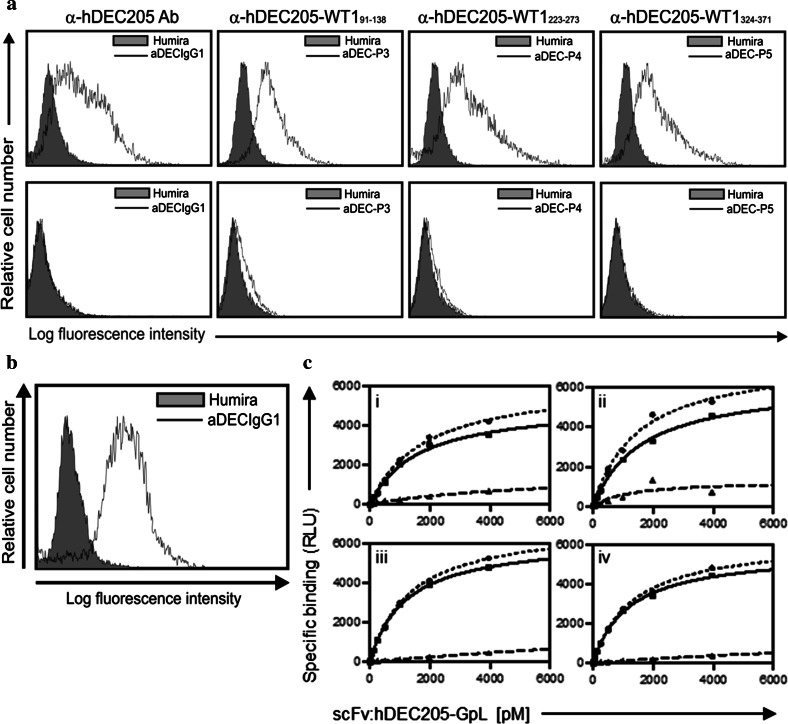
Binding capacity of anti-hDEC205-WT1small antibody fusion proteins
Antigen-specific binding of the anti-hDEC205 antibody fusion proteins was confirmed by flow cytometry analysis using wild type and hDEC205 stably expressing CHO cells (Fig. 2a) as well as human immature moDCs (Fig. 2b). To quantify the binding affinity of anti-hDEC205, we performed a series of equilibrium binding and heterologous competition studies using a highly traceable fusion protein of the scFv version of the anti-DEC205 antibody with the luciferase of Gaussia princeps (scFv:hDEC205-GpL). A high affinity of 8.1 × 10−10 M for the anti-hDEC205 antibody was calculated from the K d of scFv:hDEC205-GpL for DEC205 binding determined in equilibrium binding studies and an IC50-value determined for the anti-hDEC205 antibody in heterologous competition experiments with scFv:hDEC205-GpL (Sup. Fig. 2). Specific binding of anti-hDEC205-WT191–138, anti-hDEC205-WT1223–273, and anti-hDEC205-WT1324–373 antibody fusion proteins was comparable to that of the parental antibody (Fig. 2c). Thus, a C-terminal extension of the heavy chain with various WT1small fragments does not alter the binding characteristics of anti-hDEC205 antibody.
Fig. 2.
Functional evaluation of anti-hDEC205 and anti-hDEC205-WT1small antibody fusion proteins. a FACS analyses to assess binding capacities of the antibody fusion proteins to human DEC205. Top CHO-hDEC205 cells. Bottom parental CHO cells. Humira, anti-hTNF-α antibody served as a negative control. An internal identity of the anti-hDEC205-WT191–138, -WT1223–273, and -WT1324–371 antibody fusion proteins: aDEC-P3, -P4, and -P5. b Specific binding of anti-hDEC205 antibody to the DEC205 on human immature moDCs detected by FACS analysis. c Equilibrium binding studies confirming the binding capability of the anti-hDEC205-WT1small antibody fusion proteins to hDEC205.  total binding,
total binding,  specific binding,
specific binding,  nonspecific binding of the scFv:hDEC205-GpL by blocking with the parental antibody or the respective anti-hDEC205-WT1small fusion proteins: anti-hDEC205 antibody (i), anti-hDEC205-WT191–138 (ii), anti-hDEC205-WT1223–273 (iii), anti-hDEC205-WT1324–371 (iv). RLU relative light unit, pM picomolar
nonspecific binding of the scFv:hDEC205-GpL by blocking with the parental antibody or the respective anti-hDEC205-WT1small fusion proteins: anti-hDEC205 antibody (i), anti-hDEC205-WT191–138 (ii), anti-hDEC205-WT1223–273 (iii), anti-hDEC205-WT1324–371 (iv). RLU relative light unit, pM picomolar
Immunogenic capacities of anti-hDEC205-WT1small antibody fusion proteins
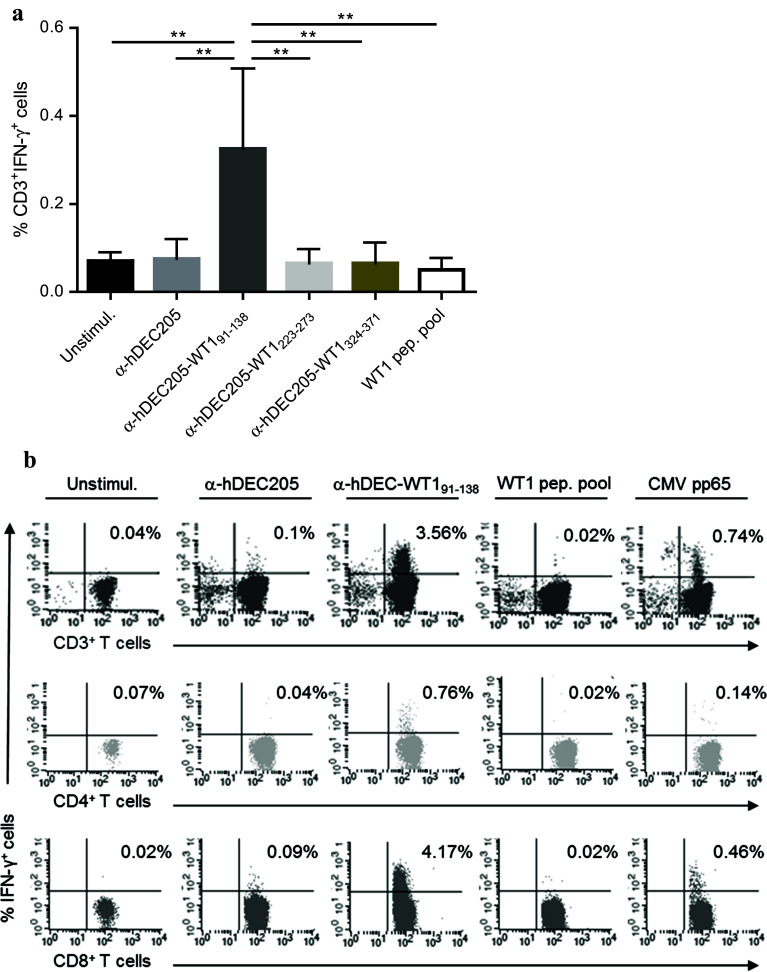
As DCs were barely detectable in freshly isolated PBMCs (Sup. Fig. 3), priming and activation of T-cells ex vivo were performed with moDCs in the following experiments. Maturation of moDCs was not affected by DEC205-targeting (Sup. Fig. 4). To investigate T-cell stimulatory capacities of anti-hDEC205-WT1, antibody fusion protein-loaded mature moDCs and autologous T-cells were co-incubated for 16 h before measuring the frequency of IFN-γ+ T-cells by flow cytometry. Of the three antibody fusion proteins tested, only the anti-hDEC205-WT191–138 showed a significant T-cell stimulatory effect directly ex vivo (Fig. 3a). In detail, the anti-hDEC205-WT191–138-loaded mature moDCs were able to prime and/or activate T-cells, while unloaded and loaded mature moDCs with the parental anti-hDEC205 antibody showed no effect (Fig. 3b). Furthermore, direct ex vivo IFN-γ+ T-cell responses to mature moDCs loaded with a WT1 15-mer peptide pool were not seen in the tested cases except for one patient sample. Consistent with published results, the mean frequency of IFN-γ+ WT1-specific T-cells activated by the targeted WT1 selfprotein was 2.3-fold lower in comparison with the frequency of IFN-γ+ CMV-specific T-cells stimulated by the nontargeted viral protein CMVpp65. However, in one case, the frequency of WT1-specific T-cells was higher than that of CMV-specific counterparts as shown in Fig. 3b.
Fig. 3.
DC-mediated T-cell response to anti-hDEC205-WT1small antibody fusion proteins. a Compiled data from all individuals (n = 16) analyzed for IFN-γ+ T-cell responses by ICS. Shown are median frequencies with interquartile range (IQR), one-way ANOVA, Tukey’s test **p < 0.01. b IFN-γ ICS and FACS analysis of cells from one patient (PN2). Top CD3+IFN-γ+ T-cell frequencies gated on lymphocytes, middle and bottom frequencies of CD3+-gated CD4+IFN-γ+ and CD8+IFN-γ+ T-cells. T-cells were co-incubated with unloaded moDCs (unstimul.), with moDCs loaded with anti-hDEC205 antibody, anti-hDEC205-WT191–138 antibody fusion protein, WT1 peptide pool, or CMV pp65 protein
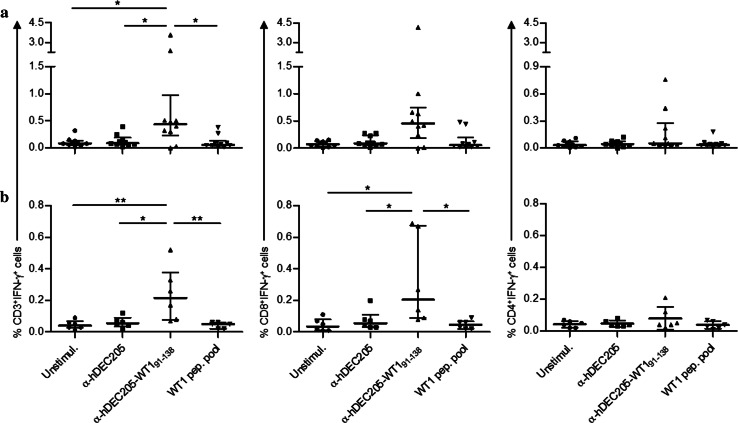
Immunogenicity of the anti-hDEC205-WT191–138 was tested with CD3+ T-cells obtained from 10 HSC transplanted patients (Sup. Table 2). In eight cases, we detected T-cell responses against the fusion protein mainly in the CD8+ subset. The median frequencies of CD3+IFN-γ+, CD8+IFN-γ+, and CD4+IFN-γ+ cells upon overnight stimulation with anti-hDEC205-WT191–138-loaded moDCs were 0.44, 0.45, and 0.05%, respectively (Fig. 4a). It is broadly accepted that GvL effect in allo-HSCT can be exerted from donor T-cells recognizing TAAs on leukemic cells. Thus, we also tested blood samples of healthy donors to evaluate whether DEC205-targeted WT191–138 is able to stimulate WT1-specific T-cells for healthy individuals. Albeit lower frequencies compared to patient-derived cells, T-cell responses were markedly detected in five of six healthy donors (Fig. 4b).
Fig. 4.
Summary of IFN-γ ICS results obtained from a all patients (n = 10) and b healthy individuals (n = 6). Plots show frequencies of IFN-γ+CD3+ (top), IFN-γ+CD8+ (middle), and IFN-γ+CD4+ (bottom) T-cells. Each data point represents one individual, bars indicate median frequencies with IQR, Tukey’s test *p < 0.05; **p < 0.01
Most responding patients and donors were positive for HLA-A*0201 in at least one allele. However, one patient (PN8) and two healthy donors with T-cell responses were HLA-A*0201 negative, suggesting that WT191–138-derived peptides can be presented by different HLA molecules. When CD4+ subset is activated in addition to CD8+ counterpart, the T-cell response would be more sustained. Of note, both CD8+ and CD4+ T-cell subsets mounted IFN-γ responses in four patients and two healthy donors of all responding individuals. In these cases, either CD8+IFN-γ+ or CD4+IFN-γ+ T-cell frequencies detected in response to anti-hDEC205-WT191–138-loaded moDC stimulation was significantly higher compared to that under parental antibody or WT1 peptide pool stimulation (Sup. Fig. 5). Notably, we did not use co-stimulating agents such as anti-CD40 for CD4+ T-cell activation to avoid unspecific effects.
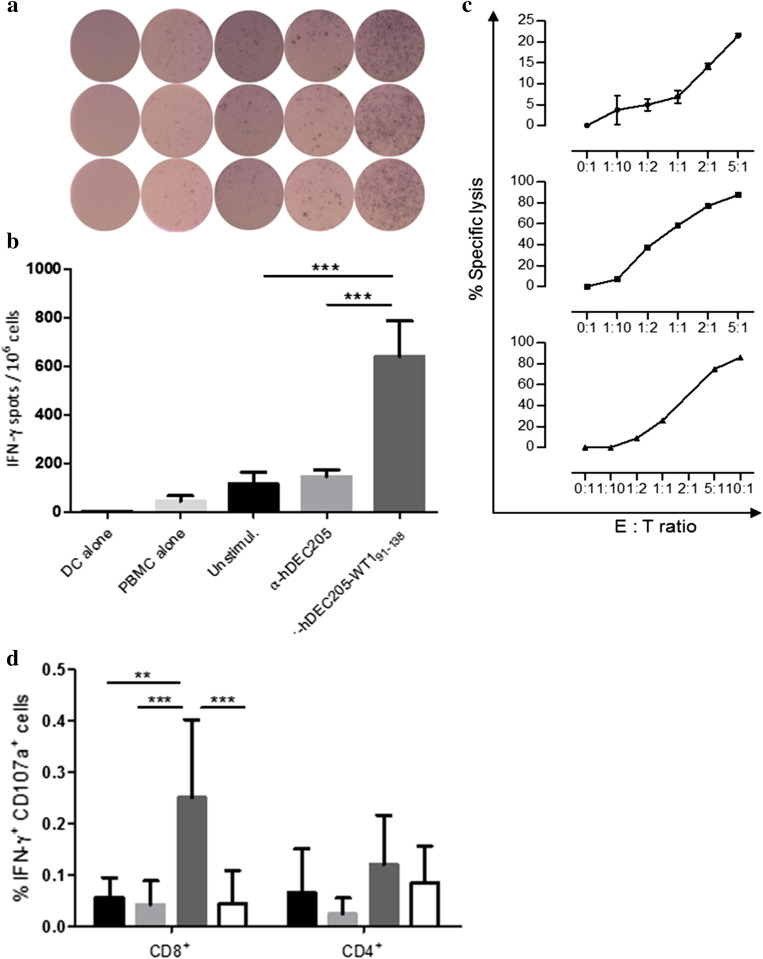
Since the use of PBMCs is less laborious, more time-saving, and closer to the in vivo situation compared to purified CD3+ T-cells, we tested whether PBMCs could be activated by this approach. Therefore, anti-hDEC205-WT191–138-loaded autologous mature moDCs and PBMCs from two patients after allo-HSCT were co-incubated to measure IFN-γ induction by ELISPOT assay (Fig. 5a). The average number of IFN-γ spots per 1 x 106 PBMCs induced by anti-hDEC205-WT191–138 was more than fourfold higher (957.2) than that of the negative controls (unstimulated 209.2; parental antibody 197.2; Fig. 5b). Thus, either isolated T-cells or whole PBMCs can be activated by mature moDCs loaded with anti-hDEC205-WT191–138.
Fig. 5.
IFN-γ response of whole PBMCs from two AML patients after allo-HSCT assessed by IFN-γ ELISPOT assay. a Triplicate of a representative IFN-γ ELISPOT assay. b Summary analysis of the IFN-γ ELISPOT assay of two patients. Shown are means with SEM, unpaired Student t test ***p < 0.001. c Cytotoxic effector function of in vitro expanded WT1-specific T-cells induced by anti-hDEC205-WT191–138-loaded mature moDCs. Each VITAL-FarRed cytotoxicity experiment was performed in duplicates and is shown by means with SEM from three different patients (from top PN 2, 4, 3). d Cytotoxicity of T-cells ex vivo by IFN-γ ICS combined with CD107a degranulation assay.  unstimulated,
unstimulated,  anti-hDEC205 Ab,
anti-hDEC205 Ab,  anti-hDEC205-WT191–138,
anti-hDEC205-WT191–138,  WT1 peptide pool stimulation. Shown are mean ± SD from five patients including PN 2, 3, 4. Tukey’s test **p < 0.01; ***p < 0.001
WT1 peptide pool stimulation. Shown are mean ± SD from five patients including PN 2, 3, 4. Tukey’s test **p < 0.01; ***p < 0.001
T-cells activated by DC-targeted WT191–138 lyse WT1-overexpressing leukemia cell line
To demonstrate the cytotoxic function of T-cells activated by anti-hDEC205-WT191–138-treated mature moDCs, T-cells were expanded for 10–14 days resulting in a 1.3–2 fold increase in WT1-specific T-cells up to frequencies of 1.6–10.2% CD137+ cells that were dominated by CD8+ T-cells. The cytotoxicity of expanded T-cells from three HLA-A*0201 positive patients was investigated by a VITAL-FR cytotoxicity assay. An HLA-A*0201-restricted lysis of THP-1 cells could be observed at relatively low E:T ratios, indicating that the WT191–138-specific T-cells mediated strong cytotoxic activity (Fig. 5c, Sup. Fig. 6). It is worth mentioning that expanded T-cells from one patient (PN 4) analyzed in this experiment showed the strongest cytotoxicity although a direct ex vivo T-cell response was not detected. This suggests that WT1-specific T-cells even with undetectably low frequencies can be expanded or naïve T-cells can be primed as a result of frequent stimulation with anti-hDEC205-WT191–138 antibody fusion protein, which then showed functional activity. Of importance, the cytotoxic capacity of T-cells evidenced by our previously performed ex vivo IFN-γ ICS combined with CD107a degranulation assay (Fig. 5d) could be confirmed by VITAL-FR cytotoxicity assay. Thus, when targeted to DEC205 on mature moDCs, WT191–138 antibody fusion protein induces cytotoxic T-cells that are even able to lyse endogenously WT1-expressing THP-1 leukemia cells.
Discussion
Antileukemia vaccination and adoptive transfer of leukemia-specific T-cells following allogeneic transplantation have been considered as therapeutic options for the control of high-risk leukemia [26]. WT1 has been used for both strategies after allo-HSCT to evoke a selective T-cell response enhancing GvL effect and reducing relapse of the disease. Certainly, HLA restrictions of the known peptides and difficulties of WT1 protein expression impair the advance of such promising strategies. Under these circumstances, DC-targeting holds a potential to overcome the aforementioned barriers in WT1-based immunotherapy. Thus, we have developed hDEC205-specific WT1 antibody fusion proteins as antileukemia vaccines and explored their T-cell stimulatory capacities directly ex vivo and in vitro.
To improve the stability and solubility of full-length WT1 protein, which are major obstacles in intracellular protein recombinant production, various expression systems and buffers as well as soluble tags have been tested. By means of huge efforts, sufficient amounts of WT1 could be purified from inclusion bodies of E. coli or eukaryotic HEK293T cell lysates [15, 27, 28]. Accordingly, anti-hDEC205 antibody fusion proteins containing full-length and major fragments of the isoform D of WT1 possessed poor secretory characteristics. Fulfilling our aim, which was to yield the anti-hDEC205-WT1 antibody fusion proteins from cell culture supernatant in a soluble and secreted form, anti-hDEC205-WT110–35, anti-hDEC205-WT191–138, anti-hDEC205-WT1223–273, and anti-hDEC205-WT1324–371 antibody fusion proteins were identified. WT1 protein fragments in the four antibody fusion proteins consist of 26 to 51 amino acids suggesting an advantage of biochemical properties compared to the full-length protein or peptides covering large major parts of the protein. Despite the relative shortness of the WT1small fragments, more than ten potentially immunogenic peptides are possibly generated by the proteasome and presented by MHC molecules of DCs.
Out of the tested three antibody fusion proteins, anti-hDEC205-WT191–138 was the only fusion protein that improved direct ex vivo T-cell responses in healthy donors and allo-HSCT patients with AML. The contained WT191–138 fragment comprises five previously determined immunogenic epitopes [29, 30] including the well-documented WT1126–134 peptide [31, 32]. Responders to anti-hDEC205-WT191–138 stimulation were not exclusively HLA-A*0201 positive, demonstrating that immunogenicity of anti-hDEC205-WT191–138 is mediated by a variety of epitopes presented on different HLA molecules of efficiently targeted moDCs.
It is known that DEC205-targeting approaches strongly enhance cross-presentation [33, 34]. Accordingly, the strongest T-cell response was composed of CD8+ subsets, but there was also a slight but significant activation of CD4+ helper T-cells of some individuals after DEC205-targeted WT191–138 stimulation. This finding confirms previous studies showing that a combination of HLA class I- and II-restricted peptides induces both, cytotoxic and helper T-cell responses [8, 35]. Due to natural processing by DCs, peptides derived from the endocytosed WT1 protein fragment were presented via HLA class I and II molecules resulting in improved T-cell responses.
We were unable to explain why anti-hDEC205-WT1223–273 and anti-hDEC205-WT1324–371 antibody fusion proteins did not induce a detectable response directly ex vivo. Of note, sequences of the two antibody fusion proteins also contain previously identified HLA class I- and II-restricted epitopes [31] [36]. Thus, anti-hDEC205-WT1223–273 and anti-hDEC205-WT1324–371 need to be further investigated to reveal their immune stimulatory capacity. Repeated stimulations of T-cells by moDCs loaded with these WT1 fusion proteins or in vivo stimulation might be an option to increase low or undetectable frequencies of specific T-cells. Specifically, an optimized stimulation with various epitopes may improve T-cell responses even though the single-epitope stimulation is only of inferior immunogenicity.
To augment therapeutic efficacy of HSCT, strengthening GvL effect independently of concurrent GvHD is essential. Therefore, targeting of these small WT1 protein fragments to DCs should be further explored in vivo after an allo-HSCT for their potential to support the GvL effect by WT1-specific T-cells and their ability to induce an effective immunity against the underlying disease. Comparable to classic vaccinations, anti-hDEC205-WT1small antibody fusion proteins may directly reach DCs in vivo and show their immune stimulatory effect in the presence of an adjuvant. Thereby, their potential clinical application could be a simple subcutaneous vaccination leading to lower costs and less ex vivo manipulation [37]. However, in individuals with insufficient frequency or function of DCs as a consequence of the disease or treatment, an improvement in the immune response could possibly be achieved by donor-derived moDC vaccination loaded with these proteins. This strategy may allow an efficient in vivo T-cell stimulation even early after allo-HSCT. Of importance, using CMV-derived peptide-loaded moDCs, we previously showed that DC vaccination can be performed safely in the allo-HSCT setting [38].
An adoptive transfer of donor-derived high-avidity WT1-specific CTL clones in the presence of IL-21 induced a long-lived memory CD8+ T-cell response correlating with an improved survival of allo-HSCT patients [39]. Our strategy can also be a basis for induction of such WT1-specific CTLs. Although genetically modified T-cells with the advantages of HLA independence and high avidity to cell surface antigens are of great interest in current adoptive T-cell therapy approaches, malignancies lacking “good” tumor-specific surface antigens are hardly targeted by T-cells with CAR. With regard to T-cells transduced by genetically modified TCRs, which also address intracellular tumor-associated antigens such as WT1, HLA-restricted antigen recognition hampers their broad use. In these cases, DC-based approaches are promising as a generally applicable immunization strategy toward the intracellular WT1 antigen. We and others support the concept that DC vaccination after allo-HSCT holds a strong promise in immunotherapy for relapsed hematological malignancies [40, 41].
Ultimately, three major findings support efforts to improve WT1-based vaccination strategies and combine them with other treatment options: first, the growing data of clinical studies demonstrating that vaccination using WT1-derived peptides is safe and feasible for patients with advanced MDS/AML [42], second, distinct data confirming the significant correlation between WT1 overexpression and worse clinical outcomes in either hematological [43–45] or solid tumor patients [46], and last, promising data indicating that patients with detectable WT1-specific T-cells show a better progression-free survival after HSCT [32, 45, 47, 48]. In light of these data, our alternative approach offers an attractive perspective to be translated into clinical practice.
Finally, delivering immunogenic WT1 protein fragments to DC by targeting them to the endocytic receptor DEC205 opens the possibility of immunizing patients directly without the step of in vitro antigen loading. As mentioned above, the feasibility of this approach has been shown in numerous rodent studies [22, 33]. If successful, such a strategy would simplify anti-WT1 vaccination enormously, allowing the immunization also of patients who do not have access to the high-end technologies required for cellular therapies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank our colleagues in the Division of Molecular Internal Medicine for their assistance and Professor Matthias Eyrich for his critical discussion. We also thank Dr. Torsten Steinbrunn for proofreading of the manuscript. Nergui Dagvadorj was supported by scholarships from the Government of Mongolia, the Fritz Thyssen Foundation of Germany, the Interdisciplinary Center for Clinical Research (IZKF) of the University of Würzburg, Germany and the German Academic Exchange Service (DAAD), Germany.
Abbreviations
- aa
Amino acid
- AML
Acute myeloid leukemia
- CAR
Chimeric antigen receptor
- CHO
Chinese hamster ovary
- CHO-hDEC205
hDEC205-stably expressing CHO
- CMV
Cytomegalovirus
- CTL
Cytotoxic T lymphocyte
- ET
Effector to target ratio
- ELISPOT
Enzyme-linked immuno spot
- FCS
Fetal calf serum
- GM-CSF
Granulocyte–macrophage colony stimulating factor
- GpL
Gaussia princeps luciferase
- GvL
Graft-versus-leukemia
- hDEC205
Human DEC205 endocytic receptor
- HEK293
Human embryonic kidney
- HLA
Human leukocyte antigen
- HSCT
Hematopoietic stem cell transplantation
- IC50
50% inhibitory concentration
- ICS
Intracellular cytokine staining
- IFN
Interferon
- IL
Interleukin
- Kd
Dissociation constant
- Ki
Dissociation constant of inhibitor
- LAL
Limulus amebocyte lysate
- MDS
Myelodysplastic syndrome
- moDCs
Monocyte-derived dendritic cells
- NBT/BCIP
Nitro blue tetrazolium/5-Bromo-4-chloro-3-indolyl phosphate
- PBMCs
Peripheral blood mononuclear cells
- PHA-L
Phytohemagglutinin-L
- pM
Picomolar
- PN
Patient number
- RT-PCR
Reverse transcriptase polymerase chain reaction
- scFv
Single chain variable fragment
- SDS-PAGE
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- TAA
Tumor-associated antigen
- TCR
T-cell receptor
- TNF
Tumor necrosis factor
- WT1
Wilms tumor protein
- WT1_D
Isoform D of WT1 protein
- WT1full
WT1 in full-length
- WT1major and WT1small
Major and small fragments of WT1 protein
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Savani BN, Mielke S, Reddy N, Goodman S, Jagasia M, Rezvani K. Management of relapse after allo-SCT for AML and the role of second transplantation. Bone Marrow Transplant. 2009;44(12):769–777. doi: 10.1038/bmt.2009.300. [DOI] [PubMed] [Google Scholar]
- 2.Lichtenegger FS, Krupka C, Kohnke T, Subklewe M. Immunotherapy for acute myeloid leukemia. Semin Hematol. 2015;52(3):207–214. doi: 10.1053/j.seminhematol.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL, Weiner LM, Matrisian LM. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15(17):5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benteyn D, Anguille S, Van Lint S, Heirman C, Van Nuffel AM, Corthals J, Ochsenreither S, Waelput W, Van Beneden K, Breckpot K, Van Tendeloo V, Thielemans K, Bonehill A. Design of an optimized Wilms’ tumor 1 (WT1) mRNA construct for enhanced WT1 expression and improved immunogenicity in vitro and in vivo. Mol Ther Nucleic Acids. 2013;2:e134. doi: 10.1038/mtna.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuball J, de Boer K, Wagner E, Wattad M, Antunes E, Weeratna RD, Vicari AP, Lotz C, van Dorp S, Hol S, Greenberg PD, Heit W, Davis HL, Theobald M. Pitfalls of vaccinations with WT1-, Proteinase3- and MUC1-derived peptides in combination with MontanideISA51 and CpG7909. Cancer Immunol Immunother. 2011;60(2):161–171. doi: 10.1007/s00262-010-0929-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaise C, Buchan SL, Rice J, Marquet J, Rouard H, Kuentz M, Vittes GE, Molinier-Frenkel V, Farcet JP, Stauss HJ, Delfau-Larue MH, Stevenson FK. DNA vaccination induces WT1-specific T-cell responses with potential clinical relevance. Blood. 2008;112(7):2956–2964. doi: 10.1182/blood-2008-02-137695. [DOI] [PubMed] [Google Scholar]
- 7.Van Driessche A, Van de Velde AL, Nijs G, Braeckman T, Stein B, De Vries JM, Berneman ZN, Van Tendeloo VF. Clinical-grade manufacturing of autologous mature mRNA-electroporated dendritic cells and safety testing in acute myeloid leukemia patients in a phase I dose-escalation clinical trial. Cytotherapy. 2009;11(5):653–668. doi: 10.1080/14653240902960411. [DOI] [PubMed] [Google Scholar]
- 8.Maslak PG, Dao T, Krug LM, Chanel S, Korontsvit T, Zakhaleva V, Zhang R, Wolchok JD, Yuan J, Pinilla-Ibarz J, Berman E, Weiss M, Jurcic J, Frattini MG, Scheinberg DA. Vaccination with synthetic analog peptides derived from WT1 oncoprotein induces T-cell responses in patients with complete remission from acute myeloid leukemia. Blood. 2010;116(2):171–179. doi: 10.1182/blood-2009-10-250993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weber G, Gerdemann U, Caruana I, Savoldo B, Hensel NF, Rabin KR, Shpall EJ, Melenhorst JJ, Leen AM, Barrett AJ, Bollard CM. Generation of multi-leukemia antigen-specific T cells to enhance the graft-versus-leukemia effect after allogeneic stem cell transplant. Leukemia. 2013;27(7):1538–1547. doi: 10.1038/leu.2013.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brayer J, Lancet JE, Powers J, List A, Balducci L, Komrokji R, Pinilla-Ibarz J. WT1 vaccination in AML and MDS: a pilot trial with synthetic analog peptides. Am J Hematol. 2015;90(7):602–607. doi: 10.1002/ajh.24014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kramarzova K, Stuchly J, Willasch A, Gruhn B, Schwarz J, Cermak J, Machova-Polakova K, Fuchs O, Stary J, Trka J, Boublikova L. Real-time PCR quantification of major Wilms’ tumor gene 1 (WT1) isoforms in acute myeloid leukemia, their characteristic expression patterns and possible functional consequences. Leukemia. 2012;26(9):2086–2095. doi: 10.1038/leu.2012.76. [DOI] [PubMed] [Google Scholar]
- 12.Niksic M, Slight J, Sanford JR, Caceres JF, Hastie ND. The Wilms’ tumour protein (WT1) shuttles between nucleus and cytoplasm and is present in functional polysomes. Hum Mol Genet. 2004;13(4):463–471. doi: 10.1093/hmg/ddh040. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton TB, Barilla KC, Romaniuk PJ. High affinity binding sites for the Wilms’ tumour suppressor protein WT1. Nucleic Acids Res. 1995;23(2):277–284. doi: 10.1093/nar/23.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ladomery MR, Slight J, Mc Ghee S, Hastie ND. Presence of WT1, the Wilm’s tumor suppressor gene product, in nuclear poly(A)(+) ribonucleoprotein. J Biol Chem. 1999;274(51):36520–36526. doi: 10.1074/jbc.274.51.36520. [DOI] [PubMed] [Google Scholar]
- 15.Geng J, Carstens RP. Two methods for improved purification of full-length mammalian proteins that have poor expression and/or solubility using standard Escherichia coli procedures. Protein Expr Purif. 2006;48(1):142–150. doi: 10.1016/j.pep.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 16.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 17.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449(7161):419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 18.Romao S, Gannage M, Munz C. Checking the garbage bin for problems in the house, or how autophagy assists in antigen presentation to the immune system. Semin Cancer Biol. 2013;23(5):391–396. doi: 10.1016/j.semcancer.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Talarn C, Urbano-Ispizua A, Martino R, Perez-Simon JA, Batlle M, Herrera C, Granell M, Gaya A, Torrebadell M, Fernandez-Aviles F, Aymerich M, Marin P, Sierra J, Montserrat E. Kinetics of recovery of dendritic cell subsets after reduced-intensity conditioning allogeneic stem cell transplantation and clinical outcome. Haematologica. 2007;92(12):1655–1663. doi: 10.3324/haematol.11076. [DOI] [PubMed] [Google Scholar]
- 20.Birkholz K, Schwenkert M, Kellner C, Gross S, Fey G, Schuler-Thurner B, Schuler G, Schaft N, Dorrie J. Targeting of DEC-205 on human dendritic cells results in efficient MHC class II-restricted antigen presentation. Blood. 2010;116(13):2277–2285. doi: 10.1182/blood-2010-02-268425. [DOI] [PubMed] [Google Scholar]
- 21.Tsuji T, Matsuzaki J, Kelly MP, Ramakrishna V, Vitale L, He LZ, Keler T, Odunsi K, Old LJ, Ritter G, Gnjatic S. Antibody-targeted NY-ESO-1 to mannose receptor or DEC-205 in vitro elicits dual human CD8+ and CD4+ T cell responses with broad antigen specificity. J Immunol. 2011;186(2):1218–1227. doi: 10.4049/jimmunol.1000808. [DOI] [PubMed] [Google Scholar]
- 22.Wang B, Zaidi N, He LZ, Zhang L, Kuroiwa JM, Keler T, Steinman RM. Targeting of the non-mutated tumor antigen HER2/neu to mature dendritic cells induces an integrated immune response that protects against breast cancer in mice. Breast Cancer Res BCR. 2012;14(2):R39. doi: 10.1186/bcr3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan SM, Kapp M, Flechsig C, Kapp K, Rachor JE, Eyrich M, Loeffler J, Einsele H, Grigoleit GU. Stimulating surface molecules, Th1-polarizing cytokines, proven trafficking—a new protocol for the generation of clinical-grade dendritic cells. Cytotherapy. 2013;15(4):492–506. doi: 10.1016/j.jcyt.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Lamoreaux L, Roederer M, Koup R. Intracellular cytokine optimization and standard operating procedure. Nat Protoc. 2006;1(3):1507–1516. doi: 10.1038/nprot.2006.268. [DOI] [PubMed] [Google Scholar]
- 25.Stanke J, Hoffmann C, Erben U, von Keyserling H, Stevanovic S, Cichon G, Schneider A, Kaufmann AM. A flow cytometry-based assay to assess minute frequencies of CD8+ T cells by their cytolytic function. J Immunol Methods. 2010;360(1–2):56–65. doi: 10.1016/j.jim.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Rezvani K. Posttransplantation vaccination: concepts today and on the horizon. Hematology Am Soc Hematol Educ Program. 2011;2011:299–304. doi: 10.1182/asheducation-2011.1.299. [DOI] [PubMed] [Google Scholar]
- 27.Nurmemmedov E, Thunnissen M. Expression, purification, and characterization of the 4 zinc finger region of human tumor suppressor WT1. Protein Expr Purif. 2006;46(2):379–389. doi: 10.1016/j.pep.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 28.Fagerlund RD, Ooi PL, Wilbanks SM. Soluble expression and purification of tumor suppressor WT1 and its zinc finger domain. Protein Expr Purif. 2012;85(2):165–172. doi: 10.1016/j.pep.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Doubrovina E, Carpenter T, Pankov D, Selvakumar A, Hasan A, O’Reilly RJ. Mapping of novel peptides of WT-1 and presenting HLA alleles that induce epitope-specific HLA-restricted T cells with cytotoxic activity against WT-1(+) leukemias. Blood. 2012;120(8):1633–1646. doi: 10.1182/blood-2011-11-394619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobayashi H, Nagato T, Aoki N, Sato K, Kimura S, Tateno M, Celis E. Defining MHC class II T helper epitopes for WT1 tumor antigen. Cancer Immunol Immunother. 2006;55(7):850–860. doi: 10.1007/s00262-005-0071-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oka Y, Elisseeva OA, Tsuboi A, Ogawa H, Tamaki H, Li H, Oji Y, Kim EH, Soma T, Asada M, Ueda K, Maruya E, Saji H, Kishimoto T, Udaka K, Sugiyama H. Human cytotoxic T-lymphocyte responses specific for peptides of the wild-type Wilms’ tumor gene (WT1) product. Immunogenetics. 2000;51(2):99–107. doi: 10.1007/s002510050018. [DOI] [PubMed] [Google Scholar]
- 32.Rezvani K, Yong AS, Mielke S, Savani BN, Musse L, Superata J, Jafarpour B, Boss C, Barrett AJ. Leukemia-associated antigen-specific T-cell responses following combined PR1 and WT1 peptide vaccination in patients with myeloid malignancies. Blood. 2008;111(1):236–242. doi: 10.1182/blood-2007-08-108241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonifaz LC, Bonnyay DP, Charalambous A, Darguste DI, Fujii S, Soares H, Brimnes MK, Moltedo B, Moran TM, Steinman RM. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med. 2004;199(6):815–824. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheong C, Choi JH, Vitale L, He LZ, Trumpfheller C, Bozzacco L, Do Y, Nchinda G, Park SH, Dandamudi DB, Shrestha E, Pack M, Lee HW, Keler T, Steinman RM, Park CG. Improved cellular and humoral immune responses in vivo following targeting of HIV Gag to dendritic cells within human anti-human DEC205 monoclonal antibody. Blood. 2010;116(19):3828–3838. doi: 10.1182/blood-2010-06-288068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koido S, Homma S, Okamoto M, Takakura K, Gong J, Sugiyama H, Ohkusa T, Tajiri H. Chemoimmunotherapy targeting Wilms’ tumor 1 (WT1)-specific cytotoxic T lymphocyte and helper T cell responses for patients with pancreatic cancer. Oncoimmunology. 2014;3(10):e958950. doi: 10.4161/21624011.2014.958950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fujiki F, Oka Y, Tsuboi A, Kawakami M, Kawakatsu M, Nakajima H, Elisseeva OA, Harada Y, Ito K, Li Z, Tatsumi N, Sakaguchi N, Fujioka T, Masuda T, Yasukawa M, Udaka K, Kawase I, Oji Y, Sugiyama H. Identification and characterization of a WT1 (Wilms Tumor Gene) protein-derived HLA-DRB1*0405-restricted 16-mer helper peptide that promotes the induction and activation of WT1-specific cytotoxic T lymphocytes. J Immunother. 2007;30(3):282–293. doi: 10.1097/01.cji.0000211337.91513.94. [DOI] [PubMed] [Google Scholar]
- 37.Sehgal K, Dhodapkar KM, Dhodapkar MV. Targeting human dendritic cells in situ to improve vaccines. Immunol Lett. 2014;162(1 Pt A):59–67. doi: 10.1016/j.imlet.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grigoleit GU, Kapp M, Hebart H, Fick K, Beck R, Jahn G, Einsele H. Dendritic cell vaccination in allogeneic stem cell recipients: induction of human cytomegalovirus (HCMV)-specific cytotoxic T lymphocyte responses even in patients receiving a transplant from an HCMV-seronegative donor. J Infect Dis. 2007;196(5):699–704. doi: 10.1086/520538. [DOI] [PubMed] [Google Scholar]
- 39.Chapuis AG, Ragnarsson GB, Nguyen HN, Chaney CN, Pufnock JS, Schmitt TM, Duerkopp N, Roberts IM, Pogosov GL, Ho WY, Ochsenreither S, Wolfl M, Bar M, Radich JP, Yee C, Greenberg PD. Transferred WT1-reactive CD8+ T cells can mediate antileukemic activity and persist in post-transplant patients. Sci Transl Med. 2013;5(174):174ra127. doi: 10.1126/scitranslmed.3004916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Plantinga M, de Haar C, Nierkens S, Boelens JJ. Dendritic cell therapy in an allogeneic-hematopoietic cell transplantation setting: an effective strategy toward better disease control? Front Immunol. 2014;5:218. doi: 10.3389/fimmu.2014.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sundarasetty BS, Kloess S, Oberschmidt O, Naundorf S, Kuehlcke K, Daenthanasanmak A, Gerasch L, Figueiredo C, Blasczyk R, Ruggiero E, Fronza R, Schmidt M, von Kalle C, Rothe M, Ganser A, Koehl U, Stripecke R. Generation of lentivirus-induced dendritic cells under GMP-compliant conditions for adaptive immune reconstitution against cytomegalovirus after stem cell transplantation. J Transl Med. 2015;13:240. doi: 10.1186/s12967-015-0599-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Di Stasi A, Jimenez AM, Minagawa K, Al-Obaidi M, Rezvani K. Review of the results of WT1 peptide vaccination strategies for myelodysplastic syndromes and acute myeloid leukemia from nine different studies. Front Immunol. 2015;6:36. doi: 10.3389/fimmu.2015.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woehlecke C, Wittig S, Arndt C, Gruhn B. Prognostic impact of WT1 expression prior to hematopoietic stem cell transplantation in children with malignant hematological diseases. J Cancer Res Clin Oncol. 2015;141(3):523–529. doi: 10.1007/s00432-014-1832-y. [DOI] [PubMed] [Google Scholar]
- 44.Yi-Ning Y, Xiao-rui W, Chu-xian Z, Chun W, You-wen Q. Prognostic significance of diagnosed WT1 level in acute myeloid leukemia: a meta-analysis. Ann Hematol. 2015;94(6):929–938. doi: 10.1007/s00277-014-2295-6. [DOI] [PubMed] [Google Scholar]
- 45.Casalegno-Garduño R, Schmitt A, Spitschak A, Greiner J, Wang L, Hilgendorf I, Hirt C, Ho AD, Freund M, Schmitt M. Immune responses to WT1 in patients with AML or MDS after chemotherapy and allogeneic stem cell transplantation. Int J Cancer. 2016;138(7):1792–1801. doi: 10.1002/ijc.29909. [DOI] [PubMed] [Google Scholar]
- 46.Qi XW, Zhang F, Wu H, Liu JL, Zong BG, Xu C, Jiang J. Wilms’ tumor 1 (WT1) expression and prognosis in solid cancer patients: a systematic review and meta-analysis. Sci Rep. 2015;5:8924. doi: 10.1038/srep08924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oka Y, Tsuboi A, Taguchi T, Osaki T, Kyo T, Nakajima H, Elisseeva OA, Oji Y, Kawakami M, Ikegame K, Hosen N, Yoshihara S, Wu F, Fujiki F, Murakami M, Masuda T, Nishida S, Shirakata T, Nakatsuka S, Sasaki A, Udaka K, Dohy H, Aozasa K, Noguchi S, Kawase I, Sugiyama H. Induction of WT1 (Wilms’ tumor gene)-specific cytotoxic T lymphocytes by WT1 peptide vaccine and the resultant cancer regression. Proc Natl Acad Sci USA. 2004;101(38):13885–13890. doi: 10.1073/pnas.0405884101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keilholz U, Letsch A, Busse A, Asemissen AM, Bauer S, Blau IW, Hofmann WK, Uharek L, Thiel E, Scheibenbogen C. A clinical and immunologic phase 2 trial of Wilms tumor gene product 1 (WT1) peptide vaccination in patients with AML and MDS. Blood. 2009;113(26):6541–6548. doi: 10.1182/blood-2009-02-202598. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.