Abstract
Hypersensitivity reactions (HSRs) to chemotherapy drugs, such as taxanes and platins, and to monoclonal antibodies limit their therapeutic use due to the severity of some reactions and the fear of inducing a potentially lethal reaction in highly sensitized patients. Patients who experience hypersensitivity reactions face the prospect of abandoning first-line treatment and switching to a second-line, less effective therapy. Some of these reactions are mast cell-mediated hypersensitivity reactions, a subset of which occur through an immunoglobulin (IgE)-dependent mechanism, and are thus true allergies. Others involve mast cells without a demonstrable IgE mechanism. Whether basophils can participate in these reactions has not been demonstrated. Rapid drug desensitization (RDD) is a procedure that induces temporary tolerance to a drug, allowing a medication allergic patient to receive the optimal agent for his or her disease. Through RDD, patients with IgE and non-IgE HSRs can safely be administered important medications while minimizing or completely inhibiting adverse reactions. Due to the clinical expansion and success of RDD, the molecular mechanisms inducing the temporary tolerization have been investigated and are partially understood, allowing for safer and more effective protocols. This article reviews the current literature on molecular mechanisms of RDD with an emphasis in our recent contributions to this field as well as the indications, methods and outcomes of RDD for taxanes, platins, and monoclonal antibodies.
Keywords: Desensitization, Hypersensitivity reactions, Chemotherapy drugs, Monoclonals, AllergoOncology Symposium in Writing
General principles and proposed mechanisms of rapid drug desensitization
Hypersensitivity reactions to drugs can result in anaphylaxis, which is a life-threatening condition linked to IgE activation of mast cells with subsequent release of powerful preformed inflammatory mediators such as histamine, arachidonic acid metabolites such as leukotrienes and prostaglandins, proteases and proteoglycans such as heparin. These factors participate in the development of classic symptoms involving cutaneous, respiratory, cardiovascular, and gastrointestinal systems. In the early phase of mast cell activation the release of granules mediators is quickly followed by increased synthesis from membrane arachidonic acid of prostaglandins (PGD2) and leukotrienes (LTC/D4 and LTB4) that have additional roles in clinical symptoms [1, 2]. During the late phase of mast cell activation, cytokines such as TNFα and IL-6 are released along with chemokines and other factors. Late-phase mast cell activation contributes to the recruitment of other effector cells, notably eosinophils, basophils, and Th2 cells, which contribute significantly to the immunopathology of the allergic response, the increase in serum IgE, and the allergic sensitization. IgE sensitization to platins is increasing in cancer survivors who have been exposed to multiple courses of chemotherapy, resulting in hypersensitivity reactions upon re-exposure. This can lead to significant morbidity and mortality when first-line treatments cannot be utilized; there is no alternative treatment or second-line treatments are less effective. The best option in such cases is to desensitize the patient to the chemotherapy agent.
Rapid drug desensitization (RDD) is a procedure that allows for temporary clinical tolerance to a drug, by administering gradually increasing small doses to complete the total therapeutic dose of drug allergens [3–5]. IgE-sensitized patients can present a positive skin test to the medication, indicating that mast cells (likely through drug specific IgE) are the main cells responsible for these reactions. After rapid desensitization, the specific skin test reactivity is abolished, implying a profound change in mast cell reactivity with inhibition of the mechanisms that induced mast cell activation for that specific drug antigen [6]. Because RDD induces transient unresponsiveness, patients need to be re-desensitized each time they are exposed to the allergenic medication.
Currently, there is a considerable interest in the study of the molecular mechanisms of desensitization to provide pharmacological targets that will allow safer and effective desensitizations. Mast cells are key effector cells in IgE-dependent immediate hypersensitivity because they express large amounts of a high-affinity tretrameric receptor (FcεRI) for the Fc region of IgE. Multivalent allergen activates mast cells through binding to IgE and aggregating IgE–FcεRI complexes. FcεRI-mediated signaling induces the activation of Src family tyrosine kinases Lyn and Fyn followed by the recruitment and activation of tyrosine kinase Syk. Phosphorylation of LAT by Syk induces the recruitment and activation of PLCγ, leading to calcium mobilization and mast cell degranulation [7]. How does RDD induce mast cell tolerization to antigen? Several mechanisms have been postulated to explain mast cell unresponsiveness to specific activating doses of allergen such as the internalization of FcεRI through progressive cross-linking at low antigen concentration. Subthreshold depletion of mediators and depletion of activating signal transduction components such as Syk kinase [8, 9] have also been implicated. Syk-deficient peripheral blood basophils and lung mast cells failed to degranulate, suggesting a critical role for Syk in non-specific desensitizations, but no molecular target has been found for specific desensitizations.
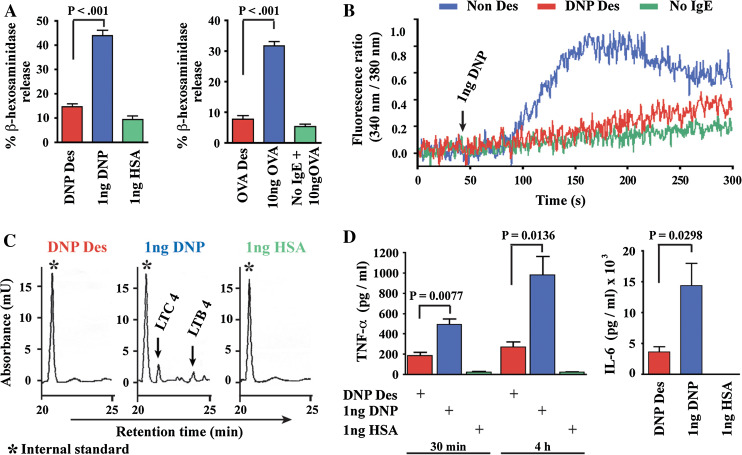
We have recently investigated the molecular mechanisms underlying specific mast cell desensitization using a reproducible in vitro model of antigen-specific, rapid mast cell/IgE desensitization in the presence of physiologic levels of calcium [10] (Fig. 1). Increasing doses of antigen delivered at fixed time intervals induced a highly specific and prolonged hypo-responsiveness to triggering doses of the desensitizing antigen. Mast cells desensitized to DNP or OVA antigens demonstrated almost complete inhibition of β-hexosaminidase and preformed TNF-α release, calcium flux, and arachidonic acid metabolism, suggesting a complete abolition of the acute phase of mast cell activation and demonstrating that the subclinical release of mediators was unlikely during human desensitizations. Desensitized mast cells did not release significant amounts of newly generated IL-6 or TNF-α, thus suggesting that during rapid desensitization patients probably have no risk of a delayed reaction, due to the lack of late-phase mediators generation. When mast cells were sensitized to both DNP and OVA antigens, DNP-desensitized cells responded fully to OVA and vice versa, proving antigen specificity and providing evidence that the activating signal transduction pathways are intact for a second allergen. Therefore, the hypothesis that activating signaling molecules are exhausted during rapid desensitization is not supported.
Fig. 1.
Rapid desensitization inhibits mast cell responses during early- and late-phase activation. a % β-hexosaminidase release induced by rapid desensitization (DNPDes or OVADes) compared to DNP-HSA or OVA-induced activation (1 ng DNP or 10 ng OVA) and controls. b Influx of calcium induced by activation and rapid desensitization when 1 ng of DNP-HSA is added to mast cells. c Arachidonic acid products LTC4 and LTB4, induced by activation and rapid desensitization of mast cells (RP-HPLC analysis). d TNF-α and IL-6 secretion from mast cells during the early (30 min) and late (4 h) phase of mast cell activation and rapid desensitization. Adapted from Sancho-Serra et al. [10]
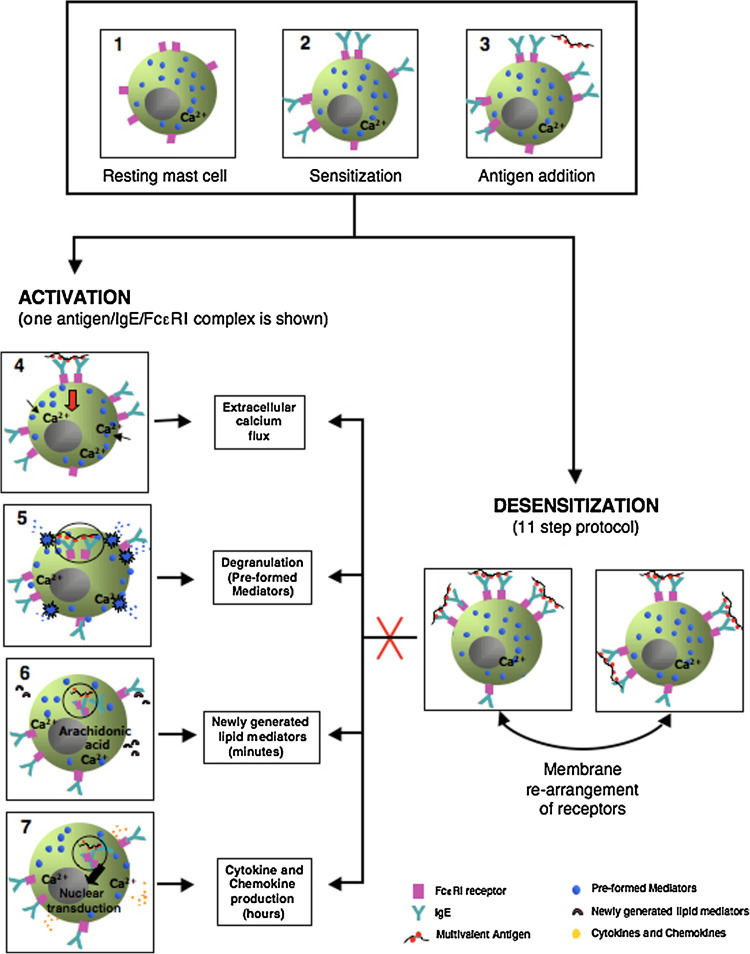
Importantly, antigen-specific IgE bound to the alpha chain of FcεRI remained at the membrane level after rapid desensitization, indicating that the lack of reactivity during desensitization was not due to the disappearance of surface IgE and FcεRI when bound to small doses of antigen (Fig. 2). Thus, the biochemical mechanism(s) by which RDD induces specific mast cell tolerance are likely to be associated with the molecular stabilization of membrane bound IgE receptors carrying the antigen being desensitized. This in vitro model provided an optimal dose–time relationship, leading to almost complete abrogation of early- and late-phase activation events. Because this model showed the lack of mediators released during rapid desensitization, it provided the basis for a modified human rapid desensitization protocol that has been used successfully in hundreds of desensitizations, illustrating the profound inhibition of acute and delayed mast cell responses and the protection against anaphylactic reactions [4, 5].
Fig. 2.
Simplified cartoon comparing activation and desensitization outcomes as well as a possible explanation of how rapid desensitization works and the re-arrangement of the FcεRI receptors at the cell membrane
A universal protocol for human rapid desensitizations to chemotherapy agents and monoclonal antibodies
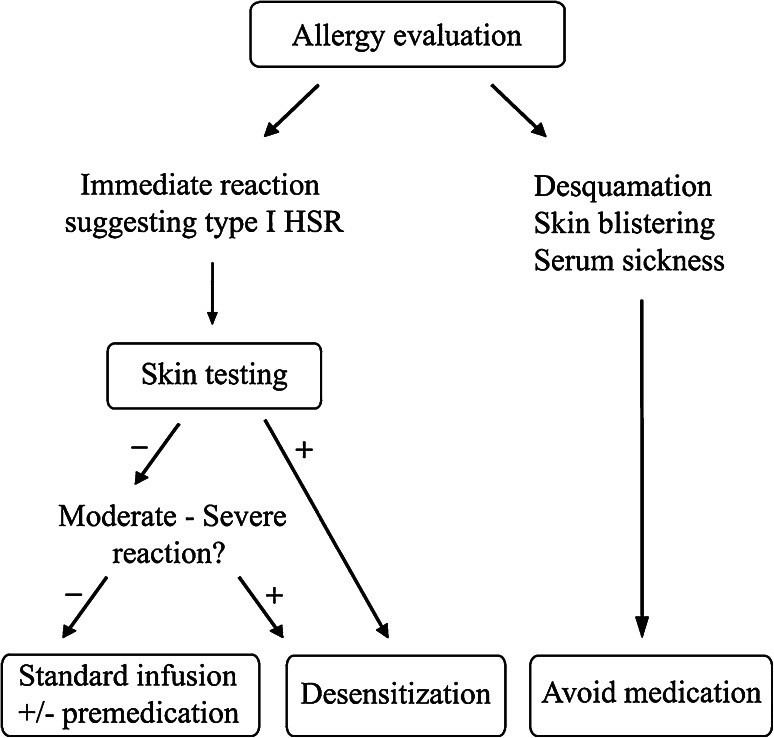
Based on the above in vitro mouse mast cell model, the BWH Desensitization Program produced a 12-step standard protocol in which unresponsiveness to a triggering antigen dose was achieved by delivering doubling doses of antigen at fixed time intervals starting at 1/1,000 to 1/10,000 the final dose [11]. The protocol is based on 3 solutions administered sequentially starting with the solution containing a 1/100 dilution, then a 1/10 dilution and a normal dilution of any chemotherapy agent to which a patient has presented a hypersensitivity reaction (Table 1). Patients who have had severe anaphylactic reactions to the agent of choice or who have reacted early in the standard 12-step desensitization may experience fewer symptoms if desensitized using a 16-step protocol, which adds another bag containing a 1/1,000 dilution of the full concentration. The use of a 16-step (4 bags) or 20-step (5 bags) protocol is reserved for high-risk patients (see below). Before desensitization, each patient is evaluated for suitability as a candidate for rapid desensitization (Fig. 3), including an in-depth historical analysis of the patient’s HSR, skin testing when available, design and testing of an initial desensitization protocol, and adjustment of this protocol based on the patient’s response to the first desensitization. Risk stratification is paramount to the allergy evaluation. High-risk patients are considered the ones with a severe initial reaction involving hypotension, oxygen desaturation, cardiovascular collapse or high cardiac risk, and on beta blockers. Beta blockers and ACE inhibitors are risk factors for poor response to epinephrine during the treatment for anaphylaxis and should be avoided during desensitization. Patients who are candidates for desensitization are educated on the procedure, the risks and benefits are explained, and the risk for anaphylaxis during desensitization is emphasized. Only trained allergists and nurses trained in desensitization can perform RDDs. High-risk patients are desensitized in an intensive care setting.
Table 1.
A protocol consisting of 3 tenfold increasing solutions and 12 steps is used as the standard desensitization protocol at the Brigham and Women’s Hospital Desensitization Program in Boston Lee et al. [6]
| Total dose | 500 mg | Solution concentration (mg/ml) | Total dose in each solution (mg) |
|---|---|---|---|
| Solution A | 250 ml | 0.02 | 5.0* |
| Solution B | 250 ml | 0.20 | 50.0* |
| Solution C | 250 ml | 2.00 | 500.0* |
| Step | Solution | Rate (ml/h) | Time (min) | Administered dose (mg) | Cumulative dose infused |
|---|---|---|---|---|---|
| 1 | A | 2 | 15 | 0.010 | 0.010 |
| 2 | A | 5 | 15 | 0.025 | 0.035 |
| 3 | A | 10 | 15 | 0.050 | 0.085 |
| 4 | A | 20 | 15 | 0.100 | 0.185 |
| 5 | B | 5 | 15 | 0.250 | 0.435 |
| 6 | B | 10 | 15 | 0.500 | 0.935 |
| 7 | B | 20 | 15 | 1.000 | 1.935 |
| 8 | B | 40 | 15 | 2.000 | 3.935 |
| 9 | C | 10 | 15 | 5.000 | 8.935 |
| 10 | C | 20 | 15 | 10.000 | 18.935 |
| 11 | C | 40 | 15 | 20.000 | 38.935 |
| 12 | C | 75 | 184.4 | 461.065 | 500.000 |
| Total time = 5.82 h | Total dose infused = 500 mg* |
* The sum of doses in the solutions A, B, and C equals 555 mg. Total dose infused is 500 mg
Fig. 3.
Evaluation of patients with hypersensitivity reactions to medications as potential candidates for rapid desensitization (Brennan et al. [40])
Rapid desensitization to taxanes
Paclitaxel, docetaxel, and other taxenes are widely used in the treatment for ovarian, breast, non-small-cell lung and other solid tumors. Hypersensitivity reactions to taxanes are common: in early trials of paclitaxel, up to 30 % of patients developed acute infusion reactions and premedication with antihistamines and glucocorticoids as well as slower infusion rates reduced the rate of severe hypersensitivity reactions to less than 10 % [12–15]. In recent years, an increase in the proportion of patient presenting HSR has occurred, some of which are severe and not responsive to premedications. Similarly, approximately 30 % of patients receiving docetaxel without premedication developed acute hypersensitivity reactions, and premedication reduces this rate to less than 10 % [16] but similar to paclitaxel, there is an increase in docetaxel reactive patients in recent years.
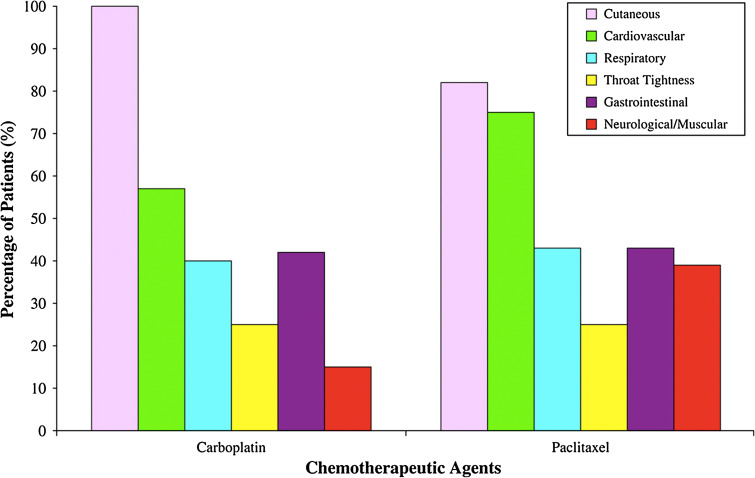
Acute hypersensitivity reactions to taxanes are characterized by dyspnea, urticaria, flushing, back or chest severe pain, gastrointestinal symptoms, hypo- or hypertension, and urticaria or erythematous rashes (Fig. 4). Symptoms typically develop within the first few minutes of the infusion and most often occur on the first or second exposure to the drug [14, 17]. The mechanisms of taxane infusion reactions are not completely understood and may be multifactorial. Proposed mechanisms include complement activation, direct mast cell and/or basophil activation, and IgE-mediated anaphylaxis and tryptase levels, a product of mast cell degranulation as been found elevated in serum of reactive patients shortly after the reaction [18]. Taxane reactions are unlikely to be due solely to an IgE response, because a majority of reactions (56 % in one study) occur with the first exposure to paclitaxel, without IgE sensitization [17]. There is evidence that both the taxane moiety itself and the vehicles in which these lipophilic agents are solubilized can contribute to infusion reactions. Specifically, paclitaxel is stabilized with Cremophor, which is derived from castor oil and is also used as the vehicle for other drugs, such as cyclosporine and vitamin K, which have been associated with similar adverse reactions [17, 19–22]. Actually, Cremophor has been described as producing acute hypersensitivity reactions that do not involve IgE but arises from activation of complement system. It activates complement through both the classical and alternative pathways, giving rise to C3a and C5a anaphylatoxins that trigger mast cells and basophils for secretory response that underlies HSRs [22, 23]. Thus, Cremophor could account, at least in part, for the adverse reactions induced by paclitaxel. However, it is important to mention that an albumin-based formulation of paclitaxel, devoid of Cremophor, has also been implicated in hypersensitivity reactions, providing evidence for taxane moiety-based hypersensitivity reactions. Delayed reactions to taxanes presenting as erythematous rashes up to 10 days after the taxane infusion can lead to IgE sensitization and severe HSR upon re-exposure even in the presence of premedication. Patients presenting such reactions should be evaluated for rapid desensitization.
Fig. 4.
Symptoms of hypersensitivity to chemotherapy agents: carboplatin and paclitaxel (Castells et al. [4])
Rapid Desensitization to taxanes has been done by others and us with great success. In a series of 17 patients who underwent a total of 77 desensitizations to paclitaxel or docetaxel, 72 desensitizations occurred without reactions. Four patients had a total of 5 reactions during desensitization, all of which were much less severe than their original reactions. On the other hand, 5 patients who underwent re-challenge (i.e., re-administration of the culprit taxane by regular infusion) prior to desensitization experienced recurrent reactions, despite additional premedication and a reduced infusion rate [24]. In our series of 98 patients undergoing a total of 413 desensitizations to various chemotherapeutic agents, the majority of desensitizations had mild or no reactions, and most reactions occurred during the final, most concentrated solution and specifically during the last step of the protocol [4].
Rapid desensitization to platins
Platinum-containing compounds are the first line of therapy for the treatment for ovarian cancer and other solid malignancies. Cisplatin was the first to be used, but due to relatively low toxicity the second-generation carboplatin has been more popular and widely used in the past decade [25]. The third-generation platinum oxaliplatin is first-line therapy for the treatment for metastatic colorectal cancer. As the use of platinum-containing compounds has increased, there has been a dramatic increase in the incidence of HSRs: cisplatin hypersensitivity varies from 5 to 20 %, carboplatin from 9 to 27 %, and oxaliplatin from 10 to 19 % [26–28]. Unlike the taxanes, repeated exposures are typically required prior to the onset of hypersensitivity to platins. In one study, 50 % of the initial HSRs to a platin occurred during the eighth course [29]. Likewise, we found that 40 out of 55 patients with carboplatin HSRs reacted between the 7th to the 10th exposure [4]. Cisplatin and oxaliplatin have similar characteristics in that reactions mostly occur between the 4th and the 8th course or after the 6th exposure, respectively [28].
The characteristics of HSRs to platinum agents are typical symptoms of IgE/mast cell-mediated hypersensitivity reactions and can range from cutaneous symptoms, notably palmar or facial flushing to shortness of breath, nausea, vomiting, and diarrhea, and patients may progress to severe reactions with cardiac arrest and deaths have been reported [4] (Fig. 4). In our report of 413 desensitizations, of the 60 patients who had carboplatin HSR, 100 % had cutaneous symptoms, 57 % had cardiovascular symptoms, 40 % had respiratory symptoms, and 42 % had gastrointestinal manifestations [4].
Oxaliplatin HSRs are often similar to those seen in response to carboplatin and cisplatin, but there have been fewer reports of severe anaphylaxis. However, in contrast to carboplatin, respiratory symptoms are common, and other reactions such as Gell and Coombs type II–mediated thrombocytopenia and Gell and Coombs type III immune complex–mediated symptoms of chronic urticaria have been reported in response to oxaliplatin [30–32]. Idiosyncratic reactions to oxaliplatin, including cytokine release syndrome and pulmonary fibrosis, make adverse responses to oxaliplatin heterogeneous and unpredictable [28, 30, 33].
There is a well-recognized association between the interval of carboplatin-free period and the risk of HSR, especially a severe reaction. Schwartz et al. [34] in a study looking at 126 patients with HSR to carboplatin noted that the risk of severe reactions was 47 % if the platinum-free interval was >24 months, versus only 6.5 % if it was <12 months. All 8 patients receiving their third carboplatin regimen had severe reactions.
Skin testing has been used to predict platinum hypersensitivity. We skin tested 60 patients referred for previous HSRs to carboplatin. Of these, 53 were skin test positive. Of the 7 with negative skin tests, 2 patients converted to positive skin tests after several infusions, one skin test was considered delayed positive, and 4 patients experienced hypersensitivity reactions during infusion [4]. Hesterberg et al. [35] published a report of 38 women with carboplatin HSR who were skin tested and desensitized. Thirteen patients were skin test negative to carboplatin, and 7 of those patients had reactions during a “rapid desensitization protocol” other than the 12-step Brigham and Women’s rapid desensitization protocol. Interestingly, they found that when dividing the negative skin test group using the time from the HSR to skin testing, those with recent history of HSR (<3 mo) and negative skin tests did not react, whereas all 7 of the reactors had remote history of HSR (>9 mo). Of note, this group uses a maximum carboplatin skin test dose of 3 mg/ml, while our group uses 10 mg/ml.
Evaluation of patients with hypersensitivity to a platinum-containing compound and/or with a positive skin test determines discontinuation of the agent or re-administration through rapid desensitization. Other treatment options such as the re-administration with increased premedication have not been validated for safety in comprehensive studies and deaths have been reported. The decision to change to a different platinum drug must be considered if the efficacy is similar based on the cancer sensitivity. Polyzos et al. [36] reported a series of 32 patients re-challenged with carboplatin after HSRs. Four of the 20 patients with mild reactions again had erythema but were able to finish the medication infusions. However, 12 patients with initial severe reactions including hyper- or hypotension were unable to complete subsequent carboplatin infusions despite the prophylaxis. Interestingly, in this report 4 of the 12 were switched to cisplatin and tolerated infusions, but the true incidence of cross-reactivity among platinum-based chemotherapeutic agents is not known. Attempts to circumvent a reaction by switching to another platinum-based chemotherapeutic can be dangerous, as exemplified by Dizon et al. [37] who reported the death of one patient due to anaphylaxis in a series of 7 patients switched from carboplatin to cisplatin. Severe hypersensitivity reactions are not a contraindication for rapid desensitization with the Brigham and Women’s rapid desensitization protocol based on several hundred cases.
Rapid desensitization to monoclonal antibodies
Monoclonal antibodies are generally well-tolerated treatments for a broad array of diseases, including malignancies and chronic inflammatory conditions, but due to prolonged exposure and repeated treatments, more patients have been reported to experience HSR in the last 5 years. Patients experiencing acute HSR following administration of these drugs present symptoms such as shaking chills, fever, rash, pruritus, flushing, shortness of breath, wheezing, back or chest pain, nausea, vomiting, hypotension, and severe life-threatening anaphylaxis [38]. In addition, administration of monoclonal antibodies can cause other HSRs such as drug-induced immune thrombocytopenia (which can be attributed to type II HRS), serum sickness (which represents a type III HRS), and T cell-dependent responses (type IV HSR) [39].
The rates of HSRs clinically consistent with immediate hypersensitivity to specific monoclonal antibodies have been reported to be 5–10 % for rituximab, 2–3 % for infliximab, and 0.6–5 % for trastuzumab [40]. Immediate HSRs have also been reported for omalizumab, natalizumab, basiliximab, abciximab, and cetuximab. Almost 70 % of initial HSRs to monoclonal antibodies include a cutaneous component, the most frequently observed type of reaction overall, followed by cardiovascular, respiratory, and throat tightness [38]. The intensity of reactions to monoclonal antibodies infusions is variable. Recent studies have reported that 26 % of initial reactions are mild, 48 % are moderate, and 26 % are severe [40].
Patients with a history suggestive of a mast cell, possibly IgE-mediated HSR should be skin tested with the offending agent to provide evidence of the IgE/mast cell sensitization and the potential for anaphylaxis if re-exposed to the medication [6]. HSRs can be mild, moderate, or severe [41]. Signs and symptoms of HSRs are classified as cutaneous (flushing, pruritus, urticaria, and angioedema), cardiovascular (chest pain, tachycardia, sense of impending doom, presyncope, syncope, and hypotension), respiratory (dyspnea, wheezing, and oxygen desaturation), throat tightness, gastrointestinal (nausea, vomiting, diarrhea, and abdominal pain), neurological/muscular (vision disturbances, back and neck pain, and numbness/weakness), and fever/chills [41].
Protocols for monoclonal antibodies are the same as for platins and taxanes, and we use the 12 steps, 3 bags protocol for all monoclonal-induced HSR. A minority of patients experience HSRs during RDD. In general, these reactions are less intense than the patient’s original reaction. Treatment for such HSRs is aimed at blocking mast cell mediators including histamine, prostaglandins, and leukotrienes [40]. In the event of a reaction during RDD, the infusion is promptly held and the reaction treated. Once the reaction resolves, the protocol can be resumed and completed.
Safety and efficacy of rapid desensitizations for the treatment for hypersensitivity reactions to chemotherapy drugs and therapeutic monoclonal antibodies
In 2008, our group reported the largest case series of rapid desensitizations, in which 98 patients with HSRs to chemotherapy underwent 413 desensitizations [4]. In this series, 67 % of desensitizations proceeded without HSR, and 27 % had only mild reactions (classified as absence of chest pain, changes in blood pressure, dyspnea, oxygen desaturation, or throat tightness), even though 77 % of patients had experienced a severe initial HSR. The remaining 6 % of desensitizations were characterized by severe HSRs; however, epinephrine was only administered during one of the desensitizations, and there were no transfers to a more acute-care setting, intubations, or deaths. All patients in the case series were able to receive their full target dose.
We subsequently published a case series of 105 desensitizations to monoclonal antibodies in 23 patients [40]. Seventy-four percent of the initial HSRs were moderate to severe. During desensitization, reactions were observed in 29 % of desensitizations and 90 % of these were mild. Antibiotic desensitization using our protocol is also exceedingly safe [42]: in our case series of 52 antibiotic desensitizations in 15 patients with cystic fibrosis (and a mean FEV1 of 44.1 % of predicted), 96.2 % of desensitizations were completed without severe adverse events. One patient did develop severe acute respiratory failure requiring intubation; however, this was felt to be secondary to worsening pulmonary infection and not a manifestation of a severe HSR during his desensitizations. All desensitizations in these series were completed, suggesting that even markedly impaired baseline lung function is not a contraindication to rapid desensitization.
Reactions during desensitization
In our experience, reactions during desensitization manifest as a wide range of symptoms that replicate the symptoms presented by the patient during the initial hypersensitivity reaction but typically with less severity, as if turning a dial down from a 10 during the initial reaction to a 1–2 during desensitization [40]. Cutaneous reactions may include flushing, pruritus, urticaria, and angioedema. More severe reactions may encompass cardiovascular manifestations, such as chest pain, tachycardia, a sense of impending doom, presyncope, syncope and hypotension, as well as respiratory symptoms, including sneezing, nasal congestion, dyspnea, coughing, wheezing, and oxygen desaturation. Severe reactions may also be characterized by throat tightness or gastrointestinal complaints, including nausea, vomiting, diarrhea, and abdominal pain. Less common signs and symptoms may include neuromuscular symptoms, such as visual changes, back and neck pain, and numbness/weakness, or, in some cases, fever and chills.
In our 2008 case series of 413 desensitizations in 98 patients, there were a total of 180 reactions, all of which subsided when the infusion was paused and treated appropriately [4]. The majority of reactions (75 %) occurred during infusion of solution 3, and 51 % of reactions occurred during Step 12 of the desensitization protocol. In our monoclonal antibody case series, in which a similar rate of reactions was reported (29 %), cutaneous reactions were the most common and, again, the majority of reactions (70 %) occurred during Step 12. Our approach to treating reactions during desensitization is aimed at blocking local and systemic effects of mast cell mediators, including histamine, prostaglandins, and leukotrienes [40].
At our institution, all reactions during desensitization are treated by pausing the infusion and by administering diphenhydramine or hydroxyzine (25–50 mg administered intravenously) and/or ranitidine (50 mg intravenously). For severe reactions, methylprednisolone sodium succinate (0.5 mg/kg administered intravenously) has been used. Epinephrine, 0.3 ml (1 mg/ml) should at the bedside for hypotension or desaturation. On resolution of the reaction, we restart the protocol from the step at which it had been paused. For patients who react during a prior desensitization [40], additional premedications prior to the start of the protocol or between specific steps during desensitization is the common approach. Most commonly, these are H1 and sometimes H2 blockers and/or methylprednisolone. These are generally added at least one full step before the point at which the reaction occurred. Protocol modification involves adding or lengthening steps before the step at which a reaction has occurred, and this approach is appropriate when a patient reacts despite the additional premedications.
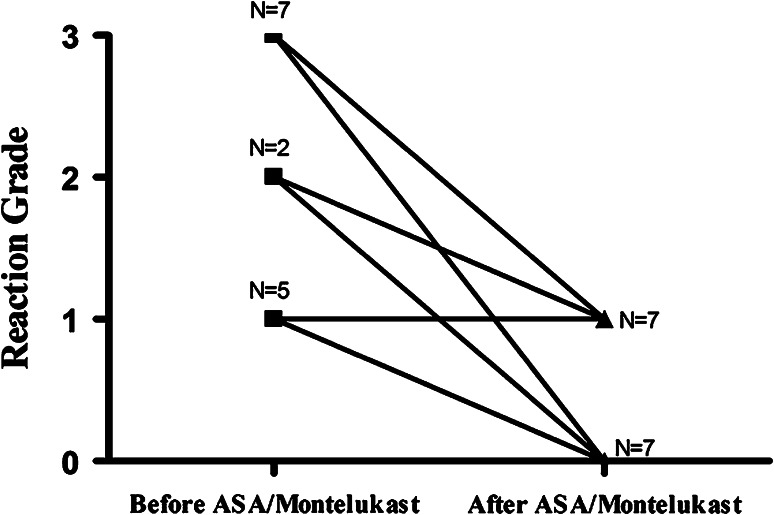
A subset of patients may continue to react during desensitization despite the protocol modification and addition of high-dose histamine receptor blockade and corticosteroids. In another case series, we prophylactically treated these patients with oral acetylsalicylic acid (325 mg) and oral montelukast (10 mg) and were able to successfully treat those patients with refractory mast cell mediator-related symptoms during rapid desensitization [43]. In this study, 78 desensitizations were performed in 14 patients with HSR to platinum chemotherapy that had cutaneous symptoms, many also with associated systemic reactions, during rapid desensitization. Pretreatment with ASA and montelukast 2 days before and on the day of RDD allowed 86 % of the patients to tolerate subsequent desensitizations with a less severe or no HSR (Fig. 5). Interestingly, only 62 % of patients in a control group that received adjunctive methylprednisolone premedication were able to tolerate further desensitizations with a less severe or with no reaction. The greatest benefit of ASA/montelukast pretreatment was seen in patients with skin and respiratory symptoms, suggesting a dominant role for prostaglandins and leukotrienes in these manifestations of HSR to platinum chemotherapies. We have subsequently also treated patients with only one dose of ASA/montelukast 60 min prior to RDD, expanded this treatment for use during monoclonal antibody and antibiotic desensitization, and successfully blocked refractory skin and systemic reactions using this regimen [40, 42].
Fig. 5.
The use of acetylsalicylic acid (ASA) and montelukast pretreatment reduces the severity of reactions during desensitization improving their safety. Patients with prior severe reactions during desensitization were premedicated with ASA and montelukast and 86 % were able to tolerate further desensitizations, with few or no symptoms (grade 2.14 vs grade 0.5, P < .001), providing evidence of improved safety (Breslow et al. [43])
Conclusions
Rapid desensitization has proven to be a safe and effective way to allow patients with hypersensitivity reactions to be treated with their first-line chemotherapy agents and monoclonal antibodies. This has permitted an improved quality of life and possibly a prolonged survival. Variability in the success rates of desensitization is believed to be due to heterogeneity of methods and protocols. With the 12-step rapid desensitization protocol from the Brigham and Women’s Desensitization Program over the past 10 years, more than 99.9 % of nearly 800 patients have received the full dose of their first-line medication in thousands of desensitizations to a wide variety of agents with no deaths. Although the molecular basis of rapid desensitizations is not completely understood, an in vitro mast cell model has provided evidence of profound inhibitory mechanisms of mast cell activation during desensitization, which correlates with the remarkable success of the desensitization protocols when used by trained allergists. These safety and efficacy outcomes provide grounds for the continued and expanded use of this approach for all patients for whom drug hypersensitivity would prevent the administration of first-line pharmacologic therapy.
Markedly reduced rate of reactions over multiple successive desensitizations has been observed, which could indicate the appearance of true tolerance due to repeated drug allergen exposures through desensitization as in allergen immunotherapy [4, 40]. Whether there is an increase in IL-10 or regulatory specific T cells in peripheral blood of desensitized patients is not known. Research is needed to increase insight into the mechanisms of rapid desensitization for the development of pharmacological therapeutic targets.
Footnotes
This paper is part of the Symposium in Writing: AllergoOncology: The Role of Th2 responses in cancer.
References
- 1.Schwartz LB, Metcalfe DD, Miller JS, Earl H, Sullivan T. Tryptase levels as an indicator of mast-cell activation in systemic anaphylaxis and mastocytosis. N Engl J Med. 1987;316:1622–1626. doi: 10.1056/NEJM198706253162603. [DOI] [PubMed] [Google Scholar]
- 2.Vadas P, Gold M, Perelman B, et al. Platelet-activating factor, PAF acetylhydrolase, and severe anaphylaxis. N Engl J Med. 2008;358:28–35. doi: 10.1056/NEJMoa070030. [DOI] [PubMed] [Google Scholar]
- 3.Castells M. Desensitization for drug allergy. Curr Opin Allergy Clin Immunol. 2006;6:476–481. doi: 10.1097/01.all.0000235900.57182.15. [DOI] [PubMed] [Google Scholar]
- 4.Castells MC, Tennant NM, Sloane DE, et al. Hypersensitivity reactions to chemotherapy: outcomes and safety of rapid desensitization in 413 cases. J Allergy Clin Immunol. 2008;122:574–580. doi: 10.1016/j.jaci.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 5.Lee CW, Matulonis UA, Castells MC. Rapid inpatient/outpatient desensitization for chemotherapy hypersensitivity: standard protocol effective in 57 patients for 255 courses. Gynecol Oncol. 2005;99:393–399. doi: 10.1016/j.ygyno.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 6.Lee CW, Matulonis UA, Castells MC. Carboplatin hypersensitivity: a 6-h 12-step protocol effective in 35 desensitizations in patients with gynecological malignancies and mast cell/IgE-mediated reactions. Gynecol Oncol. 2004;95:370–376. doi: 10.1016/j.ygyno.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Wu LC. Immunoglobulin E receptor signaling and asthma. J Biol Chem. 2011;286:32891–32897. doi: 10.1074/jbc.R110.205104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kepley CL. Antigen-induced reduction in mast cell and basophil functional responses due to reduced Syk protein levels. Int Arch Allergy Immunol. 2005;138:29–39. doi: 10.1159/000087355. [DOI] [PubMed] [Google Scholar]
- 9.Shalit M, Levi-Schaffer F. Challenge of mast cells with increasing amounts of antigen induces desensitization. Clin Exp Allergy. 1995;25:896–902. doi: 10.1111/j.1365-2222.1995.tb00033.x. [DOI] [PubMed] [Google Scholar]
- 10.Sancho-Serra Mdel C, Simarro M, Castells M. Rapid IgE desensitization is antigen specific and impairs early and late mast cell responses targeting FcepsilonRI internalization. Eur J Immunol. 2011;41:1004–1013. doi: 10.1002/eji.201040810. [DOI] [PubMed] [Google Scholar]
- 11.Morales AR, Shah N, Castells M. Antigen-IgE desensitization in signal transducer and activator of transcription 6-deficient mast cells by suboptimal doses of antigen. Ann Allergy Asthma Immunol. 2005;94:575–580. doi: 10.1016/S1081-1206(10)61136-2. [DOI] [PubMed] [Google Scholar]
- 12.Eisenhauer EA, ten Bokkel Huinink WW, Swenerton KD, et al. European-Canadian randomized trial of paclitaxel in relapsed ovarian cancer: high-dose versus low-dose and long versus short infusion. J Clin Oncol. 1994;12:2654–2666. doi: 10.1200/JCO.1994.12.12.2654. [DOI] [PubMed] [Google Scholar]
- 13.Kwon JS, Elit L, Finn M, Hirte H, Mazurka J, Moens F, Trim K. A comparison of two prophylactic regimens for hypersensitivity reactions to paclitaxel. Gynecol Oncol. 2002;84:420–425. doi: 10.1006/gyno.2001.6546. [DOI] [PubMed] [Google Scholar]
- 14.Markman M, Kennedy A, Webster K, Kulp B, Peterson G, Belinson J. Paclitaxel-associated hypersensitivity reactions: experience of the gynecologic oncology program of the Cleveland Clinic Cancer Center. J Clin Oncol. 2000;18:102–105. doi: 10.1200/JCO.2000.18.1.102. [DOI] [PubMed] [Google Scholar]
- 15.Wiernik PH, Schwartz EL, Strauman JJ, Dutcher JP, Lipton RB, Paietta E. Phase I clinical and pharmacokinetic study of taxol. Cancer Res. 1987;47:2486–2493. [PubMed] [Google Scholar]
- 16.Schrijvers D, Wanders J, Dirix L, Prove A, Vonck I, van Oosterom A, Kaye S. Coping with toxicities of docetaxel (Taxotere) Ann Oncol. 1993;4:610–611. doi: 10.1093/oxfordjournals.annonc.a058599. [DOI] [PubMed] [Google Scholar]
- 17.Weiss RB, Donehower RC, Wiernik PH, et al. Hypersensitivity reactions from taxol. J Clin Oncol. 1990;8:1263–1268. doi: 10.1200/JCO.1990.8.7.1263. [DOI] [PubMed] [Google Scholar]
- 18.Price KS, Castells MC. Taxol reactions. Allergy Asthma Proc. 2002;23:205–208. [PubMed] [Google Scholar]
- 19.Decorti G, Bartoli Klugmann F, Candussio L, Baldini L. Effect of paclitaxel and Cremophor EL on mast cell histamine secretion and their interaction with adriamycin. Anticancer Res. 1996;16:317–320. [PubMed] [Google Scholar]
- 20.Eschalier A, Lavarenne J, Burtin C, Renoux M, Chapuy E, Rodriguez M. Study of histamine release induced by acute administration of antitumor agents in dogs. Cancer Chemother Pharmacol. 1988;21:246–250. doi: 10.1007/BF00262779. [DOI] [PubMed] [Google Scholar]
- 21.Essayan DM, Kagey-Sobotka A, Colarusso PJ, Lichtenstein LM, Ozols RF, King ED. Successful parenteral desensitization to paclitaxel. J Allergy Clin Immunol. 1996;97:42–46. doi: 10.1016/S0091-6749(96)70281-6. [DOI] [PubMed] [Google Scholar]
- 22.Szebeni J, Muggia FM, Alving CR. Complement activation by Cremophor EL as a possible contributor to hypersensitivity to paclitaxel: an in vitro study. J Natl Cancer Inst. 1998;90:300–306. doi: 10.1093/jnci/90.4.300. [DOI] [PubMed] [Google Scholar]
- 23.Weiszhar Z, Czucz J, Revesz C, Rosivall L, Szebeni J, Rozsnyay Z. Complement activation by polyethoxylated pharmaceutical surfactants: Cremophor-EL, Tween-80 and Tween-20. Eur J Pharm Sci. 2012;45:492–498. doi: 10.1016/j.ejps.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 24.Feldweg AM, Lee CW, Matulonis UA, Castells M. Rapid desensitization for hypersensitivity reactions to paclitaxel and docetaxel: a new standard protocol used in 77 successful treatments. Gynecol Oncol. 2005;96:824–829. doi: 10.1016/j.ygyno.2004.11.043. [DOI] [PubMed] [Google Scholar]
- 25.Aabo K, Adams M, Adnitt P, et al. Chemotherapy in advanced ovarian cancer: four systematic meta-analyses of individual patient data from 37 randomized trials. Advanced Ovarian Cancer Trialists’ Group. Br J Cancer. 1998;78:1479–1487. doi: 10.1038/bjc.1998.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gadducci A, Tana R, Teti G, Zanca G, Fanucchi A, Genazzani AR. Analysis of the pattern of hypersensitivity reactions in patients receiving carboplatin retreatment for recurrent ovarian cancer. Int J Gynecol Cancer. 2008;18:615–620. doi: 10.1111/j.1525-1438.2007.01063.x. [DOI] [PubMed] [Google Scholar]
- 27.Gomez R, Harter P, Luck HJ, Traut A, Kommoss S, Kandel M, du Bois A. Carboplatin hypersensitivity: does introduction of skin test and desensitization reliably predict and avoid the problem? A prospective single-center study. Int J Gynecol Cancer. 2009;19:1284–1287. doi: 10.1111/IGC.0b013e3181a418ff. [DOI] [PubMed] [Google Scholar]
- 28.Makrilia N, Syrigou E, Kaklamanos I, Manolopoulos L, Saif MW (2010) Hypersensitivity reactions associated with platinum antineoplastic agents: a systematic review. Met Based Drugs. doi: 10.1155/2010/207084 [DOI] [PMC free article] [PubMed]
- 29.Markman M, Kennedy A, Webster K, Elson P, Peterson G, Kulp B, Belinson J. Clinical features of hypersensitivity reactions to carboplatin. J Clin Oncol. 1999;17:1141. doi: 10.1200/JCO.1999.17.4.1141. [DOI] [PubMed] [Google Scholar]
- 30.Maindrault-Goebel F, Andre T, Tournigand C, Louvet C, Perez-Staub N, Zeghib N, De Gramont A. Allergic-type reactions to oxaliplatin: retrospective analysis of 42 patients. Eur J Cancer. 2005;41:2262–2267. doi: 10.1016/j.ejca.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 31.Polyzos A, Tsavaris N, Gogas H, et al. Clinical features of hypersensitivity reactions to oxaliplatin: a 10-year experience. Oncology. 2009;76:36–41. doi: 10.1159/000178163. [DOI] [PubMed] [Google Scholar]
- 32.Sakaeda T, Kadoyama K, Yabuuchi H, Niijima S, Seki K, Shiraishi Y, Okuno Y. Platinum agent-induced hypersensitivity reactions: data mining of the public version of the FDA adverse event reporting system, AERS. Int J Med Sci. 2011;8:332–338. doi: 10.7150/ijms.8.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee C, Gianos M, Klaustermeyer WB. Diagnosis and management of hypersensitivity reactions related to common cancer chemotherapy agents. Ann Allergy Asthma Immunol. 2009;102:179–187. doi: 10.1016/S1081-1206(10)60078-6. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz JR, Bandera C, Bradley A, Brard L, Legare R, Granai CO, Dizon DS. Does the platinum-free interval predict the incidence or severity of hypersensitivity reactions to carboplatin? The experience from Women and Infants’ Hospital. Gynecol Oncol. 2007;105:81–83. doi: 10.1016/j.ygyno.2006.10.047. [DOI] [PubMed] [Google Scholar]
- 35.Hesterberg PE, Banerji A, Oren E, Penson RT, Krasner CN, Seiden MV, Wong JT. Risk stratification for desensitization of patients with carboplatin hypersensitivity: clinical presentation and management. J Allergy Clin Immunol. 2009;123(1262–7):e1. doi: 10.1016/j.jaci.2009.02.042. [DOI] [PubMed] [Google Scholar]
- 36.Polyzos A, Tsavaris N, Kosmas C, et al. Hypersensitivity reactions to carboplatin administration are common but not always severe: a 10-year experience. Oncology. 2001;61:129–133. doi: 10.1159/000055363. [DOI] [PubMed] [Google Scholar]
- 37.Dizon DS, Sabbatini PJ, Aghajanian C, Hensley ML, Spriggs DR. Analysis of patients with epithelial ovarian cancer or fallopian tube carcinoma retreated with cisplatin after the development of a carboplatin allergy. Gynecol Oncol. 2002;84:378–382. doi: 10.1006/gyno.2001.6519. [DOI] [PubMed] [Google Scholar]
- 38.Chung CH, O’Neil BH. Infusion reactions to monoclonal antibodies for solid tumors: immunologic mechanisms and risk factors. Oncology (Williston Park) 2009;23:14–17. [PubMed] [Google Scholar]
- 39.Hansel TT, Kropshofer H, Singer T, Mitchell JA, George AJ. The safety and side effects of monoclonal antibodies. Nat Rev Drug Discov. 2010;9:325–338. doi: 10.1038/nrd3003. [DOI] [PubMed] [Google Scholar]
- 40.Brennan PJ, Rodriguez Bouza T, Hsu FI, Sloane DE, Castells MC. Hypersensitivity reactions to mAbs: 105 desensitizations in 23 patients, from evaluation to treatment. J Allergy Clin Immunol. 2009;124:1259–1266. doi: 10.1016/j.jaci.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 41.Brown SG. Clinical features and severity grading of anaphylaxis. J Allergy Clin Immunol. 2004;114:371–376. doi: 10.1016/j.jaci.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 42.Legere HJ, 3rd, Palis RI, Rodriguez Bouza T, Uluer AZ, Castells MC. A safe protocol for rapid desensitization in patients with cystic fibrosis and antibiotic hypersensitivity. J Cyst Fibros. 2009;8:418–424. doi: 10.1016/j.jcf.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Breslow RG, Caiado J, Castells MC. Acetylsalicylic acid and montelukast block mast cell mediator-related symptoms during rapid desensitization. Ann Allergy Asthma Immunol. 2009;102:155–160. doi: 10.1016/S1081-1206(10)60247-5. [DOI] [PubMed] [Google Scholar]