Abstract
Intratumoral B lymphocytes are an integral part of the lung tumor microenvironment. Interrogation of the antibodies they express may improve our understanding of the host response to cancer and could be useful in elucidating novel molecular targets. We used two strategies to explore the repertoire of intratumoral B cell antibodies. First, we cloned VH and VL genes from single intratumoral B lymphocytes isolated from one lung tumor, expressed the genes as recombinant mAbs, and used the mAbs to identify the cognate tumor antigens. The Igs derived from intratumoral B cells demonstrated class switching, with a mean VH mutation frequency of 4 %. Although there was no evidence for clonal expansion, these data are consistent with antigen-driven somatic hypermutation. Individual recombinant antibodies were polyreactive, although one clone demonstrated preferential immunoreactivity with tropomyosin 4 (TPM4). We found that higher levels of TPM4 antibodies were more common in cancer patients, but measurement of TPM4 antibody levels was not a sensitive test for detecting cancer. Second, in an effort to focus our recombinant antibody expression efforts on those B cells that displayed evidence of clonal expansion driven by antigen stimulation, we performed deep sequencing of the Ig genes of B cells collected from seven different tumors. Deep sequencing demonstrated somatic hypermutation but no dominant clones. These strategies may be useful for the study of B cell antibody expression, although identification of a dominant clone and unique therapeutic targets may require extensive investigation.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-015-1787-0) contains supplementary material, which is available to authorized users.
Keywords: Non-small cell lung cancer, Intratumoral B lymphocytes, Tumor-infiltrating lymphocytes, Target discovery, Tropomyosin 4
Introduction
Lung cancer is a heterogeneous disease, composed of cancer cells within a complex microenvironment containing blood vessels, fibroblasts, and immune cells, including B and T lymphocytes, and an assortment of myeloid cells such as natural killer cells, neutrophils, myeloid derived suppressor cells, macrophages, and DCs [1, 2]. Some immune cells have pro-tumorigenic activity, whereas others are anti-tumorigenic. B lymphocytes have been ascribed both pro- and anti-tumorigenic activities [3–5]. Many functional types of B cells that affect tumor growth or dissemination have now been identified, including B cells that support Th1 cells and cytotoxic T lymphocytes, B cells that promote angiogenesis, B regulatory cells that both suppress T effector cells and support T regulatory cells, and B cells that express class-switched, affinity-matured antibodies [5].
When bound to their targets, antibodies can activate the innate immune system by engagement of Fcγ receptors on effector cells and by activation of the classical complement cascade, and it is likely that these activities account for the observed pro-tumorigenic properties of B lymphocytes. Circulating immune complexes are responsible for activating FcγR-dependent signaling in myeloid cells and instigation of a pro-tumorigenic program [6–8], and are associated with poor prognosis in several types of human cancer [9]. These data support a hypothesis that B cells promote an inflammatory state that promotes malignant progression.
However, the presence of intratumoral B lymphocytes is associated with increased survival in several types of cancers, including non-small cell lung cancer (NSCLC) [10–12] and others [13–16], suggestive of anti-tumorigenic effects. These effects may at least be partly target specific. Analysis of B cells from tumors and metastatic lymph nodes found hypermutated antibody genes and expansion of class-switched memory cells and plasmablasts in patients with bladder cancer [17]. Intratumoral germinal centers (GCs) have been reported to be present in NSCLC [18–20], breast cancer [21–24], colon cancer [25], and germ cell cancers [26]. In NSCLC, GCs are associated with early-stage disease [19], suggesting that a humoral immune response may hold these tumors in check. Dieu-Nosjean and colleagues have extensively characterized “tertiary lymphoid structures” at the invasive margins of NSCLC tumors that contain a T cell zone with mature DCs, and GCs with proliferating B cells and follicular DCs (reviewed in [27]). The combination of high densities of both follicular B cells and mature DCs is associated with survival [20].
Although some tumor antigens have been identified (e.g., LAGE-1, MAGE family, p53, NY-ESO-1 in NSCLC [20]), the targets of intratumoral B cell antibodies are largely unknown. Further characterization of antigen-stimulated intratumoral B cells and identification of tumor antigens will be useful in studying B cell function, but also in potentially developing new diagnostic tests and identifying novel therapeutic targets. Here we explored two strategies for characterizing intratumoral B cell antibodies expressed in lung tumors. The first strategy—cloning and expression of Igs from isolated intratumoral lymphocytes (ITLs)—while very effective at generating recombinant antibodies from individual B cells, is labor intensive. The second strategy focuses recombinant antibody production only on those B cells that show evidence of antigen-stimulated clonal expansion, so may permit the identification of antibodies that arise specifically in response to tumor antigens. This strategy uses deep sequencing to identify expanded nucleotide sequences associated with B cell clones.
Materials and methods
Patients
This study was approved by our Institutional Review Board, and all patients signed an informed consent prior to enrollment in the study. None of the patients had a primary immune deficiency. ITLs used for antibody cloning were isolated from tumor tissue, freshly resected at Duke University Medical Center, from a 75-year-old female 40 pack-year smoker with a 1.8-cm stage 1A adenocarcinoma in the right upper lobe. For the TPM4 ELISA study, we isolated serum from blood collected in red-top Vacutainer tubes from 26 patients with a new diagnosis of NSCLC and 21 without evidence of cancer (Table 1). The NSCLC group included seven patients with stage I disease, two with stage II, four with stage III, and two with stage IV. Sera were stored at −80 °C until use. For the deep sequencing study, we collected freshly resected tumor samples from seven additional patients with NSCLC. Six out of seven of these patients’ tumors contained GCs by pathological analysis, conducted as described in Ref. [19]. The age, gender, histology, and stage of these patients are shown in Table 2.
Table 1.
TPM4 ELISA study: patient characteristics
| Characteristic | Cancer | No cancer |
|---|---|---|
| Age (years) | ||
| Mean ± SD | 70.5 ± 8.7 | 58.8 ± 10.7 |
| Gender | ||
| Female | 11 | 12 |
| Male | 15 | 9 |
| Tobacco use | ||
| Pack-years | 35.1 ± 24.8 | 31.5 ± 29.3 |
| Pathologic stage | ||
| IA | 9 | |
| IB | 5 | |
| IIA | 0 | |
| IIB | 4 | |
| IIIA | 3 | |
| IIIB | 1 | |
| IV | 4 | |
| Cell type | ||
| Adenocarcinoma | 16 | |
| Squamous cell | 5 | |
| Large cell, neuroendocrine | 1 | |
| NSCLC | 3 | |
| Large cell lung cancer | 1 | |
Table 2.
Deep sequencing study: patient characteristics
| Characteristic | Value |
|---|---|
| Age (years) | |
| Mean ± SD | 71.7 ± 12.5 |
| Gender | |
| Female | 3 |
| Male | 4 |
| Tobacco use | |
| Pack-years | 32.6 ± 18.3 |
| Pathologic stage | |
| IA | 1 |
| IB | 3 |
| IIA | 0 |
| IIB | 1 |
| IIIA | 1 |
| IIIB | 0 |
| IV | 1 |
| Cell type | |
| Adenocarcinoma | 4 |
| Squamous cell | 2 |
| Large cell lung cancer | 1 |
Cell lines
Human embryonic kidney 293T cells were maintained in DMEM containing 10 % (v/v) heat-inactivated FBS and 50 µg/ml gentamicin. Lung carcinoma H460 cells (ATCC) were maintained in RPMI-1640 medium containing 10 % (v/v) FBS. Media and supplements were obtained from Gibco (Invitrogen, Carlsbad, CA), and cells were maintained in a humidified atmosphere at 37 °C and 5 % CO2.
B cell isolation and FACS
We isolated ITLs from lung tumor specimens by first placing the tissue in a Petri dish containing RPMI-1640 medium supplemented with 20 mM HEPES (RPMI/HEPES) and teasing the tissue into very small fragments with an 18-gauge needle. We then filtered the tissue fragment suspension sequentially through 100- and 40-µm pore-size nylon membranes and pelleted the cells by centrifugation for 10 min at 400×g at 18 °C. We resuspended the cell pellet in 2 ml RPMI/HEPES and isolated the lymphocytes over a Ficoll-Paque Plus (GE Healthcare, Uppsala, Sweden) cushion according to the manufacturer’s instructions. We resuspended the final lymphocyte pellet in Bambanker (Wako Chemicals, Richmond, VA) cell freezing medium and stored the cells at −80 °C until FACS.
For sorting of memory B cells and plasma cells, we first thawed the ITLs and washed them in PBS. We then stained the cells with Aqua vital dye (Invitrogen, Carlsbad, CA) and a combination of the following antihuman antibodies: CD3 phycoerythrin (PE)-Cy5, CD14 PE-Cy5, CD16 PE-Cy5, CD235a PE-Cy5, CD45 PE-Texas Red, CD19 allophycocyanin (APC)-Cy7, CD27 PE-Cy7, CD38 APC-Cy5.5, IgM FITC, and IgD PE (BD Biosciences, Mountain View, CA; Beckman Coulter, and Invitrogen). During the sort, we used forward- versus side-scatter gating to select for lymphocytes, and geometric gates to eliminate doublet events. We gated B cells as CD45+, CD3−, CD14−, CD16−, CD235a−, and CD19+; total memory B cells were further identified as IgD negative (IgD−). B cells were sorted individually into 96-well PCR plates containing ice-cold PBS, dithiothreitol, and RNAsin (Promega, Madison, WI) and stored at −80 °C until further processing. We performed FACS on a BD FACSAria (BD Biosciences, San Jose, CA) and analyzed the data with FlowJo (Tree Star, Ashland, OR).
Antibody cloning and expression
We amplified the Ig heavy and light chain variable (VH and VL) gene regions from the sorted single B cells by RT-PCR using a method first described by Tiller et al. [28] and modified by Liao et al. [29]. Briefly, we reverse-transcribed total RNA from single B cells using Superscript III reverse transcriptase (Invitrogen, Carlsbad, CA) and human IgG, IgM, IgD, IgA, Ig kappa (κ), and Ig lambda (λ) constant region primers; the sequences of these primers are in Supplementary Table 2 of Liao et al. [29]. We then used the resulting cDNA in nested PCRs to amplify the heavy chain (VH) and light chain (VLκ and λ) variable regions as previously described [29]. Single-cell RT-PCR yielded eight heavy chain gene products from eight wells; in three wells, two different light chains were isolated. The VH and VL genes were sequenced and annotated for Ig gene families, somatic mutations, CDRs, and clonality using methods previously described [30, 31]. One light chain gene was not further characterized due to poor subsequent expression; two light chain genes from different wells were predicted to encode identical proteins at the amino acid level, and only one of these was used further. Therefore, there were nine light chain genes. The characteristics of the eight distinct heavy chain genes and nine light chain genes are given in Supplementary Table 1.
Using tag sequences that were part of the second-round nested PCR primers, we cloned the VH and VL genes into human Ig(γ) and Ig(λ) pcDNA3.1(+) (Invitrogen) expression vectors and sequenced the inserts to confirm identity with the original PCR product [29].
Recombinant antibody purification
For production of recombinant antibodies, we co-transfected 293T cells with pairs of pcDNA3.1(+) plasmids containing VH and VL genes. Each plasmid pair contained VH and VL genes obtained from sorted single memory B cells. Plasmids encoding each of the two heavy chain genes originally associated with two light chain genes in the PCR well were transfected in heavy-light pairs to produce both possible antibodies for further characterization and were assigned individual recombinant antibody designations (Supplementary Table 1). Cells were cultured for 4 days in Pro293a-CDM serum-free medium (Lonza, Walkersville, MD) containing 1X GlutaMAX and 50 µg/ml gentamicin, after which we collected the culture medium containing the recombinant antibodies. We concentrated the medium using Centricon Plus-70 30 K MWCO centrifugal concentrators (Millipore, Billerica, MA) and purified each recombinant antibody using protein G agarose (Pierce, Thermo Scientific, Rockford, IL).
Immunoblotting
We tested recombinant antibodies for reactivity against H460 lung carcinoma cell lysate proteins by immunoblot. Proteins were separated by 1D-PAGE on a gel with a single preparative well and blotted to polyvinylidene fluoride membrane (PVDF, Millipore), and the PVDF inserted into a Surf-Blot apparatus (Idea Scientific Co., Minneapolis, MN) that creates individual channels that permits multiple different primary antibodies to be run simultaneously. We allowed the primary antibodies to interact with the blot for 2 h at room temperature, washed the membrane, and detected bound antibody with goat antihuman IgGγ chain-HRP conjugate. This was followed by incubation in chemiluminescent substrate and X-ray film exposure.
Antigen identification
We identified the protein antigens responsible for immunoreactive bands in 1D-PAGE immunoblots by first probing 2D-PAGE blots of H460 lysate proteins with recombinant antibodies as described above. We then used the location of the immunoreactive spot as a guide to locate the protein of interest on a duplicate Coomassie-stained 2D-PAGE gel of the same lysate sample. Proteins in the stained spot were identified at the Duke Proteomics Facility by in-gel tryptic digestion, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MS) peptide fingerprinting, and nano-liquid chromatography MS/MS.
We carried out 2D-PAGE by loading the protein sample (125 µg of H460 lysate protein in 7 M urea, 2 M thiourea, 2 % (w/v) 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate, 20 mM dithiothreitol, 0.2 % (w/v) Bio-Lyte 3-10 ampholytes, and bromophenol blue (trace)) by passive rehydration into 7 cm, pH 3–10 NL Ready-Strip IPG strips (Bio-Rad, Hercules, CA). Isoelectric focusing was carried out at 4000 V for a total of 10,000 V-h using a Protean IEF Cell (Bio-Rad). We then incubated the IPG strips sequentially in equilibration buffers consisting of 1X NuPAGE LDS sample buffer (Invitrogen) containing 50 mM dithiothreitol or 125 mM iodoacetamide for 15 min each. We carried out second dimension separation by SDS-PAGE on 4–12 % NuPAGE Bis–Tris ZOOM gels (Invitrogen). Gels to be stained were incubated in SimplyBlue SafeStain (Invitrogen) for 1 h followed by destaining with water. Blotting to PVDF was carried out as for 1D-PAGE immunoblotting.
TPM4 ELISA
We evaluated the presence of antibodies against TPM4 in sera from patients with or without lung cancer by ELISA. Recombinant human TPM4 containing a 6x-His tag (Fitzgerald Industries, Acton, MA) was immobilized for 1 h at room temperature in the wells of His-Select nickel-coated 96-well plates (Sigma-Aldrich, St. Louis, MO) that had been pre-blocked with IgG-free BSA. After removing unbound proteins by washing in PBS containing 0.1 % (v/v) Tween-20 (PBST), we added serum that had been diluted 1:50 in PBST to the wells and incubated the plates for 1 h at room temperature. After washing the wells, bound antibody was detected with antihuman IgGγ chain-HRP (1:1000) followed by the development in 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) and hydrogen peroxide. Absorbance was determined at 405 nm in a plate reader. The final absorbance values were calculated by subtracting the absorbance of wells without TPM4 from those with TPM4. The p value was calculated according to Student’s t test.
Next generation sequencing (NGS) of IgVH genes
We isolated ITLs from seven additional lung cancer patients as described above. The ITLs from each tumor were first divided into two aliquots: one being subjected to sorting individual B cells as described above, and the other aliquot used for sequencing. For sequencing, VH gene amplicons were generated from total RNAs of the isolated ITLs by RT-PCR using the same VH1-6 primers as those used for isolation of Ig VH genes from the sorted single B cells and subjected to NGS using the Illumina platform (http://res.illumina.com/documents/products/illumina_sequencing_introduction.pdf). Sequences were annotated for Ig gene families, somatic mutations, CDRs, and clonality using methods previously described [31]. The clonal relationship of sequences generated by NGS was determined using the Cloanalyst software suite (http://www.bu.edu/computationalimmunology/research/software/) as described [32, 33].
Results
Antibody cloning and expression, purification, and confirmation of reactivity with tumor antigens
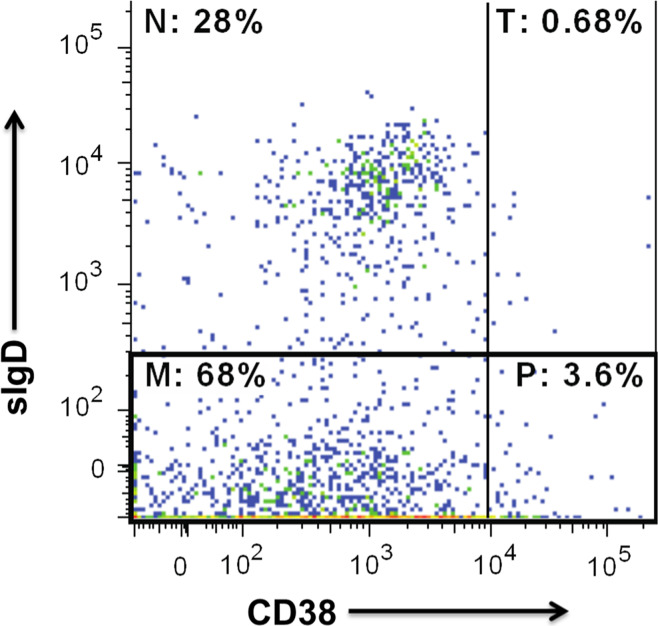
The overall work flow for the current study is shown in Supplementary Fig. 1. We performed FACS on 2.6 × 106 total ITLs isolated from a 0.133 g (wet weight) piece of freshly resected early-stage lung adenocarcinoma. Total B cells, which constituted 17.6 % of the ITL population, were further delineated into transitional, naïve, and memory B cells (Fig. 1). Total B cells (CD19+) or memory B cells (CD19+ sIgD−) were sorted into individual wells of three 96-well PCR plates.
Fig. 1.
B cell populations among ITLs isolated from a lung tumor. ITLs are sorted as described in Patients and Methods. The percentages in each quadrant refer to the proportions of naïve (N), transitional (T) and memory B cells (M), and plasmablasts (P). The image is generated with FlowJo (Tree Star, Ashland, OR)
Single-cell RT-PCR yielded heavy and light chain gene products from eight wells containing memory B cells, and these products were sequenced and cloned into expression vectors.
For expression of recombinant antibodies, we co-transfected appropriately matched pairs of VH and VL Ig gene-containing plasmids into 293T cells and harvested the recombinant antibody-containing cell culture supernatant after 96 h of culture. Although there were eight heavy chain genes synthesized, because of the unexpected synthesis of additional PCR products encoding light chain genes, two additional VH-VL pairs of plasmids were transfected, yielding a total of ten pairs of plasmids transfected into 293T cells. (See Supplementary Table 1.) The resulting ten recombinant antibodies were purified from the cell culture supernatants using protein G agarose. An image of a silver-stained polyacrylamide gel containing all ten purified recombinant antibodies is shown in Supplementary Fig. 2. Both heavy Ig chains (bands above the 55-kDa marker) and light Ig chains (bands between the 25- and 35-kDa markers) are visible in each lane.
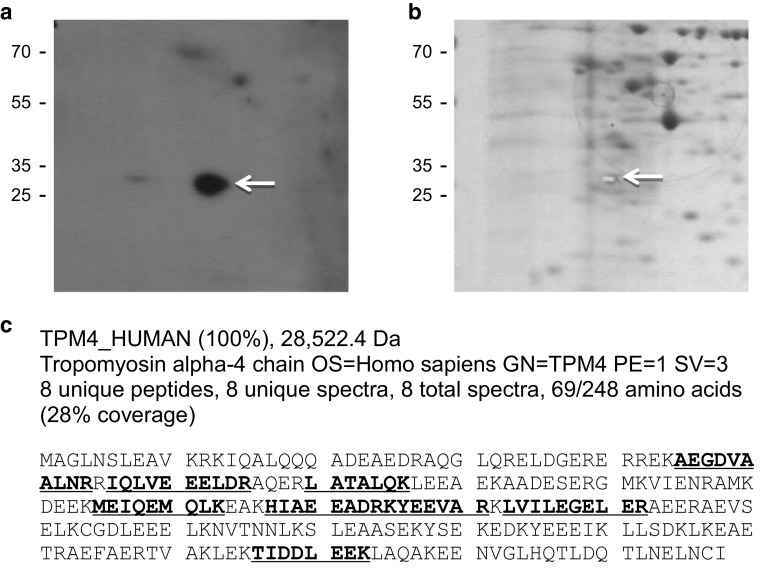
Fig. 2.
Identification of the immunoreactive 2D gel spot as TPM4. Proteins from the lung cancer cell line H460 are separated on duplicate 2D gels. One gel is blotted to PVDF and probed with recombinant antibody T009, and the other gel is stained with Coomassie blue. The immunoreactive spot on the PVDF (arrow in a) is aligned with a spot on the stained gel, which is excised (arrow in b) and subjected to in-gel trypsin digestion and nano-liquid chromatography MS/MS. All eight peptides identified from the MS analysis (shown in bold and underlined in c) mapped to TPM4. Molecular weight markers, in kDa, are shown to the left
Since these recombinant antibodies had been cloned from tumor-infiltrating B cells, we were interested in examining their immunoreactivity against lung cancer proteins. We did this by probing blots of proteins from the lung carcinoma cell line H460 with each recombinant antibody. One-dimensional immunoblots showed the recombinant antibodies to be immunoreactive against multiple proteins, suggesting polyreactivity [34]. Two-dimensional immunoblots confirmed this but also showed that one antibody, T009, was exceptionally immunoreactive against a protein of approximately 35 kDa (data not shown). This raised the possibility that T009 might exhibit a functional monoreactivity toward this antigen in vivo and might even exhibit tumor selectivity. Hence, we set out to determine the identity of the protein responsible for this immunoreactivity.
Antigen identification and confirmation
In order to identify the ~35-kDa immunoreactive protein seen in immunoblots, we used T009 to perform an additional 2D immunoblot of an H460 lysate and then aligned the resulting immunoreactive spot with a spot on a duplicate gel stained with Coomassie (Fig. 2). The spot was cut out of the stained gel and submitted to the Duke Proteomics Facility for identification by nano-liquid chromatography MS/MS. This analysis identified tropomyosin alpha-4 (TPM4) as the putative immunoreactive protein.
To determine whether recombinant antibody T009 indeed recognized TPM4, we performed an immunoblot analysis of purified recombinant TPM4 protein (Supplementary Fig. 3a). As shown in the figure, T009 is clearly immunoreactive against TPM4. We also confirmed that serum antibodies, from the same patient from whose tumor lymphocytes the TPM antibody genes were cloned, recognize purified TPM4 protein (Supplementary Fig. 3b).
TPM4 is a member of the tropomyosin family of actin binding proteins. Anaplastic lymphoma kinase (ALK) is a receptor-type protein tyrosine kinase that is rendered oncogenic as a result of its fusion to TPM4 in inflammatory myofibroblastic tumor [35]. We investigated whether the antibody to TPM4 may have arisen due to the presence of such a fusion event by performing fluorescence in situ hybridization analysis on the tumor tissue that provided the B cells from which recombinant antibody T009 was cloned. However, no evidence for a translocation involving TPM4 was found in this tissue (data not shown).
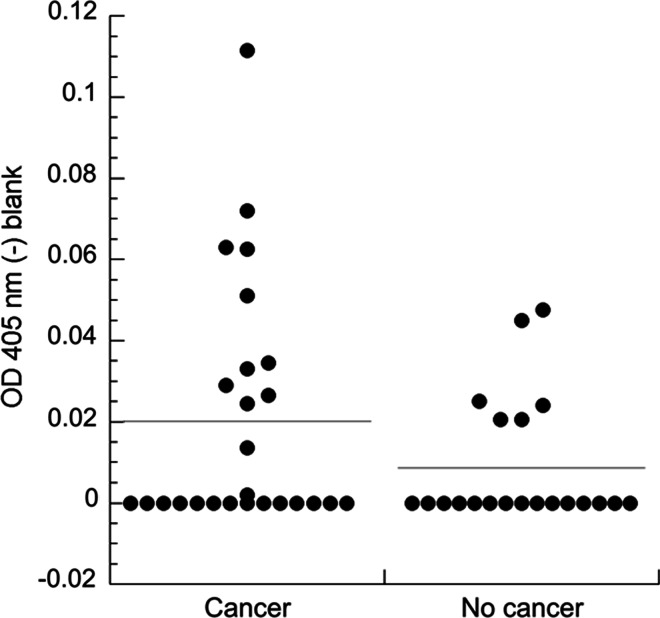
Since it was possible that anti-TPM4 antibodies in this patient’s serum arose due to a tumor-specific immune response, we were interested in comparing the prevalence of the antibodies in serum from lung cancer patients and individuals without cancer. We surveyed the serum from 26 patients with lung cancer and 21 without lung cancer using a direct ELISA developed in our laboratory. As shown in Fig. 3, antibodies against TPM4 were detected in serum from patients both with and without lung cancer. Approximately 42 % (11/26) of patients with lung cancer had the antibody compared to 29 % (6/21) without cancer. Although these differences are not statistically significant (p = 0.118), by ROC analysis the sera exhibiting the five highest ELISA values (>47.74) were all from patients with lung cancer (specificity = 100 %, sensitivity = 20 %).
Fig. 3.
Prevalence of anti-TPM4 antibodies in lung cancer patients and controls. Serum from 26 patients with lung cancer and 21 without are tested by ELISA against immobilized recombinant TPM4. All sera are diluted 1:50. Optical density values at 405 nm from wells containing only blocking agent are subtracted from those with TPM4 plus blocking agent. All negative blank-corrected values are reported in the plot as zero. The horizontal lines show the average blank-corrected values
No restricted clonal expansion associated with ITLs
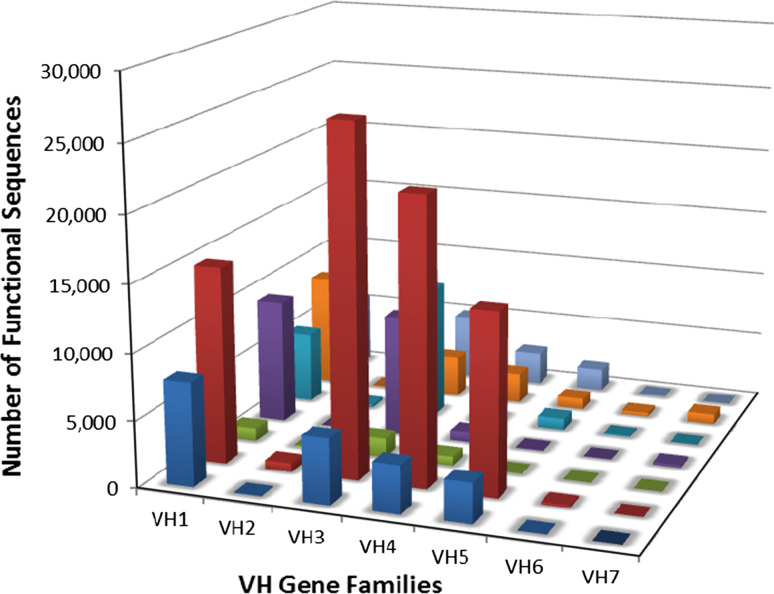
We performed in-depth analysis of Ig VH genes from RNA isolated from ITLs from seven additional lung cancer patients by using Illumina NGS technology with the aim of obtaining evidence for clonal expansion of antigen-stimulated B cells. Since we had also sorted individual B cells from these same seven ITL samples, if evidence for clonal expansion was obtained, we could later identify individual B cell members of these clones by single-cell RT-PCR and sequencing and use only those B cells for recombinant antibody production. Sequence analysis identified a total of 155,901 functional VH gene sequences with individual patient samples containing from 3327 to 77,182 functional sequences (Fig. 4, Supplementary Table 2). Sequences representing all VH gene families were identified and exhibited a distribution typically found in peripheral B cells [30].
Fig. 4.
VH gene sequences in the intratumoral B lymphocytes from 7 NSCLC patients. Depicted are the number of unique, functional VH gene sequences from each of seven individuals (each represented by a different color bar on the z-axis) as determined by deep sequencing of their intratumoral B lymphocyte DNA
We have further determined the clonal relationship of sequences generated by NGS using the Cloanalyst software suite. We examined the top 20 clonal lineages from each of seven individuals analyzed. These top 20 lineages have VH members ranging from 252 members to as many as 2243 members on average. We found these lineages distributed in wide ranges of VH/JH gene families with various heavy chain CDR3 lengths and found no biased use of a particular VH gene family or subfamilies among these seven individuals (Supplementary Fig. 4). These results clearly demonstrated that analysis of the VH repertoire in seven lung cancer specimens revealed no skewing in VH usage.
Discussion
Tumor growth reflects a dynamic, multidimensional relationship between cancer cells and host cells, including stromal, endothelial, and immune cells [36, 37]. Tumors can be infiltrated with many types of immune cells, some that may foster and others that may inhibit tumor growth and progression [38]. GCs, normally found in secondary lymphoid organs such as the spleen and lymph nodes, can also develop in tumors. They are well-defined loci of B cells, T cells, and DCs in which B cells proliferate and undergo differentiation, somatic hypermutation, and affinity maturation [39]. The presence of GCs in tumors is consistent with an in situ immune response to tumor antigens. We aimed to identify the targets of antibodies produced directly by intratumoral B cells in lung cancer. This would allow us to explore the possibility that tumor antigens targeted by these intratumoral B cells may be relevant therapeutic targets or that the antibodies they produce may be diagnostic biomarkers.
Many TAAs have been identified in the serum by techniques such as SEREX [40]. However, serum antibodies have unknown etiology; e.g., they may be preexisting autoantibodies that weakly recognize tumor antigens. Nevertheless, it is clear that some intratumoral B cells express antibodies against tumor antigens. Punt et al. [41] isolated and cultured tumor-infiltrating B cells and tested the IgG from culture supernatants: the IgG from 25 of 36 B cells from tumor cell suspensions showed reactivity with autologous tumor targets. Ten of thirteen IgGs reacted with allogeneic tumor targets of the same histological diagnosis, but no reactivity was found with tumor targets of a different histology. Antibodies produced by B cells within a tumor may be more likely to be directed against relevant targets than those produced by peripheral B cells, particularly if they demonstrate antigenic stimulation, class switching, affinity maturation, and somatic hypermutation. To date, very few targets of antibodies produced by B cells located within a tumor have been directly identified. Kotlan et al. [23] constructed an scFv library from IgG variable chain genes derived from human medullary breast carcinoma-infiltrating B lymphocytes and isolated an scFv that binds to gangliosides, which had been previously identified as TAAs. Also, Yasuda et al. [42] engrafted human lung tumor tissue in SCID mice and identified antibodies to mutated p53 in the mouse serum. These antibodies had presumably originated from B cells in the engrafted tumor tissue.
Germain et al. [20] identified intratumoral B cells that express antibodies that bind well-known tumor antigens, such as the cancer-testis (CT) antigens LAGE-1, MAGE family, and NY-ESO-1, as well as p53. Some of these antibodies bound to more than one antigen, thus demonstrating polyreactivity.
Here, we have used methodology originally developed to study the role of antigen in the progression of chronic lymphocytic leukemia [43] and later modified by researchers in the HIV field to permit the cloning of human antibodies from antigen-secreting B cells [29, 44]. Our study represents the first attempt to adapt this methodology to characterize single B cells isolated from a lung cancer and to identify their stimulating antigens. In this approach, Ig VH and VL genes are amplified by RT-PCR from single sorted B cells and separately inserted adjacent to sequences encoding constant regions in the expression vector pcDNA3.1(+). Transient transfection of the resulting heavy chain and light chain plasmids into human 293T cells yields mature antibody, which is then purified. The purified antibody is used to probe two-dimensional immunoblots of lung tumor cell lysates. The protein is excised from a corresponding stained gel and identified by peptide fingerprint analysis and sequencing by tandem mass spectrometry. Antibody reactivity with purified target protein is then confirmed.
In the current study, we focused on lung cancer where the majority of patients have advanced stage disease at presentation when cure is unlikely. Hence, new therapeutic and diagnostic strategies are clearly needed [45]. This approach provided a unique opportunity to take cues from the host immune response to the tumors and possibly discover new therapeutic or diagnostic targets. From this study, several interesting observations were made:
First, we showed that the ITLs demonstrated class switching as only one of the mAbs tested was originally IgM; the remaining mAbs were IgG1, IgG3, and IgA1 (Supplementary Table 1). The mean heavy chain mutation frequency was 4.00 % ± 1.61 % (range 1.72–6.16 %), a mutation frequency typical of antibodies isolated from vaccine recipients [46, 47]. None of the recovered mAbs were clonally related to each other, and a range of VH genes was used (Supplementary Table 1). In addition, heavy chain complementarity-determining region 3 loops ranged from 9 to 25 amino acids (median 13 amino acids) with no single length predominating. These data are consistent with an antigen-driven maturation, although recruitment of preexisting memory B cells to the tumor cannot be excluded.
Second, all ten antibodies produced from ITLs in our study appear to be polyreactive. Polyreactive antibodies are often low affinity (Kd of 10−3–10−7 M) that react with a variety of totally unrelated antigens [34]. In newborns, 50 % of cord B cells express polyreactive antibodies [48], and in adults, 15–20 % of peripheral B cells express polyreactive antibodies [34]. Interestingly, polyreactive antibodies in the circulation appear to have broad antimicrobial properties [49]. It is intriguing to speculate that polyreactive antibodies in tumors may, in the aggregate, have antitumor properties. However, some investigations have suggested that they may cause tumor progression by forming immune complexes and activating the complement system [6, 8]. Polyreactive antibodies resemble germline antibodies, and thus, a more fruitful approach to identifying monospecific antibodies expressed by B cells in tumors might be to focus on those cells that have undergone affinity maturation and clonal expansion. Our approach would also be feasible using B cells in tumor-draining lymph nodes.
Third, we identified an antibody to TPM4 that was present in both cancer and non-cancer sera, although higher levels of the antibody were associated with the sera of cancer patients. The gene encoding TPM4 is one of four tropomyosin genes that are expressed in most normal tissues (www.genecards.org); however, alternative RNA splicing produces approximately 40 diverse tropomyosin isoforms. The functions of the tropomyosins are to (a) regulate actin–myosin interaction and thus muscle contraction, and (b) regulate cytoskeletal functions such as cell adhesion and motility. The number and diversity of tropomyosin forms contribute to tissue specificity. With regard to cancer, a switch in expression from high to low molecular weight isoforms is correlated with cell transformation and acquisition of metastatic properties [50]. In breast cancer, increased expression of TPM4 is correlated with lymph node metastasis [51].
Since TPM4 is an intracellular protein that interacts with the cytoskeleton, it is not apparent how an antibody was generated against it; possibilities include that it was released from necrotic or apoptotic cells or it was presented on the cell membrane as part of an aberrant gene fusion event. However, we found no evidence for a TPM4 fusion event in the tumor from which the antibody had been cloned. This result and the fact that the antibody is found in non-cancer sera makes it more likely that TPM4 is antigenic upon release from dying cells [52].
These results, while demonstrating the power of recombinant antibody cloning from individual B cells, underscored the fact that choosing B cells randomly from isolated ITLs is labor intensive for large-scale interrogation of the B cell response within tumors. Hence, we employed NGS in an attempt to first identify clonally amplified B cells that could then be used for recombinant antibody cloning and expression. Since we had also sorted individual B cells from these same seven ITL samples, we could identify individual B cell members of these clones by single-cell RT-PCR and sequencing and use only those B cells for recombinant antibody production. In our study, however, deep sequencing revealed a pattern of VH gene families not substantially different from that expected for peripheral B cells. Although this could be reflective of true tumor-infiltrating B cells, it is possible that there is a component of peripheral blood lymphocytes in an ITL sample. It is possible that tumor-draining lymph nodes may provide a more productive starting population of B cells.
The current study resulted in identifying predominately polyreactive antibodies, but an insufficient number of antibodies were characterized to allow conclusions to be drawn regarding the antigen specificity of ITLs. While this study succeeded in demonstrating a distribution of VH gene families equivalent to that found in peripheral blood B cells, we could find no evidence of a dominant clone within the tumor milieu. While further interrogation of individual clones could potentially identify tumor targets, it may be that one aspect of the intratumoral humoral response is a generalized host response to dying cancer cells and the presentation of intracellular antigens; additional studies are needed to confirm this hypothesis as we continue to explore and develop a comprehensive understanding of the host response to cancer. This area of research is essential to harness the full potential of immunotherapy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank Thaddeus C. Gurley and Minyue Wang for expert technical assistance, and gratefully acknowledge the Duke Human Vaccine Institute Flow Cytometry Shared Resource Facility for the isolation of tumor B cells. This work was supported by Grants from the LUNGevity Foundation and the Department of Defense (W81XWH-13-1-0189) to Edward F. Patz, Jr.
Abbreviations
- CDR
Complementarity-determining region
- CT antigens
Cancer-testis antigens
- GC
Germinal center
- ITL
Intratumoral lymphocyte
- MS
Mass spectrometry
- NGS
Next-generation sequencing
- NSCLC
Non-small cell lung cancer
- PVDF
Polyvinylidene fluoride
- ROC analysis
Receiver-operating characteristic analysis
- TPM4
Tropomyosin 4
- VH
Variable heavy chain immunoglobulin domain
- VL
Variable light chain immunoglobulin domain
Compliance with ethical standards
Conflict of interest
None of the authors of this paper have a conflict of interest.
References
- 1.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 2.Heuvers ME, Aerts JG, Cornelissen R, Groen H, Hoogsteden HC, Hegmans JP. Patient-tailored modulation of the immune system may revolutionize future lung cancer treatment. BMC Cancer. 2012;12:580. doi: 10.1186/1471-2407-12-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gunderson AJ, Coussens LM. B cells and their mediators as targets for therapy in solid tumors. Exp Cell Res. 2013;319:1644–1649. doi: 10.1016/j.yexcr.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 5.Silina K, Rulle U, Kalnina Z, Line A. Manipulation of tumour-infiltrating B cells and tertiary lymphoid structures: A novel anti-cancer treatment avenue? Cancer Immunol Immunother CII. 2014;63:643–662. doi: 10.1007/s00262-014-1544-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andreu P, Johansson M, Affara NI, et al. FcRgamma activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell. 2010;17:121–134. doi: 10.1016/j.ccr.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbera-Guillem E, May KF, Jr, Nyhus JK, Nelson MB. Promotion of tumor invasion by cooperation of granulocytes and macrophages activated by anti-tumor antibodies. Neoplasia. 1999;1:453–460. doi: 10.1038/sj.neo.7900054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7:411–423. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Tan TT, Coussens LM. Humoral immunity, inflammation and cancer. Curr Opin Immunol. 2007;19:209–216. doi: 10.1016/j.coi.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Al-Shibli KI, Donnem T, Al-Saad S, Persson M, Bremnes RM, Busund LT. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res. 2008;14:5220–5227. doi: 10.1158/1078-0432.CCR-08-0133. [DOI] [PubMed] [Google Scholar]
- 11.Riemann D, Wenzel K, Schulz T, Hofmann S, Neef H, Lautenschlager C, Langner J. Phenotypic analysis of T lymphocytes isolated from non-small-cell lung cancer. Int Arch Allergy Immunol. 1997;114:38–45. doi: 10.1159/000237640. [DOI] [PubMed] [Google Scholar]
- 12.Pelletier MP, Edwardes MD, Michel RP, Halwani F, Morin JE. Prognostic markers in resectable non-small cell lung cancer: a multivariate analysis. Can J Surg. 2001;44:180–188. [PMC free article] [PubMed] [Google Scholar]
- 13.Mahmoud SM, Lee AH, Paish EC, Macmillan RD, Ellis IO, Green AR. The prognostic significance of B lymphocytes in invasive carcinoma of the breast. Breast Cancer Res Treat. 2012;132:545–553. doi: 10.1007/s10549-011-1620-1. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt M, Bohm D, von Torne C, et al. The humoral immune system has a key prognostic impact in node-negative breast cancer. Cancer Res. 2008;68:5405–5413. doi: 10.1158/0008-5472.CAN-07-5206. [DOI] [PubMed] [Google Scholar]
- 15.Hiraoka N, Ino Y, Yamazaki-Itoh R, Kanai Y, Kosuge T, Shimada K. Intratumoral tertiary lymphoid organ is a favourable prognosticator in patients with pancreatic cancer. Br J Cancer. 2015;112:1782–1790. doi: 10.1038/bjc.2015.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milne K, Kobel M, Kalloger SE, Barnes RO, Gao D, Gilks CB, Watson PH, Nelson BH. Systematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic factors. PLoS ONE. 2009;4:e6412. doi: 10.1371/journal.pone.0006412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zirakzadeh AA, Marits P, Sherif A, Winqvist O. Multiplex B cell characterization in blood, lymph nodes, and tumors from patients with malignancies. J Immunol. 2013;190:5847–5855. doi: 10.4049/jimmunol.1203279. [DOI] [PubMed] [Google Scholar]
- 18.Dieu-Nosjean MC, Antoine M, Danel C, et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol. 2008;26:4410–4417. doi: 10.1200/JCO.2007.15.0284. [DOI] [PubMed] [Google Scholar]
- 19.Gottlin EB, Bentley RC, Campa MJ, Pisetsky DS, Herndon JE, 2nd, Patz EF., Jr The association of intratumoral germinal centers with early-stage non-small cell lung cancer. J Thorac Oncol. 2011;6:1687–1690. doi: 10.1097/JTO.0b013e3182217bec. [DOI] [PubMed] [Google Scholar]
- 20.Germain C, Gnjatic S, Tamzalit F, et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am J Respir Crit Care Med. 2014;189:832–844. doi: 10.1164/rccm.201309-1611OC. [DOI] [PubMed] [Google Scholar]
- 21.Coronella JA, Spier C, Welch M, Trevor KT, Stopeck AT, Villar H, Hersh EM. Antigen-driven oligoclonal expansion of tumor-infiltrating B cells in infiltrating ductal carcinoma of the breast. J Immunol. 2002;169:1829–1836. doi: 10.4049/jimmunol.169.4.1829. [DOI] [PubMed] [Google Scholar]
- 22.Kotlan B, Simsa P, Foldi J, Fridman WH, Glassy M, McKnight M, Teillaud JL. Immunoglobulin repertoire of B lymphocytes infiltrating breast medullary carcinoma. Hum Antibod. 2003;12:113–121. [PubMed] [Google Scholar]
- 23.Kotlan B, Simsa P, Teillaud JL, Fridman WH, Toth J, McKnight M, Glassy MC. Novel ganglioside antigen identified by B cells in human medullary breast carcinomas: the proof of principle concerning the tumor-infiltrating B lymphocytes. J Immunol. 2005;175:2278–2285. doi: 10.4049/jimmunol.175.4.2278. [DOI] [PubMed] [Google Scholar]
- 24.Nzula S, Going JJ, Stott DI. Antigen-driven clonal proliferation, somatic hypermutation, and selection of B lymphocytes infiltrating human ductal breast carcinomas. Cancer Res. 2003;63:3275–3280. [PubMed] [Google Scholar]
- 25.Pages F, Galon J, Dieu-Nosjean MC, Tartour E, Sautes-Fridman C, Fridman WH. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2010;29:1093–1102. doi: 10.1038/onc.2009.416. [DOI] [PubMed] [Google Scholar]
- 26.Willis SN, Mallozzi SS, Rodig SJ, et al. The microenvironment of germ cell tumors harbors a prominent antigen-driven humoral response. J Immunol. 2009;182:3310–3317. doi: 10.4049/jimmunol.0803424. [DOI] [PubMed] [Google Scholar]
- 27.Dieu-Nosjean MC, Goc J, Giraldo NA, Sautes-Fridman C, Fridman WH. Tertiary lymphoid structures in cancer and beyond. Trends Immunol. 2014;35:571–580. doi: 10.1016/j.it.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Tiller T, Meffre E, Yurasov S, Tsuiji M, Nussenzweig MC, Wardemann H. Efficient generation of monoclonal antibodies from single human B cells by single cell RT-PCR and expression vector cloning. J Immunol Methods. 2008;329:112–124. doi: 10.1016/j.jim.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao HX, Levesque MC, Nagel A, et al. High-throughput isolation of immunoglobulin genes from single human B cells and expression as monoclonal antibodies. J Virol Methods. 2009;158:171–179. doi: 10.1016/j.jviromet.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liao HX, Chen X, Munshaw S, et al. Initial antibodies binding to HIV-1 gp41 in acutely infected subjects are polyreactive and highly mutated. J Exp Med. 2011;208:2237–2249. doi: 10.1084/jem.20110363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liao HX, Lynch R, Zhou T, et al. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature. 2013;496:469–476. doi: 10.1038/nature12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kepler TB (2013) Reconstructing a B-cell clonal lineage. I. Statistical inference of unobserved ancestors. F1000Res 2:103. doi:10.12688/f1000research.2-103.v1 eCollection 2013 [DOI] [PMC free article] [PubMed]
- 33.Kepler TB, Munshaw S, Wiehe K, et al. Reconstructing a B-Cell clonal lineage. II. Mutation, selection, and affinity maturation. Front Immunol. 2014;5:170. doi: 10.3389/fimmu.2014.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Notkins AL. Polyreactivity of antibody molecules. Trends Immunol. 2004;25:174–179. doi: 10.1016/j.it.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 35.Mano H. ALKoma: a cancer subtype with a shared target. Cancer Discov. 2012;2:495–502. doi: 10.1158/2159-8290.CD-12-0009. [DOI] [PubMed] [Google Scholar]
- 36.Christensen JD, Colby TV, Patz EF., Jr Correlation of [18F]-2-fluoro-deoxy-d-glucose positron emission tomography standard uptake values with the cellular composition of stage I nonsmall cell lung cancer. Cancer. 2010;116:4095–4102. doi: 10.1002/cncr.25302. [DOI] [PubMed] [Google Scholar]
- 37.Mantovani A, Garlanda C, Allavena P. Molecular pathways and targets in cancer-related inflammation. Ann Med. 2010;42:161–170. doi: 10.3109/07853890903405753. [DOI] [PubMed] [Google Scholar]
- 38.DeNardo DG, Andreu P, Coussens LM. Interactions between lymphocytes and myeloid cells regulate pro- versus anti-tumor immunity. Cancer Metastasis Rev. 2010;29:309–316. doi: 10.1007/s10555-010-9223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.MacLennan IC. Germinal centers. Annu Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 40.Chen Y (2004) Identification of human tumor antigens by serological expression cloning: an online review on SEREX. Cancer Immunity. http://www.cancerimmunity.org/serex. Accessed 2 Dec 2015
- 41.Punt CJ, Barbuto JA, Zhang H, Grimes WJ, Hatch KD, Hersh EM. Anti-tumor antibody produced by human tumor-infiltrating and peripheral blood B lymphocytes. Cancer Immunol Immunother. 1994;38:225–232. doi: 10.1007/BF01533513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yasuda M, Takenoyama M, Obata Y, Sugaya M, So T, Hanagiri T, Sugio K, Yasumoto K. Tumor-infiltrating B lymphocytes as a potential source of identifying tumor antigen in human lung cancer. Cancer Res. 2002;62:1751–1756. [PubMed] [Google Scholar]
- 43.Volkheimer AD, Weinberg JB, Beasley BE, Whitesides JF, Gockerman JP, Moore JO, Kelsoe G, Goodman BK, Levesque MC. Progressive immunoglobulin gene mutations in chronic lymphocytic leukemia: evidence for antigen-driven intraclonal diversification. Blood. 2007;109:1559–1567. doi: 10.1182/blood-2006-05-020644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morris L, Chen X, Alam M, et al. Isolation of a human anti-HIV gp41 membrane proximal region neutralizing antibody by antigen-specific single B cell sorting. PLoS ONE. 2011;6:e23532. doi: 10.1371/journal.pone.0023532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Birring SS, Peake MD. Symptoms and the early diagnosis of lung cancer. Thorax. 2005;60:268–269. doi: 10.1136/thx.2004.032698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moody MA, Yates NL, Amos JD, et al. HIV-1 gp120 vaccine induces affinity maturation in both new and persistent antibody clonal lineages. J Virol. 2012;86:7496–7507. doi: 10.1128/JVI.00426-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moody MA, Zhang R, Walter EB, et al. H3N2 influenza infection elicits more cross-reactive and less clonally expanded anti-hemagglutinin antibodies than influenza vaccination. PLoS ONE. 2011;6:e25797. doi: 10.1371/journal.pone.0025797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen ZJ, Wheeler CJ, Shi W, Wu AJ, Yarboro CH, Gallagher M, Notkins AL. Polyreactive antigen-binding B cells are the predominant cell type in the newborn B cell repertoire. Eur J Immunol. 1998;28:989–994. doi: 10.1002/(SICI)1521-4141(199803)28:03<989::AID-IMMU989>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 49.Zhou ZH, Zhang Y, Hu YF, Wahl LM, Cisar JO, Notkins AL. The broad antibacterial activity of the natural antibody repertoire is due to polyreactive antibodies. Cell Host Microbe. 2007;1:51–61. doi: 10.1016/j.chom.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gunning P, O’Neill G, Hardeman E. Tropomyosin-based regulation of the actin cytoskeleton in time and space. Physiol Rev. 2008;88:1–35. doi: 10.1152/physrev.00001.2007. [DOI] [PubMed] [Google Scholar]
- 51.Li DQ, Wang L, Fei F, et al. Identification of breast cancer metastasis-associated proteins in an isogenic tumor metastasis model using two-dimensional gel electrophoresis and liquid chromatography-ion trap-mass spectrometry. Proteomics. 2006;6:3352–3368. doi: 10.1002/pmic.200500617. [DOI] [PubMed] [Google Scholar]
- 52.Lleo A, Invernizzi P, Gao B, Podda M, Gershwin ME. Definition of human autoimmunity–autoantibodies versus autoimmune disease. Autoimmun Rev. 2010;9:A259–A266. doi: 10.1016/j.autrev.2009.12.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.