Abstract
Macrophage-activating lipopeptide-2 (MALP-2) is a potent inducer of proinflammatory cytokine secretion by macrophages, monocytes, and dendritic cells. MALP-2 was reported to be involved in natural killer (NK) cell activation and ensuing tumor rejection. However, the mechanism of MALP-2-mediated NK cell activation remained unclear. Therefore, we studied the effects of MALP-2 on cultured human NK cells. We found that MALP-2 had no direct effect on NK cells. Instead, MALP-2 acted on monocytes and triggered the release of different molecules such as interleukin (IL)-1β, IL-6, IL-10, IL-12, IL-15, interferon gamma-induced protein (IP-10), and prostaglandin (PG)-E2. Our data show that monocyte-derived IP-10 could significantly induce NK cell cytotoxicity as long as the immunosuppression by PGE2 is specifically inhibited by cyclooxygenase (COX)-2 blockade. In summary, our results show that MALP-2-mediated stimulation of monocytes results in the production of several mediators which, depending on the prevailing conditions, affect the activity of NK cells in various ways. Hence, MALP-2 administration with concurrent blocking of COX-2 can be considered as a promising approach in MALP-2-based adjuvant tumor therapies.
Keywords: MALP-2, Monocytes, NK cell activation, IP-10, COX-2 upregulation, PGE2
Introduction
Mycoplasmas have been implicated in a wide variety of human diseases ranging from pneumonia to urethritis (reviewed in [1–4]). Persistent mycoplasma infection can elicit chronic inflammatory cytokine secretion in the infected milieu [5, 6]. This can contribute to the propagation of life-threatening secondary diseases such as acquired immunodeficiency syndrome (AIDS) and cancer [7, 8]. Although chronic mycoplasma-triggered inflammation proves to be detrimental to the host, the immune-activating property of mycoplasma-derived lipopeptides can principally be exploited as adjuvants for tumor immunotherapy [9–11]. MALP-2 is one of such lipopeptides. It was initially derived from the membrane of Mycoplasma fermentans [12]. However, it is possible to chemically synthesize the endotoxin-free MALP-2 peptide without any loss of its macrophage-activating property [12, 13]. MALP-2 acts as an agonist for Toll-like receptor (TLR)-2 and TLR-6 [14] which further activate the nuclear factor ‘kappa-light-chain-enhancer’ of activated B cells (NF-κB) signaling cascade [15]. It is a potent inducer of proinflammatory cytokine secretion by macrophages, monocytes, and dendritic cells [12, 16–18].
In a mouse model of pancreatic carcinoma, MALP-2 was found to suppress tumor growth, possibly by stimulation of T cell and NK cell activities [9]. The efficacy of MALP-2 as an immunotherapeutic adjuvant has also been documented in a phase I/II clinical trial in patients with pancreatic carcinoma [11]. This study demonstrated that in patients with incompletely resectable pancreatic carcinomas, postoperative NK cell activity was significantly higher in MALP-2-treated patients than in controls. In a rat metastasis model, MALP-2 treatment led to a significant increase in monocyte and NK cell accumulation at metastatic sites [10]. These reports suggest that NK cell activation could be an essential element of MALP-2-induced tumor suppression. NK cells play an important role in cancer surveillance; they kill malignant metastatic cells and produce several immunomodulatory cytokines such as interferon (IFN)-γ and tumor necrosis factor (TNF)-α [19]. However, there is little information about how MALP-2 affects NK cell functions.
Although NK cells are known to express TLR-2 on their surface (reviewed in [20]), a recent study showed that MALP-2 fails to stimulate murine NK cell cytotoxicity [21]. In the present study, we also examined whether MALP-2 can directly activate human NK cell cytotoxicity and found that NK cell cytotoxicity was unaltered by MALP-2. In murine models, TLR-2 agonists can enhance NK cell activity indirectly by activating dendritic cells [22, 23]. Moreover, the effect of MALP-2 on monocytes/macrophages has been studied in some detail [12, 16, 24]. Several molecules are induced in monocytes/macrophages during MALP-2 stimulation, and COX-2 is one of these molecules [24–26]. COX-2 is well known as the rate-limiting enzyme for NK cell suppressive PGE2 production [27–29]. However, in MALP-2-stimulated monocytes, several NK cell-activating molecules are also released simultaneously. Therefore, we further investigated whether the reported MALP-2-associated human NK cell activation [9–11] was mediated indirectly by activated monocytes.
Materials and methods
Cells
The study was approved by the local ethical committee of Hannover Medical School. Heparinized blood samples were collected from healthy consenting volunteers at the Institute for Transfusion Medicine, Hannover Medical School. The blood samples were diluted 1:2 with phosphate-buffered saline (PBS) and separated by Ficoll–Hypaque centrifugation (20 min/1000g) to collect peripheral blood mononuclear cells (PBMCs) as previously described [30]. NK cells were isolated from PBMCs by negative enrichment using an NK cell isolation kit (BD Biosciences) according to the manufacturer’s protocol. Monocytes were enriched using their adherence capability to plastic surfaces. Briefly, PBMC were incubated in cell culture flasks for 45 min (37 °C, 5 % CO2) in horizontal position. After removing the supernatant, monocytes were detached from the flask surface by Accutase treatment for 15 min. Fluorescence-activated cell sorting (FACS) analysis revealed the purity of isolated NK cells and monocytes to be ≥89 and ≥85 %, respectively. For the purpose of coculture, NK cells (CD56+CD3−, purity ≥98 %) were sorted by FACS using fluorochrome-conjugated anti-CD56 and anti-CD3 antibodies. Monocytes (CD14+, purity ≥92 %) were also sorted from the same donor by FACS using their forward scatter (FSC) and side scatter (SSC) profiles. The ratio of NK cells to monocytes in the PBMCs were used for the coculture of sorted NK cells and sorted monocytes.
Cell culture
Cells were suspended in Roswell Park Memorial Institute (RPMI) 1640 supplemented with 10 % fetal calf serum (FCS), penicillin/streptomycin (100 U/ml), sodium pyruvate (1 mM), and glutamine (2 mM). PBMCs, monocytes, or NK cells were stimulated with 500 pg/ml MALP-2 or solvent control and incubated for 16 h (37 °C, 5 % CO2) before being harvested for further analysis. Lipopolysaccharide (LPS, 100 ng/ml) was used as positive stimulation control. COX-2 inhibitors (NS-398 or indomethacin; Cayman Chemicals) were added to cell cultures at a final concentration of 5 and 20 µM, respectively. To analyze the soluble factors released by MALP-2 activated cells, supernatants from stimulated and unstimulated (control) cell cultures were collected after 16 h of stimulation and stored at −20 °C. For detection of intracellular cytokines by FACS, brefeldin A (2.5 mM) was added to the cultures after 1 h of stimulation to prevent the secretion of the induced cytokines.
MALP-2 preparation
MALP-2 was synthesized as described previously [13, 31] and kept as a stock solution of 1 mg/ml in 33 % isopropanol/water at 4 °C. For in vitro use, stock solutions were first diluted 1:100 with pure FCS Superior (Biochrome, Berlin, Germany) to prevent adhesion to plastic surfaces and were then further diluted in several steps with culture medium. As solvent control, 33 % isopropanol water-diluted the same way was used and had no effects on the cell cultures.
Antibodies
The following antibodies were used for analysis of surface and intracellular molecules: anti-CD3-PerCP, anti-CD14-APC/PerCP, anti-CD20-PE, anti-CD56-FITC, anti-IL-12p70-BV421/PE, anti-TNF-α-FITC/PE (BD PharMingen), anti-CD3-FITC, anti-CD56-APC, anti-CD69-PE-Cy7, anti-CD107a-PE, anti-IFN-γ-FITC/PE, anti-IL-1β-FITC, anti-IP-10-PE, anti-TNF-α-BV421/PE-Cy7 (BioLegend), anti-COX-2-FITC (Cayman Chemicals), anti-IL-6-APC (ImmunoTools), and anti-IL-15-FITC (R&D Systems). For all flow cytometry experiments, appropriate isotype control antibodies were utilized. Surface and intracellular staining was performed as described before [30]. All samples were measured using FACSCalibur or FACSCantoII. Neutralizing antibodies against IP-10 and macrophage inflammatory protein (MIP)-1α were obtained from PeproTech Inc.
51Cr release assay
Standard 4-h 51Cr release cytotoxicity assays were performed using PBMC and NK cells as effectors against 51Cr-labelled K562 target cells as described elsewhere [32]. Different effector-to-target cell (E/T) ratios were used (60:1, 30:1, 15:1, and 7.5:1 for PBMCs; 10:1, 5:1, 2.5:1, and 1.25:1 for NK cells). Following the 4-h incubation, target cell lysis was measured by 51Cr released into the supernatant using a gamma counter (PerkinElmer, Rodgau, Germany). Specific lysis was calculated as follows: [(experimental release − spontaneous release)/(maximum release − spontaneous release)] × 100. Maximum and spontaneous releases were determined by lysing target cells in 1 % Triton X-100 and incubating targets without effectors, respectively. Lytic units (LU20/107 cells) were calculated in reference to Bryant et al. [33].
CD107a degranulation assay
To assess CD107a degranulation in NK cells, purified NK cells were cocultured with purified monocytes for 16 h in the presence or absence of MALP-2 (500 pg/ml) or NS-398 (5 µM) prior to the assay. NK cells were then washed and coincubated with the K562 target cells at an E/T ratio of 10:1 in the presence of anti-CD107a antibody. Monensin (BD Biosciences) was added after 1 h at a final concentration of 6 μg/ml. Following the total 4 h of coincubation, the cells were stained for surface markers, washed, and analyzed for the surface expression of CD107a by flow cytometry.
Detection of intracellular proteins
For detection of intracellular cytokines in CD14+ monocytes, PBMCs, or enriched monocytes (purity ≥89 %) were cultured with or without MALP-2 (5 pg/ml, 500 pg/ml or 50 ng/ml) or LPS (100 ng/ml). Brefeldin A (2.5 mM) was added to the cultures after 1 h of stimulation to prevent the secretion of the induced cytokines. After final 16 h of incubation, intracellular expressions of different cytokines in CD14+ monocytes were analyzed by flow cytometry as described earlier [29]. For detection of intracellular COX-2 in PBMCs, cells were cultured with or without MALP-2 (500 pg/ml). Brefeldin A (2.5 mM) was added to the cultures after 1 h of stimulation. After final 16 h of incubation, intracellular expression of COX-2 was analyzed in CD3+ T cells, CD56+CD3− NK cells, CD20+ B cells, and CD14+ monocytes by flow cytometry as described earlier [29]. Furthermore, to assess the IFN-γ and TNF-α in NK cells, NK cells were cocultured with monocytes for 16 h in the presence or absence of MALP-2 (500 pg/ml) or NS-398 (5 µM). Cells were then incubated with brefeldin A (Sigma) for 3 h, and intracellular expressions of IFN-γ and TNF-α were analyzed by flow cytometry as described earlier [19].
Cytokine array
Cell supernatants were collected from PBMC and monocyte cultures as described. Cytokines present in pooled supernatants (PBMC: n = 14; monocytes: n = 5) were detected using an inflammation antibody array 3 according to manufacturer’s protocol (RayBiotech, Norcross GA). Briefly, samples were applied to an antibody-coated membrane provided with the kit. Biotinylated antibodies were added, followed by horseradish peroxidase (HRP)-conjugated streptavidin. After treatment with detecting agents, the membrane was placed on a BioMax MR film (Kodak, Rochester, USA) and exposed for 10 min. Dots on the developed film were analyzed for density and diameter using the free software ImageJ 1.46 (http://imagej.nih.gov/ij/). After subtracting the background (determined from negative controls), mean values of the spotted duplicates were calculated and normalized against mean values of internal positive controls. Results were presented as percentages of maximum density.
PGE2 enzyme-linked immunosorbent assay (ELISA)
We determined PGE2 content in the supernatants using a commercially available kit (EnzoLifeScience, Lörrach, Germany) according to the instructions provided by the manufacturer. PGE2 concentrations were calculated by comparison with known PGE2 standards using a freely available five-parameter logistic curve-fitting program (www.readerfit.com).
Statistical analysis
GraphPad Prism v5.0 software (GraphPad Inc.) was utilized for statistical analysis. Paired t tests (to compare two groups) or one-way analysis of variance (ANOVA) followed by Bonferroni posttests (to compare three or more groups) were applied to evaluate the statistical significance of the observed differences. Asterisks indicate a statistically significant difference (*p < 0.05; **p < 0.01 and ***p < 0.001), and the plotted data represent mean ± standard error of mean (SEM).
Results
Stimulation of monocytes by MALP-2
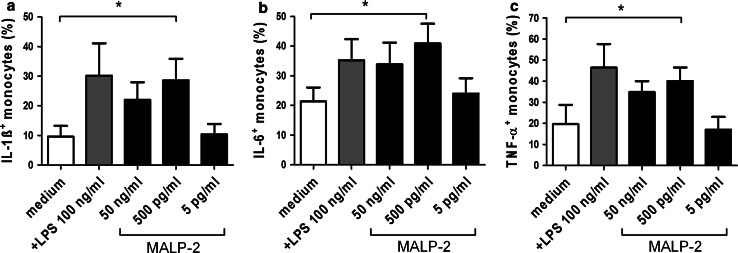
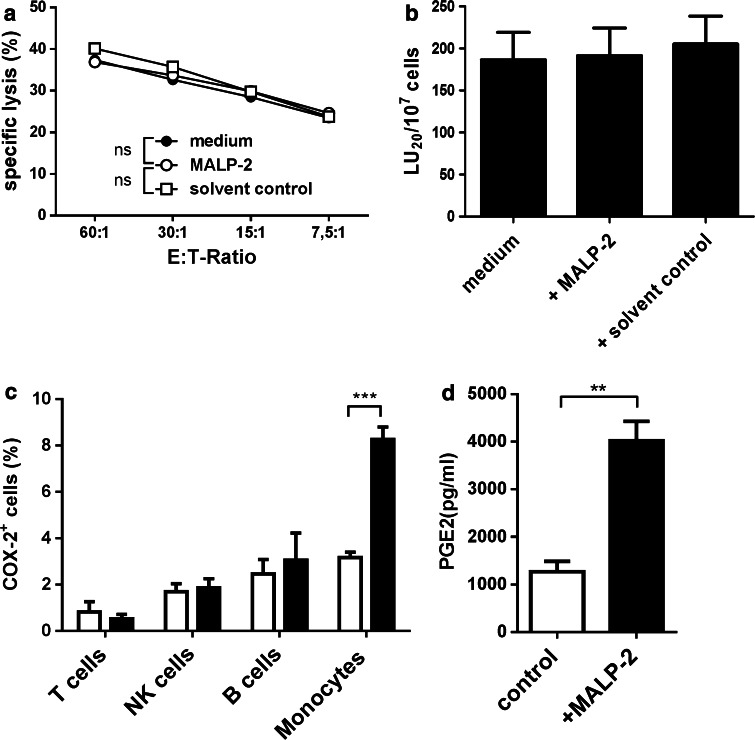
Monocytes are known to release an array of inflammatory cytokines such as IL-1β, IL-6, and TNF-α upon stimulation with MALP-2 [12]. To determine the concentration of MALP-2 leading to maximal production of cytokines, we stimulated monocytes with different concentrations of MALP-2. LPS stimulation was used as positive control. After 16 h of stimulation, cytokine production was assessed by intracellular staining followed by FACS analysis. In a dose-dependent manner, MALP-2 treatment led to significant increase in the production of IL-1β, IL-6, and TNF-α (Fig. 1a–c). The maximal effect of MALP-2 was achieved at a concentration of 500 pg/ml. Thus, this concentration was used in all subsequent experiments. To test whether MALP-2 affects NK cell cytotoxicity, we cultured PBMC in the presence or absence of MALP-2 for 16 h and performed chromium release assay with K562 target cells. Under normal condition, we found no difference in the lytic potential of NK cells in PBMCs stimulated with MALP-2 compared with unstimulated controls (Fig. 2a, b).
Fig. 1.
MALP-2 induced cytokine production in monocytes. To determine the most effective concentration of MALP-2 for cytokine induction, enriched monocytes were stimulated with various concentrations of MALP-2 for 16 h. LPS (100 ng/ml) stimulation was used as positive control. Intracellular expressions of IL-1β, IL-6, and TNF-α were analyzed by flow cytometry, and the percentages of IL-1β+ (a), IL-6+ (b), or TNF-α+ (c) monocytes were shown. Bars display mean ± SEM (*p < 0.05, 1 way ANOVA, n = 5)
Fig. 2.
Effect of MALP-2 on the cytotoxicity of NK cells and PGE2 production by monocytes. PBMCs were incubated for 16 h in media in the presence or absence of MALP-2 or solvent control. a Specific lysis was determined by chromium release assays using PBMC as effectors (E) and K562 targets (T) at different E/T ratios (n = 8/group). b The results of the cytotoxicity assays were calculated and displayed as lytic units (LU20/107 cells) (n = 8/group). c Following a 16-h stimulation with MALP-2, intracellular expression of cyclooxygenase (COX-)2 was analyzed in CD3+ T cells, CD56+CD3− NK cells, CD20+ B cells, and CD14+ monocytes by flow cytometry (***p < 0.001, 2 way ANOVA, n = 3/group). d Synthesis of PGE2 by PBMC was quantified by ELISA. Open bars represent controls and solid bars MALP-2-stimulated samples (**p < 0.01, paired t test, n = 3/groups). Bars display mean ± SEM
COX-2 expression in MALP-2-stimulated PBMCs
We hypothesized that MALP-2 stimulation in our system could be leading to PGE2 production by a subpopulation of the PBMCs, and this masks any potential effect of MALP-2 on NK cells. To verify our hypothesis, we examined whether MALP-2 induced COX-2 expression in PBMCs. We found that MALP-2 stimulation significantly upregulated COX-2 expression in CD14+ monocytes but not in T, B, and NK cells (Fig. 2c). Next, we tested whether the observed COX-2 upregulation actually leads to increased PGE2 release by MALP-2-stimulated monocytes. We detected significantly higher PGE2 production by stimulated monocytes compared with unstimulated controls. The amount of PGE2 in the supernatants of stimulated monocytes was about three times higher than that in unstimulated controls (Fig. 2d).
MALP-2-stimulated NK cell activation under COX-2 inhibition
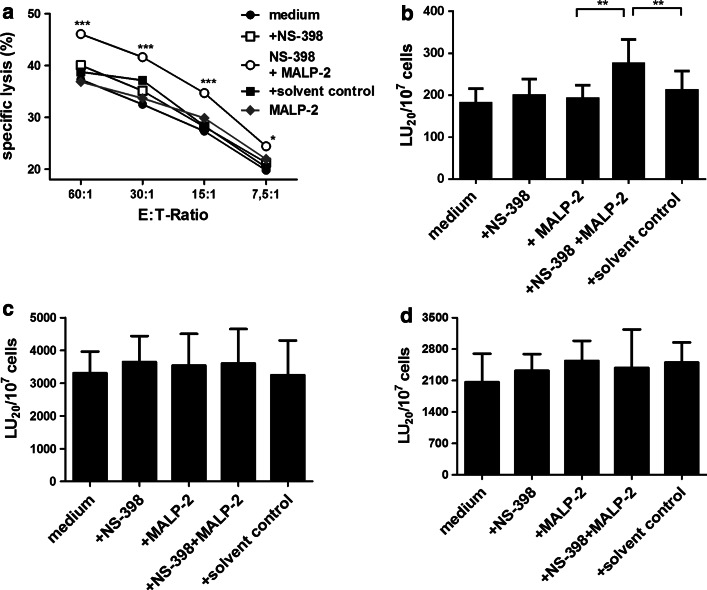
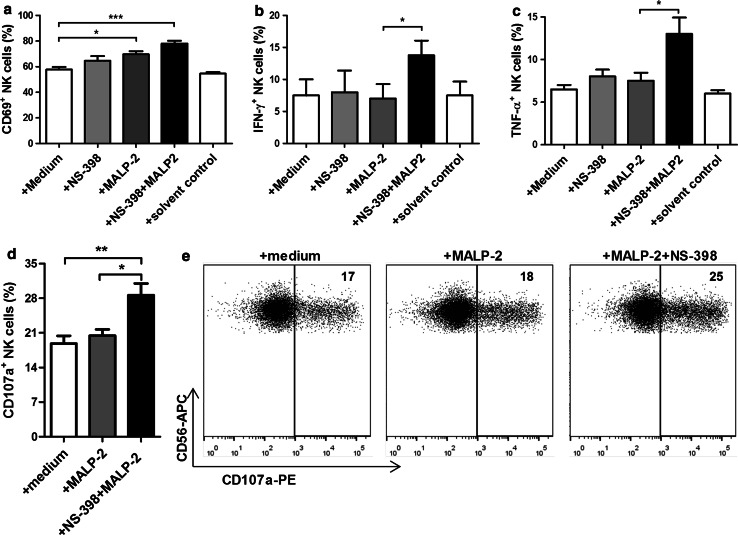
To eliminate the effect of PGE2, we stimulated PBMCs with MALP-2 in the presence of NS-398, a pharmacological inhibitor of COX-2. Under COX-2 blockade, MALP-2 stimulation enhanced the cytotoxicity of NK cells present in the stimulated PBMCs at all E/T ratios tested. NS-398 alone had no direct effect on NK cell cytotoxicity (Fig. 3a). The data from the cytotoxicity assays were further used to calculate the lytic units. We found that PBMCs treated with MALP-2 in combination with NS-398 led to significantly higher lytic units than PBMC treated with NS-398 alone or the solvent control (Fig. 3b). We further investigated whether MALP-2-mediated increase in cytotoxicity was caused by a direct effect of MALP-2 on NK cells under COX-2 inhibition. We stimulated NK cell line (KHYG-1) or purified NK cells with MALP-2 in the presence or absence of NS-398 for 16 h. Following the stimulation, chromium release assay was performed. MALP-2 produced no change in cytolytic activity of enriched NK cells (Fig. 3c) or KHYG-1 cells (Fig. 3d). In the coculture of purified NK cells and monocytes, MALP-2-stimulated monocytes also upregulated CD69 expression on NK cells under PGE2 inhibition (Fig. 4a). These NK cells also produced significantly higher IFN-γ (Fig. 4b) and TNF-α (Fig. 4c). They also showed significantly higher CD107a degranulation activity after contact with K562 target cells (Fig. 4d, e).
Fig. 3.
Effect of MALP-2 on NK cytotoxicity in the presence of COX-2 blocking. PBMCs, enriched NK cells or KHYG-1 cell line were cultured for 16 h in media (controls) in presence or absence of NS-398, MALP-2 or solvent control. Cytotoxicity of PBMC (a, b n = 12/group), enriched NK cells (c n = 5/group) and KHYG-1 cell line (d n = 4/group) against K562 cell line was measured by chromium release assay. a Means of specific lysis determined by chromium release assay are shown. b–d The bars representing lytic units (LU20/107 cells) calculated from the results of the cytotoxicity assays are shown. Bar graphs represent the mean ± SEM of calculated lytic units per 107 effector cells (*p < 0.05; **p < 0.01; ***p < 0.001, 1 way ANOVA)
Fig. 4.
Effect of MALP-2 on NK activity in the presence of COX-2 blocking. Fresh FACS-sorted NK cells (purity ≥98 %) were cocultured with sorted monocytes (purity ≥92 %) for 16 h in the presence or absence of NS-398, MALP-2, or solvent control. a NK cells were stained for CD69 expression, and the percentage of CD69+ cells is shown (n = 6/group). b NK cells were stained for intracellular IFN-γ expression, and the percentage of IFN-γ+ cells is shown (n = 4/group). c NK cells were stained for intracellular TNF-α expression, and the percentage of TNF-α+ cells is shown (n = 4/group). d CD107a degranulation assay was performed using K562 targets, and the percentage of CD107a+ NK cells is shown (n = 5/group). e CD107a degranulation assay was performed using K562 target cells, and dot plots are shown. Data are representative of five independent experiments. Values represent the percentages of CD107a+ NK cells. a–d Data are shown as bar graphs representing mean ± SEM (*p < 0.05; **p < 0.01; ***p < 0.001, 1 way ANOVA)
NK cell-activating cytokines released by PBMCs upon MALP-2 stimulation
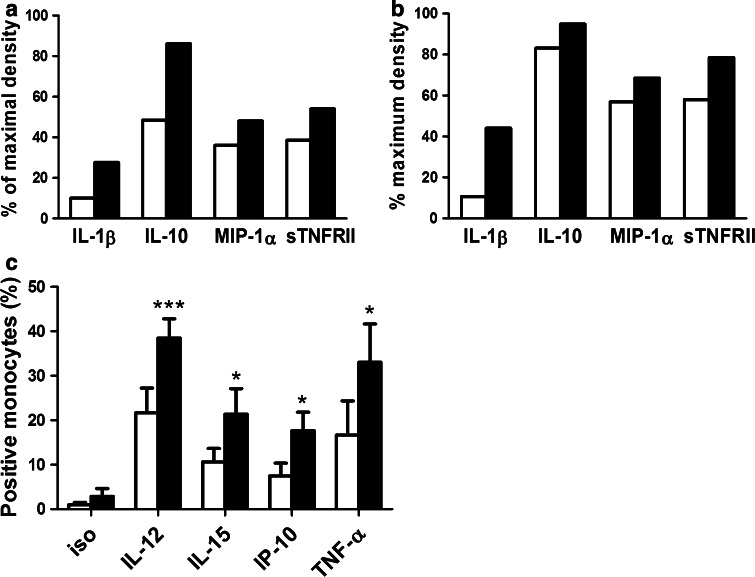
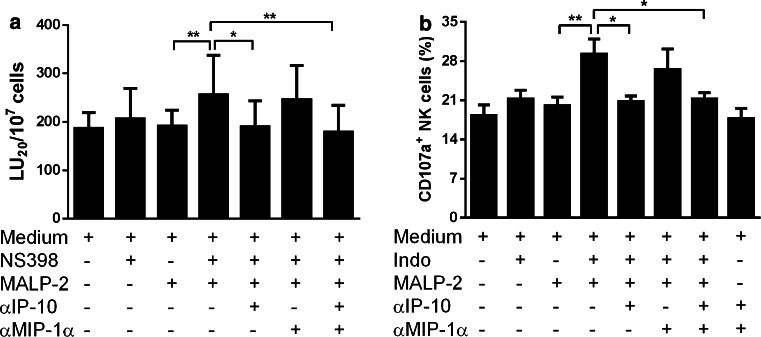
Our results and those of others had already demonstrated that MALP-2-stimulated monocytes, present in PBMCs, produce TNF-α, IL-1β, and IL-6 [12]. To further identify MALP-2-stimulated cytokines, we performed a cytokine array analysis. We found that MALP-2 stimulation resulted in considerable induction of IL-1β, IL-10, MIP-1α, and soluble TNF receptor II (sTNFRII) in both PBMC (Fig. 5a) and monocyte (Fig. 5b) supernatants. Using FACS analysis, we found that the intracellular production of IL-12, IL-15, IP-10, and TNF-α was significantly induced by MALP-2 in monocytes (Fig. 5c). MIP-1α and IP-10 are known to increase NK cell cytotoxicity [34]. We checked whether these cytokines played any role in activating NK cells under our culture conditions. In the presence of COX-2 blockade, we stimulated PBMCs with MALP-2. As mentioned above, this led to an increase in NK cell cytotoxicity. However, no such increase was seen when IP-10 was neutralized using a blocking antibody (Fig. 6a). Our coculture experiments also showed the ability of MALP-2-stimulated monocytes to enhance CD107a degranulation of NK cells which is also mediated by IP-10 (Fig. 6b). Interestingly, MIP-1α-neutralizing antibodies had no such effect (Fig. 6a, b).
Fig. 5.
Detailed analyses of soluble factors produced by MALP-2-activated PBMCs and monocytes. PBMCs or enriched monocytes (purity ≥89 %) were cultured for 16 h with or without MALP-2. a Supernatants from PBMC cultures were pooled (n = 14/group) and analyzed using a cytokine array, and the extent of MIP-1α, IL-10, IL-1β, and sTNFRII released by unstimulated and stimulated PBMCs is shown. b Supernatants from enriched monocyte cultures were pooled (n = 5/group) and analyzed using a cytokine array, and the extent of MIP-1α, IL-10, IL-1β, and sTNFRII released by unstimulated and stimulated monocytes is shown. a, b The bar graphs (display means) represent the amounts of cytokines released relative to the positive controls embedded in the array. c MALP-2-stimulated and MALP-2-unstimulated enriched monocytes (purity ≥89 %) were collected. Intracellular IL-12, IL-15, IP-10, and TNF-α were analyzed using FACS. Open bars represent controls, solid bars are MALP-2-stimulated samples. Bars represent mean ± SEM (*p < 0.05; ***p < 0.001, 2 way ANOVA, n = 7/group)
Fig. 6.
Role of IP-10 and MIP-1α in MALP-2-mediated increase in NK cytotoxicity. a PBMCs were incubated for 16 h with or without MALP-2, NS-398, or neutralizing antibodies against IP-10 and/or MIP-1α as indicated. Lytic units (LU) were calculated based on cytotoxicity values assessed by standard chromium release assay using K562 targets. Bar graphs represent the mean ± SEM of calculated lytic units per 107 effector cells (*p < 0.05; **p < 0.01, 1 way ANOVA, n = 8/group). b Fresh FACS-sorted NK cells (purity ≥98 %) were cocultured with sorted monocytes (purity ≥92 %) for 16 h in the presence or absence of indomethacin (Indo), MALP-2, or neutralizing antibodies against IP-10 and/or MIP-1α as indicated. CD107a degranulation assay was performed using K562 targets, and the percentage of CD107a+ cells is shown. Bars display mean ± SEM (*p < 0.05; **p < 0.01, 1 way ANOVA, n = 8/group)
Discussion
Different species of mycoplasmas have been implicated in a wide variety of human diseases ranging from pneumonia to urethritis (reviewed in [1–4]). Persistent mycoplasma infection elicits chronic inflammatory cytokine secretion, which proves to be detrimental to the host [5–8]. Nevertheless, the immune-activating property of mycoplasma-derived lipopeptides can principally be exploited as adjuvants for tumor immunotherapy [9–11]. MALP-2 is one of such lipopeptides initially derived from the membrane of M. fermentans [12]. Currently, the chemically synthesized endotoxin-free MALP-2 peptide is also available with its macrophage-activating property [12, 13]. MALP-2 acts as an agonist for TLR-2 and TLR-6 [14] which further activates the NF-κB signaling cascade [15]. It is a potent inducer of proinflammatory cytokine secretion by macrophages, monocytes, and dendritic cells [12, 16–18]. However, there is little information about how MALP-2 affects NK cell functions. In this study, we examined whether MALP-2 can directly activate human NK cell cytotoxicity.
Stimulation of NK cell line (KHYG-1) or purified NK cells with MALP-2 produced no change in cytolytic activity of both cells, suggesting that MALP-2 has no direct effect on NK cells. We also found no difference in the lytic potential of NK cells in PBMCs stimulated with MALP-2 compared with unstimulated controls under normal condition. This is apparently contradictory to the reports that suggest that MALP-2 can stimulate NK cell activity which helps to reduce the tumor burden [9–11]. We found that MALP-2 stimulation significantly upregulated COX-2 expression in CD14+ monocytes but not in T, B, and NK cells. This preferential responsiveness toward MALP-2 could be caused by the high expression of TLR-2 on monocytes [35]. It is known that MALP-2 can induce COX-2 expression in monocytes with ensuing PGE2 production [24, 25]. PGE2 is a potent suppressor of NK cell activity [28, 29]. Therefore, we speculated whether the inhibitory effect of PGE2 in our experiment masked the activating effect of MALP-2 on NK cells. Inhibition of PGE2 through COX-2 blocker in our study revealed the indirect activating effect of MALP-2 on NK cells. Our coculture experiments where we applied COX-2 blocker also revealed that MALP-2-stimulated monocytes upregulated CD69 expression on NK cells, indicating enhanced activation of NK cells. These NK cells also showed increased cytokine production and CD107a degranulation potentials. Therefore, the indirect activating effect of MALP-2 on NK cells is not only enabling the cells to acquire enhanced cytotoxicity but also stimulating the cells to produce important cytokines such as IFN-γ and TNF-α for tumor surveillance.
We found that MALP-2 stimulation resulted in considerable induction of IL-1β, IL-10, MIP-1α, and sTNFRII in both PBMC and monocyte supernatants. Of particular interest was the production of NK cell-activating molecules such as IL-12, IL-15, IP-10 and TNF-α. Using FACS analysis, we found that the intracellular production of all four molecules was significantly induced by MALP-2 in monocytes. Hence, these findings could clearly show that MALP-2 can indirectly activate NK cells by one or several of the proinflammatory cytokines produced in mixed cultures by PBMCs. In our experiments, IP-10 was found to be the most important factor that could enhance NK cell cytotoxicity. MIP-1α and IP-10 are known to induce NK cell chemotaxis with a concomitant increase in NK cell cytotoxicity [34]. However, blocking of MIP-1α did not influence the cytolytic potential of NK cells in our experiments.
In summary, TLR-2 ligands such as MALP-2 are being increasingly considered as effective adjuvants for cancer immunotherapy. Designing of new adjuvants based on the structure of MALP-2 is also under way [11, 36]. Our data revealed that NK cells are indirectly affected by MALP-2. Upon MALP-2 stimulation, monocytes produce several proinflammatory (IL-1β, IL-6, IP-10, MIP-1α, and TNF-α) as well as immunosuppressive factors (PGE2 and IL-10). The net effect of these factors on NK cells depends on the balance between the activating and inhibiting mediators. In our experiments, IP-10 was found to enhance NK cell cytotoxicity and PGE2, being the mediator which suppresses NK activity. The latter is in keeping with in vivo findings [37]. Therefore, our results underscore the importance of understanding the mechanisms behind MALP-2-mediated effects and the intricate balance between activation and inhibition of the immune system. Blocking of COX-2 simultaneously with the administration of MALP-2 could be considered in future adjuvant-mediated cancer immunotherapy protocols. Indeed, the beneficial effects of COX inhibitors on tumor development are currently investigated in clinical settings [38, 39].
As a concluding remark, the importance of tumor-associated macrophages for tumor progression and outcome of cancer therapies have been outlined in recent reviews [40, 41]. Tumor-associated macrophages have been categorized in two subpopulations: the one of an inflammatory type involved in antitumor activity (M1) and the other as producer of angiogenic and other factors which promote tumor progression (M2). PGE2 is one such products of stimulated macrophages that is associated with poor prognosis [26]. High (COX)-2 expression was found in areas of tumor tissue rich in macrophages and was again associated with a high Gleason score in prostate cancer [42]. Our experimental ex vivo system using PBMCs as source of monocytes/macrophages and NK cells and MALP-2 as an inflammatory stimulus is of necessity, a poor approximation of what occurs in the microenvironment of tumors, ignoring the influence of the individual cancer cells as well as any influence of stroma cells and angiogenesis. However, the Janus character of macrophages becomes apparent when stimulated with MALP-2. On the one hand, they generate growth factors which can induce angiogenesis, as shown in previous studies [43, 44]. Our current study also showed that monocytes can further produce PGE2 thus resembling M2 type macrophages. When PGE2 production is inhibited, they liberate typical inflammatory cytokines and hence can act as M1 type macrophages giving rise to several lymphokines which give rise to IFN-γ and TNF-α-positive NK cells and finally inducing cytotoxicity in NK cells. Typically, actinic keratosis, a precancerosis caused among other factors by UV light which in turn leads to a state of chronic inflammation, is currently successfully treated by diclofenac, a COX inhibitor [45]. Our data and the hypothesis outlined above may give a likely explanation for the underlying mechanism.
Acknowledgments
The authors would like to extend their sincere appreciation to Sabine Buyny for her help with the chromium release assays. This work was supported by grants from the Deutsche Forschungsgemeinschaft (DFG): SFB738/A5, Hannover Biomedical Research School (HBRS), REBIRTH Cluster of Excellence, Niedersächsische Krebsgesellschaft e.V. The funding agencies had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors have no financial, personal, or professional conflict of interest to declare.
Abbreviations
- AIDS
Acquired immunodeficiency syndrome
- ANOVA
Analysis of variance
- COX
Cyclooxygenase
- ELISA
Enzyme-linked immunosorbent assay
- E:T
Effector to target
- FSC
Forward scatter
- HRP
Horseradish peroxidase
- IFN
Interferon
- IL
Interleukin
- IP
Interferon-γ-induced protein
- LU
Lytic units
- MALP
Macrophage-activating lipopeptide
- MIP
Macrophage inflammatory protein
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NK
Natural killer
- PBMCs
Peripheral blood mononuclear cells
- PBS
Phosphate-buffered saline
- PG
Prostaglandin
- RPMI
Roswell Park Memorial Institute
- SEM
Standard error of the mean
- SSC
Side scatter
- sTNFR
Soluble tumor necrosis factor receptor
- TLR
Toll-like receptor
- TNF
Tumor necrosis factor
- TNFR
Tumor necrosis factor receptor
Footnotes
Christina Müller and Dejene M. Tufa have contributed equally to this work.
References
- 1.Embree J, Embil J. Mycoplasmas in diseases of humans. Can Med Assoc J. 1980;123:105–111. [PMC free article] [PubMed] [Google Scholar]
- 2.Cunha CB. The first atypical pneumonia: the history of the discovery of Mycoplasma pneumoniae . Infect Dis Clin North Am. 2010;24:1–5. doi: 10.1016/j.idc.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Viscardi R, Hasday J. Role of Ureaplasma species in neonatal chronic lung disease: epidemiologic and experimental evidence. Pediatr Res. 2009;65:84R–90R. doi: 10.1203/PDR.0b013e31819dc2f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartmann M. Genital mycoplasmas. J Dtsch Dermatol Ges. 2009;7:371–377. doi: 10.1111/j.1610-0387.2008.06965.x. [DOI] [PubMed] [Google Scholar]
- 5.McGowin CL, Annan RS, Quayle AJ, et al. Persistent Mycoplasma genitalium infection of human endocervical epithelial cells elicits chronic inflammatory cytokine secretion. Infect Immun. 2012;80:3842–3849. doi: 10.1128/IAI.00819-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang J, Hooper W. Regulation of proinflammatory cytokines in human lung epithelial cells infected with Mycoplasma pneumoniae . Infect Immun. 2002;70:3649–3655. doi: 10.1128/IAI.70.7.3649-3655.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang S, Li J, Wu J. Mycoplasma infections and different human carcinomas. World J Gastroenterol. 2001;7:266–269. doi: 10.3748/wjg.v7.i2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manhart LE, Mostad SB, Baeten JM, et al. High Mycoplasma genitalium organism burden is associated with shedding of HIV-1 DNA from the cervix. J Infect Dis. 2008;197:733–736. doi: 10.1086/526501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schneider C. Tumour suppression induced by the macrophage activating lipopeptide MALP-2 in an ultrasound guided pancreatic carcinoma mouse model. Gut. 2004;53:355–361. doi: 10.1136/gut.2003.026005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shingu K, Kruschinski C, Lührmann A, et al. Intratracheal macrophage-activating lipopeptide-2 reduces metastasis in the rat lung. Am J Respir Cell Mol Biol. 2003;28:316–321. doi: 10.1165/rcmb.2002-0106OC. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt J, Welsch T, Jäger D, et al. Intratumoural injection of the toll-like receptor-2/6 agonist “macrophage-activating lipopeptide-2” in patients with pancreatic carcinoma: a phase I/II trial. Br J Cancer. 2007;97:598–604. doi: 10.1038/sj.bjc.6603903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaufmann A, Mühlradt PF, Gemsa D, Sprenger H. Induction of cytokines and chemokines in human monocytes by Mycoplasma fermentans-derived lipoprotein MALP-2. Infect Immun. 1999;67:6303–6308. doi: 10.1128/iai.67.12.6303-6308.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mühlradt PF, Kiess M, Meyer H, et al. Isolation, structure elucidation, and synthesis of a macrophage stimulatory lipopeptide from Mycoplasma fermentans acting at picomolar concentration. J Exp Med. 1997;185:1951–1958. doi: 10.1084/jem.185.11.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takeuchi O, Kawai T, Mühlradt P. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int Immunol. 2001;13:933–940. doi: 10.1093/intimm/13.7.933. [DOI] [PubMed] [Google Scholar]
- 15.Garcia J. A Mycoplasma fermentans-derived synthetic lipopeptide induces AP-1 and NF-kB activity and cytokine secretion in macrophages via the activation of mitogen-activated protein kinase pathways. J Biol Chem. 1998;273:34391–34398. doi: 10.1074/jbc.273.51.34391. [DOI] [PubMed] [Google Scholar]
- 16.Deiters U, Mühlradt P. Mycoplasmal lipopeptide MALP-2 induces the chemoattractant proteins macrophage inflammatory protein 1α (MIP-1α), monocyte chemoattractant protein 1, and MIP-2 and promotes leukocyte infiltration in mice. Infect Immun. 1999;67:3390–3398. doi: 10.1128/iai.67.7.3390-3398.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Link C, Gavioli R, Ebensen T, et al. The Toll-like receptor ligand MALP-2 stimulates dendritic cell maturation and modulates proteasome composition and activity. Eur J Immunol. 2004;34:899–907. doi: 10.1002/eji.200324511. [DOI] [PubMed] [Google Scholar]
- 18.Shimizu T, Kida Y, Kuwano K. A dipalmitoylated lipoprotein from Mycoplasma pneumoniae activates NF-kB through TLR1, TLR2, and TLR6. J Immunol. 2005;175:4641–4646. doi: 10.4049/jimmunol.175.7.4641. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs R, Hintzen G, Kemper A, et al. CD56bright cells differ in their KIR repertoire and cytotoxic features from CD56dim NK cells. Eur J Immunol. 2001;31:3121–3127. doi: 10.1002/1521-4141(2001010)31:10<3121::AID-IMMU3121>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 20.Adib-Conquy M, Scott-Algara D, Cavaillon J-M, Souza-Fonseca-Guimaraes F. TLR-mediated activation of NK cells and their role in bacterial/viral immune responses in mammals. Immunol Cell Biol. 2014;92:256–262. doi: 10.1038/icb.2013.99. [DOI] [PubMed] [Google Scholar]
- 21.Sawahata R, Shime H, Yamazaki S, et al. Failure of mycoplasma lipoprotein MALP-2 to induce NK cell activation through dendritic cell TLR2. Microbes Infect. 2011;13:350–358. doi: 10.1016/j.micinf.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Akao Y, Ebihara T, Masuda H, et al. Enhancement of antitumor natural killer cell activation by orally administered Spirulina extract in mice. Cancer Sci. 2009;100:1494–1501. doi: 10.1111/j.1349-7006.2009.01188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azuma M, Sawahata R, Akao Y, et al. The peptide sequence of diacyl lipopeptides determines dendritic cell TLR2-mediated NK activation. PLoS ONE. 2010;5:1–12. doi: 10.1371/journal.pone.0012550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma X, You X, Zeng Y, et al. Mycoplasma fermentans MALP-2 induces heme oxygenase-1 expression via mitogen-activated protein kinases and Nrf2 pathways to modulate cyclooxygenase 2 expression in human monocytes. Clin Vaccine Immunol. 2013;20:827–834. doi: 10.1128/CVI.00716-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knorr C, Marks D, Gerstberger R, et al. Peripheral and central cyclooxygenase (COX) products may contribute to the manifestation of brain-controlled sickness responses during localized inflammation induced by macrophage-activating lipopeptide-2 (MALP-2) Neurosci Lett. 2010;479:107–111. doi: 10.1016/j.neulet.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 26.Choy H, Milas L. Enhancing radiotherapy with cyclooxygenase-2 enzyme inhibitors: a rational advance? J Natl Cancer Inst. 2003;95:1440–1452. doi: 10.1093/jnci/djg058. [DOI] [PubMed] [Google Scholar]
- 27.Simmons D, Botting R, Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol Rev. 2004;56:387–437. doi: 10.1124/pr.56.3.3. [DOI] [PubMed] [Google Scholar]
- 28.Van Elssen CH, Vanderlocht J, Oth T, et al. Inflammation restraining effects of prostaglandin E2 on natural killer–dendritic cell (NK–DC) interaction are imprinted during DC maturation. Blood. 2011;118:2473–2482. doi: 10.1182/blood-2010-09-307835. [DOI] [PubMed] [Google Scholar]
- 29.Chatterjee D, Marquardt N, Tufa D, et al. Role of gamma-secretase in human umbilical-cord derived mesenchymal stem cell mediated suppression of NK cell cytotoxicity. Cell Commun Signal. 2014;12:63. doi: 10.1186/s12964-014-0063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tufa DM, Chatterjee D, Low HZ, et al. TNFR2 and IL-12 coactivation enables slanDCs to support NK-cell function via membrane-bound TNF-α. Eur J Immunol. 2014;44:3717–3728. doi: 10.1002/eji.201444676. [DOI] [PubMed] [Google Scholar]
- 31.Morr M, Takeuchi O, Akira S. Differential recognition of structural details of bacterial lipopeptides by toll like receptors. Eur J Immunol. 2002;32:3337–3347. doi: 10.1002/1521-4141(2002012)32:12<3337::AID-IMMU3337>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt R, MacDermott R, Bartley G. Specific release of proteoglycans from human natural killer cells during target lysis. Nature. 1985;318:289–291. doi: 10.1038/318289a0. [DOI] [PubMed] [Google Scholar]
- 33.Bryant J, Day R. Calculation of lytic units for the expression of cell-mediated cytotoxicity. J Immunol Methods. 1992;146:91–103. doi: 10.1016/0022-1759(92)90052-U. [DOI] [PubMed] [Google Scholar]
- 34.Maghazachi AA, Al-aoukaty A. Chemokines activate natural killer cells through heterotrimeric G-proteins : implications for the treatment of AIDS and cancer. FASEB J. 1998;12:913–924. doi: 10.1096/fasebj.12.11.913. [DOI] [PubMed] [Google Scholar]
- 35.Hornung V, Rothenfusser S, Britsch S, et al. Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168:4531–4537. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 36.Akazawa T, Ohashi T, Nakajima H, et al. Development of a dendritic cell-targeting lipopeptide as an immunoadjuvant that inhibits tumor growth without inducing local inflammation. Int J Cancer. 2014;135:2847–2856. doi: 10.1002/ijc.28939. [DOI] [PubMed] [Google Scholar]
- 37.De Heer P, Sandel MH, Guertens G, et al. Celecoxib inhibits growth of tumors in a syngeneic rat liver metastases model for colorectal cancer. Cancer Chemother Pharmacol. 2008;62:811–819. doi: 10.1007/s00280-007-0668-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khan Z, Khan N, Tiwari RP, et al. Biology of COX-2: an application in cancer therapeutics. Curr Drug Targets. 2011;12:1082–1093. doi: 10.2174/138945011795677764. [DOI] [PubMed] [Google Scholar]
- 39.Sarkar FH, Adsule S, Li Y, Padhye S. Back to the future: COX-2 inhibitors for chemoprevention and cancer therapy. Mini Rev Med Chem. 2007;7:599–608. doi: 10.2174/138955707780859431. [DOI] [PubMed] [Google Scholar]
- 40.Jinushi M, Komohara Y. Tumor-associated macrophages as an emerging target against tumors: creating a new path from bench to bedside. Biochim Biophys Acta. 2015;1855:123–130. doi: 10.1016/j.bbcan.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 41.Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel) 2014;6:1670–1690. doi: 10.3390/cancers6031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang W, Bergh A, Damber JE. Cyclooxygenase-2 expression correlates with local chronic inflammation and tumor neovascularization in human prostate cancer. Clin Cancer Res. 2005;11:3250–3256. doi: 10.1158/1078-0432.CCR-04-2405. [DOI] [PubMed] [Google Scholar]
- 43.Grote K, Schuett H, Salguero G, et al. Toll-like receptor 2/6 stimulation promotes angiogenesis via GM-CSF as a potential strategy for immune defense and tissue regeneration. Blood. 2010;115:2543–2552. doi: 10.1182/blood-2009-05-224402. [DOI] [PubMed] [Google Scholar]
- 44.Deiters U, Barsig J, Tawil B, Mühlradt PF. The macrophage-activating lipopeptide-2 accelerates wound healing in diabetic mice. Exp Dermatol. 2004;13:731–739. doi: 10.1111/j.0906-6705.2004.00233.x. [DOI] [PubMed] [Google Scholar]
- 45.Martin GM, Stockfleth E. Diclofenac sodium 3% gel for the management of actinic keratosis: 10+ years of cumulative evidence of efficacy and safety. J Drugs Dermatol. 2012;11:600–608. [PubMed] [Google Scholar]