Abstract
The anti programmed cell death-1 (PD-1) antibodies pembrolizumab and nivolumab have been recently licensed by the Food and Drug Administration for the treatment of advanced melanoma. Immune checkpoint inhibitors such as these can induce endocrine adverse events but autoimmune diabetes has not been described to date. However, there is a strong preclinical rationale that supports this autoimmune toxicity. We describe for the first time the case of an adult patient who developed autoimmune diabetes likely as a consequence of PD-1 inhibition with pembrolizumab. The presence of high serum titres of anti-glutamic acid decarboxylase antibodies together with a suggestive clinical presentation, age of the patient and preclinical data strongly support an autoimmune aetiology of the diabetes. Moreover, the patient was found to have a well-known high-risk human leucocyte antigen type for the development of type 1 diabetes in children, so the PD-1 inhibition is very likely to have triggered the autoimmune phenomenon. Our case suggests that insulin-dependent diabetes might be a rare but important anti-PD-1 immune-related adverse event.
Keywords: Diabetes, Melanoma, MK-3475, Nivolumab, PD-1, Pembrolizumab
Introduction
Pembrolizumab is an IgG4 monoclonal antibody targeting the co-inhibitory immune checkpoint molecule programmed cell death-1 (PD-1). It was approved by the FDA for the treatment of advanced melanoma in September 2014. PD-1 is a key immune regulator with a protective role against the development of autoimmune diabetes. Forced PD-1 expression in transgenic mice significantly reduces the incidence of autoimmune diabetes, whilst blockade of PD-1 or its ligand, PD-L1, rapidly precipitates diabetes in pre-diabetic NOD mice [1–3]. Although diabetes following anti-PD-L1 treatment has been observed in one patient, no information on time course or causality was reported [4]. Here, we report a case of acute onset of insulin-dependent diabetes occurring after treatment with pembrolizumab.
Case report
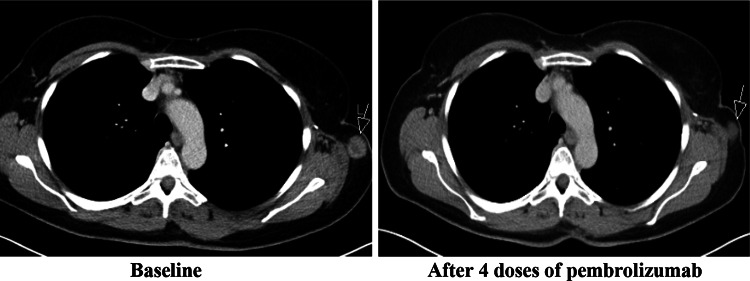
A 54-year-old female presented with a 5.1-mm-thick, BRAF wild-type cutaneous melanoma. This was resected, and axillary node dissection identified a single positive node. No adjuvant treatment was advised. Past medical history included mild asthma treated with occasional salbutamol inhalers. Her grandmother was diagnosed with type 2 diabetes in her sixties, but there was no other relevant history. One year after initial presentation, there was relapse with subcutaneous metastases. Four doses of ipilimumab at 3 mg/kg were given with disease stabilisation and without significant side effects. Eight months later, the disease progressed and pembrolizumab 2 mg/kg 3-weekly was started within an expanded access programme. Treatment was initially well tolerated, and baseline glucose levels were normal. However, after three infusions the patient presented with lethargy, vomiting, polydipsia and polyuria. Diabetic ketoacidosis was diagnosed, and intravenous fluids and insulin were initiated. Insulin therapy was converted to a subcutaneous regimen, and the patient was discharged with satisfactory glucose levels after a 3-day admission. Further investigation identified high serum titres of anti-glutamic acid decarboxylase (anti-GAD) antibodies, supporting an autoimmune aetiology of the diabetes. Other islet autoantibodies were negative (Table 1). Human leucocyte antigen (HLA) typing revealed the autoimmune diabetes high-risk genotype DRB1*04, DQB1*0302 (HLA A2 DR4 DQ8), which confers a 1:16 chance of developing type 1 childhood diabetes [5]. Re-staging investigations after four doses of pembrolizumab demonstrated response to therapy (Fig. 1). At the time of writing, two further cycles of pembrolizumab have been given without further toxicity, and there have been no changes in insulin requirements.
Table 1.
Islet autoantibodies
| Autoantibody | Results | Reference range |
|---|---|---|
| Anti-GAD | 70.1 U/mL | 0–5 |
| Pancreatic islet cell | Negative | |
| IgG insulin | 2.2 mg/L | 0–5 |
Fig. 1.
Tumour response to pembrolizumab
Discussion
This case supports a potential link between PD-1 inhibition and autoimmune diabetes in humans. In the absence of baseline sampling, it is unknown whether serum anti-GAD antibody titres increased in response to pembrolizumab; however, the clinical presentation, age of the patient and preclinical data strongly suggest an autoimmune aetiology of the diabetes. The possibility that our patient developed a spontaneous, non-pembrolizumab-induced latent autoimmune diabetes of the adult (LADA) is unlikely since this is a rare entity which is usually non-insulin dependent at presentation and clinically indistinguishable from type 2 diabetes at early stages in the majority of cases. Moreover, the patient has a well-known high-risk HLA for the development of type 1 diabetes in children, so the PD-1 inhibition is very likely to have triggered the autoimmune phenomenon.
Diabetes is not described as a potential side effect in the pembrolizumab prescribing information. However, physicians and patients should be aware that it may be a rare but important anti-PD-1 immune-related adverse event.
Conflict of interest
James Larkin has received research funding from Pfizer and Novartis. He has also been a consultant for GlaxoSmithKline, Bristol-Myers Squib, Pfizer and Novartis. The rest of the authors have no conflict of interest to disclose.
Informed consent
The patient provided written informed consent for her clinical data to be published in a medical journal.
Abbreviations
- Anti-GAD
Anti-glutamic acid decarboxylase
- FDA
Food and Drug Administration
- HLA
Human leucocyte antigen
- LADA
Latent autoimmune diabetes of the adult
- PD-1
Programmed cell death-1
References
- 1.Ansari MJ, Salama AD, Chitnis T, Smith RN, Yagita H, Akiba H, et al. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med. 2003;198(1):63–69. doi: 10.1084/jem.20022125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guleria I, Gubbels Bupp M, Dada S, Fife B, Tang Q, Ansari MJ, et al. Mechanisms of PDL1-mediated regulation of autoimmune diabetes. Clin Immunol. 2007;125(1):16–25. doi: 10.1016/j.clim.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Kochupurakkal NM, Kruger AJ, Tripathi S, Zhu B, Adams LT, Rainbow DB, et al. Blockade of the programmed death-1 (PD1) pathway undermines potent genetic protection from type 1 diabetes. PLoS One. 2014;9(2):e89561. doi: 10.1371/journal.pone.0089561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barker JM, Barriga KJ, Yu L, Miao D, Erlich HA, Norris JM, et al. Prediction of autoantibody positivity and progression to type 1 diabetes: Diabetes Autoimmunity Study in the Young (DAISY) J Clin Endocrinol Metab. 2004;89(8):3896–3902. doi: 10.1210/jc.2003-031887. [DOI] [PubMed] [Google Scholar]