Abstract
Few immunotherapy compounds have demonstrated a direct link between the predicted mode of action of the product and benefit to the patient. Since cancer vaccines are thought to have a delayed therapeutic effect, identification of the active moiety may enable the development of an early marker of efficacy. Patients with renal cancer and requiring first-line treatment for metastatic disease were randomized 1:1 to receive MVA-5T4 (TroVax®) or placebo alongside Sunitinib, IL-2 or IFN-α in a multicentre phase III trial. Antibody responses were quantified following the 3rd and 4th vaccinations. A surrogate for 5T4 antibody response (the immune response surrogate; IRS) was constructed and then used in a survival analysis to evaluate treatment benefit. Seven hundred and thirty-three patients were randomized, and immune responses were assessed in 590 patients. A high 5T4 antibody response was associated with longer survival within the MVA–5T4-treated group. The IRS was constructed as a linear combination of pre-treatment 5T4 antibody levels, hemoglobin and hematocrit and was shown to be a significant predictor of treatment benefit in the phase III study. Importantly, the IRS was also associated with antibody response and survival in an independent dataset comprising renal, colorectal and prostate cancer patients treated with MVA–5T4 in phase I–II studies. The derivation of the IRS formed part of an exploratory, retrospective analysis; however, if confirmed in future studies, the results have important implications for the development and use of the MVA–5T4 vaccine and potentially for other similar vaccines.
Keywords: Therapeutic vaccine, Renal cell carcinoma, 5T4, Antibody, Immune response surrogate
Introduction
Renal cell carcinoma (RCC) represents 5% of epithelial cancers diagnosed annually in the United States [1, 2] and 20–30% of RCC patients present with metastatic disease which has a poor prognosis. However, treatment options for patients have improved substantially over the last few years following FDA approval of sunitinib, sorafenib, temsirolimus, everolimus and bevacizumab for use against advanced RCC. Despite such advances, the management of metastatic renal cancer remains a challenge. The ability to layer a new therapeutic moiety on top of an existing therapy without any increased toxicity or reduced efficacy would be of great value. Cancer vaccines offer one possible approach to achieve this goal.
The cancer vaccine TroVax® (MVA–5T4) has been tested in multiple phase I and II trials in patients with renal cell carcinoma (RCC; 4 phase II trials), colorectal cancer (CRC; 4 phase I/II trials) and hormone refractory prostate cancer (HRPC: 1 phase II trial). TroVax consists of the attenuated vaccinia virus, modified vaccinia Ankara (MVA) which has been engineered to deliver the 5T4 tumor-associated antigen (TAA). 5T4 is an oncofetal antigen which is expressed at high levels in the placenta [3, 4], but is rarely detected in normal adult tissues. However, 5T4 is highly expressed in the most common tumors, typically in >80% of carcinomas of the kidney, breast, gastrointestinal tract, colon, prostate and ovaries [4, 5].
The completed phase I and II studies showed MVA–5T4 to be well tolerated and able to induce 5T4-specific immune responses in the majority of patients (>90%) [6]. Furthermore, correlations between 5T4-specific antibody and/or cellular responses and clinical benefit were reported in seven independent studies [7–13].
Recently, we reported results from a large randomized phase III trial (TRIST; TroVax Renal Immunotherapy Survival Trial) in which the cancer vaccine TroVax® (MVA–5T4) was tested in >700 patients with metastatic clear cell renal carcinoma [14]. Despite not meeting the primary endpoint of enhanced patient survival, exploratory analyses suggested that it may be possible to identify patients who are more likely to receive clinical benefit from this class of cancer therapy.
In order to deliver any therapeutic benefit to a patient, cancer vaccines, such as MVA–5T4, must induce an efficacious immune response. It is critical that we continue to gain a better understanding of the nature of the efficacious immune response induced by vaccination, determine how best to monitor the response and ultimately, how to improve upon it. Previously, we have demonstrated an association between the magnitude of 5T4-specific antibody responses and enhanced clinical benefit. However, such analyses were limited by the relatively small number of patients recruited to each study and the lack of a control or comparator arm. The availability of plasma samples from over 500 patients treated with either MVA–5T4 or placebo has enabled a more robust analysis of 5T4-specific antibody responses.
One of the prospectively defined secondary endpoints of the phase III TRIST study included an analysis of 5T4 antibody responses and their association with measurements of clinical benefit. Data from this study confirmed results from previous phase I/II trials of MVA–5T4 in which a strong positive association between quantitative antibody response and overall survival was observed. However, association does not necessarily imply causation, and it is possible that patients who are relatively more robust both mount a stronger antibody response and live longer i.e. they are ‘healthier’ patients. We have addressed this issue by constructing a surrogate for the immune response (immune response surrogate; IRS) using baseline (pre-treatment) factors that can be readily measured in any patient irrespective of the treatment they receive. A stronger association between overall survival and the IRS within the MVA–5T4-treated patients compared to the placebo-treated patients would be evidence of beneficial biological activity.
Materials and methods
Study design
A detailed description of the trial design has been published elsewhere [14]. In brief, patients with advanced or metastatic clear cell renal cancer who had undergone prior nephrectomy, had a good or intermediate prognosis (MSKCC score 0–2), Karnofsky performance status >80% and life expectancy of >12 weeks were eligible.
MVA–5T4 (1 × 109 TCID50/ml) or placebo were administered by intra-muscular injection into the deltoid muscle at weeks 1, 3, 6, 9, 13, 17, 21, 25, 33, 41, 49, 57 and 65. During the course of the study, plasma samples were obtained from patients prior to treatment and following the 3rd and 4th MVA–5T4/placebo vaccinations (weeks 7 and 10, respectively) for assessment of MVA- and 5T4-specific antibody responses. Furthermore, blood samples were obtained from 50 consenting and nominally healthy, individuals, aged between 55 and 73 (mean = 62) of whom 25 were men (50%); these served as controls for comparison against patients with cancer.
Measurement of antibody responses
5T4- and MVA-specific antibody responses were determined using a relative-quantitative ELISA that had been validated according to industry standard methods [15]. Microtiter plates were coated with purified recombinant 5T4 protein (0.44 μg/ml) or wild-type MVA virus (1 × 107 pfu/ml). Polyclonal plasma, known to be positive for both 5T4 and MVA antibodies, were used as a standard curve for each assay. The standard curves for each ELISA were assigned a nominal value of 5T4 or MVA antibody relative units (RU) and were titrated from 200 to 1.56 RU. A cut-point was established for each assay by analyzing 5T4- and MVA-specific antibody levels in plasma recovered from 50 healthy donors. Cut-points of 12.77RU and 5.20RU were established for 5T4 and MVA, respectively, by setting the false-positive rate to be 5%. Variation in the level of 5T4- and MVA antibody levels was assessed in cancer patients who had not received any 5T4- or MVA-targeted therapies. A 1.54-fold increase in 5T4 antibody and a 1.76-fold increase in MVA antibody was established as the level at which a 1% false-positive rate could be expected.
All plasma test samples were analyzed, in a blinded manner, at a dilution of 1:50 for 5T4 or 1:2,000 for MVA and results reported as relative units (RU) of 5T4 or MVA-specific antibodies. A positive response at baseline was reported if the pre-treatment antibody levels exceeded the cut-point. A positive response following vaccination was reported if the antibody levels exceeded the cut-point and the increase, relative to the baseline, exceeded the pre-determined fold increase for each antigen (1.54-fold for 5T4 and 1.76-fold for MVA). Samples were un-blinded once all analyses had been completed and the study had finished. A goat anti-human IgG HRP antibody (Dako) was used as the secondary antibody for both 5T4 and MVA assays.
Statistical analysis
Analysis of associations between the 5T4 antibody response induced by MVA–5T4 and clinical benefit was a prospectively defined secondary endpoint in the phase III study design. An immunological analysis set was defined comprising subjects with antibody data at baseline (week 1) and at week 10 (after the 4th MVA–5T4 vaccination). Within the MVA–5T4 group, the effect of quantitative 5T4 immune response (defined as the logarithm of the ratio of week 10 5T4 antibody level to the baseline level) on overall survival was assessed using a proportional hazards model. A similar analysis was performed looking at the association of MVA immune response with overall survival. It should be noted that the immunological analysis set included all subjects for which antibody data were available, even if the subject was defined a “non-responder” on the basis of that data.
The immune response surrogate (IRS) was constructed by finding all baseline hematological, immunological, demographic and cancer characterizing variables that predicted quantitative 5T4 immune response with a significance of P less than 0.20 within a general linear model. These variables were then used as the initial model in a backwards elimination procedure that only retained those associated with a P value of less than 0.05, all models being adjusted for standard of care (IL-2, IFN-α or Sunitinib). The variables retained in the model were used to construct a predictor of immune response (the IRS) that was calculated for all subjects in the immune response set. The IRS was then used in a proportional hazards model to assess treatment benefit on overall survival.
All survival analyses were performed three times with retrospective censoring at 12, 18 and 24 months from randomization, respectively, in order to establish the robustness of the conclusions, given uncertainty over the time period over which MVA–5T4 shows benefit. All proportional hazards models were stratified by standard of care with separate treatment effects for each standard of care.
The IRS derived from the TRIST study was also applied to a historic dataset derived from 9 separate phase I and II studies of MVA–5T4 in patients with renal, colorectal and prostate cancer [7–13, 16, 17]. In all 9 studies, antibody responses against 5T4 and MVA were assessed in the same manner as described for this study. Likewise, an immunological analysis set was defined comprising subjects with antibody response data at baseline and after the 4th MVA–5T4 vaccination. This immunological analysis set contained antibody data from 52 patients with renal cancer, 32 with colorectal cancer and 24 with prostate cancer.
Results
5T4 and MVA antibody levels
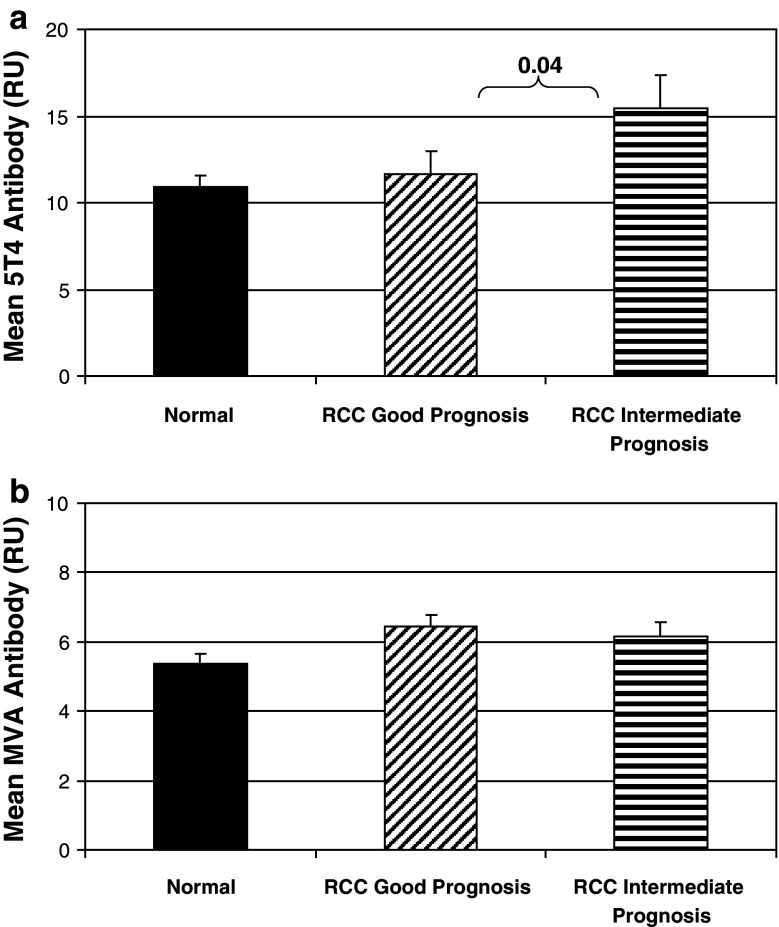
During the course of the TRIST study, antibody responses against the 5T4 tumor antigen and the MVA viral vector were determined at baseline (pre-treatment) and at weeks 7 and 10 (following the 3rd and 4th MVA–5T4/placebo vaccinations). Prior to treatment with MVA–5T4 or placebo, positive 5T4-specific antibody responses were detected in 81 (23%) and 99 (27%) patients, respectively; positive MVA-specific antibody responses were detected in 98 (27%) and 87 (24%) patients, respectively (data not shown). The magnitude of pre-treatment 5T4 and MVA antibody levels in renal cancer patients were compared to those found in plasma samples recovered from 50 nominally healthy age-matched donors (Fig. 1a, b, respectively). Pre-treatment 5T4 and MVA antibody levels were not significantly different in RCC patients compared to healthy donors (data not shown). Interestingly, patients classified as intermediate prognosis had higher 5T4 antibody levels compared to good prognosis patients (Fig. 1a; P = 0.04), but neither were significantly different from healthy donors. MVA antibody levels were higher in good and intermediate prognosis RCC patients compared to healthy donors (Fig. 1b; P = 0.08 and 0.04, respectively). However, unlike 5T4, there was no difference in pre-treatment MVA antibody levels in good prognosis patients compared to intermediate prognosis patients (P = 0.73).
Fig. 1.
Pre-treatment 5T4 (a) and MVA (b) antibody levels in healthy donors (normal; n = 50) compared to RCC patients classified as good prognosis (MSKCC = 0; n = 419) or intermediate prognosis (MSKCC = 1–2; n = 305). The figure plots the mean pre-treatment antibody levels (±0.95 CI) and shows the P value (Mann–Whitney) between groups
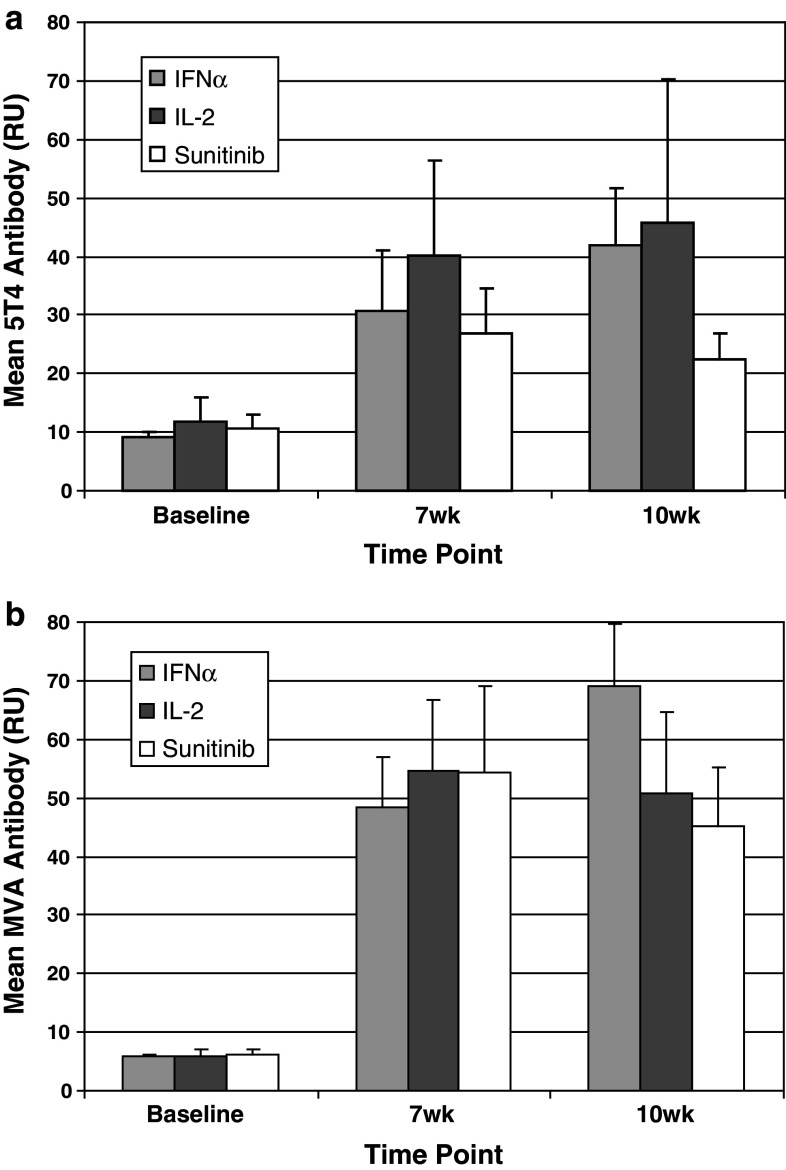
The percentage of patients who were classified as having mounted a positive 5T4-specific antibody response, relative to pre-treatment levels, following the 3rd and/or 4th vaccination with MVA–5T4 or placebo was 56 and 6%, respectively. An analysis of the magnitude of 5T4 (Fig. 2a) and MVA (Fig. 2b) antibody responses was undertaken in patients treated with MVA–5T4 and classified as 5T4 seroconverters. In this subset of patients, the magnitude of antibody responses was similar at each time point and for each SOC, with the only significant difference in the magnitude of 5T4 or MVA antibody responses being at week 10 in patients receiving IFN-α compared to sunitinib (Mann–Whitney; both P < 0.01).
Fig. 2.
Analysis of the magnitude of 5T4 and MVA antibody responses in patients classified as having seroconverted. The figures plot the mean (±0.95 CI) 5T4 (a) and MVA (b) antibody responses (by SOC) in patients treated with MVA–5T4 who were classified as having mounted a positive 5T4 antibody response (n = 161 patients)
Association between immunological response and overall survival
The immunological analysis set contained 590 individuals, 288 in the MVA–5T4-treated group and 302 in the placebo group. Table 1 shows the number of events in each group under each of the three censoring regimens. A total of 143 randomized individuals were excluded from the immunological analysis set: 140 because of missing immunological data, 2 were randomized in error and 1 had an outlying high baseline 5T4 antibody level. In order to have a non-missing week 10 assessment, the subject must have survived at least to that assessment. Fifty subjects (25 in each of the two treatment groups) either died (20 in each of the two groups) or were lost to follow-up (5 in each of the two groups) on or before day 70.
Table 1.
Censoring regimens
| Censoring cutoff (months) | Numbers of subjects | |||||||
|---|---|---|---|---|---|---|---|---|
| Placebo group | MVA–5T4 group | |||||||
| Total | LTFU | Censored | Died | Total | LTFU | Censored | Died | |
| 12 | 302 | 2 | 220 | 80 | 288 | 1 | 201 | 86 |
| 18 | 302 | 5 | 173 | 124 | 288 | 4 | 157 | 127 |
| 24 | 302 | 8 | 139 | 155 | 288 | 9 | 136 | 143 |
The table presents numbers of subjects lost to follow-up (LTFU) before cutoff date, censored (at earlier of cutoff date and end of study) and died before cutoff dates for three censoring regimens (retrospectively censored at cutoff dates 12, 18 and 24 months after randomization)
The quantitative 5T4 immune response was positively associated with longer survival (hazard ratio of approximately 0.78 and P < 0.05) at all three censoring time points (12, 18 and 24 months; Table 2). A hazard ratio less than unity indicates that the hazard decreases with increasing immune response. In contrast, the quantitative MVA immune response was not significantly associated with survival (hazard ratio ~1 and P > 0.10) at all three censoring time points. It was interesting to note that MVA–5T4-treated patients categorized as antibody responders had a greater predicted median survival than patients classified as non-responders (not reached vs. 18.94 months, respectively; P = 0.11, HR = 0.76).
Table 2.
The effect of antibody response on survival
| Censoring cutoff (months) | Hazard ratio [0.95 confidence interval] (P value) | |
|---|---|---|
| Effect of MVA response | Effect of 5T4 response | |
| 12 | 1.154 [0.940, 1.416] (0.17) | 0.789 [0.634, 0.983] (0.035) |
| 18 | 1.140 [0.964, 1.349] (0.13) | 0.783 [0.656, 0.936] (0.0071) |
| 24 | 1.060 [0.907, 1.239] (0.46) | 0.789 [0.666, 0.934] (0.0059) |
Response is defined as the natural logarithm of the ratio of antibody level at week 10 to that at baseline. A proportional hazards model for overall survival was used stratified by standard of care. The hazard ratios correspond to a doubling of the ratio of the antibody level at week 10 compared to baseline
Obtaining a surrogate for quantitative 5T4 immune response
The baseline variables that individually correlated with quantitative 5T4 immune response (within the MVA–5T4-treated group) with a P value of less than 0.10 after adjustment for standard of care are listed in Table 3. The backwards elimination algorithm applied to the variables in Table 3 resulted in three factors remaining in the model for quantitative 5T4 immune response, after adjustment for standard of care: the logarithm of the baseline 5T4 antibody level (P = 0.0004), hemoglobin level (P = 0.0071) and hematocrit (P = 0.028). The regression equation for quantitative 5T4 immune response has two components: a term depending on standard of care, and the IRS with formula:
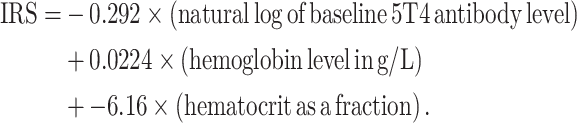 |
Table 3.
Univariate predictors of quantitative 5T4 antibody response
| Baseline variable | Direction of effect | P value |
|---|---|---|
| Hemoglobin level | Positive | 0.0031 |
| Baseline 5T4 antibody level below LLQ† | Positive | 0.0081 |
| Hematocrit | Positive | 0.026 |
| Baseline VEGF level below LLQ† | Positive | 0.042 |
| Baseline MVA antibody level below LLQ† | Positive | 0.050 |
| Bilirubin concentration* | Positive | 0.058 |
| Red blood cell count | Positive | 0.069 |
| Age | Negative | 0.099 |
| Urea concentration | Negative | 0.079 |
| Baseline VEGF Level* | Negative | 0.052 |
| Alkaline phosphatase level* | Negative | 0.015 |
| Platelet count* | Negative | 0.012 |
| Baseline 5T4 antibody level* | Negative | <0.0001 |
The direction of effect is positive if an increase in the baseline variable is associated with an increase in the immune response and negative if an increase in the baseline variable is associated with a decrease in the immune response. Only those variables that are associated with a P value of less than 0.1 are included in the table. [Key: * The natural logarithm of these baseline variables was taken after inspection of the distributions; † indicator for below the lower limit of quantitation.]
The variables with univariate P values greater than 0.1 but less than 0.2 were the following: lymphocyte count and Karnofsky score (both with positive association) and serum phosphorus, serum potassium and baseline MVA (all with negative association). The variables with univariate P values greater than 0.2 (and therefore were not included in the multivariate model) were the following: gender, the number of liver lesions, the size of target lesions at baseline, white blood cell count, basophil count, neutrophil count, eosinophil count, monocyte count, serum ALAT, serum ASAT, serum LDH, serum creatinine, serum corrected calcium, serum total calcium, serum sodium and serum chloride
It is noteworthy that the sign associated with hematocrit is negative in the IRS despite being positive when the model just contained hematocrit. The form of the IRS is indicating that, for a given level of hemoglobin, response is negatively associated with hematocrit.
Standard of care was a significant predictor of quantitative immune response. Nevertheless, terms for standard of care are not part of the IRS as the intent of the IRS is to reflect intrinsic baseline properties of the subject and standard of care is an externally imposed treatment regimen (which may not be transferable between studies).
Using the immune response surrogate to model treatment benefit
The IRS and its interaction with treatment were included in a proportional hazards model of overall survival stratified by standard of care and including separate treatment effects for each standard of care. The adjustment for standard of care was necessary for an unambiguous interpretation of the predictive effect of the IRS.
Table 4 shows the hazard ratios associated with the effect of IRS in the placebo group and in the MVA–5T4 group, together with their quotient. The hazard ratios being less than unity in the individual treatment arms shows that the IRS is prognostic within each treatment group: the higher the value of IRS, the lower the hazard (and the longer the survival).
Table 4.
The effect of the immune response surrogate (IRS) on survival
| Censoring cutoff (months) | Hazard ratio [0.95 confidence interval] (P value) | ||
|---|---|---|---|
| Prognostic effect of IRS in placebo group | Prognostic effect of IRS in MVA–5T4 group | Treatment benefit associated with IRS | |
| 12 | 0.555 [0.322, 0.956] (0.0339) | 0.153 [0.085, 0.273] (<0.0001) | 0.275 [0.124, 0.61] (0.0015) |
| 18 | 0.62 [0.394, 0.975] (0.0387) | 0.233 [0.14, 0.387] (<0.0001) | 0.375 [0.19, 0.741] (0.0048) |
| 24 | 0.704 [0.463, 1.068] (0.0991) | 0.251 [0.154, 0.41] (<0.0001) | 0.357 [0.188, 0.68] (0.0017) |
A proportional hazards model for overall survival was used, stratified by standard of care. The hazard ratios correspond to a doubling of predicted quantitative 5T4 antibody response
Treatment benefit is assessed by dividing the hazard ratio for the IRS in the MVA–5T4 group by the hazard ratio in the placebo group: a result less than unity means that the higher the IRS, the lower the hazard ratio of MVA–5T4 against placebo. Under all three censoring regimens, the IRS is a statistically significant predictor of treatment benefit.
Given the relationship between the immune response surrogate and treatment benefit, we undertook further exploratory analyses using components of the IRS to identify large subsets of the TRIST population who may have received significant clinical benefit from MVA–5T4. An exemplary subset of patients who were in both the top 50% for baseline hemoglobin-hematocrit ratio and bottom 50% for baseline 5T4 antibody was constructed. This subset of patients showed a hazard ratio of 0.52 in favor of MVA–5T4 (146 subjects, P = 0.011). Relaxing the inclusion criteria to the top and bottom 60%, respectively, yielded a hazard ratio of 0.56 (211 subjects, P = 0.0063).
Application of the IRS to the phase II studies
Following the derivation of the immune response surrogate using the TRIST dataset, the IRS was applied to historical data from previous phase I and II studies of MVA–5T4 in patients with renal, colorectal and prostate cancer. From the phase I and II studies, 108 evaluable patients contributed to the immunological and survival dataset. When the IRS was applied to these data, it was positively associated with quantitative 5T4 antibody response (P < 0.0001, adjusted for indication and study; P = 0.017, renal cancer subjects only, adjusted for study) and with overall survival (P = 0.0034, stratified by indication and study; P = 0.0023, renal cancer subjects only, stratified for study). In the renal cancer subjects, the hazard ratio was 0.076 (0.95 CI: [0.014, 0.398]), which is consistent with the hazard ratio of approximately 0.2 seen in the MVA–5T4 column of Table 4.
Discussion
This study describes a detailed analysis of antibody responses specific for the 5T4 target antigen and the MVA viral vector in >500 RCC patients treated with the investigational vaccine MVA–5T4 or placebo. Quantification of antibody responses prior to treatment showed that RCC patients had similar 5T4 and MVA antibody levels relative to age-matched healthy donors. However, it was interesting to note that patients classified as having an intermediate prognosis had significantly higher 5T4 antibody levels compared to those with a good prognosis suggesting that the more advanced the disease stage the higher the antibody response. This may fit with the observation that 5T4 protein appears to be even more highly expressed on late-stage disease and has been shown to have prognostic value in some cancers. In contrast, no differential in MVA antibody levels were seen between patients classified as good or intermediate prognosis.
Following treatment with MVA–5T4, the magnitude of 5T4-specific antibody responses in patients classified as having seroconverted was largely comparable across all 3 SOCs, although there was a trend toward higher responses in patients receiving cytokine therapy. Unfortunately, we were not able to analyze cellular response during the course of this study and therefore cannot comment on the impact of each SOC on the quantitative or qualitative nature of 5T4-specific cellular responses.
Few immunotherapy studies have demonstrated convincingly that there is a direct link between the predicted mode of action of a compound and therapeutic benefit. Phase I and II results for MVA–5T4 in renal, colorectal and prostate cancer patients were encouraging and demonstrated that immune responses were induced in almost all treated patients and associations between 5T4-specific cellular or humoral responses and clinical benefit were reported in seven of the nine phase II studies [7–13, 16, 17]. In particular, studies in RCC and colorectal cancer patients have demonstrated an association between 5T4-specific (but not MVA) antibody responses and enhanced survival [8, 11, 13].
This study provides additional strong evidence of an association between 5T4-specific immune response and clinical benefit, although it could be argued that the association is not wholly causal, in that healthy subjects might be expected to both mount a strong antibody response and survive longer. However, in the current study as in previous phase II studies, no association was detected between MVA antibody response and survival. In principle, convincing arguments that a high 5T4 antibody response is associated with treatment benefit can only be obtained from placebo-controlled studies. Logically, this is impossible as the immune response cannot by definition be obtained from unvaccinated subjects. We have coped with this difficulty by deriving a surrogate for immune response using the MVA–5T4-treated subjects and applying this to a survival analysis in MVA–5T4 and placebo-treated patients to evaluate treatment benefit.
The surrogate is a prognostic factor for survival in both placebo, and MVA–5T4-treated groups. It is noteworthy, that in the MVA–5T4-treated group, the surrogate for immune response is a stronger prognostic factor for survival than the immune response itself is. The difference in the hazard ratios between patients treated with placebo and those receiving MVA–5T4 is highly significant: the higher the value of the surrogate, the lower the value of the MVA–5T4 hazard relative to placebo. The demonstration that the immune response surrogate is a predictor of treatment benefit has several important implications:
it is indirect evidence of the biological (and, potentially, therapeutic) activity of MVA–5T4—if MVA–5T4 were biologically inert, the treatment effect relative to placebo could not be associated with a baseline covariate such as the IRS;
it establishes that some subsets of patients are more likely to obtain clinical benefit from MVA–5T4 than other subsets, with potential consequences both for the design of future clinical trials (indeed we have shown, in exploratory analyses, that choosing subsets based on the components of the IRS can result in a significantly favorable hazard ratio for MVA–5T4);
it strengthens the argument that the 5T4 immune response at week 10 is an early marker of efficacy.
To our knowledge, this is the first report in the cancer vaccine field in which it has been demonstrated convincingly that an antigen-specific immune response induced by vaccination is associated with enhanced patient survival and is not simply a function of the general “health” of a patient. Of course, these results must be treated with caution; the surrogate was derived as part of a retrospective exploratory analysis, and it is critical that the value of the surrogate is tested in future trials. Indeed, to this end, the set of candidate variables for the surrogate only included variables that were common to all studies and adjusted for, rather than included, variables specific to the phase III TRIST study (e.g. standard of care).
It is interesting and unexpected that hemoglobin and hematocrit should be identified as factors contributing to the prediction of immune response. An explanation of the mechanistic rationale to explain this is not immediately obvious. However, low levels of hemoglobin and hematocrit are indicators of anemia that is often caused by iron deficiency and is commonly seen in renal cancer patients. Although there are contradictory data regarding the role of iron in adaptive immune responses, some publications have reported that iron deficiency can impact on the quality and/or quantity of immune responses [18, 19]. Furthermore, vaccinia virus is known to induce innate immunity and dendritic cell (DC) maturation through stimulation of toll-like receptors (TLR) 2, 3 and 4 [20, 21], and hemoglobin has recently been shown to synergize with TLR agonists to potentiate innate immune responses and cytokine release [22]. Therefore, perhaps, in patients with higher baseline hemoglobin levels, immune responses may be potentiated by the synergy of hemoglobin for TLR stimulation at the site of injection, while those with lower hemoglobin (or iron) levels may have dysfunctional immune responses. This observation warrants more detailed analysis and confirmation in subsequent studies.
In the light of the unexpected constituents of the IRS, it was very encouraging that the surrogate showed a significant association with both immune response and survival when applied to data collated from phase I and II studies of MVA–5T4 in renal, colorectal and prostate cancer patients. The ability of the immune response surrogate to predict 5T4 immune response in our phase II renal cancer dataset provides some validation of the algorithm. Furthermore, the successful application of the surrogate to colorectal and prostate cancer patients treated with MVA–5T4 suggests that it may have application beyond renal cancer.
By their very nature, immunotherapy products will have a delayed therapeutic benefit leading to protracted monitoring before clinical benefit (or lack thereof) is detectable. The availability of an early marker of efficacy would be particularly beneficial for cancer immunotherapy products. Here, we have confirmed previous observations that an antibody response specific for 5T4 and generated within 10 weeks of treatment initiation is associated with enhanced survival. This represents the first steps toward the identification of an early marker of efficacy for the cancer vaccine MVA–5T4. In this respect, it is encouraging that various paradigms relating to the clinical development of immunotherapy products are currently being challenged [23, 24]. For example, Berry [23] suggests that unnecessarily restrictive requirements, such as (a) only using biomarkers that have been validated and (b) only using early endpoints that are surrogates of the primary endpoint are inhibiting progress. While recognizing that false-positive relationships may be identified, Berry concludes that all available information should be assessed and associated with the primary endpoint and used to draw stronger conclusions about the endpoint.
In conclusion, we have shown that patients with a higher value for a surrogate of immune response obtain greater benefit from MVA–5T4. This finding is an indirect confirmation of the biological activity of MVA–5T4 and has important implications for future clinical trials that will target patients with good performance status and minimize the recruitment of patients with abnormal levels of various hematology factors. Confirmation of these observations in future studies and in different cancer types is ongoing.
Acknowledgment
This paper is dedicated to the memory of Dr. Sue Kingsman who gave so much of her life to the discovery and development of novel treatments for areas of unmet medical need.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57(1):43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Costa LJ, Drabkin HA. Renal cell carcinoma: new developments in molecular biology and potential for targeted therapies. Oncologist. 2007;12(12):1404–1415. doi: 10.1634/theoncologist.12-12-1404. [DOI] [PubMed] [Google Scholar]
- 3.Hole N, Stern PL. A 72 kD trophoblast glycoprotein defined by a monoclonal antibody. Br J Cancer. 1988;57(3):239–246. doi: 10.1038/bjc.1988.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Southall PJ, Boxer GM, Bagshawe KD, Hole N, Bromley M, Stern PL. Immunohistological distribution of 5T4 antigen in normal and malignant tissues. Br J Cancer. 1990;61(1):89–95. doi: 10.1038/bjc.1990.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Starzynska T, Marsh PJ, Schofield PF, Roberts SA, Myers KA, Stern PL. Prognostic significance of 5T4 oncofetal antigen expression in colorectal carcinoma. Br J Cancer. 1994;69(5):899–902. doi: 10.1038/bjc.1994.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harrop R, Shingler W, Kelleher M, De Belin J, Treasure P (2010) Cross-trial analysis of immunological and clinical data resulting from phase I and II trials of MVA-5T4 (TroVax®) in colorectal, renal and prostate cancer patients. J Immunother (in press) [DOI] [PubMed]
- 7.Amato RJ, Drury N, Naylor S, Jac J, Saxena S, Cao A, Hernandez-McClain J, Harrop R. Vaccination of prostate cancer patients with modified vaccinia Ankara delivering the tumor antigen 5T4 (TroVax): a phase 2 trial. J Immunother. 2008;31(6):577–585. doi: 10.1097/CJI.0b013e31817deafd. [DOI] [PubMed] [Google Scholar]
- 8.Amato RJ, Shingler W, Goonewardena M, de Belin J, Naylor S, Jac J, Willis J, Saxena S, Hernandez-McClain J, Harrop R. Vaccination of renal cell cancer patients with modified vaccinia Ankara delivering the tumor antigen 5T4 (TroVax) alone or administered in combination with interferon-alpha (IFN-alpha): a phase 2 trial. J Immunother. 2009;32(7):765–772. doi: 10.1097/CJI.0b013e3181ace876. [DOI] [PubMed] [Google Scholar]
- 9.Amato RJ, Shingler W, Naylor S, Jac J, Willis J, Saxena S, Hernandez-McClain J, Harrop R. Vaccination of renal cell cancer patients with modified vaccinia Ankara delivering tumor antigen 5T4 (TroVax) administered with interleukin 2: a phase II trial. Clin Cancer Res. 2008;14(22):7504–7510. doi: 10.1158/1078-0432.CCR-08-0668. [DOI] [PubMed] [Google Scholar]
- 10.Elkord E, Dangoor A, Drury NL, Harrop R, Burt DJ, Drijfhout JW, Hamer C, Andrews D, Naylor S, Sherlock D, Hawkins RE, Stern PL. An MVA-based vaccine targeting the oncofetal antigen 5T4 in patients undergoing surgical resection of colorectal cancer liver metastases. J Immunother. 2008;31(9):820–829. doi: 10.1097/CJI.0b013e3181876ab3. [DOI] [PubMed] [Google Scholar]
- 11.Harrop R, Connolly N, Redchenko I, Valle J, Saunders M, Ryan MG, Myers KA, Drury N, Kingsman SM, Hawkins RE, Carroll MW. Vaccination of colorectal cancer patients with modified vaccinia Ankara delivering the tumor antigen 5T4 (TroVax) induces immune responses which correlate with disease control: a phase I/II trial. Clin Cancer Res. 2006;12(11 Pt 1):3416–3424. doi: 10.1158/1078-0432.CCR-05-2732. [DOI] [PubMed] [Google Scholar]
- 12.Harrop R, Drury N, Shingler W, Chikoti P, Redchenko I, Carroll MW, Kingsman SM, Naylor S, Melcher A, Nicholls J, Wassan H, Habib N, Anthoney A. Vaccination of colorectal cancer patients with modified vaccinia Ankara encoding the tumor antigen 5T4 (TroVax) given alongside chemotherapy induces potent immune responses. Clin Cancer Res. 2007;13:4487–4494. doi: 10.1158/1078-0432.CCR-07-0704. [DOI] [PubMed] [Google Scholar]
- 13.Kaufman HL, Taback B, Sherman W, Kim DW, Shingler WH, Moroziewicz D, DeRaffele G, Mitcham J, Carroll MW, Harrop R, Naylor S, Kim-Schulze S. Phase II trial of Modified Vaccinia Ankara (MVA) virus expressing 5T4 and high dose Interleukin-2 (IL-2) in patients with metastatic renal cell carcinoma. J Transl Med. 2009;7:2. doi: 10.1186/1479-5876-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amato RJ, Hawkins RE, Kaufman HL, Thompson JA, Tomczak P, Szczylik C, McDonald M, Eastty S, Shingler WH, de Belin J, Goonewardena M, Naylor S, Harrop R (2010) Vaccination of metastatic renal cancer patients with MVA-5T4: a randomized, double blind, placebo controlled phase III study. Clin Cancer Res. doi:10.1158/1078-0432.ccr-10-2082. (in press) [DOI] [PubMed]
- 15.Mire-Sluis AR, Barrett YC, Devanarayan V, Koren E, Liu H, Maia M, Parish T, Scott G, Shankar G, Shores E, Swanson SJ, Taniguchi G, Wierda D, Zuckerman LA. Recommendations for the design and optimization of immunoassays used in the detection of host antibodies against biotechnology products. J Immunol Methods. 2004;289(1–2):1–16. doi: 10.1016/j.jim.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Harrop R, Drury N, Shingler W, Chikoti P, Redchenko I, Carroll MW, Kingsman SM, Naylor S, Griffiths R, Steven N, Hawkins RE. Vaccination of colorectal cancer patients with TroVax given alongside chemotherapy (5-fluorouracil, leukovorin and irinotecan) is safe and induces potent immune responses. Cancer Immunol Immunother. 2008;57(7):977–986. doi: 10.1007/s00262-007-0428-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawkins RE, Macdermott C, Shablak A, Hamer C, Thistlethwaite F, Drury NL, Chikoti P, Shingler W, Naylor S, Harrop R. Vaccination of patients with metastatic renal cancer with modified vaccinia Ankara encoding the tumor antigen 5T4 (TroVax) given alongside interferon-alpha. J Immunother. 2009;32(4):424–429. doi: 10.1097/CJI.0b013e31819d297e. [DOI] [PubMed] [Google Scholar]
- 18.Dallman PR. Iron deficiency and the immune response. Am J Clin Nutr. 1987;46(2):329–334. doi: 10.1093/ajcn/46.2.329. [DOI] [PubMed] [Google Scholar]
- 19.Ekiz C, Agaoglu L, Karakas Z, Gurel N, Yalcin I. The effect of iron deficiency anemia on the function of the immune system. Hematol J. 2005;5(7):579–583. doi: 10.1038/sj.thj.6200574. [DOI] [PubMed] [Google Scholar]
- 20.Zhu J, Martinez J, Huang X, Yang Y. Innate immunity against vaccinia virus is mediated by TLR2 and requires TLR-independent production of IFN-beta. Blood. 2007;109(2):619–625. doi: 10.1182/blood-2006-06-027136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hutchens MA, Luker KE, Sonstein J, Nunez G, Curtis JL, Luker GD. Protective effect of toll-like receptor 4 in pulmonary vaccinia infection. PLoS Pathog. 2008;4(9):e1000153. doi: 10.1371/journal.ppat.1000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin T, Kwak YH, Sammy F, He P, Thundivalappil S, Sun G, Chao W, Warren HS. Synergistic inflammation is induced by blood degradation products with microbial toll-like receptor agonists and is blocked by hemopexin. J Infect Dis. 2010;202(4):624–632. doi: 10.1086/654929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berry DA. The hazards of endpoints. J Natl Cancer Inst. 2010;102(18):1376–1377. doi: 10.1093/jnci/djq334. [DOI] [PubMed] [Google Scholar]
- 24.Hoos A, Eggermont AM, Janetzki S, Hodi FS, Ibrahim R, Anderson A, Humphrey R, Blumenstein B, Old L, Wolchok J. Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst. 2010;102(18):1388–1397. doi: 10.1093/jnci/djq310. [DOI] [PMC free article] [PubMed] [Google Scholar]