Abstract
Dendritic cell (DC) vaccination has demonstrated potential in clinical trials as a new effective cancer treatment, but objective and durable clinical responses are confined to a minority of patients. Interferon (IFN)-α, a type-I IFN, can bolster anti-tumor immunity by restoring or increasing the function of DCs, T cells and natural killer (NK) cells. Moreover, type-I IFN signaling on DCs was found to be essential in mice for tumor rejection by the innate and adaptive immune system. Targeted delivery of IFN-α by DCs to immune cells could boost the generation of anti-tumor immunity, while avoiding the side effects frequently associated with systemic administration. Naturally circulating plasmacytoid DCs, major producers of type-I IFN, were already shown capable of inducing tumor antigen-specific T cell responses in cancer patients without severe toxicity, but their limited number complicates their use in cancer vaccination. In the present work, we hypothesized that engineering easily generated human monocyte-derived mature DCs to secrete IFN-α using mRNA electroporation enhances their ability to promote adaptive and innate anti-tumor immunity. Our results show that IFN-α mRNA electroporation of DCs significantly increases the stimulation of tumor antigen-specific cytotoxic T cell as well as anti-tumor NK cell effector functions in vitro through high levels of IFN-α secretion. Altogether, our findings mark IFN-α mRNA-electroporated DCs as potent inducers of both adaptive and innate anti-tumor immunity and pave the way for clinical trial evaluation in cancer patients.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-015-1688-2) contains supplementary material, which is available to authorized users.
Keywords: Dendritic cells, Cytotoxic T cells, Natural killer cells, Interferon-alpha, mRNA electroporation, Cancer immunotherapy
Introduction
The understanding that the immune system can specifically recognize and eliminate tumor cells has fueled the development of cancer immunotherapy. Based on the potent immune stimulatory capacity of dendritic cells (DCs) and the promising results from early clinical trials, DC vaccination is currently widely investigated for its therapeutic potential in various solid and hematological malignancies [1]. We recently demonstrated in a phase I/II clinical trial that DCs, loaded with the tumor-associated antigen Wilms’ tumor 1 (WT1) by mRNA electroporation, could induce clinical responses in acute myeloid leukemia patients, which correlated with the expansion of WT1-specific T cells and the activation of natural killer (NK) cells [2]. Yet, in general, objective and durable clinical responses are limited to a minority of cancer patients, spurring the exploration of strategies that could improve the therapeutic efficacy of DC vaccination [3–5].
Interferon (IFN)-α has long been known for its direct and immune-mediated anti-proliferative action in different malignancies, but is nowadays rarely used in cancer treatment because of heterogeneous clinical results and significant toxicity following systemic administration [6–9]. Nevertheless, the recent observation that type-I IFN signaling on DCs is essential in mice for tumor rejection by the innate and adaptive immune system rekindles interest in IFN-α in this era of immunotherapy [10, 11]. Targeted delivery of IFN-α by DCs to immune cells in the context of cancer vaccination could limit the troublesome side effects while still allowing the exploitation of its natural immune adjuvant properties [12–14]. Notably, given its ability to stimulate IFN-γ production and cytotoxicity by CD8+ T cells and NK cells [14], IFN-α secretion by DCs could facilitate the generation of strong adaptive as well as innate anti-tumor immunity. Enlisting innate immunity might be particularly relevant in the context of DC cancer vaccination, since NK cells are increasingly recognized as important mediators of anti-tumor immunity and their activation following DC vaccination has been linked to favorable clinical outcome [2, 15, 16].
Naturally circulating plasmacytoid DCs, major producers of type-I IFN, already demonstrated the ability to induce tumor antigen-specific T cell responses in cancer patients [17], but their limited availability complicates their use in cancer vaccination. We hypothesized that engineering easily generated human monocyte-derived DCs to secrete IFN-α increases their capacity for stimulating both anti-tumor T cell and NK cell immunity and can boost the therapeutic efficacy of DC cancer vaccination. The latter is supported by mouse studies in which DCs modified to express IFN-α through viral gene transduction improved clinical outcome in different tumor models [18–20]. This approach has not translated into clinical trials, however, which could be related to the reluctance to use viral transduction in a patient setting. Therefore, in the present work, we engineered human monocyte-derived mature DCs to secrete IFN-α using mRNA electroporation, an efficient and clinically safe transfection method implemented worldwide in clinical grade DC vaccine preparation [21–23].
In summary, we show that mature DCs electroporated with IFN-α mRNA secrete high levels of IFN-α and can be effectively co-electroporated with WT1 mRNA, while preserving IFN-α secretion and WT1 antigen presentation. Moreover, we demonstrate that IFN-α mRNA electroporation significantly enhances the ability of DCs to promote tumor antigen-specific CD8+ T cell and anti-tumor NK cell effector functions in vitro through IFN-α signaling.
Materials and methods
Experimental conditions and cell material
This study and reporting thereof is MIATA compliant (supplementary MIATA information) [24]. Experiments were approved by the ethics committee of the University of Antwerp, Antwerp, Belgium and performed using buffy coats derived from anonymous healthy volunteers’ peripheral blood (with citrate phosphate dextrose anticoagulant) donated the day before and stored overnight at room temperature (Red Cross Blood Transfusion Center, Antwerp, Edegem, Belgium). Peripheral blood mononuclear cells (PBMCs) were isolated from buffy coats by Ficoll density gradient separation (Ficoll-Paque PLUS, GE Healthcare, Diegem, Belgium). The K562 (American Type Culture Collection, Rockville, MD, USA) and Daudi (gift from Dr. K. Thielemans, Free University of Brussels (VUB), Brussels, Belgium) cell lines were cultured in complete medium consisting of Iscove’s modified Dulbecco’s medium (IMDM) with 10 % fetal bovine serum (FBS; microorganism pretested and qualified, cat# 10270-106, Life Technologies, Merelbeke, Belgium). The cytotoxic CD8+ T cell clone specific for the human leukocyte antigen (HLA)-A*0201-restricted WT1126 epitope (WT1 amino acids 126–134: RMFPNAPYL) was kindly provided by Dr. P. Greenberg (University of Washington, Seattle, WA, USA). In all experiments, cells were counted using an automated ABX Micros 60 cell counter (Horiba ABX, Deurne, Belgium), and (co-)cultures were maintained at 37 °C. Cryopreserved cell samples were frozen down in FBS with 10 % dimethyl sulfoxide (Sigma-Aldrich, Diegem, Belgium) in a Nalgene Mr. Frosty™ Cryo 1 °C freezing container (Thermo Fisher Scientific, Erembodegem, Belgium) at −80 °C. All experiments were conducted in a biosafety level 2-certified laboratory that operates under exploratory research principles using established laboratory protocols and investigative research assays.
mRNA
The human IFN-α2b gene was subcloned into a pST1 plasmid vector, putting it under the control of a T7 promoter and providing it with a poly(A)tail [25]. mRNA transcripts were generated using a mMessage mMachine T7 in vitro transcription kit (Life Technologies) according to the manufacturer’s protocol. Clinical grade in vitro transcribed WT1 mRNA was produced by eTheRNA (Kortenberg, Belgium) from a T7 promoter-driven plasmid containing the codon-optimized human WT1 gene, lacking the 5′ and 3′ untranslated regions and the nuclear localization signal (aa292–348), and flanked by the signal sequence and HLA class II-targeting sequence of DC-lysosome-associated membrane protein [26].
DC generation
DCs were generated according to our acute myeloid leukemia clinical trial protocol (clinicaltrials.gov—NCT00834002, NCT00965224 and NCT01686334) with adaptations for research [2, 27]. Briefly, CD14+ monocytes were positively selected from fresh PBMCs using CD14 MicroBeads according to the manufacturer’s instructions (Miltenyi Biotec, Leiden, The Netherlands) and added to T175 culture flasks [Becton–Dickinson (BD), Erembodegem, Belgium] at a final concentration of 1.0–1.4 × 106 cells/mL in 50–70 mL Roswell Park Memorial Institute (RPMI) 1640 medium with 2.5 % heat-inactivated human AB serum (microorganism pretested and qualified, cat# 34005-100, Life Technologies), 800 U/mL granulocyte–macrophage colony-stimulating factor (Gentaur, Brussels, Belgium) and 20 ng/mL interleukin (IL)-4 (Life Technologies). After 5 days, 20 ng/mL tumor necrosis factor-α (Gentaur) and 2.5 µg/mL prostaglandin E2 (Pfizer, Puurs, Belgium) were added to induce maturation. DCs were harvested 40–44 h later and used as such (non-EP DCs) or following electroporation without mRNA (mock EP DCs), with 10 µg WT1 mRNA (WT1 EP DCs), with 10 µg IFN-α mRNA (IFN-α EP DCs), or with both 10 µg WT1 mRNA and 10 µg IFN-α mRNA (WT1/IFN-α EP DCs) in 200 µL Opti-MEM reduced serum medium without phenol red (Life Technologies). Immediately following electroporation, DCs were collected and washed in RPMI 1640 medium supplemented with 2.5 % human AB serum and used in further experiments. In specific experiments, DCs were labeled with the cytoplasmic fluorescent CellTracker Violet BMQC dye (Violet; Life Technologies) prior to electroporation, as described previously [28].
NK cells
CD3−CD56+ NK cells were isolated from fresh CD14+ monocyte-depleted PBMCs using a negative selection NK cell isolation kit (Miltenyi Biotec) and then cryopreserved. After 7 days, NK cells were thawed and washed in preheated (37 °C) complete medium and cultured alone or together with autologous DCs at a 1:1 ratio in complete medium with or without 104 U/mL (~30–50 ng/mL [29, 30]) exogenously added rIFN-α2 (Peprotech, Rocky Hill, NJ, USA).
IFN-α and IFN-γ ELISA
IFN-α and IFN-γ secretion was determined by ELISA using a human IFN-α-matched antibody pairs kit (eBioscience, Vienna, Austria) and IFN-γ ELISA kit (Peprotech), respectively, according to the manufacturers’ protocols. Standards and samples were measured in duplicate and triplicate, respectively, in a 96-well flat-bottom microplate (Nunc, Rochester, NY, USA) on a Victor3 multilabel counter (PerkinElmer, Waltham, MA, USA). IFN-α levels were calculated in IU according to a conversion factor of 3 pg/IU [29, 30].
WT1 staining
DCs were stained with LIVE/DEAD® Fixable Green Stain (Life Technologies) prior to fixation and permeabilization for intracellular staining using the Foxp3/Transcription Factor Staining Buffer Set (eBioscience) according to the manufacturer’s instructions. Subsequently, DCs were incubated with mouse anti-WT1 monoclonal antibody (mAb; clone 6F-H2, Dako, Heverlee, Belgium) followed by PE-labeled rabbit anti-mouse immunoglobulin polyclonal antibody (Dako). WT1 expression in viable (LIVE/DEAD−) cells was determined on a FACScan flow cytometer (BD; Suppl. Fig. 1).
Immunophenotyping
DC and NK cell phenotypes were examined using combinations of fluorochrome-conjugated mAbs (BD) followed by analysis on a FACSAria II flow cytometer (BD). DCs were surface stained 48 h after electroporation with FITC-, V450-, APC- and APC-H7-conjugated mAbs against CCR7, CD14, CD40, CD80, CD83, CD86 and HLA-DR. DCs were gated on side scatter/forward scatter properties (Suppl. Fig. 2). NK cells were surface stained 48 h after initiation of DC/NK cell co-cultures with FITC-, PE-, V450-, APC-/AF647- and APC-Cy7-/APC-H7-conjugated mAbs against CD16, CD56, CD69, HLA-DR, NKG2D, NKp30 and NKp46. NK cells were gated on lymphocyte side scatter/forward scatter properties and CD56 expression (Suppl. Fig. 2). DC and NK cell viability was determined based on 7-amino actinomycin D (7-AAD; BD) staining (Suppl. Fig. 2). Dead (7-AAD+) cells were excluded from phenotypic analysis. Mean fluorescence intensity was used for statistical comparison.
Mixed lymphocyte reaction
Thawed CD14+ monocyte-depleted PBMCs were labeled with CFSE (5 µM; Life Technologies) according to the manufacturer’s instructions and used as responder cells in a mixed lymphocyte reaction (MLR) at a DC:responder cell ratio of 1:10, as also described previously [31]. Specifically, 2 × 105 allogeneic responder cells were cultured with 2 × 103 non-EP DCs, mock EP DCs or IFN-α EP DCs in 200 µL complete medium. In specific conditions, DCs were incubated with anti-IFN-α neutralizing IgA mAb or corresponding IgA isotype control mAb (2 µg/104 DCs; InvivoGen, Toulouse, France) for 1 h prior to the addition of responder cells. Medium stimulation and PHA/IL-2 served as negative and positive controls, respectively. After 5 days, samples were stained with LIVE/DEAD® Fixable Aqua Stain (Life Technologies), CD3–PerCP–Cy5.5 (BD), CD4–APC–H7 (BD) and CD8–PB (Life Technologies) and measured on a FACSAria II flow cytometer. CD4+ and CD8+ T cell proliferation was assessed by quantifying the percentage of proliferating (CFSE-diluted) cells within the viable (LIVE/DEAD−) CD3+CD4+ and CD3+CD8+ lymphocyte population, respectively (Suppl. Fig. 3).
WT1126-specific cytotoxic CD8+ T cell clone activation
The WT1126-specific T cell clone was cultured in triplicate with mock EP DCs, WT1 EP DCs, IFN-α EP DCs or WT1/IFN-α EP DCs from HLA-A*0201+ donors at a DC:T cell ratio of 4:1 in IMDM supplemented with 1 % human AB serum in 96-well round-bottom microplates (Corning, Amsterdam, The Netherlands). Cultures of DCs or T cells alone and cultures of T cells with mock EP DCs and 50 µg/mL WT1126 peptide (Eurogentec, Seraing, Belgium) served as negative and positive controls, respectively. After overnight culture, supernatants were collected and cryopreserved at −20 °C for IFN-γ quantification.
NK cell functional assay
K562 and Daudi tumor cells were labeled with the green fluorescent membrane dye PKH67 according to the manufacturer’s instructions (Sigma-Aldrich) and served as target cells in a flow cytometry-based cytotoxicity assay as described previously with minor adaptations [28, 31]. In short, PKH67-labeled tumor cells were cultured alone or added to 48-h cultures of NK cells and/or Violet-stained DCs at a DC:NK cell:tumor cell ratio of 5:5:1. After 4 h, supernatants were collected for IFN-γ quantification and cells were acquired on a FACSAria II flow cytometer following staining with annexin V-APC (BD) and propidium iodide (PI; Life Technologies). Killing was calculated based on the percentages of viable (annexin V−/PI−) cells within the Violet−/PKH67+ tumor cell population (Suppl. Fig. 4) using the following equation: % killing = 100 % − (% viable tumor cells with effector cells/% viable tumor cells without effector cells). In specific experiments, anti-IFN-α neutralizing IgA mAb or corresponding IgA isotype control mAb (InvivoGen) was added to Violet-stained DCs (10 µg/5 × 105 DCs) 1 h prior to the addition of NK cells.
Data analysis
Raw data were routinely checked for consistency and plausibility and can be provided upon motivated request. All samples were compared empirically to their respective negative and positive controls to determine reactivity and whether they fell within the ranges of the assay. Flow cytometry data were analyzed using FlowJo v9.3 or vX.0.6 (Treestar, Ashland, OR, USA). GraphPad Prism 5 software (GraphPad, San Diego, CA, USA) was used for data comparison, statistical calculations and artwork. Statistical analysis was performed using repeated-measures one-way analysis of variance (ANOVA) with Bonferroni post hoc test. Differences were predefined to be considered as statistically significant when p < 0.05.
Results
Electroporation of DCs with IFN-α mRNA results in substantial IFN-α secretion and can be combined with WT1 mRNA co-electroporation
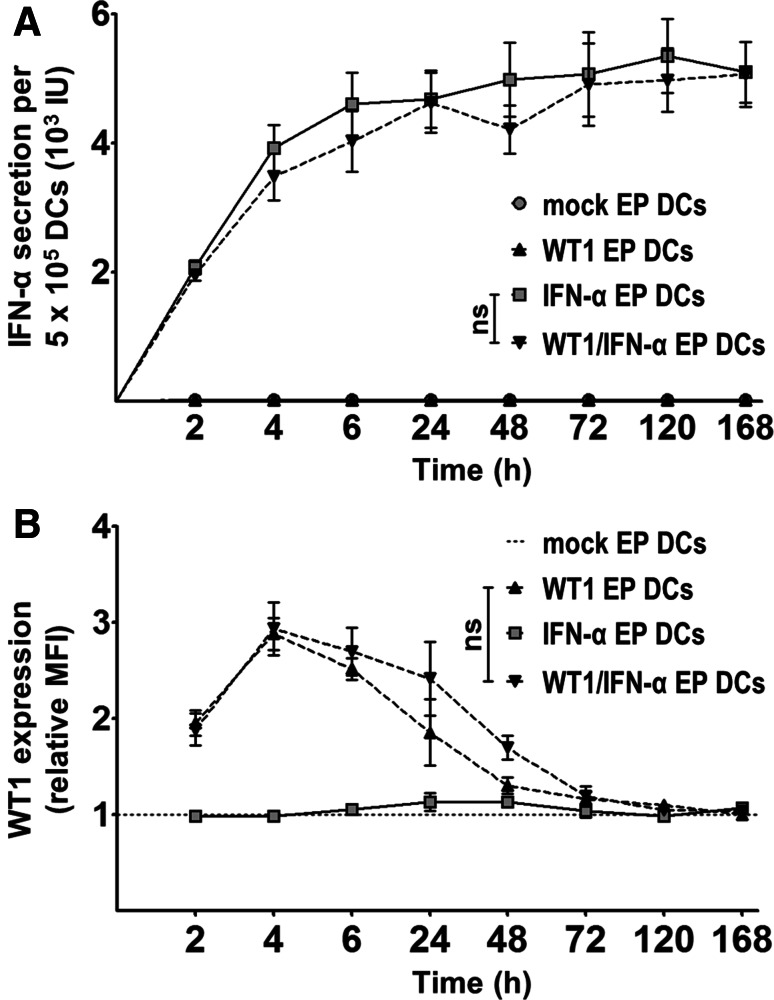
To determine whether IFN-α mRNA-electroporated DCs produce IFN-α, we quantified IFN-α levels in the supernatants of mock EP DCs, WT1 EP DCs, IFN-α EP DCs and WT1/IFN-α EP DCs at different time points (2, 4, 6, 24, 48, 72, 120 and 168 h) after electroporation. Simultaneously, we examined WT1 protein expression in these DCs to verify that IFN-α mRNA electroporation does not interfere with tumor antigen translation following mRNA co-electroporation. Whereas mock EP DCs and WT1 EP DCs did not secrete detectable levels of IFN-α (<2.6 IU/5 × 105 DCs; n = 6; Fig. 1a), IFN-α EP DCs and WT1/IFN-α EP DCs secreted comparably high levels of this cytokine (mean 5.9 [range 2.8–10.8] and 5.6 [range 2.3–10.7] × 103 IU/5 × 105 DCs, respectively; n = 6; Fig. 1a). Results further showed that IFN-α secretion was highest within the first 4 h after electroporation and virtually fell to zero after 24 h (n = 6; Fig. 1a). WT1 protein expression in IFN-α EP DCs did not differ significantly from background levels found in mock EP DCs at the evaluated time points (n = 6; Fig. 1b). Conversely, WT1 EP DCs and WT1/IFN-α EP DCs demonstrated increased WT1 expression with comparable expression levels and kinetics (n = 6; Fig. 1b). Altogether, these data show that electroporation of DCs with IFN-α mRNA results in high IFN-α secretion, independent of co-electroporation with WT1 mRNA, and that, in turn, WT1 expression is not hampered by IFN-α mRNA co-electroporation.
Fig. 1.
Electroporation of DCs with IFN-α mRNA results in substantial IFN-α secretion and can be combined with WT1 mRNA co-electroporation. a IFN-α levels were quantified by ELISA in culture supernatants of mock EP DCs, WT1 EP DCs, IFN-α EP DCs and WT1/IFN-α EP DCs 2, 4, 6, 24, 48, 72, 120 and 168 h after electroporation. b Simultaneously, DCs were examined for WT1 protein expression by intracellular staining. Expression levels (in mean fluorescence intensity) were transformed to relative levels compared to those of the corresponding mock EP DCs, which were set to one. Data are depicted as mean (±SEM) of six independent donors. ns not significant, repeated-measures one-way ANOVA with Bonferroni post hoc test. MFI mean fluorescence intensity
IFN-α mRNA electroporation slightly increases DC viability and maturation
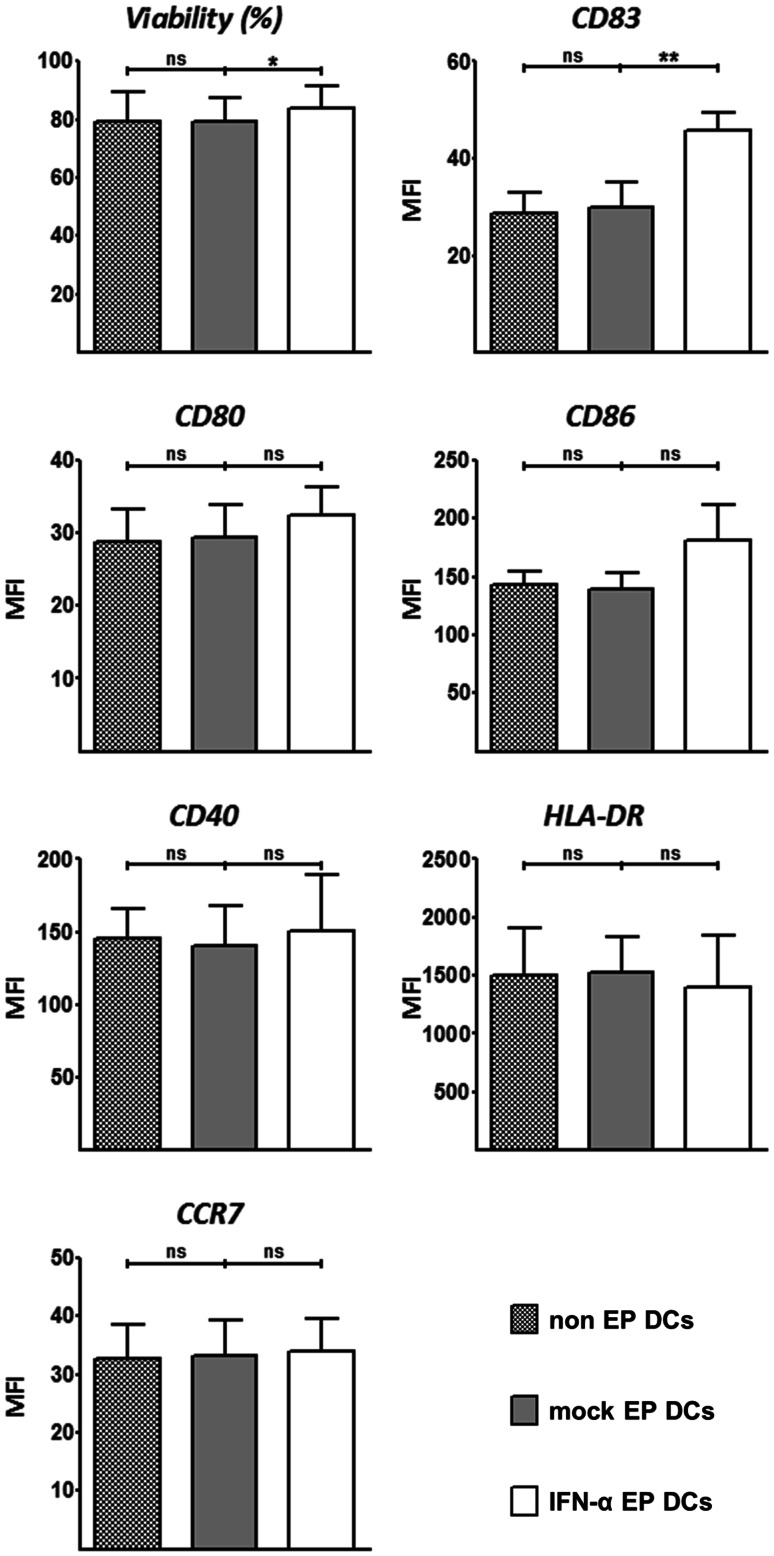
To investigate the effects of IFN-α mRNA electroporation on DC viability and phenotype, we compared non-EP DCs, mock EP DCs and IFN-α EP DCs for these parameters after 48 h of culture. There was no difference in viability or surface marker expression of any of the tested markers between non-EP DCs and mock EP DCs (Fig. 2). Viability of IFN-α EP DCs was marginally higher than that of non-EP DCs and mock EP DCs (83.9 vs. 79.1 and 79.3 %, respectively; p < 0.05; n = 4; Fig. 2). Non-EP DCs, mock EP DCs and IFN-α EP DCs all expressed CD83, CD80, CD86, CD40, HLA-DR and CCR7, as fluorescence levels following staining for these markers always exceeded those obtained following corresponding isotype staining (data not shown). Yet, CD83 was appreciably up-regulated on IFN-α EP DCs compared to non-EP DCs and mock EP DCs (p < 0.01; n = 4; Fig. 2). Surface expression of other markers was slightly increased (CD80, CD86 and CD40) or decreased (HLA-DR), or virtually unchanged (CCR7) on IFN-α EP DCs compared to non-EP DCs and mock EP DCs, but these differences were not statistically significant (Fig. 2). Non-EP DCs, mock EP DCs and IFN-α EP DCs did not express CD14 (data not shown), as fluorescence levels following CD14 staining were lower than those following corresponding isotype staining. For each of the isotype stainings, fluorescence levels did not differ between non-EP DCs, mock EP DCs and IFN-α EP DCs (data not shown). These results demonstrate that IFN-α mRNA electroporation of DCs does not negatively affect and even to some degree increases viability and phenotypic maturation.
Fig. 2.
IFN-α mRNA electroporation slightly increases DC viability and maturation. Viability and phenotype of non-EP DCs, mock EP DCs and IFN-α EP DCs were determined by flow cytometry 48 h after electroporation. Data are depicted as mean (+SD) of four independent donors. *p < 0.05, **p < 0.01, ns not significant, repeated-measures one-way ANOVA with Bonferroni post hoc test. MFI mean fluorescence intensity
IFN-α mRNA electroporation of DCs reduces the induction of allogeneic CD4+ and CD8+ T cell proliferation in an IFN-α-dependent manner
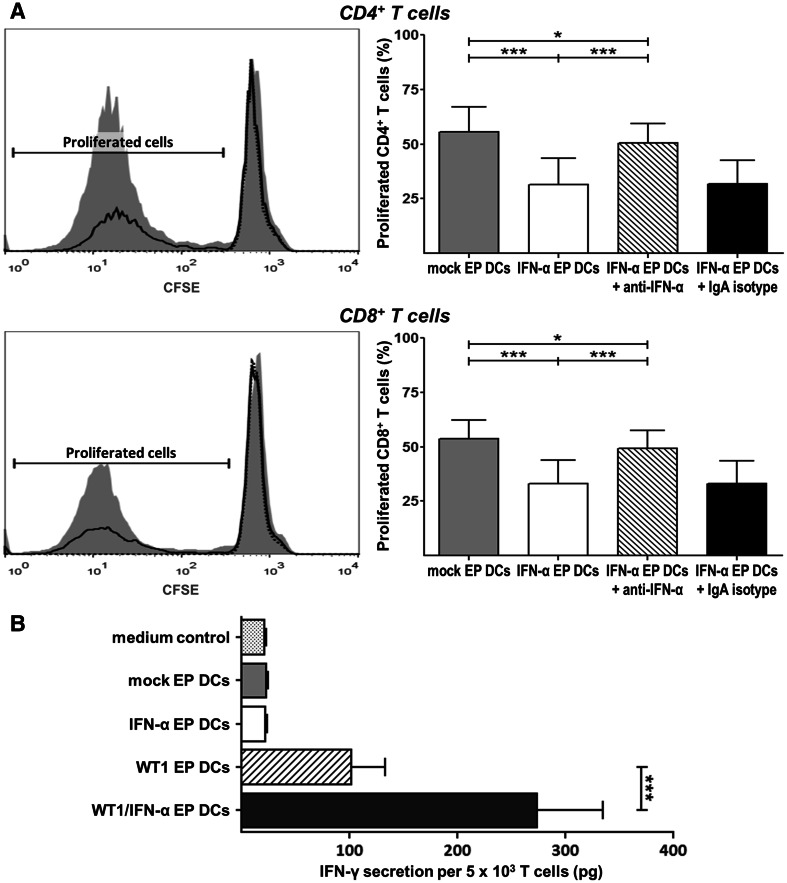
To assess whether IFN-α mRNA electroporation influences DC-induced CD4+ and CD8+ T cell proliferation, we compared non-EP DCs, mock EP DCs and IFN-α EP DCs in an MLR. There were no differences in allogeneic CD4+ or CD8+ T cell proliferation between conditions stimulated with non-EP DCs versus mock EP DCs (data not shown). Whereas mock EP DCs and IFN-α EP DCs both stimulated allogeneic CD4+ and CD8+ T cell proliferation, as evidenced by CFSE dilution, the former did so to a significantly higher extent than the latter (Fig. 3). Addition of anti-IFN-α mAb, but not its isotype control, increased CD4+ and CD8+ T cell proliferation in conditions stimulated with IFN-α EP DCs to levels almost equal to those found in conditions stimulated with mock EP DCs (Fig. 3a). These data show that IFN-α EP DCs retain allo-stimulatory properties, but induce significantly less proliferation of allogeneic CD4+ and CD8+ T cells than their mock EP DC counterparts, and that this effect depends on IFN-α signaling.
Fig. 3.
IFN-α mRNA electroporation of DCs reduces the induction of allogeneic T cell proliferation, but enhances WT1-specific cytotoxic CD8+ T cell activation. a Mock EP DCs and IFN-α EP DCs served as stimulators for CFSE-labeled allogeneic, CD14+ monocyte-depleted PBMC responder cells at a DC:responder cell ratio of 1:10. In specific conditions, anti-IFN-α neutralizing IgA mAb or corresponding IgA isotype control mAb (2 µg/104 DCs) was added to DCs 1 h prior to the addition of responder cells. After 5 days, the percentage of proliferated (CFSE-diluted) cells within the viable (LIVE/DEAD−) CD3+CD4+ and CD3+CD8+ lymphocyte populations was determined by flow cytometry. Histogram overlays show CFSE dilution and gating of proliferated cells after stimulation with medium (dotted black line), mock EP DCs (grey filled area), or IFN-α EP DCs (full black line) for one representative donor from one experiment out of two experiments on four independent donors. The percentages of proliferated CD4+ and CD8+ T cells are depicted as mean (+SD) of two experiments on four independent donors. b The WT1126-specific T cell clone was cultured overnight without DCs (medium control), with mock EP DCs, with IFN-α EP DCs, with WT1 EP DCs or with WT1/IFN-α EP DCs from HLA-A*0201+ donors at a DC:T cell ratio of 4:1. IFN-γ levels were quantified by ELISA in culture supernatants. Data are depicted as mean (+SD) of three independent donors. *p < 0.05, ***p < 0.001, repeated-measures one-way ANOVA with Bonferroni post hoc test. MFI mean fluorescence intensity
IFN-α mRNA electroporation of DCs enhances WT1-specific cytotoxic CD8+ T cell clone activation
To evaluate the effects of IFN-α mRNA electroporation of DCs on WT1-specific T cell activation, we tested the capacity of mock EP DCs, IFN-α EP DCs, WT1 EP DCs and WT1/IFN-α EP DCs for inducing IFN-γ secretion by a WT1126-specific cytotoxic CD8+ T cell clone. T cells stimulated with mock EP DCs or IFN-α EP DCs did not secrete more IFN-γ than T cells cultured alone (medium control; Fig. 3b). In contrast, IFN-γ secretion by T cells stimulated with WT1 EP DCs or WT1/IFN-α EP DCs exceeded medium control baseline levels (Fig. 3b). Remarkably, stimulation with WT1/IFN-α EP DCs resulted in 2.7-fold higher IFN-γ secretion compared to stimulation with WT1 EP DCs (273.8 vs. 101.3 pg/5 × 103 T cells, respectively; p < 0.001; n = 3; Fig. 3b). These results indicate that IFN-α mRNA co-electroporation of WT1 mRNA-loaded DCs enhances their ability to activate cytotoxic CD8+ T cells specific for this tumor-associated antigen.
IFN-α EP DCs promote autologous NK cell survival and activation
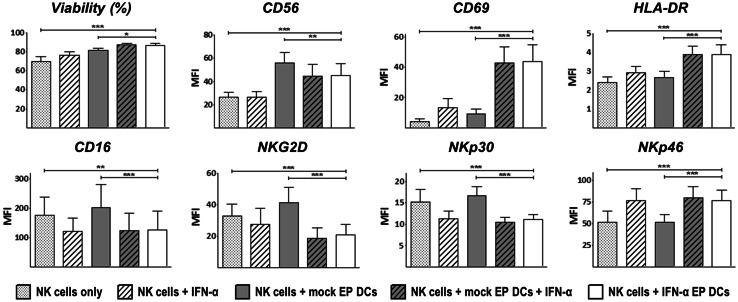
To investigate the NK cell stimulatory capacity of IFN-α EP DCs, we compared viability and phenotype of NK cells cultured alone or with autologous IFN-α EP DCs for 48 h. In addition, NK cells were cultured with rIFN-α, with mock EP DCs, or with mock EP DCs plus rIFN-α to define whether effects depended on IFN-α, DCs or both. Culture with IFN-α EP DCs significantly improved NK cell survival compared to NK cells cultured alone (86.7 vs. 70.0 %, respectively; p < 0.001; n = 4; Fig. 4). While rIFN-α and mock EP DCs each also increased survival of NK cells (76.1 and 81.3 %, respectively; p < 0.01 and p < 0.001, respectively; n = 4), the combined signal provided by IFN-α EP DCs was superior to both (p < 0.001 and p < 0.05, respectively; Fig. 4).
Fig. 4.
IFN-α EP DCs promote autologous NK cell survival and activation. NK cells were cultured alone or together with autologous mock EP DCs or IFN-α EP DCs at a DC:NK cell ratio of 1:1. As additional controls, NK cells were cultured with 104 U/mL (~30–50 ng/mL) rIFN-α or with mock EP DCs plus 104 U/mL IFN-α. After 48 h, NK cell viability and phenotype were determined by flow cytometry. NK cells were discriminated from DCs based on lymphocyte side scatter/forward scatter properties and CD56 expression. Data are expressed as mean (+SD) of four independent donors. *p < 0.05, **p < 0.01, ***p < 0.001, repeated-measures one-way ANOVA with Bonferroni post hoc test. MFI mean fluorescence intensity
Besides superior survival, NK cells cultured with IFN-α EP DCs showed a marked up-regulation of the phenotypic activation marker CD69 (p < 0.001; n = 4; Fig. 4). Strikingly, neither rIFN-α nor mock EP DCs alone significantly up-regulated CD69 expression on NK cells (Fig. 4). Furthermore, IFN-α EP DCs also increased the expression of CD56, HLA-DR and NKp46 on NK cells (p < 0.001 each; n = 4; Fig. 4). Conversely, CD16, NKG2D and NKp30 expression was down-regulated on NK cells cultured with IFN-α EP DCs (p < 0.01, p < 0.001 and p < 0.001, respectively; n = 4; Fig. 4). Of note, CD16, NKp30 and NKp46 expression did not differ between NK cells cultured with IFN-α EP DCs or rIFN-α (Fig. 4). Altogether, these data demonstrate that IFN-α EP DCs improve survival and induce activation of autologous NK cells.
IFN-α EP DCs boost autologous NK cell cytotoxicity and IFN-γ secretion against tumor cells
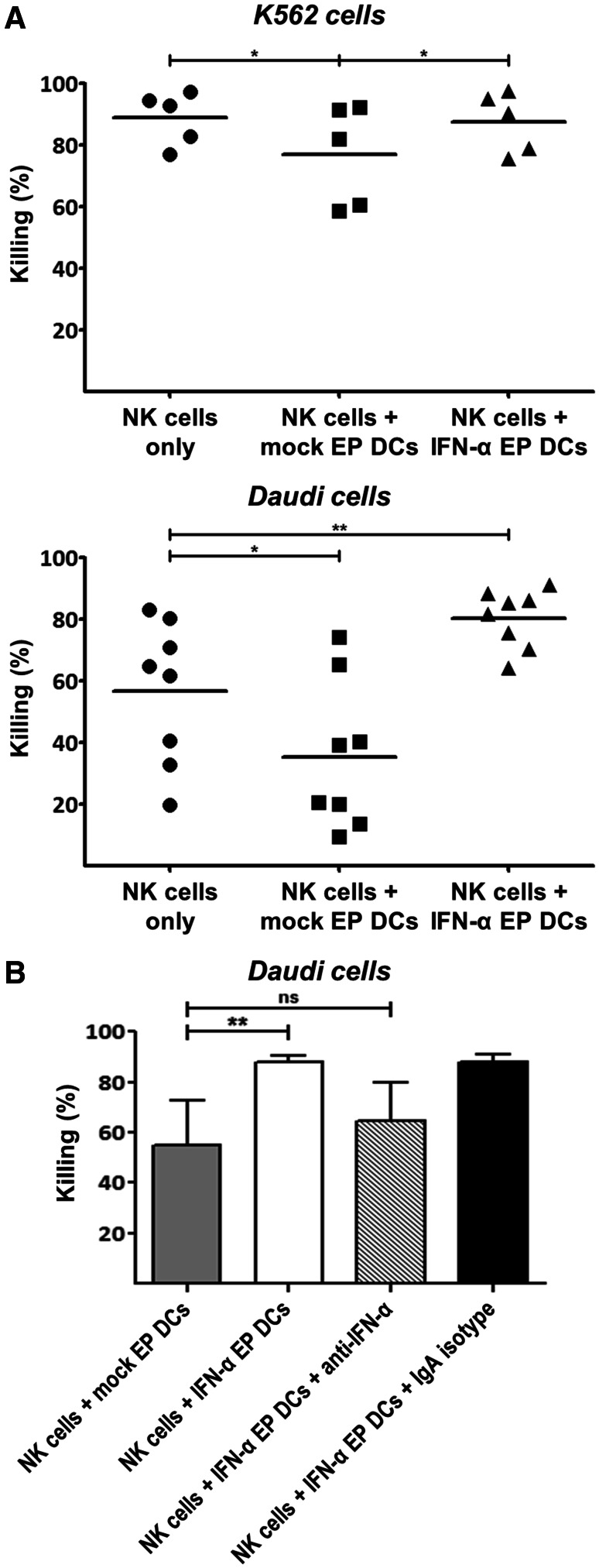
To assess whether IFN-α EP DCs stimulate the anti-tumor effector functions of autologous NK cells, we determined the cytotoxicity and IFN-γ secretion by NK cells and/or DCs against K562 or Daudi tumor cells. The fact that mock EP DCs and IFN-α EP DCs alone did not show any cytotoxicity against K562 or Daudi cells implies that IFN-α secretion by DCs did not directly affect tumor cell viability (data not shown). Surprisingly, compared to NK cells cultured alone, mock EP DCs reduced NK cell cytotoxicity against K562 and Daudi cells (Fig. 5). In contrast, IFN-α EP DCs preserved NK cell cytotoxicity against K562 cells and improved cytotoxicity against Daudi cells (Fig. 5). IFN-α secretion by IFN-α EP DCs was crucial for their stimulatory effect on NK cell cytotoxicity against Daudi cells, since antibody neutralization of IFN-α reduced mean killing by NK cells from 87.9 to 64.6 % (p > 0.05; n = 4; Fig. 5b).
Fig. 5.
IFN-α EP DCs sustain or improve autologous NK cell cytotoxicity against tumor cells. NK cells were cultured alone or together with Violet-stained autologous mock EP DCs or IFN-α EP DCs at a DC:NK cell ratio of 1:1. After 48 h, PKH67-labeled K562 or Daudi tumor cells were added at a DC:NK cell:tumor cell ratio of 5:5:1. Tumor cells cultured alone served as controls. After 4 h, the percentage of viable (annexin V−/PI−) cells within the Violet−/PKH67+ tumor cell population was determined by flow cytometry. Killing of tumor cells was calculated as follows: % killing = 100 % − (% viable tumor cells with effector cells/% viable tumor cells without effector cells). a Killing of K562 or Daudi cells is expressed as individual values and means of 5–8 independent donors. b One hour prior to the addition of NK cells, anti-IFN-α neutralizing IgA or corresponding IgA isotype control antibody (10 µg/5 × 105 DCs) was added to DCs for the remainder of the co-culture. Killing of Daudi cells is depicted as mean (+SD) of four independent donors. *p < 0.05, **p < 0.01, ns not significant, repeated-measures one-way ANOVA with Bonferroni post hoc test
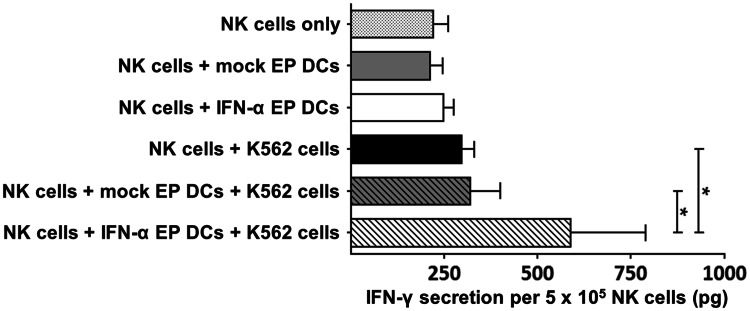
In addition to enhanced cytotoxicity against Daudi cells, NK cells cultured with IFN-α EP DCs also secreted significantly more IFN-γ in response to K562 cells than NK cells cultured alone or with mock EP DCs (p < 0.05 each; n = 4; Fig. 6). Strikingly, only the dual signal from IFN-α EP DCs and K562 cells prompted a statistically significant increase in IFN-γ secretion, whereas either IFN-α EP DCs or K562 cells did not (Fig. 6). Collectively, these results show that IFN-α EP DCs enhance the anti-tumor effector functions of autologous NK cells, as illustrated by improved cytotoxicity and IFN-γ secretion against tumor cells.
Fig. 6.
IFN-α EP DCs boost IFN-γ secretion by autologous NK cells in response to tumor cells. NK cells and mock EP DCs or IFN-α EP DCs were cultured alone or together at a DC:NK cell ratio of 1:1. After 48 h, medium or K562 tumor cells were added to the DC/NK cell cultures at a DC:NK cell:tumor cell ratio of 5:5:1. Tumor cells cultured alone served as controls. After 4 h, supernatants were collected for IFN-γ quantification by ELISA. Data are depicted as mean (+SD) of four independent donors. *p < 0.05, repeated-measures one-way ANOVA with Bonferroni post hoc test
Discussion
Clinical trials by our group and others demonstrated that DC vaccination can induce objective clinical responses in cancer patients without significant toxicity [2–5]. However, such responses currently only occur in a minority of patients. Integrating IFN-α into DC cancer vaccination could improve therapeutic efficacy, because of the favorable effects of type-I IFN on innate and adaptive anti-tumor immunity [10–14].
In this study, we engineered human monocyte-derived mature DCs to produce IFN-α using mRNA electroporation and found that this approach resulted in the transient secretion of high levels of IFN-α, limited almost entirely to the first 24 h after electroporation. Although it has been described that individual transgene protein expression might decrease when cells are simultaneously electroporated with different mRNAs [32], we demonstrated that both IFN-α secretion and WT1 expression were sustained following co-electroporation of DCs with IFN-α and WT1 mRNA. Furthermore, we showed that IFN-α mRNA electroporation did not negatively affect and even slightly increased viability and phenotypic maturation of DCs, which corresponds to earlier studies documenting phenotypic changes on DCs in response to IFN-α/type-I IFN [33–35].
We showed that IFN-α EP DCs induced less allogeneic CD4+ and CD8+ T cell proliferation than their mock EP DC counterparts and established that IFN-α was responsible for this impaired proliferation. This could have been due to direct anti-proliferative action of IFN-α in high doses as well as indirect effects influencing DC/T cell interaction and warrants future research [6]. Even though IFN-α secreted by IFN-α EP DCs reduced allogeneic T cell proliferation, WT1/IFN-α EP DCs proved to be efficient antigen-presenting cells and more potent activators of WT1-specific CD8+ T cells than WT1 EP DC controls, as evidenced by significantly higher IFN-γ secretion by the WT1126-specific CD8+ T cell clone. Possibly, IFN-α stimulated IFN-γ secretion by providing a third signal—next to the T cell receptor and co-stimulatory signaling by DCs—directly to CD8+ T cells, as reported earlier [36, 37]. Another explanation might be that IFN-α enhanced HLA class-I antigen presentation by DCs, similar to a study describing the superiority of DCs generated with IFN-α instead of IL-4 in terms of antigen survival and HLA class-I presentation [38].
In addition, we found that electroporation of DCs with IFN-α mRNA promoted autologous NK cell survival, activation, cytotoxicity and IFN-γ secretion against tumor cells. This corresponds to other studies underscoring the significance of type-I IFN in NK cell homeostasis, activation and anti-tumor function and confirms the two-signal requirement for inducing IFN-γ secretion by NK cells described by Kalinski and others [39–43]. The pronounced effects on survival and phenotypic activation seen after stimulation with both IFN-α and DCs could result from effects working directly and/or indirectly on NK cells, the latter being mediated by DCs in response to type-I IFN [44]. In our study, phenotypic activation and increased function of NK cells were associated with a decreased expression of the activating receptors NKG2D and NKp30, which we hypothesize to be part of a negative feedback mechanism to prevent excessive activation [45]. In addition, we demonstrated through antibody neutralization experiments that the enhancing effect of IFN-α mRNA-electroporated DCs on NK cell cytotoxicity depended at least in part on IFN-α signaling, thereby further supporting the integration of IFN-α into DC vaccination.
As mentioned earlier, the systemic administration of IFN-α to cancer patients is greatly restricted by frequently associated side effects, including asthenia, depression, flu-like symptoms, gastrointestinal complaints and hematological toxicity [6–9]. In patients with metastatic renal cell cancer, IFN-α treatment consists of 3–20 × 106 IU of IFN-α subcutaneously 3 days per week, which leads to severe adverse events in up to 80 % of patients [8, 9]. Considering that we administer at most 10 × 106 DCs/vaccine once every 2 weeks in our clinical trials [2], and taking into account a conversion factor of 3–5 pg/IU for human IFN-α [29, 30] and the observed inter-donor variability, one vaccination with IFN-α mRNA-electroporated DCs is estimated to expose patients to only 0.125–0.2 × 106 IU of IFN-α. This variability in IFN-α secretion by DCs following mRNA electroporation might be regarded as a drawback for clinical application, but can easily be factored into future trials by quantifying IFN-α secretion by thawed DCs for each vaccine batch using ELISA. In any case, we expect that the much lower doses will be better tolerated but will still be able to improve anti-tumor immune induction following DC vaccination, because, in contrast to systemic administration or even co-injection with DCs, IFN-α mRNA electroporation ensures the presence of IFN-α at the immunological synapse between DCs and other immune cells. From a further clinical viewpoint, IFN-α mRNA electroporation also offers important advantages over viral transduction, as applied by others to generate IFN-α-producing DCs in mice [46]. The former results in transient, more controlled IFN-α secretion, does not disrupt the host genome and can be combined in a single electroporation step with other mRNAs, which is already widely used and considered safe for clinical grade DC vaccine preparation [2, 23, 32].
In conclusion, we demonstrated that IFN-α mRNA-electroporated human monocyte-derived mature DCs secrete high levels of IFN-α and that they can be effectively co-electroporated with WT1 mRNA, without compromising IFN-α secretion or WT1 antigen presentation. Moreover, we showed that these DCs are not only excellent stimulators of tumor antigen-specific CD8+ T cells, but also significantly enhance autologous NK cell effector functions via IFN-α signaling. The latter is particularly interesting because the interaction between DCs and NK cells receives increasing appreciation for its role in establishing effective anti-tumor immunity following DC vaccination [2, 16, 47]. Collectively, our findings mark these DCs engineered to secrete IFN-α using mRNA electroporation as potent activators of both adaptive and innate anti-tumor immunity in vitro and pave the way for clinical trial evaluation in cancer patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors would like to thank Maaike W.G. Looman for excellent technical advice. This work was supported in part by grants from the Dutch Cancer Society (KWF; 2009-4402), the Research Foundation—Flanders (FWO), the Foundation against Cancer (STK), and the Methusalem program of the Flemish Government (attributed to Prof. H. Goossens). Y. Willemen is a PhD fellow of the agency for Innovation by Science and Technology (IWT). E.L.J. Smits was supported by an FWO postdoctoral fellowship and a training grant from the Belgian Hematological Society. S. Anguille was funded by an Emmanuel van der Schueren grant from the Flemish League against Cancer (VLK).
Conflict of interest
The authors declare that they have no conflict of interest.
Abbreviations
- 7-AAD
7-Amino actinomycin D
- ANOVA
Analysis of variance
- CFSE
Carboxyfluorescein succinimidyl ester
- DC
Dendritic cell
- EP
Electroporated
- FBS
Fetal bovine serum
- HLA
Human leukocyte antigen
- IFN
Interferon
- IL
Interleukin
- IMDM
Iscove’s modified Dulbecco’s medium
- mAb
Monoclonal antibody
- MLR
Mixed lymphocyte reaction
- NK
Natural killer
- PBMC
Peripheral blood mononuclear cells
- PHA
Phytohemagglutinin
- PI
Propidium iodide
- RPMI
Roswell Park Memorial Institute
- WT1
Wilms’ tumor 1
References
- 1.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Tendeloo VF, Van de Velde A, Van Driessche A, et al. Induction of complete and molecular remissions in acute myeloid leukemia by Wilms’ tumor 1 antigen-targeted dendritic cell vaccination. Proc Natl Acad Sci U S A. 2010;107:13824–13829. doi: 10.1073/pnas.1008051107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anguille S, Smits EL, Lion E, van Tendeloo VF, Berneman ZN. Clinical use of dendritic cells for cancer therapy. Lancet Oncol. 2014;15:e257–e267. doi: 10.1016/S1470-2045(13)70585-0. [DOI] [PubMed] [Google Scholar]
- 5.Anguille S, Willemen Y, Lion E, Smits EL, Berneman ZN. Dendritic cell vaccination in acute myeloid leukemia. Cytotherapy. 2012;14:647–656. doi: 10.3109/14653249.2012.693744. [DOI] [PubMed] [Google Scholar]
- 6.Anguille S, Lion E, Willemen Y, Van Tendeloo VF, Berneman ZN, Smits EL. Interferon-alpha in acute myeloid leukemia: an old drug revisited. Leukemia. 2011;25:739–748. doi: 10.1038/leu.2010.324. [DOI] [PubMed] [Google Scholar]
- 7.Kirkwood J. Cancer immunotherapy: the interferon-alpha experience. Semin Oncol. 2002;29:18–26. doi: 10.1053/sonc.2002.33078. [DOI] [PubMed] [Google Scholar]
- 8.Atzpodien J, Kirchner H, Rebmann U, et al. Interleukin-2/interferon-alpha2a/13-retinoic acid-based chemoimmunotherapy in advanced renal cell carcinoma: results of a prospectively randomised trial of the German Cooperative Renal Carcinoma Chemoimmunotherapy Group (DGCIN) Br J Cancer. 2006;95:463–469. doi: 10.1038/sj.bjc.6603271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 10.Diamond MS, Kinder M, Matsushita H, et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 2011;208:1989–2003. doi: 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuertes MB, Kacha AK, Kline J, Woo SR, Kranz DM, Murphy KM, Gajewski TF. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8α+ dendritic cells. J Exp Med. 2011;208:2005–2016. doi: 10.1084/jem.20101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arico E, Belardelli F. Interferon-alpha as antiviral and antitumor vaccine adjuvants: mechanisms of action and response signature. J Interferon Cytokine Res. 2012;32:235–247. doi: 10.1089/jir.2011.0077. [DOI] [PubMed] [Google Scholar]
- 13.Kirkwood JM, Butterfield LH, Tarhini AA, Zarour H, Kalinski P, Ferrone S. Immunotherapy of cancer in 2012. CA Cancer J Clin. 2012;62:309–335. doi: 10.3322/caac.20132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hervas-Stubbs S, Perez-Gracia JL, Rouzaut A, Sanmamed MF, Le Bon A, Melero I. Direct effects of type I interferons on cells of the immune system. Clin Cancer Res. 2011;17:2619–2627. doi: 10.1158/1078-0432.CCR-10-1114. [DOI] [PubMed] [Google Scholar]
- 15.Smits EL, Lee C, Hardwick N, Brooks S, Van Tendeloo VF, Orchard K, Guinn BA. Clinical evaluation of cellular immunotherapy in acute myeloid leukaemia. Cancer Immunol Immunother. 2011;60:757–769. doi: 10.1007/s00262-011-1022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lion E, Smits EL, Berneman ZN, Van Tendeloo VF. NK cells: key to success of DC-based cancer vaccines? Oncologist. 2012;17:1256–1270. doi: 10.1634/theoncologist.2011-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tel J, Aarntzen EH, Baba T, et al. Natural human plasmacytoid dendritic cells induce antigen-specific T-cell responses in melanoma patients. Cancer Res. 2013;73:1063–1075. doi: 10.1158/0008-5472.CAN-12-2583. [DOI] [PubMed] [Google Scholar]
- 18.Okada H, Tsugawa T, Sato H, et al. Delivery of interferon-alpha transfected dendritic cells into central nervous system tumors enhances the antitumor efficacy of peripheral peptide-based vaccines. Cancer Res. 2004;64:5830–5838. doi: 10.1158/0008-5472.CAN-04-0130. [DOI] [PubMed] [Google Scholar]
- 19.Kuwashima N, Nishimura F, Eguchi J, et al. Delivery of dendritic cells engineered to secrete IFN-alpha into central nervous system tumors enhances the efficacy of peripheral tumor cell vaccines: dependence on apoptotic pathways. J Immunol. 2005;175:2730–2740. doi: 10.4049/jimmunol.175.4.2730. [DOI] [PubMed] [Google Scholar]
- 20.Huang C, Ramakrishnan R, Trkulja M, Ren X, Gabrilovich DI. Therapeutic effect of intratumoral administration of DCs with conditional expression of combination of different cytokines. Cancer Immunol Immunother. 2012;61:573–579. doi: 10.1007/s00262-011-1198-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Tendeloo VF, Ponsaerts P, Lardon F, Nijs G, Lenjou M, Van Broeckhoven C, Van Bockstaele DR, Berneman ZN. Highly efficient gene delivery by mRNA electroporation in human hematopoietic cells: superiority to lipofection and passive pulsing of mRNA and to electroporation of plasmid cDNA for tumor antigen loading of dendritic cells. Blood. 2001;98:49–56. doi: 10.1182/blood.V98.1.49. [DOI] [PubMed] [Google Scholar]
- 22.Van Tendeloo VF, Ponsaerts P, Berneman ZN. mRNA-based gene transfer as a tool for gene and cell therapy. Curr Opin Mol Ther. 2007;9:423–431. [PubMed] [Google Scholar]
- 23.Smits EL, Anguille S, Cools N, Berneman ZN, Van Tendeloo VF. Dendritic cell-based cancer gene therapy. Hum Gene Ther. 2009;20:1106–1118. doi: 10.1089/hum.2009.145. [DOI] [PubMed] [Google Scholar]
- 24.Britten CM, Janetzki S, Butterfield LH, et al. T cell assays and MIATA: the essential minimum for maximum impact. Immunity. 2012;37:1–2. doi: 10.1016/j.immuni.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Holtkamp S, Kreiter S, Selmi A, Simon P, Koslowski M, Huber C, Tureci O, Sahin U. Modification of antigen-encoding RNA increases stability, translational efficacy, and T-cell stimulatory capacity of dendritic cells. Blood. 2006;108:4009–4017. doi: 10.1182/blood-2006-04-015024. [DOI] [PubMed] [Google Scholar]
- 26.Benteyn D, Anguille S, Van Lint S, et al. Design of an optimized Wilms’ Tumor 1 (WT1) mRNA construct for enhanced WT1 expression and improved immunogenicity in vitro and in vivo. Mol Ther Nucleic Acids. 2013;2:e134. doi: 10.1038/mtna.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Driessche A, Van de Velde AL, Nijs G, Braeckman T, Stein B, De Vries JM, Berneman ZN, Van Tendeloo VF. Clinical-grade manufacturing of autologous mature mRNA-electroporated dendritic cells and safety testing in acute myeloid leukemia patients in a phase I dose-escalation clinical trial. Cytotherapy. 2009;11:653–668. doi: 10.1080/14653240902960411. [DOI] [PubMed] [Google Scholar]
- 28.Lion E, Anguille S, Berneman ZN, Smits EL, Van Tendeloo VF. Poly(I:C) enhances the susceptibility of leukemic cells to NK cell cytotoxicity and phagocytosis by DC. PLoS ONE. 2011;6:e20952. doi: 10.1371/journal.pone.0020952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubinstein M, Levy WP, Moschera JA, Lai CY, Hershberg RD, Bartlett RT, Pestka S. Human leukocyte interferon: isolation and characterization of several molecular forms. Arch Biochem Biophys. 1981;210:307–318. doi: 10.1016/0003-9861(81)90194-6. [DOI] [PubMed] [Google Scholar]
- 30.Hobbs DS, Pestka S. Purification and characterization of interferons from a continuous myeloblastic cell line. J Biol Chem. 1982;257:4071–4076. [PubMed] [Google Scholar]
- 31.Anguille S, Lion E, Tel J, de Vries IJ, Coudere K, Fromm PD, Van Tendeloo VF, Smits EL, Berneman ZN. Interleukin-15-induced CD56(+) myeloid dendritic cells combine potent tumor antigen presentation with direct tumoricidal potential. PLoS ONE. 2012;7:e51851. doi: 10.1371/journal.pone.0051851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonehill A, Tuyaerts S, Van Nuffel AM, Heirman C, Bos TJ, Fostier K, Neyns B, Thielemans K. Enhancing the T-cell stimulatory capacity of human dendritic cells by co-electroporation with CD40L, CD70 and constitutively active TLR4 encoding mRNA. Mol Ther. 2008;16:1170–1180. doi: 10.1038/mt.2008.77. [DOI] [PubMed] [Google Scholar]
- 33.Luft T, Pang KC, Thomas E, Hertzog P, Hart DN, Trapani J, Cebon J. Type I IFNs enhance the terminal differentiation of dendritic cells. J Immunol. 1998;161:1947–1953. [PubMed] [Google Scholar]
- 34.Santini SM, Lapenta C, Logozzi M, Parlato S, Spada M, Di Pucchio T, Belardelli F. Type I interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in Hu-PBL-SCID mice. J Exp Med. 2000;191:1777–1788. doi: 10.1084/jem.191.10.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montoya M, Schiavoni G, Mattei F, Gresser I, Belardelli F, Borrow P, Tough DF. Type I interferons produced by dendritic cells promote their phenotypic and functional activation. Blood. 2002;99:3263–3271. doi: 10.1182/blood.V99.9.3263. [DOI] [PubMed] [Google Scholar]
- 36.Nguyen KB, Watford WT, Salomon R, Hofmann SR, Pien GC, Morinobu A, Gadina M, O’Shea JJ, Biron CA. Critical role for STAT4 activation by type 1 interferons in the interferon-gamma response to viral infection. Science. 2002;297:2063–2066. doi: 10.1126/science.1074900. [DOI] [PubMed] [Google Scholar]
- 37.Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J. Immunol. 2005;174:4465–4469. doi: 10.4049/jimmunol.174.8.4465. [DOI] [PubMed] [Google Scholar]
- 38.Spadaro F, Lapenta C, Donati S, Abalsamo L, Barnaba V, Belardelli F, Santini SM, Ferrantini M. IFN-alpha enhances cross-presentation in human dendritic cells by modulating antigen survival, endocytic routing, and processing. Blood. 2012;119:1407–1417. doi: 10.1182/blood-2011-06-363564. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen KB, Salazar-Mather TP, Dalod MY, Van Deusen JB, Wei XQ, Liew FY, Caligiuri MA, Durbin JE, Biron CA. Coordinated and distinct roles for IFN-alpha beta, IL-12, and IL-15 regulation of NK cell responses to viral infection. J Immunol. 2002;169:4279–4287. doi: 10.4049/jimmunol.169.8.4279. [DOI] [PubMed] [Google Scholar]
- 40.Mailliard RB, Son YI, Redlinger R, Coates PT, Giermasz A, Morel PA, Storkus WJ, Kalinski P. Dendritic cells mediate NK cell help for Th1 and CTL responses: two-signal requirement for the induction of NK cell helper function. J Immunol. 2003;171:2366–2373. doi: 10.4049/jimmunol.171.5.2366. [DOI] [PubMed] [Google Scholar]
- 41.Swann JB, Hayakawa Y, Zerafa N, Sheehan KC, Scott B, Schreiber RD, Hertzog P, Smyth MJ. Type I IFN contributes to NK cell homeostasis, activation, and antitumor function. J Immunol. 2007;178:7540–7549. doi: 10.4049/jimmunol.178.12.7540. [DOI] [PubMed] [Google Scholar]
- 42.Lion E, Smits EL, Berneman ZN, Van Tendeloo VF. Acute myeloid leukemic cell lines loaded with synthetic dsRNA trigger IFN-gamma secretion by human NK cells. Leuk Res. 2009;33:539–546. doi: 10.1016/j.leukres.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 43.Boudreau JE, Stephenson KB, Wang F, et al. IL-15 and type I interferon are required for activation of tumoricidal NK cells by virus-infected dendritic cells. Cancer Res. 2011;71:2497–2506. doi: 10.1158/0008-5472.CAN-10-3025. [DOI] [PubMed] [Google Scholar]
- 44.Jinushi M, Takehara T, Kanto T, et al. Critical role of MHC class I-related chain A and B expression on IFN-alpha-stimulated dendritic cells in NK cell activation: impairment in chronic hepatitis C virus infection. J Immunol. 2003;170:1249–1256. doi: 10.4049/jimmunol.170.3.1249. [DOI] [PubMed] [Google Scholar]
- 45.Elpek KG, Rubinstein MP, Bellemare-Pelletier A, Goldrath AW, Turley SJ. Mature natural killer cells with phenotypic and functional alterations accumulate upon sustained stimulation with IL-15/IL-15Ralpha complexes. Proc Natl Acad Sci U S A. 2010;107:21647–21652. doi: 10.1073/pnas.1012128107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salguero G, Daenthanasanmak A, Munz C, et al. Dendritic cell-mediated immune humanization of mice: implications for allogeneic and xenogeneic stem cell transplantation. J Immunol. 2014;192:4636–4647. doi: 10.4049/jimmunol.1302887. [DOI] [PubMed] [Google Scholar]
- 47.Walzer T, Dalod M, Robbins SH, Zitvogel L, Vivier E. Natural-killer cells and dendritic cells: “l’union fait la force”. Blood. 2005;106:2252–2258. doi: 10.1182/blood-2005-03-1154. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.