Abstract
B7-H4 (B7x/B7S1), a B7 family inhibitor of T cell activity, is expressed in multiple human cancers and correlates with decreased infiltrating lymphocytes and poor prognosis. In murine models, tumor-expressed B7-H4 enhances tumor growth and reduces T cell immunity, and blockade of tumor-B7-H4 rescues T cell activity and lowers tumor burden. This implicates B7-H4 as a target for cancer immunotherapy, yet limits the efficacy of B7-H4 blockade exclusively to patients with B7-H4+ tumors. Given the expression of B7-H4 on host immune cells, we have previously shown that BALB/c mice lacking host B7-H4 have enhanced anti-tumor profiles, yet similar 4T1 tumor growth relative to control. Given that T cell-mediated immunotherapies work best for tumors presenting tumor-associated neoantigens, we further investigated the function of host B7-H4 in the growth of a more immunogenic derivative, 4T1-12B, which is known to elicit strong anti-tumor CD8 T cell responses due to expression of a surrogate tumor-specific antigen, firefly luciferase. Notably, B7-H4 knockout hosts not only mounted greater tumor-associated anti-tumor T cell responses, but also displayed reduced tumors. Additionally, B7-H4-deficiency synergized with gemcitabine to further inhibit tumor growth, often leading to tumor eradication and the generation of protective T cell immunity. These findings imply that inhibition of host B7-H4 can enhance anti-tumor T cell immunity in immunogenic cancers, and can be combined with other anti-cancer therapies to further reduce tumor burden regardless of tumor-B7-H4 positivity.
Keywords: B7-H4, Cancer immunotherapy, T cell immunity, Immune checkpoint blockade
Introduction
Immune evasion is a hallmark of cancer [1], and immune checkpoint blockade has been shown to enhance T cells’ ability to destroy cancer cells, sometimes leading to durable cancer remission [2, 3]. B7-H4, an inhibitory B7 protein, negatively regulates T cell activity and is expressed on APCs and numerous human tumors [4–8]. Several groups have shown that tumors overexpressing B7-H4 have enhanced growth, and that blockade of tumor-expressed B7-H4 could rescue T cell responses and suppress tumor progression [9, 10]. These findings suggest therapeutic value for B7-H4 blockade in human cancer patients, yet its efficacy may be limited to patients whose tumors express B7-H4. Further, accumulating evidence has revealed cytosolic or nuclear localization of B7-H4 in multiple cancers [10–14]. Data suggest that this intracellular pool of tumor B7-H4 may somehow promote cellular proliferation and tumor growth in an immune-independent manner, and likely remains inaccessible to conventional antibody-based blocking agents, raising the possibility that tumor-expressed B7-H4 blockade may not be as effective.
Multiple studies have correlated the overexpression of B7-H4 in human cancer with decreased tumor infiltrating lymphocytes and poor prognosis [15–19]. Although host B7-H4 plays an inhibitory role for immune responses against infection [20, 21], the function of host B7-H4 has seldom been addressed in the context of cancer. To date, contradicting data have surfaced regarding the anti- or pro-tumor role of host B7-H4. On one hand, B7-H4 expression in non-immune host cells was associated with elevated anti-tumor T cell responses in MMTV-PyMT mammary tumors, and subsets of human breast cancer also revealed a positive correlation between B7-H4 mRNA levels and recurrence-free survival [22]. In contrast, B7-H4-deficient mice had fewer lung nodules and elevated T cell responses relative to wild type (WT) mice when 4T1 mammary carcinoma cells (proven to be B7-H4-negative) were directly injected into the bloodstream [23]. Thus, a greater understanding of the function of host B7-H4 during tumor growth will be invaluable when considering the merit of blocking B7-H4 in human cancer patients.
We previously showed that B7-H4 knockout (KO) mice displayed stronger anti-tumor cytokine profiles, yet exhibited similar growth of lowly immunogenic 4T1 tumor cells compared with WT controls [24]. Considering the emerging concept that the responsiveness of tumor cells to checkpoint blockades heavily relies on the presence of T cell neoantigens [25, 26], we investigated the function of host B7-H4 in the development of anti-tumor T cell responses using a model in which tumor cells express known T cell epitopes. To this end, we chose a clonal derivative of 4T1 termed 4T1-12B, which expresses firefly luciferase [27, 28]. Using the 4T1-12B transplantation model, we show that host B7-H4 inhibits anti-tumor T cell responses, promoting the growth of primary mammary tumors. Additionally, we reveal that treatment with the chemotherapeutic drug gemcitabine can lead to complete tumor rejection in B7-H4 KO mice, but not in WT hosts. These “cured” B7-H4 KO mice are resistant to rechallenge of the same and related tumor cells in a CD8 T cell-dependent manner, suggesting a role for host B7-H4 in the development of protective T cell immunity against tumor-associated antigens.
Materials and methods
In vivo tumor experiments
Early passages of the murine mammary carcinoma cell lines 4T1-12B (a gift from Gary Sahagian, Tufts University, Boston, USA) or 4T1 tumor cells (ATCC) were harvested from culture by trypsinization and were washed twice with PBS. One million viable 4T1-12B cells or 5 × 104 4T1 cells were subcutaneously injected into the third mammary gland of female 6- to 10-week-old BALB/cJ (Jackson Laboratory) and B7-H4 KO mice in BALB/cJ background (N10) [20]. Tumor volume was calculated as (length × width2)/2. Luciferase activity was measured using the Xenogen IVIS 200 weekly. In some experiments, mice were treated with gemcitabine (1.5 mg/mouse, Sigma Aldrich) intraperitoneally starting on week 1, as previously described [24]. For T cell depletion, WT or KO mice were injected i.p. with 100 μg anti-CD4 (clone GK1.5; Bio X Cell), anti-CD8 (clone 2.43; Bio X Cell) or isotype control (clone LTF-2; Bio X Cell) on days −2, −1, +2, +6, and +9 prior to or after tumor injection (day 0) to deplete CD4 or CD8 T cells. Depletion of targeted cell populations was confirmed by FACS analysis using peripheral blood and splenocytes. CD4 and CD8 T cell depletion was >99% effective, as previously described [24]. All animal use protocols were approved by the Animal Care Committee of the IRCM.
Flow cytometry
Tumors, spleens and draining lymph nodes were harvested and prepared into single-cell suspensions as previously described [24]. For flow cytometry analysis, cells were treated with Fc-block (eBioscience) prior to antibody and 7-AAD staining, and subsequently analyzed using the BD LSR Fortessa, as previously described [24]. For intracellular cytokine staining, fixable viability dye was used to distinguish dead cells, and cells were fixed and permeabilized with BD Cytofix/Cytoperm according to the manufacturer’s instructions (BD). 7AAD−CD45+cells were gated as live host hematopoietic cells, and 7AAD−CD45−populations were defined as tumor cells. Anti-CD45 (30-F11), anti-CD11b (M1/70), anti–Gr-1 (RB6-8C5), anti-CD3 (145-2C11), anti-MHC I (34-1-2S), anti-MHC II (M5/114.15.2), anti–PD-L1 (MIH5), anti-CD8 (53–6.7), anti-CD4 (GK1.5), anti-IFN-γ (XMG1.2), Fixable Viability Dye eFluor 450 were purchased from eBioscience. AH1-H-2Ld tetramers were generated by NIH Tetramer Core Facility.
Quantitative PCR
Total RNA was isolated from tumor single-cell suspensions using the RNeasy Mini Kit (Qiagen), and cDNA was synthesized using SuperScript II Reverse Transcriptase (Invitrogen) according to the manufacturer’s instructions. All reactions were performed as previously described [24].
In vitro stimulation and suppression assays
For peptide stimulation, collagenase-digested cells were cultured with peptides (10 µM) or PMA (50 ng/ml) and ionomycin (500 ng/ml) for 5 h in the presence of GolgiPlug (BD). Cells were then processed for cytokine staining. For long-term in vitro cultures, 1 × 106 collagenase-digested splenocytes were plated in 1 ml of complete media supplemented with 100 U of IL-2 (Peprotech) and 10 µM of peptide. After ~1 week in culture, cells used for cytokine staining. AH1 peptides (SPSYVYHQF) were purchased from AnaSpec, and dominant and minor luciferase peptides (GFQSMYTFV and VPFHHGFGM, VALPHRTAC, respectively) were synthesized by Peptron Inc (Daejeon, Republic of Korea). Splenic MDSCs from tumor-bearing mice were co-cultured with naïve WT splenocytes, and T cell proliferation was measured as previously described [24].
Statistical analyses
Prism software was used to determine statistical significance by unpaired Student’s t tests (two-tailed).
Results
4T1-12B tumors have delayed growth and reduced luciferase activity in the absence of host B7-H4
4T1-12B cells were clonally derived from the 4T1 mammary carcinoma cell line, and stably express firefly luciferase. In T cell-deficient BALB/c hosts, 4T1-12B has been shown to readily grow and maintain high levels of luciferase activity, while its growth is substantially delayed in WT hosts [27]. This is presumably due to the strong immunogenic nature of luciferase epitopes, since outgrowing tumors in WT hosts are observed to have progressively diminished luciferase activity [27], and BALB/c mice have been demonstrated to elicit IFN-γ-producing CD8 T cells recognizing luciferase-derived peptide epitopes [28]. To confirm that the presence of luciferase epitopes in 4T1-12B cells elicit greater tumor-suppressive T cell responses relative to the parental 4T1 cell line, we compared the growth of both 4T1-12B and 4T1 tumors in BALB/c mice with or without depletion of CD8 or CD4 T cells during the early stages of tumor growth. Despite injecting 20x more 4T1-12B cells than 4T1 cells (1 × 106 4T1-12B vs. 5 × 104 4T1), 4T1-12B tumors tended to grow slower (isotype, Fig. 1a), consistent with the notion that stronger T cell responses elicited by 4T1-12B cells may inhibit tumor growth. Indeed, 4T1-12B tumors grew much faster in the absence of CD8 T cells, and to some extent, in the absence of CD4 T cells, compared to 4T1-12B tumors grown in mice with intact T cell populations (Fig. 1a). In contrast, growth of 4T1 tumors was not affected by CD8 or CD4 T cell depletion. Thus, 4T1-12B cells can mimic cancer cells that express tumor-associated neoantigens which elicit tumor-suppressive T cells responses, an emerging feature of human cancers that are responsive to immune checkpoint blockades [25, 26].
Fig. 1.

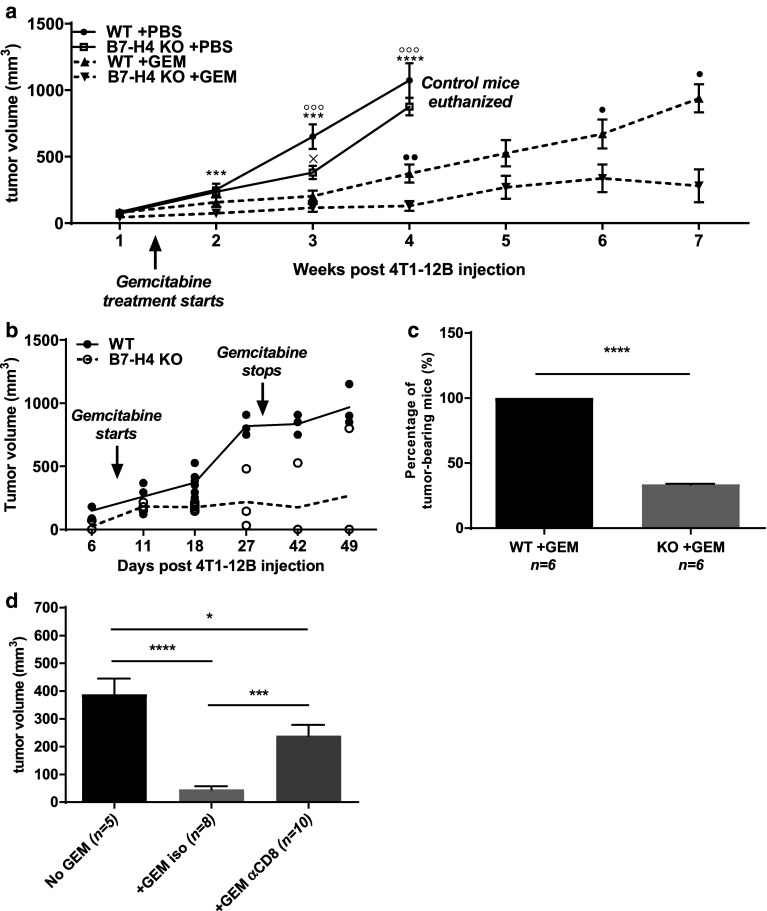
B7-H4 KO mice exhibit slower 4T1-12B tumor growth relative to WT mice. a Tumor weight measured after 2 weeks from WT mice bearing 4T1 or 4T1-12B tumors administered with isotype, anti-CD8, or anti-CD4 depleting antibodies. b–e WT and B7-H4 KO mice were injected with 1 × 106 4T1-12B cells and b tumor volume and c luciferase activity were measured weekly. d Bioluminescence intensity of growing tumors measured on week 4. Tumor contours were shaded in black to denote the total tumor area. e Final tumor weight was measured after 5 weeks. Data presented as mean ± standard error. The presented data were either pooled from two to three experiments, or represent two or more independent experiments with similar results. *P < 0.05; **P < 0.01; ***P < 0.001
To determine the contribution of host B7-H4 in the growth of an immunogenic cancer, we injected B7-H4-negative 4T1-12B cells into WT and B7-H4 KO mice and monitored tumor growth and luciferase activity. Strikingly, B7-H4-deficient mice showed markedly reduced 4T1-12B growth kinetics relative to WT mice throughout the course of the experiment (Fig. 1b). As expected, luciferase activity peaked at 3 week post injection and progressive loss of luciferase activity was observed concurrent with steady tumor growth (Fig. 1b, c). Disappearance of luciferase-positive tumor cells was more pronounced in B7-H4 KO hosts compared to WT counterparts within a dynamic window of time (Fig. 1d), suggesting elevated anti-tumor T cell responses in the absence of host B7-H4. Consistently, the final weight of 4T1-12B tumors grown in B7-H4 KO hosts was approximately two folds less than that of tumors from WT hosts (Fig. 1e).
Taken together, these observations imply a pro-tumor role for host B7-H4, and suggest that the impact of host B7-H4 is more pronounced in the presence of a highly immunogenic antigen, since we observed that lowly immunogenic parental 4T1 tumors grew similarly with or without host B7-H4 [24].
B7-H4-deficient mice show signs of enhanced anti-tumor immunity
Since B7-H4-deficient mice were seen to have reduced tumor burden relative to WT mice, we next sought to characterize the host anti-tumor response. When tumor-bearing mice were killed at week 3 in which tumor-immune interactions were most dynamic (data not depicted), B7-H4 KO mice exhibited enhanced CD8+ T cell infiltration in the tumor, and greater numbers of CD4+ and CD8+ T cells in the spleen (Fig. 2a, b). Further, ex vivo 4T1-12B cells from KO mice revealed an elevated expression of MHC I, MHC II and B7-H1 protein compared to controls (Fig. 2c, d). These features have been previously shown to reflect the level of local bioactive IFN-γ [29]. Consistently, qPCR analysis confirmed a twofold enhancement of IFN-γ transcripts in ex vivo 4T1-12B tumors coming from B7-H4-deficient hosts relative to WT hosts (Fig. 2e). This predicts a role for host B7-H4 in the regulation of IFN-γ-producing T cells during tumor growth, and links 4T1-12B tumor regression with enhanced levels of IFN-γ secreting T cells.
Fig. 2.
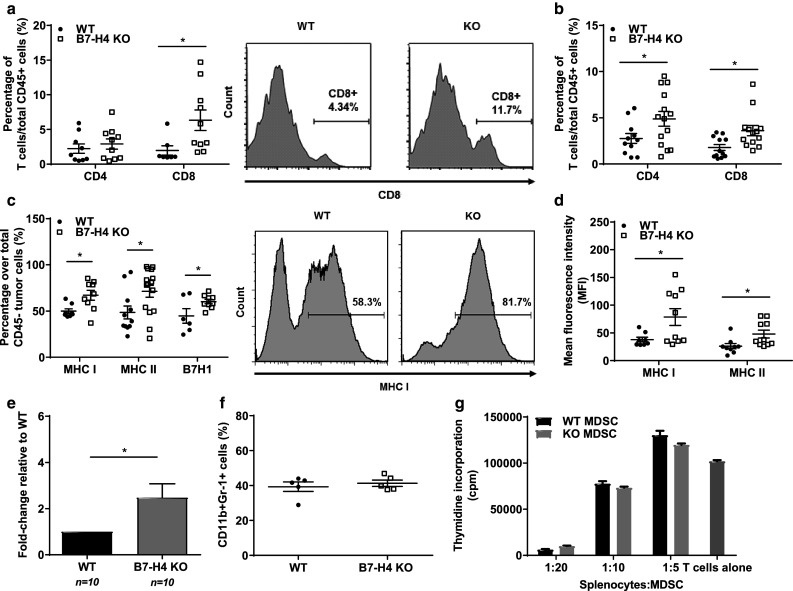
B7-H4 KO mice display enhanced anti-tumor T cell responses relative to WT mice. After 5 weeks, a tumor cells and b splenocytes from WT or B7-H4 KO mice were harvested, stained and analyzed via flow cytometry for CD4+ and CD8+ T cells, gated on live, CD45+ cells. c Percentage of tumor cells positive for MHC I, MHC II and B7-H1, gated on live, CD45- cells. d Mean fluorescence intensity of MHC I and MHC II on tumor cells. e mRNA was extracted from WT and KO tumors, and qPCR was performed to quantitate the abundance of IFN-γ transcripts in the tumor microenvironment. f Percentage of CD11b+Gr-1+MDSCs gated over total CD45+cells from tumor and spleen. g Ex vivo myeloid-derived suppressor cells were isolated from splenocytes of WT and B7-H4 KO tumor-bearing mice, and co-cultured with CD3 and CD28-stimulated naïve splenocytes. Thymidine incorporation assay was performed to measure T cell proliferation and MDSC suppression after 2 days. Data presented as mean ± standard error. The presented data were either pooled from two to three experiments, or represent two or more independent experiments with similar results. *P < 0.05; **P < 0.01; ***P < 0.001
In addition to modulating T cell activity, B7-H4 has also been demonstrated to regulate myeloid cells. Using the 4T1 tumor model, we have previously shown that B7-H4 can inhibit the immunosuppressive capacity of myeloid-derived suppressor cells (MSDCs) [24], and others have also revealed an inhibitory role for B7-H4 in mediating neutrophil expansion [21, 23]. To test these findings in the 4T1-12B model, we analyzed MDSC infiltration and found similar levels in the tumor milieu of B7-H4-deficient and sufficient mice (Fig. 2f). To investigate the function of these cells, we next co-cultured ex vivo splenic MDSCs from WT and B7-H4 KO tumor-bearing mice with naive splenocytes stimulated with anti-CD3 and anti-CD28 antibodies. We also observed no differences in the ability of either MDSC subsets to suppress T cell proliferation (Fig. 2g). We speculate that the discrepancy in B7-H4-dependent MDSC functions between 4T1 and 4T1-12B tumor model could be due to differences in dynamic tumor-immune interactions that are heavily influenced by the immunogenicity of growing tumor cells.
B7-H4 KO hosts exhibit increased tumor-associated T cell responses compared to WT hosts
In cancer immunotherapy, the existence of prior or ongoing immune responses predicts better survival and patient outcome [30, 31]. In particular, patients whose tumors exhibit high mutation loads and the presence of T cell epitopes tend to respond better to immune checkpoint blockade, implying the significance of antigen-specific T cell immunity [25, 26]. To further examine the role of B7-H4 in the anti-tumor response induced by 4T1-12B, we assessed the tumor-associated T cell response elicited in WT and B7-H4 KO hosts. 4T1 and 4T1-12B tumors express AH1, a MHC I-restricted tumor-associated antigen from gp70 of an endogenous murine leukemia virus [32]. As AH1-tetramers detect T cells specific for the AH1 epitope, we proceeded to stain ex vivo tumor samples. Notably, B7-H4 KO tumors at week 3 contained more AH1-specific CD8+ T cells compared with WT samples (Fig. 3a, b). We next sought to determine the functional capacity of AH1-specific T cells coming from mice deficient or sufficient for B7-H4. To this end, we acutely stimulated lymphocytes from draining LN of tumor-bearing mice with AH1 peptides and quantitated the number of IFN-γ-producing T cells. In line with the previous finding, we observed an increase in the number of IFN-γ-secreting CD8+ T cells from B7-H4 KO hosts (Fig. 3c). Consistently, when splenocytes from tumor-bearing WT and KO mice were cultured for one week in vitro with IL-2 and AH1 peptides, KO splenocytes had more AH1-specific T cells relative to WT (Fig. 3d).
Fig. 3.
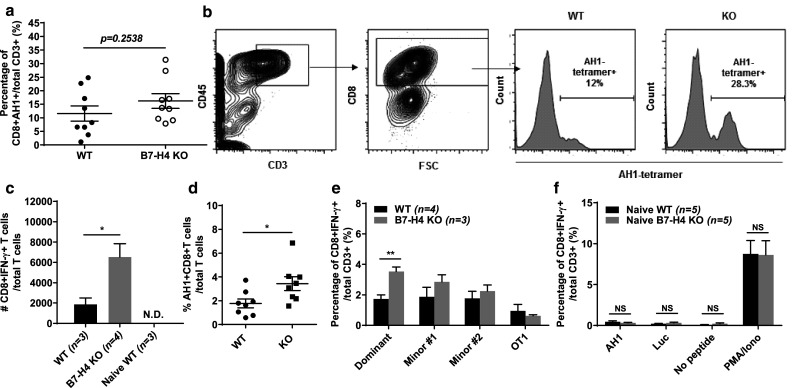
B7-H4 deficiency augments tumor-associated T cell immunity. a Ex vivo tumors were stained with AH1 tetramers to determine percentage of AH1-specific T cells at 3 weeks post injection. b Gating strategy for detection of AH1-tetramer+CD8+ T cells in the tumor. c Ex vivo cells from the draining lymph node of tumor-bearing WT, KO, or naïve mice were acutely stimulated in vitro for 5 h with 10 µg/ml of AH1 peptide in the presence of GolgiPlug (BD). Cells were then collected and stained to determine the absolute number of CD8+IFN-γ+T cells. d Splenocytes from WT and KO tumor-bearing mice cultured for 7 days with IL-2 and AH1 peptides in vitro prior to staining with AH1-tetramers. e Splenocytes were acutely stimulated in vitro for 5 h with 10 µg/ml of luciferase peptides corresponding to the dominant or minor epitopes (#1 and 2), or with irrelevant control peptide (OT1) in the presence of GolgiPlug. Cells were then stained to quantitate the percentage of CD8+IFN-γ+ T cells. f Splenocytes from naïve WT (n = 3) or KO (n = 3) mice were stimulated for 5 h with luciferase (dominant epitope) or AH1 peptides, or with PMA and ionomycin in the presence of GolgiPlug. Cells were then stained to quantitate the percentage of CD8+IFN-γ+T cells. Data presented as mean ± standard error. The presented data were either pooled from two to three experiments, or represent two or more independent experiments with similar results. *P < 0.05; **P < 0.01; ***P < 0.001
Since 4T1-12B cells express firefly luciferase, we also sought to determine if B7-H4 could influence the generation of T cells recognizing luciferase epitopes. So far, one dominant and two minor T cell epitopes, restricted to H2-Kd, have been identified in BALB/c mice [28]. As no commercial tetramers are available to stain for luciferase-specific T cells, we used synthetic peptides representing these epitopes to stimulate ex vivo splenocytes harvested from tumor-bearing WT and KO mice, and assessed the percentage of IFN-γ+CD8+ T cells recognizing luciferase epitopes on 4T1-12B tumors. Importantly, splenocytes coming from B7-H4-deficient hosts had significantly higher percentages of IFN-γ+CD8+ T cells responding to the dominant luciferase epitope (Fig. 3e). Likewise, a similar trend was observed in KO splenocytes responding to the minor luciferase epitopes (Fig. 3e).
Given that in vitro peptide restimulation of KO splenocytes from tumor-bearing mice display greater cytokine secretion relative to WT, we wanted to exclude the possibility that B7-H4 deficiency leads to spontaneous T cell activation regardless of antigen driven tumor-immune interactions. To this end, splenocytes from naïve WT and B7-H4 KO mice were thus cultured with AH1 or luciferase peptides to assess acute cytokine production. After a 5-hour peptide stimulation, very few T cells produced IFN-γ in both groups (Fig. 3f). Moreover, when splenocytes from WT and KO mice were treated with PMA and ionomycin, CD8 T cells from B7-H4 KO mice displayed no difference in IFN-γ secretion compared to WT (Fig. 3f). These results indicate that the enhanced anti-tumor T cell response in B7-H4 deficient mice was not a result of pre-existing activated T cells but rather depends on immune priming by tumor-associated antigens (Fig. 3c–e).
Taken together, these data imply an inhibitory role for B7-H4 in the generation of 4T1-12B tumor-specific CD8 T cells. This also suggests that B7-H4 blockade may be effective in reducing cancer burden regardless of tumor B7-H4 positivity, since despite the absence of B7-H4 on 4T1-12B cells, B7-H4 KO animals were nonetheless able to show augmented tumor-associated T cell responses.
B7-H4-deficiency synergizes with gemcitabine treatment to further reduce 4T1-12B growth
In light of accumulating clinical studies reporting the success of combinatorial therapies comprising radiation, chemotherapy, and immunotherapies [3, 33, 34], we wondered if B7-H4 blockade could also be combined with other therapeutic options. We chose gemcitabine, a nucleoside analog currently in use as a chemotherapeutic drug for multiple cancers including breast cancer [35]. Gemcitabine not only slows tumor growth but also suppresses MDSCs and promotes T cell responses against transplanted 4T1 tumors [24, 36]. To test whether gemcitabine and B7-H4 deficiency can cooperate in controlling tumor growth, we treated 4T1-12B tumor-bearing WT and KO mice with gemcitabine. While gemcitabine treatment was able to delay 4T1-12B growth in both WT and B7-H4-deficient hosts, the absence of B7-H4 resulted in a substantially pronounced reduction of tumor burden relative to all other groups (Fig. 4a). In some experiments, around 20% of KO mice completely rejected tumor or maintained static residual tumors up to 2 weeks following removal from gemcitabine treatment (data not depicted). Importantly, when we reduced the tumor inoculum size by two-fold (0.5 × 106 cells per mouse), two thirds of gemcitabine-treated KO mice were able to reject 4T1-12B tumors (Fig. 4b, c). In contrast, this was not observed in WT mice. Similar synergistic effects of gemcitabine and B7-H4 deficiency has been observed in the 4T1 transplantation model, but tumor rejection was not achieved presumably due to the poorly immunogenic nature of 4T1 cells [24].
Fig. 4.
B7-H4 deficiency synergizes with gemcitabine (GEM) to further reduce 4T1-12B tumor burden. a WT and KO mice were injected with 1 × 106 4T1-12B cells as described in “Materials and methods”. In some groups, gemcitabine (1.5 mg/mouse, intraperitoneal) was administered 9 days after tumor inoculation. Treatment was repeated every 3–4 days up to ten times. Tumor volume was measured weekly and statistical significance was evaluated pairwise: x = WT +PBS vs. KO +PBS, º = WT +PBS vs. WT +GEM, * = KO +PBS vs. KO +GEM, • = WT +GEM vs. KO +GEM. Each data point represents at least three mice per group. b WT and B7-H4 KO mice were injected with 0.5 × 106 4T1-12B cells, and treated with gemcitabine after 9 days. Each circle represents the tumor volume of an individual mouse. c After 7 weeks, the percentage of tumor-bearing mice was quantitated. d B7-H4 KO mice bearing 4T1-12B tumors were treated with gemcitabine following administration of isotype or anti-CD8 T cell depleting antibodies. Tumor volume was measured after 2 weeks. Data presented as mean ± standard error. The presented data were either pooled from two to three experiments, or represent two or more independent experiments with similar results. *P < 0.05; **P < 0.01; ***P < 0.001
To assess the contribution of CD8 T cells in gemcitabine-mediated tumor regression in the absence of B7-H4, we treated 4T1-12B tumor-bearing B7-H4 KO mice with gemcitabine with or without CD8 T cell depletion. Interestingly, we observed that depletion of CD8 T cells substantially reduced the synergistic effects of gemcitabine and B7-H4 deficiency (Fig. 4d). Thus, at least in part, the enhanced efficacy of gemcitabine on 4T1-12B tumors in B7-H4 KO mice depends on CD8 T cell responses.
Combinatorial therapy elicits protective anti-tumor immunity against tumor re-challenge
Since B7-H4 deficiency and gemcitabine treatment led to complete 4T1-12B tumor rejection in some cases, we predicted that the adaptive immune response elicited could also protect against a subsequent 4T1-12B inoculation. To test this, KO mice that had previously eliminated 4T1-12B tumors were re-challenged with 4T1-12B tumors. Consistent with the features of adaptive immunity, previously challenged KO mice were resistant to 4T1-12B growth relative to naïve KO hosts (Fig. 5a, b), indicating the development of immunological memory. To confirm the role of T cells in 4T1-12B tumor rejection, a third inoculation of 4T1-12B cells was performed following CD8+ T cell depletion in KO mice that had previously rejected 4T1-12B cells (Fig. 5c). In the absence of CD8+ T cells, 4T1-12B tumors grew undeterred relative to T cell sufficient KO mice (Fig. 5d), indicating that CD8+ T cells are a necessary component of protective anti-tumor immunity in our model.
Fig. 5.
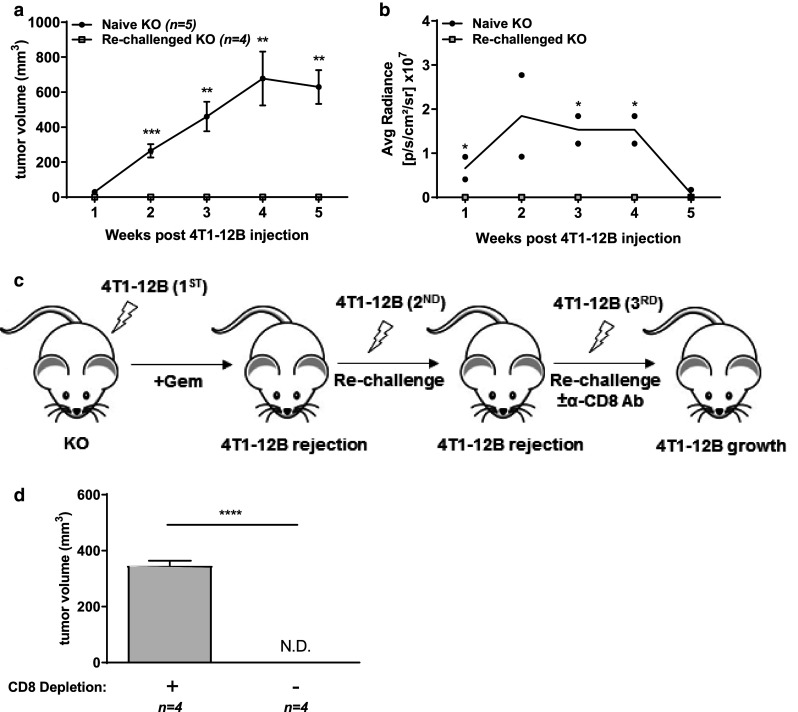
B7-H4 KO mice generate protective CD8 T cell immunity against 4T1-12B cells following gemcitabine treatment. B7-H4 KO mice that had previously eliminated or maintained stable tumor size (less than 3 mm in diameter) were re-challenged with 1 × 106 4T1-12B cells on the opposite mammary gland. a Tumor volume and b luciferase activity were measured every week post re-challenge. c Diagram depicting series of tumor inoculation and CD8 T cell depletion. Second injection more than 2 weeks after rejection of primary tumor; third injection more than 2 weeks after rejection of second tumor. d Final tumor volume of a representative CD8 T cell-depleted KO mouse re-challenged with 4T1-12B. Data presented as mean ± standard error. The presented data were either pooled from two to three experiments, or represent two or more independent experiments with similar results. *P < 0.05; **P < 0.01; ***P < 0.001
One major advantage of checkpoint blockade is the induction of durable resistance to recurrence which probably relies on the generation of a broad T cell repertoire against tumor-associated antigens [2, 34]. We reasoned that gemcitabine-treated B7-H4 KO mice that rejected 4T1-12B tumors may have developed a sustainable T cell repertoire against other tumor-specific antigens that are shared between 4T1 and 4T1-12B (such as AH1). To test this idea, following gemcitabine administration and 4T1-12B tumor rejection, B7-H4 KO mice were re-challenged with 4T1 cells. Indeed, 4T1 tumors grew much slower in the “cured” mice (Fig. 6a) compared to naïve B7-H4 KO hosts, and the final tumor weight was more than eightfold reduced (Fig. 6b). Consistently, splenocytes isolated from cured mice showed a robust expansion of CD8 T cells specific for the shared AH1 epitope upon restimulation in vitro (Fig. 6c). Collectively, these data indicate that the presence of potent T cell antigens can elevate protective anti-tumor T cell responses targeting other tumor-associated antigens, and suggest that combination of gemcitabine and B7-H4 blockade is able to promote this process.
Fig. 6.
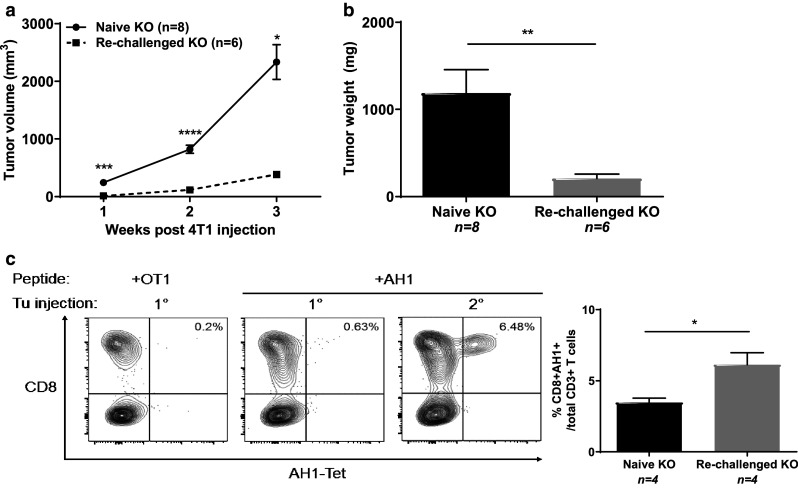
Eradication of 4T1-12B tumors upon gemcitabine treatment in KO mice provides partial protection against 4T1 tumor growth. a KO mice that had previously rejected 4T1-12B tumors upon gemcitabine treatment and naïve KO mice controls were challenged with 5 × 104 4T1 cells. Tumor volume was measured weekly, and b final tumor weight was quantitated after 4 weeks. c Splenocytes from KO naïve or re-challenged mice [1° vs. 2° tumor (Tu) injection] were cultured for 7 days with IL-2 and AH1 peptides in vitro prior to staining with AH1-tetramers. Data presented as mean ± standard error. The presented data were either pooled from two to three experiments, or represent two or more independent experiments with similar results. *P < 0.05; **P < 0.01; ***P < 0.001
Discussion
While there is evidence supporting both anti- and pro-tumor role of host B7-H4 in cancer development, our results indicate that in an immunogenic transplantable mammary carcinoma model, host B7-H4 inhibits T cell anti-tumor immunity. Consistent with its known ability to negatively regulate adaptive immune responses, we detected increased infiltration of T cells and a greater capacity to respond to 4T1-12B antigens in B7-H4-deficient animals, resulting in diminished tumor growth. These results are in contrast with our previous work [24], as the parental, lowly immunogenic 4T1 cells displayed similar tumor growth despite signs of enhanced anti-tumor immunity in the tumor microenvironment of B7-H4 KO mice.
Our data suggest that the impact of B7-H4 blockade can be most readily observed in circumstances where a robust T cell response can be elicited, such as in murine models involving strong T cell antigens, or in cancer patients with high mutation loads and new T cell epitopes. These findings are consistent with a recent cancer genome sequencing of melanoma patients treated with anti-CTLA-4 antibodies that demonstrated an association between the presence of T cell neoantigens and the degree of clinical benefit, implicating the significance of neoantigen availability and patient response to checkpoint blockades [25, 26]. This further correlates with enhanced responsiveness to checkpoint blockade therapies in patients showing ongoing immune responses, such as those displaying tumor-specific T cells, sustained ICOS expression and the upregulation of T cell activation markers [31, 37–39].
Given that the absence of host B7-H4 has been shown to enhance adaptive anti-tumor immunity in B7-H4-negative tumor models such as 4T1 [23] and 4T1-12B (current study), B7-H4 blockade may benefit patients regardless of tumor-expressed B7-H4. However, further work is required to determine whether B7-H4-positive tumors respond better to B7-H4 blockade and whether or not the subcellular location of tumor B7-H4 affects the outcome.
We also revealed a synergistic effect of B7-H4 deficiency and gemcitabine treatment in the reduction of 4T1-12B growth, and showed that this combination could induce complete tumor rejection in B7-H4 KO mice and elicit protective T cell immunity. Since gemcitabine has been established to reduce 4T1 growth and have proven activity in advanced and metastatic breast cancer patients [35], our study argues that B7-H4 blockade may help to reduce tumor load in patients being administered gemcitabine. Notably, considering that B7-H4 acts as a co-inhibitor of T cell response, we wonder if B7-H4 deficiency could also synergize with other immune checkpoint blockades to maximize the rescue of adaptive anti-tumor immunity. The promising clinical efficacy of dual anti-CTLA-4 and anti-PD-1 therapy in advanced melanoma [40, 41] suggests that targeting both early and late phase immune checkpoints is advantageous. Certainly, combinatorial regimens involving CTLA-4, PD-1, B7-H4, may prove to elicit stronger efficacy.
While we showed that lack of host B7-H4 can augment T cell anti-tumor immunity, the source of B7-H4 in our 4T1-12B model remains unclear. In normal individuals, B7-H4 mRNA is ubiquitously expressed in non-hematopoietic tissues and protein expression is restricted to immune cells and certain tissues [4–6, 42]; however, it is conceivable that during cancer development, aberrations in the regulation of B7-H4 expression may occur such that peripheral tissues may upregulate B7-H4 protein and further promote cancer immune evasion. Indeed, it has been demonstrated that IL-6 and IL-10 can induce the expression of B7-H4 in tumor-associated macrophages [12], and more recently, HIF-1α has also been shown to enhance B7-H4 expression in cancer cells, albeit this expression was restricted to the cytoplasm [14]. Since IL-6 and HIF-1α are crucial mediators of inflammation, it is plausible that long-term exposure to these factors could promote the translation of B7-H4 transcripts in stromal cells, and facilitate tumor development.
Overall, our results suggest that in cancer patients with strong tumor-associated T cell epitopes, B7-H4 blockade may prove beneficial regardless of the level of tumor-expressed B7-H4, and may be combined with chemotherapy or other immunotherapies to improve its efficacy in eliciting tumor clearance and long-term protection against tumor recurrence.
Acknowledgements
We thank G. Sahagian for 4T1-12B cell line, M.C. Lavallée and B. Locas for help with animal experiments, and E. Massicotte and J. Leconte for flow cytometry assistance. This research was funded by the Canadian Cancer Society (#702047, W.-K. Suh) and the Canadian Institutes of Health Research (#243998, J. Stagg).
Abbreviations
- 7-AAD
7-Amino-actinomycin D
- IRCM
Institut de recherches cliniques de Montréal
- KO
Knockout
- MDSC
Myeloid-derived suppressor cell
- MMTV-PyMT
Mouse mammary tumor virus-polyoma middle T
- N. D.
Not detected
- qPCR
Quantitative polymerase chain reaction
- WT
Wild type
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348(6230):56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 3.Leung J, Suh WK. The CD28-B7 family in anti-tumor immunity: emerging concepts in cancer immunotherapy. Immune Netw. 2014;14(6):265–276. doi: 10.4110/in.2014.14.6.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sica GL, Choi IH, Zhu G, Tamada K, Wang SD, Tamura H, Chapoval AI, Flies DB, Bajorath J, Chen L. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity. 2003;18(6):849–861. doi: 10.1016/S1074-7613(03)00152-3. [DOI] [PubMed] [Google Scholar]
- 5.Prasad DV, Richards S, Mai XM, Dong C. B7S1, a novel B7 family member that negatively regulates T cell activation. Immunity. 2003;18(6):863–873. doi: 10.1016/S1074-7613(03)00147-X. [DOI] [PubMed] [Google Scholar]
- 6.Zang X, Loke P, Kim J, Murphy K, Waitz R, Allison JP. B7x: a widely expressed B7 family member that inhibits T cell activation. Proc Natl Acad Sci USA. 2003;100(18):10388–10392. doi: 10.1073/pnas.1434299100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salceda S, Tang T, Kmet M, Munteanu A, Ghosh M, Macina R, Liu W, Pilkington G, Papkoff J. The immunomodulatory protein B7-H4 is overexpressed in breast and ovarian cancers and promotes epithelial cell transformation. Exp Cell Res. 2005;306(1):128–141. doi: 10.1016/j.yexcr.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 8.Yi KH, Chen L. Fine tuning the immune response through B7-H3 and B7-H4. Immunol Rev. 2009;229(1):145–151. doi: 10.1111/j.1600-065X.2009.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeon H, Vigdorovich V, Garrett-Thomson SC, Janakiram M, Ramagopal UA, Abadi YM, Lee JS, Scandiuzzi L, Ohaegbulam KC, Chinai JM, Zhao R, Yao Y, Mao Y, Sparano JA, Almo SC, Zang X. Structure and cancer immunotherapy of the B7 family member B7x. Cell Rep. 2014;9(3):1089–1098. doi: 10.1016/j.celrep.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dangaj D, Lanitis E, Zhao A, Joshi S, Cheng Y, Sandaltzopoulos R, Ra HJ, Danet-Desnoyers G, Powell DJ, Jr, Scholler N. Novel recombinant human b7-h4 antibodies overcome tumoral immune escape to potentiate T-cell antitumor responses. Cancer Res. 2013;73(15):4820–4829. doi: 10.1158/0008-5472.CAN-12-3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang L, Wu H, Lu D, Li G, Sun C, Song H, Li J, Zhai T, Huang L, Hou C, Wang W, Zhou B, Chen S, Lu B, Zhang X. The costimulatory molecule B7-H4 promote tumor progression and cell proliferation through translocating into nucleus. Oncogene. 2013;32(46):5347–5358. doi: 10.1038/onc.2012.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kryczek I, Zou L, Rodriguez P, Zhu G, Wei S, Mottram P, Brumlik M, Cheng P, Curiel T, Myers L, Lackner A, Alvarez X, Ochoa A, Chen L, Zou W. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. 2006;203(4):871–881. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quandt D, Fiedler E, Boettcher D, Marsch WC, Seliger B. B7-h4 expression in human melanoma: its association with patients’ survival and antitumor immune response. Clin Cancer Res. 2011;17(10):3100–3111. doi: 10.1158/1078-0432.CCR-10-2268. [DOI] [PubMed] [Google Scholar]
- 14.Jeon YK, Park SG, Choi IW, Lee SW, Lee SM, Choi I. Cancer cell-associated cytoplasmic B7-H4 is induced by hypoxia through hypoxia-inducible factor-1alpha and promotes cancer cell proliferation. Biochem Biophys Res Commun. 2015;459(2):277–283. doi: 10.1016/j.bbrc.2015.02.098. [DOI] [PubMed] [Google Scholar]
- 15.Mugler KC, Singh M, Tringler B, Torkko KC, Liu W, Papkoff J, Shroyer KR. B7-h4 expression in a range of breast pathology: correlation with tumor T-cell infiltration. Appl Immunohistochem Mol Morphol. 2007;15(4):363–370. doi: 10.1097/01.pai.0000213159.79557.71. [DOI] [PubMed] [Google Scholar]
- 16.Kryczek I, Wei S, Zhu G, Myers L, Mottram P, Cheng P, Chen L, Coukos G, Zou W. Relationship between B7-H4, regulatory T cells, and patient outcome in human ovarian carcinoma. Cancer Res. 2007;67(18):8900–8905. doi: 10.1158/0008-5472.CAN-07-1866. [DOI] [PubMed] [Google Scholar]
- 17.Zang X, Thompson RH, Al-Ahmadie HA, Serio AM, Reuter VE, Eastham JA, Scardino PT, Sharma P, Allison JP. B7-H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc Natl Acad Sci USA. 2007;104(49):19458–19463. doi: 10.1073/pnas.0709802104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krambeck AE, Thompson RH, Dong H, Lohse CM, Park ES, Kuntz SM, Leibovich BC, Blute ML, Cheville JC, Kwon ED. B7-H4 expression in renal cell carcinoma and tumor vasculature: associations with cancer progression and survival. Proc Natl Acad Sci USA. 2006;103(27):10391–10396. doi: 10.1073/pnas.0600937103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arigami T, Uenosono Y, Hirata M, Hagihara T, Yanagita S, Ishigami S, Natsugoe S. Expression of B7-H4 in blood of patients with gastric cancer predicts tumor progression and prognosis. J Surg Oncol. 2010;102(7):748–752. doi: 10.1002/jso.21722. [DOI] [PubMed] [Google Scholar]
- 20.Suh WK, Wang S, Duncan GS, Miyazaki Y, Cates E, Walker T, Gajewska BU, Deenick E, Dawicki W, Okada H, Wakeham A, Itie A, Watts TH, Ohashi PS, Jordana M, Yoshida H, Mak TW. Generation and characterization of B7-H4/B7S1/B7x-deficient mice. Mol Cell Biol. 2006;26(17):6403–6411. doi: 10.1128/MCB.00755-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu G, Augustine MM, Azuma T, Luo L, Yao S, Anand S, Rietz AC, Huang J, Xu H, Flies AS, Flies SJ, Tamada K, Colonna M, van Deursen JM, Chen L. B7-H4-deficient mice display augmented neutrophil-mediated innate immunity. Blood. 2009;113(8):1759–1767. doi: 10.1182/blood-2008-01-133223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rahbar R, Lin A, Ghazarian M, Loo YH, Paramathas S, Lang P, Schildknecht A, Elford A, Garcia-Batres C, Martin B, Berman HK, Leong W, McCready D, Reedijk MJ, Done SJ, Miller N, Youngson B, Suh WK, Mak TW, Ohashi PS. B7-H4 expression by nonhematopoetic cells in the tumor microenvironment promotes anti-tumor immunity. Cancer Immunol Res. 2015;3(2):184–195. doi: 10.1158/2326-6066.CIR-14-0113. [DOI] [PubMed] [Google Scholar]
- 23.Abadi YM, Jeon H, Ohaegbulam KC, Scandiuzzi L, Ghosh K, Hofmeyer KA, Lee JS, Ray A, Gravekamp C, Zang X. Host b7x promotes pulmonary metastasis of breast cancer. J Immunol. 2013;190(7):3806–3814. doi: 10.4049/jimmunol.1202439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leung J, Suh WK. Host B7-H4 regulates antitumor T cell responses through inhibition of myeloid-derived suppressor cells in a 4T1 tumor transplantation model. J Immunol. 2013;190(12):6651–6661. doi: 10.4049/jimmunol.1201242. [DOI] [PubMed] [Google Scholar]
- 25.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, Hollmann TJ, Bruggeman C, Kannan K, Li Y, Elipenahli C, Liu C, Harbison CT, Wang L, Ribas A, Wolchok JD, Chan TA. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371(23):2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, Miller ML, Rekhtman N, Moreira AL, Ibrahim F, Bruggeman C, Gasmi B, Zappasodi R, Maeda Y, Sander C, Garon EB, Merghoub T, Wolchok JD, Schumacher TN, Chan TA. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tao K, Fang M, Alroy J, Sahagian GG. Imagable 4T1 model for the study of late stage breast cancer. BMC Cancer. 2008;8:228. doi: 10.1186/1471-2407-8-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Limberis MP, Bell CL, Wilson JM. Identification of the murine firefly luciferase-specific CD8 T-cell epitopes. Gene Ther. 2009;16(3):441–447. doi: 10.1038/gt.2008.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dupre’ SA, Redelman D, Hunter KW., Jr Microenvironment of the murine mammary carcinoma 4T1: endogenous IFN-gamma affects tumor phenotype, growth, and metastasis. Exp Mol Pathol. 2008;85(3):174–188. doi: 10.1016/j.yexmp.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Gajewski TF. Cancer immunotherapy. Mol Oncol. 2012;6(2):242–250. doi: 10.1016/j.molonc.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, Chen L. Colocalization of inflammatory response with b7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4(127):127ra137. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McWilliams JA, Sullivan RT, Jordan KR, McMahan RH, Kemmler CB, McDuffie M, Slansky JE. Age-dependent tolerance to an endogenous tumor-associated antigen. Vaccine. 2008;26(15):1863–1873. doi: 10.1016/j.vaccine.2008.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161(2):205–214. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi PM, Herati RS, Mansfield KD, Patsch D, Amaravadi RK, Schuchter LM, Ishwaran H, Mick R, Pryma DA, Xu X, Feldman MD, Gangadhar TC, Hahn SM, Wherry EJ, Vonderheide RH, Minn AJ. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520(7547):373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heinemann V. Role of gemcitabine in the treatment of advanced and metastatic breast cancer. Int Soc Cell. 2003;64(3):191–206. doi: 10.1159/000069315. [DOI] [PubMed] [Google Scholar]
- 36.Le HK, Graham L, Cha E, Morales JK, Manjili MH, Bear HD. Gemcitabine directly inhibits myeloid derived suppressor cells in BALB/c mice bearing 4T1 mammary carcinoma and augments expansion of T cells from tumor-bearing mice. Int Immunopharmacol. 2009;9(7–8):900–909. doi: 10.1016/j.intimp.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 37.Gajewski TF. Cancer immunotherapy. Mol Oncol. 2012;6(2):242–250. doi: 10.1016/j.molonc.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuan J, Ginsberg B, Page D, Li Y, Rasalan T, Gallardo HF, Xu Y, Adams S, Bhardwaj N, Busam K, Old LJ, Allison JP, Jungbluth A, Wolchok JD. CTLA-4 blockade increases antigen-specific CD8(+) T cells in prevaccinated patients with melanoma: three cases. Cancer Immunol Immunother. 2011;60(8):1137–1146. doi: 10.1007/s00262-011-1011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liakou CI, Kamat A, Tang DN, Chen H, Sun J, Troncoso P, Logothetis C, Sharma P. CTLA-4 blockade increases IFNgamma-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc Natl Acad Sci USA. 2008;105(39):14987–14992. doi: 10.1073/pnas.0806075105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, Burke MM, Caldwell A, Kronenberg SA, Agunwamba BU, Zhang X, Lowy I, Inzunza HD, Feely W, Horak CE, Hong Q, Korman AJ, Wigginton JM, Gupta A, Sznol M. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, Wolchok JD. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hofmeyer KA, Scandiuzzi L, Ghosh K, Pirofski LA, Zang X. Tissue-expressed B7x affects the immune response to and outcome of lethal pulmonary infection. J Immunol. 2012;189(6):3054–3063. doi: 10.4049/jimmunol.1200701. [DOI] [PMC free article] [PubMed] [Google Scholar]