Abstract
The role of human intraepithelial Vδ1+ γδ T cell cytotoxic effectors in the immune surveillance against metastatic colon cancer has never been addressed, despite their reported capacity to infiltrate colon carcinomas and to kill colonic cancer cells in vitro. We previously showed that Vδ1+ γδ T cells are enriched in blood in response to cytomegalovirus (CMV) infection, and that such increase may be protective against epithelial cancers. The objective of the present study was to investigate whether CMV-induced Vδ1+ γδ T lymphocytes could inhibit the propagation of human colon tumors in vivo, in order to evaluate their immunotherapeutic potential in this context. Even though metastases are an important cause of death in various cancers including colorectal cancer (CRC), the anti-metastatic effect of immune effectors has been poorly analyzed. To this purpose, we set up a reliable model of metastatic colon cancer through orthotopic implantation of luciferase-expressing human HT29 cells in immunodeficient mice. Using bioluminescence imaging to follow the outcome of colonic cancer cells, we showed that a systemic treatment with CMV-induced Vδ1+ γδ T cells could not only inhibit primary colon tumor growth but also the emergence of secondary tumor foci in the lungs and liver. Finally, our data lead to propose that Vδ1+ γδ T lymphocytes may directly influence the appearance of metastases independently from their control of primary tumor size. These findings, which extend our previous work, pave the road for the potential manipulation of Vδ1+ γδ T lymphocytes in novel anti-CRC immunotherapeutic protocols.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-013-1402-1) contains supplementary material, which is available to authorized users.
Keywords: Invasive colon carcinoma, Immunotherapy, Human γδ T cells, Orthotopic mouse xenograft model, Bioluminescence imaging
Introduction
Colorectal carcinoma (CRC) remains one of the leading causes of cancer death worldwide. CRC develops slowly from colorectal hyperplasia to adenoma and then to invasive carcinoma, but progress rapidly to the metastatic phase. The most frequent sites of metastases are the lungs and the liver, due to the influence of definite circulation patterns and specific molecular interactions that favor the retention of colonic carcinoma cells in these organs [1].
Recent investigation of the CRC primary tumor microenvironment revealed that the type, density, and in situ location of immune cells could predict the clinical outcome of CRC patients [2, 3]. The intratumoral density of CD8+ αβ T cell cytotoxic effectors and the expression of genes related to cytotoxicity were associated with a better clinical outcome in CRC patients [4, 5]. Such discoveries encourage the development of novel anti-CRC treatments that aim at manipulating own patients’ cytotoxic effectors.
Among cytotoxic effectors, γδ T lymphocytes participate uniquely to the host protection against cancers by their capacity to quickly react against stress-induced cellular alterations [6, 7]. In physiological context, γδ T lymphocytes represent 0.5–5 % of T cells in the blood, while they constitute a substantial T cell fraction (30 %) in epithelial surfaces [8]. A majority of human peripheral blood γδ T cells express Vδ2+ γ9+ T cell-receptors (TCRs), in contrast to intraepithelial γδ T cells which are Vδ2− (Vδ1+ for most of them). In pathological situations, Vδ1+ γδ T cells may represent a substantial fraction of circulating T lymphocytes [9]. The reported capacities of Vδ1+ γδ T lymphocytes to infiltrate colon carcinomas [10] and to kill colon cancer cells in vitro [11, 12] make them potential candidates for novel immunotherapeutic protocols against CRC. However, their role in the immune surveillance against metastatic colon cancer has never been addressed.
Our previous data convey compelling evidence for a cytomegalovirus (CMV)-induced increase of circulating Vδ2− γδ T cells in both immune-suppressed and immune-competent individuals [13–15]. CMV-induced Vδ2− γδ T lymphocytes were associated with a rapid anti-viral response in vivo [16], and Vδ2− γδ T cell clones were reactive against CMV-infected cells in vitro [17]. Interestingly, some of these clones also displayed a TCR-dependent cytotoxic activity against HT29 colonic cancer cells in vitro, in contrast to normal intestinal epithelial cells [17]. When injected in the peritonea (i.p.) of immunodeficient mice, Vδ5+ (γ4+) γδ T cell clones specifically delayed the growth of subcutaneous HT29 tumors [18]. These results are consistent with the observation that CMV+ renal transplant recipients with high numbers of circulating Vδ2− lymphocytes appeared less susceptible to immunosuppression-associated skin cancer [19]. Taken together, our recent findings suggest that Vδ2− γδ T cells induced during CMV-infection may also be involved in tumor surveillance in vivo.
The present study was undertaken to analyze the potential role of CMV-induced Vδ1+ γδ T cells in the immune surveillance against metastatic colon cancer. To this purpose, we set up an orthotopic mouse xenograft model of human colon carcinoma that allows the development of metastases. Using bioluminescence imaging (BLI) to follow the outcome of cancer cells, we showed that a systemic treatment with human Vδ1+ γδ T cells could inhibit the growth of intracaecal HT29 tumors. More importantly, our results argue for a crucial role of γδ T cells in preventing the dissemination of colonic cancer cells, leading to a substantial reduction of distant (hepatic and pulmonary) metastases in γδ-treated mice when compared to control mice. Considering that metastases represent a major cause of death in cancer patients, our findings, which extend our previous work [18], lead to propose Vδ2− γδ T cells as attractive candidates for novel anti-tumor immunotherapeutic protocols [20, 21].
Materials and methods
Animals and human cells
We used 7–10 weeks old NOD-scid-gamma (NSG) mice from Jackson laboratories [22] and occasionally rag −/− γc −/− mice (a gift from Dr. Di Santo, Institut Pasteur, Paris, France). HT29 cells were from the American Type Culture Collection (ATCC). Mouse embryonic fibroblasts (MEF) were prepared by trypsinization of 18 days old BALB/C foetuses. Fibroblasts and cancer cells were cultured at 37 °C, 5 % CO2, in DMEM (Invitrogen, Cergy Pontoise, France) 8 % heat-inactivated fetal bovine serum (FBS) (PAA laboratories GmbH, Haidmannweg, Austria). Vδ1 line and Vδ1 line 2 were obtained through immunomagnetic positive sorting (Dynabeads) with an anti-Vδ1 mAb (clone R9.12, Beckman Coulter), using peripheral blood mononuclear cells (PBMC) from two children with a neonatal CMV-infection (blood samples were kindly provided by Dr. Le Deist (INSERM U429, Hôpital Necker-Enfants Malades, Paris, France). They were expanded 2–3 weeks before use with Leucoagglutinin (Leucoagglutinin, Invitrogen, NY) and irradiated allogeneic PBMC as described previously [17], in RPMI 1640 (Invitrogen), 10 % human serum (HS), and 1,000 IU/mL recombinant human interleukin-2 (rhIL-2, Peprotech France, Neuilly-sur–Seine, France).
Generation of firefly luciferase-expressing HT29 cells (HT29 luc)
Firefly luciferase (fluc) cDNA was amplified by PCR from pGL3 luciferase reporter vector (Promega, Charbonnières les Bains, France) and cloned into pRRLSIN.cPPT.MNDU3.MCS.WPRE vector (plateforme de vectorologie, université Bordeaux Segalen, France). Lentiviral particles were produced by transient transfection of 293 T cells as described previously [23]; HT29 cells were transduced at a multiplicity of infection (MOI) of 40.
In vitro detection of luciferase activity
Organs were dislocated using an Ultra Turax T25 (IKA, Staufen, Germany); luciferase activity was determined with the Luciferase Assay System (LAS) (Promega, Madison, WC); photons were counted for 10 s with a luminometer (LUMAT 9501, Berthold Technology, Bad Wildbad, Germany).
Cellular cytotoxicity assay
HT29, HT29 luc, or MEF cells were labelled with 51Cr (1.85 MBq/106 cells) and incubated with γδ T lymphocytes at the indicated effector/target ratios. In Fig. 1c, γδ T cells were pre-treated 2 h with 200 nM concanamycin A (Calbiochem, Darmstadt, Germany), or with same amount of dimethylsulfoxide (DMSO). In Fig. 1d, γδ T cells were incubated with 10 μg/mL of anti-CD3 (clone OKT3, eBioscience, Montrouge, France), anti-NKG2D (clone 149810, R&D systems, Lille, France), or isotype control IgG2A (R&D systems). After 4 h at 37 °C, 51Cr released in supernatant was measured and percentage of specific 51Cr release was calculated [24].
Fig. 1.
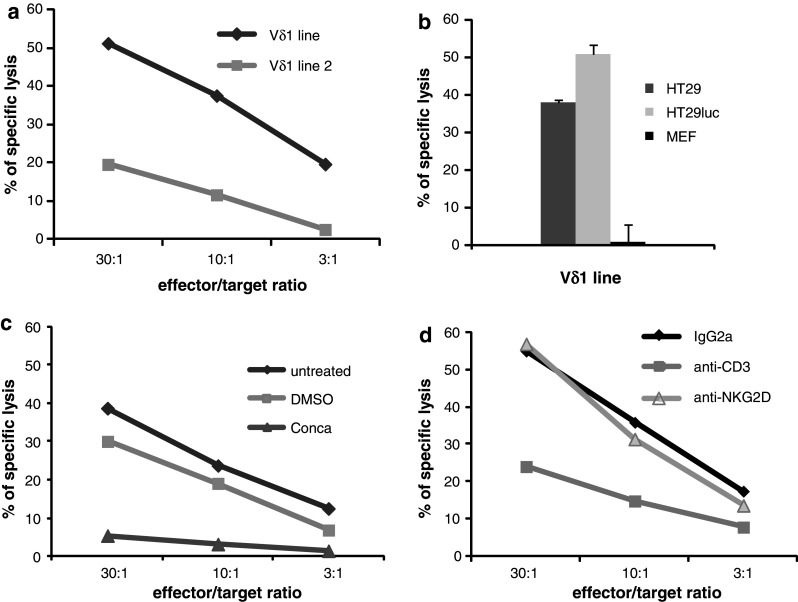
Mechanism of colonic cancer cells killing by CMV-induced Vδ1+ γδ T lymphocytes. a HT29 cells were labelled with 51Cr and incubated with two different Vδ1+ γδ T cell lines. Results are from one representative experiment among three. b HT29, HT29 luc cells, and MEF were labelled with 51Cr and incubated with CMV-induced Vδ1+ γδ T cells. Results are expressed as mean ± SD of specific 51Cr release in culture triplicates (effector/target ratio 10/1) and are representative of three independent experiments. c HT29 luc cells were labeled with 51Cr and incubated with Vδ1+ γδ T cells untreated, treated with Concanamycin (Conca), or incubated with similar dilution of DMSO (negative control). d In a series of similar experiments, effectors were untreated, or treated with anti-NKG2D, anti-CD3 or isotype control IgG2A mAbs. Results are from one representative experiment among three
Histopathology
Tissues were fixed in 10 % formalin and embedded in paraffin. 4-μm tissue sections were mounted on glass slides and dried at 56 °C before dewaxing in xylene and rehydration in alcohol. Sections were stained with Hematoxylin Eosin Safranin (HES) according to standard histological procedures. Photographs were taken with the X4 objective of a Coolscope microscope (Nikon).
Bioluminescence imaging (BLI)
For in vitro imaging of HT29 luc cells, 4 × 10−3 M of d-luciferin (Promega, Madison, WC) were added 5 min before imaging with a Photon Imager (Biospace, Paris, France). For in vivo imaging, mice were anesthetized using an inhalation system with 5 % isofluoran for induction and 2 % for maintenance (TEM-SEGA, Lormont, France). Mice were injected i.p with 150 mg/kg d-luciferin and Light Emission was recorded in real time during 5–25 min with the Photon Imager. For ex vivo imaging, tissues were rapidly excised, immersed 5 min into 500 μL of PBS containing 4.5 mg/mL d-luciferin and imaged. Bioluminescent pseudocolor images were obtained with a compilation of 30 s of maximal signal emission and were adjusted to the same threshold/smoothing for comparison. They are shown superimposed on gray-scale photographic images of the mice, with the most intense bioluminescent signal shown as red and the weakest signal shown as blue.
Implantations of human HT29 luc cells in mice and adoptive transfer of γδ T cells
To produce subcutaneous (s.c.) tumors mice received 1 × 105 HT29 luc cells into the right flank. Local s.c. injections of γδ T cells were performed by the simultaneous inoculation of 107 Vδ1+ γδ T cells. Tumor volume (mm3) was estimated using the formula: [length (mm) × width2 (mm)]/2. The experimental metastasis assay was achieved by intravenous (i.v) inoculation of HT29 luc cells into the retro-orbital plexus of mice. To induce orthotopic tumors, mice were anesthetized and placed under a binocular microscope (Nikon); 50 μL of medium containing 105 HT29 or HT29 luc cells were injected subserously in the caecum. Animals recovered in a warm environment and received i.p. 1 μg/animal of Buprenorphine (Vetergesic®, Centravet SA Cooperative, Dinan France). When a systemic γδ-treatment was applied, mice received i.p injections of 4 × 106 Vδ1+ lymphocytes 3 times a week, from day 0 to day 36. In vivo experiments were performed in accordance to ethical principles and were arrested before day 40 in order to avoid animals’ sufferance.
Analysis of tumor development using BLI
For quantitative analyses of primary tumor sizes, an elliptical region of interest (ROI) was drawn on bioluminescent images over the intracaecal tumor location. The surface area of the ROI was kept constant. A time activity curve for every acquisition was achieved by analyzing images using M3 Vision software (Biospace). Results were obtained with a compilation of 30 s of maximal signal emission and were expressed as photons/second/steradian (ph/s/sr) using the conversion factor (1 cpm = 28 ph/s/sr) provided by the camera manufacturer (Biospace). The minimal signal emitted by intracaecal tumors was ~1 × 104 ph/s/sr. For metastases detection the threshold was set up 3 times above background. Bioluminescent spots in lungs and liver areas were notified, while signals close to the caecum were not considered because of possible interference with the primary tumor.
Statistical analysis
To analyze the relationship between the volume and Light Emission of subcutaneous HT29 luc tumors, the Spearman correlation coefficient was calculated on the whole data set (every mouse at each time point). To compare the time of metastasis apparition in control and treated mice, as well as to compare primary tumors sizes when metastasis appeared, results from experiments 1 and 2 were pooled and analyzed using a version of the Wilcoxon–Mann–Whitney test for interval censored data. For all experiments, a P value <0.05 was considered as significant.
Results
Characterization of CMV-induced Vδ1+ γδ T cell lines with high killing potential against HT29 colonic cancer cells
We previously showed that CMV-induced Vδ1+ γδ T cell clones isolated from kidney allograft recipients killed HT29 cells in vitro [17]. To extend these data to other clinical contexts and to select fast-growing Vδ1+ γδ T lymphocytes reliable for adoptive transfer, we tested the cytotoxic potential of two oligoclonal Vδ1+ γδ T cell lines isolated from children infected with CMV. Both killed HT29 cells although with different intensity (Fig. 1a) thus the most efficient cell line was used subsequently. We next generated luciferase-expressing HT29 (HT29 luc) cells to follow colon cancer development in vivo. A positive correlation was shown between Photon Emission and HT29 luc cell counts (Fig. 2a) and luciferase expression was stable over time (not shown). CMV-induced Vδ1+ γδ T cells exhibit a cytolytic effect against both HT29 and HT29 luc cells but not against MEF in vitro (Fig. 1b). Most of Vδ1+ γδ T cells expressed intracellular perforin (80 %) and, as measured by the induced membrane expression of CD107a, 25–35 % released cytolytic granules when incubated with HT29 luc cells (not shown). Accordingly to the above finding, HT29 killing was abolished by Concanamycin A (Fig. 1c), a potent inhibitor of degranulation [25]. Finally, the cytotoxic activity of Vδ1+ γδ T lymphocytes toward HT29 luc cells was inhibited by anti-CD3 monoclonal antibody (mAb), whereas addition of anti-NKG2D mAb had no effect (Fig. 1d). In conclusion, CMV-induced Vδ1+ γδ T lymphocytes are able to kill HT29 colonic cancer cells through the release of cytolytic granules by a mechanism that involves γδ TCR-engagement, and represent reliable material for adoptive transfer in mice.
Fig. 2.
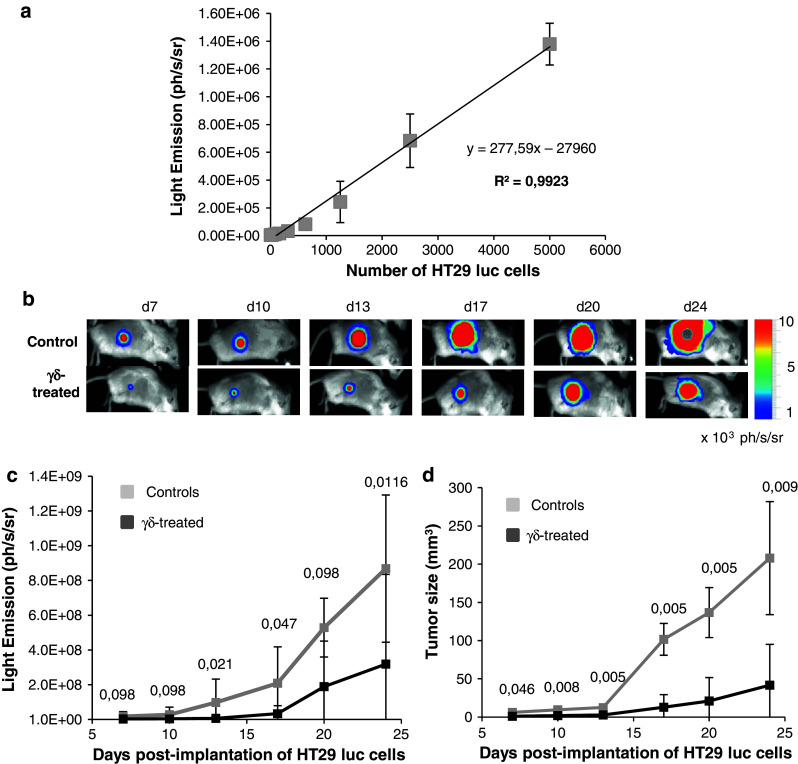
HT29 luc cells allow following the antitumor effects of γδ T cell therapy in vivo. a ½ serial dilutions of HT29 luc cells were distributed in 96-well plates. Light Emission was quantified with the Photon Imager and plotted against HT29 luc cell counts (from 5,000 to 9.75 cells). This experiment was done twice with similar results. b–d Tumor growth in groups of 6–8 mice receiving subcutaneous injections of 1 × 105 HT29 luc cells in the absence (controls) or the presence (γδ-treated) of 107 Vδ1+ T cells. b Bioluminescent images of one representative untreated (control) and γδ-treated mouse taken from day 7 (d7) to day 24 (d24). c Tumor growth was monitored using BLI every 3–4 days for 24 days; bioluminescent emission is expressed in photons/s/steradian (ph/s/sr). d Tumor sizes were estimated concomitantly by volumetric caliper measurements and are expressed in mm3. Values are mean ± SD. Numbers above curves represent P values obtained at indicated times from comparative analyses of Light Emission (left) or tumors sizes (right) between controls and γδ-treated mice (Wilcoxon test)
Local treatment with Vδ1+ γδ T lymphocytes delays the development of HT29 luc hypodermal tumors
We first tested the effect of local γδ-treatment on tumor growth, by subcutaneous and simultaneous inoculations of HT29 luc cells and Vδ1+ lymphocytes (single-shot). Tumor growth was monitored every 3–4 days until day 24, using BLI and concomitant volumetric measurements of tumor mass. Figure 2b shows the kinetic of tumor development visualized by BLI, in representative control and treated mice. Owing to the high sensitivity of BLI, HT29 luc cells were detected as early as day 2 in control mice (not shown). Mean photon outputs were smaller in γδ-treated mice than in control mice, but the differences did not always reach statistical significance (Fig. 2c), likely because BLI remains dependent on substrate pharmacokinetics. In contrast, highly significant differences were observed between mean tumor sizes in untreated and γδ-treated mice throughout the experiment (Fig. 2d). Finally, a relationship between tumor size and Light Emission could be clearly established (Spearman rank correlation coefficient = 0.91, p = 2.2 × 10−16, “Materials and methods”). These results suggest that CMV-induced Vδ1+ γδ T cells have an inhibitory effect on the development of colon cancer in vivo and show that BLI is a viable option to follow tumor development.
The experimental metastasis assay generates bioluminescent colon tumor foci in lungs
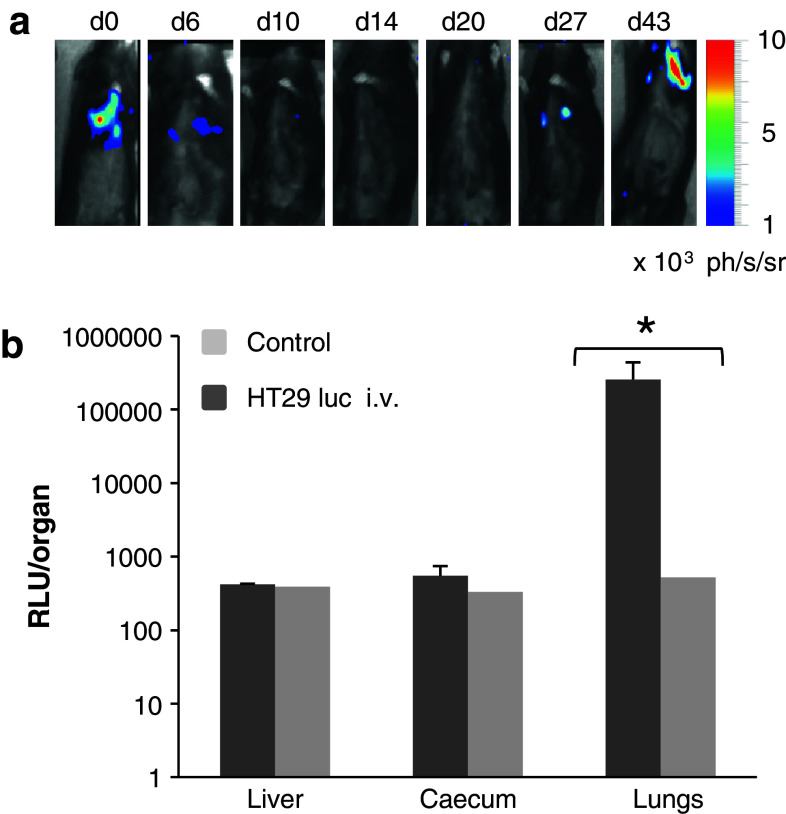
Subcutaneous xenografts of human colon carcinomas (HCC) are scarcely metastatic [26]. To generate colonic metastases, we tried at first to use the previously described experimental metastasis assay consisting in intravenous (i.v.) injections of cancer cells [27, 28]. Figure 3a depicts representative bioluminescent images obtained from mice receiving 106 HT29 luc cells retro-orbitally. Human cancer cells were detected around the lung area as early as 10 min post-i.v. inoculation at day 0; they were still visible at day 6, disappeared at day 10 and reappeared at day 27. When 105 HT29 luc cells were inoculated, a minimum of 40 days was necessary to recover bioluminescent data (not shown). In vitro measurement of luciferase activity on ground tissues confirmed the presence of colonic cancer cells in the lungs of HT29 luc i.v.-infused mice at the end of experiment, in contrast to gut and liver (Fig. 3b).
Fig. 3.
Intravenous injection of HT29 luc cells. a Bioluminescent images from day 0 to day 43, of one representative mouse among five, which received i.v injections of 106 HT29 luc cells at day 0. b In vitro detection of luciferase activity in livers, caecum, and lungs withdrawn at day 43, from mice receiving i.v injections of 106 HT29 luc cells (HT29 luc i.v) or one healthy mouse (control). Results are from 3 different mice, and values are shown as means Relative Light Unit (RLU) ± SD for each organ (Kruskal–Wallis test *P < 0.05)
Setting up a mouse model for human metastatic colon carcinoma
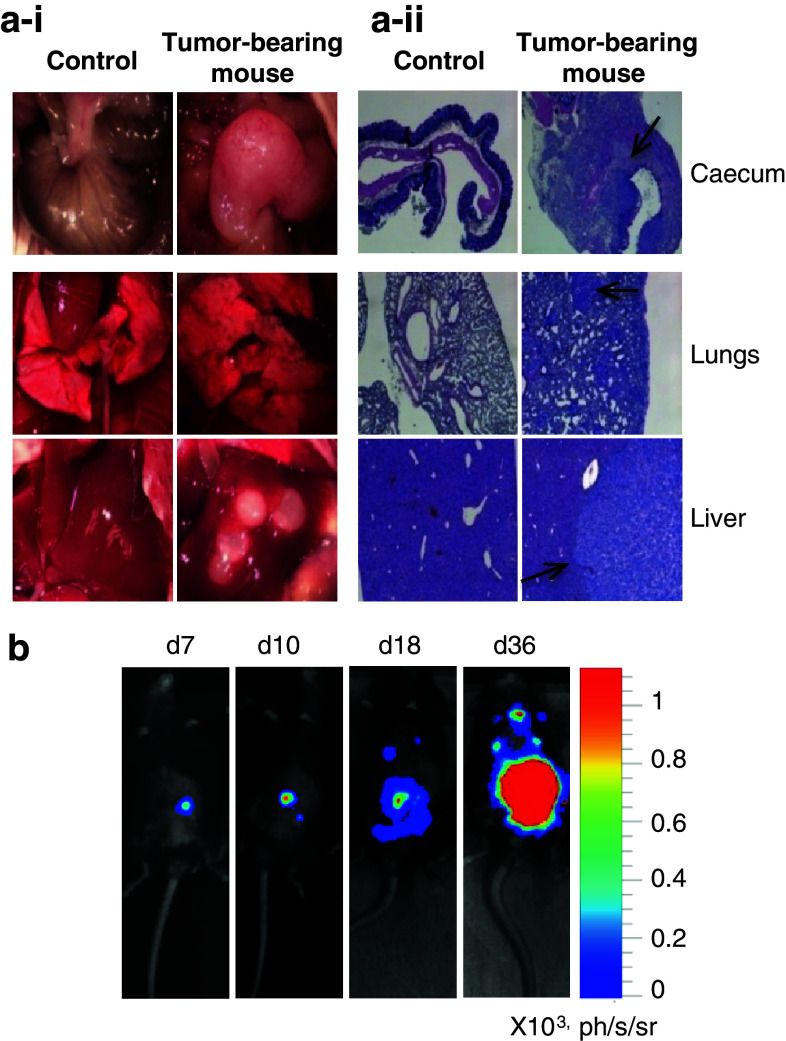
Because no luciferase activity was detected in the liver with the experimental metastases assay, we switched for an orthotopic implantation model (Fig. 4). Primary tumors developed locally and, after 2 months, formed huge white masses with disorganized vascularization (Fig. 4a–i). Tumor secondary foci were also present as semi-translucent spots in lungs and white masses in livers. The presence of human colonic cancer cells was ascertained on histological sections of organs (Fig. 4a–ii). When BLI was used to follow tumor development, the signal emitted by the primary tumor decreased during the first week (not shown), then increased progressively until day 36 (Fig. 4b). Distant metastases apparition occurred at days 13–20 with inter-animal variations. At that time, bioluminescent signals were frequently observed in the liver area in contrast to the lung area where they appeared later on (day 36 on Fig. 4b). Luminometric analyses of organs proved the presence of luciferase activity in caecum, lungs and liver, and, more occasionally, in other organs like the stomach (not shown). On the whole, tumor take occurred in >90 % of mice and 100 % of mice bearing intracaecal tumors developed metastases.
Fig. 4.
Orthotopic microinjection of human colonic cancer cells in mice and visualization of secondary tumor foci in the lungs and liver. a–i Pictures of caecum, lungs, and liver from one healthy (control) and one tumor-bearing mouse (among five). Organs were withdrawn 2 months after orthotopic injections of human HT29 cancer cells. a–ii Histological examination of the same organs. Human colonic cancer cells are shown by black arrows. b Bioluminescent images of one representative mouse among eight at various times post-orthotopic injection of 105 HT29 luc cells
Continuous i.p. infusions of Vδ1+ γδ T lymphocytes inhibit intracaecal tumor growth in the mouse orthotopic xenograft model
We recently showed that HT29 cancer cells secrete pro-inflammatory chemokines able to attract Vδ2− γδ T lymphocytes [18]. To test whether Vδ1+ γδ T cells could exert their anti-tumor activity against HT29 luc intracaecal tumors, we tested the effect of repeated γδ-infusion on primary tumor growth. Two independent experiments were performed where the kinetic of primary tumor development was followed by BLI (Supplementary Fig.). Tumor take occurred in > 90 % of mice (10/10 controls and 9/10 γδ-treated in experiment 1, 9/10 controls and 10/10 γδ-treated in experiment 2), but some mice died before the end of experiment due to extensive anesthesia upon imaging (2 γδ-treated mice in experiment 1, 1 γδ-treated mouse and 2 controls in experiment 2). Neither mice that did not develop intracaecal tumors nor mice that died before day 36 were exploited. For each control and γδ-treated mouse bearing intracaecal tumors, BLI signals in the caecum area were quantified, and primary tumor sizes were tabulated (Table 1). Overall, primary tumor sizes increased with time, although individual BLI signals rippled sporadically probably due to repositioning of the caecum. No significant differences were observed between the two groups of mice at day 7. At days 13 and 15, differences between means tumor sizes of controls and γδ-treated mice reached significance in experiment 1 in contrast to experiment 2. From day 20 to the end of experiment, however, primary tumors were statistically smaller in γδ-infused mice than in control mice for both experiments (Table 1). Figure 5a shows the evolution of means primary tumor sizes in pooled controls and γδ-treated mice from pooled experiments 1 + 2. Primary tumor growth was clearly delayed in γδ-treated mice when compared to untreated mice, in agreement with an effective anti-tumor role of human Vδ1+ γδ T cells against colorectal tumors in vivo.
Table 1.
Analysis of primary tumor sizes and metastases development in NSG mice treated or not with CMV-induced Vδ1+ γδ T lymphocytes
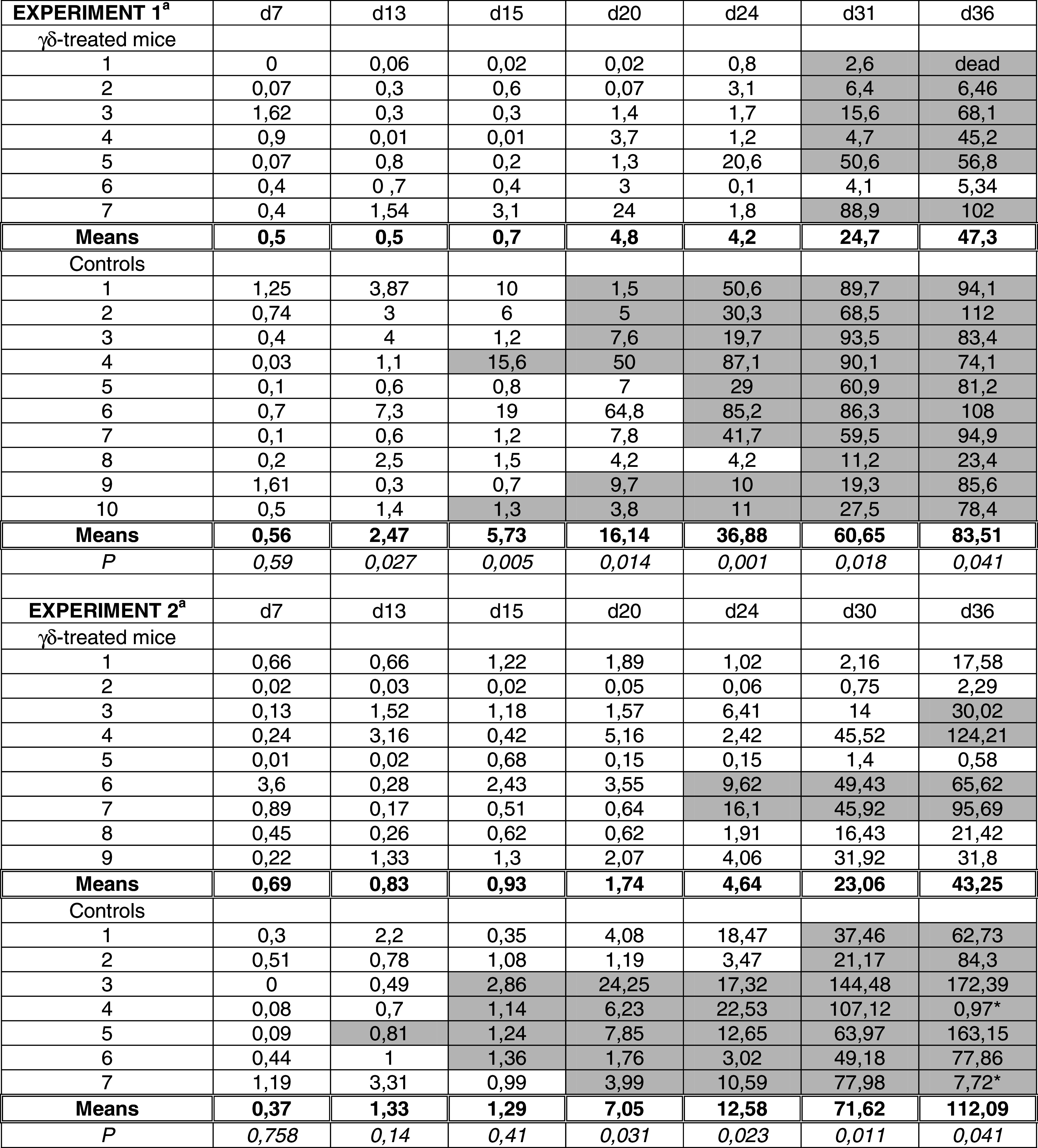
a For each control and γδ-treated mouse from experiment 1 (top) and 2 (bottom), primary tumor sizes were determined based on Light Emission at indicated days. For simplification, results are expressed as percentages of an arbitrary maximal tumor size of 1.109 ph/s/sr. Bolded values indicate means tumor sizes for each (untreated and γδ-treated) group of mice. Values with * (d36, experiment 2) were not considered because unexpectedly low when compared to d30. P values result from comparative analysis of means tumor sizes between controls and treated mice using the Wilcoxon test. Grey boxes indicate that at that time the mouse was holding at least one bioluminescent signal in liver and/or lungs area, whose intensity was at least 3 times above background
Fig. 5.
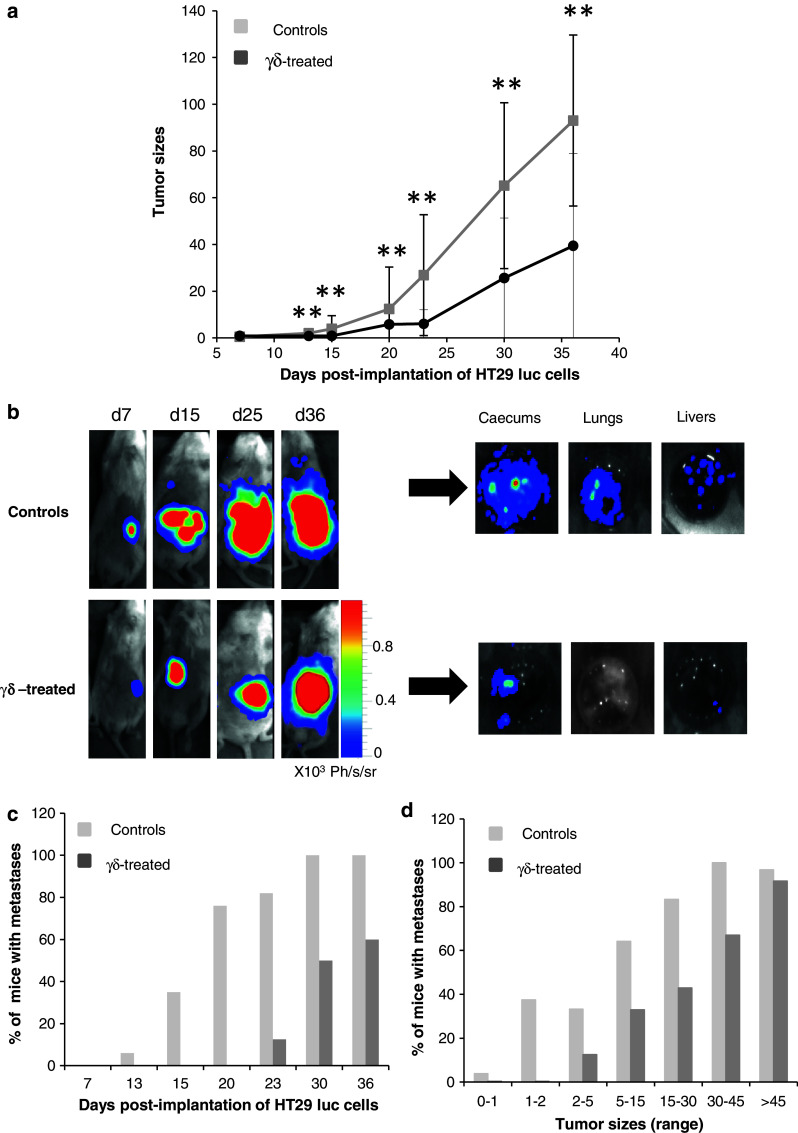
Effect of continuous i.p injections of Vδ1+ γδ T lymphocytes on the development of HT29 luc intracaecal tumors and metastases. a Means tumor sizes (determined as described in Table 1) of 16 γδ-treated mice (7 from experiment 1 + 9 from experiment 2) were plotted against time and compared at indicated times to means tumor sizes of 17 controls (10 from experiment 1 + 7 from experiment 2) (Wilcoxon test **P < 0.005). b Bioluminescent images of primary tumors and metastases from representative untreated (control) or γδ-treated NSG mice. Images were collected at days 7 (d7), 15 (d15), 25 (d25), and 36 (d36) post-tumor implantation in the caecum. Following necropsy of controls and γδ-treated mice at day 36 (n = 3 for each group), caecum, lungs, and liver were excised and bioluminescent signals were analyzed ex vivo. c The percentages of mice with detectable metastases in the lungs and/or livers areas in pooled controls and treated mice from experiments 1 + 2 were determined at indicated days d Different ranges of intracaecal tumor sizes were fixed (between two arbitrary values, Table 1), and the proportion of mice with metastases among the 17 controls and 16 γδ-treated mice was determined
Vδ1+ γδ T cells present anti-metastatic properties
The kinetic of metastases development for each control and γδ-treated mouse from experiment 1 and 2 was followed by BLI concomitantly to the development of HT29 luc intracaecal tumors. The presence of metastases was notified at indicated times (Table 1, grey boxes). As can be observed, the time of metastases appearance was clearly delayed by the γδ-treatment (statistical test for censored data gave p = 0.00004, “Materials and methods”). Consequently, the proportion of mice bearing metastases at each time point was smaller in the treated than in the control group (Fig. 5c). Dissection of mice at necropsy proved the presence of secondary tumor foci in lungs and livers from untreated mice, while fewer if any HT29 luc cells were detected in the γδ-treated group at these hits (Fig. 5b). Importantly, metastases were never observed in absence of a primary tumor (not shown). In fact, metastases incidence increased with time, concomitantly to primary tumor development (Table 1). Yet, when considering equivalent intracaecal tumor sizes, the proportion of mice bearing metastases was lower in the γδ-treated group than in controls (Fig. 5d). Moreover, when the first metastases appeared, mice receiving the immunotherapy had larger intracaecal tumors than control mice (statistical test for censored data gave P = 0.01714). From these findings, we consider that the observed anti-metastatic effect of γδ-T cell immunotherapy is not only due to the generation of lower numbers of metastases from smaller primary tumors, but also to a direct action of γδ T cells on metastatic cancer cells.
Discussion
The concept for a protective role of murine intraepithelial γδ T cells against carcinomas was demonstrated using γδ-T cell deficient mice that appeared to be more susceptible to cutaneous carcinogenesis as compared to control mice [29]. γδ T cells were also suggested to suppress the formation and progression of azoxymethane-induced colorectal adenocarcinoma [30], while the protective role of human intraepithelial Vδ1+ γδ T cells against colon cancer has not been reported so far. Although Vδ1+ γδ T lymphocytes were shown to infiltrate epithelial tumors from various origins including colon carcinomas, correlative analyses between the presence/frequency of intratumoral Vδ1+ γδ T cells and the outcome of disease are scarce [31] and remain tricky [9]. Besides, human studies mostly rely on late stage tumors, and the early effects of γδ T cells on emerging cancer cells cannot be investigated.
Murine xenograft tumor models offer an alternative for testing the capacity of human lymphocytes to inhibit tumor growth and evaluate the efficiency of novel immunotherapeutic protocols. Even if the present analysis was restricted to one Vδ1+ γδ T cells line, we have indications that the observed anti-colon cancer activity could be generalized to other CMV-induced Vδ2− γδ T cells. Indeed: (1) in a previous study, we showed that a systemic treatment of immunodeficient mice with CMV-induced Vδ5+ γδ T cell clones, in contrast to Vδ2+ T cells, delayed the development of HT29 tumors implanted subcutaneously [18]; (2) we have been generating for many years different lines and clones of Vδ2− γδ T cells that show efficient reactivity against colon cancer cells ([17] and data not shown); and we showed that the efficiency of γδ T cells in anti-tumor therapy correlated with their cytotoxic activity in vitro [18].
In a first step toward the evaluation of the therapeutic potential of CMV-induced Vδ1+ γδ T lymphocytes against colon carcinomas, we showed that CMV-induced Vδ1+ γδ T cell lines were capable of colonic cancer cell killing. Recognition of target cells involved TCR-engagement and the release of cytolytic granules. However, despite γδ T cells expressed NKG2D (not shown) and consistently with our previous observations [17], HT29-killing by Vδ1+ γδ T cells was not inhibited by anti-NKG2D mAbs even though HT29 cells expressed ULBP2 and low levels of MICA. CMV-infection thus appears as a favorable context for the amplification of Vδ2− γδ T cells with TCR-specific anti-tumor reactivities.
A single subcutaneous injection of Vδ1+ γδ T lymphocytes significantly delayed the growth of HT29 hypodermal tumors as previously observed with Vδ5+γ4+ T cell clones [18]. When HT29 luc cells were injected intravenously in an attempt to generate a metastatic colon cancer model, bioluminescent signals were rapidly detected in the lung area. The ensuing signal decline is consistent with death of most of trapped cells as shown earlier with radiolabelled HT29 cells [27]. Therefore, although numerous cells pass through the lungs after introduction into the venous circulation, only few of them will give rise to tumor foci in lungs. Moreover, no liver metastases were evidenced by sensitive measurement of luciferase activity in vitro. These findings confirm that ectopic and intravenous injections of human colonic cancer cells in mice do not faithfully replicate the physiological spread of human CRC [26].
The local microenvironment regulates tumor growth, angiogenesis, and the expression of pro-metastatic proteins thus influencing tumor spreading [32, 33]. We show here that intracaecal injection of HT29 cells in mice results in the formation of a solid tumor that invades the caecum and generates both pulmonary and hepatic metastases. The apparition of secondary foci: (1) was always associated with the development of an intracaecal primary tumor and (2) occurred earlier than observed after i.v. injection of HT29 luc cells. This implies a specific phenotype of metastatic cells beyond the only capacity to reach the circulation.
The orthotopic mouse model described in the present study allows evaluating alongside the action of the γδ-treatment on both the intracaecal colon tumor and emerging metastases. We show that a continuous i.p. treatment with CMV-induced Vδ1+ γδ T cells delays the growth of primary colon tumors xenografted into immunodeficient mice. Despite repeated γδ T cells infusion, the antitumor effect was short lived because once formed, the intracaecal tumors developed with equivalent rate in untreated and γδ-treated mice. Concordant results were observed in orthotopic mouse models of human glioblastoma multiforme (GBM) and bladder cancer, where the γδ-based immunotherapeutic treatment had no effect when introduced 1 week post-implantation of U251-MG and UM-UC-3 cancer cells, respectively [34, 35]. In both studies, the infused γδ T cells were highly cytotoxic against the human cancer cell lines. The transient effect of the adoptive immunotherapy is likely due to the lack of maintenance/proliferation of human γδ T cells in the mouse microenvironment, although we cannot formally exclude that tumor cells acquire resistance to cytotoxicity. Among the human growth factors tested for in vitro amplification of Vδ2− γδ T cells from CMV+ donors, we showed that IL2 was critical (not shown). However, injection of rIL2 did not improve γδ T cell immunotherapy in our previous study [18]. In the mouse xenograft tumor model, human γδ T cells probably never reach sufficient numbers to overcome tumor growth except at the initial steps of tumor development, when the effector/target ratio is in their favor.
Subsequently, we hypothesized that γδ T cells may also control the dissemination of colonic cancer cells. Indeed, Vδ1+ γδ T cells delayed the apparition of metastases in the liver and/or lungs. Since distant metastases incidence increased concomitantly to primary tumor sizes in HT29-bearing mice (Table 1 and [36]), the anti-metastatic potential of γδ T cells is likely due, at least in part, to the generation of smaller intracaecal tumors in γδ-treated mice. However, our results strongly suggest that the treatment also directly influence the appearance of metastases independently from its control of primary tumor size (Fig. 5d).
While the origin of peripheral Vδ1+ γδ T cells from CMV+ donors remains elusive, the cell surface expression of homeostatic gut homing receptors suggests that they may come from the intestinal tract where CMV replicates [17]. Once activated by CMV, Vδ1+ γδ T cells may be prompt to induce local anti-CRC surveillance, or recirculate and migrate back to the intestine in response to inflammatory chemokines secreted by colon cancer cells [18]. CMV-infection on the one hand [37] and elevated percentages of Vδ1+ γδ on the other hand [38] have both been associated with reduced cancer risk in leukemia patients after bone marrow transplantation. Whether CMV+ individuals with increased numbers of Vδ1+ γδ T cells are less subjected to CRC development than CMV− controls is under consideration and would extend our recent findings on cutaneous cancer [19]. If this holds true, CMV+ CRC patients could be good candidates for adaptive Vδ1+ γδ-T cell immunotherapy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We are very grateful to Isabelle Duluc (INSERM U682, Strasbourg, France) for technical assistance for orthotopic injection of HT29 cells. We thank Michel Cogné (Animalerie de l’Université de Limoges, France) and Raphaël Pineau (Animalerie de l’Université de Bordeaux 1, France) for providing some rag−/−γc−/− immunodeficient mice. We are grateful to Maria Mamani, Marianne Guenot, and Charlotte Behr for their helpful advices. We thank the cytometry core facility and the vectorology platform (Université Bordeaux Ségalen, France). This work was supported by the Ligue Contre le Cancer comité départemental du Lot et Garonne, the Fondation pour la Recherche Médicale, the Association pour la Recherche contre le Cancer, the Conseil Régional d’Aquitaine, and the Institut National du Cancer, France.
Conflict of interest
None.
References
- 1.Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 2.Pages F, Berger A, Camus M, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 3.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 4.Camus M, Tosolini M, Mlecnik B, et al. Coordination of intratumoral immune reaction and human colorectal cancer recurrence. Cancer Res. 2009;69:2685–2693. doi: 10.1158/0008-5472.CAN-08-2654. [DOI] [PubMed] [Google Scholar]
- 5.Tosolini M, Kirilovsky A, Mlecnik B, et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011;71:1263–1271. doi: 10.1158/0008-5472.CAN-10-2907. [DOI] [PubMed] [Google Scholar]
- 6.Hayday AC. [gamma][delta] cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 7.Hayday AC. Gammadelta T cells and the lymphoid stress-surveillance response. Immunity. 2009;31:184–196. doi: 10.1016/j.immuni.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Hayday A, Theodoridis E, Ramsburg E, et al. Intraepithelial lymphocytes: exploring the third way in immunology. Nat Immunol. 2001;2:997–1003. doi: 10.1038/ni1101-997. [DOI] [PubMed] [Google Scholar]
- 9.Behr C, Capone M, Couzi L, et al. Vdelta2neg gamma-delta T cells, a multi-reactive tissue subset: from innate to adaptive altered-self-surveillance. Open Immunol J. 2009;2:106–118. doi: 10.2174/1874226200902020106. [DOI] [Google Scholar]
- 10.Maeurer MJ, Martin D, Walter W, et al. Human intestinal Vdelta1 + lymphocytes recognize tumor cells of epithelial origin. J Exp Med. 1996;183:1681–1696. doi: 10.1084/jem.183.4.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Groh V, Steinle A, Bauer S, et al. Recognition of stress-induced MHC molecules by intestinal epithelial gammadelta T cells. Science. 1998;279:1737–1740. doi: 10.1126/science.279.5357.1737. [DOI] [PubMed] [Google Scholar]
- 12.Groh V, Rhinehart R, Secrist H, et al. Broad tumor-associated expression and recognition by tumor-derived gamma delta T cells of MICA and MICB. Proc Natl Acad Sci USA. 1999;96:6879–6884. doi: 10.1073/pnas.96.12.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dechanet J, Merville P, Berge F, et al. Major expansion of gammadelta T lymphocytes following cytomegalovirus infection in kidney allograft recipients. J Infect Dis. 1999;179:1–8. doi: 10.1086/314568. [DOI] [PubMed] [Google Scholar]
- 14.Dechanet J, Merville P, Lim A, et al. Implication of gammadelta T cells in the human immune response to cytomegalovirus. J Clin Invest. 1999;103:1437–1449. doi: 10.1172/JCI5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pitard V, Roumanes D, Lafarge X, et al. Long-term expansion of effector/memory Vdelta2-gammadelta T cells is a specific blood signature of CMV infection. Blood. 2008;112:1317–1324. doi: 10.1182/blood-2008-01-136713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lafarge X, Merville P, Cazin MC, et al. Cytomegalovirus infection in transplant recipients resolves when circulating gammadelta T lymphocytes expand, suggesting a protective antiviral role. J Infect Dis. 2001;184:533–541. doi: 10.1086/322843. [DOI] [PubMed] [Google Scholar]
- 17.Halary F, Pitard V, Dlubek D, et al. Shared reactivity of V{delta}2(neg) gamma}{delta} T cells against cytomegalovirus-infected cells and tumor intestinal epithelial cells. J Exp Med. 2005;201:1567–1578. doi: 10.1084/jem.20041851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devaud C, Bilhere E, Loizon S, et al. Antitumor activity of gammadelta T cells reactive against cytomegalovirus-infected cells in a mouse xenograft tumor model. Cancer Res. 2009;69:3971–3978. doi: 10.1158/0008-5472.CAN-08-3037. [DOI] [PubMed] [Google Scholar]
- 19.Couzi L, Levaillant Y, Jamai A, et al. Cytomegalovirus-Induced {gamma}{delta} T Cells Associate with reduced cancer risk after Kidney transplantation. J Am Soc Nephrol. 2010;21:181–188. doi: 10.1681/ASN.2008101072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zocchi MR, Poggi A. Role of gammadelta T lymphocytes in tumor defense. Front Biosci. 2004;9:2588–2604. doi: 10.2741/1419. [DOI] [PubMed] [Google Scholar]
- 21.Kabelitz D, Wesch D, He W. Perspectives of gammadelta T cells in tumor immunology. Cancer Res. 2007;67:5–8. doi: 10.1158/0008-5472.CAN-06-3069. [DOI] [PubMed] [Google Scholar]
- 22.Shultz LD, Lyons BL, Burzenski LM, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174:6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 23.Geronimi F, Richard E, Redonnet-Vernhet I, et al. Highly efficient lentiviral gene transfer in CD34 + and CD34 +/38-/lin- cells from mobilized peripheral blood after cytokine prestimulation. Stem Cells. 2003;21:472–480. doi: 10.1634/stemcells.21-4-472. [DOI] [PubMed] [Google Scholar]
- 24.Davodeau F, Peyrat MA, Hallet MM, et al. Close correlation between Daudi and mycobacterial antigen recognition by human gamma delta T cells and expression of V9JPC1 gamma/V2DJC delta-encoded T cell receptors. J Immunol. 1993;151:1214–1223. [PubMed] [Google Scholar]
- 25.Kataoka T, Shinohara N, Takayama H, et al. Concanamycin A, a powerful tool for characterization and estimation of contribution of perforin- and Fas-based lytic pathways in cell-mediated cytotoxicity. J Immunol. 1996;156:3678–3686. [PubMed] [Google Scholar]
- 26.Giavazzi R, Campbell DE, Jessup JM, et al. Metastatic behavior of tumor cells isolated from primary and metastatic human colorectal carcinomas implanted into different sites in nude mice. Cancer Res. 1986;46:1928–1933. [PubMed] [Google Scholar]
- 27.Price JE, Daniels LM, Campbell DE, et al. Organ distribution of experimental metastases of a human colorectal carcinoma injected in nude mice. Clin Exp Metastasis. 1989;7:55–68. doi: 10.1007/BF02057181. [DOI] [PubMed] [Google Scholar]
- 28.Garofalo A, Chirivi RG, Scanziani E, et al. Comparative study on the metastatic behavior of human tumors in nude, beige/nude/xid and severe combined immunodeficient mice. Invasion Metastasis. 1993;13:82–91. [PubMed] [Google Scholar]
- 29.Girardi M, Oppenheim DE, Steele CR, et al. Regulation of cutaneous malignancy by gammadelta T cells. Science. 2001;294:605–609. doi: 10.1126/science.1063916. [DOI] [PubMed] [Google Scholar]
- 30.Matsuda S, Kudoh S, Katayama S. Enhanced formation of azoxymethane-induced colorectal adenocarcinoma in gammadelta T lymphocyte-deficient mice. Jpn J Cancer Res. 2001;92:880–885. doi: 10.1111/j.1349-7006.2001.tb01176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma C, Zhang Q, Ye J, et al. Tumor-Infiltrating gammadelta T lymphocytes predict clinical outcome in human breast cancer. J Immunol. 2012;189:5029–5036. doi: 10.4049/jimmunol.1201892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fidler IJ. Critical factors in the biology of human cancer metastasis: twenty-eighth G.H.A. Clowes memorial award lecture. Cancer Res. 1990;50:6130–6138. [PubMed] [Google Scholar]
- 33.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuasa T, Sato K, Ashihara E, et al. Intravesical administration of gammadelta T cells successfully prevents the growth of bladder cancer in the murine model. Cancer Immunol Immunother. 2009;58:493–502. doi: 10.1007/s00262-008-0571-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bryant NL, Gillespie GY, Lopez RD, et al. Preclinical evaluation of ex vivo expanded/activated gammadelta T cells for immunotherapy of glioblastoma multiforme. J Neurooncol. 2011;101:179–188. doi: 10.1007/s11060-010-0245-2. [DOI] [PubMed] [Google Scholar]
- 36.Jojovic M, Schumacher U. Quantitative assessment of spontaneous lung metastases of human HT29 colon cancer cells transplanted into SCID mice. Cancer Lett. 2000;152:151–156. doi: 10.1016/S0304-3835(99)00443-7. [DOI] [PubMed] [Google Scholar]
- 37.Elmaagacli AH, Steckel NK, Koldehoff M, et al. Early human cytomegalovirus replication after transplantation is associated with a decreased relapse risk: evidence for a putative virus-versus-leukemia effect in acute myeloid leukemia patients. Blood. 2011;118:1402–1412. doi: 10.1182/blood-2010-08-304121. [DOI] [PubMed] [Google Scholar]
- 38.Lamb LS, Jr, Musk P, Ye Z, et al. Human gammadelta(+) T lymphocytes have in vitro graft vs leukemia activity in the absence of an allogeneic response. Bone Marrow Transpl. 2001;27:601–606. doi: 10.1038/sj.bmt.1702830. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.