Abstract
Appropriate activation of dendritic cells (DC) is essential for successful active vaccination and induction of cell-mediated immunity. The scarcity of precursor cells, as well as long culture methods, have hampered wide-scale application of DC vaccines derived from CD34+ precursors, despite their suggested superior efficacy over the more commonly applied monocyte-derived DC (MoDC). Here, employing the CD34+/CD14+ AML-derived human DC progenitor cell line MUTZ3, we show that cytostatic anthraquinone-derivatives (i.e., the anthracenedione mitoxantrone and the related anthracyclin doxorubicin) induce rapid differentiation of CD34+ DC precursors into functional antigen-presenting cells (APC) in a three-day protocol. The drugs were found to act specifically on CD34+, and not on CD14+ DC precursors. Importantly, these observations were confirmed for primary CD34+ and CD14+ DC precursors from peripheral blood. Mitoxantrone-generated DC were fully differentiated within three days and after an additional 24 h of maturation, were as capable as standard 9-day differentiated and matured DC to migrate toward the lymph node-homing chemokines CCL19 and CCL21, to induce primary allogeneic T cell proliferation, and to prime functional MART1-specific CD8+ T lymphocytes. Our finding that anthraquinone-derivatives like mitoxantrone support rapid high-efficiency differentiation of DC precursors may have consequences for in vitro production of DC vaccines as well as for novel immunochemotherapy strategies.
Keywords: CD34+ precursors, Dendritic cell, Differentiation, Immunotherapy, Mitoxantrone, Cytostatic drugs
Introduction
Antigen presenting cells (APC) are key players in the initiation of an effective immune response [2]. Dendritic cells (DC), which reside in peripheral tissues, are professional APC. DC take up antigens and present derived epitopes to naïve T cells in the context of MHC class I or class II molecules. If appropriate danger signals are present at the site of antigen uptake, DC will undergo maturation and migrate from the tissue to draining lymph nodes (LN), where they encounter, and subsequently activate, antigen-specific naïve T cells. Unfortunately, these processes are often hampered in cancer patients due to prevailing tumor-induced immune suppression, which interferes with the generation of an effective anti-tumor response [9, 11].
Although chemotherapeutic agents at high systemic levels are invariably lethal to immune effector cells, we previously reported that they can actually activate DC when applied locally and might thus act as an adjuvant in vaccination settings [13]. A similar observation was made by Yu et al. who combined DC vaccination with paclitaxel treatment, resulting in increased anti-tumor responses [41]. More recently, low-dose chemotherapeutic agents were reported to induce enhanced expression of activation markers and proteins involved in the antigen-presenting machinery on murine bone marrow-derived DC (BM-DC) and human monocyte-derived DC (MoDC) [10, 32]. Aiming to develop new immunotherapeutic regimens for cancer treatment in humans, the direct effect of cytostatic anthraquinone-derivatives, i.e., mitoxantrone and doxorubicin, on DC precursor cells was studied. Both mitoxantrone and doxorubicin are used in the clinic to treat various types of cancer [5, 14, 17–19, 21, 39], while mitoxantrone is also used to treat multiple sclerosis [31]. As a source of DC precursor cells we made use of blood-derived CD14+ monocytes and CD34+ precursors as well as of the human acute myeloid leukemia cell line MUTZ3. MUTZ3 consists of a CD34+ proliferating fraction that develops into a CD14+ fraction with direct DC differentiation potential [15, 27]. These MUTZ3 progenitor cells can be differentiated into interstitial DC (MUTZ3-IDC) in a 7–9 day culture protocol with granulocyte macrophage-colony-stimulating factor (GM-CSF), interleukin-4 (IL-4), and tumor necrosis factor α (TNFα) or Langerhans Cells (MUTZ3-LC) in a 10–12 day culture protocol with GM-CSF, transforming growth factor β (TGFβ) and TNFα, as previously described [15, 27]. In extensive studies, MUTZ3-IDC and -LC were shown to accurately reflect their in vivo primary skin counterparts—both in terms of phenotype and of function [26, 29, 36]. Here, we show that in vitro exposure of CD34+ precursors to mitoxantrone in the presence of appropriate cytokine cocktails resulted in accelerated DC/LC differentiation. These DC were fully functional with respect to migration and T cell stimulation and priming. These data suggest that short-term exposure to anthraquinone-derivatives like mitoxantrone accelerates DC differentiation from CD34+ precursor cells and may be applied as a fast differentiation stimulus in DC cultures for the efficient in vitro production of clinical DC vaccines for immunotherapy regimens. This is of particular interest for the use of CD34+ precursors, since their general application has been hampered due to the relatively long culture periods required, despite their suggested superiority over monocyte-derived DC (MoDC) in terms of vaccination efficacy [22]. Moreover, our findings present anthraquinone-derivatives as attractive cytostatics to be applied in immunochemotherapeutic regimens.
Materials and methods
Chemicals
Unless otherwise stated, all chemicals and drugs were obtained from Sigma Chemical Co. (St. Louis, MO) except for Ko-143 which was kindly provided by Dr. Allen (Netherlands Cancer Institute, Amsterdam, The Netherlands) and has been described before [37].
Cell culture
The AML-derived CD34+ MUTZ3 cell line was cultured as previously described [15]. In brief, MUTZ3 progenitors were cultured in MUTZ3 routine maintenance medium, consisting of MEM-α (Minimum essential medium, Lonza, Verviers, Belgium) containing 20% fetal calf serum (FCS) (Hyclone, Perbio Science, Etten-Leur, The Netherlands), 100 IU/ml sodium-penicillin (pen), 100μg/ml streptomycin (strep), 2 mM l-glutamine (glut), 50μM β-mercaptoethanol (2ME), and 10% conditioned medium (CM) from cultures of the 5637 renal cell carcinoma cell line, in 12-well plates (Co-star) at a concentration of 0.2 million cells/ml and were passaged twice weekly. For mitoxantrone and doxorubicin effects on progenitor cell cultures, 0.1–0.2 million cells/ml of unseparated MUTZ3 cells or CD34+, CD14+ and double negative (DN) magnetic-bead sorted (MACS) MUTZ3 subpopulations (Miltenyi Biotec, Bergisch Gladbach, Germany) were seeded in 12-well plates in MUTZ3 routine medium in the absence or presence of 1–16.7 nM mitoxantrone (IC30-IC70) or 16.7–100 nM doxorubicin (IC30-IC70) and were cultured for 72 h before quantification of viable cells by trypan blue exclusion and phenotypic characterization by flow cytometry. For inhibition of the ABC transporter breast cancer resistance protein (BCRP), 200 nM Ko-143 was added.
MUTZ3-derived Langerhans cells (MUTZ3-LC) were cultured in MEM-α containing 20% FCS, pen/strep/glut, 2ME supplemented with 10 ng/ml TGF-β1 (Biovision, Mountain View, CA), 1,000 IU/ml rhGM-CSF (Sagramostim, Berlex) and 120 IU/ml TNFα (Strathmann Biotec) for 10 days in 12-well plates at a concentration of 0.1 million cells/ml, adding fresh cytokines on days 4 and 7. Interstitial DC derived from MUTZ3 (MUTZ3-IDC) were cultured in MEM-α containing 20% FCS, pen/strep/glut, 2ME supplemented with 20 ng/ml IL-4 (R&D systems Europe, Abingdon, United Kingdom), 1,000 IU/ml rhGM-CSF, and 120 IU/ml TNFα (MUTZ3-IDC medium) in 12 well plates for 6 days, adding fresh cytokines on day 3. Mitox-IDC were generated by culture of MUTZ3 progenitor cells in the presence of 2.1 nM mitoxantrone for 3 days. Mitox –LC received 2.1–16.7 nM mitoxantrone for 4 days before phenotypic analysis. Immature cells were matured by adding 2,400 IU/ml TNFα, 100 ng/ml IL-6 (Strathmann Biotec), 25 ng/ml IL-1β (Strathmann Biotec) and 1 μg/ml prostaglandin E2 (PGE2) (Sigma–Aldrich) for 24 h. Monocyte-derived DC (MoDC) were generated from monocytes isolated from healthy donor buffy-coats (Sanquin, Amsterdam, The Netherlands) in IMDM supplemented with 10% FCS, pen/strep/glut, 2ME, 1,000 IU/ml GM-CSF, and 10 ng/ml IL4 in the absence or presence of 16.7 nM mitoxantrone for 4–5 days.
CD34+ haematopoietic progenitor cells
CD34+ haematopoietic progenitor cells were isolated from blood of healthy donors and expanded for 2–5 weeks with 25 ng/ml fms-like tyrosine kinase-3 ligand (Flt3-L) and 10 ng/ml stem cell factor (SCF) as described previously [4]. To study the effect of mitoxantrone on these cells, thawed expanded CD34+ progenitor cells were cultured with or without 16.7 nM mitoxantrone for 72 h in the presence of 10 ng/ml Flt3-L and SCF. To study effects on LC differentiation, CD34+ progenitors were cultured with or without 16.7 nM mitoxantrone for 72 h in the presence of 1,000 IU/ml rhGM-CSF, 10 ng/ml TGFβ, and 120 IU/ml TNFα. After 72 h, phenotypic analysis was performed by flow cytometry.
Flowcytometric immunophenotypic analyses
Cells were immunophenotyped using the following FITC-conjugated and/or PE-conjugated Mabs reactive against: CD1a (1:25), CD54 (1:25), CD80 (1:25), CD86 (1:25), CD40 (1:10) (PharMingen, San Diego, CA), CD14 (1:25), HLA-DR (1:25), DC-SIGN (1:10) (BD Biosciences, San Jose, CA), CD83 (1:10), CD34 (1:10), Langerin (1:10) (Immunotech, Marseille, France). In short, 2.5–5·104 cells were washed in PBS supplemented with 0.1% BSA and 0.02% NaN3 and incubated with specific or corresponding control Mabs for 30 min at 4°C. Cells were washed and analyzed on a FACS–Calibur flow cytometer (Becton and Dickinson, San Jose, CA) equipped with CellQuest analysis software. Results were expressed as the percentage of positive cells or histogram plots were shown.
Allogeneic mixed leukocyte reaction (alloMLR)
Titrated DC(1·102–3·104) were cocultured with 1·105 peripheral blood lymphocytes (PBL) for 4 days in 96-wells plates in IMDM containing 10% human pooled serum (Sanquin, Amsterdam, The Netherlands), pen/strep/glut and 2ME. At day 4, 2.5 μCi/ml [3H]-thymidine (6.7 Ci/mmol, MP Biomedicals, Irvine, CA) was added per well for 16 h. Plates were harvested onto glass fiber filtermats (Packard Instruments, Groningen, The Netherlands) using a Skatron cell harvester (Skatron Instruments, Norway), and [3H]-thymidine incorporation was quantified using a Topcount NXT Microbetacounter (Packard, Meriden, CT).
Trans-well migration toward CCL19 and CCL21
For in vitro trans-well migration assays, 10^5 mature d7 MUTZ3-IDC or d4 mitox-DC were seeded in the upper compartment of Costar 24-well trans-wells with a pore size of 6 μm. The lower compartment contained 600 μl serum free MEM-α supplemented with pen/strep/glut, and 250 ng/ml CCL19 (Peprotech, Huissen, The Netherlands) or CCL21 (Invitrogen, Carlsbad, CA). Cells were allowed to migrate for 4 h at 37°C. After migration, 500 μl medium was harvested from the lower compartment and migrated cells were quantified with flow-count fluorospheres (Beckman Coulter, Fullerton, CA) by flow cytometry.
In vitro CD8+ T cell priming
The in vitro priming of MART-1 specific CD8+ T cells was performed as described previously [28]. In short, mature d7 MUTZ3-DC and d4 mitox-DC, at a concentration of 1.0 million cells/ml, were loaded with 1 μg/ml MART-126–35L peptide in serum free IMDM for 3–4 h in the presence of 3 μg/ml β2-microglobulin (β2 M). After loading, cells were irradiated at 5,000 rad, washed, and seeded at 0.2 million cells/ml in Yssels medium [40] supplemented with 2% hAB serum (ICN Biochemicals), pen/strep/glut, 2ME, 10 ng/ml IL6, and 10 ng/ml IL12 in 24-well plates. About 0.1 million loaded DC were co-cultured with 1.0 million CD8β+ T cells, isolated from an HLA-A2+ donor by magnetic-bead sorting and 0.75–1.0 million, irradiated (5,000 rad) CD8β− cells from the same donor, both diluted in Yssels medium. For each DC condition, 6 priming wells were started and the experiment was performed with 3 different HLA-A2+ donors. On days 10 and 19, CD8+ T cells were restimulated with 10 ng/ml MART-126–35L loaded mature d7 MUTZ3-DC or d4 mitox-DC in the presence of 10 ng/ml IL-7. On days 12 and 21, 10 IU/ml IL-2 was added per well. MART-1 tetramer (Tm) analysis was performed on CD8+ T cells on days 10 (1st restimulation) and 24 (2nd restimulation) using PE- and APC-labeled MART-126–35L Tm.
Intracellular IFNγ CD8+ T cell avidity assay
To determine whether the primed MART-126–35L specific CD8+ T effector cells could recognize and respond to target cells, an intracellular IFNγ staining was performed as described previously [28]. As target cells, HLA-A2+ JY cells were pulsed with serial tenfold dilutions of MART-126–35L peptide in the range of 1 pM–100 nM in the presence of 3 μg/ml β2 M. CD8+ T cells were cultured with the JY cells in a 2:1 E:T ratio (effector/target cell) for 4 h. 0.5 μl Golgiplug (BD Biosciences) was added to each well after 1 h of stimulation. After 4 h, cells were harvested, washed, and stained with APC-labeled MART-1 Tm and PE-labeled anti-CD8 antibodies. After fixation with cytofix/cytoperm (BD Biosciences) and permeabilization with 1 × BD perm/wash solution (BD Biosciences), cells were stained with FITC-labeled anti-IFNγ. Stained cells were analyzed by flow cytometry.
Statistical analysis
Statistical analysis of the data was performed using the paired two-tailed Student’s T-test. Differences were considered statistically significant when P < 0.05.
Results
Mitoxantrone induces differentiation of MUTZ3 progenitors
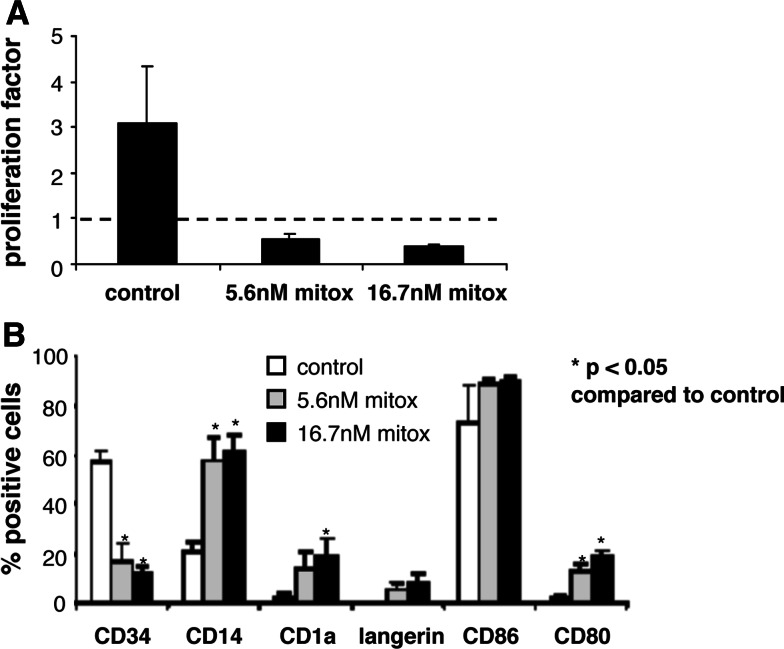
MUTZ3 progenitor cells were analyzed for their sensitivity to mitoxantrone, in a 3 day toxicity assay, MUTZ3 progenitor cells were found to be sensitive to mitoxantrone with an IC50 value of 1.5 ± 0.5 nM (n = 3, data not shown). In studying the cytotoxic effects of mitoxantrone on MUTZ3 progenitors, morphological changes reflecting DC differentiation became apparent at concentrations exceeding 5.6 nM (IC60). MUTZ3 progenitors were therefore cultured in the presence of 5.6 nM or 16.7 nM (IC70) mitoxantrone and subsequently analyzed for expansion (Fig. 1a) and DC marker expression (Fig. 1b). These analyses revealed that at these dose levels, mitoxantrone dramatically compromised cell division and drove the surviving MUTZ3 progenitors to DC differentiation, reflected by an altered CD34+/CD14+ ratio in favor of the direct CD14+ DC precursor subset and low-level de novo expression of the LC markers CD1a and Langerin, and the co-stimulatory molecules CD86 and CD80 on the viable cell population (Fig. 1b). Similar experiments were carried out with the related drug doxorubicin. Like mitoxantrone, the IC70 concentration of doxorubicin (~100 nM) induced LC differentiation of MUTZ3 progenitors (Fig. 2), whereas no such effects were observed at lower concentrations of mitoxantrone or doxorubicin (data not shown), suggesting that drug-induced differentiation of CD34+ precursors relates to low expansion levels and a high induction of cell death within the precursor cultures.
Fig. 1.
Mitoxantrone induces LC differentiation of MUTZ3 precursor cells. a MUTZ-3 precursor cell proliferation was determined in the presence 0 nM (control), 5.6 nM or 16.6 nM mitoxantrone. Shown is the fold expansion over 3 days of culture, which was determined by dividing the number of cells present on day 3 by the standard input amount of 0.4 million cells/well that was plated on day 0. b MUTZ-3 precursor cells cultured in the presence of 0 nM (control), 5.6 nM or 16.7 nM mitoxantrone were phenotyped by flow cytometry for typical DC/LC markers on day 3 (n = 3). P < 0.05 compared to the control cultures
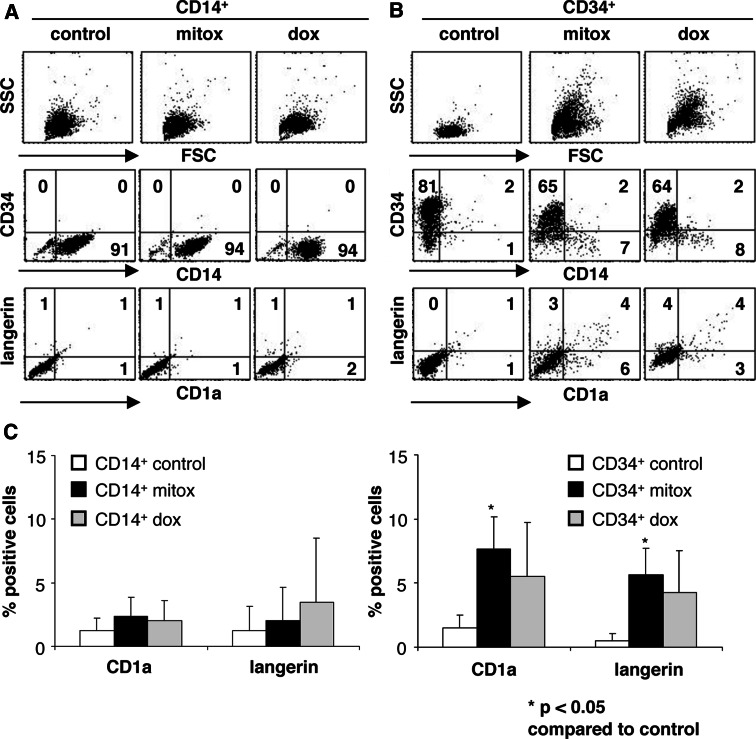
Fig. 2.
Cytostatic anthraquinone-derivatives induce differentiation of CD34+, but not of CD14+ MUTZ3 precursors. a CD14+ and b CD34+ MUTZ-3 precursor cells were isolated and cultured in the presence of 16.7 nM mitoxantrone or 100 nM doxorubicin for 3 days. Shown are the FSC/SSC plots, CD14, CD34, CD1a, and Langerin expression on the control and drug-treated precursor cells. c The percentages of CD1a and Langerin expressing cells in CD14+ and CD34+ MUTZ-3 precursor cells after 3 days in the presence of 0 nM, 16.7 nM mitoxantrone or 100 nM doxorubicin are shown (n = 3). P < 0.05 compared to the control cultures
CD34+, but not CD14+, MUTZ3 cells undergo drug-induced differentiation
Isolated CD34+, CD34−CD14− double negative (DN) and CD14+ MUTZ3 cells were incubated with 16.7 nM mitoxantrone or 100 nM doxorubicin (both IC70) to analyze which population was drug-responsive, i.e., was induced to differentiate. Table 1 shows the percentage of viable cells after 72 h relative to the amount of viable cells present in the control cultures, as determined by trypan blue exclusion. CD14+ cells were not reduced in viable cell numbers upon mitoxantrone or doxorubicin exposure, whereas CD34+ and CD34CD14 DN cells were. Further analysis revealed that the phenotypic effects of mitoxantrone and doxorubicin on the MUTZ3 progenitor cells were attributable to effects on the CD34+ population (Fig. 2). The DN population was excluded from this analysis as this population was often contaminated with remaining CD34+ cells. The CD14+ cell phenotype was not affected by incubation with these anthraquinone-derivatives (Fig. 2a, c). In contrast, higher frequencies of CD14+ cells, as well as de novo arising fully differentiated CD1a+Langerin+ DC, were found in cultures of CD34+ sorted cells upon treatment with both drugs (Fig. 2b, c). This differentiation effect could also be visualized by alterations in the forward/side scatter (FSC/SSC) as a clear shift in SSC in the drug-treated CD34+ cells, which was not present in drug-treated CD14+ cells (Fig. 2a, b). The graphs in Fig. 2c show the average of induced CD1a and Langerin expression rates within the two isolated subsets after control, mitoxantrone, or doxorubicin treatment (n = 3; P = 0.02 for CD1a and P = 0.03 for Langerin, comparing the CD34+ mitoxantrone-treated and control cells). Similarly to mitoxantrone, doxorubicin induced CD1a and Langerin expression on CD34+, but not CD14+, MUTZ3 cells.
Table 1.
Percentage of viable cells after 72 h drug-treatment (IC70), relative to non-drug control
| Percentage viable cells compared to non-treated control | P-value | |
|---|---|---|
| CD14+ | ||
| Mitoxantrone | 120 ± 27 | P > 0.05 |
| Doxorubicin | 100 ± 71 | P > 0.05 |
| CD34+ | ||
| Mitoxantrone | 4 ± 3 | P < 0.01 |
| Doxorubicin | 6 ± 6 | P < 0.01 |
| Double negative (DN) | ||
| Mitoxantrone | 20 ± 4 | P < 0.03 |
| Doxorubicin | 27 ± 7 | P < 0.05 |
Cytostatic anthraquinone-derivatives accelerate LC differentiation
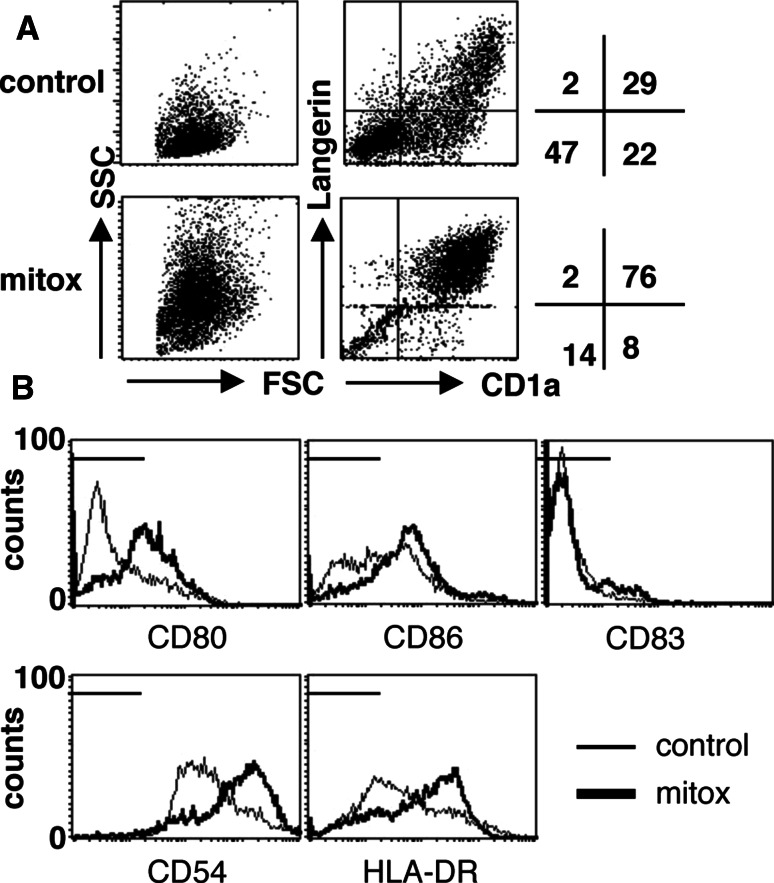
Next, we tested whether the addition of the anthraquinone-derivatives mitoxantrone or doxorubicin at the start of MUTZ3-LC differentiation cultures (i.e., in the presence of GM-CSF, TNFα, and TGFβ) could boost differentiation. Addition of a single dose of 16.7 nM mitoxantrone at day 0 of MUTZ3-LC differentiation, resulted in fully differentiated cells with high expression levels of specific LC markers on day 4, whereas control cultures usually take 8–10 days to induce fully fledged LC with all the typical phenotypic hallmarks. In Fig. 3a, FSC/SSC and CD1a/Langerin plots of day 4 LC control or mitoxantrone cultures are shown. Clearly, the mitoxantrone-treated cells were more differentiated as they displayed a more typical dendritic morphology in the FSC/SSC plot (i.e., high SSC levels) and threefold higher CD1a and Langerin expression rates as compared to the control culture. Mitoxantrone-treated cells also showed enhanced expression of the costimulatory molecules CD80 and CD86 and of HLA-DR and CD54, and to a lesser extent CD83, (Fig. 3b). The percentages of CD14+ and CD34+ cells were decreased upon mitoxantrone treatment compared to control (P = 0.04 and P = 0.02, respectively, data not shown). Comparable results were obtained for doxorubicin (data not shown).
Fig. 3.
MUTZ3 Langerhans cell (LC) differentiation is accelerated by mitoxantrone. a MUTZ3-LC differentiation was performed in the presence of no drug or 16.7 nM mitoxantrone. At day 4, flow cytometric analysis was performed for DC markers. FSC/SSC and CD1a/Langerin dotplots are shown, revealing enhanced differentiation in the mitoxantrone-treated sample. b Histogram plots for the markers CD80, CD86, CD83, CD54, and HLA-DR are shown. Control culture expression levels are indicated with thin lines, whereas expression levels on mitoxantrone-treated LC are indicated with bold lines. Markers indicate isotype fluorescence range. (a and b, experiment representative of three)
Primary CD34+ haematopoietic progenitors, but not CD14+ monocytes, respond to mitoxantrone
We subsequently established whether primary human CD14+ and CD34+ precursors from blood responded in a similar manner to anthraquinone-derivatives as the CD34+ MUTZ3 cells. In analogy with the CD14+ MUTZ3 data, mitoxantrone treatment of CD14+ monocytes isolated from blood had no effect on cell viability (data not shown), nor was any accelerated differentiation observed in the presence of DC differentiation-inducing cytokines (i.e., GM-CSF, IL-4 and TNFα) (Fig. 4a). In contrast, in cultures from CD34+ cells, where mitoxantrone (16.7 nM) was added together with LC differentiation inducing cytokines (i.e., GM-CSF, TGFβ, and TNFα), an increased percentage of CD1a+ Langerin+ cells was observed in donor-1 as well as enhanced CD1a expression in donor-2 and induced expression of the co-stimulatory markers CD80 and CD86 in both donors (Fig. 4b) (n = 2).
Fig. 4.
CD34+ blood precursors respond to mitoxantrone. a Human blood CD14+ monocytes were differentiated into MoDC in the absence (white bars) or presence (black bars) of mitoxantrone. Shown are average percentages (n = 4) of CD1a+, DC-SIGN+ and CD80+ cells. b Expanded CD34+ blood precursor cells were cultured in the presence of LC-differentiating cytokines supplemented with 0 nM or 16.7 nM mitoxantrone and were analyzed for LC marker expression after 3 days. Shown are CD1a/Langerin and CD80/CD86 dot plots for 2 different donors
High yields of rapidly differentiated and fully functional DC upon exposure to low-dose mitoxantrone
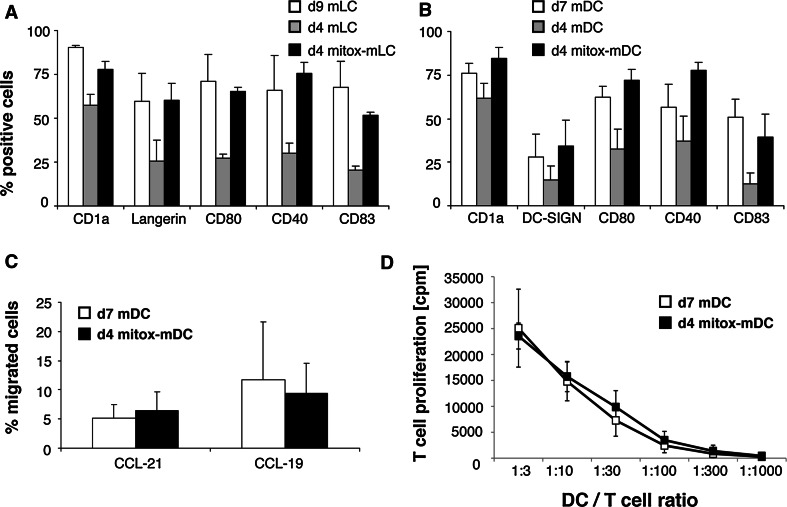
Their differentiation-accelerating capacity might make anthraquinone-derivatives attractive supplements to culture media formulated for the in vitro differentiation of DC from CD34+ progenitors for immunotherapeutic purposes. Hence, we analyzed whether the concentration of mitoxantrone could be reduced in order to increase the yield of viable cells without losing the rapid differentiation advantage. A concentration range of mitoxantrone (1–16.7 nM) was tested to identify the least toxic concentration with maintained stimulatory effects on DC differentiation in the presence of DC-differentiating cytokines. The phenotype of the cells was analyzed for DC characteristics after 3 days. A robust accelerating effect on MUTZ3-LC (Fig. 5a) and MUTZ3–IDC (Fig. 5b) differentiation was still observed when 2 nM mitoxantrone was added (n = 5), with eighty percent viability of the cells in both the control and the mitoxantrone-exposed cultures. The percentages of CD1a, Langerin (LC), DC-SIGN (IDC), CD80, CD40, and CD83 expressing cells were determined among matured MUTZ3-LC/IDC from conventional cultures (i.e., d9 mLC/d7 mDC) and from DC cultured for 3 days in the absence (i.e., d4-mLC/d4 mDC) or presence of 2 nM mitoxantrone and matured for 24 h (i.e., d4 mitox-mLC/d4 mitox-mDC) (Fig. 5a, b). The short culture protocol in the presence of 2 nM mitoxantrone resulted in a comparable phenotype to longer, conventional cultures (Fig. 5a, b), without the need for further addition of fresh cytokines during culture —as is the case for the conventional LC/IDC cultures. In the absence of mitoxantrone, LC and IDC could not fully develop within 4 days. Additionally, if mitox-DC are to be useful for future DC vaccination strategies for immunotherapy, they need to be functionally active to at least a comparable level to conventional DC. Indeed, with half the culture time and half the amount of differentiation-inducing cytokines, d4 mitox-mDC were as able as conventional d7 MUTZ3-mDC to migrate toward the chemokines CCL19 and CCL21 in a trans-well assay (Fig. 5c) and to stimulate allogeneic T cell proliferation in an allogeneic mixed leukocyte reaction (MLR) (Fig. 5d).
Fig. 5.
Day 4 MUTZ3-derived mitox-DC are equally functional as day 7 conventional DC. a, b, MUTZ3 mitox-LC/DC differentiated for 3 days in the presence of 2 nM mitoxantrone and matured for 24 h with a maturation cocktail (d4 mitox-mLC/mDC) were phenotyped by flow cytometry and compared to LC/DC differentiated in the absence of mitoxantrone for 3 days and matured for 24 h (d4 mDC) and conventional day 9 mature MUTZ3-LC (d9 mLC) or day 7 mature MUTZ3-DC (d7 mDC) (n = 5). c d4 mitox-mDC were compared to d7 MUTZ3-mDC for their migratory capacity toward CCL19 and CCL21 in a trans-well migration assay (n = 3). d d4 mitox-mDC were compared to d7 MUTZ3-mDC for their T cell stimulatory capacity in an alloMLR (n = 3 experiments, with 3 replicates per experiment)
Efficient priming of MART-1 specific CD8+ T cells by mitox-DC
A crucial function of DC in anti-tumor vaccination strategies is their ability to prime tumor antigen-specific CD8+ effector T cells. Hence, d4 mitox-mDC and d7 MUTZ3-mDC were compared in their capacity to in vitro prime CD8+ T cells against the immunodominant HLA-A2 restricted aa26–35L epitope of the well characterized melanoma antigen MART-1. Upon peptide loading, both DC types displayed a similar priming efficiency, as revealed by the frequencies of MART-1 specific tetramer (Tm)-positive cells among CD8+ T cell bulk cultures from three separate HLA-A2 matched donors. Figure 6 shows the percentage of Tm+ CD8+ T cells for 6 bulk cultures from the three tested HLA-A2+ donors after the first and the second restimulation (Fig. 6a) with peptide-loaded DC. Unloaded (d7) DC were taken along as a negative control. From the total of 18 bulk cultures (n = 6 per donor) in which CD8+ T cell priming was initiated, MART-1 Tm+ T cells could be detected in 16/18 cultures primed with d4 mitox-DC, compared to 17/18 cultures primed with d7 standard MUTZ3-mDC. Figure 6b shows the capacity of the primed CD8+ T cells to secrete IFNγ upon recognition of the MART-1 25-36L epitope. Avidity analysis showed that the d7 mDC- and the d4 mitox-mDC-generated CD8+ T cells displayed a similar, intermediate avidity for the MART-126–35L peptide (Fig. 6b). These data show that rapidly differentiated mitox-DC are fully functional and equivalent to their conventionally differentiated counterparts with respect to their capacity to prime functional antigen-specific CD8+ T cells.
Fig. 6.
Day 4 MUTZ3 mitox-DC can prime antigen-specific CD8+ T cells. a d4 MUTZ3 mitox-mDC and d7 MUTZ3-mDC were loaded with MART-126–35L peptides and were used to prime MART-1 specific CD8+ T cells from HLA-A2+ donors. Shown are the percentages of MART1-tetramer positive CD8+ T cells within 6 bulk cultures generated from three HLA-A2+ donors (experiment −1, −2 and −3 respectively) after two and three rounds of stimulation with peptide-loaded DC. B, MART-125-36L Tm-positive T cells were analyzed for IFNγ release upon target recognition. CD8+ T cell avidity was analyzed by stimulating two d7 mDC- and two d4 mitox-mDC generated CD8+ T cells bulk cultures with JY target cells loaded with tenfold dilutions of MART-126–35L peptide (range 1 pM–100 nM). Average percentages of the 2 bulk cultures are shown ± standard deviation. The bulk cultures used were the bulks with the highest percentage of Tm-positive cells after the 2nd restimulation of experiment 3 shown in Fig. 6a
Discussion
In this study, we explored whether anthraquinone-based cytostatic drugs could be used to improve in vitro human DC differentiation. We have shown that, in the absence of DC differentiation-inducing cytokines, the cytostatic drugs mitoxantrone and doxorubicin induce cell death of DC precursors on the one hand, but differentiation of the surviving CD34+ DC precursors on the other. Moreover, when added at the start of differentiation in combination with DC or LC differentiation-inducing cytokines, these drugs dramatically promoted differentiation resulting in accelerated maturation of DC/LC from CD34+ precursors without inducing excessive cell death.
In the past, systemic and local administration of carefully selected and optimally dosed cytostatic agents was shown to enhance cellular immunity [30]. Whereas systemic administration of cyclophosphamide (CY) had severe effects like B cell depletion [34], Limpens et al. demonstrated that local administration of the active CY-derivative Z 7557 could prevent this B cell depletion while immunostimulatory effects were maintained [12]. In a later paper, the authors showed that local administration of Z-7557 resulted in more activated DC within the regional lymph nodes [13]. In our in vitro studies of DC precursor cultures without added differentiation-inducing cytokines, dose levels at which excessive cell death occurred (IC70) seemed to be essential for DC differentiation induction. Possibly, the massive cell death in the cultures provoked an endogenous danger signal (e.g., endogenous toll-like receptor ligands [1, 16]) or in-culture cytokine production, inducing differentiation of the surviving cells. Importantly, the sensitivity to mitoxantrone-induced cytotoxicity was reduced when differentiation-inducing cytokines were present from the start of the culture (likely due to halted proliferation of CD34+ progenitors) and a window was established at lower dosages of mixantrone, in which accelerated DC differentiation was achieved without excess cell death. This finding makes it possible to apply cytostatic anthraquinone-derivates (like e.g., doxorubicin and mitoxantrone) in CD34+ precursor-derived DC differentiation cultures for clinical vaccination, with the express purpose to reduce the required culture time and thus increase the feasibility of this approach.
In murine bone marrow-derived DC, Shurin et al. showed that a variety of cytostatic agents at non-toxic concentrations induced expression of molecules related to antigen presentation and enhanced expression of activation markers such as CD80 and CD40 [32]. The same group also showed that human monocyte-derived DC displayed a more mature phenotype, with enhanced HLA-DR and CD83 expression levels after 5 days of exposure to various chemotherapeutic drugs, with vinblastine, vincristine, paclitaxel, and methotrexate having the largest effect [10]; of note mitoxantrone was not tested, nor were DC derived from CD34+ precursor cells. Whereas they did show some effect on CD83 expression on MoDC when adding 10 nM doxorubicin to their cultures for 5 days, they also did not detect changes in CD1a or CD80 expression nor did doxorubicin increase the ability of these MoDC to stimulate T cell proliferation in an allogeneic MLR. A small, but potentially crucial difference between those studies and ours, is that in our study the drugs were added from the start of culture, whereas they were added from day 1 in the studies by Shurin et al., resulting in a longer drug exposure at earlier differentiation stages in our studies, which could have conceivably effected the observed accelerated differentiation kinetics. Indeed, those previous studies did not assess differentiation kinetics, nor migration capacity and T cell priming abilities of the drug-exposed DC.
Unfortunately, we have thus far not been able to unravel the mechanism(s) that underlie the observed drug-induced differentiation-accelerating effects. One possible cause of the observed induction and acceleration of DC differentiation could be the drug-induced expression of the ABC transporter BCRP (ABCG2) [38], as mitoxantrone is a highly efficient BCRP substrate and other ABC transporters, e.g., MRP1 [35] and P-gp [23] have previously been linked to DC differentiation. However, no induction of BCRP expression was observed in either CD14+ or CD34+ MUTZ3 cells upon mitoxantrone treatment, nor could inhibition of BCRP activity with the antagonist Ko-143 prevent mitoxantrone-mediated DC differentiation of precursor cells or accelerated differentiation in the presence of cytokines (data not shown).
Another possible underlying mechanism could involve the induction of intracellular diacylglycerol (DAG). Various cytostatic drugs, e.g., mitoxantrone, daunorubicin, doxorubicin, and cisplatin have been shown to induce rapid synthesis of DAG from sphingomyelin [3, 24, 25]. DAG is known to activate protein kinase C (PKC), which was previously shown to be sufficient for the induction of DC differentiation from CD34+ haematopoietic progenitor cells upon their culture with the DAG-analog phorbol 12-myristate 13-acetate (PMA) [8] or with DC-inducing cytokines [33]. In this context, it is of particular interest that PKC has been implicated in the activation of the nuclear factor κB (NFκB) sub-component RelB, which in turn is linked to DC differentiation and activation [6, 7, 20]. Whether these signal transduction events are indeed induced downstream of cytostatic anthraquinone-derivatives like mitoxantrone and affect the DC differentiation-promoting effects remains to be established.
Functional capacities of in vitro cultured DC to be used for vaccination purposes are vitally important. The mitoxantrone-generated DC, although only differentiated for 3 days and matured with a standard maturation cocktail for 24 h, were functionally equivalent to their conventionally cultured counterparts (i.e., for 7–9 days) in every way tested: they were capable of migration toward LN-homing chemokines and capable of the induction of allogeneic T cell proliferation as well as of tumor antigen-specific CD8+ T cells able to secrete IFNγ upon recognition of their specific epitope. Previously we have shown that such tumor-specific CD8+ T cells induced by MUTZ3-DC are functional with respect to their recognition and elimination of the targeted tumor cells [28]. The optimized in vitro DC culture system, using low-dose mitoxantrone as a differentiation-accelerating supplement in clinical-grade media, is currently under further development for the generation of in vitro cultured clinical-grade DC for tumor vaccination purposes for AML patients. The advantage of this system is that it is quick, that the drug-reagent has already been clinically approved, and that it is relatively cheap due to shorter culture time and the reduced use of clinical-grade recombinant cytokines.
Finally, our data and those of others [10, 13, 41] also suggest that local administration of cytostatic anthraquinone-derivatives like anthracyclins or anthracenediones at vaccination sites with resident DC precursors, e.g., the skin, might lead to their rapid differentiation and maturation. As such, these cytostatic drugs might act as DC potentiating vaccine adjuvants. In addition, the combination of anthraquinone-based chemotherapy and the administration of DC differentiation-inducing cytokines (e.g., GM-CSF) might lead to the simultaneous rapid maturation of functional DC and the release of tumor-associated antigens from dying tumor cells: a seemingly ideal scenario for in vivo tumor immunization. In line with this, Apetoh et al. previously showed that anthracyclins (i.e., doxorubicin) can induce immunogenic tumor cell death due to the release of the high mobility group box 1 (HMGB1) protein from dying cells, which can induce DC activation through interaction with TLR4 [1]. Combined, these observations warrant further investigation of the use of anthraquinones in various combined chemo-immunotherapy strategies.
Acknowledgments
This work was supported by a grant from the Dutch Cancer Society: KWF2003-2830 to GLS, TDG and RJS.
Conflict of interest
DCPrime B.V. is a spin-off company from the Pathology Department at the VU University medical center, Amsterdam developing allogeneic DC vaccines for clinical use. AW Reurs, PGJTB Wijnands, S van Wetering, and AM Kruisbeek are employees, and the latter CEO of DCPrime B.V. RJ Scheper and AM Kruisbeek are co-founders of DCPrime B.V.
References
- 1.Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 3.Bettaieb A, Plo I, Mansat-de Mas V, et al. Daunorubicin- and mitoxantrone-triggered phosphatidylcholine hydrolysis: implication in drug-induced ceramide generation and apoptosis. Mol Pharmacol. 1999;55:118–125. doi: 10.1124/mol.55.1.118. [DOI] [PubMed] [Google Scholar]
- 4.Bontkes HJ, de Gruijl TD, Schuurhuis GJ, et al. Expansion of dendritic cell precursors from human CD34(+) progenitor cells isolated from healthy donor blood; growth factor combination determines proliferation rate and functional outcome. J Leukoc Biol. 2002;72:321–329. [PubMed] [Google Scholar]
- 5.Bosch F, Ferrer A, Villamor N, et al. Fludarabine, cyclophosphamide, and mitoxantrone as initial therapy of chronic lymphocytic leukemia: high response rate and disease eradication. Clin Cancer Res. 2008;14:155–161. doi: 10.1158/1078-0432.CCR-07-1371. [DOI] [PubMed] [Google Scholar]
- 6.Cejas PJ, Carlson LM, Kolonias D, et al. Regulation of RelB expression during the initiation of dendritic cell differentiation. Mol Cell Biol. 2005;25:7900–7916. doi: 10.1128/MCB.25.17.7900-7916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark GJ, Gunningham S, Troy A, et al. Expression of the RelB transcription factor correlates with the activation of human dendritic cells. Immunology. 1999;98:189–196. doi: 10.1046/j.1365-2567.1999.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis TA, Saini AA, Blair PJ, et al. Phorbol esters induce differentiation of human CD34+ hemopoietic progenitors to dendritic cells: evidence for protein kinase C-mediated signaling. J Immunol. 1998;160:3689–3697. [PubMed] [Google Scholar]
- 9.Gabrilovich DI, Corak J, Ciernik IF, et al. Decreased antigen presentation by dendritic cells in patients with breast cancer. Clin Cancer Res. 1997;3:483–490. [PubMed] [Google Scholar]
- 10.Kaneno R, Shurin GV, Tourkova IL, et al. Chemomodulation of human dendritic cell function by antineoplastic agents in low noncytotoxic concentrations. J Transl Med. 2009;7:58. doi: 10.1186/1479-5876-7-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kusmartsev S, Gabrilovich DI. Effect of tumor-derived cytokines and growth factors on differentiation and immune suppressive features of myeloid cells in cancer. Cancer Metastasis Rev. 2006;25:323–331. doi: 10.1007/s10555-006-9002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Limpens J, Garssen J, Germeraad WT, et al. Enhancing effects of locally administered cytostatic drugs on T effector cell functions in mice. Int J Immunopharmacol. 1990;12:77–88. doi: 10.1016/0192-0561(90)90070-4. [DOI] [PubMed] [Google Scholar]
- 13.Limpens J, Van Meijer M, Van Santen HM, et al. Alterations in dendritic cell phenotype and function associated with immunoenhancing effects of a subcutaneously administered cyclophosphamide derivative. Immunology. 1991;73:255–263. [PMC free article] [PubMed] [Google Scholar]
- 14.Long HJ, III, Nelimark RA, Podratz KC, et al. Phase III comparison of methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) versus doxorubicin and cisplatin (AC) in women with advanced primary or recurrent metastatic carcinoma of the uterine endometrium. Gynecol Oncol. 2006;100:501–505. doi: 10.1016/j.ygyno.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 15.Masterson AJ, Sombroek CC, de Gruijl TD, et al. MUTZ-3, a human cell line model for the cytokine-induced differentiation of dendritic cells from CD34+ precursors. Blood. 2002;100:701–703. doi: 10.1182/blood.V100.2.701. [DOI] [PubMed] [Google Scholar]
- 16.Messmer D, Yang H, Telusma G, et al. High mobility group box protein 1: an endogenous signal for dendritic cell maturation and Th1 polarization. J Immunol. 2004;173:307–313. doi: 10.4049/jimmunol.173.1.307. [DOI] [PubMed] [Google Scholar]
- 17.Michallet AS, Coiffier B. Recent developments in the treatment of aggressive non-Hodgkin lymphoma. Blood Rev. 2008;23:11–23. doi: 10.1016/j.blre.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Mike S, Harrison C, Coles B et al (2006) Chemotherapy for hormone-refractory prostate cancer. Cochrane Database Syst Rev CD005247 [DOI] [PMC free article] [PubMed]
- 19.Onyenadum A, Gogas H, Kosmidis P, et al. Mitoxantrone plus gemcitabine in pretreated patients with metastatic breast cancer. J Chemother. 2006;18:192–198. doi: 10.1179/joc.2006.18.2.192. [DOI] [PubMed] [Google Scholar]
- 20.Ouaaz F, Arron J, Zheng Y, et al. Dendritic cell development and survival require distinct NF-kappaB subunits. Immunity. 2002;16:257–270. doi: 10.1016/S1074-7613(02)00272-8. [DOI] [PubMed] [Google Scholar]
- 21.Oyan B, Koc Y, Ozdemir E, et al. High dose sequential chemotherapy and autologous stem cell transplantation in patients with relapsed/refractory lymphoma. Leuk Lymphoma. 2006;47:1545–1552. doi: 10.1080/10428190600570958. [DOI] [PubMed] [Google Scholar]
- 22.Palucka AK, Ueno H, Fay JW, et al. Taming cancer by inducing immunity via dendritic cells. Immunol Rev. 2007;220:129–150. doi: 10.1111/j.1600-065X.2007.00575.x. [DOI] [PubMed] [Google Scholar]
- 23.Pendse SS, Behjati S, Schatton T, et al. P-glycoprotein functions as a differentiation switch in antigen presenting cell maturation. Am J Transplant. 2006;6:2884–2893. doi: 10.1111/j.1600-6143.2006.01561.x. [DOI] [PubMed] [Google Scholar]
- 24.Posada J, Vichi P, Tritton TR. Protein kinase C in adriamycin action and resistance in mouse sarcoma 180 cells. Cancer Res. 1989;49:6634–6639. [PubMed] [Google Scholar]
- 25.Rubin E, Kharbanda S, Gunji H, et al. cis-Diamminedichloroplatinum(II) induces c-jun expression in human myeloid leukemia cells: potential involvement of a protein kinase C-dependent signaling pathway. Cancer Res. 1992;52:878–882. [PubMed] [Google Scholar]
- 26.Santegoets SJ, Bontkes HJ, Stam AG, et al. Inducing antitumor T cell immunity: comparative functional analysis of interstitial versus Langerhans dendritic cells in a human cell line model. J Immunol. 2008;180:4540–4549. doi: 10.4049/jimmunol.180.7.4540. [DOI] [PubMed] [Google Scholar]
- 27.Santegoets SJ, Masterson AJ, van der Sluis PC, et al. A CD34+ human cell line model of myeloid dendritic cell differentiation: evidence for a CD14+ CD11b+ Langerhans cell precursor. J Leukoc Biol. 2006;80:1337–1344. doi: 10.1189/jlb.0206111. [DOI] [PubMed] [Google Scholar]
- 28.Santegoets SJ, Schreurs MW, Masterson AJ, et al. In vitro priming of tumor-specific cytotoxic T lymphocytes using allogeneic dendritic cells derived from the human MUTZ-3 cell line. Cancer Immunol Immunother. 2006;55:1480–1490. doi: 10.1007/s00262-006-0142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santegoets SJ, van den Eertwegh AJ, van de Loosdrecht AA, et al. Human dendritic cell line models for DC differentiation and clinical DC vaccination studies. J Leukoc Biol. 2008;84:1364–1373. doi: 10.1189/jlb.0208092. [DOI] [PubMed] [Google Scholar]
- 30.Scheper RJ, Limpens J, Tan BT, et al. Immunotherapeutic effects of local chemotherapy with an active metabolite of cyclophosphamide. Methods Find Exp Clin Pharmacol. 1987;9:611–615. [PubMed] [Google Scholar]
- 31.Scott LJ, Figgitt DP. Mitoxantrone: a review of its use in multiple sclerosis. CNS Drugs. 2004;18:379–396. doi: 10.2165/00023210-200418060-00010. [DOI] [PubMed] [Google Scholar]
- 32.Shurin GV, Tourkova IL, Kaneno R, et al. Chemotherapeutic agents in noncytotoxic concentrations increase antigen presentation by dendritic cells via an IL-12-dependent mechanism. J Immunol. 2009;183:137–144. doi: 10.4049/jimmunol.0900734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.St Louis DC, Woodcock JB, Franzoso G, et al. Evidence for distinct intracellular signaling pathways in CD34+ progenitor to dendritic cell differentiation from a human cell line model. J Immunol. 1999;162:3237–3248. [PubMed] [Google Scholar]
- 34.Stockman GD, Heim LR, South MA, et al. Differential effects of cyclophosphamide on the B and T cell compartments of adult mice. J Immunol. 1973;110:277–282. [PubMed] [Google Scholar]
- 35.van de Ven R, de Jong MC, Reurs AW, et al. Dendritic cells require multidrug resistance protein 1 (ABCC1) transporter activity for differentiation. J Immunol. 2006;176:5191–5198. doi: 10.4049/jimmunol.176.9.5191. [DOI] [PubMed] [Google Scholar]
- 36.van Helden SF, van Leeuwen FN, Figdor CG. Human and murine model cell lines for dendritic cell biology evaluated. Immunol Lett. 2008;117:191–197. doi: 10.1016/j.imlet.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 37.van Loevezijn A, Allen JD, Schinkel AH, et al. Inhibition of BCRP-mediated drug efflux by fumitremorgin-type indolyl diketopiperazines. Bioorg Med Chem Lett. 2001;11:29–32. doi: 10.1016/S0960-894X(00)00588-6. [DOI] [PubMed] [Google Scholar]
- 38.van de Ven R, Lindenberg JJ, Reurs AW et al (2011) Preferential Langerhans cell differentiation from CD34(+) precursors upon introduction of ABCG2 (BCRP). Immunol Cell Biol (in press) [DOI] [PubMed]
- 39.Yardley DA, Burris HA, III, Farley CP, et al. A phase II feasibility trial of dose-dense docetaxel followed by doxorubicin/cyclophosphamide as adjuvant or neoadjuvant treatment for women with node-positive or high-risk node-negative breast cancer. Clin Breast Cancer. 2008;8:242–248. doi: 10.3816/CBC.2008.n.027. [DOI] [PubMed] [Google Scholar]
- 40.Yssel H, De Vries JE, Koken M, et al. Serum-free medium for generation and propagation of functional human cytotoxic and helper T cell clones. J Immunol Methods. 1984;72:219–227. doi: 10.1016/0022-1759(84)90450-2. [DOI] [PubMed] [Google Scholar]
- 41.Yu B, Kusmartsev S, Cheng F, et al. Effective combination of chemotherapy and dendritic cell administration for the treatment of advanced-stage experimental breast cancer. Clin Cancer Res. 2003;9:285–294. [PubMed] [Google Scholar]