Abstract
Interactions between pancreatic cancer cells and inflammatory cells play crucial roles in the biological behavior of pancreatic cancer. Abundant infiltration of immunoglobulin G4 (IgG4)-positive plasma cells in the pancreas is the most significant feature of autoimmune pancreatitis; however, the clinical significance of IgG4-positive plasma cell infiltration in pancreatic cancer has not previously been reported. Herein, we analyzed intratumoral and peritumoral infiltrations of IgG4-positive plasma cells in 95 pancreatic cancer cases after curative resection. The correlations between IgG4-positive plasma cell infiltration and the clinicopathologic traits and overall survival of pancreatic cancer were investigated. IgG4-positive plasma cells were found in 86 % of tumor tissue samples compared with 69 % of peritumoral tissue samples (P = 0.0063). The high-level infiltration of intratumoral IgG4-positive plasma cells was positively correlated with poor histological grade (P = 0.017). The high-level infiltration of intratumoral IgG4-positive plasma cells was significantly correlated with worse prognosis (P = 0.01) in multivariate analysis. We further found that intratumoral M2-polarized tumor-associated macrophages (TAMs) were positively, linearly correlated with IgG4-positive plasma cells. In conclusion, IgG4-positive plasma cell infiltration is correlated with the clinicopathologic traits and overall survival of pancreatic cancer. High-level intratumoral infiltration of IgG4-positive plasma cells is an independent predictor for poor overall survival in pancreatic cancer patients after curative resection. Intratumoral M2-polarized TAMs probably induce IgG4-positive plasma cells.
Keywords: Pancreatic cancer, Prognosis, Overall survival, Clinicopathologic traits, IgG4-positive plasma cell, Tumor-associated macrophages
Introduction
Abundant infiltration of immunoglobulin G4 (IgG4)-positive plasma cells in affected organs and high IgG4 serum levels are representative characteristics of IgG4-related sclerosing diseases (IRSD) [1]. Autoimmune pancreatitis (AIP) is the most common clinical manifestation of IRSD [2]. Recently, some malignancies accompanying IRSD have also been reported to have significant intratumoral infiltration of IgG4-positive plasma cells [3–5]. The potential roles of IgG4-positive plasma cells in cancer are receiving increasing attention. Karagiannis et al. [6] reported that infiltration of IgG4-positive plasma cells, induced by melanoma cell-promoted type 2 helper T cells (Th2), biased the inflammatory environment and might restrict the effector cell functions against melanoma, as well as promote immune escape. Pancreatic cancer is the fourth leading cause of cancer-related death in the Western world, and it has a rising incidence [7]. Pancreatic cancer is one of the most well-known inflammatory malignancies; it is characterized by a scarcity of cancer cells, excessive desmoplasia and abundant infiltration of a variety of inflammatory cells [8]. The inflammatory cells in the tumor microenvironment have been extensively reported to play crucial roles in promoting pancreatic carcinogenesis, progression and metastasis by inducing immune suppression, stimulating angiogenesis, promoting tumor cell migration and invasion and maintaining pancreatic cancer stem cells [9–13]. Although there is substantial infiltration of IgG4-positive plasma cells in AIP and approximately 10 % of pancreatic cancer patients are reported to have an elevated IgG4 serum level [14], the distributions and roles of IgG4-positive plasma cells in pancreatic cancer have never previously been reported. This study is the first to evaluate a large cohort of matched tumor and peritumoral tissue samples from 95 pancreatic cancer patients who underwent curative resection and retrospectively analyze the correlations between intratumoral and peritumoral infiltrations of IgG4-positive plasma cells and the clinicopathologic traits and overall survival for pancreatic cancer. Since IgG4 is mainly induced by interleukin-10 (IL-10) and M2-polarized tumor-associated macrophages (TAMs) are the main source of intratumoral IL-10, we further explored the potential correlation between M2-polarized TAMs and IgG4-positive plasma cells.
Patients and methods
Patient information and follow-up
Pancreatic cancer tumor tissues and peritumoral tissues were collected from 95 patients using the following inclusion criteria: (1) R0 curative resection was achieved; (2) pancreaticoduodenectomy (Whipple procedure) or distal pancreatectomy was performed; (3) pancreatic cancer samples were histologically proven to be ductal adenocarcinoma by hematoxylin–eosin staining; (4) paired tumoral and peritumoral tissues were obtained; and (5) patients were not undergoing neoadjuvant chemotherapy. Patients who had a medical history of autoimmune disease that required immunosuppressive drugs or who were unable to provide informed consent were excluded. The diagnosis and staging were based on the Staging Manual of the American Joint Committee on Cancer, 7th edition. After the operation, the patients were followed up with an outpatient clinic visit or telephone interview every 3–6 months. Overall survival was defined as the survival time after surgery. This study was approved by the Ethics Committee of Peking Union Medical College Hospital and adhered to the tenets of the Declaration of Helsinki. Written informed consents for tissue sample storage and study publication were obtained before patients underwent surgery.
Tissue microarray construction and immunohistochemistry
The tissue microarray (TMA) was constructed with a manual TMA (Beecher Instruments, Sun Prairie, WI, USA) using formalin-fixed paraffin-embedded blocks. Two tumoral and peritumoral tissue cores for each case were sampled from representative areas (without obvious hemorrhage or necrosis) using a 1.5-mm punch. Immunohistochemistry was performed to detect IgG4-positive plasma cells. A mouse antihuman IgG4 Fc monoclonal antibody (No. 3677M-14, Cell Marque Corporation, Rocklin, California, USA) or a mouse antihuman CD163 monoclonal antibody (ab17051, Abcam, UK) and an EnVision+ two-step staining kit (Dako, Glostrup, Denmark) were used for staining. Briefly, the 4-μm-thick formalin-fixed, paraffin-embedded tumor sections were mounted, deparaffinized in xylene and rehydrated in a graded alcohol series. The slides were washed with phosphate-buffered saline (PBS), immersed in a 0.01 mol/L citrate buffer solution (pH 6.0) and heated in a microwave for 10 min to achieve antigen retrieval. Peroxidase was quenched using 3 % hydrogen peroxide for 10 min. After washing again with PBS, the slides were incubated at 4 °C overnight with the primary antibody (IgG4: 1:40; CD163: 1:100). After washing three times with PBS, the horseradish peroxidase-conjugated secondary antibody was added for 30 min and diaminobenzidine was used as a chromogen. Finally, the slides were counter-stained with hematoxylin (Sigma-Aldrich Co., LLC, Munich, Germany) to visualize the nuclei. Preimmune mouse serum was used at the same dilution as the negative control.
Evaluation of the immunohistochemical results
The numbers of IgG4-positive plasma cells and CD163-positive M2-polarized TAMs were counted using a computerized image analysis system consisting of a Hitachi HV-C20A CCD camera (Hitachi, Tokyo, Japan) installed on a Leica DMLA light microscope (Leica Microsystems, Wetar, Germany) and attached to a personal computer. A blinded staining evaluation was performed by two experienced pathologists who did not have access to the clinicopathologic or follow-up data, and the average value was used for analysis. The IgG4-positive plasma cells were defined as cells that had immunochemically IgG4-positive staining and exclusive morphological characteristics of plasma cells, including a considerable nucleus-to-cytoplasm ratio and eccentric nucleus. After primary evaluation of the scanned slides, we found that IgG4-positive plasma cells were scarce in many peritumoral tissue cores. As a result, we counted all IgG4-positive plasma cells in each scanned tissue core using Imagescope Software. There was substantially more infiltration of M2-polarized TAMs than IgG4-positive plasma cells in both tumor and peritumoral tissue cores. Each pathologist selected 5 views of high infiltration of M2-polarized TAMs under a high-resolution field (×400, magnification), 10 views were recorded, and the average value was considered representative of each tissue core.
Statistical analysis
Microsoft office software 2010 version was used for primary data recording. IBM SPSS Statistics software 22.0 version and GraphPad Prism software 5.0 version were used for statistical analysis and drawing the graphs. The cumulative survival time was calculated using the Kaplan–Meier method and analyzed by the log-rank test. Univariate and multivariate analyses were based on the Cox proportional hazards regression model. The cutoff for defining subgroups was the median value. A secondary analysis was performed to assess the relations among IgG4-positive plasma cell infiltration, clinicopathologic characteristics, overall survival for pancreatic cancer and M2-polarized TAMs. To compare individual variables, Wilcoxon signed-rank test and Chi-square tests were performed as appropriate. Two-tailed P < 0.05 was considered to be significant.
Results
Distributions of intratumoral and peritumoral IgG4-positive plasma cells in pancreatic cancer
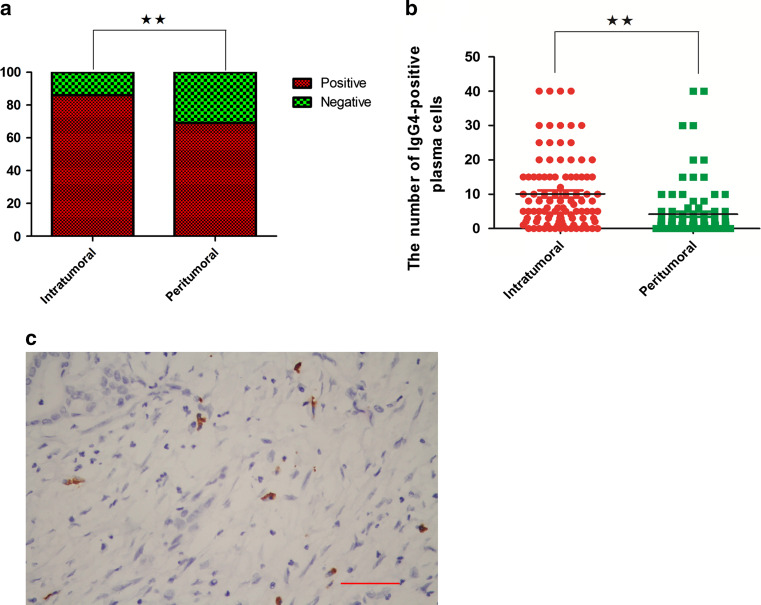
In the 95 matched cases, 86 % of the tumor tissues had IgG4-positive plasma cells; however, only 69 % of the peritumoral tissues had IgG4-positive plasma cells; these were significantly different (Chi-square test, P = 0.0063; Fig. 1a). The total number of IgG4-positive plasma cells in each tissue core was counted by using Imagescope software at a ×200 magnification. The total number of intratumoral IgG4-positive plasma cells in each tissue core was also significantly higher than the number in peritumoral tissue (Wilcoxon signed-rank test, P < 0.0001; Fig. 1b). Representative figures of IgG4-positive plasma cells are shown in Fig. 1c. Although IgG4-positive plasma cells were found in most of the tumor and peritumoral tissue samples, none of the cases met the pathologic diagnostic criteria for autoimmune pancreatitis (AIP), which is the form of pancreatic IRSD. This meant that none of the pancreatic patients had IRSD when they underwent the operation.
Fig. 1.
Distributions of IgG4-positive plasma cells in tumor and peritumoral tissues are different. a IgG4-positive plasma cells are found in 86 % of tumor tissue samples compared with 69 % of the peritumoral tissue samples (Chi-square test, **P < 0.01). b The infiltrations of intratumoral and peritumoral IgG4-positive plasma cell in each case are compared (Wilcoxon signed-rank test, **P < 0.01). c Representative figures of IgG4-positive plasma cells, which are immunochemically stained brown and had a large eccentric nucleus, are shown (the scale bar represented 200 μm, 200×, magnification)
Correlations of the intratumoral and peritumoral IgG4-positive plasma cells with clinicopathologic traits in pancreatic cancer
According to the median number of intratumoral or peritumoral IgG4-positive plasma cells in each tissue core, the cases were subdivided into high-level and low-level infiltration groups, respectively. Since the median value of the intratumoral group was 6.0, cases with a value less than or equal to 6.0 were enrolled into the low-level intratumoral infiltration group. The cases with a value higher than 6.0 were enrolled into the high-level intratumoral infiltration group. The median value for peritumoral group was 2.0. The cases with a value less than or equal to 2.0 were enrolled into the low-level peritumoral infiltration group. The cases with a value higher than 2.0 were enrolled into the high-level peritumoral infiltration group. The results are summarized in detail in Table 1. Briefly, the high-level intratumoral infiltration of IgG4-positive plasma cells was positively correlated with poor differentiation of pancreatic cancer cells (Chi-square test, P = 0.017). The other clinicopathologic characters were not correlated with the intratumoral infiltration of IgG4-positive plasma cells. The ratio of high-level peritumoral infiltration of IgG4-positive plasma cells in the tumors that lacked lymph node metastasis was significantly higher than that of the tumors with lymph node metastasis (Chi-square test, 43.86 vs. 13.95 %, P = 0.019). The other clinicopathologic characteristics were not correlated with the peritumoral infiltration of IgG4-positive plasma cells.
Table 1.
Association between the infiltrations of IgG4-positive plasma cells and the clinicopathologic variables of pancreatic cancer
| No. of patients | Intratumoral IgG4-positive plasma cells | Peritumoral IgG4-positive plasma cells | |||||
|---|---|---|---|---|---|---|---|
| Low | High | P value* | Low | High | P value* | ||
| Age (years) | 0.557 | 0.452 | |||||
| ≤65 | 63 | 31 | 32 | 41 | 22 | ||
| >65 | 32 | 16 | 16 | 22 | 10 | ||
| Sex | 0.361 | 0.263 | |||||
| Male | 62 | 32 | 30 | 43 | 19 | ||
| Female | 33 | 15 | 18 | 20 | 13 | ||
| Location in pancreas | |||||||
| Head | 67 | 31 | 36 | 0.307 | 40 | 27 | 0.058 |
| Body/tail | 33 | 18 | 15 | 26 | 7 | ||
| Tumor size | 0.385 | 0.284 | |||||
| >4 cm | 38 | 20 | 18 | 27 | 11 | ||
| ≤4 cm | 57 | 27 | 30 | 36 | 21 | ||
| Histological grade | 0.017 | 0.17 | |||||
| G1–2 | 64 | 37 | 27 | 45 | 19 | ||
| G3 | 31 | 10 | 21 | 18 | 13 | ||
| pT stage | 0.522 | 0.594 | |||||
| T1–2 | 74 | 37 | 27 | 49 | 25 | ||
| T3–4 | 21 | 10 | 11 | 14 | 7 | ||
| pN stage | 0.534 | 0.019 | |||||
| N0 | 56 | 28 | 28 | 32 | 24 | ||
| N1 | 39 | 19 | 20 | 31 | 8 | ||
| Perineural invasion | 0.533 | 0.509 | |||||
| Positive | 37 | 18 | 19 | 25 | 12 | ||
| Negative | 58 | 29 | 29 | 38 | 20 | ||
The boldface type indicates a significant difference. G1, well differentiated; G2, moderately differentiated; G3, poorly differentiated; T, tumor; N, lymph node. * Chi-square test
Correlations between IgG4-positive plasma cell infiltration and clinicopathologic traits and the overall survival of pancreatic cancer patients after curative resection
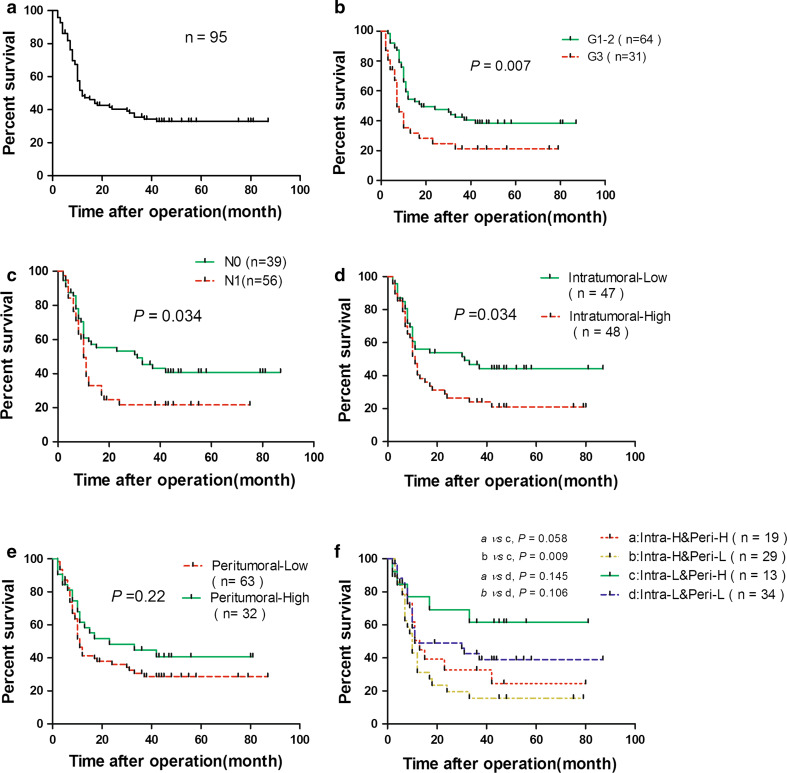
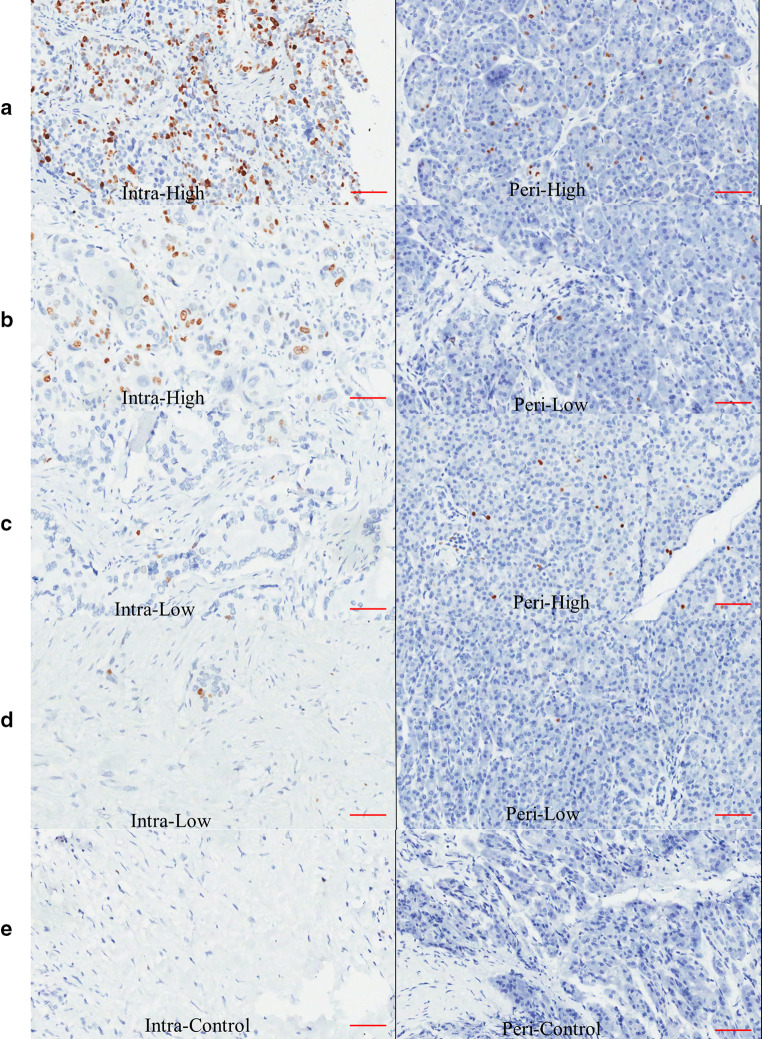
None of the patients were lost to follow-up. While sixty of 95 cases died, the remaining 35 cases were still alive until the end of the follow-up period, which ranged from 36 to 87 months after resection. The overall survival rates for this cohort were 48.34 and 32.8 % at 1 and 3 years after the operation, respectively (Fig. 2a). First, using univariate analysis, we found that poor pancreatic cancer cell differentiation, lymph node metastasis and high-level intratumoral IgG4-positive plasma cell infiltration were negatively correlated with the overall survival of pancreatic cancer patients (P = 0.007, P = 0.034, P = 0.034, respectively; Fig. 2b–d). Peritumoral infiltration of IgG4-positive plasma cells was not correlated with the prognosis. Considering the peritumoral IgG4-positive plasma cell infiltration was correlated with lymph node metastasis, we further stratified the combinations of intratumoral and peritumoral IgG4-positive plasma cell infiltration into the following four groups: a—combination of a high level of both intratumoral and peritumoral infiltrations (Intra-H&Peri-H); b—combination of a high level of intratumoral infiltration and low level of peritumoral infiltration (Intra-H&Peri-L); c—combination of a low level of intratumoral infiltration and high level of peritumoral infiltration (Intra-L&Peri-H); and d—combination of a low level of both intratumoral and peritumoral infiltrations (Intra-L&Peri-L). Unexpectedly, the c group had the best prognosis with a 2-year overall survival up to 61.54 % compared with the b group, which had a 2-year overall survival of 15.65 % (P = 0.009; Figs. 2f, 3). Then, we analyzed the histological grade, lymph node metastasis and intratumoral infiltration of IgG4-positive plasma cells by multivariate analysis; we found that poor differentiation, lymph node metastasis and high-level intratumoral IgG4-plasma cell infiltration were significantly correlated with poor prognosis (P = 0.002, P = 0.008 and P = 0.01, respectively), and the details are listed in Table 2.
Fig. 2.
Kaplan–Meier analysis of the overall survival for the entire cohort (a), differentiation grade of pancreatic cancer cells (b), lymph node metastasis (c), intratumoral infiltration of IgG4-positive plasma cells (d), peritumoral infiltration of IgG4-positive plasma cells (e) and different combinations of intratumoral and peritumoral infiltrations of IgG4-positive plasma cells (f) are shown, respectively (log-rank test, *P < 0.05, **P < 0.01)
Fig. 3.
Representative figures of the different combinations of immunohistochemical staining of peritumoral and intratumoral IgG4-positive plasma cells are shown: a high-level infiltration of both intratumoral and peritumoral IgG4-positive plasma cells; b high-level infiltration of intratumoral and low-level infiltration of peritumoral IgG4-positive plasma cells; c low-level infiltration of intratumoral and high-level infiltration of peritumoral IgG4-positive plasma cells; and d low-level infiltration of both intratumoral and peritumoral IgG4-positive plasma cells. e Control staining of the tumor and peritumoral tissues. IgG4-positive plasma cells are immunochemically stained brown and had a considerable nucleus-to-cytoplasm ratio, as well as an eccentric nucleus (the scale bar represented 150 μm, 200×, magnification)
Table 2.
Univariate and multivariate Cox regression analyses of the association of the prognosis with the clinicopathological parameters and the infiltrations of IgG4-positive plasma cells in pancreatic cancer
| Variable | No. of patients | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|
| Median ± SE | 95 % CI | P value# | RR | 95 % CI | P value* | ||
| Age (years) | 0.667 | ||||||
| ≥65 | 63 | 13.00 ± 2.96 | 7.21–18.80 | ||||
| <65 | 32 | 12.00 ± 2.68 | 6.75–17.25 | ||||
| Sex | 0.105 | ||||||
| Male | 62 | 11.00 ± 1.16 | 8.72–13.28 | ||||
| Female | 33 | 24.00 ± 19.97 | 0–63.145 | ||||
| Location in pancreas | 0.328 | ||||||
| Head | 67 | 13.00 ± 3.81 | 5.533–20.47 | ||||
| Body and tail | 33 | 11.00 ± 1.55 | 7.96–14.04 | ||||
| Tumor size | 0.586 | ||||||
| >4 cm | 38 | 12.00 ± 13.52 | 0–38.51 | ||||
| ≤4 cm | 57 | 12.00 ± 3.04 | 6.04–17.96 | ||||
| Histological grade | 0.007 | 0.002 | |||||
| G1–2 | 64 | 18.00 ± 8.38 | 1.57–34.42 | 0.425 | 0.248–0.730 | ||
| G3 | 31 | 7.00 ± 1.16 | 4.72–9.28 | 1.000 | |||
| pT stage | 0.727 | ||||||
| T1–2 | 74 | 11.00 ± 1.10 | 8.84–13.16 | ||||
| T3–4 | 21 | 23.00 ± 13.06 | 0–48.60 | ||||
| pN stage | 0.034 | 0.008 | |||||
| N0 | 56 | 31.00 ± 12.51 | 6.49–55.52 | 0.491 | 0.290–0.832 | ||
| N1 | 39 | 10.00 ± 0.74 | 8.54–11.46 | 1.000 | |||
| Perineural invasion | 0.086 | ||||||
| Positive | 37 | 10.00 ± 0.66 | 8.70–11.30 | ||||
| Negative | 58 | 17.00 ± 3.56 | 10.03–23.97 | ||||
| Intratumoral IgG4(+) cells | 0.034 | 0.01 | |||||
| Low | 47 | 31.00 ± 16.36 | 0–63.07 | 0.504 | 0.299–0.851 | ||
| High | 48 | 11.00 ± 0.99 | 9.06–12.94 | 1.000 | |||
| Peritumoral IgG4(+) cells | 0.218 | ||||||
| Low | 63 | 10.00 ± 0.94 | 8.15–11.85 | ||||
| High | 32 | 23.00 ± 15.73 | 0–53.84 | ||||
The boldface type indicates a significant difference. SE standard error, RR relative risk, CI confidence interval, G1 well differentiated, G2 moderately differentiated, G3 poorly differentiated, T tumor, N lymph node, # log-rank test, * Cox regression test
M2-polarized TAMs predicted poor prognosis and potentially induced IgG4-positive plasma cells
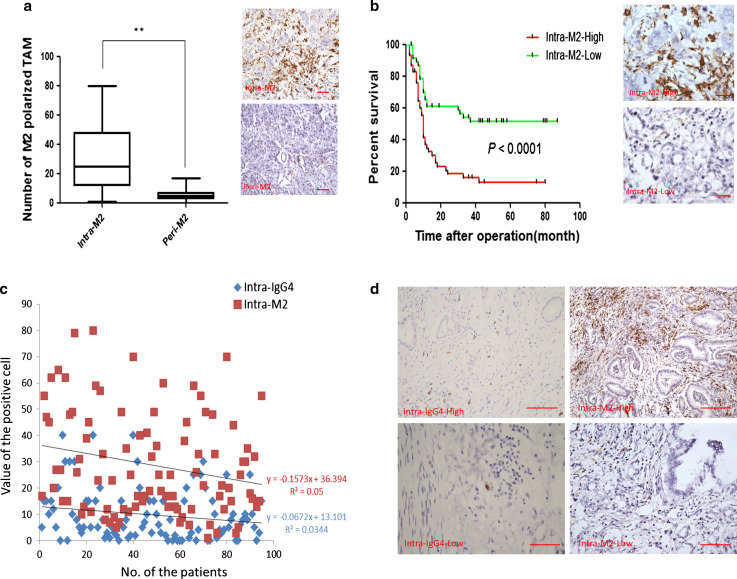
Since the M2-polarized TAMs are the main source of IL-10 in pancreatic cancer tissue and could induce IgG4, we further explored the M2 distribution in tumor and peritumoral tissue samples, as well as the significance of M2, in predicting the pancreatic cancer prognosis. Then, we evaluated the potential correlation of M2-polarized TAMs with IgG4. The number of M2-polarized TAMs in tumor tissue was significantly higher than that in peritumoral tissue (Wilcoxon signed-rank test, P < 0.0001; Fig. 4a). The median value of M2-polarized TAMs in tumor tissue was 25.0; the samples with values higher than 25.0 were defined as the intra-M2-high group. By contrast, the samples with values lower than 25.0 were defined as the intra-M2-low group. The survival curve indicated that high infiltration of M2-polarized TAMs predicted a significantly worse prognosis for pancreatic cancer patients (P < 0.0001; Fig. 4b). The multivariate analysis with differentiation grade, lymph node metastasis, IgG4-positive plasma cells and M2-polarized TAMs also indicated that differentiation grade (RR 0.477, 95 % CI 0.261–0.702, P = 0.0043), lymph node metastasis (RR 0.501, 95 % CI 0.213–0.867, P = 0.0095), IgG4-positive plasma cells (RR 0.657, 95 % CI 0.307–0.891, P = 0.023) and M2-polarized TAMs (RR 0.357, 95 % CI 0.179–0.456, P < 0.0001) were independent risk factors for poor prognosis of pancreatic cancer after curative resection, respectively. The distribution of M2-polarized TAMs and IgG4-positive plasma cells was further evaluated in detail, and they were positively linear correlated (Fig. 4c, d). These results indicated that M2-polarized TAMs probably contributed to inducing intratumoral IgG4-positive plasma cells.
Fig. 4.
High-level infiltration of intratumoral M2-polarized TAMs predicted poor prognosis of pancreatic cancer after curative resection and the intratumoral M2-polarized TAMs and IgG4-positive plasma cells are positively, linearly correlated. a There are more M2-polarized TAMs in tumor tissue than those in peritumoral tissue (Wilcoxon signed-rank test, **P < 0.01; the scale bar represented 50 μm, 200×, magnification). b High-level infiltration of intratumoral M2-polarized TAMs predicted poor prognosis of pancreatic cancer (log-rank test, **P < 0.01; the scale bar represented 25 μm, 400×, magnification). c Linear regression analysis indicated that the intratumoral M2-polarized TAMs and IgG4-positive plasma cells are positively, linearly correlated. d Representative figures of the immunohistochemical staining of the positive, linear relationships between intratumoral M2-polarized TAMs and IgG4-positive plasma cells are shown (the scale bar represented 200 μm, 100×, magnification)
Discussion
In 1995, Yoshida et al. [15] defined a new type of chronic pancreatitis, which is characterized by an elevated serum IgG4 concentration, high level of IgG4-positive plasma cell infiltration, extensive pancreatic tissue fibrosis and good response to steroids therapy. This type is well known as type I AIP. Later, similar clinical manifestations in the lacrimal gland, salivary gland, lung, liver, bile duct, gallbladder, retroperitoneal tissue and other sites were reported. These clinical manifestations have been summarized and called IgG4-related sclerosing diseases (IRSD) [1]. Several cases of IRSD have had accompanying malignancies originating from organs that were not affected by IRSD; these cases have been reported to have significantly more IgG4-positive plasma cell infiltration in the intratumoral tissue than in the peritumoral tissue [3, 5, 16]. Therefore, the presence of IgG4-positive plasma cells may not be an exclusive hallmark of IRSD. IgG4 reactions are also speculated to be nonspecific in several pathological conditions, including malignancies. Ngwa et al. [14] reported that 10.1 % of 548 patients with pancreatic cancer have elevated serum IgG4, but they found that serum IgG4 elevation did not have prognostic significance for pancreatic cancer. However, the peritumoral and intratumoral distributions of IgG4-positive plasma cells and the correlation of IgG4-positive plasma cell infiltration with clinicopathologic traits and pancreatic cancer prognosis have not been previously shown. Pancreatic cancer has exclusive pathological features, including massive inflammatory cell infiltration and scarcity of cancer cells [17]. These inflammatory cells in the tumor microenvironment play important roles in regulating the biological behavior of pancreatic cancer cells [18]. In 2013, after analyzing the infiltrations of inflammatory cells in 212 pancreatic cancer specimens, Ino et al. [19] found that typing the specimens according to the combinations of intratumoral inflammatory cell populations helped determine the pancreatic cancer prognosis. However, IgG4-positive plasma cell infiltration was not mentioned in that article. In this study, using a large cohort of 95 cases of matched tumor and peritumoral pancreatic cancer tissues, we analyzed the distributions and correlations of IgG4-positive plasma cells with the clinicopathologic traits and overall survival of pancreatic cancer after curative resection. IgG4-positive plasma cells were found in 86 % of the tumor tissue samples, and they were found in 69 % of peritumoral tissue samples. Although IgG4-positive plasma cells were found in most of the tumor tissue and peritumoral tissue samples, none of the 95 cases met the pathological diagnostic criteria or developed clinical manifestation of IRSD throughout the study course. A high-level of intratumoral IgG4-positive plasma cell infiltration was also correlated with poor pancreatic cancer cell differentiation. Multivariate analysis demonstrated that high-level intratumoral IgG4-positive cell infiltration was a significant, independent risk factor for poor overall survival. Karagiannis et al. [20] also found that IgG4 could impair the roles of other IgGs in activating macrophages and promoting immune escape of malignant melanoma. According to our results, higher intratumoral IgG4-positive plasma cell infiltration predicted poor prognosis of pancreatic cancer patients, which could also imply that IgG4-positive plasma cells impaired immunity against pancreatic cancer. In univariate analysis, peritumoral IgG4-positive plasma cell infiltration alone was not correlated with the overall survival of pancreatic cancer patients; however, a higher rate of high-level peritumoral IgG4 positive infiltration was found in tumor samples from cases that lacked lymph node metastasis. Therefore, we stratified the combinations of peritumoral and intratumoral IgG4-positive plasma cell infiltration and found that the combination of low-level intratumoral and high-level peritumoral IgG4-positive plasma cell infiltration indicated the best overall survival.
A Th2-polarized inflammatory microenvironment, which is rich in interleukin-10 (IL-10), has been demonstrated as a key factor inducing B cells to secrete IgG4. When co-cultured with melanoma cells, B cells secreted more IgG4 and tumor cells enhanced IL-10 expression [6]. Harada et al. [21, 22] reported that 43 % of 54 cholangiocarcinoma specimens were abundant in IgG4-positive plasma cells and the cultured cholangiocarcinoma cells expressed IL-10, which could potentially induce IgG4 reactions. These two articles suggested that IL-10 secreted from cancer cells could induce IgG4 reaction, which potentially impaired the anti-tumor immune function of macrophages. Consistent with the above results, our data demonstrated a higher infiltration of IgG4-positive plasma cells in tumor tissue than in peritumoral tissue. However, although IL-10 secreted from cancer cells is suggested to induce an IgG4 reaction in cholangiocarcinoma and malignant melanoma, the conditions in pancreatic cancer are not necessarily the same. There is a scarcity of pancreatic cancer cells in tumor tissue, while inflammatory cells are abundant, including M2-polarized macrophages [23, 24]. Because M2-polarized TAMs can secrete significantly more IL-10 than pancreatic cancer cells, M2-polarized TAMs, instead of pancreatic cancer cells, are probably the main source of intratumoral IL-10. As a result, M2-polarized TAMs could be the main inducers of IgG4 reactions in pancreatic cancer. We further analyzed the potential correlations of M2-polarized TAMs and IgG-4 positive plasma cells. There was significantly more infiltration of M2-polarized TAMs in tumor tissue than in peritumoral tissue. A higher infiltration of intratumoral M2-polarized TAMs predicted poorer prognosis of pancreatic cancer patients. Linear regression analysis also indicated that M2-polarized TAMs and IgG4-positive plasma cells were positively, linearly correlated. According to these results, intratumoral M2-polarized TAMs probably induced IgG4-positive plasma cells.
Conclusions
We present the first evidence of intratumoral and peritumoral IgG4-positive plasma cell infiltration in pancreatic cancer, which was associated with the clinicopathologic traits and overall survival after curative resection. Our data also suggest that in the pancreatic cancer tumor microenvironment, M2-polarized TAMs probably induce B cells to develop into IgG4-positive plasma cells. High-level intratumoral IgG4-positive plasma cell infiltration is a definitive predictor for poor pancreatic cancer prognosis. However, because this is only a retrospective study and IgG4 sera data were not available, further studies are still needed to explore the correlations between the sera IgG4 and intratumoral IgG4-related conditions, as well as the exact molecular mechanisms for the interactions of IgG4-positive plasma cells with pancreatic cancer cells and other intratumoral inflammatory cells. IgG4-positive plasma cells could have therapeutic value for pancreatic cancer.
Acknowledgments
This work was supported by grants from the National High Technology Research and Development Program (No. 2012AA02A212) and National Natural Science Foundation of China (81272573, 81502068).
Abbreviations
- AIP
Autoimmune pancreatitis
- IL
Interleukin
- IRSD
IgG4-related sclerosing diseases
- MDSC
Myeloid-derived suppressor cells
- TAM
Tumor-associated macrophage
- Th2
Type 2 T helper cells
- TMA
Tissue microarray
- Treg
Regulatory T cell
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interests.
Contributor Information
Quan Liao, Phone: +861069156007, Email: lqpumc@126.com.
Yupei Zhao, Phone: +861069156007, Email: zhao8028@263.net.
References
- 1.Kamisawa T, Zen Y, Pillai S, Stone JH. IgG4-related disease. Lancet. 2015;385(9976):1460–1471. doi: 10.1016/S0140-6736(14)60720-0. [DOI] [PubMed] [Google Scholar]
- 2.Hart PA, Zen Y, Chari ST. Recent advances in autoimmune pancreatitis. Gastroenterology. 2015;149(1):39–51. doi: 10.1053/j.gastro.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Inoue T, Hayama M, Kobayashi S, Oyaizu T, Nakazato Y, Honma K, Chida M. Lung cancer complicated with IgG4-related disease of the lung. Ann Thorac Cardiovasc Surg. 2014;20(Suppl):474–477. doi: 10.5761/atcs.cr.12.02208. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y, Chen L, Li F. Predominant IgG4 disease and concurrent early-stage rectal cancer. Clin Nucl Med. 2011;36(12):1135–1136. doi: 10.1097/RLU.0b013e3182336247. [DOI] [PubMed] [Google Scholar]
- 5.Ito M, Naruke Y, Mihara Y, So K, Miyashita T, Origuchi T, Nakashima M, Livolsi V. Thyroid papillary carcinoma with solid sclerosing change in IgG4-related sclerosing disease. Pathol Int. 2011;61(10):589–592. doi: 10.1111/j.1440-1827.2011.02701.x. [DOI] [PubMed] [Google Scholar]
- 6.Karagiannis P, Gilbert AE, Josephs DH, Ali N, Dodev T, Saul L, Correa I, Roberts L, Beddowes E, Koers A, Hobbs C, Ferreira S, Geh JL, Healy C, Harries M, Acland KM, Blower PJ, Mitchell T, Fear DJ, Spicer JF, Lacy KE, Nestle FO, Karagiannis SN. IgG4 subclass antibodies impair antitumor immunity in melanoma. J Clin Invest. 2013;123(4):1457–1474. doi: 10.1172/JCI65579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegel R, Ma J, Zou Z. Jemal A (2014) Cancer statistics. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 8.Vonderheide RH, Bajor DL, Winograd R, Evans RA, Bayne LJ, Beatty GL. CD40 immunotherapy for pancreatic cancer. Cancer Immunol Immunother. 2013;62(5):949–954. doi: 10.1007/s00262-013-1427-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shevchenko I, Karakhanova S, Soltek S, Link J, Bayry J, Werner J, Umansky V, Bazhin AV. Low-dose gemcitabine depletes regulatory T cells and improves survival in the orthotopic Panc02 model of pancreatic cancer. Int J Cancer. 2013;133(1):98–107. doi: 10.1002/ijc.27990. [DOI] [PubMed] [Google Scholar]
- 10.Storz P. The crosstalk between acinar cells with mutations and M1-polarized macrophages leads to initiation of pancreatic precancerous lesions. Oncoimmunology. 2015;4(6):e1008794. doi: 10.1080/2162402X.2015.1008794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karakhanova S, Link J, Heinrich M, Shevchenko I, Yang Y, Hassenpflug M, Bunge H, von Ahn K, Brecht R, Mathes A, Maier C, Umansky V, Werner J, Bazhin AV. Characterization of myeloid leukocytes and soluble mediators in pancreatic cancer: importance of myeloid-derived suppressor cells. Oncoimmunology. 2015;4(4):e998519. doi: 10.1080/2162402X.2014.998519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sainz B, Jr, Alcala S, Garcia E, Sanchez-Ripoll Y, Azevedo MM, Cioffi M, Tatari M, Miranda-Lorenzo I, Hidalgo M, Gomez-Lopez G, Canamero M, Erkan M, Kleeff J, Garcia-Silva S, Sancho P, Hermann PC, Heeschen C. Microenvironmental hCAP-18/LL-37 promotes pancreatic ductal adenocarcinoma by activating its cancer stem cell compartment. Gut. 2015;64(12):1921–1935. doi: 10.1136/gutjnl-2014-308935. [DOI] [PubMed] [Google Scholar]
- 13.Panni RZ, Sanford DE, Belt BA, Mitchem JB, Worley LA, Goetz BD, Mukherjee P, Wang-Gillam A, Link DC, Denardo DG, Goedegebuure SP, Linehan DC. Tumor-induced STAT3 activation in monocytic myeloid-derived suppressor cells enhances stemness and mesenchymal properties in human pancreatic cancer. Cancer Immunol Immunother. 2014;63(5):513–528. doi: 10.1007/s00262-014-1527-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ngwa T, Law R, Hart P, Smyrk TC, Chari ST. Serum IgG4 elevation in pancreatic cancer: diagnostic and prognostic significance and association with autoimmune pancreatitis. Pancreas. 2015;44(4):557–560. doi: 10.1097/MPA.0000000000000297. [DOI] [PubMed] [Google Scholar]
- 15.Yoshida K, Toki F, Takeuchi T, Watanabe S, Shiratori K, Hayashi N. Chronic pancreatitis caused by an autoimmune abnormality. Proposal of the concept of autoimmune pancreatitis. Dig Dis Sci. 1995;40(7):1561–1568. doi: 10.1007/BF02285209. [DOI] [PubMed] [Google Scholar]
- 16.Gill J, Angelo N, Yeong ML, McIvor N. Salivary duct carcinoma arising in IgG4-related autoimmune disease of the parotid gland. Hum Pathol. 2009;40(6):881–886. doi: 10.1016/j.humpath.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 17.Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The pancreas cancer microenvironment. Clin Cancer Res. 2012;18(16):4266–4276. doi: 10.1158/1078-0432.CCR-11-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, Torigian DA, O’Dwyer PJ, Vonderheide RH. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331(6024):1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ino Y, Yamazaki-Itoh R, Shimada K, Iwasaki M, Kosuge T, Kanai Y, Hiraoka N. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer. 2013;108(4):914–923. doi: 10.1038/bjc.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karagiannis P, Gilbert AE, Nestle FO, Karagiannis SN. IgG4 antibodies and cancer-associated inflammation: insights into a novel mechanism of immune escape. Oncoimmunology. 2013;2(7):e24889. doi: 10.4161/onci.24889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harada K, Shimoda S, Kimura Y, Sato Y, Ikeda H, Igarashi S, Ren XS, Sato H, Nakanuma Y. Significance of immunoglobulin G4 (IgG4)-positive cells in extrahepatic cholangiocarcinoma: molecular mechanism of IgG4 reaction in cancer tissue. Hepatology. 2012;56(1):157–164. doi: 10.1002/hep.25627. [DOI] [PubMed] [Google Scholar]
- 22.Kimura Y, Harada K, Nakanuma Y. Pathologic significance of immunoglobulin G4-positive plasma cells in extrahepatic cholangiocarcinoma. Hum Pathol. 2012;43(12):2149–2156. doi: 10.1016/j.humpath.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Gardian K, Janczewska S, Olszewski WL, Durlik M. Analysis of pancreatic cancer microenvironment: role of macrophage infiltrates and growth factors expression. J Cancer. 2012;3:285–291. doi: 10.7150/jca.4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]