Abstract
Premalignant lesions for many cancers have been identified, and efforts are currently directed toward identification of antigens expressed on these lesions that would provide suitable targets for vaccines for cancer prevention. Intraductal papillary mucinous neoplasms (IPMNs) are premalignant pancreatic cysts of which a subset has the potential to progress to cancer. Currently, there are no validated predictive markers for progression to malignancy. We hypothesized that the presence or absence of immune surveillance of these lesions would be one such factor. Here we show that the tumor antigen MUC1, which is abnormally expressed on pancreatic cancer and is a target for cancer immunosurveillance, is also abnormally expressed on premalignant IPMN. We show that some IPMN patients make MUC1-specific IgG. Moreover, we show evidence of CD4 and CD8 T cell infiltration into IPMN areas of high dysplasia suggesting an ongoing immune response within the lesions. We also found, however, increased levels of circulating myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs) in some IPMN patients as well as evidence of T cell exhaustion. Further studies correlating immunosurveillance or immunosuppression with IPMN progression to malignancy will help define the immune response as a biomarker of risk, leading potentially to a vaccine to boost spontaneous immunity and prevent progression to cancer.
Keywords: MUC1, Cancer vaccines, Pancreatic cancer, IPMN, Immunosurveillance, CITIM 2015
Introduction
Cancer immunotherapy is a rapidly evolving field with several new agents showing unprecedented results in clinical trials [1–3]. In spite of the latest successes, immunotherapy is not a definitive answer to the cancer problem, being effective in only a subset of patients. This is considered to be largely due to multiple and often redundant mechanisms that developing tumors use to thwart an effective antitumor immune surveillance. In addition, the cost of cancer therapy in general and immunotherapy in particular is rising twice as fast as overall healthcare costs making these treatments unavailable for most patients [4]. An alternative strategy to combat the existing cancer crisis would be to prevent cancer, primarily in the form of prophylactic vaccines.
The most appropriate candidates for initial testing of prophylactic vaccines are patients diagnosed with premalignant disease that can rapidly progress to cancer, or individuals with known cancer-causing mutations and thus at high risk of developing cancer. Administration of cancer vaccines to individuals without cancer would circumvent many tumor-suppressive mechanisms that are reducing the potential for success of many, if not all immunotherapies [5–8]. Advancements in cancer screening have allowed identification of premalignant lesions for many cancers and have provided new opportunities to examine and confirm that many well-known tumor antigens are also expressed in premalignancy, thus providing suitable targets for the immune system to recognize and potentially eliminate premalignant lesions and/or prevent their recurrence and progression to cancer.
IPMNs are a recognized precursor lesion for pancreatic adenocarcinoma. IPMNs can present as cystic lesions of the main pancreatic duct or one of its branches and they are often found incidentally on abdominal imaging prior to invasive cancer development [9]. IPMNs present a clinical challenge since only 15–25 % of IPMNs will progress to invasive adenocarcinoma. Risk factors have been reported that predict the presence of high-grade dysplasia or microscopic invasive adenocarcinoma, but they are imperfect, and there are no validated predictive factors for progression to malignancy [9–11]. In addition, patients with IPMNs are at an increased risk of not only cancer arising from the cyst itself but elsewhere in the pancreas as a result of field carcinogenesis [12, 13]. The field carcinogenesis may also give rise to pancreatic adenocarcinoma even after resection of invasive or noninvasive IPMN and thus requires lifelong close surveillance [11]. Patients with IPMN would greatly benefit from immunoprevention strategies.
To consider a prophylactic vaccine as an alternative approach to intercepting IPMN progression to cancer, an antigen needed to be identified that is present on IPMN and on pancreatic cancer but not on normal epithelial cells of the pancreatic ducts. Mucin 1 (MUC1) is a transmembrane glycoprotein that is expressed on most glandular and ductal epithelial cells, including that of the pancreas [14]. In normal epithelial cells, MUC1 is highly glycosylated, expressed at low levels and polarized to the apical cell surface. Cancer cells hypoglycosylate MUC1 resulting in truncated sugars on a more exposed protein backbone susceptible to processing into new peptides and glycopeptides that are different from normal and thus recognized by the immune system as foreign. This, in addition to its loss of polarization and overexpression in cancer compared to normal cells, has made MUC1 an attractive target for cancer immunotherapy [15]. MUC1-specific cytotoxic T lymphocytes (CTLs) have been found in pancreatic [16], breast [17] and ovarian [18] cancer patients, and MUC1-specific antibodies have been associated with a survival benefit in cancer patients [19–21]. The abnormal form of MUC1 has also been detected in chronic inflammation and some premalignant lesions including colonic polyps, precursors to colon cancer [22–25]. We have previously reported that individuals with colonic polyps have anti-MUC1 IgG showing that these premalignant lesions are under immune surveillance. In addition, a MUC1 peptide vaccine was administered to healthy individuals who had colon adenomas removed but were at high risk of adenoma recurrence and progression to colon cancer [26]. The vaccine was highly immunogenic and safe and is currently being tested in a multicenter placebo-controlled trial for efficacy, i.e., prevention of adenoma recurrence. Considering that MUC1 is abnormally expressed on pancreatic cancer, we sought to provide information on MUC1 expression in IPMNs and the immune microenvironment of IPMN that would suggest either ongoing immunosurveillance or immunosuppression, both important for the success of a vaccine.
Here we confirm expression of abnormal MUC1 on IPMNs and show evidence of immune surveillance. We also found that some patients with premalignant IPMN already had an increased level of immunosuppressive cell populations in their circulation and elevated levels of inhibitory receptors on their T cells indicating T cell exhaustion, similar to what has been found in cancer patients.
Materials and methods
Blood and tissue collection
Serum and peripheral blood mononuclear cells (PBMCs) were collected at diagnosis from IPMN and pancreatic cancer patients seen in the Department of Medicine, Division of Gastroenterology, Hepatology and Nutrition at the University of Pittsburgh Medical Center. All patients signed an informed consent that was approved by the Institutional Review Board of the University of Pittsburgh. IPMN patients with a family history of pancreatic cancer were excluded from this study. Serum and PBMC samples from healthy donors were collected under a separate protocol.
Histology and immunohistochemistry
IPMNs for histopathology and immunohistochemistry were obtained from patients who underwent surgery. For MUC1-specific staining, 5-µm-thick sections of paraffin-embedded tissue were deparaffinized by baking overnight at 59 °C. Endogenous peroxidase activity was eliminated by treatment with 30 % H2O2 for 15 min at room temperature. Antigen retrieval was achieved by microwave heating in 0.1 % citrate buffer. Nonspecific binding sites were blocked with 2 % BSA. Anti-MUC1 antibody HMPV was purchased from BD Pharmingen, and anti-MUC1 antibody VU-4H5 was purchased from Santa Cruz Biotechnology. Staining was performed by the avidin–biotin–peroxidase complex method with a commercial kit (Vectastain ABC kit; Vector Laboratories). Color development was performed using a 3,3′-diaminobenzidine kit (BD Pharmingen).
Evaluation of IPMN infiltrating leukocytes was performed on Ventana BenchMark Ultra automated system (Ventana Medical Systems, Inc), using monoclonal antibodies for CD3, CD4, CD8, and CD20. Density of immune cells and their spatial distribution in neoplastic tissue was assessed by manual count at 200×.
The results were evaluated independently by two investigators blinded to tissue origin.
Anti-MUC1 ELISA
MUC1-specific antibody levels were measured by ELISA. Immulon 4HBX microtiter plates (Thermo Scientific) were coated overnight at 4 °C with 1 μg MUC1 100-mer peptide (H2N-(GVTSAPDTRPAPGSTAPPAH)5-CONH2) in 50 μL phosphate-buffered saline (PBS). The plates were washed three times with PBS and subsequently blocked with 100 μL 2.5 % bovine serum albumin (Sigma-Aldrich) in PBS for 1 h at room temperature to prevent nonspecific antibody binding. MUC1-negative control plates were prepared similarly. After removal of the blocking reagent, 50 μL of plasma dilutions (1:40 in 2.5 % BSA in PBS) was added to the wells and incubated for 1 h at room temperature. Subsequently, the plates were washed five times with 0.1 % TWEEN 20 (Sigma-Aldrich) in PBS; 50 μL of alkaline phosphatase-conjugated goat antihuman IgG (Sigma-Aldrich; diluted 1:1000 in 2.5 % BSA in PBS) was then added to each well. Following 1-h incubation at room temperature, the plates were washed five times with 0.1 % TWEEN 20 in PBS. After washing, 100 μL of substrate solution (SIGMAFAST p-nitrophenyl phosphate tablets; Sigma-Aldrich) was added to the plates. The reaction was terminated after 1 h by adding 50 μL 0.5 M NaOH. Using a spectrophotometer, the optical density at 405 nm was then measured. To correct for non-specific antibody binding, the OD values of the MUC1-negative control wells were subtracted from the OD values of the test wells coated with MUC1. Assays were performed in triplicates.
Flow cytometry
PBMCs were obtained as described above. Before staining for surface markers, cells were incubated with human BD Fc Block (BD Biosciences). For the analysis of circulating MDSCs and Tregs, the following antihuman antibodies were used: CD11b-APC (clone: ICRF44; BD Biosciences), CD33-PE (clone: WM53; BD Biosciences), HLA-DR-FITC (clone: G46-6; BD Biosciences), CD4-FITC (clone: RPA-T4; BD Biosciences), CD25-APC (clone: BC96; BioLegend) and FOXP3-PE (clone: PCH101; eBioscience). For intracellular staining of FOXP3, cells were first stained for surface markers and subsequently fixed and permeabilized in fixation/permeabilization buffer according to manufacturer’s instruction (eBioscience). After washing, cells were resuspended in permeabilization buffer and stained for FOXP3. Flow cytometry analysis was performed using an LSR II (BD Biosciences), and data were analyzed using FlowJo (Tree Star). MDSCs were defined as CD11b+ CD33+ HLA-DR− cells. Tregs were defined as CD4+ CD25+ FOXP3+ cells. T cell exhaustion was defined using antihuman antibodies for lymphocyte-activated gene-3 (LAG-3) (clone: 3DS223H; eBioscience), programed cell death protein-1 (PD-1) (clone: MlH4; eBioscience) and T cell immunoglobulin and mucin-containing protein-3 (TIM-3) (clone: 344823; R&D systems).
Data analysis
Data represents means + individual data points. Cutoff values for antibody titers and MDSCs were calculated as the mean + 3 standard deviations of the healthy donor population [27]. Subjects that could be clearly distinguished from the rest of the healthy donor population as having a high anti-MUC1 antibody titer were excluded for calculation of the cutoff values. Statistical analyses were performed with GraphPad Prism (GraphPad) using a one-way ANOVA and Tukey’s post hoc test. P values <0.05 were considered significant.
Results
IPMNs overexpress the hypoglycosylated abnormal form of MUC1
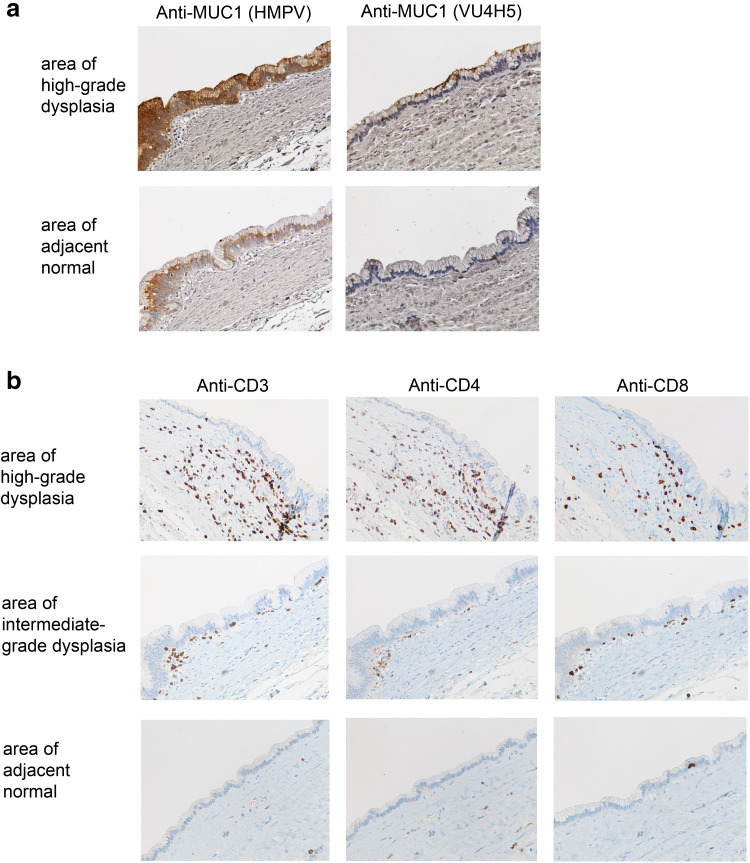
Tissue sections of intermediate- or high-grade IPMN were stained with two MUC1-specific monoclonal antibodies. Anti-MUC1 antibody clone HMPV is glycosylation independent and recognizes both normal and abnormal MUC1. In contrast, anti-MUC1 antibody clone VU4H5 is glycosylation dependent and recognizes only the hypoglycosylated tumor form of MUC1. Analysis of stained IPMN biopsies revealed increased expression and loss of polarization of MUC1 on epithelial cells in areas of dysplasia compared to adjacent normal ductal epithelium (Fig. 1a). The majority of MUC1 expressed in areas of dysplasia is the abnormal hypoglycosylated form of MUC1 that is not found on the adjacent normal epithelium (Fig. 1a).
Fig. 1.
MUC1 expression and T cell infiltration in IPMN. a Consecutive tissue sections are stained with antibodies directed against normal MUC1 (left panel) or hypoglycosylated tumor MUC1 (right panel). b Consecutive tissue sections are stained with antibodies directed against CD3, CD4 and CD8 showing the relationship between T cell infiltration and high-grade dysplasia, intermediate-grade dysplasia and adjacent normal epithelium
Given the expression of abnormal MUC1 in areas of dysplasia, which would be expected to be recognized by the immune system, sequential tissue sections were stained with antibodies against CD3, CD4, CD8 and CD20 to determine whether abnormal MUC1 expression on IPMNs elicited a local immune response. Increased T cell infiltration as detected using anti-CD3 antibody was seen in the stroma surrounding areas with high-grade dysplasia compared to areas with intermediate-grade dysplasia (Fig. 1b). The T cell infiltrate comprised both CD4 and CD8 T cells with CD4 T cells predominantly associated with the stroma, and CD8 T cells found within the stroma and also within the epithelial layer. Little to no T cell infiltration was detected in the stroma surrounding adjacent normal epithelia. Staining with anti-CD20 revealed very few if any B cells (not shown).
Spontaneously generated MUC1-specific IgG in sera of patients with IPMN
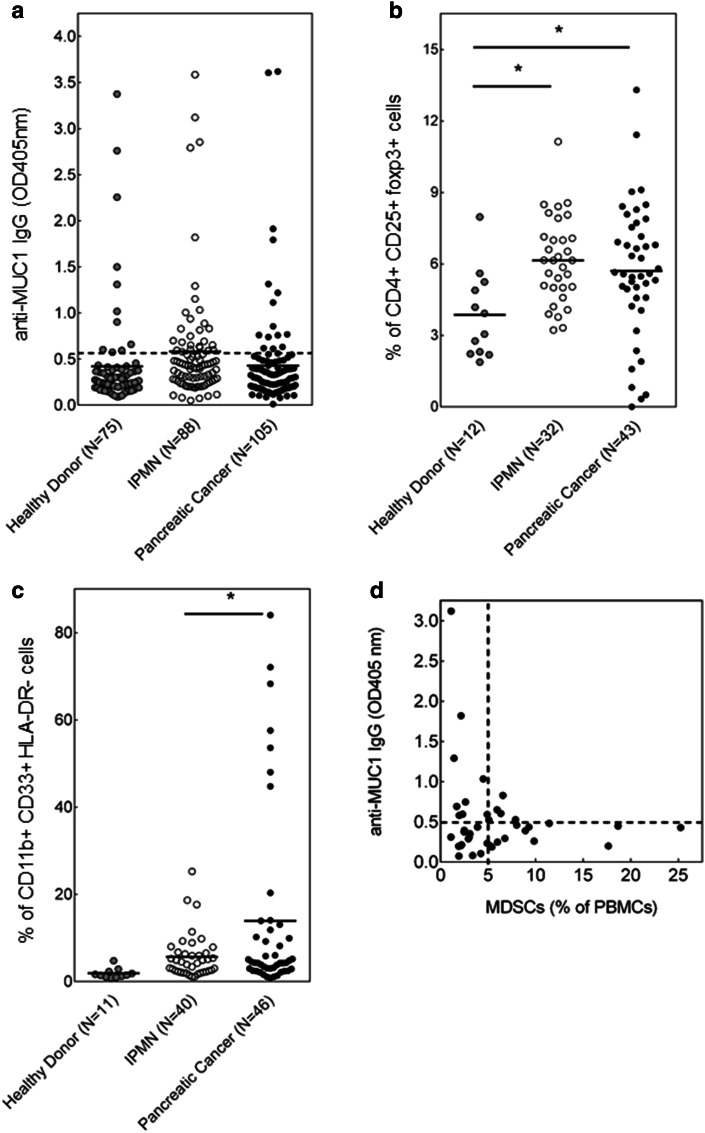
Using serum ELISA, we analyzed anti-MUC1 antibody responses in patients with premalignant IPMNs. We specifically focused on IgG antibodies, as the MUC1-specific B cell isotype switching from IgM to IgG requires T cell help and thus indirectly indicates the presence of MUC1-specific T cell immunity. Anti-MUC1 IgG responses were found in 30 of 88 patients with IPMN (34.1 %) compared to 11 of 75 healthy subjects (14.7 %; Fig. 2a). In agreement with the profound immunosuppression that is commonly observed in cancer patients, anti-MUC1 IgG was found in only 19 out of 105 (18.1 %) pancreatic cancer patients (Fig. 2a).
Fig. 2.
Anti-MUC1 IgG and levels of circulating Treg and MDSC in IPMN. a Detection by ELISA of anti-MUC1 IgG in sera of patients with IPMN and pancreatic cancer compared to healthy donor controls. Absorbance at 405 nm. b Treg as percent of CD4+ cells; c MDSC as percent of PBMC; and d levels of anti-MUC IgG correlate inversely with the levels of MDSCs in the circulation of patients with IPMN. *p < 0.01; **p < 0.05
Evidence for systemic immunosuppression in some patients with IPMN
Tregs and MDSCs have been shown to accumulate in the tumor microenvironment and in circulation and to suppress immune effector responses of cancer patients and thus facilitate cancer growth and lower effectiveness of cancer immunotherapy [5, 7]. Very little is known about the role of these cells in premalignancy. As expected, Tregs (+48 %; p < 0.05) and MDSCs (7.5-fold; p = 0.0512) were markedly elevated in patients with pancreatic cancer compared to healthy subjects (Fig. 2b, c). These immunosuppressive cells were also present in higher percentages in IPMN patients compared to healthy subjects with the major increase in Treg (+59 %; p < 0.05), comparable to the levels found in cancer. MDSCs were also present in some patients with IPMN and showing a trend toward higher percentages than in healthy donors. Significantly, lower percentages of MDSC were seen in IPMN patients compared to pancreatic cancer patients. It thus seems that at least in a fraction of patients with premalignant IPMN the immunosuppressive microenvironment has already been established and might facilitate progression of these IPMNs to cancer.
We also explored the possibility that the same immunosuppressive environment might be responsible for the lack of or low levels of anti-MUC1 IgG in some IPMN patients. We examined the correlation between the levels of Tregs and MDSCs and anti-MUC1 IgG in patients where we had matched serum and PBMC samples. There was no correlation between Tregs and anti-MUC1 IgG (data not shown). However, the levels of anti-MUC IgG showed a strong inverse relationship with the levels of MDSCs (Fig. 2d).
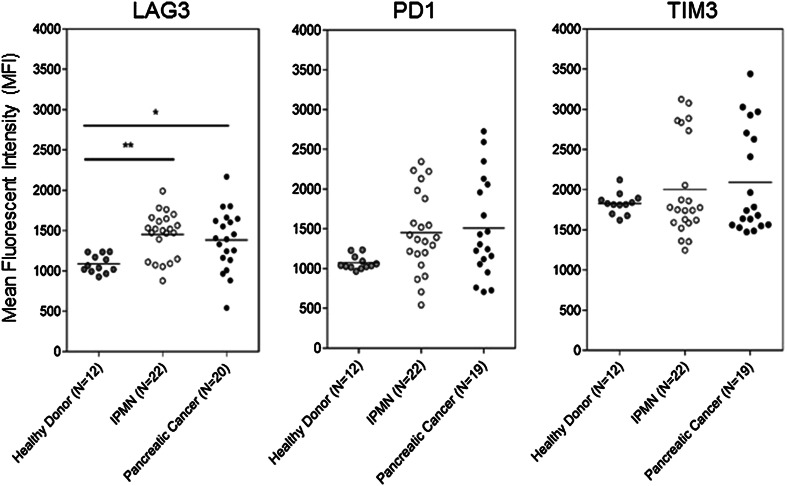
We also looked for evidence of suppression of the effector T cells by evaluating surface expression of inhibitory receptors LAG-3, PD-1 and TIM-3, typically expressed on chronically activated and “exhausted” T cells. T cell exhaustion is thought to be a natural mechanism for limiting immune pathology as a result of an overzealous immune response to chronic antigen exposure. Tumors can hijack these same mechanisms for limiting activity of tumor-specific T cells by turning them into exhausted T cells that fail to proliferate, secrete cytokines or lyse target cells [28–30]. Compared to healthy controls, T cells in PBMC from IPMN patients had significantly elevated expression of one of the exhaustion markers, LAG-3 (Fig. 3). Expression levels of PD-1 and TIM-3 were also higher in IPMN patients and closer to the levels found on T cells from pancreatic cancer patients, even though the difference from normal did not reach statistical significance.
Fig. 3.
Surface expression of markers of T cell exhaustion. Flow cytometry analysis of circulating T cells stained with antibodies against LAG-3, PD-1 and TIM-3. *p < 0.01; **p < 0.05
Discussion
The process of tumorigenesis is complex, and it can take many years before tumor is clinically detectable. Most tumors possess antigens that can elicit an immune response resulting in tumor elimination or long-term control of tumor growth [31, 32]. Many of these target antigens have been identified based on their abnormal expression on tumor cells compared to normal cells. It is postulated that many molecules would also be abnormally expressed on premalignant lesions as the cells are undergoing initial changes in the progression from normal to malignant phenotype. There is still only limited information on such molecules that could be very important in targeting these early lesions for cancer immunoprevention.
We show here that IPMN, one of pancreatic cancer precursors, expresses the abnormal tumor form of MUC1, a well-defined and studied tumor antigen [33]. We also show that immune surveillance occurs early in the development of pancreatic cancer, as seen by the presence of anti-MUC1 IgG antibodies in patients’ sera and greater infiltration of CD4 and CD8 T cells in the IPMN stroma in areas of dysplasia compared to adjacent normal epithelium. This finding, especially the presence of anti-MUC1 antibody, supports attempts at prevention of progression of IPMNs to pancreatic cancer by administering a MUC1 vaccine to boost this preexisting immunity. The presence of anti-MUC1 IgG in some patients should also be further evaluated as a possible biomarker for risk of progression from premalignant IPMN to malignant cancer. It is known that breast and pancreatic cancer patients have survival advantage if they present with anti-MUC1 antibody at the time of diagnosis [19]. It would be reasonable to postulate that the presence of anti-MUC1 IgG would similarly control IPMN progression and lower the risk of cancer not only in the cyst itself but elsewhere in the pancreatic parenchyma.
Under the pressure from the immune system, some tumors build an elaborate, multi-component, multi-mechanism immunosuppressive environment that facilitates their growth, invasion and metastasis and affects negatively both passive and active cancer immunotherapy [34]. It is now appreciated that some immunosuppressive mechanisms might be already present in premalignancy. We have previously published that T cells from patients with pancreatic cancer have a reduced ability to be activated [6, 35]. We also recently reported that in the setting of premalignant disease in the colon some patients already harbor high levels of MDSCs that are known to suppress T cell function and facilitate cancer progression [26]. Similar to this finding, we discovered that some patients with IPMN also show evidence of immune suppression seen in elevated levels primarily of Tregs and less so of MDSCs, compared to healthy subjects. While the specific effect of Tregs is not yet clear, patients with increased MDSCs were those negative for anti-MUC1 IgG.
In addition to an increase in circulating Tregs and MDSCs, we also found elevated levels of inhibitory receptors LAG-3 [36], PD-1 [30, 36] and TIM-3 [29, 30] on the surface of circulating T cells, markers of T cell exhaustion.
Collectively, this work demonstrates that tumor antigen MUC1 is expressed in premalignant IPMN and that MUC1-specific immune surveillance does in fact occur early during the progression from normal epithelium to premalignant IPMN. In addition, the profound immune suppression that we and others have observed in most patients with pancreatic cancer begins already in premalignancy in some patients.
This work suggests that in patients with IPMN the presence of anti-MUC1 antibodies contrasted with the presence of Treg and MDSC could be used to discriminate between cysts with a high malignant potential and cysts with a low malignant potential. If this can be validated prospectively in a larger cohort of patients, it could reduce the number of patients who are unnecessarily exposed to invasive procedures.
In a much broader sense, this work suggests that patients with premalignant IPMNs might benefit from preventive immunotherapy, an example being a MUC1 vaccine. Administration of a MUC1 vaccine to patients with IPMN could enhance MUC1-specific immunity in those who already have an ongoing MUC1-specific immune response, and might also counteract the immune suppression observed in some patients resulting in new antibody production.
We previously reported that T cells obtained from pancreatic cancer patients, pre- and post-MUC1 vaccine have a different ability to produce cytokines [35]. Freshly isolated T cells from the majority of patients prior to the vaccine were completely impaired in their ability to make either IFN-g or IL-4. After receiving at least two doses of the MUC1 vaccine, their ability to produce cytokines was significantly increased. In addition to the reversal of suppression, a MUC1 vaccine would have the advantage of generating immunological memory to prevent the development of future IPMN in these patients.
Acknowledgments
We thank Dr. Aatur Singhi for providing IPMN tissue for immunohistochemistry and Dr. John McKolanis for help with ELISA. This work was supported by the National Institute of Health Grant CA168392-05 (Finn) and the University of Pittsburgh CTSI Grant # 5UL1TR000005 (Finn and Brand).
Abbreviations
- CTL
Cytotoxic T cell
- IFN-g
Interferon gamma
- IL-4
Interleukin-4
- IPMN
Intraductal papillary mucinous neoplasm(s)
- LAG-3
Lymphocyte-activated gene-3
- MDSC
Myeloid-derived suppressor cell
- MUC1
Mucin 1
- PBMC
Peripheral blood mononuclear cell
- PD-1
Programed cell death protein-1
- TIM-3
T cell immunoglobulin and mucin-containing protein-3
- Treg
Regulatory T cell
Compliance with ethical standards
Conflict of interest
None of the authors has conflict of interest to declare.
Footnotes
Pamela L. Beatty and Rick van der Geest have contributed equally to this work.
References
- 1.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lipson EJ, Drake CG. Ipilimumab: an anti-CTLA-4 antibody for metastatic melanoma. Clin Cancer Res. 2011;17(22):6958–6962. doi: 10.1158/1078-0432.CCR-11-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ledford H. Immunotherapy’s cancer remit widens. Nature. 2013;497(7451):544. doi: 10.1038/497544a. [DOI] [PubMed] [Google Scholar]
- 5.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmielau J, Finn OJ. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res. 2001;61(12):4756–4760. [PubMed] [Google Scholar]
- 7.Whiteside TL. Induced regulatory T cells in inhibitory microenvironments created by cancer. Expert Opin Biol Ther. 2014;14(10):1411–1425. doi: 10.1517/14712598.2014.927432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woodford D, Johnson SD, De Costa AM, Young MR. An inflammatory cytokine milieu is prominent in premalignant oral lesions, but subsides when lesions progress to squamous cell carcinoma. J Clin Cell Immunol. 2014;5(3):230. doi: 10.4172/2155-9899.1000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi C, Hruban RH. Intraductal papillary mucinous neoplasm. Hum Pathol. 2012;43(1):1–16. doi: 10.1016/j.humpath.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Klibansky DA, Reid-Lombardo KM, Gordon SR, Gardner TB. The clinical relevance of the increasing incidence of intraductal papillary mucinous neoplasm. Clin Gastroenterol Hepatol. 2012;10(5):555–558. doi: 10.1016/j.cgh.2011.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanaka M. International consensus on the management of intraductal papillary mucinous neoplasm of the pancreas. Ann Transl Med. 2015;3(19):286. doi: 10.3978/j.issn.2305-5839.2015.11.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dakubo GD, Jakupciak JP, Birch-Machin MA, Parr RL. Clinical implications and utility of field cancerization. Cancer Cell Int. 2007;7:2. doi: 10.1186/1475-2867-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matthaei H, Norris AL, Tsiatis AC, Olino K, Hong SM, dal Molin M, Goggins MG, Canto M, Horton KM, Jackson KD, Capelli P, Zamboni G, Bortesi L, Furukawa T, Egawa S, Ishida M, Ottomo S, Unno M, Motoi F, Wolfgang CL, Edil BH, Cameron JL, Eshleman JR, Schulick RD, Maitra A, Hruban RH. Clinicopathological characteristics and molecular analyses of multifocal intraductal papillary mucinous neoplasms of the pancreas. Ann Surg. 2012;255(2):326–333. doi: 10.1097/SLA.0b013e3182378a18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vlad AM, Kettel JC, Alajez NM, Carlos CA, Finn OJ. MUC1 immunobiology: from discovery to clinical applications. Adv Immunol. 2004;82:249–293. doi: 10.1016/S0065-2776(04)82006-6. [DOI] [PubMed] [Google Scholar]
- 15.Kimura T, Finn OJ. MUC1 immunotherapy is here to stay. Expert Opin Biol Ther. 2013;13(1):35–49. doi: 10.1517/14712598.2012.725719. [DOI] [PubMed] [Google Scholar]
- 16.Barnd DL, Lan MS, Metzgar RS, Finn OJ. Specific, major histocompatibility complex-unrestricted recognition of tumor-associated mucins by human cytotoxic T cells. Proc Natl Acad Sci USA. 1989;86(18):7159–7163. doi: 10.1073/pnas.86.18.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jerome KR, Barnd DL, Bendt KM, Boyer CM, Taylor-Papadimitriou J, McKenzie IF, Bast RC, Jr, Finn OJ. Cytotoxic T-lymphocytes derived from patients with breast adenocarcinoma recognize an epitope present on the protein core of a mucin molecule preferentially expressed by malignant cells. Cancer Res. 1991;51(11):2908–2916. [PubMed] [Google Scholar]
- 18.Ioannides CG, Fisk B, Jerome KR, Irimura T, Wharton JT, Finn OJ. Cytotoxic T cells from ovarian malignant tumors can recognize polymorphic epithelial mucin core peptides. J Immunol. 1993;151(7):3693–3703. [PubMed] [Google Scholar]
- 19.Hamanaka Y, Suehiro Y, Fukui M, Shikichi K, Imai K, Hinoda Y. Circulating anti-MUC1 IgG antibodies as a favorable prognostic factor for pancreatic cancer. Int J Cancer. 2003;103(1):97–100. doi: 10.1002/ijc.10801. [DOI] [PubMed] [Google Scholar]
- 20.Pinheiro SP, Hankinson SE, Tworoger SS, Rosner BA, McKolanis JR, Finn OJ, Cramer DW. Anti-MUC1 antibodies and ovarian cancer risk: prospective data from the Nurses’ Health Studies. Cancer Epidemiol Biomarkers Prev. 2010;19(6):1595–1601. doi: 10.1158/1055-9965.EPI-10-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von Mensdorff-Pouilly S, Verstraeten AA, Kenemans P, Snijdewint FG, Kok A, Van Kamp GJ, Paul MA, Van Diest PJ, Meijer S, Hilgers J. Survival in early breast cancer patients is favorably influenced by a natural humoral immune response to polymorphic epithelial mucin. J Clin Oncol. 2000;18(3):574–583. doi: 10.1200/JCO.2000.18.3.574. [DOI] [PubMed] [Google Scholar]
- 22.Ajioka Y, Watanabe H, Jass JR. MUC1 and MUC2 mucins in flat and polypoid colorectal adenomas. J Clin Pathol. 1997;50(5):417–421. doi: 10.1136/jcp.50.5.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beatty PL, Plevy SE, Sepulveda AR, Finn OJ. Cutting edge: transgenic expression of human MUC1 in IL-10-/- mice accelerates inflammatory bowel disease and progression to colon cancer. J Immunol. 2007;179(2):735–739. doi: 10.4049/jimmunol.179.2.735. [DOI] [PubMed] [Google Scholar]
- 24.Kadayakkara DK, Beatty PL, Turner MS, Janjic JM, Ahrens ET, Finn OJ. Inflammation driven by overexpression of the hypoglycosylated abnormal mucin 1 (MUC1) links inflammatory bowel disease and pancreatitis. Pancreas. 2010;39(4):510–515. doi: 10.1097/MPA.0b013e3181bd6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vlad AM, Diaconu I, Gantt KR. MUC1 in endometriosis and ovarian cancer. Immunol Res. 2006;36(1–3):229–236. doi: 10.1385/IR:36:1:229. [DOI] [PubMed] [Google Scholar]
- 26.Kimura T, McKolanis JR, Dzubinski LA, Islam K, Potter DM, Salazar AM, Schoen RE, Finn OJ. MUC1 vaccine for individuals with advanced adenoma of the colon: a cancer immunoprevention feasibility study. Cancer Prev Res (Phila) 2013;6(1):18–26. doi: 10.1158/1940-6207.CAPR-12-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cramer DW, Titus-Ernstoff L, McKolanis JR, Welch WR, Vitonis AF, Berkowitz RS, Finn OJ. Conditions associated with antibodies against the tumor-associated antigen MUC1 and their relationship to risk for ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2005;14(5):1125–1131. doi: 10.1158/1055-9965.EPI-05-0035. [DOI] [PubMed] [Google Scholar]
- 28.Baitsch L, Baumgaertner P, Devevre E, Raghav SK, Legat A, Barba L, Wieckowski S, Bouzourene H, Deplancke B, Romero P, Rufer N, Speiser DE. Exhaustion of tumor-specific CD8(+) T cells in metastases from melanoma patients. J Clin Invest. 2011;121(6):2350–2360. doi: 10.1172/JCI46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferris RL, Lu B, Kane LP. Too much of a good thing? Tim-3 and TCR signaling in T cell exhaustion. J Immunol. 2014;193(4):1525–1530. doi: 10.4049/jimmunol.1400557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V, Zarour HM. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207(10):2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3(11):991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 32.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 33.Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL, Weiner LM, Matrisian LM. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15(17):5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pardoll D. Cancer and the Immune System: Basic Concepts and Targets for Intervention. Semin Oncol. 2015;42(4):523–538. doi: 10.1053/j.seminoncol.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramanathan RK, Lee KM, McKolanis J, Hitbold E, Schraut W, Moser AJ, Warnick E, Whiteside T, Osborne J, Kim H, Day R, Troetschel M, Finn OJ. Phase I study of a MUC1 vaccine composed of different doses of MUC1 peptide with SB-AS2 adjuvant in resected and locally advanced pancreatic cancer. Cancer Immunol Immunother. 2005;54(3):254–264. doi: 10.1007/s00262-004-0581-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, Bettini ML, Gravano DM, Vogel P, Liu CL, Tangsombatvisit S, Grosso JF, Netto G, Smeltzer MP, Chaux A, Utz PJ, Workman CJ, Pardoll DM, Korman AJ, Drake CG, Vignali DA. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72(4):917–927. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]