Abstract
Targeted therapy with sunitinib, pazopanib or everolimus has improved treatment outcome for patients with metastatic renal cell carcinoma patients (RCC). However, despite considerable efforts in sequential or combined modalities, durable remissions are rare. Immunotherapy like cytokine therapy with interleukin-2, T cell checkpoint blockade or adoptive T cell therapies can achieve long-term benefit and even cure. This raises the question of whether combining targeted therapy with immunotherapy could also be an effective treatment option for RCC patients. Sunitinib, one of the most frequently administered therapeutics in RCC patients has been implicated in impairing T cell activation and proliferation in vitro. In this work, we addressed whether this notion holds true for expansion of tumor-infiltrating lymphocytes (TILs) in sunitinib-treated patients. We compared resected primary RCC tumor material of patients pretreated with sunitinib with resection specimen from sunitinib-naïve patients. We found improved TIL expansion from sunitinib-pretreated tumor digests. These TIL products contained more PD-1 expressing TIL, while the regulatory T cell infiltration was not altered. The improved TIL expansion was associated with reduced intratumoral myeloid-derived suppressor cell (MDSC) content. Depletion of MDSCs from sunitinib-naïve RCC tissue-digest improved TIL expansion, proving the functional relevance of the MDSC alteration by sunitinib. Our in vivo results do not support previous in vitro observations of sunitinib inhibiting T cell function, but do provide a possible rationale for the combination of sunitinib with immunotherapy.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-015-1735-z) contains supplementary material, which is available to authorized users.
Keywords: Sunitinib, Tumor-infiltrating lymphocytes, Myeloid-derived suppressor cells, Renal cell carcinoma, Adoptive cell therapy
Introduction
Renal cell carcinoma (RCC) accounts for approximately 3 % of all adult malignancies with a rising incidence. About 25–30 % of patients suffer from metastatic disease at the time of diagnosis. An additional 20–30 % of patients will develop metastases following radical nephrectomy [1]. Metastatic RCC (mRCC) has a 5-year survival rate of approximately 10 % [2]. Targeted therapies with vascular endothelial growth factor receptor (VEGF-R) inhibitors such as sunitinib, sorafenib, axitinib and pazopanib or mammalian target of rapamycin (mTOR) inhibitors like everolimus or temsirolimus achieve objective response rates of up to 40 %. However, durable complete remissions are rare [3]. Therefore, novel therapeutic options that potentially offer long-term benefit are in high demand.
Interestingly, complete responses in mRCC have been observed after cytokine therapies with interleukin (IL)-2 or interferon (IFN)-alpha and responses of up to 20 % are described in strongly selected patient populations [4, 5]. In randomized setups, however, only IFN-alpha achieved a moderate 3-month survival benefit [2]. Nevertheless, together with the observed rare spontaneous complete remissions, these results strongly argue that RCC is an immunogenic tumor type in which other immunotherapeutic approaches should be explored.
In the last decade, T cell checkpoint blockade (TcCB) has had a major impact on the treatment of melanoma, another immunogenic tumor type. Meanwhile, blockade of CTLA-4 has become standard of care in melanoma, and targeting PD-1/PD-L1 or combining CTLA-4 and PD-1 blockade show even more promising results in phase 1/2 trials (reviewed in [6]). In RCC, targeting PD-1 has demonstrated activity and is currently being studied in comparison with everolimus as second-line treatment and in combination with ipilimumab in comparison with sunitinib in first-line treatment (www.clincialtrials.gov, and [7]).
In addition to TcCB, adoptive cell therapies (ACT) have proven to be a promising tool in the treatment of melanoma. Ex vivo expanded tumor-infiltrating lymphocytes (TIL) have revealed response rates in up to 72 % of selected melanoma patient populations [8]. Like melanoma, RCC is a malignancy with a (relatively) high mutational load [9]. Targeting neoantigens evolving from such mutations by gene-modified T cells might improve the outcome of ACT [10]. In melanoma such neoantigen-specific T cells have already been found [11], which analogously also could be assumed for RCC.
Whether combining tyrosine kinase inhibitors (TKIs), like sunitinib, with immune therapeutic approaches in renal cell carcinoma can be synergistic remains unknown. In animal studies, sunitinib results in increased TIL infiltration, dendritic cell (DC) maturation and decreased infiltration of immune-suppressive cells into the tumor [12–14]. Subsequently, in the MC38 murine colon carcinoma model, the 4T1 breast cancer model and the B16.OVA melanoma model, synergistic effects between sunitinib or sorafenib and poxvirus-based cancer vaccines, or peptide pulsed dendritic cell vaccination, respectively, have been described [15, 16].
However, when testing the effect of sunitinib on human lymphocytes in vitro, sunitinib was shown to impair lymphocyte proliferation and function [17, 18]. Analysis of peripheral blood from mRCC patients treated with sunitinib revealed a decrease in myeloid and myeloid-derived suppressor cells (MDSC) in peripheral blood at week 4 of treatment [19, 20]. If this also occurs within the tumor environment, sunitinib might improve TIL function, and thus serve as an interesting combination partner for immunotherapy.
In this work, we performed a structured analysis of the effect of sunitinib on the proliferative capacity of RCC tumor-infiltrating lymphocytes. We found that sunitinib pretreatment increases tumor leukocyte infiltration, does not significantly alter the amount of tumor T cell infiltration in vivo, but improves in vitro TIL expansion from tumor digests. Sunitinib markedly decreased intratumoral MDSC content and removal of MDSC from digests of sunitinib-naïve RCC tumors improved TIL expansion. PD-1 expression was increased on CD8+ TIL from sunitinib-exposed RCC tissue indicating their previously induced activation. We therefore propose that in RCC patients, sunitinib is a promising combination partner for immunotherapeutic treatments like adoptive cell therapies or T cell checkpoint blockade.
Materials and methods
Patients
Patients with clear cell renal cell carcinoma presenting at the Netherlands Cancer Institute (NKI) between 2010 and 2012 were included in this study. Sunitinib-pretreated tumor material was obtained from participants of the phase two (N06SUN, EudraCT number 2006-006491-38) or phase three (EORTC30073/NCT01099423) trials. The additional analysis of the tumor material unrelated to the primary objective of these studies was approved by the ethical committee of the NKI in line with the Dutch regulation for research on human material (WMO). Selection bias was reduced by including all patients available without any predetermined selection. As a tertiary referral center, our Department of Urology does not receive many patients for nephrectomy only. Only material that was considered by the pathologist not relevant for routine diagnostics was used.
Patient characteristics, including MSKCC risk profile, are summarized in Table 1. Further individual data and TNM stage are shown in supplemental Table 1.
Table 1.
Patient characteristics
| No pTx | Sunitinib | |
|---|---|---|
| Sex (%) | ||
| Male | 10 (59) | 8 (62) |
| Female | 7 (41) | 5 (38) |
| Average age (range) | 60 (40–78) | 60 (43–72) |
| Tumor size in cm (range) | 9 (4–19) | 7 (2.3–19) |
| Metastasized (%) | 9 (53) | 12 (92) |
| Fuhrman grade (%) | ||
| Unknown | 0 (0) | 1 (8) |
| 1 | 4 (24) | 1 (8) |
| 2 | 6 (35) | 3 (23) |
| 3 | 2 (12) | 5 (38) |
| 4 | 5 (29) | 3 (23) |
| WHO status (%) | ||
| 0 | 10 (59) | 5 (38) |
| 1 | 7 (41) | 5 (38) |
| 2 | 0 (0) | 3 (23) |
| MSKCC criteria (%) | ||
| N.a. | 7 (41) | 1 (8) |
| Favorable | 0 (0) | 1 (8) |
| Intermediate | 7 (41) | 7 (54) |
| Unfavorable | 3 (18) | 4 (31) |
Immunohistochemistry and immunofluorescence
CD45 (clone 2B11&PD7/26, Roche), CD3 (clone SP7, Spring Bioscience), CD4 (clone SP35, Cell Marque) and CD8 (clone C8/144B, DAKO) staining was performed according to standard protocols using the BenchMark Ultra autostainer (Ventana Medical Systems) and heat-induced antigen retrieval using Cell Conditioning 1 (CC1, Ventana Medical Systems). CD45 was detected using the Ultraview Universal DAB Detection Kit (Ventana Medical Systems). CD3, CD4 and CD8 were detected using the Optiview Universal DAB Detection Kit (Ventana Medical Systems). Counterstaining was performed using Hematoxylin. CD11b (clone 2Q902, Abcam) was stained manually at a 1:40 concentration after EDTA antigen retrieval. For secondary Ab staining, Brightvision (ImmunoLogic) and DAB immune complex visualization were used, followed by Hematoxylin counterstaining. CD45-, CD3-, CD4- and CD8-positive cells were scored relative to the tumor cells from low (+) to very high (++++) by a pathologist in a blinded manner. Tumor-infiltrating CD11b positive cells were counted in 10 high power fields (HPF).
Fluorescent immunohistochemistry was performed according to standard protocols using a confocal laser scanning microscope (LSM 510, ZEISS) in a multitrack setting. The following primary antibodies were used: anti-CD33 (1:50, clone PWS44, Leica Biosystems), anti-HLA-DR (1:100, clone TAL.1B5, Dako), anti-CD3 (1:100, ab828, Abcam), anti-CD8 (1:200, clone 4B11, Leica) and anti-FOXP3 (1:100, clone 236A/E7, Abcam). Secondary antibodies used were as follows: Alexa 647 goat-anti-mouse-IgG2b (A-21242, Life Technologies), Alexa 546 goat-anti-mouse-IgG1 (A-21123, Life Technologies) and Alexa 488 goat-anti-mouse-IgG1 (A-21121, Life Technologies). Per slide, 3–5 randomly selected images were captured. Negative control slides, omitting the primary antibody, were included. Tumor cell nests and stroma were measured using the ZEISS LSM Image Examiner, and subsets were manually counted and presented as number/mm2.
Flow cytometry
Cells were stained with PD-1-PE (clone eBioJ105, eBioscience), CD8-FITC/PE/PerCpCy5.5 (clone SK1, BD Biosciences), CD4-FITC/PE (clone SK3, BD Biosciences), CD56-PE (clone MEM188, eBioscience), CD56-FITC (clone CMSSB, eBioscience), CD3-FITC/PE/APC (clone SK7, BD Biosciences), CD16-FITC (clone eBioCB16, ebioscience), CD19-FITC (clone HIB19, BD Biosciences), CD11b-PE (clone ICRF44, BD Biosciences), CD33-PerCpCy5.5 (clone WM-53, eBioscience), HLA-DR-APC (clone LN3, eBioscience), CD25-PE (clone M-A251, BD Biosciences), FOXP3-PE (clone PCH101, eBioscience), IFN-γ-APC (clone B27, BD Biosciences), CD107a-PE (clone H4A3, BD Biosciences), CD90-FITC (clone 5E10, BD Biosciences) and G250-PE (clone 303123, R&D Systems) and analyzed using BD FACSCalibur and Cell-Quest or FlowJo software. Cell Sorting was performed using the FACSAria I (BD biosciences).
Digestion of tumor material
Sterile patient tumor material (not required for pathological routine diagnosis) was digested for 3 h at room temperature in 50 ml of TIL medium: RPMI-1640 (52400041, Life Technologies), 10 % heat-inactivated human albumin serum (N0398000, Sanquin), 100 U/ml penicillin–streptomycin (11074440001, Roche), 2 mM l-glutamine (25030-024, Life Technologies), supplemented for digestion with 1 mg/ml collagenase from Clostridium histolyticum type IV (C5138, Sigma-Aldrich], 30 U/ml deoxyribonuclease I from bovine pancreas type IV (D5025 Sigma-Aldrich), and 0.1 mg/ml hyaluronidase from sheep testes type V (H6254, Sigma-Aldrich). The digested tissue was filtered and viable lymphocytes were isolated by Ficoll-Hypaque (17-1440-03, GE Healthcare). Final numbers were determined by microscopic cell count and trypan blue exclusion.
TIL culture
Tumor digests were seeded at a concentration of 1 × 106 live cells/well. The leukocyte content in the digests varied between 14 and 83 % (mean 60 ± 19 %), and the mean was identical for sunitinib-pretreated and sunitinib-naïve specimens. The fold expansions were calculated based on the real baseline leukocyte numbers. The cells were cultured for 25 days (±3 days) in 2 ml of the abovedescribed TIL medium, supplemented with 6000 IU/ml IL-2 (Proleukin 18 × 106 IE Novartis) at 37 °C and 5 % CO2, and split when the density reached 2 × 106 cells/ml.
Tumor cell culture
Spare tumor digest was maintained in flasks with tumor medium (RPMI-1640 supplemented with 10 % heat-inactivated FCS [758093, Greiner Bio-One], 100 U/ml penicillin–streptomycin and 2 mM l-glutamine) for 25 days. Tumor cell cultures were harvested by trypsinization, washed, resuspended and frozen in 10 % DMSO. Fibroblast contamination was excluded by staining for anti-G250 (clone 303123, R&D Systems) and anti-CD90 (clone 5E10, BD Biosciences).
Cytokine and lysis assays
Cytokine production (intracellular IFN-γ) and lytic function (CD107 surface expression) were determined after co-culture with autologous tumor cells (ratio 1:1) in a 96-well plate for 4 h at 37 °C in the presence of an exocytosis inhibitor (555029, BD Biosciences), or unspecific stimulation using the Cytofix/Cytoperm kit following the manufacturer’s guidelines (345763, BD Biosciences).
Statistical analysis
Two-tailed student t test using GraphPad Prism (version 6.01 for Windows, GraphPad Software) was performed for statistical analysis.
Results
Establishment of tumor lines and TIL cultures from ccRCC
Thirty tissue samples were obtained from nephrectomies of clear cell renal cell carcinoma patients. Seventeen patients had received no prior systemic treatment (no pTx) and 13 were pretreated with sunitinib. Most of the specimens were from stage IV RCC [12/13], although 9/17 patients in the no pTx group were from earlier tumor stages (Table 1, supplemental Table 1).
Sunitinib pretreatment did not impair the ability to grow RCC tumor lines, as we managed to grow RCC tumor lines in 11 out of 17 (65 %) sunitinib-naïve specimens and in 11 out of 13 (85 %) from the sunitinib-pretreated tumors. In three out of the eight failing cultures, this was due to early fungal contamination; the other cases failed due to too low tumor cell numbers. Disease stage did not correlate with the ability to grow tumor lines as we managed to grow lines from both metastatic and non-metastatic RCC (supplemental Table 1). We established TIL cultures in 24 out of 30 patient samples. The number of TIL obtained after digestion and 25 days of culture was higher than after fragmentation (3.3- to 119-fold higher, supplemental Figure 1). Thus, we proceeded with tumor digestions only. TIL cultures or tumor lines were successfully established independent of tumor size or disease stage (supplemental Table 1).
Effect of sunitinib pretreatment on TIL infiltration in RCC
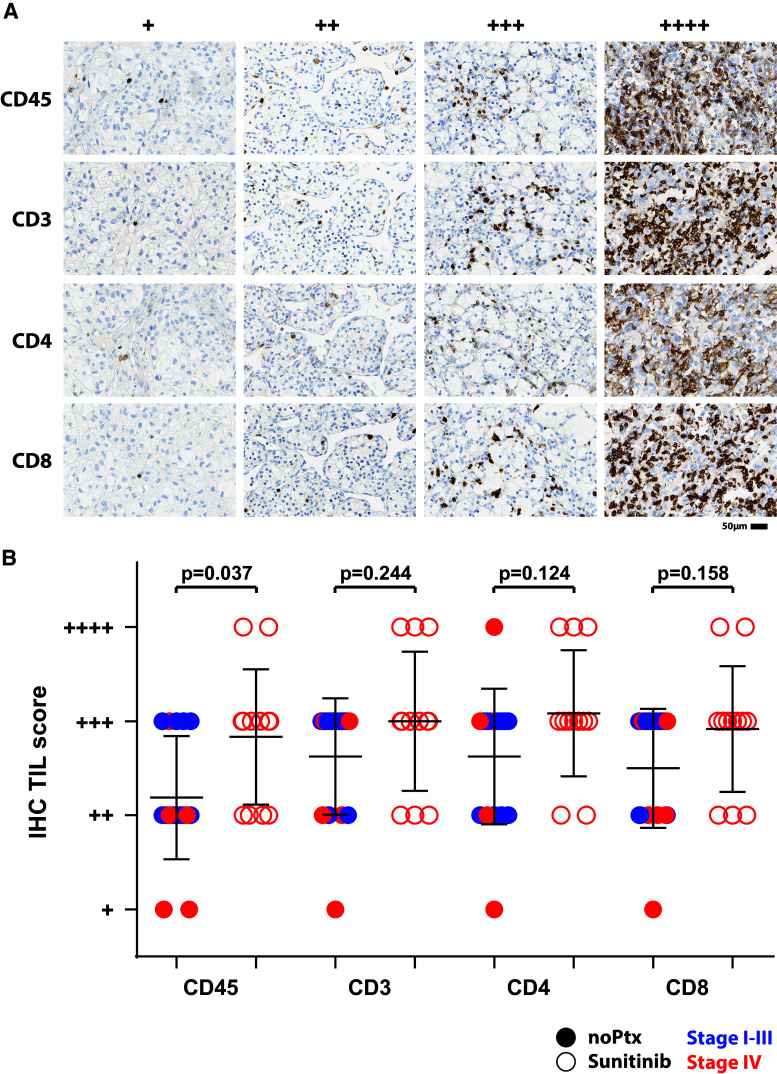
We investigated the effect of sunitinib treatment on TIL infiltration into the primary tumor. We analyzed 16 sunitinib-pretreated and 12 non-pretreated RCC tumors for CD45+, CD3+, CD4+ and CD8+ cell content by immunohistochemistry (IHC) (one tumor block from sunitinib pretreatment and one from naïve RCC was not available for IHC). Samples were scored in a blinded manner by our pathologist and categorized according to low, intermediate, high and very high (+ to ++++) infiltration (Fig. 1a). We found a strong variation between the individual tumors, and no influence by the presence or absence of distant metastases (Fig. 1b, stage I-III blue symbols, stage IV red symbols). Sunitinib pretreatment (open symbols) increased leukocyte content significantly, while modestly improving CD3, CD4 and CD8 TIL infiltration (Fig. 1b). Thus, sunitinib pretreatment increases leukocyte infiltration into the primary tumor, but fails to affect lymphocyte infiltration.
Fig. 1.
Sunitinib pretreatment increases intratumoral leukocytes. Representative IHC images of the scoring categories’ from low (+) to very high (++++) infiltration of CD45+, CD3+, CD4+ and CD8+ cells. Shown are examples from non-pretreatment tumor samples, except for the ++++ staining examples, which were sunitinib samples (a). Sixteen sunitinib-naïve (filled symbols) and twelve sunitinib-pretreated (open symbols) RCC tissues were scored in a blinded fashion for content of CD45+, CD3+, CD4+ and CD8+ cells. Stage I–III and Stage IV RCC are indicated by blue and red symbols, respectively (b)
Sunitinib pretreatment improves the expansion of RCC TIL and alters their phenotypic composition
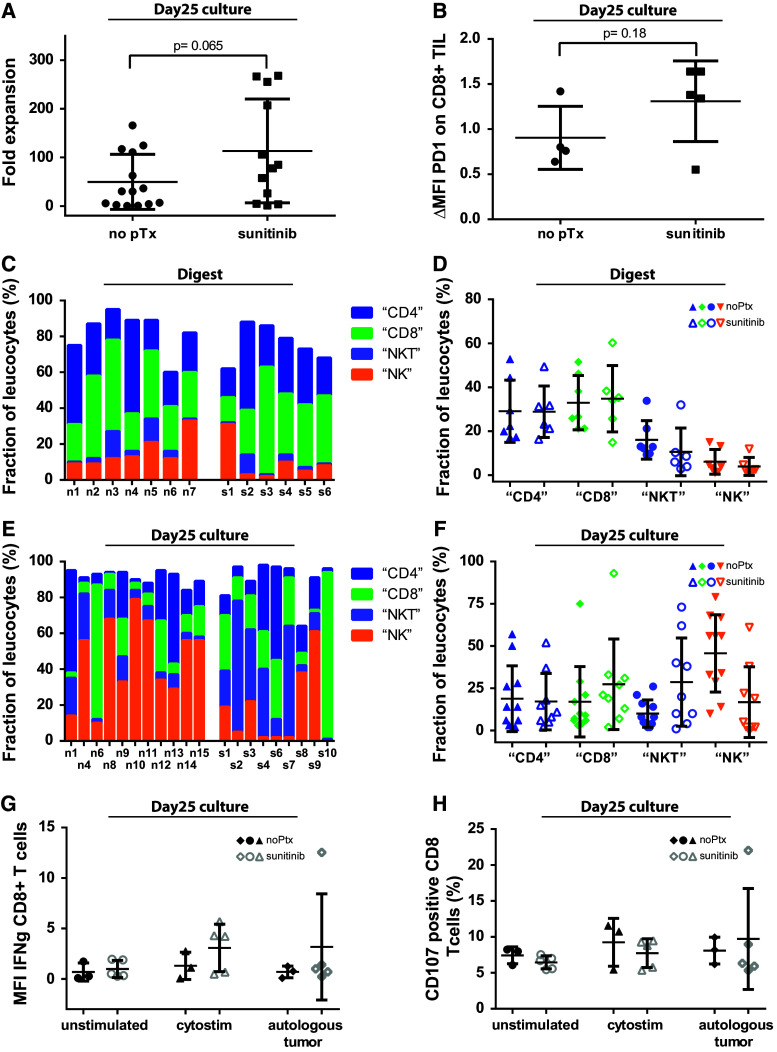
TIL were expanded from 13 out of 17 (76 %) sunitinib-naïve tumors and from 11 out of 13 (85 %) sunitinib-pretreated tumors. After 25 days of TIL culture, we found that TIL expanded more readily from sunitinib-pretreated RCC digests as compared with sunitinib-naïve RCC digests, namely 113-fold ± 107 versus 50-fold ± 57, p = 0.065, which was not statistically significant due to the strong variation within the groups (Fig. 2a).
Fig. 2.
Sunitinib pretreatment improves TIL outgrowth and alters TIL composition. Tumor digests from sunitinib-naïve and sunitinib-pretreated ccRCC tissues were cultured 25 ± 3 days according to our standard TIL protocol. The outgrowth of TILs from 106 tumor digest is represented as fold expansion of baseline TILs (a). PD-1 expression on CD3+CD8+ T cells was analyzed by FACS staining on day 25. Shown here is the ΔMFI (mean fluorescence intensity (MFI) of PD-1 mAb staining divided by MFI of the isotype mAb) (b). CD4 T cell, CD8 T cell, NKT cell and NK cell frequencies were performed in baseline digests from sunitinib-naïve and sunitinib-pretreated RCC specimens and after 25d TIL expansion (c–f). Cytokine production (intracellular IFN-γ) and lytic function (CD107 surface expression) was determined after co-culture with autologous tumor cells (ratio 1:1) (g, h)
Next, we compared PD-1 expression and the cellular composition of the baseline digests with ex vivo expanded TIL cultures for content of “CD4+ T cells” (CD3+/CD8−/CD56−), “CD8+ T cells” (CD3+/CD8+/CD56−), “NK T cells” (CD3+/CD56+) and “NK cells” (CD3−/CD56+) (Fig. 2b–f). We found a tendency toward higher PD-1 expression on expanded CD8+ TIL from sunitinib-pretreated tissue (Fig. 2b), indicating the presence of activated TIL and the induction of an inflammatory environment by sunitinib, as similarly described for melanoma [21]. Blocking PD-L1 by adding functional grade anti-PD-L1 mAb did not alter TIL expansion (data not shown), possibly due to the required presence of IL-2 in the TIL culture medium. IL-2 has been shown to overcome PD-L1/PD-1-mediated inhibitory signals [22].
Concerning the immune cell composition itself, we found no striking differences between sunitinib-pretreated versus non-pretreated tumor infiltrates in baseline digest material (Fig. 2c, d). However, we found a higher content of NK cells in day 25 cultures of sunitinib-naïve samples (p = 0.009), versus more NKT cells in sunitinib pretreatment samples (p = 0.037, Fig. 2e, f).
Next, we analyzed the functionality of the expanded TIL product upon unspecific re-stimulation with cytostim or specific re-stimulation by autologous tumor cells. We found no significant differences, neither in IFN-γ production nor in lytic activity (as determined by CD107/Lamp1 surface expression) (Fig. 2g, h).
Thus, sunitinib pretreatment has a quantitative impact on leukocyte infiltration into the primary tumor, but no impact on the lymphocyte composition or function.
However, ex vivo TIL expansion was improved and subpopulations were impacted upon sunitinib treatment, arguing for the alteration of a non-lymphocyte compartment in the primary tumor.
Sunitinib pretreatment reduces intratumoral MDSC content
Several cell types including regulatory T cells and MDSC contribute to tumor-mediated immune suppression [23]. Considering the absence of the significant TIL-intrinsic differences after sunitinib treatment (Fig. 2g, h), we investigated whether the observed tendency for improved outgrowth of TIL after in vivo sunitinib exposure may result from alterations in such intratumoral inhibitory cells.
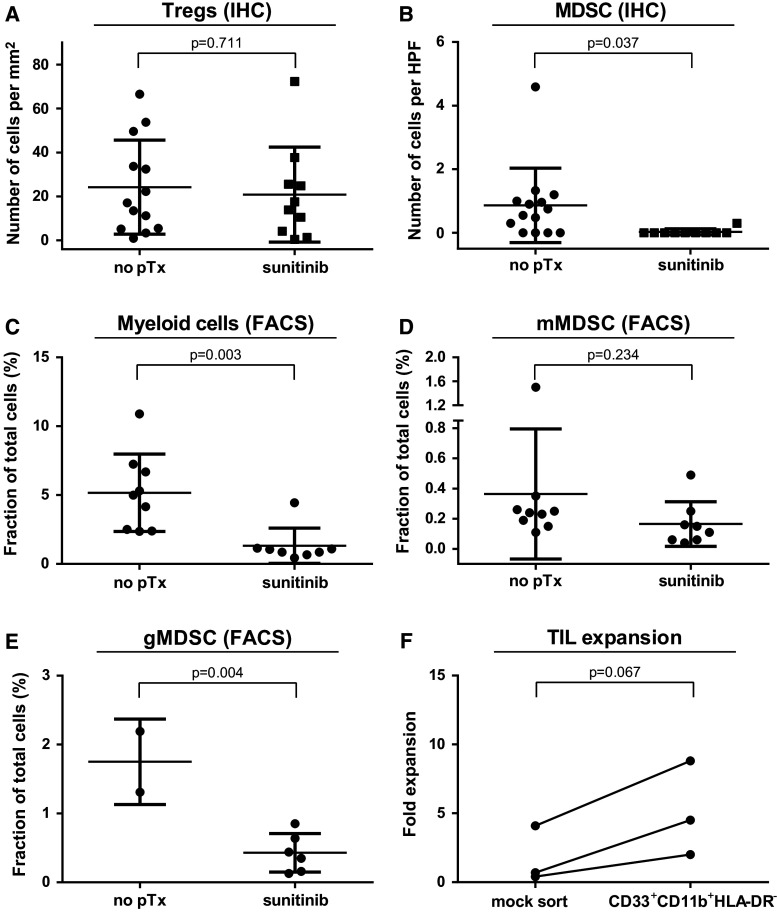
While we found no difference in the number of Tregs (defined by CD3+CD8−FOXP3+), sunitinib pretreatment induced a complete absence of MDSC (defined by CD11b+CD33+HLA-DR−) within the tumor microenvironment in almost all samples as analyzed by immunofluorescence (Fig. 3a, b). This microscopy-based observation could be confirmed by FACS analysis of tumor digests for myeloid cells (Fig. 3c). Additionally, MDSC subtypes were analyzed in samples where sufficient material was still available. The following subsets were analyzed: granulocytic MDSC (gMDSC, CD15+CD33+HLADR−), lymphocyte-linage-negative MDSC (lin-MDSC, CD14−CD15−CD33+HLA-DR−) and monocytic MDSC (mMDSC, CD14+CD33+HLADR−) [24]. We found that the gMDSC fraction likely is the most affected group by sunitinib treatment (Fig. 3d, e). However, due to lack of additional digested material, we were only able to test these subgroups in a minority of patients.
Fig. 3.
Sunitinib pretreatment reduced MDSC tumor content. Infiltration by Treg and MDSC was analyzed by immune fluorescence microscopy of RCC tissue from sunitinib-naïve or sunitinib-pretreated patients (a, b). To confirm IHC counts and look in depth in MDSC subsets, tumor digests from sunitinib-naïve and sunitinib-pretreated patients were analyzed by flow cytometry (c–e). The suppressive capacity of the infiltrating mMDSC was assessed upon TIL outgrowth from tumor digest of sunitinib-naïve RCC samples after prior removal, or not, of the mMDSC cell population by flow sorting (f)
The functional relevance of MDSCs was proven by removing the CD33+CD11b+HLA-DR− population from sunitinib-naïve RCC digests. This resulted in improved TIL expansion (Fig. 3f). Of note, the FACS-sorting itself strongly impaired the TIL expansion as shown by the reduced fold expansion after mock sorting (Fig. 3f as compared to Fig. 2a), indicating that FACS-sorting of pre-TIL cultures is seemingly too detrimental as an approach for MDSC removal. Thus, the observed differences during TIL culture can be attributed (at least in part) to the sunitinib-mediated reduction in MDSC within the tumor environment.
Discussion
Targeted therapies, like sunitinib, have become standard first-line treatment for RCC, but cure has not been realized and a plateau has been reached in terms of benefit and outcome [25]. By contrast, novel immunotherapeutic approaches hold the promise of cure in immunogenic tumor types. In melanoma, cell-based immunotherapy can induce high response rates and durable responses [8]. Targeting coinhibitory molecules on T cells is particularly promising. Blockade of PD-1 has been shown to induce long-lasting responses not only in melanoma but also in RCC, non-small cell lung cancer and even transitional cell carcinoma [7, 26].
This raises the question of whether the combination of sunitinib with immunotherapy can act synergistically to improve the long-term outcome of RCC patients. Sunitinib reduces the Treg and MDSC content in tumor animal models, while in a xenograft RCC model, sunitinib increases the MDSC content [13, 14, 27]. Studies on peripheral blood of TKI-treated patients revealed reduced MDSC and Treg numbers after sunitinib but not sorafenib [19, 20, 28, 29]. In addition, synergistic effects between tyrosine kinase inhibitors (TKIs) and vaccination (tumor control, and depth of MDSC tumor infiltration) have been shown in different (non-RCC) murine cancer models [15, 16, 30, 31].
In the only analysis published so far from RCC patients treated with sunitinib in a neoadjuvant setting, only three out of the eight patients had lower levels of intratumoral MDSC (0.7, 2 and 0.9 % as defined by CD33+HLADR−) as compared to untreated tumors (5.9 ± 1.1 %) [24]. Here, we structurally analyzed the effect of sunitinib on RCC intratumor TIL content, TIL phenotypic changes, TIL expansion and alteration of inhibitory cells.
Nevertheless, our work inherits still some relevant limitations. Due to the large amount of tissue required in the standard TIL manufacturing protocol, our analyses were restricted to inter-patient comparison. Another limitation that challenges our observations was the clinical differences in our cohorts. There were less metastasized patients in the non-pretreatment group, whereas all sunitinib-pretreated patients classified as stage IV RCC. However, we found no differences in baseline TIL infiltration into the primary tumor within the untreated group comparing stage I-III versus stage IV RCC. Thus, it seems that RCC stage does not influence the TIL infiltration into the primary tumor. Sunitinib pretreatment did not impair the ability to grow TIL or tumor lines, and had no impact on the TIL subtypes found in fresh tumor digests at baseline.
Sunitinib pretreatment did increase tumor leukocyte infiltration, and, although not significantly, also the lymphocyte infiltration. During TIL culture, sunitinib pretreatment resulted in improved yield. The NKT subset showed a stronger expansion rate, while in sunitinib-naïve tumors, NK cell expansion was dominant. PD-1 expression was increased in CD8+ TIL from sunitinib-exposed RCC indicating the induction of an immune inflammatory tumor environment by sunitinib [21]. Our data suggest that sunitinib pretreatment could be beneficial for ACT as it allows a stronger initial TIL expansion. This could hypothetically be accompanied by a broader T cell repertoire within the TIL culture, which is known to be a prerequisite for clinical response [32, 33].
In contrast to a human RCC xenograft model, but in line with the small study on human RCC receiving neoadjuvant sunitinib, we found sunitinib to decrease the intratumoral MDSC content [20, 27]. More strikingly, we observed that sunitinib alters preferentially the gMDSC subset in line with data from a mouse tumor model [13]. Removal of the CD11+CD33+HLA-DR− population from untreated RCC digests improved the TIL outgrowth, indicating that sunitinib-mediated MDSC alteration could be the major factor for the observed improved TIL outgrowth in sunitinib-pretreated specimens. Indeed, several animal models have also led to the hypothesis that inhibiting MDSC by TKIs might be an effective combination partner for immunotherapies [14, 16, 23, 34].
In addition, it has been shown that sunitinib can not only reduce the amount of MDSC [20] but also alter the IL-10, CTLA-4, PD-1, FOXP3 and PD-L1 levels in the tumor microenvironment [14]. We have previously identified PD-L1 as a major immunosuppressive molecule in RCC [35], and Thompson et al. [36] have identified PD-L1 as an independent prognostic factor in RCC. Recently, it has been shown that under hypoxic conditions, PD-L1 is upregulated on MDSC in a HIF-1α-dependent manner [37]. These data in combination with our observations give a rationale for combining sunitinib (known to induce hypoxia within the tumor) with PD-1/PD-L1 blockade and possibly adoptive cell transfer therapy, either synchronously or sequentially.
Current trials combining sunitinib, axitinib or pazopanib with PD-1 blockade are recruiting patients. Provided that these trials report positively, addition of cell-based therapies generated from TKI-pretreated tumor material might be an approach to further improving RCC treatment.
Major challenges to our work remain the nature of this non-randomized inter-patient analysis, the small patient numbers and the different patient cohorts. Therefore, further analyses are needed to confirm our hypothesis-generating observations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was funded by an in house grant (start-up funding) of the Netherlands Cancer Institute.
Conflict of interest
The authors declare that they have the following conflicts of interest in context of this manuscript: Axel Bex and John Haanen are principal investigators of the SURTIME study that is financed by an educational grant from Pfizer to the European Organisation EORTC. Axel Bex and John Haanen received compensation for advisory roles from Pfizer. All other authors declare that they have no conflict of interest.
Abbreviations
- ACT
Adoptive cell therapy
- CD
Cluster of differentiation
- CTLA-4
Cytotoxic T-lymphocyte-associated protein 4
- DCs
Dendritic cells
- IFN
Interferon
- IHC
Immunohistochemistry
- IL
Interleukin
- FACS
Fluorescence-activated cell sorting
- HPF
High power field
- MDSCs
Myeloid-derived suppressor cells
- MFI
Mean fluorescent intensity
- mRCC
Metastasized renal cell carcinoma
- mTOR
Mammalian target of rapamycin
- NK
Natural killer
- NKT
Natural killer T
- NKI
The Netherlands Cancer Institute
- PD-1
Programed death receptor-1
- PD-L1
Programed death receptor ligand-1
- pTx
Pre-treatment
- RCC
Renal cell carcinoma
- TcCB
T cell checkpoint blockade
- TILs
Tumor-infiltrating lymphocytes
- TKIs
Tyrosine kinase inhibitors
- Treg
Regulatory T cells
- VEGF-R
Vascular endothelial growth factor receptor
References
- 1.Golimbu M, Joshi P, Sperber A, Tessler A, Al-Askari S, Morales P. Renal cell carcinoma: survival and prognostic factors. Urology. 1986;27:291–301. doi: 10.1016/0090-4295(86)90300-6. [DOI] [PubMed] [Google Scholar]
- 2.Coppin C, Le L, Porzsolt F, Wilt T (2008) Targeted therapy for advanced renal cell carcinoma. Cochrane Database Syst Rev CD006017. doi:10.1002/14651858.CD006017.pub2 [DOI] [PMC free article] [PubMed]
- 3.Albiges L, Choueiri T, Escudier B, et al. A systematic review of sequencing and combinations of systemic therapy in metastatic renal cancer. Eur Urol. 2014;67:100–110. doi: 10.1016/j.eururo.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Klapper JA, Downey SG, Smith FO, et al. High-dose interleukin-2 for the treatment of metastatic renal cell carcinoma: a retrospective analysis of response and survival in patients treated in the surgery branch at the National Cancer Institute between 1986 and 2006. Cancer. 2008;113:293–301. doi: 10.1002/cncr.23552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Mulder PH, Oosterhof G, Bouffioux C, van Oosterom AT, Vermeylen K, Sylvester R. EORTC (30885) randomised phase III study with recombinant interferon alpha and recombinant interferon alpha and gamma in patients with advanced renal cell carcinoma. The EORTC Genitourinary Group. Br J Cancer. 1995;71:371–375. doi: 10.1038/bjc.1995.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blank CU. The perspective of immunotherapy: new molecules and new mechanisms of action in immune modulation. Curr Opin Oncol. 2014;26:204–214. doi: 10.1097/CCO.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 7.Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J Clin Oncol. 2014 doi: 10.1200/JCO.2014.59.0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009;21:233–240. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexandrov LB, Nik-Zainal S, Wedge DC, Campbell PJ, Stratton MR. Deciphering signatures of mutational processes operative in human cancer. Cell Rep. 2013;3:246–259. doi: 10.1016/j.celrep.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heemskerk B, Kvistborg P, Schumacher TN. The cancer antigenome. EMBO J. 2013;32:194–203. doi: 10.1038/emboj.2012.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Rooij N, van Buuren MM, Philips D, et al. Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. J Clin Oncol. 2013;31:e439–e442. doi: 10.1200/JCO.2012.47.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shrimali RK, Yu Z, Theoret MR, Chinnasamy D, Restifo NP, Rosenberg SA. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. 2010;70:6171–6180. doi: 10.1158/0008-5472.CAN-10-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ko JS, Rayman P, Ireland J, Swaidani S, Li G, Bunting KD, Rini B, Finke JH, Cohen PA. Direct and differential suppression of myeloid-derived suppressor cell subsets by sunitinib is compartmentally constrained. Cancer Res. 2010;70:3526–3536. doi: 10.1158/0008-5472.CAN-09-3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ozao-Choy J, Ma G, Kao J, et al. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res. 2009;69:2514–2522. doi: 10.1158/0008-5472.CAN-08-4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farsaci B, Higgins JP, Hodge JW. Consequence of dose scheduling of sunitinib on host immune response elements and vaccine combination therapy. Int J Cancer. 2012;130:1948–1959. doi: 10.1002/ijc.26219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bose A, Lowe DB, Rao A, Storkus WJ. Combined vaccine + axitinib therapy yields superior antitumor efficacy in a murine melanoma model. Melanoma Res. 2012;22:236–243. doi: 10.1097/CMR.0b013e3283538293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu Y, Zhao W, Meng F, Qu B, Zhu X, Sun Y, Shu Y, Xu Q. Sunitinib impairs the proliferation and function of human peripheral T cell and prevents T-cell-mediated immune response in mice. Clin Immunol. 2010;135:55–62. doi: 10.1016/j.clim.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 18.Alfaro C, Suarez N, Gonzalez A, et al. Influence of bevacizumab, sunitinib and sorafenib as single agents or in combination on the inhibitory effects of VEGF on human dendritic cell differentiation from monocytes. Br J Cancer. 2009;100:1111–1119. doi: 10.1038/sj.bjc.6604965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Cruijsen H, van der Veldt AA, Vroling L, et al. Sunitinib-induced myeloid lineage redistribution in renal cell cancer patients: CD1c+ dendritic cell frequency predicts progression-free survival. Clin Cancer Res. 2008;14:5884–5892. doi: 10.1158/1078-0432.CCR-08-0656. [DOI] [PubMed] [Google Scholar]
- 20.Ko JS, Zea AH, Rini BI, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15:2148–2157. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 21.Taube JM, Anders RA, Young GD et al (2012) Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 4:127ra37. doi:10.1126/scitranslmed.3003689 [DOI] [PMC free article] [PubMed]
- 22.Carter L, Fouser LA, Jussif J, et al. PD-1:PD-L inhibitory pathway affects both CD4(+) and CD8(+) T cells and is overcome by IL-2. Eur J Immunol. 2002;32:634–643. doi: 10.1002/1521-4141(200203)32:3<634::AID-IMMU634>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 23.Najjar YG, Finke JH. Clinical perspectives on targeting of myeloid derived suppressor cells in the treatment of cancer. Front Oncol. 2013;3:49. doi: 10.3389/fonc.2013.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finke J, Ko J, Rini B, Rayman P, Ireland J, Cohen P. MDSC as a mechanism of tumor escape from sunitinib mediated anti-angiogenic therapy. Int Immunopharmacol. 2011;11:856–861. doi: 10.1016/j.intimp.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584–3590. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panka DJ, Liu Q, Geissler AK, Mier JW. Effects of HDM2 antagonism on sunitinib resistance, p53 activation, SDF-1 induction, and tumor infiltration by CD11b+/Gr-1+ myeloid derived suppressor cells. Mol Cancer. 2013;12:17. doi: 10.1186/1476-4598-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Finke JH, Rini B, Ireland J, et al. Sunitinib reverses type-1 immune suppression and decreases T-regulatory cells in renal cell carcinoma patients. Clin Cancer Res. 2008;14:6674–6682. doi: 10.1158/1078-0432.CCR-07-5212. [DOI] [PubMed] [Google Scholar]
- 29.Florcken A, Takvorian A, Van Lessen A, Singh A, Hopfenmuller W, Dorken B, Pezzutto A, Westermann J. Sorafenib, but not sunitinib, induces regulatory T cells in the peripheral blood of patients with metastatic renal cell carcinoma. Anticancer Drugs. 2012;23:298–302. doi: 10.1097/CAD.0b013e32834ee2b1. [DOI] [PubMed] [Google Scholar]
- 30.Bose A, Taylor JL, Alber S, et al. Sunitinib facilitates the activation and recruitment of therapeutic anti-tumor immunity in concert with specific vaccination. Int J Cancer. 2011;129:2158–2170. doi: 10.1002/ijc.25863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farsaci B, Donahue RN, Coplin MA, Grenga I, Lepone LM, Molinolo AA, Hodge JW. Immune consequences of decreasing tumor vasculature with antiangiogenic tyrosine kinase inhibitors in combination with therapeutic vaccines. Cancer Immunol Res. 2014;2:1090–1102. doi: 10.1158/2326-6066.CIR-14-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Linnemann C, Mezzadra R, Schumacher TN. TCR repertoires of intratumoral T-cell subsets. Immunol Rev. 2014;257:72–82. doi: 10.1111/imr.12140. [DOI] [PubMed] [Google Scholar]
- 33.Kvistborg P, Shu CJ, Heemskerk B, et al. TIL therapy broadens the tumor-reactive CD8(+) T cell compartment in melanoma patients. Oncoimmunology. 2012;1:409–418. doi: 10.4161/onci.18851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kujawski M, Kortylewski M, Lee H, Herrmann A, Kay H, Yu H. Stat3 mediates myeloid cell-dependent tumor angiogenesis in mice. J Clin Invest. 2008;118:3367–3377. doi: 10.1172/JCI35213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blank C, Kuball J, Voelkl S, et al. Blockade of PD-L1 (B7-H1) augments human tumor-specific T cell responses in vitro. Int J Cancer. 2006;119:317–327. doi: 10.1002/ijc.21775. [DOI] [PubMed] [Google Scholar]
- 36.Thompson RH, Kuntz SM, Leibovich BC, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66:3381–3385. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 37.Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, Bronte V, Chouaib S. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211:781–790. doi: 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.