Abstract
At present, there are no vaccines approved for the prevention or treatment of malignant melanoma, despite the amount of time and resources that has been invested. In this study, we aimed to develop a self-contained vaccine capable of directly stimulating anticancer CD8+ T-cell immune responses. To achieve this, three whole-cell melanoma vaccines were developed expressing 4-1BBL or B7.1 T-cell co-stimulatory molecules individually or in combination. The ability of engineered vaccine cell lines to stimulate potent anticancer immune responses in C57BL/6 mice was assessed. Mice vaccinated with cells overexpressing both 4-1BBL and B7.1 (B16-F10-4-1BBL-B7.1-IFNγ/β anticancer vaccine) displayed the greatest increases in CD8+ T-cell populations (1.9-fold increase versus control within spleens), which were efficiently activated following antigenic stimulation, resulting in a 10.7-fold increase in cancer cell cytotoxicity relative to control. The enhanced immune responses in B16-F10-4-1BBL-B7.1-IFNγ/β-vaccinated mice translated into highly efficient rejection of live tumour burdens and conferred long-term protection against repeated tumour challenges, which were likely due to enhanced effector memory T-cell populations. Similar results were observed when dendritic cell (DC)-deficient LTα−/− mice were treated with the B16-F10-4-1BBL-B7.1-IFNγ/β anticancer vaccine, suggesting that the vaccine can directly stimulate CD8+ T-cell responses in the context of severely reduced DCs. This study shows that the B16-F10-4-1BBL-B7.1-IFNγ/β anticancer vaccine acted as a highly effective antigen-presenting cell and is likely to be able to directly stimulate CD8+ T-cells, without requiring co-stimulatory signals from either CD4+ T-cells or DCs, and warrants translation of this technology into the clinical setting.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-015-1695-3) contains supplementary material, which is available to authorized users.
Keywords: Vaccine, Melanoma, Immune response, Cytotoxic T-cells, Effector memory CD8+ T-cells
Introduction
Cancer remains to be one of the leading causes of morbidity and mortality worldwide with malignant melanoma being one type that significantly contributes to these statistics [1, 2]. It is partly due to this sizeable global burden that research into cancer is so prolific [1, 3]. A plethora of research investigating the pathogenesis, molecular mechanisms and potential treatment options of malignant melanoma has been undertaken [4, 5]. However, despite the amount of research that has been conducted, there is still no vaccine that has been approved for use in preventing or treating malignant melanoma.
The development of potent cancer vaccines is an active area of research, and numerous methods of generating effective vaccines to target specific cancers have been established. One such methodology relies on using the patient’s own cells, either melanoma cells or dendritic cells (DCs), to generate customised vaccines in order to boost their immune responses against their own cancer cells [6–9]. However, a concern with personalised therapies, including treatments based on autologous cancer cell or DC vaccines, is the highly time-consuming nature of the process and the substantial cost of manufacture. These two characteristics represent significant impediments for the timely and cost-effective production of personalised cancer vaccines and may explain the disinterest of pharmaceutical companies to proactively engage in the development of such therapeutics [10–12]. A closely related technology that might be more commercially viable is the use of allogeneic whole-cell-based vaccines. These vaccines are increasing in popularity, and recent research has provided useful information about the relationship between cancer and patients’ immune responses [5, 13].
The most successful whole-cell vaccine to date has been Canvaxin™, which was comprised of 3 irradiated, allogeneic melanoma cell lines that between them expressed over 20 different melanoma antigens [14]. The vaccine reached Phase III clinical testing; however, the trial was halted after the Data Safety Monitoring Board determined that Canvaxin™-treated patients were unlikely to show any survival benefit [11]. This trial did provide a useful methodology for vaccine development, which has been utilised in studies since [15, 16]. A separate study by Dezfouli et al. [13] identified that engineering melanoma cells to express B7.1 on the cell surface and subsequently treating with a combination of IFNγ/β not only enhanced B7.1 expression, but also induced specific cytotoxic T lymphocyte (CTL) responses to melanoma cells. Furthermore, this study concluded that the addition of B7.1 to the melanoma vaccine mediated the observed CD8+ T-cell responses independently of CD4+ helper T-cells [13] and provided inspiration for our study.
The purpose of our study was to determine whether the addition of a third co-stimulatory molecule to a whole-cell anticancer vaccine could replace or reduce the requirement for DCs to aid in the stimulation and activation of anticancer CD8+ T-cell responses. We identified CD137L (4-1BBL) as a promising candidate, as the ligand is expressed on antigen-presenting cells (APCs), including DCs, and is known to preferentially regulate CD8+ T-cells over CD4+ T-cells by activating and expanding antigen-specific CD8+ T-cells [17]. Furthermore, we wanted to determine whether co-expression of B7.1 and 4-1BBL on an anticancer vaccine could enhance its immune-stimulating capacity over vaccines expressing either 4-1BBL or B7.1 alone. To answer these questions, three vaccines were developed and tested for their ability to stimulate immune responses in syngeneic, wild-type and DC-deficient mouse models.
Materials and methods
Plasmids
Murine 4-1BBL cDNA was PCR-amplified from pcDNA3-4-1BBL [18] using the primers: 5′-CTCGAATTCGGTAATGGACCAGCACA-3′ (forward) and 5′-CCTCTAGATCATTCCCATGGGTTGTC-3′ (reverse), cloned into p-GEMT Easy Vector as an intermediate and then ligated into the XbaI and EcoRI sites of pEFIRES-Puro. Murine B7.1 cDNA was excised from pSRI-neo-mB7.1 and ligated into the KpnI and ClaI sites of pEF-MC1neoN9 containing the more powerful elongation factor-1 alpha promoter.
Cell lines, gene transfer and development of stably expressing sublines
B16-F10 cells and murine lymphocytes were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco) supplemented with 10 % heat-inactivated calf serum (6 % foetal, 4 % new born), 50 U/mL penicillin, 50 µg/mL streptomycin and 25 mM HEPES buffer. B16-F10 cells were transfected with pEFIRES-Puro/4-1BBL vector using Fugene HD transfection reagent. A second sample of B16-F10 cells was transfected with pEF-MC1neoN9/B7.1 vector using Lipofectamine LTX with Plus reagent. Stably transfected cell lines were selected and maintained with 3 µg/mL puromycin or 2 mg/mL G418. To generate cells stably overexpressing both 4-1BBL and B7.1, B16-F10-4-1BBL cells were transfected with pEF-MC1neoN9/B7.1 vector as described above. Stable, highly expressing sublines were selected via repeated rounds of fluorescence-activated cell sorting.
Vaccine preparation
Cells were grown in 150 cm2 culture dishes for 3 days, supplemented with 1000 IU/mL of IFNγ for 44 h and 1000 IU/mL of IFNβ for 20 h to stimulate MHC class I expression. Cells were harvested using 0.02 % EDTA/PBS, thoroughly washed with PBS and then irradiated on ice with 200 grey using a gamma source irradiator (Premion Cancer Care, Southport, QLD, Australia). The three resulting vaccine cell line preparations will be referred throughout as B16-F10-4-1BBL-IFNγ/β, B16-F10-B7.1-IFNγ/β and B16-F10-4-1BBL-B7.1-IFNγ/β.
In vivo vaccination studies
Male C57BL/6 and C57BL/6 LTα−/− mice (age 5–8 weeks) were utilised for experiments. C57BL/6 mice were purchased from the Animal Resources Centre (WA, Australia) and were housed in a specific pathogen-free environment within the Griffith University Gold Coast Campus Animal Facility. C57BL/6 LTα−/− mice were bred and housed within the same facility, and all procedures on both strains were approved by the Griffith University Animal Ethics Committee. Mice, irrespective of genotype, were vaccinated with 1 × 107 cells/200 µL PBS as an intraperitoneal injection or remained unvaccinated as controls. The vaccine schedule involved injecting a priming dose of vaccine on day 0, a boosting dose on day 7, and 2 additional booster doses on days 34 and 38. Tumour challenge studies involved injecting 5 × 105 B16-F10-B7.1 cells/150 µL PBS subcutaneously into the rear left flank of mice 4 days following the last vaccine dose. Tumour growth was monitored every 2–3 days, and mice were humanely euthanased when any dimension of the tumour reached 20 mm in length. Mice surviving the initial tumour challenge were boosted with an additional 2 doses of vaccine 4 days apart, re-challenged 4 days later and then monitored for tumour development. Memory CD8+ T-cell populations were assessed by harvesting mice 6–8 days post-re-challenge and examining T-cell profiles.
In vitro lymphocyte assays
Peripheral blood, spleens and lymph nodes were harvested 4 days after mice received their final vaccination. Peripheral blood was collected and mixed 1:1 with anticoagulant solution (45 mM sodium citrate, 44 mM citric acid and 82 mM glucose, pH 7.0). Lymphocytes were isolated from blood using Ficoll-Paque PLUS (GE Healthcare). Spleens and lymph nodes were disrupted mechanically and passed through a 70-µm filter. A hemolysis buffer containing a 9:1 ratio of 0.16 M NH4Cl and 0.01 M Tris–HCl was added to the spleen cells and incubated for 10 min at room temperature. Samples were divided and assessed for changes in T-cell populations via flow cytometry, or grown in mixed lymphocyte cultures (MLCs). For MLCs, spleen cells from vaccinated and unvaccinated mice were grown on a monolayer of antigenic stimulator cells to promote CD8+ T-cell activation [13, 19]. After 5 days in MLC, T-cell populations were characterised via flow cytometry and also assessed for their cytotoxic activity in CTL assays. CTL assays were performed as per Stannard et al. [19]. Briefly, lymphocytes (effectors) were carefully washed off stimulator cells and separated from debris using Ficoll-Paque PLUS. Target cells were seeded in black-sided 96-well tissue culture plates (Corning), at a density of 5 × 103 cells/well and treated with the same IFNγ/β schedule as previously described. Target cells were cultured for 3 days, expanding in number to approximately 2 × 104 cells/well. Effector cells were seeded onto target cells at a range of ratios from 0.3:1 to 10:1 (effectors to targets), and the dead cell detecting dye, SYTOX Green, was immediately added at a final concentration of 1 µM. Culture well fluorescence intensity was measured 4 h later in a spectrophotometer with excitation at 485 nm and emission at 525 nm. The proportion of cell death for each effector-to-target ratio was calculated by comparing sample well fluorescence to that of 100 % cell death controls. The number of effector cells (×104) required to produce 30 % target cell lysis and the relative fold increase in lytic units/effector cell population of vaccinated mice compared to unvaccinated mice were calculated.
Flow cytometry
Flow cytometric analyses were performed using a BD LSRFortessa cell analyser. The cell surface expression of B7.1, 4-1BBL and MHC class I molecules in stably transfected cells lines were assessed using CD80, 4-1BBL and H-2Kb antibodies, respectively. Staining was conducted according to manufactures’ instructions using a staining medium comprising 2 % FCS in PBS. Lymphocytes from all animal samples were incubated with an Fc receptor blocker (Innovex Biosciences) for 10 min, stained using a cocktail of CD3, CD4 and CD8 fluorescently conjugated antibodies and analysed via flow cytometry. Gating of lymphocytes was performed before individual T-cell subsets were calculated as a percentage of total lymphocytes. T-cells from MLCs were also analysed in this fashion and additionally for the activation markers CD107a and IFNγ. Briefly, samples from MLCs were collected and stained with CD107a for 1 h at 37 °C and then subsequently incubated for a further 4 h at 37 °C with GolgiStop (BD Biosciences). Samples were then stained with T-cell markers, fixed in 2 % paraformaldehyde at room temperature for 20 min, permeabilised with ice-cold 100 % methanol for 30 min and stained with IFNγ on ice for 30 min before analysis via flow cytometry. Memory CD8+ T-cells were detected using the suite of T-cell markers in addition to CCR7 and CD62L. All data were analysed using FACS Diva software.
Statistics
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) program version 17.0. GraphPad Prism v4.0 was used to graph results, displayed as mean ± standard error of the mean (SEM). ANOVA with LSD or Games-Howell (to adjust for unequal group variances) post hoc tests were used to compare means between treatment groups. General linear models with LSD post hoc tests were performed on CTL assay results, and Kaplan–Meier survival analyses were used to assess survival of vaccinated mice. A significance level of 0.05 was used for all analyses.
Results
The B16-F10-4-1BBL-B7.1-IFNγ/β vaccine generates enhanced cell-mediated immune responses in C57BL/6 mice
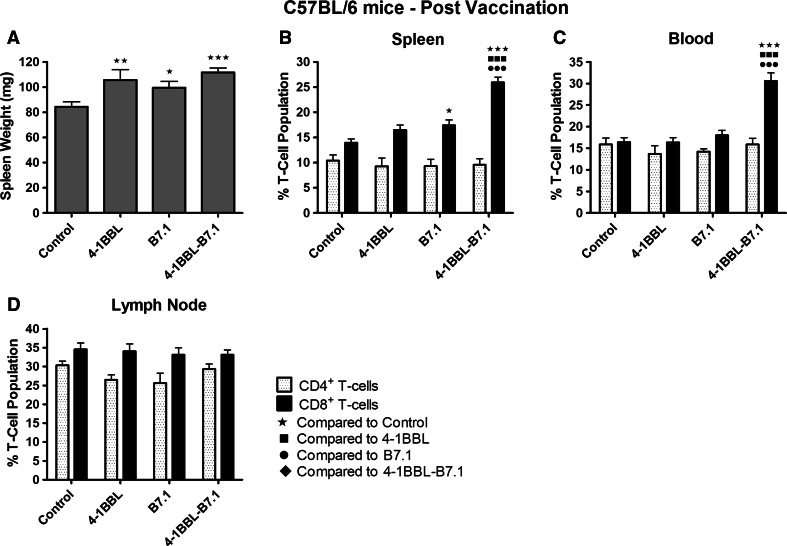
Expression of 4-1BBL and/or B7.1 on vaccine cells was increased by a minimum of 450- and/or 24-fold, respectively, compared to untransfected parental cells (p values <0.001; Supplementary Table 1). Treatment with IFNγ/β mediated an average 22-fold increase in MHC class I expression across cell lines (range 18- to 24-fold). Mice treated with any vaccine presented with significantly heavier spleens compared to unvaccinated mice (maximum p value of 0.027; Fig. 1a). B16-F10-4-1BBL-B7.1-IFNγ/β-vaccinated mice displayed the greatest increase in spleen weight being, on average, 30 % heavier than control spleens (p < 0.001).
Fig. 1.
B16-F10-4-1BBL-B7.1-IFNγ/β-vaccinated C57BL/6 mice produce highly elevated % CD8+ T-cells in the spleen and peripheral blood. C57BL/6 mice remained untreated as controls (n = 12) or were injected with either B16-F10-4-1BBL-IFNγ/β (n = 9), B16-F10-B7.1-IFNγ/β (n = 10) or B16-F10-4-1BBL-B7.1-IFNγ/β (n = 11) vaccine cells. Four days after the mice received their final vaccine dose, the spleens were harvested and weighed (a), and then, lymphocytes from spleens (b), peripheral blood (c) and lymph nodes (d) were assessed for changes in CD4+ and CD8+ T-cell populations. Data are representative of two independent experiments, and the mean ± SEM is shown. Significance levels are denoted by: ★p < 0.05; ★★p < 0.01; ★★★ p < 0.005
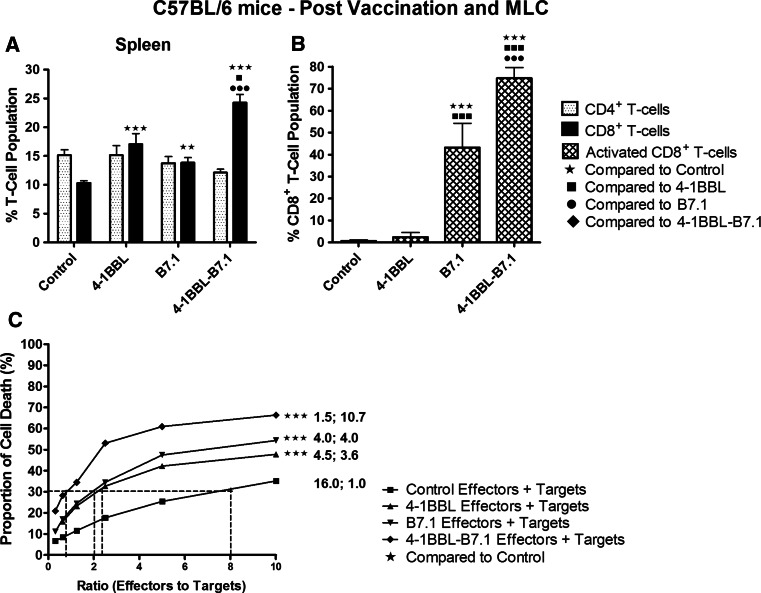
B16-F10-4-1BBL-B7.1-IFNγ/β-vaccinated mice were associated with significantly elevated CD8+ T-cell populations in spleens and peripheral blood compared to all other vaccinated and unvaccinated mice (p < 0.001; Fig. 1b, c). Treatment of mice with any vaccine did not alter CD4+ T-cell populations in spleen, blood or lymph nodes nor did any vaccine alter CD8+ T-cell populations within lymph nodes (Fig. 1b–d). In MLCs, lymphocytes derived from all vaccinated mice maintained significantly higher proportions of CD8+ T-cells compared to unvaccinated control mice (Fig. 2a). B16-F10-4-1BBL-B7.1-IFNγ/β-vaccinated mice retained significantly higher CD8+ T-cell populations compared to B16-F10-4-1BBL-IFNγ/β (p = 0.034)- and B16-F10-B7.1-IFNγ/β (p < 0.001)-vaccinated mice (Fig. 2a).
Fig. 2.
Spleen cells from B16-F10-4-1BBL-B7.1-IFNγ/β-vaccinated C75BL/6 mice maintained significantly increased % CD8+ T-cells with the largest % activated after culture in MLC and were most effective at killing target melanoma cells in CTL assays. C57BL/6 mice were used as controls (n = 12) or received either B16-F10-4-1BBL-IFNγ/β (n = 9), B16-F10-B7.1-IFNγ/β (n = 10) or B16-F10-4-1BBL-B7.1-IFNγ/β (n = 11) vaccines. Splenic-derived lymphocytes were grown in MLCs for 5 days, and T-cell profiles were assessed including CD4+ and CD8+ T-cell populations (a) and activated CD8+ T-cell populations (b). Lymphocytes were also assessed for their ability to kill target melanoma cells in CTL assays (c). For (c), the numbers on the right of the graph indicate the quantity of effector cells (×104) required to produce 30 % target cell lysis and the fold increase in lytic units per effector cell population relative to the CTL response from unvaccinated mice. Data were collected from four independent experiments. The mean ± SEM are plotted for each part, and significance levels are denoted by: ★p < 0.05; ★★p < 0.01; ★★★p < 0.005
The % activated CD8+ T-cells were highest in cells derived from B16-F10-4-1BBL-B7.1-IFNγ/β-vaccinated mice (Fig. 2b), increased 107-fold (p < 0.001) compared to unvaccinated mice, and 30-fold (p < 0.001) and 1.7-fold (p = 0.004) relative to B16-F10-4-1BB-IFNγ/β and B16-F10-B7.1-IFNγ/β-vaccinated mice, respectively. CTL assays showed that the proportion of live IFNγ/β-treated effector cells killed by lymphocytes derived from the B16-F10-4-1BBL-B7.1-IFNγ/β-vaccinated mice was significantly increased compared to lymphocytes derived from all other vaccines (p < 0.001; Fig. 2c). Cells derived from B16-F10-4-1BBL-B7.1-IFNγ/β-vaccinated mice displayed the greatest increase in cell death per unit increase in effector-to-target cell ratio presenting with a 10.7-fold increase in lytic units per effector cell population compared to lymphocytes from control mice. Lymphocytes derived from B16-F10-B7.1-IFNγ/β- and B16-F10-4-1BBL-IFNγ/β-vaccinated mice each showed approximately fourfold increase in cytotoxic activity relative to control (Fig. 2c).
Prophylactic treatment of C57BL/6 mice with either B16-F10-B7.1-IFNγ/β or B16-F10-4-1BBL-B7.1-IFNγ/β vaccines is protected from the development of tumours
With tumour challenge studies, all control, unvaccinated mice succumbed to the tumour burden by day 31 (Supplementary Fig. 1A). In contrast, only 60 % of mice vaccinated with B16-F10-4-1BBL-IFNγ/β succumbed to tumour, and no deaths were observed in mice vaccinated with either B16-F10-B7.1-IFNγ/β (p = 0.014 compared to B16-F10-4-1BBL-IFNγ/β) or B16-F10-4-1BBL-B7.1-IFNγ/β (p = 0.014 compared to B16-F10-4-1BBL-IFNγ/β). For re-challenge studies, 100 % of the unvaccinated control mice again succumbed to tumour burden (by day 19, Supplementary Fig. 1B). However, all re-vaccinated and re-challenged mice remained completely protected against tumour development.
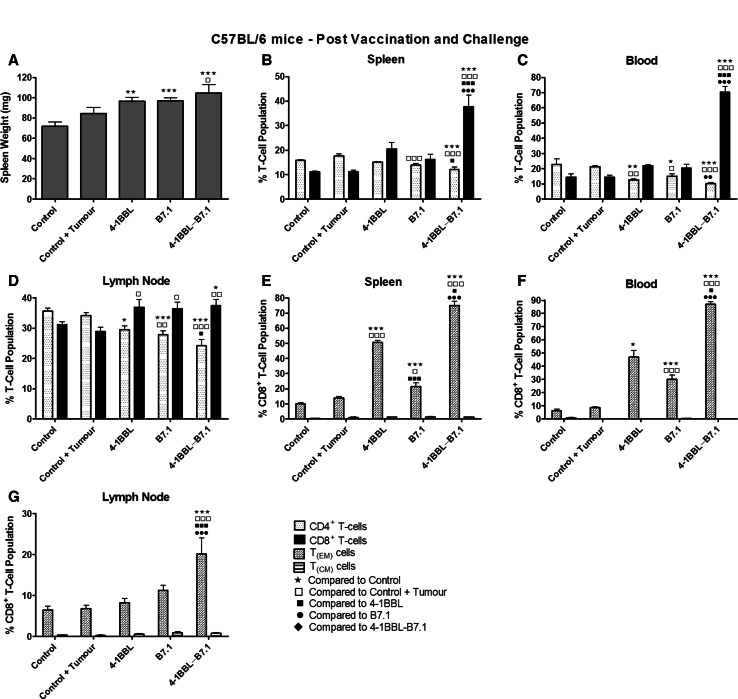
B16-F10-4-1BBL-B7.1-IFNγ/β-vaccinated C57BL/6 mice generated highly elevated CD8+ and effector memory T-cell (TEM) populations after conferring protection against repeated tumour challenges
All vaccinated mice that were protected against tumour development for at least 190 days were assessed for changes in immune cell populations. B16-F10-4-1BBL-B7.1-IFNγ/β-vaccinated mice recorded the heaviest mean spleen weight (Fig. 3a). Splenic- and peripheral blood-derived CD8+ T-cell populations from B16-F10-4-1BBL-B7.1-IFNγ/β-vaccinated mice were at least 1.5-fold higher in the spleen and at least threefold higher in the peripheral blood compared to any other treatment or control group (Fig. 3b, c). By comparison, splenic- and peripheral blood-derived % CD4+ T-cell populations from B16-F10-4-1BBL-B7.1-IFNγ/β-vaccinated mice were significantly reduced compared to control, unvaccinated mice that had or had not been injected with tumour cells (p values <0.005; Fig. 3b, c). Furthermore, all vaccinated mice had higher mean lymph node derived % CD8+ T-cell populations and lower mean % CD4+ T-cell populations compared to control mice with and without tumours (Fig. 3d).
Fig. 3.
B16-F10-4-1BBL-B7.1-IFNγ/β-vaccinated C57BL/6 mice that demonstrated long-term protection against tumour development produced highly elevated % CD8+ and TEM cell populations. Vaccinated C57BL/6 mice that remained tumour free after initial tumour challenge were boosted twice with the same vaccine, either B16-F10-4-1BB-IFNγ/β (n = 3), B16-F10-B7.1-IFNγ/β (n = 7) or B16-F10-4-1BBL-B7.1-IFNγ/β (n = 6), and re-challenged with tumour cells. A group of non-vaccinated mice (n = 4) were also challenged (control + tumour), and second group of control mice remained untreated (control; n = 4). Spleens were collected and weighed (a), and then, lymphocytes from spleens (b), peripheral blood (c) and lymph nodes (d) were analysed for % CD4+ and CD8+ T-cell populations. CD8+ T-cell populations in spleens (e), peripheral blood (f) and lymph nodes (g) were further analysed for the presence of TEM and TCM CD8+ T-cell subpopulations. The mean ± SEM is displayed with significance levels represented by: ★p < 0.05; ★★p < 0.01; ★★★p < 0.005
The levels of effector (TEM) and central memory (TCM) CD8+ T-cells were assessed in vaccinated mice. Significantly higher % TEM CD8+ cells were detected in the spleen and peripheral blood of all vaccinated mice compared to the untreated control mice (Fig. 3e, f). The most significant increases in % TEM CD8+ cells were observed in samples from B16-F10-4-1BBL-B7.1-IFNγ/β-vaccinated mice, with 75 and 87 % of CD8+ T-cells in the spleen and blood, respectively, identified as TEM cells (Fig. 3e, f). By comparison, only 14 and 9 % of CD8+ T-cells in the spleen and blood, respectively, were identified as TEM cells in control mice with tumours. Furthermore, B16-F10-4-1BBL-B7.1-IFNγ/β-vaccinated mice were the only group to display significantly elevated % TEM CD8+ cells isolated from their lymph nodes compared to control mice with, or without, tumour (p < 0.005 compared to all other treatments; Fig. 3g). No significant changes in TCM CD8+ cells were observed in mice from any treatment group.
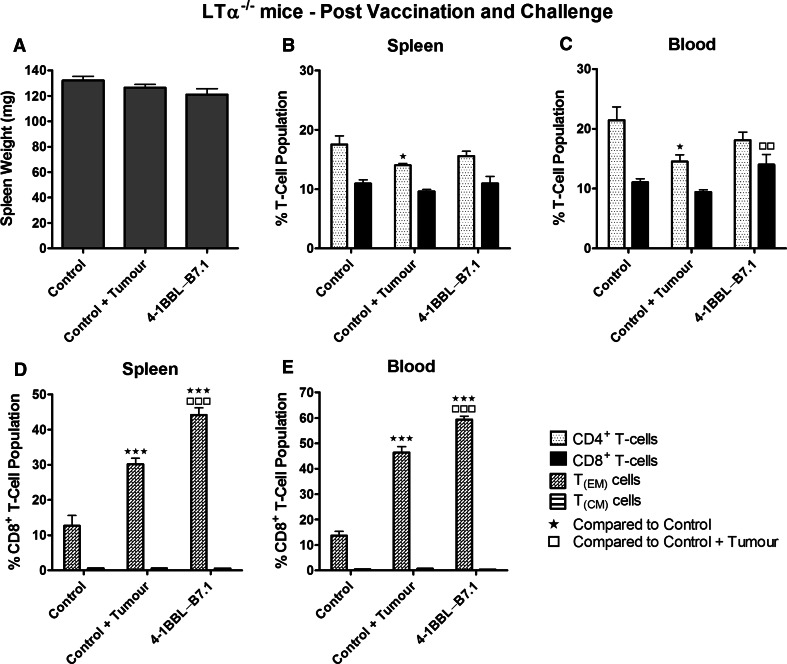
LTα−/− mice injected with the B16-F10-4-1BBL-B7.1-IFNγ/β vaccine generated enhanced CD8+ T-cell responses in the absence of DCs
The immune response of LTα−/− mice vaccinated with either B16-F10-4-1BBL-B7.1-IFNγ/β or B16-F10-B7.1-IFNγ/β was examined to assess the capacity of 4-1BBL to overcome severely reduced DC co-stimulatory activity. LTα−/− mice vaccinated with B16-F10-4-1BBL-B7.1-IFNγ/β had significantly heavier spleens compared to unvaccinated mice (p = 0.002) and mice vaccinated with B16-F10-B7.1-IFNγ/β vaccine (p = 0.022; Fig. 4a). Increased % CD8+ T- and decreased % CD4+ T-cell populations were produced in the spleens from B16-F10-4-1BBL-B7.1-IFNγ/β-vaccinated LTα−/− mice compared to control and B16-F10-B7.1-IFNγ/β-vaccinated mice (Fig. 4b). B16-F10-4-1BBL-B7.1-IFNγ/β-vaccinated mice also displayed an increased % CD8+ T-cell population in peripheral blood compared to control mice, but at borderline significance (p = 0.057; Fig. 4c).
Fig. 4.
LTα−/− mice produced elevated splenic CD8+ T-cell populations following injection with the B16-F10-4-1BBL-B7.1-IFNγ/β cell vaccine. LTα−/− mice were injected with either the B16-F10-B7.1-IFNγ/β (n = 4) or B16-F10-4-1BBL-B7.1-IFNγ/β (n = 8) anticancer vaccine, or remained unvaccinated as controls (n = 8). Four days following the final vaccination, spleens were collected and weighed (a), and then, spleens (b) and peripheral blood (c) were analysed for differences in CD4+ and CD8+ T-cell populations. Data are representative of two independent experiments, and the mean ± SEM is shown. Significance levels are represented by: ★p < 0.05; ★★p < 0.01; ★★★p < 0.005
In MLCs, T-cells derived from B16-F10-4-1BBL-B7.1-IFNγ/β-vaccinated LTα−/− mice maintained significantly increased % CD8+ T-cell population compared to either control (p = 0.003) or B16-F10-B7.1-IFNγ/β-vaccinated mice (p = 0.048; Fig. 5a). Subsequently, 39 % of the CD8+ T-cells in MLCs derived from B16-F10-4-1BBL-B7.1-IFNγ/β-vaccinated LTα−/− mice were characterised as activated, a more than fourfold increase compared to unvaccinated mice (Fig. 5b). CTL analysis showed that lymphocytes derived from B16-F10-4-1BBL-B7.1-IFNγ/β-vaccinated LTα−/− mice induced significantly greater cell death than either unvaccinated or B16-F10-B7.1-IFNγ/β-vaccinated LTα−/− mice (p < 0.001; Fig. 5c), displaying a 5.63-fold increase in lytic units per effector cell population compared to control.
Fig. 5.
% CD8+ T-cells and % activated CD8+ T-cells derived from B16-F10-4-1BBL-B7.1-IFNγ/β-vaccinated LTα−/− mice were enhanced following culture in MLC and were most effective at killing target melanoma cells in CTL assays. LTα−/− mice were used as controls (n = 8) or injected with either B16-F10-B7.1-IFNγ/β (n = 4) or B16-F10-4-1BBL-B7.1-IFNγ/β (n = 8) vaccines. Splenic-derived lymphocytes were grown in MLCs for 5 days and then analysed for changes in CD4+ and CD8+ T-cell populations (a) and activated CD8+ T-cell populations (b). Lymphocytes were assessed for their ability to kill target melanoma cells in CTL assays (c). For (c), the numbers on the right of the graph indicate the quantity of effector cells (×104) required to produce 30 % target cell lysis and the fold increase in lytic units per effector cell population relative to the CTL response from unvaccinated mice. The data are derived from four independent experiments, and the mean ± SEM is shown. Significance levels are denoted by: ★p < 0.05; ★★p < 0.01; ★★★p < 0.005
In in vivo tumour challenge studies, 75 % of B16-F10-B7.1-IFNγ/β-vaccinated LTα−/− mice survived live tumour challenge for a period of 60 days and 67 % of B16-F10-4-1BBL-B7.1-IFNγ/β-vaccinated mice survived for a total of 131 days (p = 0.001 and p < 0.001 for B16-F10-B7.1-IFNγ/β and B16-F10-4-1BBL-B7.1-IFNγ/β, respectively, compared to unvaccinated control mice; Supplementary Fig. 2). In re-challenge studies, the mean spleen weight of the re-challenged B16-F10-4-1BBL-B7.1-IFNγ/β mice was not significantly different to control mice (no tumour challenge) or control mice challenged with tumour (Fig. 6a). Furthermore, no differences in % splenic CD8+ T-cell populations were observed (Fig. 6b). A higher mean % CD8+ T-cell population was detected within the peripheral blood of B16-F10-4-1BBL-B7.1-IFNγ/β-vaccinated LTα−/− mice; however, this difference was only significantly higher than unvaccinated mice injected with live tumour cells (Fig. 6c). In contrast, TEM CD8+ T-cell populations of re-challenged B16-F10-4-1BBL-B7.1-IFNγ/β-vaccinated mice were significantly elevated compared to control unvaccinated mice, with or without tumour burden, with 45 % of splenic T-cells (Fig. 6d) and 60 % of peripheral blood T-cells (Fig. 6e) classified as TEM CD8+ T-cells.
Fig. 6.
B16-F10-4-1BBL-B7.1-IFNγ/β anticancer vaccine protects LTα−/− mice against tumour challenge and increases % TEM cell population. Vaccinated LTα−/− mice that remained tumour free after initial tumour challenge were boosted twice with the B16-F10-4-1BBL-B7.1-IFNγ/β cell vaccine (n = 3) and re-challenged with B16-F10-B7.1 tumour cells. Control, unvaccinated mice challenged with tumour cells (control + tumour; n = 3) and unvaccinated mice that remained untreated (control; n = 4) were also assessed. Spleens were collected and weighed (a), and then, lymphocytes from spleens (b) and peripheral blood (c) were analysed for CD4+ and CD8+ T-cell populations. CD8+ T-cell populations in spleens (d) and peripheral blood (e) were further analysed for the presence of TEM and TCM CD8+ T-cell subpopulations. The mean ± SEM is presented, and significance levels are denoted by: ★p < 0.05; ★★p < 0.01; ★★★p < 0.005
Discussion
A large number of preclinical studies assessing the development of specific cancer immunotherapeutics, including vaccines, have been shown to enhance anticancer immune responses [13, 19, 20]. One study of particular relevance [13] highlighted that a melanoma cell line stimulated with IFNγ/β to upregulate MHC class I expression and engineered to highly express the T-cell co-stimulatory molecule B7.1 was capable of inducing similar CTL responses in wild-type C57BL/6 mice and CD4+ helper T-cell-deficient MHC II−/− C57BL/6 mice [21], suggesting B7.1 expression on the vaccine could directly stimulate CTL responses independently of CD4+ T-cells. In the present study, we expanded on this knowledge and characterised the ability of numerous engineered melanoma cell lines, stably overexpressing 4-1BBL and B7.1, to induce potent immune responses when administered as whole-cell vaccines. We showed that vaccine cell lines overexpressing 4-1BBL and B7.1, either individually or simultaneously, produced significantly increased CD8+ T-cell populations in spleen and blood, displayed enhanced immunogenic properties in MLC and CTL assays, and protected against tumour development in vivo. Importantly, the vaccine overexpressing both 4-1BBL and B7.1 induced the most effective anti-tumour responses. Furthermore, the favourable immune-stimulating properties of the vaccine cells overexpressing both 4-1BBL and B7.1 extended to LTα−/− mice, which have severely reduced numbers of DCs but are able to generate functional T-cell responses [22, 23], indicating that the requirement for DCs in establishing long-term immunity could be circumvented. However, we note that immune responses with vaccination were less potent in LTα−/− mice relative to C57BL/6 mice, suggesting that DC deficiency could not be fully overcome with 4-BBL overexpression.
DCs play a vital role in the anti-tumour immune response by presenting tumour antigens to T-cells and supplying co-stimulatory molecules in order for T-cells to mature and differentiate [24, 25]. When DCs do not mature or fail to present antigens correctly, tumours can more easily grow and metastasise [26, 27]. Of concern, several clinical studies have shown reduced numbers of DCs in the peripheral blood and lower numbers of mature DCs in the blood and lymph nodes of cancer patients [27–29]. It is uncertain whether reduced DC function occurs in melanoma patients, but the inclusion of 4-1BBL in a whole-cell vaccine offers a way to guard against this possibility, increasing the likelihood of generating robust and effective anticancer immune responses.
We chose to use whole cells engineered to express CD8+ T-cell co-stimulatory molecules as our vaccine, which has numerous advantages compared to more traditional vaccine technologies, such as DC vaccines. Clinical trials have highlighted that DC vaccines are well tolerated because they usually involve taking the patient’s own DCs, manipulating them to express tumour antigens or peptides, and then returning them to the patient to induce tumour-specific responses. However, patients’ clinical outcomes have been varied [30–32]. The limited success could be due to the type of antigen and/or adjuvant used, the route of administration, the timing and dose of the vaccine and more recently the targeting of specific DC subsets in vivo [33, 34]. Our vaccine based directly on whole cells provides a self-contained package, which expresses the necessary co-stimulatory molecules and antigens to stimulate potent immune responses. Such technology bypasses the need to tailor cells to express cancer antigens, which is time-consuming and expensive, and also avoids modulating specific DC subtype activation, which has recently been demonstrated to be important for optimising patient responses [34].
In recent years, treatments for melanoma have advanced to include methods of alleviating or reducing immunosuppression [4, 5]. Immunotherapeutics including Ipilimumab and Novolumab, which block cytotoxic lymphocyte-T-associated antigen 4 (CTLA-4) expressed on regulatory T-cells and programmed cell death-1 (PD-1) receptor expressed on activated T-cells, respectively, are capable of inhibiting anti-tumour immune responses. Clinical trials using the monoclonal antibodies have identified a reduction in tumour size in some patients as well as minor increases in median overall survival [35, 36]. However, these agents alone are not able to promote the generation of anti-tumour immune responses. This highlights the requirement for and importance of our vaccine, which in its preclinical model has shown highly elevated anticancer immune responses capable of preventing tumour development in normal and DC-deficient mice. Therefore, we hope to translate this technology into a self-contained human melanoma vaccine that can be administered to a wider population, which in turn would reduce time taken to manufacture and be more cost-effective.
The efficient anti-tumour immune responses observed in our in vivo vaccination investigations are consistent with several published studies. A similar pattern of CD8+ T-cell stimulation and expansion was reported in a study that generated an artificial APC (aAPC) comprising K32 erythromyeloid cells engineered to express the human Fcγ receptor and 4-1BBL and coated with anti-CD3 and anti-CD28 antibodies (an alternative to B7.1) [37]. The study determined that K32 cells without 4-1BBL or beads coated with CD3 and CD28 antibodies only were not able to induce sustained proliferation of CD8+ T-cells and were not as effective in preventing apoptosis of T-cells compared to aAPCs including 4-1BBL [37]. A separate study using gamma-irradiated spontaneous sarcoma cells expressing B7.1 and 4-1BBL provided the greatest CTL response with respect to percentage of specific lysis of target cells compared to cells expressing either marker alone [38]. These data suggest that 4-1BBL expression on an aAPC is important for the expansion of effector CD8+ T-cell populations capable of killing antigen-specific tumour cells, which is what we observed in our in vivo and in vitro vaccine analyses.
Previous studies have shown that cancer vaccines expressing B7.1 or 4-1BBL were able to prevent tumour growth after injection with live cancer cells [13, 38]. Our results determined that both the B16-F10-B7.1-IFNγ/β and B16-F10-4-1BBL-B7.1-IFNγ/β vaccines were able to protect all tumour-challenged mice from developing tumours, even after repeated injections of live cancer cells. Although there was no difference in terms of survival outcome for these mice, when long-term memory responses were assessed, B16-F10-4-1BBL-B7.1-IFNγ/β-vaccinated mice generated significantly higher CD8+ T-cells, of which a high proportion were identified as TEM cells. This result indicated that the most potent, long-term memory responses were generated in B16-F10-4-1BBL-B7.1-IFNγ/β-vaccinated mice and that the resulting T-cells were responsible for activating the immune responses against the invading tumour cells to prevent tumour development.
In conclusion, we have highlighted the numerous advantages of incorporating 4-1BBL into a whole-cell anticancer vaccine also expressing B7.1 and MHC class I molecules. C57BL/6 mice treated with the B16-F10-4-1BBL-B7.1-IFNγ/β anticancer vaccine displayed greatly enhanced CD8+ T-cell responses, which translated into highly efficient live tumour cell rejection and enhanced cancer cell cytotoxicity. Furthermore, for the first time, we have been able to show that a whole-cell vaccine can directly stimulate CD8+ T-cell responses without the requirement for DC help, as was evidenced when LTα−/− mice generated potent immune responses after vaccination. This indicated that the vaccine was acting as a highly effective APC and is likely to be able to directly stimulate CD8+ T-cells, without requiring co-stimulatory signals from DCs (this study) or CD4+ T-cells [13]. However, the potential contribution of these immune cell populations to vaccination in an intact immune system requires further investigation. Therefore, it is highly recommended that 4-1BBL be incorporated into any whole-cell vaccines currently being tested on melanoma patients in clinical trials. Such a vaccine has the potential to enhance CD8+ T-cell responses and help prevent the development of secondary metastases leading to greater and more significant patient responses.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors would like to acknowledge Dr. Brendan Hill and Nigel Middlebrooke at Premion Cancer Care, Southport, for assisting with the use of the irradiator. This study was supported by Genvax Pty. Ltd. Katie L. Powell was supported by an Australian Postgraduate Award.
Conflict of interest
S.J. Ralph is the Director of Genvax Pty. Ltd., and this study was supported by funding from Genvax Pty. Ltd. S.J. Ralph is a consultant and inventor on patents related to the commercialisation of cancer vaccines including some technology described herein. S.J. Ralph and K.L. Powell have filed a patent relating to the material presented. A.S. Stephens has no conflict of interest to declare.
Abbreviations
- APC
Antigen-presenting cell
- CTL
Cytotoxic T lymphocyte
- DC
Dendritic cell
- IFN
Interferon
- MLC
Mixed lymphocyte culture
- TCM
Central memory CD8+ T-cell
- TEM
Effector memory CD8+ T-cell
References
- 1.Trotter SC, Sroa N, Winkelmann RR, Olencki T, Bechtel M. A global review of melanoma follow-up guidelines. J Clin Aesthet Dermatol. 2013;6:18–26. [PMC free article] [PubMed] [Google Scholar]
- 2.Bray F, Jemal A, Grey N, Ferlay J, Forman D. Global cancer transitions according to the Human Development Index (2008–2030): a population-based study. Lancet Oncol. 2012;13:790–801. doi: 10.1016/S1470-2045(12)70211-5. [DOI] [PubMed] [Google Scholar]
- 3.Azoury SC, Lange JR. Epidemiology, risk factors, prevention, and early detection of melanoma. Surg Clin North Am. 2014;94:945–962. doi: 10.1016/j.suc.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 4.Lee C, Collichio F, Ollila D, Moschos S. Historical review of melanoma treatment and outcomes. Clin Dermatol. 2013;31:141–147. doi: 10.1016/j.clindermatol.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 5.Fang L, Lonsdorf AS, Hwang ST. Immunotherapy for advanced melanoma. J Invest Dermatol. 2008;128:2596–2605. doi: 10.1038/jid.2008.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berd D, Maguire HC, Jr, Schuchter LM, Hamilton R, Hauck WW, Sato T, Mastrangelo MJ. Autologous hapten-modified melanoma vaccine as postsurgical adjuvant treatment after resection of nodal metastases. J Clin Oncol. 1997;15:2359–2370. doi: 10.1200/JCO.1997.15.6.2359. [DOI] [PubMed] [Google Scholar]
- 7.Soiffer R, Hodi FS, Haluska F, et al. Vaccination with irradiated, autologous melanoma cells engineered to secrete granulocyte-macrophage colony-stimulating factor by adenoviral-mediated gene transfer augments antitumor immunity in patients with metastatic melanoma. J Clin Oncol. 2003;21:3343–3350. doi: 10.1200/JCO.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 8.O’Rourke MG, Johnson M, Lanagan C, et al. Durable complete clinical responses in a phase I/II trial using an autologous melanoma cell/dendritic cell vaccine. Cancer Immunol Immunother. 2003;52:387–395. doi: 10.1007/s00262-003-0375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Rosa F, Ridolfi L, Ridolfi R, et al. Vaccination with autologous dendritic cells loaded with autologous tumor lysate or homogenate combined with immunomodulating radiotherapy and/or preleukapheresis IFN-alpha in patients with metastatic melanoma: a randomised “proof-of-principle” phase II study. J Transl Med. 2014;12:209. doi: 10.1186/1479-5876-12-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaffee EM, Hruban RH, Biedrzycki B, et al. Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol. 2001;19:145–156. doi: 10.1200/JCO.2001.19.1.145. [DOI] [PubMed] [Google Scholar]
- 11.Sondak VK, Sabel MS, Mule JJ. Allogeneic and autologous melanoma vaccines: where have we been and where are we going? Clin Cancer Res. 2006;12:2337s–2341s. doi: 10.1158/1078-0432.CCR-05-2555. [DOI] [PubMed] [Google Scholar]
- 12.Ralph SJ. An update on malignant melanoma vaccine research: insights into mechanisms for improving the design and potency of melanoma therapeutic vaccines. Am J Clin Dermatol. 2007;8:123–141. doi: 10.2165/00128071-200708030-00001. [DOI] [PubMed] [Google Scholar]
- 13.Dezfouli S, Hatzinisiriou I, Ralph SJ. Enhancing CTL responses to melanoma cell vaccines in vivo: synergistic increases obtained using IFNgamma primed and IFNbeta treated B7-1+ B16-F10 melanoma cells. Immunol Cell Biol. 2003;81:459–471. doi: 10.1046/j.0818-9641.2003.01189.x. [DOI] [PubMed] [Google Scholar]
- 14.Hsueh EC, Morton DL. Antigen-based immunotherapy of melanoma: canvaxin therapeutic polyvalent cancer vaccine. Semin Cancer Biol. 2003;13:401–407. doi: 10.1016/j.semcancer.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Faries MB, Hsueh EC, Ye X, Hoban M, Morton DL. Effect of granulocyte/macrophage colony-stimulating factor on vaccination with an allogeneic whole-cell melanoma vaccine. Clin Cancer Res. 2009;15:7029–7035. doi: 10.1158/1078-0432.CCR-09-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lotem M, Kadouri L, Merims S, et al. HLA-B35 correlates with a favorable outcome following adjuvant administration of an HLA-matched allogeneic melanoma vaccine. Tissue Antigens. 2011;78:203–207. doi: 10.1111/j.1399-0039.2011.01709.x. [DOI] [PubMed] [Google Scholar]
- 17.Mogi S, Sakurai J, Kohsaka T, Enomoto S, Yagita H, Okumura K, Azuma M. Tumour rejection by gene transfer of 4-1BB ligand into a CD80(+) murine squamous cell carcinoma and the requirements of co-stimulatory molecules on tumour and host cells. Immunology. 2000;101:541–547. doi: 10.1046/j.1365-2567.2000.t01-1-00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeBenedette MA, Wen T, Bachmann MF, Ohashi PS, Barber BH, Stocking KL, Peschon JJ, Watts TH. Analysis of 4-1BB ligand (4-1BBL)-deficient mice and of mice lacking both 4-1BBL and CD28 reveals a role for 4-1BBL in skin allograft rejection and in the cytotoxic T cell response to influenza virus. J Immunol. 1999;163:4833–4841. [PubMed] [Google Scholar]
- 19.Stannard KA, Collins PM, Ito K, et al. Galectin inhibitory disaccharides promote tumour immunity in a breast cancer model. Cancer Lett. 2010;299:95–110. doi: 10.1016/j.canlet.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Townsend SE, Allison JP. Tumor rejection after direct costimulation of CD8+ T cells by B7-transfected melanoma cells. Science. 1993;259:368–370. doi: 10.1126/science.7678351. [DOI] [PubMed] [Google Scholar]
- 21.Cosgrove D, Gray D, Dierich A, Kaufman J, Lemeur M, Benoist C, Mathis D. Mice lacking MHC class II molecules. Cell. 1991;66:1051–1066. doi: 10.1016/0092-8674(91)90448-8. [DOI] [PubMed] [Google Scholar]
- 22.De Togni P, Goellner J, Ruddle NH, et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. 1994;264:703–707. doi: 10.1126/science.8171322. [DOI] [PubMed] [Google Scholar]
- 23.Wu Q, Wang Y, Wang J, Hedgeman EO, Browning JL, Fu YX. The requirement of membrane lymphotoxin for the presence of dendritic cells in lymphoid tissues. J Exp Med. 1999;190:629–638. doi: 10.1084/jem.190.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 25.Ardavin C, del Hoyo GM, Martin P, Anjuere F, Arias CF, Marin AR, Ruiz S, Parrillas V, Hernandez H. Origin and differentiation of dendritic cells. Trends Immunol. 2001;22:691–700. doi: 10.1016/S1471-4906(01)02059-2. [DOI] [PubMed] [Google Scholar]
- 26.Gabrilovich DI, Nadaf S, Corak J, Berzofsky JA, Carbone DP. Dendritic cells in antitumor immune responses. II. Dendritic cells grown from bone marrow precursors, but not mature DC from tumor-bearing mice, are effective antigen carriers in the therapy of established tumors. Cell Immunol. 1996;170:111–119. doi: 10.1006/cimm.1996.0140. [DOI] [PubMed] [Google Scholar]
- 27.Almand B, Resser JR, Lindman B, Nadaf S, Clark JI, Kwon ED, Carbone DP, Gabrilovich DI. Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res. 2000;6:1755–1766. [PubMed] [Google Scholar]
- 28.Tas MP, Simons PJ, Balm FJ, Drexhage HA. Depressed monocyte polarization and clustering of dendritic cells in patients with head and neck cancer: in vitro restoration of this immunosuppression by thymic hormones. Cancer Immunol Immunother. 1993;36:108–114. doi: 10.1007/BF01754410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gabrilovich DI, Corak J, Ciernik IF, Kavanaugh D, Carbone DP. Decreased antigen presentation by dendritic cells in patients with breast cancer. Clin Cancer Res. 1997;3:483–490. [PubMed] [Google Scholar]
- 30.O’Rourke MG, Johnson MK, Lanagan CM, et al. Dendritic cell immunotherapy for stage IV melanoma. Melanoma Res. 2007;17:316–322. doi: 10.1097/CMR.0b013e3282c3a73b. [DOI] [PubMed] [Google Scholar]
- 31.Trepiakas R, Berntsen A, Hadrup SR, et al. Vaccination with autologous dendritic cells pulsed with multiple tumor antigens for treatment of patients with malignant melanoma: results from a phase I/II trial. Cytotherapy. 2010;12:721–734. doi: 10.3109/14653241003774045. [DOI] [PubMed] [Google Scholar]
- 32.Lesterhuis WJ, Schreibelt G, Scharenborg NM, et al. Wild-type and modified gp100 peptide-pulsed dendritic cell vaccination of advanced melanoma patients can lead to long-term clinical responses independent of the peptide used. Cancer Immunol Immunother. 2011;60:249–260. doi: 10.1007/s00262-010-0942-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nestle FO, Farkas A, Conrad C. Dendritic-cell-based therapeutic vaccination against cancer. Curr Opin Immunol. 2005;17:163–169. doi: 10.1016/j.coi.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 34.Radford KJ, Tullett KM, Lahoud MH. Dendritic cells and cancer immunotherapy. Curr Opin Immunol. 2014;27:26–32. doi: 10.1016/j.coi.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 35.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus Ipilimumab in Advanced Melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maus MV, Thomas AK, Leonard DG, Allman D, Addya K, Schlienger K, Riley JL, June CH. Ex vivo expansion of polyclonal and antigen-specific cytotoxic T lymphocytes by artificial APCs expressing ligands for the T-cell receptor, CD28 and 4-1BB. Nat Biotechnol. 2002;20:143–148. doi: 10.1038/nbt0202-143. [DOI] [PubMed] [Google Scholar]
- 38.Melero I, Bach N, Hellstrom KE, Aruffo A, Mittler RS, Chen L. Amplification of tumor immunity by gene transfer of the co-stimulatory 4-1BB ligand: synergy with the CD28 co-stimulatory pathway. Eur J Immunol. 1998;28:1116–1121. doi: 10.1002/(SICI)1521-4141(199803)28:03<1116::AID-IMMU1116>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.