Abstract
Adoptive transfer of immune cells, such as T lymphocytes and NK cells, has potential to control cancer growth. However, this can be counteracted by immune escape mechanisms within the tumor microenvironment, including those mediated by reactive oxygen species (ROS). Here, we determined the levels of anti-oxidant molecules in NK cells and their capacity to overcome ROS-induced immune suppression. We investigated the effect of H2O2 on resting NK cells, IL-2-activated NK cells and NK cells expanded by coculture with the K562 leukemia cell line genetically modified to express membrane-bound IL-15 and 4-1BB ligand (K562-mb15-41BBL). Expression of anti-oxidant and anti-apoptotic genes was evaluated by expression array, and protein levels of anti-oxidant molecules by Western blot. Activated NK cells, IL-2-activated NK cells and NK cells expanded by K562-mb15-41BBL were significantly more resistant to H2O2-induced cell death than resting NK. Thioredoxin-1 (TXN1) and peroxiredoxin-1 (PRDX1) were also up-regulated in activated NK cells. Moreover, H2O2-induced cell death after IL-2 activation was significantly induced in the presence of an anti-TXN1-neutralising antibody. Collectively, these data document that activated NK cells can resist to H2O2-induced cell death by up-regulation of TXN1.
Keywords: IL-2, NK cells, Thioredoxin-1, Hydrogen Peroxide, Immune surveillance
Introduction
We are now entering a new era of immunotherapy for treating cancer [1–3]. It has been shown that adoptive transfer of T lymphocytes and NK cells with anti-tumor capacity has the potential to control cancer growth and improve patient survival [4, 5]. We have recently shown that NK cells expanded with K562-mb15-41BBL are highly cytotoxic against gastric cancer [6]. On the other hand, a number of immune escape mechanisms within the tumor microenvironment may block anti-cancer effects. We and others previously reported that ROS such as H2O2 produced within a tumor can shut off T cell and NK cell function, which eventually leads to the immune suppression that is frequently observed in the tumor microenvironment [7–9]. Therefore, to overcome immune escape mechanisms including ROS, it would be desirable to render transferred NK cells more resistant to oxidative stress.
There are several reports which explain the mechanisms behind T cell apoptosis when exposed to oxidative stress [10, 11]; however, to date, there is very limited related information on NK cells except for a study which compared CD56bright and CD56dim NK cells and showed that CD56bright NK cells expressed higher levels of cell-surface thiols, and were able to neutralize H2O2 more efficiently than CD56dim NK cells [12]. The clinical utility of IL-2 is well known as its role in the activation of NK cells [13, 14]; hence it is important to evaluate its influence on the intracellular anti-oxidant capacity of NK cells. In this study, we investigated the sensitivity to H2O2 of resting NK, IL-2-activated NK cells, and NK cells expanded with K562-mb15-41BBL, with a particular focus on thioredoxin levels.
Materials and methods
NK cell preparation, culture and treatment
PBMC were isolated from buffy coats from anonymous healthy donors (National University Hospital, Singapore) by density gradient separation through Ficoll-Paque (GE healthcare, Uppsala, Sweden). For resting NK, NK cells were purified from PBMC by negative selection using Dynabeads Untouched Human NK cells (Invitrogen, Carlsbad, CA), according to the manufacturer’s protocol. IL-2-activated NK cells were generated by culturing the isolated resting NK cells in RPMI-1640 medium (Invitrogen) containing 10% FCS and 1-1000 IU/mL of IL-2 (Novartis Pharmaceuticals Corporation, Basel, Switzerland) for 7 days (IL-2 NK). Newly prepared IL-2 medium was replenished every 2 days. Whereas for expanded NK cells, 3 × 106 of PBMCs and 2 × 106 genetically modified, irradiated (100 Gy) K562-mb15-41BBL cell line (provided by Dr. D. Campana, National University of Singapore, Singapore) were cocultured in a 6-well plate with RPMI-1640 medium containing 10% FCS and 10 IU/mL human IL-2 (expanded NK) [15]. Newly prepared IL-2 medium was replenished every 2 days. After 7 days of coculturing, the residual T cells were depleted using Dynabeads CD3 (Invitrogen). After CD3 depletion, NK cells were cultured in 100 IU/mL of IL-2 [6].
Western blot
NK cells were lysed in CellLytic lysis buffer (Sigma–Aldrich, St. Louis, MO) with a protease inhibitor cocktail (Sigma–Aldrich) and a phosphatase inhibitor cocktail (Sigma–Aldrich), and the protein concentrations were measured using a Bicinchoninic Acid protein assay (Thermo Scientific, Rockford, IL). Proteins (5 µg/well) were resolved using a 4–15% gradient SDS–polyacrylamide gel electrophoresis (Invitrogen) followed by transfer to a polyvinylidene fluoride microporous membrane blocked in PBS with 5% milk. The membrane was then probed using a rabbit anti-human primary antibody. The following primary antibodies (from Cell Signaling Technology, Danvers, MA) were used: p44/42 MAPK (extracellular signal–regulated kinases (Erk)1/2), phospho-p44/42 MAPK (Erk1/2), protein kinase B (Akt), phospho-Akt, superoxide dismutase (SOD)1, SOD2, TXN1, PRDX1, and β-actin. After washing, the membranes were incubated with HRP-linked anti-rabbit IgG (Cell Signaling Technology). Blots were imaged by enhanced chemiluminescence (ECL) reagents (ECL Prime; Amersham Pharmacia Biotech AB, Uppsala, Sweden) and by a film processor (Konica SRX 101).
Cell treatment with inhibitor and oxidant
For blocking experiments, 0.2 × 106 of NK cells, which were stimulated with 500 IU/mL of IL-2 for 7 days, were cocultured with catalase (1000 U/mL; Sigma Aldrich) or neutralising anti-TXN1 antibody (1 mg/mL; IMCO Corporation, Stockholm, Sweden) and several concentrations of hydrogen peroxide (H2O2 30% w/w in H2O; Sigma Aldrich) in 200 µL of X-vivo medium (Invitrogen) on 96-well plate for 24 h at 37 °C and 5% CO2. H2O2 was diluted in X-vivo medium and used within 5 min of preparation. Flow cytometric analysis for cell viability was then performed after a 24-h incubation.
Microarray
RNA in NK cells was isolated by phenol–chloroform. cDNA preparation and microarray hybridization were performed according to the Affymetrix protocols (Affymetrix, Santa Clara, CA). Gene expression was analyzed using GeneChip Human Genome U133 Plus 2.0 arrays (Affymetrix). The arrays were read with a laser confocal scanner (Agilent). Probe intensities were measured using the MicroArray Suite 5.0 algorithm and log-transformed. Unsupervised hierarchical clustering analysis of resting NK, IL-2 NK (200 or 6000 IU/mL of IL-2), and expanded NK was performed using Cluster 3.0 software. Visualization and generation of a heat map were performed using Java TreeView software.
Flow cytometry
NK cells were stained using anti-CD56-FITC (NCAM 16.2) at a saturating concentration for 30 min at 4 °C. In addition, dead cells were labeled with 7-aminoactinomycin D (7-AAD) according to the manufacturer’s protocol. Both antibodies were obtained from BD Biosciences (San Jose, CA). The percentage of living cells at 0 µM of H2O2 was used as a control.
Cytotoxic assay
The cytotoxic activity of expanded NK and resting NK treated with 0 and 80 µM of H2O2 was measured using a calcein-release assay, as previously described [3, 16]. Briefly, K562 cells were stained with 5 µM of calcein-acetoxymethyl (DOJINDO LABORATORIES, Kumamoto, Japan) for 30 min at 37 °C and 5% CO2. After staining, the stained K562 cells and treated NK cells were counted for cytotoxic assay and trypan blue negative cells were used as living cells. Stained targets (5 × 103 / well) were cocultured with various ratios of H2O2-treated NK cells in 200 µL of culture medium for 4 h. Assays were performed in triplicate in a 96-well U-bottomed plate. After incubation, 100 µL of the supernatant was collected and transferred to a 96-well flat-bottomed plate. Fluorescence of each supernatant was measured at 485 nm excitation and 528 nm emission using Infinite 200 (Tecan Group Ltd., Männedorf, Switzerland). Spontaneous release was obtained from K562 cells incubated without effector cells, and maximum release was obtained from detergent-released K562 cells. The percentage of specific lysis was calculated according to the following formula: % specific lysis = 100 × (experimental release − spontaneous release)/(maximum release − spontaneous release).
Statistics
The statistical analysis was performed using a two-tailed paired t test. The brackets in the figures indicate which groups were compared and *, **, and *** indicate values of p < 0.05, <0.01, and <0.001, respectively.
Results
IL-2 NK and expanded NK are more resistant to H2O2 than resting NK
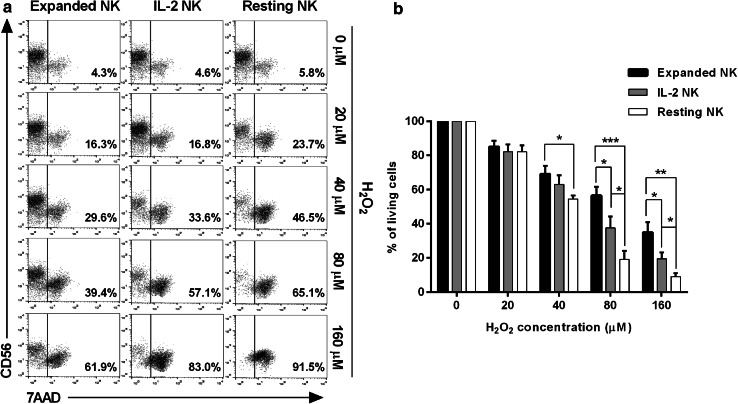
We prepared resting NK, IL-2 NK (500 IU/mL of IL-2), and expanded NK from the same healthy donor [15], and compared their sensitivity to H2O2 by 7-AAD staining of CD56 (+) NK cells. Ando et al. showed that activated granulocytes release H2O2 in concentrations that would be sufficient to induce NK cell death (100–200 µM/1 × 106 cells/100µL) [17], an observation that has also been confirmed by other groups [7, 18–20]. Based on these studies, we believe that the concentrations of H2O2 used in this study are similar to levels observed at inflammatory sites. As shown in a representative 7-AAD staining (Fig. 1a), numbers of apoptotic cells in IL-2 NK or expanded NK were markedly lower than in resting NK, where the fraction of apoptotic cells present was dependent on the concentration of H2O2. Data summarized from several different donors (n = 8) indicate that expanded NK possessed the greatest resistance towards H2O2 treatment among the three groups of NK cells. There were significant differences in sensitivity to H2O2 at 80 and 160 µM between the three groups of NK cells (Fig. 1b). To confirm whether the cell death observed was H2O2-dependent, the IL-2 NK were treated in the same way but in the presence of catalase, a scavenger of H2O2 [8, 17, 21]. The H2O2-induced cell death was significantly inhibited with catalase treatment, also in resting NK and expanded NK (data not shown).
Fig. 1.
Apoptosis of NK cells after treatment with H2O2. a Purified NK cells were incubated with H2O2 for 24 h at indicated doses within physiological levels, and then subjected to apoptosis analysis with 7-AAD staining in combination with CD56 mAb. We prepared resting NK, IL-2 NK (500 IU/mL of IL-2), and expanded NK from the same healthy donor. Representative flow cytometric data from the same healthy donor are shown. b Summarized data of the apoptosis analysis with 7-AAD staining in NK cells from different donors (n = 8) are shown. ***p < 0.001, **p < 0.01, *p < 0.05
Gene expression array of IL-2 NK, expanded NK and resting NK
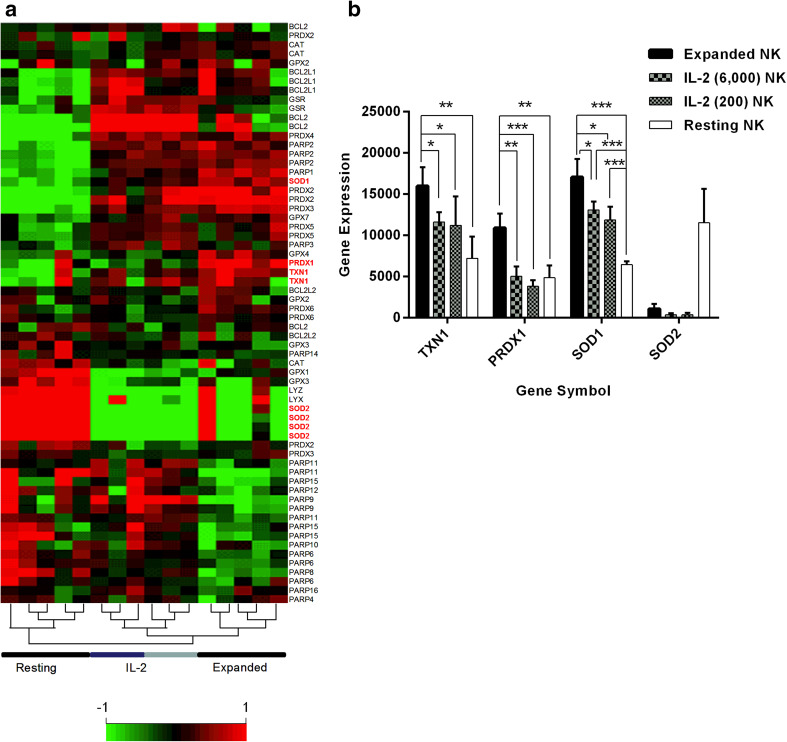
To evaluate the factors responsible for H2O2-resistance in activated NK cells, gene expression was assessed in resting NK, IL-2 NK, and expanded NK. As shown in the heat map of gene expression data specific for anti-oxidant and anti-apoptotic genes (Fig. 2a), unsupervised hierarchical clustering analysis accurately clustered the three distinct groups of resting NK, IL-2 NK, and expanded NK. The cluster of IL-2 NK samples comprised two IL-2 concentrations (dark blue, 200 IU/mL; light blue, 6000 IU/mL). Although IL-2 NK and expanded NK showed a similar pattern of anti-oxidant and anti-apoptotic gene expression, this was completely different in resting NK and expanded NK, which exhibited marked up-regulation of these genes (Fig. 2a). Amongst the different gene expression patterns, we focused on TXN1, PRDX1, and SOD-1 and −2, since it has previously been reported that TXN1, PRDX1, and SODs are closely related to anti-oxidant activity [22–24]. We found that the expression of TXN1, PRDX1, and SOD1 was significantly up-regulated in both IL-2 NK and expanded NK relative to resting NK; the greatest degree of up-regulation was observed in expanded NK (Fig. 2b). In contrast, the expression of SOD2 was down-regulated in IL-2 NK and expanded NK compared to in resting NK (Fig. 2b).
Fig. 2.
Gene expression array of resting NK, IL-2 NK and expanded NK. a Resting NK (n = 5) were evaluated immediately after immunomagnetic separation of CD56 + CD3-cells. IL-2 NK (n = 6) and expanded NK (n = 5) were evaluated after 7 days of coculture. For the IL-2 NK, two concentrations of IL-2 were used: 200 IU/mL (n = 3) and 6000 IU/mL (n = 3). A representative heat map showed three major clusters derived from the unsupervised hierarchical clustering analysis, namely resting NK, IL-2 NK, and expanded NK samples. IL-2 NK samples were in one cluster comprising of two IL-2 concentrations used (dark blue, 200 IU/mL; light blue, 6000 IU/mL). b A representative bar graph shows the gene expression of four anti-oxidant genes, namely TXN1, PRDX1, SOD1, and SOD2, selected from the microarray data. Comparisons were made for TXN1, PRDX1, SOD1 between each group of NK cells with expanded NK versus IL-2 NK; expanded NK versus resting NK; IL-2 NK versus resting NK. No comparison was made for SOD2 since the expression showed opposite pattern. ***p < 0.001, **p < 0.01, *p < 0.05
Up-regulation of PRDX1 and TXN1 protein in activated NK cells
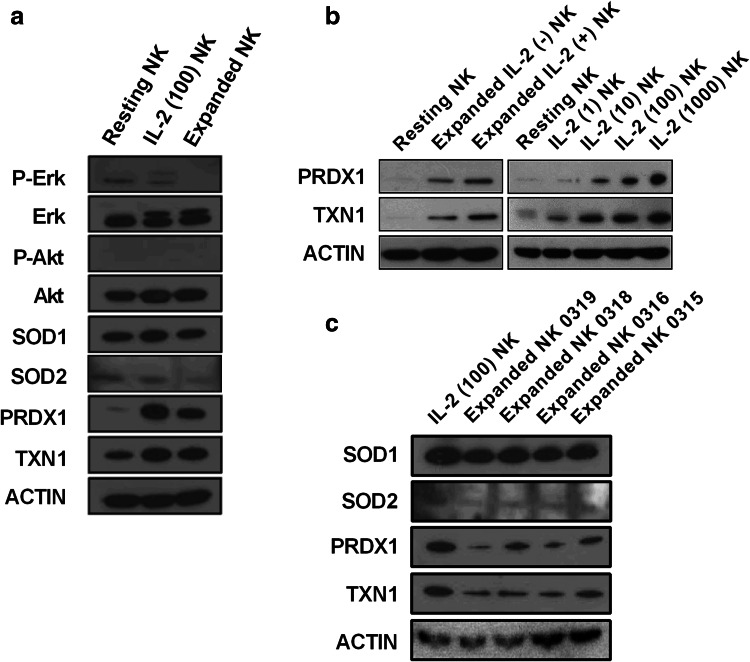
Next, we examined the protein levels of anti-oxidant molecules including SOD1, SOD2, TXN1, and PRDX1 in resting NK, IL-2 NK, and expanded NK. The results were shown to be in line with the gene expression levels (Fig. 2a), as the protein levels of TXN1 and PRDX1 were also up-regulated in IL-2 NK and expanded NK compared to those in resting NK. There was no significant alteration of SOD1 and SOD2 protein seen in NK cells (Fig. 3a).
Fig. 3.
Expression of anti-oxidant molecules in activated NK cells. a Erk, Akt and anti-oxidant molecules were evaluated in three populations of NK cells: resting NK, IL-2 NK stimulated at 100 IU/mL of IL-2 and expanded NK. b Evaluation of PRDX1 and TXN1 protein expressions were carried out in resting NK with two populations of expanded NK stimulated with or without 500 IU/mL of IL-2, respectively (left); and with four populations of IL-2 NK stimulated with 1, 10, 100, and 1000 IU/mL of IL-2, respectively (right). c The SOD1, SOD2, TXN1, and PRDX1 protein expressions were evaluated in IL-2 NK and expanded NK from four different donors. These observations were obtained from three independent experiments
The up-regulation of TXN1 and PRDX1 by IL-2 NK was dependent on the dose of IL-2 (Fig. 3b, on the right), demonstrating that IL-2 plays an important role in the regulation of TXN1 and PRDX1. On the other hand, up-regulation of TXN1 and PRDX1 was also observed in expanded NK without IL-2 (Fig. 3b, on the left), indicating that other stimulating signals such as IL-15 or 41BBL may also cause up-regulation of TXN1 and PRDX1 levels. However, since the addition of IL-2 together with the K562-mb15-41BBL cell line induces higher expression of TXN1 and PRDX1 (Fig. 3b), it is likely that IL-2 is a main inducer of up-regulation of TXN1 and PRDX1 molecules. We further evaluated SOD1, SOD2, TXN1, and PRDX1 expression in expanded NK from four different donors. The same tendency was observed in all donors (Fig. 3c).
Erk 1/2 and PI3K-Akt expression in activated NK cells
Because IL-2 and IL-15 activate multiple signaling pathways in NK cells, including the Ras-MAP kinase [25, 26] and PI3K-Akt-p70 S6 kinase [26], we evaluated the expressions of Erk1/2 for the MAPK pathway and Akt for the PI3K-Akt pathway by Western blot. However, no significant changes to phosphorylated or total Erk 1/2 and Akt expression were seen in the three types of NK cells (Fig. 3a).
Effect of anti-TXN1-neutralising Ab on H2O2-induced cell death in NK cells
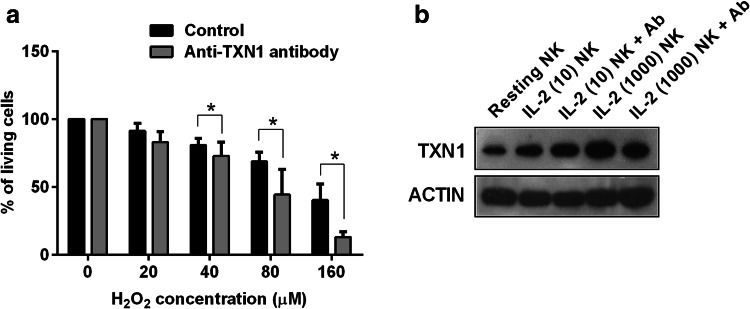
It has been reported that T cell resistance to H2O2-induced cell death could be antagonized by anti-TXN1-neutralising antibodies [27]. To confirm that resistance to H2O2-induced cell death in activated NK cells is dependent on up-regulation of TXN1, IL-2 NK were subjected to an apoptosis assay in the presence of anti-TXN1-neutralising Ab. As shown in Fig. 4a, H2O2-induced cell death in IL-2 NK was significantly increased with anti-TXN1-neutralising Ab, suggesting that up-regulation of TXN1 plays an important role in conferring resistance against ROS in activated NK cells.
Fig. 4.
TXN1 is responsible for NK cell resistance to H2O2-induced cell death. a IL-2 NK stimulated at 100 IU/mL of IL-2 were treated with H2O2 for 24 h with anti-TXN1-neutralising antibody (1 mg/mL) or without (Control). Representative viability data of IL-2 NK from four independent experiments is shown. *p < 0.05. b TXN1 protein expression was evaluated in five populations of NK cells: resting NK; IL-2 NK stimulated at 10 or 1000 IU/mL of IL-2 with or without anti-TXN1-neutralising Ab antibody. These observations were obtained from three independent experiments
We also determined the effect of anti-TXN1-neutralising Ab on TXN1 expression in the IL-2 NK. TXN1 expression was evaluated by Western blot in NK cells treated with IL-2 at 10 and 1000 IU/mL in the presence of anti-TXN1-neutralising Ab. As shown in Fig. 4b, TXN1 expression showed no difference between those with or without anti-TXN1-neutralising Ab, a finding in line with previous studies [27].
NK cell cytotoxicity is inhibited by H2O2 exposure
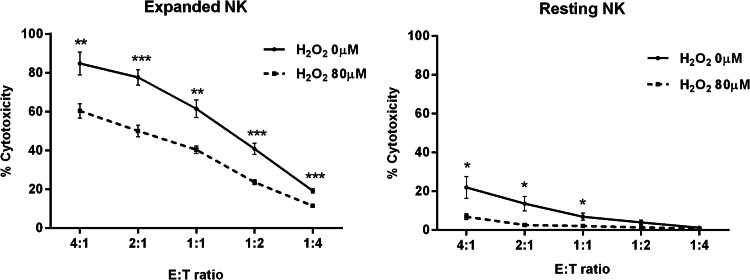
To evaluate the functional consequence of H2O2-exposed NK cells, we treated expanded NK (n = 5) and resting NK (n = 5) with 0 and 80 µM of H2O2 for 24 h and then assessed their cytotoxicity. NK cell cytotoxicity was significantly reduced after H2O2 exposure, although the expanded NK treated with H2O2 were relatively more cytotoxic against K562 compared to resting NK (Fig. 5).
Fig. 5.
Cytotoxic assay in NK cells treated with H2O2. Resting NK (n = 5) and expanded NK (n = 5) were treated with H2O2 (0 and 80 µM) for 24 h, and then subjected to cytotoxic assay against K562 cells in a calcein-release assay. E:T ratio, effector:K562 ratio. ***p < 0.001, **p < 0.01, *p < 0.05
Discussion
This study has shown that IL-2 NK and expanded NK were more resistant to H2O2-induced cell death than resting NK, and that IL-2 confers protection on NK cells against oxidative stress mainly by up-regulation of TXN1, amongst several other anti-oxidant molecules.
We have previously shown that resting NK are sensitive to apoptosis in the presence of physiological levels of H2O2 [7, 17, 19], which is produced abundantly within the tumor microenvironment of gastric and esophageal cancer [7, 28]. It is possible that the H2O2 produced within tumor cells has the capability to switch off NK function, leading to NK cell dysfunction which is frequently observed in the tumor microenvironment [7, 17]. We have also reported that expanded NK were significantly more cytotoxic to gastric cancer than resting NK [6]. Hence, we wanted to evaluate the resistance of expanded NK to ROS when transferred into cancer patients. This study has clearly demonstrated that the expanded NK are able to resist the effects of ROS better than resting NK, suggesting that expanded NK transferred into patients can exert cytotoxic function despite the presence of ROS within the tumor microenvironment.
With regard to the candidate molecules that mediate the increased tolerance to oxidative stress in activated NK cells, and based on the function of SODs [29], PRDX1 and catalase [30], it is plausible that anti-oxidant molecules or anti-apoptotic molecules are involved in this mechanism. Additionally, it has also been recently reported that PRDX1 are responsible for redox balance in the NK cell system [31], and thiols such as glutathione and TXN1 have been documented as being important in the anti-oxidative systems [12, 24].
Our data suggest a novel mechanism by which IL-2 confers protection on activated NK cells against oxidative stress by up-regulation of TXN1. Furthermore, we found that other signals such as IL-15 and, to a lesser extent, 4-1BBL also contributed to the up-regulation of TXN1. While there are reports that describe the relationship between IL-2 and TXN1 in T cells and NKT cells, very little was previously known about the role of IL-2 and TXN1 in NK cells. IL-2 may contribute to the effect of the TXN1 binding protein on NKT cell populations [32], and overexpression of TXN1 suppresses oxidative stress damage [33]. As IL-2R and IL-15R share the same β- and γ-subunits, they both activate downstream signaling pathways including the PI3K and the MAPK pathway, and finally the nuclear factor-κB (NF-κB) pathway [34–36]. In the present study, there was no significant difference in the expression of the PI3K and MAPK pathway molecules between resting NK and activated NK cells stimulated either with IL-2 or K562-mb15-41BBL. Taken together, these data show that activated NK cells are resistant to H2O2-induced cell death, while IL-2 mainly conferred protection on NK cells by up-regulation of TXN1 without the involvement of the MAPK or PI3K pathway. Of note, it has been shown that the thioredoxin pathways have the greatest selectivity for NF-κB relative to the other pathways [37].
This study revealed that IL-2 NK and expanded NK were resistant to H2O2-induced cell death by up-regulation of TXN1. Extracellular TXN1 is responsible for suppressing endothelial cell damage induced by H2O2 [38], while secreted TXN1 also exhibits potential anti-apoptotic and oncogenic effects beyond its antioxidative function [27, 39, 40]. This study showed that H2O2-induced cell death in IL-2 NK was significantly increased with anti-TXN1-neutralising Ab, which has no effect on TXN1 expression in IL-2 NK, indicating that extracellular TXN1 on IL-2 NK and/or secreted TXN1 from IL-2 NK plays an important role in conferring resistance to ROS. We intend to investigate this further in future studies.
Much effort has been focused on the characterization and functional significance of NK cell dysfunction in the tumor microenvironment [41]. Our findings provide a novel explanation suggesting that an existing redox balance system in NK cells may account for how activated NK cells can survive in such hostile microenvironments. Therefore, a better understanding of how NK cells overcome oxidative stress within the tumor microenvironment will improve the clinical efficacy of NK cell therapy for solid tumors. This study also shows that we can generate the expanded NK with 10 IU/mL of IL-2, but to overcome oxidative stress in the tumor microenvironment the use of 100 IU/mL of IL-2 would be of greater benefit.
Acknowledgements
This work was supported by a Clinician Scientist Award (CSA) and Clinician Scientist-Individual Research Grant (CS-IGR) from the National Medical Research Council of Singapore. We would like to thank Dr. Dario Campana (National University of Singapore, Singapore) for providing us with the K562-mb15-41BBL cell line.
Abbreviations
- 7-AAD
7-Aminoactinomycin D
- Akt
Protein kinase B
- ECL
Enhanced chemiluminescence
- Erk
Extracellular signal–regulated kinase
- Expanded NK
NK cells expanded with K562 leukemia cell line genetically modified to express membrane-bound IL-15 and 4-1BB ligand for 7 days
- IL-2 NK
NK cells activated by several doses of IL-2 for 7 days
- K562-mb15-41BBL
K562 leukemia cell line genetically modified to express membrane-bound IL-15 and 4-1BB ligand
- PRDX1
Peroxiredoxin-1
- Resting NK
Resting NK cells
- ROS
Reactive oxygen species
- SOD
Superoxide dismutase
- TXN1
Thioredoxin-1
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
K. Mimura and L.-F. Kua equally contributed to this study.
References
- 1.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 2.Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mimura K, Kua LF, Shiraishi K, Kee Siang L, Shabbir A, Komachi M, et al. Inhibition of mitogen-activated protein kinase pathway can induce upregulation of human leukocyte antigen class I without PD-L1-upregulation in contrast to interferon-gamma treatment. Cancer Sci. 2014;105:1236–1244. doi: 10.1111/cas.12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng M, Chen Y, Xiao W, Sun R, Tian Z. NK cell-based immunotherapy for malignant diseases. Cell Mol Immunol. 2013;10:230–252. doi: 10.1038/cmi.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalos M, June CH. Adoptive T cell transfer for cancer immunotherapy in the era of synthetic biology. Immunity. 2013;39:49–60. doi: 10.1016/j.immuni.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mimura K, Kamiya T, Shiraishi K, Kua LF, Shabbir A, So J, et al. Therapeutic potential of highly cytotoxic natural killer cells for gastric cancer. Int J Cancer. 2014;135:1390–1398. doi: 10.1002/ijc.28780. [DOI] [PubMed] [Google Scholar]
- 7.Izawa S, Kono K, Mimura K, Kawaguchi Y, Watanabe M, Maruyama T, et al. H(2)O(2) production within tumor microenvironment inversely correlated with infiltration of CD56(dim) NK cells in gastric and esophageal cancer: possible mechanisms of NK cell dysfunction. Cancer Immunol Immunother. 2011;60:1801–1810. doi: 10.1007/s00262-011-1082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kono K, Salazar-Onfray F, Petersson M, Hansson J, Masucci G, Wasserman K, et al. Hydrogen peroxide secreted by tumor-derived macrophages down-modulates signal-transducing zeta molecules and inhibits tumor-specific T cell-and natural killer cell-mediated cytotoxicity. Eur J Immunol. 1996;26:1308–1313. doi: 10.1002/eji.1830260620. [DOI] [PubMed] [Google Scholar]
- 9.Hansson M, Asea A, Ersson U, Hermodsson S, Hellstrand K. Induction of apoptosis in NK cells by monocyte-derived reactive oxygen metabolites. J Immunol. 1996;156:42–47. [PubMed] [Google Scholar]
- 10.Martner A, Aurelius J, Rydstrom A, Hellstrand K, Thoren FB. Redox remodeling by dendritic cells protects antigen-specific T cells against oxidative stress. J Immunol. 2011;187:6243–6248. doi: 10.4049/jimmunol.1102138. [DOI] [PubMed] [Google Scholar]
- 11.Gostner JM, Becker K, Fuchs D, Sucher R. Redox regulation of the immune response. Redox Rep. 2013;18:88–94. doi: 10.1179/1351000213Y.0000000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thoren FB, Romero AI, Hermodsson S, Hellstrand K. The CD16-/CD56bright subset of NK cells is resistant to oxidant-induced cell death. J Immunol. 2007;179:781–785. doi: 10.4049/jimmunol.179.2.781. [DOI] [PubMed] [Google Scholar]
- 13.Bernstein ZP, Porter MM, Gould M, Lipman B, Bluman EM, Stewart CC, et al. Prolonged administration of low-dose interleukin-2 in human immunodeficiency virus-associated malignancy results in selective expansion of innate immune effectors without significant clinical toxicity. Blood. 1995;86:3287–3294. [PubMed] [Google Scholar]
- 14.Meropol NJ, Barresi GM, Fehniger TA, Hitt J, Franklin M, Caligiuri MA. Evaluation of natural killer cell expansion and activation in vivo with daily subcutaneous low-dose interleukin-2 plus periodic intermediate-dose pulsing. Cancer Immunol Immunother. 1998;46:318–326. doi: 10.1007/s002620050493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujisaki H, Kakuda H, Shimasaki N, Imai C, Ma J, Lockey T, et al. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009;69:4010–4017. doi: 10.1158/0008-5472.CAN-08-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mimura K, Shiraishi K, Mueller A, Izawa S, Kua LF, So J, et al. The MAPK pathway is a predominant regulator of HLA-A expression in esophageal and gastric cancer. J Immunol. 2013;191:6261–6272. doi: 10.4049/jimmunol.1301597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ando T, Mimura K, Johansson CC, Hanson MG, Mougiakakos D, Larsson C, et al. Transduction with the antioxidant enzyme catalase protects human T cells against oxidative stress. J Immunol. 2008;181:8382–8390. doi: 10.4049/jimmunol.181.12.8382. [DOI] [PubMed] [Google Scholar]
- 18.Test ST, Weiss SJ. Quantitative and temporal characterization of the extracellular H2O2 pool generated by human neutrophils. J Biol Chem. 1984;259:399–405. [PubMed] [Google Scholar]
- 19.Harlin H, Hanson M, Johansson CC, Sakurai D, Poschke I, Norell H, et al. The CD16CD56(bright) NK cell subset is resistant to reactive oxygen species produced by activated granulocytes and has higher antioxidative capacity than the CD16 + CD56(dim) subset. J Immunol. 2007;179:4513–4519. doi: 10.4049/jimmunol.179.7.4513. [DOI] [PubMed] [Google Scholar]
- 20.Mellqvist UH, Hansson M, Brune M, Dahlgren C, Hermodsson S, Hellstrand K. Natural killer cell dysfunction and apoptosis induced by chronic myelogenous leukemia cells: role of reactive oxygen species and regulation by histamine. Blood. 2000;96:1961–1968. [PubMed] [Google Scholar]
- 21.Takahashi A, Kono K, Ichihara F, Sugai H, Amemiya H, Iizuka H, et al. Macrophages in tumor-draining lymph node with different characteristics induce T-cell apoptosis in patients with advanced stage-gastric cancer. Int J Cancer. 2003;104:393–399. doi: 10.1002/ijc.10973. [DOI] [PubMed] [Google Scholar]
- 22.Hole PS, Zabkiewicz J, Munje C, Newton Z, Pearn L, White P, et al. Overproduction of NOX-derived ROS in AML promotes proliferation and is associated with defective oxidative stress signaling. Blood. 2013;122:3322–3330. doi: 10.1182/blood-2013-04-491944. [DOI] [PubMed] [Google Scholar]
- 23.Nadeem A, Chhabra SK, Masood A, Raj HG. Increased oxidative stress and altered levels of antioxidants in asthma. J Allergy Clin Immunol. 2003;111:72–78. doi: 10.1067/mai.2003.17. [DOI] [PubMed] [Google Scholar]
- 24.Kaur N, Naga OS, Norell H, Al-Khami AA, Scheffel MJ, Chakraborty NG, et al. T cells expanded in presence of IL-15 exhibit increased antioxidant capacity and innate effector molecules. Cytokine. 2011;55:307–317. doi: 10.1016/j.cyto.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu TK, Caudell EG, Smid C, Grimm EA. IL-2 activation of NK cells: involvement of MKK1/2/ERK but not p38 kinase pathway. J Immunol. 2000;164:6244–6251. doi: 10.4049/jimmunol.164.12.6244. [DOI] [PubMed] [Google Scholar]
- 26.Malek TR, Castro I. Interleukin-2 receptor signaling: at the interface between tolerance and immunity. Immunity. 2010;33:153–165. doi: 10.1016/j.immuni.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mougiakakos D, Johansson CC, Jitschin R, Bottcher M, Kiessling R. Increased thioredoxin-1 production in human naturally occurring regulatory T cells confers enhanced tolerance to oxidative stress. Blood. 2011;117:857–861. doi: 10.1182/blood-2010-09-307041. [DOI] [PubMed] [Google Scholar]
- 28.Izawa S, Mimura K, Watanabe M, Maruyama T, Kawaguchi Y, Fujii H, et al. Increased prevalence of tumor-infiltrating regulatory T cells is closely related to their lower sensitivity to H2O2-induced apoptosis in gastric and esophageal cancer. Cancer Immunol Immunother. 2013;62:161–170. doi: 10.1007/s00262-012-1327-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim JH, Chu SC, Gramlich JL, Pride YB, Babendreier E, Chauhan D, et al. Activation of the PI3K/mTOR pathway by BCR-ABL contributes to increased production of reactive oxygen species. Blood. 2005;105:1717–1723. doi: 10.1182/blood-2004-03-0849. [DOI] [PubMed] [Google Scholar]
- 30.Rhee SG, Yang KS, Kang SW, Woo HA, Chang TS. Controlled elimination of intracellular H(2)O(2): regulation of peroxiredoxin, catalase, and glutathione peroxidase via post-translational modification. Antioxid Redox Signal. 2005;7:619–626. doi: 10.1089/ars.2005.7.619. [DOI] [PubMed] [Google Scholar]
- 31.Siernicka M, Winiarska M, Bajor M, Firczuk M, Muchowicz A, Bobrowicz M, et al. Adenanthin, a new inhibitor of thiol-dependent antioxidant enzymes, impairs the effector functions of human natural killer cells. Immunology. 2015;146:173–183. doi: 10.1111/imm.12494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okuyama H, Yoshida T, Son A, Oka S, Wang D, Nakayama R, et al. Thioredoxin binding protein 2 modulates natural killer T cell-dependent innate immunity in the liver: possible link to lipid metabolism. Antioxid Redox Signal. 2009;11:2585–2593. doi: 10.1089/ars.2009.2691. [DOI] [PubMed] [Google Scholar]
- 33.Mitsui A, Hamuro J, Nakamura H, Kondo N, Hirabayashi Y, Ishizaki-Koizumi S, et al. Overexpression of human thioredoxin in transgenic mice controls oxidative stress and life span. Antioxid Redox Signal. 2002;4:693–696. doi: 10.1089/15230860260220201. [DOI] [PubMed] [Google Scholar]
- 34.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 35.Becknell B, Caligiuri MA. Interleukin-2, interleukin-15, and their roles in human natural killer cells. Adv Immunol. 2005;86:209–239. doi: 10.1016/S0065-2776(04)86006-1. [DOI] [PubMed] [Google Scholar]
- 36.Fehniger TA, Cooper MA, Caligiuri MA. Interleukin-2 and interleukin-15: immunotherapy for cancer. Cytokine Growth Factor Rev. 2002;13:169–183. doi: 10.1016/S1359-6101(01)00021-1. [DOI] [PubMed] [Google Scholar]
- 37.Jin DY, Chae HZ, Rhee SG, Jeang KT. Regulatory role for a novel human thioredoxin peroxidase in NF-kappaB activation. J Biol Chem. 1997;272:30952–30961. doi: 10.1074/jbc.272.49.30952. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura H, Masutani H, Yodoi J. Extracellular thioredoxin and thioredoxin-binding protein 2 in control of cancer. Semin Cancer Biol. 2006;16:444–451. doi: 10.1016/j.semcancer.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 39.Nilsson J, Soderberg O, Nilsson K, Rosen A. Thioredoxin prolongs survival of B-type chronic lymphocytic leukemia cells. Blood. 2000;95:1420–1426. [PubMed] [Google Scholar]
- 40.Backman E, Bergh AC, Lagerdahl I, Rydberg B, Sundstrom C, Tobin G, et al. Thioredoxin, produced by stromal cells retrieved from the lymph node microenvironment, rescues chronic lymphocytic leukemia cells from apoptosis in vitro. Haematologica. 2007;92:1495–1504. doi: 10.3324/haematol.11448. [DOI] [PubMed] [Google Scholar]
- 41.Waldhauer I, Steinle A. NK cells and cancer immunosurveillance. Oncogene. 2008;27:5932–5943. doi: 10.1038/onc.2008.267. [DOI] [PubMed] [Google Scholar]