Abstract
Pancreatic cancer (PC) is an aggressive disease with dismal prognosis. Surgical resection is the recommended treatment for long-term survival, but patients with resectable PC are in the minority (with a 5-year survival rate of 20 %). Therefore, development of novel therapeutic strategies, such as anti-PC immunotherapy, is crucial. α-Enolase (ENO1) is an enzyme expressed on the surface of pancreatic cancer cells and is able to promote cell migration and cancer metastasis. The capacity of ENO1 to induce an immune response in PC patients renders it a true tumor-associated antigen. In this study, we characterized the effector functions of ENO1-specific T cells isolated from PC patients, and we specifically evaluated the successful role of intra-tumoral T helper 17 (Th17) cells and the inhibitory role of regulatory T (Tregs) cells in respectively promoting or reducing the cancer-specific immune response. In this ex vivo study, we have demonstrated, for the first time, that ENO1-specific Th17 cells have a specific anti-cancer effector function in PC patients, and that there are decreased levels of these cells in cancer compared to healthy mucosa. Conversely, there are elevated levels of ENO1-specific Tregs in PC patients which lead to inhibition of the antigen-specific effector T cells, thus highlighting a possible role in promoting PC progression. These results may be relevant for the design of novel immunotherapeutic strategies in pancreatic cancer.
Keywords: Pancreatic cancer, Tumor-infiltrating lymphocytes (TILs), Regulatory T cells (Tregs), T helper 17 (Th17), α-Enolase (ENO1)
Introduction
Pancreatic cancer (PC) is an aggressive disease with dismal prognosis and an overall 5-year survival rate of 5 % [1]. Although surgical resection is the only treatment for long-term survival, patients with resectable PC are in the minority [2] and have a 5-year survival rate of just 20 % [3]. Therefore, the development of novel therapeutic modalities such as anti-PC immunotherapy is crucial.
In pancreatic cancer, some tumor-associated antigens (TAAs) such as MUC1, Kras, coactosin-like protein and mesothelin [4–7] have been identified, but their use as therapeutic agents has been unsuccessful. Case-report studies have indicated that vaccination with survivin and human telomerase reverse transcriptase (h-TERT) elicited an immune response and clinical remission of pancreatic cancer [8, 9]. Thus, selection of appropriate TAAs is essential for the induction of a strong integrated (humoral and cellular) immune response and cancer remission. Over-expression of the recently identified PC-associated antigen α-enolase (ENO1) in tumors and its ability to induce an immune response in cancer patients classifies it as a suitable TAA candidate [10].
The plasminogen receptor, ENO1, is expressed on the cell surface of many cancer cells, including PC [10], where it plays a role in promoting cell migration and cancer metastasis [11]. Notably, in PC, ENO1 elicits a T cell response, and the presence of anti-ENO1 autoantibodies correlates with a significantly better clinical outcome in PC patients [12]. In addition, ENO1 vaccination delays tumor progression and significantly extends survival in a genetic model of pancreatic carcinoma [13].
The role of CD4+ T cells in tumor immunity is poorly understood. Naive CD4+ T cells differentiate into mature T helper 1 (Th1), Th2, Th17 or T regulatory cells (Tregs). Various studies have shown that Tregs suppress immune responses and induce tolerance at tumor sites, particularly in PC [14, 15]. There is an intricate reciprocal regulation between Th17/Treg cells [16], and the final differentiation into suppressor versus effector cells at the tumor site may tip the balance between tolerance and tumor rejection. Moreover, mature Tregs can be reprogrammed into competent Th17 effector cells, and the plasticity of the T cell lineage may be an important mechanism by which immune homeostasis is maintained [17].
Th17 cells have been implicated in many autoimmune diseases, but their role in cancer has not been fully elucidated [18]. Some studies report that higher levels of Th17 cells correlate with advanced cancer [19, 20], while other data suggest that Th17 may have a potent antitumor effect, due to its presence in patients with long-term survival [21].
Conversely, Tregs maintain immune homeostasis by inhibiting effector T cell proliferation and autoimmune responses [22, 23]. An increased prevalence of Tregs correlated with more advanced PC is a marker of poor prognosis [14, 24]. When Tregs are depleted, a greater antitumor effect has been demonstrated in a PC mouse model [25].
The aims of this study were to characterize the effector functions of ENO1-specific T cells isolated from the neoplastic tissue of PC patients, as well as to specifically evaluate the successful role of intra-tumoral Th17 and the inhibitory role of Treg cells in promoting and/or inhibiting the cancer-specific immune response.
Materials and methods
Patients
Fifteen patients with pancreatic cancer: 8 males and 7 females were enrolled after obtaining informed consent and approval of the local ethical committee. The mean age of the PC patients was 63 years (range 36–92 years). The characteristics of patients are summarized in Table 1. Cancer samples were classified as pancreatic ductal adenocarcinomas according to the TNM classification for pancreatic tumors [26]. All patients underwent surgical resection of the primary lesion but did not receive chemotherapy. Patients with evidence of serious illness, immunosuppression, autoimmune or infectious diseases were excluded.
Table 1.
Patient characteristics and ENO1-reactivity of T cell clones (Tcc) generated from TILs of patients with pancreatic cancer
| Code | Age | Sex | TNM | Overall survival (months) | Tcc obtained | ENO1-specific Tcc (%) | ENO1-PBMC proliferation | Anti-ENO1 IgG |
|---|---|---|---|---|---|---|---|---|
| 01. PP | 50 | Male | pT3, N1, Mx | 11 | 84 | 17 (20) | Y | ++ |
| 02. MR | 73 | Male | pT4, N1, Mx | 13 | 26 | 8 (31) | Y | ++ |
| 03. PR | 56 | Male | pT3, N0, Mx | 8 | 6 | 1 (17) | N | + |
| 04. PT | 36 | Male | pT2, N1, M1 | 7 | 16 | 5 (31) | N | + |
| 05. NN | 74 | Female | pT3, N1, Mx | 9 | 91 | 13 (14) | Y | + |
| 06. ME | 61 | Female | pT2, N1, Mx | 8 | 20 | 0 (0) | N | + |
| 07. DA | 63 | Male | pT3, N1, Mx | 12 | 37 | 2 (5) | Y | ++ |
| 08. RA | 74 | Female | pT3, N0, Mx | 8 | 74 | 4 (14) | Y | + |
| 09. MM | 54 | Female | pT3, N0, Mx | 14 | 5 | 3 (60) | Y | +++ |
| 10. PL | 92 | Male | pT3, N1, Mx | 9 | 3 | 1 (33) | N | – |
| 11. CM | 57 | Male | pT2, N1, M1 | 10 | 22 | 6 (27) | Y | ++ |
| 12. RA | 51 | Female | pT4, N1, Mx | 11 | 13 | 3 (23) | Y | ++ |
| 13. BB | 72 | Female | pT3, N1, Mx | 8 | 22 | 4 (18) | N | + |
| 14. BR | 80 | Female | pT2, N1, M1 | 12 | 58 | 14 (24) | Y | ++ |
| 15. RP | 58 | Male | pT3, N0, Mx | 13 | 28 | 9 (32) | Y | +++ |
| All PC patients | 63 | 8 M/7F | Pancreatic ductal adenocarcinoma | 10.2 | 505 | 90 (18) | 10/15 (67 %) | 14/15(93 %) |
To rank the presence of anti-ENO1-IgG, we referred as threshold the OD value + 3SD from 45 age-matched sera from healthy subjects (0.13). The range between 0.13 and the higher value from PC patient sera (0.622) was divided for 3 in order to assign (+) for OD values between 0.13 and 0.294, (++) for OD between 0.294 and 0.458 and (+++) for OD between 0.458 and 0.622
PBMC peripheral blood mononuclear cells, Y Yes, N Not
Reagents
T cell cultures were performed in RPMI 1640 culture medium (SERO-Med GmbH, Wien) supplemented with 10 % FCS Hyclone (Gibco Laboratories, Grand Island, NY) and recombinant human interleukin-2 (IL-2) (Eurocetus, Milan, Italy). Unlabeled or fluorochrome-conjugated anti-CD3, CD4, CD8, CD25 and isotype-matched control mAbs were purchased from BD Biosciences (San Jose, Calif). The fluorochrome-conjugated anti-IL-10, anti-TGF-β isoform TGF−β1) and anti-FoxP3 mAbs were purchased from eBioscience (San Diego, Calif).
Production of recombinant histidin-tagged α-enolase
Recombinant α-enolase was overexpressed in the E. coli strain BL21(DE3)/pLysS (kindly provided by A. Giallongo, Institute of Biomedicine and Molecular Immunology, National Council of Research, Palermo, Italy) and was produced as previously reported [10].
Generation of T cell clones (Tcc) from TILs of the neoplastic pancreatic tissue
Surgical specimens of PC tissue were cultured for 7 days in RPMI 1640 medium supplemented with IL-2 (50 units/ml), in order to expand in vivo–activated tumor-infiltrating lymphocytes (TILs); in detail, tissue pieces from each patient were obtained from three different sites, namely (a) central tumor, (b) marginal tumor and (c) surrounding healthy mucosa. Each specimen was then disrupted, and single T cell blasts were cloned under limiting dilution, as described previously (27). Briefly, single T cell blasts were seeded in microwells (0.3 cells/well) in the presence of 2 × 105 irradiated (9000 rad) peripheral blood mononuclear cells (PBMCs), phytohemagglutinin (PHA) (0.5 % vol/vol) and IL-2 (50 U/ml). At weekly intervals, 2 × 105 irradiated PBMC and IL-2 were added to each microculture to maintain the expansion of growing clones.
The T cell clones (Tcc) were screened for responsiveness to ENO1 by measuring [3H]thymidine (Amersham Pharmacia Biotech, Uppsala, Sweden) uptake after a 60 h co-culture with irradiated autologous mononuclear cells in the presence of medium or ENO1 (10 μg/ml). The mitogenic index (MI) was calculated as the ratio between mean values of cpm (counts per minute) obtained in stimulated cultures and those obtained in the presence of medium alone. A MI ≥3 was considered as positive.
Analysis of cell surface markers and evaluation of the cytokine profile of isolated T cell clones
We analyzed surface marker (CD3, CD4, CD8) expression in blasts of single Tcc by flow cytometry as previously described [27] along with the production of the intracellular marker (FoxP3) and cytokines (IL-10 and TGF-β) of ENO1-specific Tcc (unable to secrete IFN-γ, IL-4 and IL-17). For intracellular analysis, the blasts were stimulated with PMA (phorbol myristate acetate) (10 ng/ml) plus ionomycin (200 ng/ml), and stained with anti-IL-10, anti-TGF-β and anti-FoxP3 mAbs.
Tcc that were negative for FoxP3, IL-10 and TGF-β were defined as T null, and Tcc that were positive for FoxP3, IL-10 and TGF-β were defined as Tregs.
To evaluate the amount of secreted cytokines, T cell blasts of single Tcc were resuspended at a concentration of 106 cells/ml medium and cultured for 36 h in the presence of PMA (10 ng/ml) plus ionomycin (200 ng/ml). Cell-free supernatants were collected and assayed in duplicate for IFN-γ, IL-4 and IL-17 content by commercial ELISA kits (BioSource International, Camarillo). Supernatants presenting cytokine levels that were 5 SD above the mean levels in control supernatants derived from irradiated antigen-presenting cells (APCs) alone were regarded as positive.
Based on the cytokine profile and the CD4/CD8 expression evaluation, we divided the clones into the following groups: Th1/Tc1 (only IFN-γ), Th2/Tc2 (only IL-4), Th0/Tc0 (IFN-γ and IL-4) and Th17/Tc17 (IL-17).
Analysis of the T cell receptor (TCR) Vβ chain repertoire of ENO1-specific T cell clones
To verify the clonality of ENO1-specific Tcc, the repertoire of the TCR Vβ chain of single Tcc was analyzed with the TCR Vβ Repertoire Kit (Beckman Coulter, Fullerton, USA); isotype-matched nonspecific Igs were used as negative controls.
B-EBV cell preparation
B cells were prepared using the B cell isolation kit II (Miltenyi Biotec, Bergisch Gladbach, Germany). To obtain EBV-transformed lymphoblastoid B cell lines (EBV-B cells), B cells (isolated from B cells of the patients) were incubated for 48 h with supernatants of the EBV-producing marmoset cell line B95.8 and subsequently expanded in complete medium supplemented with 15 % FCS.
Perforin-mediated cytotoxicity and Fas–Fas ligand (L)-mediated proapoptotic activity
Perforin-mediated cytolytic activity of Tcc was assessed as reported [28]. T cell blasts of ENO1-specific clones were incubated at an effector-to-target ratio of 10-, 5- and 2.5-to-1 with 51Cr-labeled autologous (EBV)-B cells preincubated with ENO1 (10 μg/ml). After centrifugation, microplates were incubated for 8 h at 37 °C, and 0.1 ml supernatant was removed from each microculture for measurement of 51Cr release. To evaluate whether the cytotoxicity against target cells of specific Tcc was ENO1/HLA dependent, the cytotoxicity tests were also performed in two other different conditions: (a) with ENO1-pulsed 51Cr-labeled autologous EBV-B cells in the presence of anti-HLA-DR blocking mAbs (10 μg/ml) to evaluate the ENO1-specific CD4+ Tcc and (b) in the presence of anti-HLA-A-B-C to assess the ENO1-specific CD8+ Tcc. Anti-HLA-DR and anti-HLA-A-B-C mAbs were purchased from Immunotech (Beckman Coulter, Marseille, France).
The ability of ENO1-specific Tcc to induce Fas–FasL-mediated apoptosis was assessed using Fas+ Panc1 cells as targets. T cell blasts from each clone were co-cultured with 51Cr-labeled Panc1 cells at an effector-to-target ratio of 10-, 5- and 2.5-to-1 for 18 h in the presence of PMA (10 ng/ml) and ionomycin (200 ng/ml), as reported [28]. To block the Fas–FasL interaction, the anti-Fas antagonistic monoclonal antibody M3 (Amgen, Thousand Oaks, USA) was used at a final concentration of 5 μg/ml in a 30 min pre-treatment of 51Cr-labeled Panc1 cells, as reported.
Suppressive assays
To assess the ability of antigen-specific Treg clones to suppress the antigen-induced autologous cell proliferation of ENO1-specific effective Tcc, 2 × 104 of these latter cells were cultured with 4 × 103 irradiated (9000 rad) autologous ENO1-loaded or ENO1-unloaded dendritic cells (DCs) in the presence of 2 × 104 cells of Tcc specific to ENO1. The DCs were obtained using the Blood Dendritic Cell Isolation Kit II (Miltenyi Biotec, Bergisch Gladbach, Germany).
At day 4, after 8 h of pulsing with 0.5 mCi 3H-TdR/well (Amersham, Little Chalfont, United Kingdom), cultures were harvested and radionuclide uptake was measured by β-counting. Tetanic toxoid (TT) was used for T cell clone specificity control.
Human PBMC culture
In order to assess the presence of T cells specific for ENO1 in the peripheral blood of patients with pancreatic cancer, PBMCs from all 15 patients and 15 healthy controls were cultured (3 × 105) for 5 days in the presence of medium alone or ENO1 (10 μg/ml). The responsiveness to ENO1 was evaluated by measuring [3H]thymidine (as previously described).
ELISA
The levels of anti-ENO1 IgG were measured by ELISA by binding to human rGST-ENO1 (2 μg/ml in Na2CO3 0.1 M 50 μl/well) and as secondary antibody, an HRP-conjugated anti-human IgG (1:2,000 Santa Cruz). To ascertain the presence of anti-ENO1-IgG, we referred to the OD value + 3SD from 45 age-matched sera from healthy subjects (0.13) as a threshold. The range between 0.13 and the higher value from PC patient sera (0.622) was divided by 3 in order to assign (+) for OD values between 0.13 and 0.294, (++) for OD between 0.294 and 0.458 and (+++) for OD between 0.458 and 0.622.
Statistical analysis
Results are expressed as mean ± SD. An unpaired t test was used to assess the difference of Th17 and Tnull/Treg frequency between healthy mucosa (HM), central tumor (CT) and marginal tumor (MT). A p value of <0.05 was considered to be statistically significant. The cytolytic activity of ENO1-specific T cell clones was compared using the Mann–Whitney nonparametric test.
We used Spearman’s correlation coefficient to correlate the titer of anti-ENO1 IgG and the percentage of ENO1-specific T clones obtained from PC patients.
Results
Characterization of pancreatic cancer tumor-infiltrating cells (TILs)
To evaluate the intra-tumoral immune response in PC patients, we expanded and cloned in vivo-activated TILs isolated from three different sites, namely CT, MT and surrounding HM.
We obtained T cell clones from each patient (Table 1) and taking into account all patients, the number of Tcc from the different sites was very similar, with a slight increase observed in cancer tissue: 149 (HM), 135 (MT) and 221(CT).
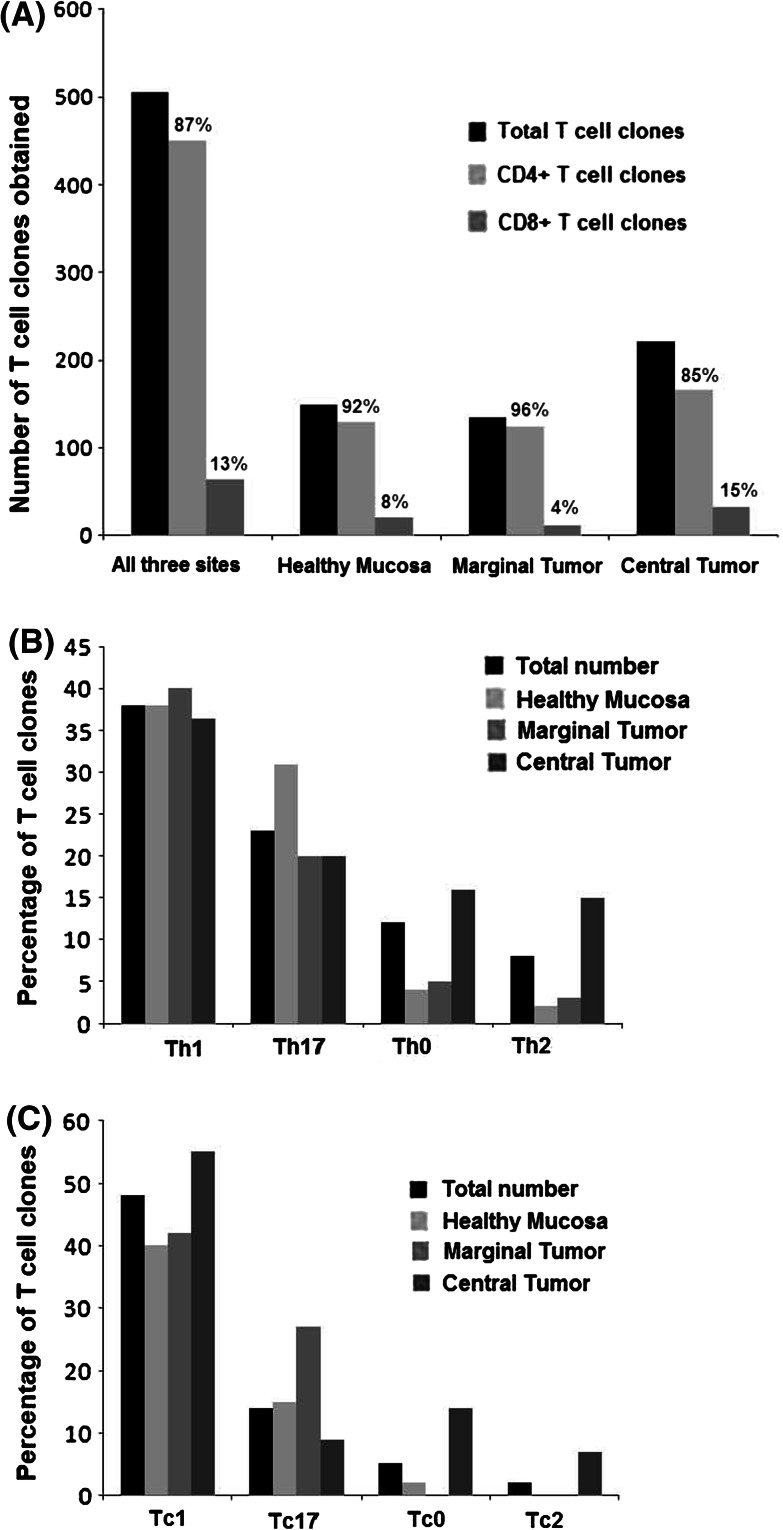
Eighty-seven percent (441/505) of Tcc were positive for CD4, and interestingly, the CD8+ population (13 %) increased from HM (8 %) to CT (15 %) (Fig. 1a).
Fig. 1.
Repertoire and cytokine production of TIL-derived T-cell clones (Tcc) obtained from patients with pancreatic cancer. a The histogram represents the total number of T cell clones isolated from single tumor tissue sites and the ratio of CD4+/CD8+ populations. b The cytokine phenotype distribution of CD4+ Tcc and c CD8+ Tcc. The percentages of clone cytokine profiles of CD4 (Th1, Th17, Th0, Th17) and of CD8 (Tc1, Tc17, Tc0, Tc17) were calculated comparing the number of Th/Tc types with the total number of CD4/CD8 clones obtained from the same tumor tissue sites (HM, MT, CT). The production of IFN-γ, IL-17 and IL-4 was measured in culture supernatants by specific ELISA tests
Most of the CD4+ Tcc (166/441, 38 %) were Th1 with a similar percentage at different sites: 38 % (HM), 40 % (MT) and 36 % (CT) (Fig. 1b). A considerable fraction (20 %) of the CD4+ Tcc was able to produce IL-4: alone (8 %) or with IFN-γ (12 %). Interestingly, the percentage of IL-4 producing T cells (Th2 + Th0) are greater in the neoplastic tissue (MT + CT) (25 %) compared with the healthy mucosa (15 %) (Fig. 1c).
A different distribution was found for the Th17 population (23 %): the highest percentage was recorded in HM (31 %), compared with only 20 % in CT and MT (p = 0.001) (Fig. 1c). It is remarkable that 78 % of Th17 clones also produce IFN-γ, in detail: 70 % (HM), 80 % (MT) and 84 % (CT) (data not shown).
Regarding the cytokine profile of the CD8+ population, we found that the overall percentage of IFN-γ producing cells (Tc1) were 48 % (31/64) and that the Tc1 percentage increases from HM (40 %) to CT (55 %), while the percentage of Tc17 (14 %) is greater in HM (15 %) compared to CT (9 %), albeit only slightly. We also isolated a high percentage of Tc17 (27 %, 3/11) from MT (Fig. 1c); again, 33 % of the total Tc17 clones secreted IFN-γ (data not shown). Five percent of the remaining CD8+ TCC (Tc0 + Tc2) were able to produce IL-4.
Intra-tumoral Tregs in PC patients
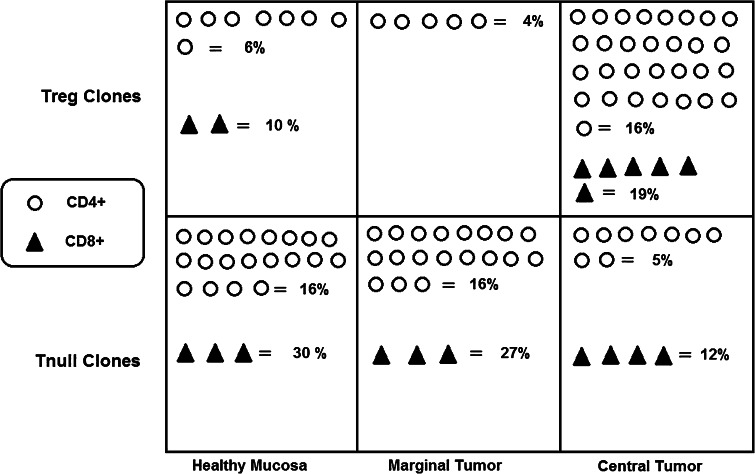
Evaluating the T cell cytokine profile, we found that a high percentage of CD4+ (20 %) and CD8+ (33 %) T cells were unable to produce any of the evaluated cytokines. To clarify the nature of these Tcc, we subsequently analyzed FoxP3 expression by Facs, along with the amounts of TGF-β and IL-10 intracytoplasmatic.
The presence of FoxP3, IL-10 and TGF-β was not revealed in 20 out of 129 (16 %) of the CD4+ Tcc isolated from healthy mucosa, while 8 (6 %) were FoxP3+ and produced IL-10 with TGF-β, and we defined them as T null and Tregs, respectively. In addition, we obtained 16 % of T null cells from MT and 5 % from CT, while 4 % of Tregs from MT and 16 % from CT were obtained.
In summary, we isolated T null and Treg cells in all three sites, but the percentage of Tregs statistically increased in CT (16 %) compared to HM (6 %) (Fig. 2) (p = 0.02).
Fig. 2.
Evaluation of Treg/Tnull populations in the three different tumor tissue sites. For single T cell clones, we evaluated the expression of CD25, TGF-β, IL-10 and FoxP3 by flow cytometry. Tregs were defined as the T cells producing IL-10, TGF-β and FoxP3+, and Tnull cells were the FoxP3− T blasts unable to synthesize IL-10 and TGF-β. The figure indicates the number of Treg and Tnull T clones isolated from the healthy mucosa (HM), marginal tumor (MT) and central tumor (CT). The percentage is calculated by referring to the total CD4+/CD8+ population isolated from the same tumor tissue site (HM, MT, CT)
Regarding the CD8+ population, the percentage of Tc null cells were lower in the CT (12 %) versus HM (30 %) and MT (27 %). Conversely, the percentage of Tc regs in CT (19 %) were twice that found in HM (10 %) (p = 0.03) (Fig. 2).
PC patients present ENO1-specific T cells
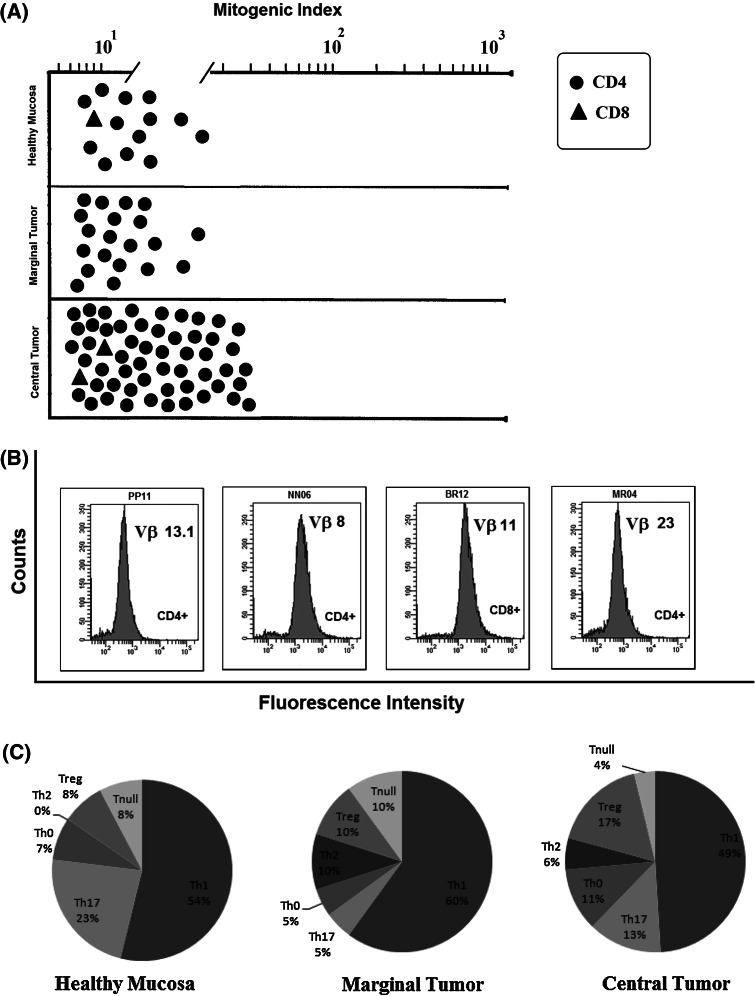
As ENO1 is a PC-associated antigen that induces an integrated humoral and cellular response in PC patients [8], T cell clones obtained were assayed for proliferation in response to ENO1. From the 505 Tcc tested, 90 (18 %) (Table 1) clonally proliferated in response to ENO1: 14/149 (9 %) from HM, 20/135 (15 %) from MT and 56/221 (25 %) from CT (Fig. 3a).
Fig. 3.
Clonality and functional profile of TIL-derived T cells reactive to ΕΝΟ1. a T cell clones were tested for proliferation in response to ENO1 in the presence of irradiated autologous APCs. Results are expressed as mean values of mitogenic index obtained in triplicate microcultures. b TCR Vβ chain collection of ENO1-specific T cell clones. The clonality of T cell clones reactive to ENO1 was analyzed by a panel of 24 monoclonal antibodies specific for human TCR Vβ chain families. T cell blasts from each clone were divided into aliquots and stained with each monoclonal antibody and the appropriate isotype controls. c Functional profile distribution of CD4+ ENO1-specific T cell clones
Evidence for clonality of ENO1-specific T cell clones was provided by the cytofluorimetric patterns of single TCR Vß expression shown by these clones. Each T cell clone was stained by only one of the TCR Vß chain-specific monoclonal antibodies, showing a single peak of fluorescence intensity (Fig. 3b).
All patients had detectable serum anti-ENO1 IgG (Table 1), and most interestingly, those with higher titers had the highest percentage of ENO1-specific T clones (in particular patient 9) with a Spearman’s correlation coefficient of 0.3. Also, the PBMCs of 10 patients (67 %) with pancreatic cancer proliferated in the presence of ENO1 (Table 1) compared to just 3 out of 15 (20 %) from healthy controls.
Of the 90 ENO1-specific Tcc, three were CD8+: one producing IL-17, IFN-γ and IL-4 from HM and two (Tc 0 and Tc null) from CT. The majority of the remaining 87 ENO1-specific CD4+ Tcc displayed a Th1 profile (52 %), with similar percentages in each of the three areas: 54 % (HM), 60 % (MT) and 49 % (CT) (Fig. 3c). Thirteen percent of these ENO1-specific CD4+ Tcc secreted IL-17 (Th17) and 73 % also produced IFN-γ; notably, the percentage of Th17-specific ENO1 Tcc were almost double in the HM (23 %) compared to the CT (13 %) (p = 0.05). Nine percent of the ENO1-specific CD4+ Tcc secreted IFN-γ and IL-4 (Th0); remarkably, 88 % of overall Th0 Tcc were isolated from CT. Seven percent were T null and 12 % Tregs, with an increasing percentage from HM (8 %) to CT (17 %, p = 0.001). The remaining 7 % of ENO1-specific CD4+ T cell clones only secreted IL-4 (Th2) and were all isolated from MT and CT (Fig. 3c).
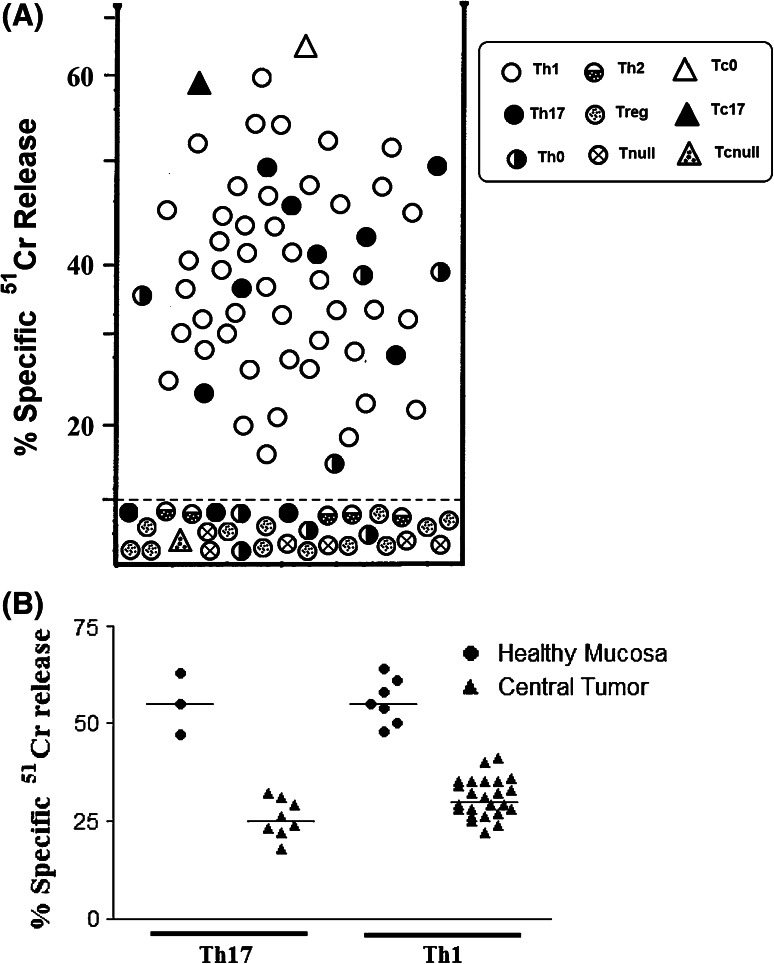
Since most of the antigen-activated T cell clones producing IFN-γ are able to exert perforin-mediated cytotoxicity against autologous APC, we assessed the cytolytic potential of ENO1-specific Tcc using ENO1-pulsed 51Cr-labeled autologous EBV-B cells as targets. At an effector-to-target ratio of 10:1, all 45 Th1 and four out of eight Th0 clones lysed their targets (Fig. 4a). Among the Th17 population, only the IL-17/IFN-γ double producers (73 %) killed the target cells. None of the five Th2, 12 Treg and six Tnull cells lysed their targets. Likewise, two out of the three CD8+ clones destroyed the EBV-B cells, while the Tc null were unable to do so. As expected, in the same experimental conditions but in the presence of anti-HLA class II blocking mAbs, all the ENO1-specific CD4+ Tcc were unable to destroy the EBV-B cells, while the two CD8+ ENO1-specific Tcc maintained cytotoxicity activity, which was only lost in the presence of anti-HLA class II blocking mAbs (data not shown).
Fig. 4.
Cytotoxic activity of ENO1-specific T cell clones. a ENO1-induced cytotoxicity by TIL-derived ENO1-specific CD4+ and CD8+ T cell clones. To assess their perforin-mediated cytotoxicity, T cell clones were co-cultured with 51Cr-labeled autologous EBV-B cells pulsed with ENO1 for 8 h. Results represent mean values of percentage-specific 51Cr release at an effector-to-target ratio of 10-to-1 in triplicate microcultures. The dotted line represents 3 SD over the mean 51Cr release in cultures with 51Cr-labeled autologous unpulsed EBV-B cells. b Evaluation of the specific cytolytic activity of T cell clones obtained from HM compared with those obtained from CT. We compared the specific release of 51Cr of Th17 and Th1 isolated from healthy mucosa and tumor tissue
Of particular interest, comparing the specific cytolytic activity of Tcc obtained from HM with those obtained from CT, we have found that the specific release of 51Cr was significantly higher in Th17 and Th1 isolated from healthy mucosa compared to that released from the tumor tissue counterparts; this suggests that the tumor microenvironment negatively influences the cytolytic activity of T lymphocytes reactive to tumor cells (Fig. 4b).
As effector T cells can also kill their targets by Fas–FasL-mediated apoptosis, the ability of ENO1-specific Tcc to induce 51Cr release of Fas+ Panc1 cells was evaluated. Upon mitogen activation, 45 of 87 CD4+ (51 %) and one of three CD8+ ENO1-specific Tcc induced apoptosis in target cells (Table 2). Among the 45 CD4+ T cells able to induce apoptosis in Panc1 cells, 37 were Th1, six were Th17 and two were Th0, but none were Th2, Tregs or T null cells. The unique CD8+ ENO1-specific clone, competent for killing Panc1 cells by apoptosis, showed a Tc0 profile (Table 2). The role of Fas–FasL interaction in 51Cr release was confirmed by its inhibition (range 38.3–57.6 %) using a blocking anti-Fas antibody (data not shown).
Table 2.
Proapoptotic activity against the pancreatic cancer cell line Panc1
| ENO1-specific Tcc profile | % Lysis with ratio of 2.5 | % Lysis with ratio of 5 | % Lysis with ratio of 10 |
|---|---|---|---|
| Th1 | 41 ± 4 | 52 ± 4 | 72 ± 5 |
| Th1 | 38 ± 5 | 47 ± 6 | 68 ± 4 |
| Th1 | 43 ± 6 | 54 ± 7 | 73 ± 6 |
| Th1 | 44 ± 3 | 51 ± 5 | 78 ± 6 |
| Th1 | 46 ± 5 | 53 ± 4 | 65 ± 7 |
| Th1 | 48 ± 6 | 56 ± 3 | 76 ± 3 |
| Th1 | 47 ± 7 | 52 ± 2 | 69 ± 4 |
| Th1 | 35 ± 2 | 48 ± 6 | 65 ± 5 |
| Th1 | 42 ± 4 | 53 ± 7 | 64 ± 4 |
| Th1 | 41 ± 5 | 58 ± 4 | 73 ± 7 |
| Th1 | 40 ± 6 | 51 ± 5 | 71 ± 7 |
| Th1 | 38 ± 7 | 56 ± 6 | 70 ± 8 |
| Th1 | 39 ± 3 | 49 ± 8 | 68 ± 7 |
| Th1 | 42 ± 5 | 51 ± 7 | 64 ± 4 |
| Th1 | 46 ± 4 | 53 ± 8 | 66 ± 5 |
| Th1 | 37 ± 6 | 49 ± 4 | 67 ± 5 |
| Th1 | 39 ± 4 | 54 ± 4 | 74 ± 3 |
| Th1 | 37 ± 5 | 55 ± 5 | 70 ± 2 |
| Th1 | 44 ± 6 | 57 ± 3 | 72 ± 4 |
| Th1 | 45 ± 4 | 61 ± 7 | 73 ± 5 |
| Th1 | 42 ± 7 | 58 ± 8 | 74 ± 6 |
| Th1 | 47 ± 8 | 62 ± 4 | 78 ± 7 |
| Th1 | 40 ± 6 | 52 ± 5 | 76 ± 8 |
| Th1 | 38 ± 4 | 46 ± 5 | 64 ± 7 |
| Th1 | 39 ± 6 | 45 ± 6 | 68 ± 4 |
| Th1 | 37 ± 4 | 53 ± 7 | 69 ± 5 |
| Th1 | 44 ± 8 | 51 ± 5 | 67 ± 3 |
| Th1 | 41 ± 5 | 56 ± 6 | 72 ± 6 |
| Th1 | 42 ± 6 | 55 ± 7 | 74 ± 6 |
| Th1 | 42 ± 7 | 61 ± 7 | 79 ± 7 |
| Th1 | 44 ± 3 | 60 ± 4 | 72 ± 5 |
| Th1 | 39 ± 4 | 54 ± 8 | 68 ± 4 |
| Th1 | 38 ± 5 | 54 ± 4 | 65 ± 3 |
| Th1 | 43 ± 6 | 58 ± 5 | 73 ± 2 |
| Th1 | 41 ± 8 | 56 ± 6 | 74 ± 5 |
| Th1 | 42 ± 3 | 54 ± 6 | 68 ± 5 |
| Th1 | 39 ± 6 | 48 ± 4 | 61 ± 6 |
| Th17 | 35 ± 4 | 45 ± 4 | 58 ± 7 |
| Th17 | 29 ± 5 | 46 ± 5 | 62 ± 8 |
| Th17 | 33 ± 6 | 42 ± 7 | 67 ± 8 |
| Th17 | 36 ± 8 | 52 ± 8 | 63 ± 4 |
| Th17 | 27 ± 4 | 41 ± 6 | 57 ± 5 |
| Th17 | 34 ± 5 | 45 ± 5 | 54 ± 5 |
| Th0 | 35 ± 6 | 44 ± 4 | 56 ± 4 |
| Th0 | 33 ± 3 | 42 ± 7 | 57 ± 3 |
| Tc0 | 37 ± 2 | 51 ± 6 | 71 ± 7 |
Ratio = effector to target. The percentage of lysis was measured as 51Cr release. Results represent mean values (±SD) of three repeated experiments
α-Enolase-specific Tregs inhibit effector T cells
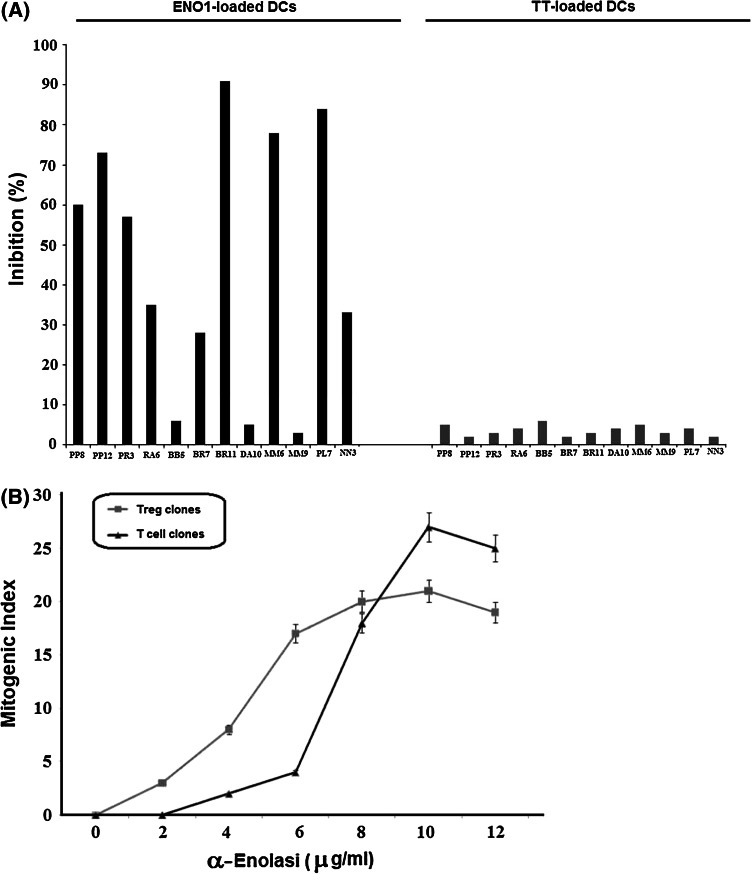
We have demonstrated that 14 % of the ENO1-specific Tcc present a Treg phenotype. For each Treg clone, we tested the ability to inhibit the proliferative response of ENO1-specific effector Tcc in autologous stimulation.
Nine out of 12 (75 %) ENO1-specific Treg clones inhibited proliferation of autologous ENO1-specific effector Tcc. In greater detail, 42 % of the ENO1-specific Treg clones exerted a suppressive activity higher than 50, and 25 % of clones showed an activity between 20 and 50 %. However, upon tetanus toxoid stimulation, none of the ENO1-specific Treg clones inhibited the ENO1-specific T cell proliferation (Fig. 5a).
Fig. 5.
Evaluation of characteristics of ENO1-specific Treg clones. a ENO1-specific Treg clones suppress the proliferation of ENO1-specific effector T cell clones. We used a suppression test with ENO1-loaded DCs and TT-loaded DCs as controls. b Dose–response curve of proliferation of ENO1-specific T cell clones. We compared the proliferation between blasts of Treg and effector T cell clones after different doses of ENO1. The results represent mean values (±SD) of three repeated experiments
To address whether TCRs of ENO1-specific Tregs derived from T cells have a high-affinity interaction with self-antigens [29], we evaluated the minimum antigen concentration capable of triggering proliferation of the ENO1-specific Treg and T effector clones. Treg clone blasts began to proliferate at low doses of ENO1: a concentration of 2 μg/ml was able to promote Treg proliferation, reaching maximum proliferation at around 8 μg/ml ENO1. In contrast, blasts of ENO1-specific effector T cell clones began to proliferate at concentrations of around 5 μg/ml ENO1, peaking at 10 μg/ml (Fig. 5b). These data suggest that ENO1-specific Tregs inhibit ENO1-specific effector T cells because of their higher affinity for the antigen.
Discussion
In this study, we analyzed the intra-tumoral T cell response specific to ENO1, a newly discovered antigen associated with PC, able to elicit an anti-PC T cell response [10]. From 15 patients with PC, we isolated and characterized TILs from three different sites: the central tumor, the peripheral tumor and surrounding healthy mucosa.
Overall analysis of TIL clonal progeny has shown a large predominance of CD4+ T cells compared to the CD8+ T cell population, whose number notably doubles from healthy mucosa to central tumor.
The TIL cytokine profile demonstrated that most CD4+ and CD8+ Tcc predominantly produced IFN-γ and accumulated in CT, while a considerable fraction (20 %) of CD4+ Tcc secreted IL-4 or IL-4 along with IFN-γ. Recent data demonstrated that not only the tumor cells alone but also cancer-associated fibroblasts and dendritic cells favor Th2 cell polarization in PC patients [30]. In other words, in human PC, there is a complex cross talk among tumor cells and cells of the PC microenvironment which influences the anti-tumor immune response and significantly correlates with poor survival. Accordingly, we observed that the percentage of T cells producing IL-4 are greater in the neoplastic tissue compared to HM: 25 versus 15 %, respectively. However, the highest percentage of IL-17-secreting CD4+ and CD8+ Tcc were obtained from HM. Conversely, the increased number of Tregs and Tc regs in CT confirms the strong immunosuppressive microenvironment in PC [26, 31] and accounts for the functional paralysis of Th1/Th17 effector cells recruited from HM.
The role of Th17 cells in cancer remains a controversial issue: an increase in Th17 cells has been detected in certain human cancers such as ovarian, breast, colon and melanoma [32, 33]. Conversely, levels of tumor-infiltrating Th17 cells were reduced in advanced ovarian cancer patients, which appeared to correlate with a positive outcome [34]. A low number of Th17 cells have been documented in HER2-positive breast cancer patients [35] as well as in the tumor microenvironment of hematopoietic tumors, where malignant B cells may up-regulate Tregs and inhibit Th17 cells [34, 36]. In this study, we demonstrated an increased number of effector Th17 cells in HM and that the specific cytotoxic activity was significantly higher in Th17/Th1 cells isolated from healthy mucosa compared to tumor tissue counterparts. Taken together, these data may suggest an active recruitment of Th17 cells from HM with a protective role, suppressed by the immunosuppressive PC microenvironment.
The role of Th17 in the cancer response is uncertain, a study in prostate cancer demonstrated that Th17 cells that infiltrate the tumor inversely correlates with the Gleason score, suggesting an antitumor effect of Th17 cells in cancer development [37]. However, it has also been shown that IL-17 promotes the tumorigenicity of human cervical tumors in nude mice while inhibiting the growth of the hematopoietic tumors, mastocytoma P815 and plasmocytoma in immunocompetent mice [36, 38]. In others words, it appears that Th17 cells are capable of both promoting, as suggested by the angiogenic role of IL-17 [39], and inhibiting cancer progression. The antitumor activity of Th17 cells may be implemented by means of a T cell-dependent mechanism [36] or by facilitating dendritic cell recruitment to tumor tissue, thus promoting activation of tumor-specific cytotoxic T lymphocytes (CTL) [40]. Th17 cells may also contribute to protective anti-tumor immunity by inducing Th1-type chemokines and stimulating CXCL9 and CXCL10 production to recruit effector cells to the tumor microenvironment. To this effect, recent data have demonstrated that Th17 cells isolated from hepatocarcinoma tissues simultaneously produced IFN-γ [41] as well as IL-17-secreting T cell clones obtained from long-term survivor patients after immunotherapy [42]. Accordingly, our data demonstrated that almost all Th17 clones isolated from pancreatic cancer tissue produce IFN-γ and, more importantly, also have cytotoxic activity, suggesting an anti-tumor protective role.
Interestingly, it has been shown that there were increased levels of Th17 cells during treatment with trastuzumab in patients with breast cancer [43] or during treatment with tremelimumab in metastatic melanoma [44]. Alvarez et al. [40] demonstrated that fusion of dendritic cells and tumor cells (FC) transduced with adenovirus encoding CD40L (Adv-CD40L) increased the Th17 response and enhanced the antitumor effect of FC vaccines in a murine lymphoma model. Moreover, Derhovanessian et al. [45] observed a highly significant correlation between an increased frequency of IL-17-producing T cells pre-vaccination and a shorter time to metastatic progression following immunotherapy. Taken together, these data suggest the important involvement of Th17 cells in response to cancer immunotherapy.
The peripheral blood of PC patients displays serum anti-α-ENO1 IgG autoantibodies and ENO1-specific T cells [9]. Here, we have provided the first evidence that PC patients present intra-tumoral ENO1-specific T cells of levels of up to 20 % of the total TIL-derived T cell clones. Of the 90 ENO1-specific T cell clones examined, only three were CD8+ and most of the remaining 87 CD4+ Tcc showed a Th1 profile with cytotoxic activity.
The Th17 ENO1-population is of considerable interest, as most of these cells synthesized IFN-γ, and was twice as much expressed in HM compared to CT. In contrast, the vast majority of the ENO1-specific Th0 cells and the entire population of Th2 cell clones were only present in CT.
In addition, we showed that all Th1 cell clones killed their targets, compared to only half of the Th0 clones and none of the Th2 clones. Among the Th17 population, only the IL-17/IFN-γ double producers killed their target cells, and of particular interest, the specific cytolytic potential of Th17 and Th1 cells of HM was significantly higher compared to that of ENO1-specific T cells isolated from CT. These data confirm that the tumor microenvironment negatively influences the cytolytic activity of T lymphocytes reactive to tumor cells [26, 31]. It is noteworthy that about 20 % of ENO1-specific CD4+ Tcc were T null or Tregs and that none of these Tcc was capable of lysing their targets. Also, the percentage of ENO1-specific Tregs significantly increase from HM (8 %) to CT (17 %).
In other words, while the levels of effector Th17 cells decrease from HM to tumor tissue, Th2 and Th0 cells, being unable to contrast cancer cells, increase in parallel with Tregs which serves to favor tumor progression [30, 31]. In fact, we demonstrated that the most ENO1-Tregs efficiently suppressed the proliferative response of effector ENO1-specific T cells in vitro and noticed that 86 % of patients with a low ENO1-specific Treg/Teffector ratio survived more than 10 months compared to those with a high ratio that survived less than 10 months (data not shown).
In conclusion, we have shown for the first time that in PC patients, the levels of ENO1-specific Treg cells increase and functionally attenuate the recruited specific effector Th17 and Th1 cells. In addition, our data allow us to hypothesize that certain approaches, such as ENO1-vaccination, in combination with strategies aimed at counteracting the effects of Tregs and improving Th17/Th1 effector functions [42, 44] in the tumor microenvironment could be an innovative and efficacious way to treat patients with pancreatic cancer.
Acknowledgments
This work is dedicated to the memory of Professor Gianfranco del Prete, who was a valuable researcher and teacher. We would like to thank Dr. Radhika Srinivasan for accurate editing of the manuscript. This work was supported by grants from the Italian Ministry of University and Research (PRIN 2009), the Italian Ministry of Health (Progetto Integrato Oncologia), the University of Florence, the Regione Piemonte (BIOTHER, IMMONC, Ricerca Sanitaria Finalizzata), the Associazione Italiana Ricerca sul Cancro (AIRC IG n. 11643 and 5 per 1000 n. 12182), the University of Torino-Progetti di Ateneo 2011 (grant Rethe-ORTO11RKTW), the Istituto Superiore di Sanità and European Community, the Seventh Framework Program, and the European Pancreatic Cancer-Tumor-Microenvironment Network (EPC-TM-Net, nr. 256974) and the Fondazione Internazionale di Medicina Sperimentale.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 2.Goonetilleke KS, Siriwardena AK. Nationwide questionnaire survey of the contemporary surgical management of pancreatic cancer in the United Kingdom & Ireland. Int J Surg. 2007;5:147–151. doi: 10.1016/j.ijsu.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Moon HJ, An JY, Heo JS, Choi SH, Joh JW, Kim YI. Predicting survival after surgical resection for pancreatic ductal adenocarcinoma. Pancreas. 2006;32:37–43. doi: 10.1097/01.mpa.0000194609.24606.4b. [DOI] [PubMed] [Google Scholar]
- 4.Laheru D, Lutz E, Burke J, Biedrzycki B, Solt S, Onners B, Tartakovsky I, Nemunaitis J, Le D, Sugar E, Hege K, Jaffee E. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin Cancer Res. 2008;14:1455–1463. doi: 10.1158/1078-0432.CCR-07-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramanathan RK, Lee KM, McKolanis J, Hitbold E, Schraut W, Moser AJ, Warnick E, Whiteside T, Osborne J, Kim H, Day R, Troetschel M, Finn OJ. Phase I study of a MUC1 vaccine composed of different doses of MUC1 peptide with SB-AS2 adjuvant in resected and locally advanced pancreatic cancer. Cancer Immunol Immunother. 2005;54:254–264. doi: 10.1007/s00262-004-0581-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jung S, Schluesener HJ. Human T lymphocytes recognize a peptide of single point-mutated, oncogenic ras proteins. J Exp Med. 1991;173:273–276. doi: 10.1084/jem.173.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakatsura T, Senju S, Ito M, Nishimura Y, Itoh K. Cellular and humoral immune responses to a human pancreatic cancer antigen, coactosin-like protein, originally defined by the SEREX method. Eur J Immunol. 2002;32:826–836. doi: 10.1002/1521-4141(200203)32:3<826::AID-IMMU826>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 8.Wobser M, Keikavoussi P, Kunzmann V, Weininger M, Andersen MH, Becker JC. Complete remission of liver metastasis of pancreatic cancer under vaccination with a HLA-A2 restricted peptide derived from the universal tumor antigen survivin. Cancer Immunol Immunother. 2006;55:1294–1298. doi: 10.1007/s00262-005-0102-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suso EM, Dueland S, Rasmussen AM, Vetrhus T, Aamdal S, Kvalheim G, Gaudernack G. hTERT mRNA dendritic cell vaccination: complete response in a pancreatic cancer patient associated with response against several hTERT epitopes. Cancer Immunol Immunother. 2011;60:809–818. doi: 10.1007/s00262-011-0991-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cappello P, Tomaino B, Chiarle R, Ceruti P, Novarino A, Castagnoli C, Migliorini P, Perconti G, Giallongo A, Milella M, Monsurrò V, Barbi S, et al. An integrated humoral and cellular response is elicited in pancreatic cancer by alpha-enolase, a novel pancreatic ductal adenocarcinoma-associated antigen. Int J Cancer. 2009;125:639–648. doi: 10.1002/ijc.24355. [DOI] [PubMed] [Google Scholar]
- 11.Capello M, Ferri-Borgogno S, Cappello P, Novelli F. α-Enolase: a promising therapeutic and diagnostic tumor target. FEBS J. 2011;278:1064–1074. doi: 10.1111/j.1742-4658.2011.08025.x. [DOI] [PubMed] [Google Scholar]
- 12.Tomaino B, Cappello P, Capello M, Fredolini C, Sperduti I, Migliorini P, Salacone P, Novarino A, Giacobino A, Ciuffreda L, Alessio M, Nisticò P, et al. Circulating autoantibodies to phosphorylated alpha-enolase are a hallmark of pancreatic cancer. J Proteome Res. 2011;10:105–112. doi: 10.1021/pr100213b. [DOI] [PubMed] [Google Scholar]
- 13.Cappello P, Rolla S, Chiarle R, Principe M, Cavallo F, Perconti G, Feo S, Giovarelli M, Novelli F (2013) Vaccination with ENO1 DNA prolongs survival of genetically engineered mice with pancreatic cancer. Gastroenterology. doi:10.1053/j.gastro.2013.01.020. [Epub ahead of print] [DOI] [PubMed]
- 14.Linehan DC, Goedegebuure PS. CD25+ CD4+ regulatory T-cells in cancer. Immunol Res. 2005;32:155–168. doi: 10.1385/IR:32:1-3:155. [DOI] [PubMed] [Google Scholar]
- 15.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, Linehan DC. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 16.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 17.Sharma MD, Hou DY, Liu Y, Koni PA, Metz R, Chandler P, Mellor AL, He Y, Munn DH. Indoleamine 2,3-dioxygenase controls conversion of Foxp3+ Tregs to TH17-like cells in tumor-draining lymph nodes. Blood. 2009;113:6102–6111. doi: 10.1182/blood-2008-12-195354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 19.Kryczek I, Wei S, Zou L, Altuwaijri S, Szeliga W, Kolls J, Chang A, Zou W. Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J Immunol. 2007;178:6730–6733. doi: 10.4049/jimmunol.178.11.6730. [DOI] [PubMed] [Google Scholar]
- 20.Zhang B, Rong G, Wei H, Zhang M, Bi J, Ma L, Xue X, Wei G, Liu X, Fang G. The prevalence of Th17 cells in patients with gastric cancer. Biochem Biophys Res Commun. 2008;374:533–537. doi: 10.1016/j.bbrc.2008.07.060. [DOI] [PubMed] [Google Scholar]
- 21.Koyama K, Kagamu H, Miura S, Hiura T, Miyabayashi T, Itoh R, Kuriyama H, Tanaka H, Tanaka J, Yoshizawa H, Nakata K, Gejyo F. Reciprocal CD4+ T-cell balance of effector CD62Llow CD4+ and CD62LhighCD25+ CD4+ regulatory T cells in small cell lung cancer reflects disease stage. Clin Cancer Res. 2008;14:6770–6779. doi: 10.1158/1078-0432.CCR-08-1156. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakaguchi S. Immunologic self-tolerance maintained by CD25+ CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 23.Shevach EM. Certified professionals: CD4(+)CD25(+) suppressor T cells. J Exp Med. 2001;193:F41–F46. doi: 10.1084/jem.193.11.F41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ikemoto T, Yamaguchi T, Morine Y, Imura S, Soejima Y, Fujii M, Maekawa Y, Yasutomo K, Shimada M. Clinical roles of increased populations of Foxp3+ CD4+ T cells in peripheral blood from advanced pancreatic cancer patients. Pancreas. 2006;33:386–390. doi: 10.1097/01.mpa.0000240275.68279.13. [DOI] [PubMed] [Google Scholar]
- 25.Viehl CT, Moore TT, Liyanage UK, Frey DM, Ehlers JP, Eberlein TJ, Goedegebuure PS, Linehan DC. Depletion of CD4+ CD25+ regulatory T cells promotes a tumor-specific immune response in pancreas cancer bearing mice. Ann Surg Oncol. 2006;13:1252–1258. doi: 10.1245/s10434-006-9015-y. [DOI] [PubMed] [Google Scholar]
- 26.Warshaw AL, Fernández-del Castillo CN. Pancreatic carcinoma. N Engl J Med. 1992;26:455–465. doi: 10.1056/NEJM199202133260706. [DOI] [PubMed] [Google Scholar]
- 27.Amedei A, Della Bella C, Niccolai E, Stanflin N, Benagiano M, Duranti R, Del Prete G, Murphy TF, D’Elios MM. Moraxella catarrhalis-specific Th1 cells in BAL fluids of chronic obstructive pulmonary disease patients. Int J Immunopathol Pharmacol. 2009;22:979–990. doi: 10.1177/039463200902200413. [DOI] [PubMed] [Google Scholar]
- 28.Amedei A, Niccolai E, Della Bella C, Cianchi F, Trallori G, Benagiano M, Bencini L, Bernini M, Farsi M, Moretti R, Del Prete G, D’Elios MM. Characterization of tumor antigen peptide-specific T cells isolated from the neoplastic tissue of patients with gastric adenocarcinoma. Cancer Immunol Immunother. 2009;58:1819–1830. doi: 10.1007/s00262-009-0693-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu P, Haymaker CL, Divekar RD, Ellis JS, Hardaway J, Jain R, Tartar DM, Hoeman CM, Cascio JA, Ostermeier A, Zaghouani H. Fetal exposure to high-avidity TCR ligand enhances expansion of peripheral T regulatory cells. J Immunol. 2008;181:73–80. doi: 10.4049/jimmunol.181.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Monte L, Reni M, Tassi E, Clavenna D, Papa I, Recalde H, Braga M, Di Carlo V, Doglioni C, Protti MP. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med. 2011;208:469–478. doi: 10.1084/jem.20101876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Bernstorff W, Voss M, Freichel S, Schmid A, Vogel I, Jöhnk C, Henne-Bruns D, Kremer B, Kalthoff H. Systemic and local immunosuppression in pancreatic cancer patients. Clin Cancer Res. 2001;7:925s–932s. [PubMed] [Google Scholar]
- 32.Miyahara Y, Odunsi K, Chen W, Peng G, Matsuzaki J, Wang RF. Generation and regulation of human CD4+ IL-17-producing T cells in ovarian cancer. Proc Natl Acad Sci USA. 2008;105:15505–15510. doi: 10.1073/pnas.0710686105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su X, Ye J, Hsueh EC, Zhang Y, Hoft DF, Peng G. Tumor microenvironments direct the recruitment and expansion of human Th17 cells. J Immunology. 2010;184:1630–1641. doi: 10.4049/jimmunol.0902813. [DOI] [PubMed] [Google Scholar]
- 34.Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, Huang E, Finlayson E, Simeone D, Welling TH, Chang A, Coukos G, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141–1149. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horlock C, Stott B, Dyson PJ, Morishita M, Coombes RC, Savage P, Stebbing J. The effects of trastuzumab on the CD4+ CD25+ FoxP3+ and CD4+ IL17A+ T-cell axis in patients with breast cancer. Br J Cancer. 2009;100:1061–1067. doi: 10.1038/sj.bjc.6604963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benchetrit F, Ciree A, Vives V, Warnier G, Gey A, Sautès-Fridman C, Fossiez F, Haicheur N, Fridman WH, Tartour E. Interleukin-17 inhibits tumor cell growth by means of a T-cell-dependent mechanism. Blood. 2002;99:2114–2121. doi: 10.1182/blood.V99.6.2114. [DOI] [PubMed] [Google Scholar]
- 37.Sfanos KS, Bruno TC, Maris CH, Xu L, Thoburn CJ, DeMarzo AM, Meeker AK, Isaacs WB, Drake CG. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res. 2008;4:3254–3261. doi: 10.1158/1078-0432.CCR-07-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tartour E, Fossiez F, Joyeux I, Galinha A, Gey A, Claret E, Sastre-Garau X, Couturier J, Mosseri V, Vives V, Banchereau J, Fridman WH, et al. Interleukin 17, a T-cell-derived cytokine, promotes tumorigenicity of human cervical tumors in nude mice. Cancer Res. 1999;59:3698–3704. [PubMed] [Google Scholar]
- 39.Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620–2627. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 40.Alvarez E, Moga E, Barquinero J, Sierra J, Briones J. Dendritic and tumor cell fusions transduced with adenovirus encoding CD40L eradicate B-cell lymphoma and induce a Th17-type response. Gene Ther. 2009;17:469–477. doi: 10.1038/gt.2009.150. [DOI] [PubMed] [Google Scholar]
- 41.Kuang DM, Peng C, Zhao Q, Wu Y, Chen MS, Zheng L. Activated monocytes in peritumoral stroma of hepatocellular carcinoma promote expansion of memory T helper 17 cells. Hepatology. 2010;51:154–164. doi: 10.1002/hep.23291. [DOI] [PubMed] [Google Scholar]
- 42.Kyte JA, Trachsel S, Risberg B, Thor Straten P, Lislerud K, Gaudernack G. Unconventional cytokine profiles and development of T cell memory in long-term survivors after cancer vaccination. Cancer Immunol Immunother. 2009;58:1609–1626. doi: 10.1007/s00262-009-0670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, Hwu P, Restifo NP, Overwijk WW, Dong C. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31:787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.von Euw E, Chodon T, Attar N, Jalil J, Koya RC, Comin-Anduix B, Ribas A. CTLA4 blockade increases Th17 cells in patients with metastatic melanoma. J Transl Med. 2009;7:35. doi: 10.1186/1479-5876-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Derhovanessian E, Adams V, Hähnel K, Groeger A, Pandha H, Ward S, Pawelec G. Pretreatment frequency of circulating IL-17+ CD4+ T-cells, but not Tregs, correlates with clinical response to whole-cell vaccination in prostate cancer patients. Int J Cancer. 2009;125:1372–1379. doi: 10.1002/ijc.24497. [DOI] [PubMed] [Google Scholar]