Abstract
The introduction of autologous stem cell transplantation (SCT) and novel drugs has improved overall survival in multiple myeloma (MM) patients. However, minimal residual disease (MRD) remains and most patients eventually relapse. Myeloma plasma cells express tumor-associated antigens (TAA), which are interesting targets for immunotherapy. In this phase 1 study, we investigated the safety and immunological effects of TAA-mRNA-loaded dendritic cell (DC) vaccination for treatment for MRD in MM after SCT. Mature monocyte-derived DCs were pulsed with keyhole limpet hemocyanin (KLH) and electroporated with MAGE3, Survivin or B-cell maturation antigen (BCMA) mRNA. Twelve patients were vaccinated three times with intravenous (5–22 × 106 DCs) and intradermal vaccines (4–11 × 106 DCs), at biweekly intervals. Immunological responses were monitored in blood and delayed-type hypersensitivity (DTH) biopsies. All patients developed strong anti-KLH T-cell responses, but not KLH antibodies. In 2 patients, vaccine-specific T cells were detected in DTH biopsies. In one patient, we found MAGE3-specific CD4+ and CD8+ T cells, and CD3+ T cells reactive against BCMA and Survivin. In the other patient, we detected low numbers of MAGE3 and BCMA-reactive CD8+ T cells. Vaccination was well tolerated with limited toxicity. These findings illustrate that TAA-mRNA-electroporated mature DCs are capable of inducing TAA-T-cell responses in MM patients after SCT.
Keywords: Dendritic cell vaccination, Multiple myeloma, MAGE3, Survivin, BCMA, Tumor-associated antigen
Introduction
Multiple myeloma remains a largely incurable disease despite the improvement in therapy after the introduction of high-dose chemotherapy and the novel immunomodulatory agents (IMIDs) and proteasome inhibitors [1, 2]. Allogeneic SCT can prolong disease remission and may even cure the disease due to the graft-versus-myeloma effect constituted by alloreactive T cells. This immune sensitivity of MM has been clearly demonstrated by the observation of clinical and even molecular remissions following donor lymphocyte infusions in patients with relapsed MM [3, 4]. However, allogeneic SCT is associated with significant morbidity and mortality and currently predominantly applied to patients with relapsed disease.
Dendritic cell (DC) therapy is a promising adjuvant immunotherapy with low toxicity in patients with stable or residual disease after chemotherapy and autologous SCT. As DCs are the most professional antigen-presenting cells, they can effectively initiate and reactivate T-cell-based immune responses [5, 6]. The awareness that the T-cell repertoires of healthy individuals and cancer patients contain potentially self- and tumor-reactive T-cell precursors has directed clinical investigation toward boosting these T-cell responses using DC vaccines pulsed with tumor-associated antigens (TAA). Thereby, selective eradication of (residual) malignant cells could be mediated, and tumor-reactive memory is formed. Various potential TAA have been identified in MM, including the clonal immunoglobulin idiotype Id protein, members of the cancer germ-line family (Mage, Gage, Lage, NY-ESO-1), MUC1, hTERT, Survivin, PRAME, Sp17, DKK1 and B-cell maturation antigen (BCMA) [7].
The feasibility and safety of TAA-loaded DC vaccination in cancer patients has been shown for a number of malignant diseases including malignant melanoma and low-grade non-Hodgkin lymphoma, where specific immune responses to the TAA and objective tumor regressions have been demonstrated [8–10]. In MM, the patient’s tumor-specific idiotype protein has been mostly explored in DC-based vaccination trials. These studies showed that Id-pulsed DCs are capable of inducing both humoral and cellular immune responses against the Id protein with limited toxicity [11–15]. However, in comparison with follicular NHL, immunological and clinical responses in MM have been disappointing. A possible explanation may be the lack of Id-specific T-cell precursors in MM patients, due to tolerance and deletion as a result of the high amount of secreted free Id protein. Furthermore, most studies have been performed using relatively immature DCs in small patient cohorts with predominantly advanced disease.
In this phase 1 study, we evaluate the safety and immunological effects of vaccination with mature DCs pulsed with keyhole limpet hemocyanin (KLH) protein and TAA mRNA in twelve MM patients with a complete response (CR) or partial response (PR) after high-dose chemotherapy and autologous SCT. We exploited the TAA MAGE3, Survivin and BCMA because of their high and selective expression on MM plasma cells, and their reported immunogenic potential [7, 16–18]. Immunological monitoring for T-cell responses included testing for KLH- and TAA-specific T cells in skin biopsies taken from delayed-type hypersensitivity (DTH) sites.
Materials and methods
Patients
Twelve patients with MM, treated with induction chemotherapy and high-dose melphalan in our centre, participated in this phase 1 study. Patient and treatment characteristics are summarized in Table 1. All eligible patients had stage II or III MM, according to the criteria of Durie and Salmon [19], with measurable M-protein at the time of diagnosis. Patients who achieved CR, very good partial response (VGPR) or PR following intensive therapy, including high-dose melphalan and autologous SCT, were considered eligible [20]. The interval between autologous SCT and participation in this study was at least 6 months to allow for immune reconstitution. Moreover, patients had to have a WHO performance score of 3 or lower. Maintenance therapy if indicated by the treatment protocol, including interferon and thalidomide, was kept unchanged. Patients with a known allergy to shellfish were excluded from participation, because of the use of KLH as adjuvant in the vaccine preparation. The study protocol had been approved by the National Medical Ethical Committee (CCMO registration NL13547.000.06), and the study was registered at the Dutch Clinical Trial Registry (NTR1086). Written informed consent was obtained from all patients prior to enrollment. The patients were vaccinated three times with intravenous (i.v.) and intradermal (i.d.) vaccines at biweekly intervals. In the absence of disease progression requiring local or systemic therapy, revaccinations could be given after 6 months from the first DC vaccination cycle. Peripheral blood samples were collected at regular time points before and after DC vaccination, and skin biopsies from DTH test sites 1 week after the last DC vaccination were obtained in case of induration. Follow-up ended at July 31, 2012.
Table 1.
Patient characteristics
| Patient | Sex | Age (y) | Response after SCT | Interval SCT-DC vaccination (months) | Maintenance treatment | M-protein (g/l)a | Total follow-up (months)b | Time to next treatment (months)c |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 57 | PR | 25/44d | No | 13.1/19.9 | 53, deceased | 24 |
| 2 | M | 63 | PR | 39 | Thalidomide | 3.6 | 57, deceased | NA |
| 3 | M | 66 | VGPR | 47/53e | No | 5.5/8.9 | 99 | 9 |
| 4 | M | 66 | PR | 47 | No | 0 | 93 | NA |
| 5 | M | 57 | CR | 53 | No | 0 | 91 | NA |
| 6 | M | 64 | CR | 46 | No | 3.2 | 89 | 8 |
| 7 | F | 58 | PR | 28 | No | 11.6 | 61 | 33 |
| 8 | M | 64 | VGPR | 22 | Thalidomide | <2 | 48 | NA |
| 9 | M | 60 | CR | 10 | Thalidomide | <2 | 36 | NA |
| 10 | M | 60 | PR | 9 | Thalidomide | 4.6 | 26 | 17 |
| 11 | M | 49 | PR | 20 | Thalidomide | 2.0 | 38 | NA |
| 12 | M | 63 | PR | 9 | No | <2 | 21 | NA |
Patients characteristics: CR complete response, VGPR very good partial response, PR partial response, N/A not applicable, all according to the criteria of the International Myeloma Working Group
aValue at time of DC vaccination, <2 indicates that the M-protein can be detected, but not quantified; b Follow-up time since SCT; c Interval between first DC vaccination and start of next treatment because of an increase in M-protein; d This patient received revaccination because of suboptimal maturation of the DC vaccines infused in the first cycle; e This patient received revaccination because of an increase in M-protein, which did not require local or systemic therapy, despite an immunological response to the first vaccination cycle
Production of DC-based vaccine
Peripheral blood mononuclear cells (PBMC) were collected from patients by 9 L leukapheresis of peripheral blood using the COBE Spectra Aphaeresis System (Gambro BCT, Breda, the Netherlands). Thrombocytes were removed by washing the aphaeresis product using the CytoMate closed fluid management system (Baxter, Utrecht, the Netherlands) in our clean room facility according to good manufacturing practice (GMP) guidelines. Subsequently, PBMC were collected and washed with cold PBS (Baxter) until a PBMC/thrombocyte ratio of 10:1 or higher was obtained. In the meantime, the percentage of CD14+ cells within the PBMC population was determined using flow cytometry (FC500, Beckman Coulter, Fullerton, CA, USA). Then, PBMC were resuspended in X-VIVO-15 medium (Lonza, Verviers, Belgium) supplemented with 2 % virus-free heat-inactivated pooled human serum (HS; Sanquin blood bank, Nijmegen, the Netherlands), and monocytes were isolated by plastic adherence using 75 × 106 CD14+ cells per T225 flask (20 mL/flask). Non-adherent cells were removed by extensive washing with PBS, and X-VIVO-15/2 % HS supplemented with 500 U/mL IL-4 and 800 U/mL GM-CSF (both from Cellgenix, Freiburg, Germany) was added. Cells were harvested at day 3, counted and cultured at 0.5 × 106 c/mL in 6-well plates (2 ml/well) in X-VIVO/2 % HS containing 500 U/mL IL-4, 800 U/mL GM-CSF, in the absence (for delayed-type hypersensitivity (DTH) test) or presence of 50 μg/ml KLH subunits (Vacmune®, Biosyn Corporation, Fellbach, Germany). At day 7, DC maturation was induced in X-VIVO-15/2 % HS containing 500 U/mL IL-4, 800 U/mL GM-CSF, 5 ng/mL IL-1β, 15 ng/mL IL-6, 20 ng/mL TNF-α (all Cellgenix) and 1 μg/mL PGE2 (Pharmacia and Upjohn, Puurs, Belgium). Mature DCs were harvested at day 9 and analyzed for microbial contamination and phenotype.
In vitro mRNA transcription and DC loading
We subcloned the cDNA encoding the tumor-associated antigens (TAA), MAGE3, Survivin and BCMA into a pGEM4Z/A64 vector. The in vitro transcribed clinical-grade mRNAs were produced by Curevac (Tübingen, Germany) and stored in aliquots at −20 °C. Before mRNA electroporation, DCs were harvested with cold PBS and washed in phenol red free Optimem medium (Gibco Invitrogen, Carlsbad, CA, USA). Cells were subsequently resuspended to a concentration of 50–60 × 106 cells/mL in Optimem medium, and one-third was used for each TAA. Per electroporation 200 μl cell suspension was transferred to a 4-mm gene pulser cuvette (Biorad, Hercules, CA, USA) containing 20 μg mRNA. DCs were electroporated at 300 V, 150 μF using a Biorad Genepulser II (Biorad). The non-KLH-loaded DCs for DTH tests were separately electroporated with or without 20 μg MAGE3, Survivin or BCMA mRNA. Directly after electroporation, cells were resuspended in 4 mL pre-warmed phenol red free X-VIVO 15/6 % HS and incubated at 37 °C for 1 h. Then, part of the DCs were analyzed for expression of the electroporated TAA. For infusion and cryopreservation, all three batches of TAA-DCs were pooled and washed twice in infusion liquid (0.9 % NaCl solution containing 5 % human serum albumin; Albuman, Sanquin Blood bank, Amsterdam, the Netherlands). DCs for the DTH test were not pooled and cryopreserved separately. Eventually, one-third of the pooled DC vaccine was used for direct injection, while remaining cells were cryopreserved separately for subsequent vaccinations.
Flow cytometry
Phenotype and maturation state of DCs was analyzed by staining with the following antibodies: CD14 (clone TÜK4, Dako, Glostrup, Denmark), CD83 (clone HB15a), CD80 (clone MAB104), CD86 (clone HA5.2B7, all from Beckman Coulter, Fullerton, CA, USA), CD11c (clone KB90, Dako), CD40 (clone MAB89, Beckman Coulter), CCR7 (clone 150503, R&D Systems, Abingdon, UK), CD25 (clone B1.49.9), HLA-ABC (clone B9.12.1), HLA-DR (clone B8.12.2, all Beckman Coulter) or IgG1 FITC/PE dual-color isotype control (Dako). Cells were analyzed using the Coulter FC500 flow cytometer (Beckman Coulter) and CXP software. Mature DCs were characterized by high expression levels of MHC class I and II, CD80, CD83 and CD86 and absence of CD14.
To determine the expression levels of the different TAA upon electroporation, unless stated otherwise DCs were stored on ice, until staining with specific antibodies using either a direct or an indirect labeling approach. BCMA-DCs were directly stained with anti-BCMA FITC antibody (clone Vicky-1; Alexis Biochemicals, Enzo Life Sciences, Breda, the Netherlands) for 20 min at 4 °C, and after washing stored at 4 °C until flow cytometrical analysis. MAGE3-DCs and Survivin-DCs were washed twice with PBS and fixed for 10 min at room temperature in 4 % cold paraformaldehyde solution. After washing with PBS, cells were resuspended in 0.1 % saponin buffer and incubated for 30 min at 4 °C with mouse anti-hMAGE3 (clone 57B, kindly provided by Prof. dr. Giulio Spagnoli, University Hospital Basel, Basel, Switzerland) or anti-survivin PE (R&D systems, Abingdon, UK), respectively. Survivin-DCs were subsequently washed with saponin buffer and stored at 4 °C until flow cytometrical analysis. MAGE3-DCs were washed with saponin buffer and subsequently incubated with 1:100 diluted goat anti-mouse PE (Biosource, Life Technologies, Bleiswijk, the Netherlands) antibody for 30 min at 4 °C. After washing of the MAGE3-DCs, all DCs were analyzed on the FC500 flow cytometer.
Proliferative response to KLH
Cellular responses against KLH were measured in a proliferation assay. Briefly, PBMC isolated from blood samples obtained at regular time points before and after DC vaccination were restimulated in vitro with or without 50 μg/mL KLH subunits. After 5 days, 1 × 105 cells were plated in sixfold in 96-well round bottom plates (Corning Costar, Amsterdam, the Netherlands) in the presence of 0.5 μCi [3H]-thymidine (Perkin Elmer, Groningen, the Netherlands). After overnight incubation, [3H]-thymidine incorporation was measured using a 1205 Wallac Betaplate counter (Perkin Elmer). The stimulation index (SI) was calculated by dividing the counts of stimulated PBMC by that of non-stimulated PBMC. A SI ≥2 was considered positive.
Humoral response to KLH
Antibodies against KLH were measured in the serum of vaccinated patients by ELISA as described by Aarntzen et al. [21]. Briefly, 96-well NUNC MaxiSorb plates (NUNC™, Roskilde, Denmark) were coated overnight at 4 °C with 20 μg/mL KLH protein (Vacmune®) in PBS. After blocking and washing, different concentrations of patient serum (range 1:100–1:500,000) were added for 1 h at room temperature. Furthermore, isotype-specific calibration curves were included in each microtiter plate. Following extensive washing, specific antibodies (total IgG, IgG1, IgG2 and IgG4) labeled with horseradish peroxidase were allowed to bind for 1 h at room temperature. Then, the substrate 3,3′ 5,5-tetramethyl benzidine was added, and subsequently absorbance was measured in a Tecan Sunrise microtiter plate reader (Tecan, Giessen, the Netherlands) at 450 nm. A signal at a ≥1:400 dilution of the patient’s serum was considered positive.
Delayed-type hypersensitivity testing and ELISPOT
One to 2 weeks after the last immunization, a DTH test was carried out as described by de Vries et al. [22]. Briefly, 1.0 × 106 DCs loaded with KLH or electroporated with MAGE3, Survivin or BCMA mRNA were injected intradermally in the skin of the back of the patient at 4 different sites. The diameter (in millimeter) was measured after 48 h, each time by the same investigator. An induration of >5 mm and erythema was considered positive. In case of one or more positive DTH responses, 6-mm punch biopsies were obtained from all DTH sites under local anesthesia. These biopsies were subsequently scrambled and cultured in 2 mL Iscove’s modified Dulbecco’s medium (IMDM; Invitrogen) supplemented with 10 % HS and 100 U/mL IL-2 and 5 ng/mL IL-15 (both from Immunotools, Friesoythe, Germany) in a 24-well plate. Cells were cultured for 3–4 weeks, and half of the medium was replaced with fresh medium containing 200 U/mL IL-2 and 10 ng/mL IL-15 twice per week. Then, as indicated, CD3+, CD4+ and CD8+ T cells were isolated from these expanded cultures using MACS MicroBeads (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer’s instructions. Subsequently, 5 × 104 T cells were stimulated in duplo with 1 × 104 target cells (DCs with KLH or TAA mRNA) in an anti-IFN-γ antibody-coated ELISPOT plate (ELISPOT Kit, Becton–Dickinson). The following day, cells were removed and biotin-labeled anti-IFN-γ antibody was added for 2 h at room temperature. Then, the plate was washed with PBS/0.05 % Tween and incubated with avidin–biotin complex solution for 1 h at room temperature. Subsequently, the plate was washed with PBS, and 3-amino-9-ethylcarbazole (AEC) was added for 30 min until red spots became visible. Then, the plate was washed extensively and analyzed using an Eli.Scan ELISPOT scanner (A.EL.VIS GmbH, Hannover, Germany). In case sufficient cell numbers were harvested following the expansion cultures, 5 × 104 of the cultured cells derived from biopsy were stimulated in duplo with 2.5 × 104 target cells (DCs with/without TAA mRNA) in 96-well round bottom plates (Corning Costar). The next day, supernatant was harvested and stored at −20 °C until cytokine analyses. Release of IL-2, IL-4, IL-5, IL-10, TNF-α and IFN-γ by the T cells was simultaneously determined in the culture supernatant using a Th1/Th2 BD™ cytometric bead array (Becton–Dickinson) following the manufacturer’s protocol and measured using flow cytometry.
Results
Patient characteristics
Twelve patients with stage II or III MM treated with intensive chemotherapy and autologous SCT were included in this study. The disease status following autologous SCT was CR in three patients (25 %), VGPR in two patients (17 %) and PR in seven patients (58 %) (Table 1). All included patients had a performance status of 0–1. The median age of patients was 61 years (range 49–66 years), and all were treated with the first TAA-DC vaccine between December 2007 and July 2011 at a median time of 26 months (range 9–53 months) after SCT. Patient 3 was treated with interferon-α 2 years before DC vaccination. Five patients received thalidomide maintenance treatment at the time of DC vaccination. Each patient was vaccinated with 3 DC vaccines at biweekly intervals. Notably, patients 1 and 3 were revaccinated because of suboptimal DC maturation in the first vaccination cycle (patient 1; only 55 % CD83+) or because of an increase in M-protein that did not yet require local or systemic therapy, and an immunological response to the first DC vaccinations (patient 3).
Dendritic cell vaccines from MM patients display a highly mature phenotype and can be efficiently loaded with TAA mRNA
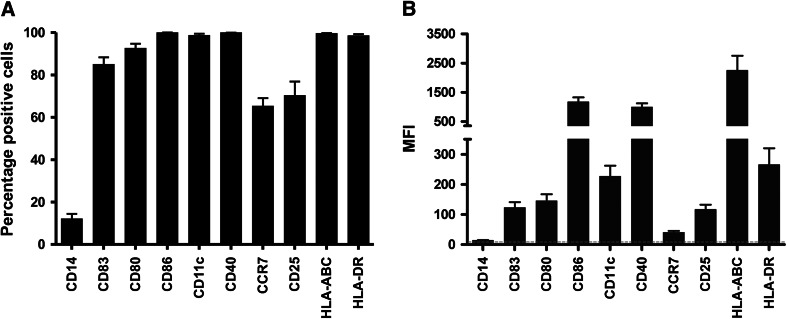
Mature DCs were cultured from monocytes isolated from autologous aphaeresis products. As shown in Fig. 1a, b, the DCs had a highly mature phenotype with >85 ± 3.2 % of the cells expressing CD83, except for patient 1 who received 55 % CD83+ DC vaccines during the first vaccination round. In all patients, the co-stimulatory molecules, CD80, CD86 and CD40, and HLA class I and II molecules were expressed at high levels. Furthermore, >65 ± 3.5 % of the cells expressed CCR7, the chemokine receptor involved in DC migration toward the lymph node. DCs used in the revaccinations of patients 1 and 3 were highly mature and had a similar phenotype as shown in Fig. 1a. Following electroporation with TAA mRNA, 55.6 ± 4.4 % of the DCs expressed MAGE3, 51.2 ± 5.2 % Survivin and 75.9 ± 4.6 % BCMA protein.
Fig. 1.
Infused DCs display a highly mature phenotype. After 48 h of maturation, expression of various maturation and co-stimulatory molecules by DCs, vaccination cycle #1—vaccine #1, was analyzed using flow cytometry. a The percentage of cells positive for the surface marker. b The median fluorescence intensity (MFI) of the surface molecule. Data are expressed as mean + SEM of 12 patients. The dotted line indicates the MFI of the isotype controls
The median number of injected DCs was 15 × 106 (range 5–23 × 106) and 8 × 106 (range 8–12 × 106) for the intravenous and intradermal routes, respectively (Table 2). The number of administered DCs was dependent on the yield after culture (vaccine #1) and thawing (vaccines #2 and #3), with a maximum of 30 × 106 DCs per vaccination (20 × 106 i.v. and 10 × 106 i.d.). DC vaccination was well tolerated; 8 patients showed induration at the i.d. DC vaccination site and 10 patients reported grade 1–2 constitutional symptoms, such as fever, chills, malaise and muscular pain within 48 h after vaccination. No grade 3–4 adverse events were reported.
Table 2.
Characteristics of DC vaccines and toxicity after vaccination
| Patient | Number of DCs injected i.v./i.d. (× 106) | TAA expression (%) | Induration at i.d. vaccination site | Constitutional symptoms | ||||
|---|---|---|---|---|---|---|---|---|
| #1 | #2 | #3 | MAGE3 | Survivin | BCMA | |||
| 1 | 20/10 | 19/10 | 20/10 | 73 | 86 | 53 | No | No |
| 1 Revaccination | 16/8 | 12/6 | 18/9 | 36 | 70 | 70 | No | Yes, grade 1–2 |
| 2 | 15/8 | 14/6 | 15/8 | 81 | 78 | 78 | No | No |
| 3 | 13/6 | 11/6 | 14/8 | 67 | 86 | 40 | Yes | Yes, grade 1–2 |
| 3 Revaccination | 9/4 | 5/2 | 8/3 | 75 | 70 | 65 | No | Yes, grade 1–2 |
| 4 | 22/11 | 14/7 | 14/7 | 41a | 29a | 21a | Yes | Yes, grade 1–2 |
| 5 | 16/8 | 15/7 | 10/5 | 68 | 64 | ND | Yes | Yes, grade 1–2 |
| 6 | 16/8 | 8/4 | 8/4 | 45 | 86 | 65 | Yes | Yes, grade 1–2 |
| 7 | 20/10 | 13/6.5 | 14/7 | 49 | 90 | 47 | Yes | Yes, grade 1–2 |
| 8 | 12/6 | 7/3 | 7/3 | 49 | 89 | 68 | No | No |
| 9 | 22/11 | 23/12 | 20/10 | 34 | 81 | 31 | Yes | No |
| 10 | 20/10 | 20/10 | 20/10 | 50 | 65 | 26 | Yes | Yes, grade 1–2 |
| 11 | 16/8 | 21/11 | 17/9 | 60 | 84 | 60 | No | No |
| 12 | 22/11 | 16/8 | 14/7 | 50 | 84 | ND | Yes | Yes, grade 1–2 |
Patients received three DC vaccines with biweekly intervals. i.v. intravenous, i.d. intradermal, TAA tumor-associated antigen
aTAA expression was analyzed after overnight storage at 4 °C due to logistic reasons
KLH-specific T-cell responses are induced upon DC vaccination in MM patients following intensive chemotherapy
DCs were loaded with the antigenic protein KLH, as an adjuvant to provide CD4+ T cell help and boost tumor-reactive CD8+ T-cell responses, as well as an immunomonitoring tool post-vaccination. PBMC obtained at various time points before and after vaccination were restimulated in vitro for 5 days with autologous KLH-pulsed DCs, after which T-cell proliferation was examined. All patients showed KLH-specific T-cell responses after the first vaccination (Fig. 2). Remarkably, in some patients, the response increased further following subsequent vaccinations, though in most patients, the size of the response remained the same or decreased. Overall, 4 patients (patients 6, 7, 10 and 11) showed a moderate response (SI < 20), patients 2, 4, 8 and 9 had a good response (SI ≥ 20 and < 200) and 4 patients (patients 1, 3, 5 and 12) had an excellent T-cell response (SI ≥ 200) to KLH. No KLH-specific antibody responses could be detected in serum (data not shown). In addition, no significant changes in T-cell subsets, including regulatory T cells, B cell and NK cell numbers, were observed (data not shown). Together, these data demonstrate that the DC vaccines were capable of inducing a specific T-cell response in MM patients more than 6 months after high-dose melphalan.
Fig. 2.
KLH-specific T-cell proliferation is induced in chemotherapy-treated MM patients by vaccination with KLH-loaded DCs. At various time points before and after vaccination, PBMC samples were obtained. Following 5-day in vitro restimulation with autologous KLH-loaded mature DCs, KLH-specific T-cell proliferation was measured by 3H-thymidine incorporation. The stimulation index (SI) was calculated by dividing the counts of stimulated PBMC by that of non-stimulated PBMC. A SI ≥2 (dotted line) was considered positive
DC vaccination results in MAGE3, Survivin and BCMA-specific T-cell responses
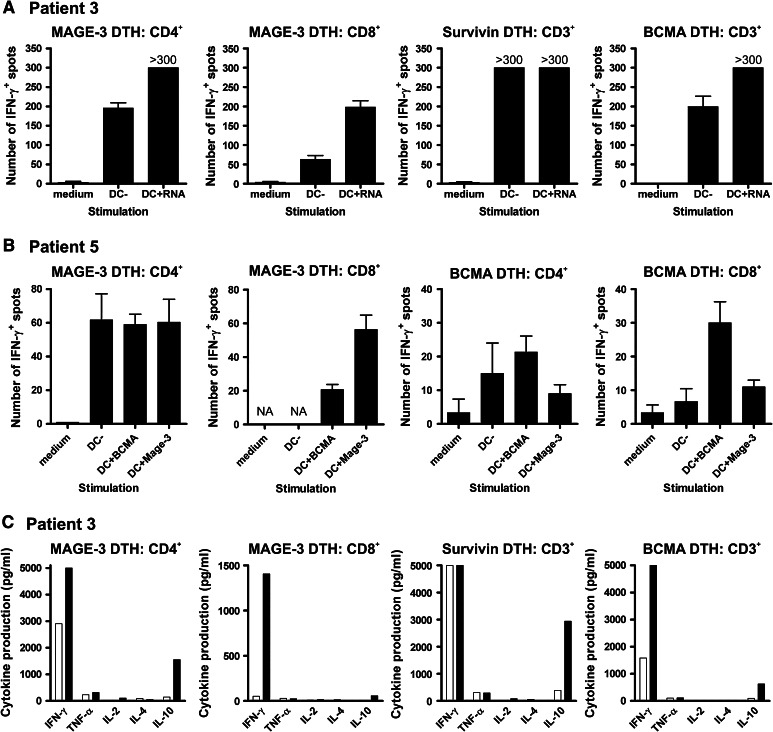
To investigate whether DC vaccination resulted in TAA-specific T-cell response in MM patients, DTH skin tests using the different TAA-pulsed or KLH-loaded DCs were performed. Only 6 patients (patients 3, 4, 5, 6, 7 and 10) showed induration and erythema at the DTH sites, and biopsies were taken from the 4 sites of the different skin tests. Subsequently, T cells were isolated and expanded, and restimulated in vitro with the corresponding KLH- or TAA-loaded DCs. The next day, supernatants were harvested for cytokine analysis. In Table 3, the data of all individuals are summarized. In 5 out of 6 patients, KLH-specific IFN-γ production was observed in an ELISPOT assay. Furthermore, 2 of the 6 patients (patients 3 and 5) had IFN-γ-producing CD4+ and CD8+ T cells upon restimulation with MAGE-loaded DCs (Fig. 3a, b). In addition, patient 3 also had CD3+ T cells responding to Survivin and BCMA. Besides antigen-specific IFN-γ secretion, these T cells also produced IL-10 (Fig. 3c). In patient 5, we could not analyze the response to Survivin as we were unable to expand these T cells from the biopsy. In contrast, although we did not observe induration at the BCMA-DTH site, BCMA-specific CD4+ and CD8+ T cells could be expanded from this biopsy and they produced IFN-γ upon antigen restimulation. Interestingly, the myeloma cells of this patient exhibited moderate BCMA and high Survivin, but not MAGE3 expression (data not shown). Together, these data indicate that vaccination with autologous TAA-mRNA-loaded DCs induced DTH responses in 6 patients, and in 2 of these 6 patients, TAA-specific T cells were isolated, which efficiently produced effector cytokines upon antigen reencounter.
Table 3.
Summary of post-vaccination DTH skin tests and immunological responses
| Patient | KLH T-cell proliferation (PB) | DC − RNA + KLH | DC + MAGE3 mRNA | DC + Survivin mRNA | DC + BCMA mRNA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DTHa | DILb | Ag recognitionc | DTHa | DILb | Ag recognitionc | DTHa | DILb | Ag recognitionc | DTHa | DILb | Ag recognitionc | ||
| 1 | +++ | No | – | No | – | – | No | – | – | No | – | – | |
| 2 | ++ | No | – | – | No | – | – | No | – | – | No | – | – |
| 3 | +++ | Yes, 7 mm | Yes | Yes | Yes, 9 mm | Yes | Yes | Yes, 9 mm | Yes | Yes d | Yes, 11 mm | Yes | Yes |
| 4 | ++ | Yes, 13 mm | Yes | Yes | Yes, 8 mm | Yes | No | Yes, 7 mm | Yes | No | Yes, 5 mm | Yes | No |
| 5 | +++ | Yes, 5 mm | Yes | No | Yes, 9 mm | Yes | Yes | Yes, 8 mm | No | – | Noe | Yes | Yes |
| 6 | + | Yes, 10 mm | Yes | Yes | Yes, 7 mm | Yes | No | Yes, 7 mm | Yes | No | Yes, 12 mm | Yes | No |
| 7 | + | Yes, ND | Yes | Yes | Yes, ND | Yes | No | Yes, ND | Yes | No | Yes, ND | Yes | No |
| 8 | ++ | No | – | – | No | – | – | No | – | – | No | – | – |
| 9 | ++ | No | – | – | No | – | – | No | – | – | No | – | – |
| 10 | + | Yes, 5 mm | Yes | Yes | Yes, 5 mm | Yes | No | Yes, 5 mm | Yes | No | Yes, 5 mm | Yes | No |
| 11 | + | No | – | – | No | – | – | No | – | – | No | – | – |
| 12 | +++ | No | – | – | No | – | – | No | – | – | No | – | – |
ND not documented, PB peripheral blood
aDTH scoring: positive skin-test reaction was defined as ≥5 mm diameter induration and erythema at 48 h after injection; b Outgrowth of >0.1 × 106 DTH-infiltrating lymphocytes (DIL) following 3–4 week culture in medium containing 200 U/mL IL-2 and 10 ng/mL IL-15; c IFN-γ production by DIL following restimulation with target cells in an ELISPOT assay. Antigen (Ag) recognition was considered positive in case the number of IFN-γ spots was higher upon DC + RNA stimulation compared to DC stimulation; d Expanded CTLs produced IFN-γ upon stimulation with Survivin mRNA-loaded DCs; however, as Survivin is already constitutively expressed by DCs, the CTLs also responded to control DCs; e As this patient showed positive responses in the three other DTH sites, also a biopsy was obtained from this BCMA-DC DTH site
Fig. 3.
Cytokine release by TAA-specific T cells upon antigen recognition. DTH-infiltrating lymphocytes were cultured for 3-4 weeks in medium containing 200 U/mL IL-2 and 10 ng/mL IL-15. Thereafter, CD3+, CD4+ and/or CD8+ T cells were isolated and overnight restimulated with target cells. a–b IFN-γ production by TAA-specific T cells of patients 3 (a) and 5 (b) was analyzed using the ELISPOT assay. c Multiple cytokine levels were measured in culture supernatants of patient 3 using a cytokine bead array and flow cytometry
Clinical follow-up
DC vaccination was well tolerated, and no severe toxicities were observed during the treatment period, only temporary grade 1–2 events were observed after vaccination. After DC vaccination, M-proteins remained unchanged (patient 2) or below detection limit (patients 4, 5, 8, 9 and 12). In the other patients, M-protein levels increased during or after DC vaccination. At last follow-up, 10 of the 12 patients were alive at a median follow-up of 55 months (range 21–99 months) after SCT and 25 months (range 9–53 months) after the first DC vaccination. Of these patients, 5 have stable disease and 5 have progressive disease for which patients 3, 6, 7 and 10 received additional treatment (Table 1). Patients 1 and 2 died because of the development of secondary acute myeloid leukemia at 29 and 18 months, respectively, after the first DC vaccination. Whereas patient 2 did not receive additional treatment after DC vaccination, patient 1 was treated with lenalidomide and dexamethasone. In addition, patient 5 developed prostate cancer 30 months after the first DC vaccination and patient 10 progressed to plasma cell leukemia at 18 months after the first DC vaccination.
Discussion
The introduction of high-dose melphalan and novel drugs like thalidomide, bortezomib and lenalidomide has significantly prolonged overall survival of MM patients [1, 2, 23–25]. Unfortunately, despite initial responses, eventually all patients relapse. Since myeloma plasma cells do show immunological responses [3, 4], exploration of cellular immunotherapies as adjuvant treatment is attractive to boost anti-myeloma immunity and allow the formation of myeloma-reactive immune memory. Previously, we and others studied the immunogenicity of the MM idiotype protein in DC-based vaccination strategies; however, both immunological and clinical responses were limited as compared to follicular NHL [9, 11–15]. Hence, it would be more interesting to explore multiple TAA simultaneously and thereby broaden the anti-myeloma immune response and more immunogenic targets. Such approaches include vaccination with peptide or RNA-loaded DCs, tumor lysate-loaded DCs and DC/tumor fusions, which are being investigated in various solid and hematological cancers [10, 26, 27]. In this phase 1 study, we investigated the safety and immunological effects of vaccination with mature DCs pulsed with KLH and MAGE3, Survivin or BCMA mRNA in MM patients in a stable remission after high-dose therapy and autologous SCT. These TAA were selected because of their high and selective expression on MM cells and their reported immunogenic potential [7, 16–18]. Importantly, in all patients, immunological responses were observed recognizing KLH or TAA following DC vaccination, which indicates immune recovery after high-dose melphalan.
Patients were vaccinated with mature DCs pulsed with TAA mRNA that have good DC migration and antigen-specific T-cell activation capacity [28]. The DC vaccines displayed a highly mature phenotype with high expression of co-stimulatory molecules, HLA molecules and CCR7. Only in one patient (no. 1), the generated DC vaccine had insufficient maturation with only 55 % of the DCs expressing CD83; therefore, this patient received a second cycle of three DC vaccines 1.5 years later. Upon electroporation with TAA mRNA, the DCs showed high expression of the MAGE3, Survivin and BCMA proteins, although levels varied between patients. Toxicity of KLH-loaded TAA-DC vaccination was limited to grade 1–2 constitutional symptoms, such as fever, perspiration, malaise, muscular pain, cold and local induration at the i.d. injection site, which is probably due to immune responses against these antigens. No severe adverse events were observed, similar to other TAA mRNA-DC vaccination studies in melanoma patients [29, 30].
The potency of our DC vaccines to induce primary immune responses was measured in DTH tests and ex vivo T-cell restimulation assays. Although it has been reported that mRNA-transfected DCs are capable of inducing CD4+ T-cell responses in the absence of KLH, we used KLH as an immunomonitoring tool to examine whether patients after high-dose melphalan and autologous SCT were capable of eliciting primary T-cell responses toward the DC vaccine. Importantly, we observed in all patients a KLH-specific T-cell response in peripheral blood after the first vaccination. Whereas in some patients this response was further boosted by subsequent vaccinations, most patients showed equal or lower KLH-specific T-cell proliferation after the second and third DC injection. In contrast to findings reported in melanoma patients [29], we could not detect KLH-specific antibody responses in serum following DC vaccination. As KLH was added to DCs at day 3, there was ample time for efficient processing of the KLH protein in order to induce KLH-specific Th1-skewed cellular responses. However, native protein was probably not present in our DC vaccines, which could explain the lack of KLH-specific antibody responses in the vaccinated patients. It could also be that due to the intensive treatment of the MM patients, B-cell functionality was affected, thereby hindering the formation of good antibody responses. During the course of DC vaccination, B cell numbers fluctuated between 0.11 and 0.18 × 106 cells/mL in our patients, which are relatively low compared to age-matched healthy individuals. This together with the KLH-mediated skewing toward Th1 responses could be another explanation for the absence of KLH-specific antibodies. At the DTH site, only in 6 of 12 patients, induration was observed upon KLH-DC injection and in 5 patients KLH-specific IFN-γ production was detected. Importantly in 2 of 6 patients with DTH reactivity, CD4+ and CD8+ T-cell responses against MAGE3 and BCMA were observed. In addition, in one patient Survivin-specific T-cell immunity was found, however, we cannot rule out that this T-cell response was already present before DC vaccination as DCs constitutively express Survivin. Furthermore, in patient 3, we were able to analyze the Th1/Th2 cytokine profile and showed that the activated TAA-specific CD4+ T cells produced both IFN-γ and, although at lower levels, IL-10. IL-10 can have immunosuppressive properties; nevertheless, the net effect on anti-tumor immunity is dependent on the ratio in immune-potentiating and immune-suppressive factors. In this patient for all three TAA, much higher levels of IFN-γ were observed than those of IL-10, indicating that our DC vaccines mainly induced Th1 cell responses, but a suppressive role of IL-10 cannot be ruled out. The TAA-specific CD8+ T cells only produced the effector cytokine IFN-γ upon antigen restimulation. In addition, we have attempted to expand TAA-specific T-cell cultures from peripheral blood and restimulated them with overlapping TAA peptides. However, expansion and detection of circulating TAA-specific T cells was unsuccessful (data not shown). Furthermore, we set up an analysis to examine TAA expression on cryopreserved MM cells, obtained at diagnosis, of patients 3 and 5. In case of patient 3, no viable MM cells could be recovered after thawing, but MM cells of patient 5 exhibited high BCMA and Survivin but no MAGE3 expression (data not shown).
Our clinical observations showed that 5 out of 12 patients vaccinated with KLH-loaded TAA DCs have stable disease after a median follow-up of 26 months (range 9–47 months). Of the two patients in whom we detected TAA-specific T-cell immunity, one received additional therapy at 10 months after vaccination because of progressing disease, and the other patient still had stable M-protein levels after 38 months. One patient progressed to plasma cell leukemia and another patient developed prostate cancer. Unfortunately, two patients died because of a secondary hematological malignancy. These secondary malignancies are not likely the result of DC vaccination, as all patients received high-dose chemotherapy prior to autologous SCT. Furthermore one of the patients (patient 1) was treated with lenalidomide after DC vaccination because of an increasing M-protein. In the literature, it has been reported that lenalidomide treatment might be associated with a higher risk to develop secondary malignancies [31, 32].
Overall our ex vivo cultured TAA DCs efficiently induced immunological responses as all patients developed KLH-specific T-cell responses in peripheral blood following vaccination. This indicates that DC function was not dysregulated in vivo by residual MM cells. In addition, overall Treg numbers did not increase following DC vaccination, suggesting that the functionality of the induced T cells is not suppressed. However, we did not analyze the Treg activation state. Notably, only 6 patients showed DTH reactivity to KLH even though all 12 patients had substantial KLH-specific T-cell immunity in peripheral blood. Moreover, the observation of KLH-specific DTH responses was independent of the size of the stimulation index measured in peripheral blood. Apparently, the KLH-specific T cells had not migrated toward the DTH site. The same could be true for the TAA-specific T cells, because in only 2 patients antigen-specific cytokine release could be demonstrated in T cells isolated from DTH biopsies. Possibly these findings under-represent the in vivo presence of TAA-specific immunity, as these T cells may have homed to the bone marrow where the myeloma cells reside. On the other hand, it may also be that the other 10 patients did not respond to TAA-DC vaccination. No correlations between lack of TAA-specific T-cell responses following DC vaccination and the potency of the immune system (reflected by the primary responses to KLH), time interval between SCT and DC vaccination, or thalidomide maintenance treatment at the time of vaccination were found. To further boost anti-myeloma immunity, TAA-DC vaccination may also be combined with strategies that augment DC immunogenicity or interfere with immunosuppressive mechanisms exploited by MM cells. In this regard, we recently showed that by silencing PD-L1 and PD-L2 on monocyte-derived DCs, and thereby interfere with negative co-signaling via PD-1, we could generate DCs with superior immunogenic potential to stimulate KLH-specific and tumor-reactive T-cell responses ex vivo [33, 34]. Another strategy would be interference with co-inhibitory signaling using blocking antibodies. In two recently published reports on anti-PD-1 (BMS-936,588) and anti-PD-L1 (BMS-936,559) blocking antibody therapy in solid cancers, durable tumor regression was observed with objective clinical responses in 18–28 % and 6–17 % of the patients, respectively [35, 36]. Hence, exploration of these immunotherapeutic strategies in MM patients would be highly interesting.
To conclude, in this study, we demonstrated that MAGE3, Survivin and BCMA mRNA-pulsed DCs are capable of stimulating TAA-specific T-cell responses in MM patients, and the application of these TAA DCs in combination with other immunotherapeutic strategies such as co-inhibitory signaling blockade may further boost anti-myeloma immunity after autologous SCT.
Acknowledgments
We would like to thank Dr. Rianne Gerritsen and Dr. Michelle van Rossum of the Department of Dermatology, Radboud University Nijmegen Medical Centre, for performing the DTH skin testing and biopsies. We thank Marij Leenders for assistance in flow cytometry analysis. This work was supported by a grant from the Dutch Cancer Society (KWF 2006-3674).
Conflict of interest
Theo de Witte is a member of the advisory board of Novartis, Celgene and Clavis. Nicolaas Schaap is a member of the advisory board of Novartis. The other authors have no conflicting financial interests.
Footnotes
Willemijn Hobo, Leonie Strobbe, Reinier Raymakers and Nicolaas Schaap contributed equally to this study.
References
- 1.Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, Casassus P, Maisonneuve H, Facon T, Ifrah N, Payen C, Bataille R. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335:91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 2.Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, Brown J, Drayson MT, Selby PJ. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 3.Lokhorst HM, Schattenberg A, Cornelissen JJ, Thomas LL, Verdonck LF. Donor leukocyte infusions are effective in relapsed multiple myeloma after allogeneic bone marrow transplantation. Blood. 1997;90:4206–4211. [PubMed] [Google Scholar]
- 4.Alyea E, Weller E, Schlossman R, Canning C, Webb I, Doss D, Mauch P, Marcus K, Fisher D, Freeman A, Parikh B, Gribben J, Soiffer R, Ritz J, Anderson K. T-cell–depleted allogeneic bone marrow transplantation followed by donor lymphocyte infusion in patients with multiple myeloma: induction of graft-versus-myeloma effect. Blood. 2001;98:934–939. doi: 10.1182/blood.V98.4.934. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5:296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 6.Palucka AK, Ueno H, Fay JW, Banchereau J. Taming cancer by inducing immunity via dendritic cells. Immunol Rev. 2007;220:129–150. doi: 10.1111/j.1600-065X.2007.00575.x. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L, Gotz M, Hofmann S, Greiner J. Immunogenic targets for specific immunotherapy in multiple myeloma. Clin Dev Immunol. 2012 doi: 10.1155/2012/820394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu FJ, Benike C, Fagnoni F, Liles TM, Czerwinski D, Taidi B, Engleman EG, Levy R. Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nat Med. 1996;2:52–58. doi: 10.1038/nm0196-52. [DOI] [PubMed] [Google Scholar]
- 9.Timmerman JM, Czerwinski DK, Davis TA, Hsu FJ, Benike C, Hao ZM, Taidi B, Rajapaksa R, Caspar CB, Okada CY, van Beckhoven A, Liles TM, Engleman EG, Levy R. Idiotype-pulsed dendritic cell vaccination for B-cell lymphoma: clinical and immune responses in 35 patients. Blood. 2002;99:1517–1526. doi: 10.1182/blood.V99.5.1517. [DOI] [PubMed] [Google Scholar]
- 10.Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 11.Wen YJ, Ling M, Bailey-Wood R, Lim SH. Idiotypic protein-pulsed adherent peripheral blood mononuclear cell-derived dendritic cells prime immune system in multiple myeloma. Clin Cancer Res. 1998;4:957–962. [PubMed] [Google Scholar]
- 12.Reichardt VL, Okada CY, Liso A, Benike CJ, Stockerl-Goldstein KE, Engleman EG, Blume KG, Levy R. Idiotype vaccination using dendritic cells after autologous peripheral blood stem cell transplantation for multiple myeloma–a feasibility study. Blood. 1999;93:2411–2419. [PubMed] [Google Scholar]
- 13.Reichardt VL, Milazzo C, Brugger W, Einsele H, Kanz L, Brossart P. Idiotype vaccination of multiple myeloma patients using monocyte-derived dendritic cells. Haematologica. 2003;88:1139–1149. [PubMed] [Google Scholar]
- 14.Rollig C, Schmidt C, Bornhauser M, Ehninger G, Schmitz M, Auffermann-Gretzinger S. Induction of cellular immune responses in patients with stage-I multiple myeloma after vaccination with autologous idiotype-pulsed dendritic cells. J Immunother. 2011;34:100–106. doi: 10.1097/CJI.0b013e3181facf48. [DOI] [PubMed] [Google Scholar]
- 15.Rasmussen T, Hansson L, Osterborg A, Johnsen HE, Mellstedt H. Idiotype vaccination in multiple myeloma induced a reduction of circulating clonal tumor B cells. Blood. 2003;101:4607–4610. doi: 10.1182/blood-2002-06-1925. [DOI] [PubMed] [Google Scholar]
- 16.van Baren N, Brasseur F, Godelaine D, Hames G, Ferrant A, Lehmann F, Andre M, Ravoet C, Doyen C, Spagnoli GC, Bakkus M, Thielemans K, Boon T. Genes encoding tumor-specific antigens are expressed in human myeloma cells. Blood. 1999;94:1156–1164. [PubMed] [Google Scholar]
- 17.Schmidt SM, Schag K, Muller MR, Weck MM, Appel S, Kanz L, Grunebach F, Brossart P. Survivin is a shared tumor-associated antigen expressed in a broad variety of malignancies and recognized by specific cytotoxic T cells. Blood. 2003;102:571–576. doi: 10.1182/blood-2002-08-2554. [DOI] [PubMed] [Google Scholar]
- 18.Bellucci R, Alyea EP, Chiaretti S, Wu CJ, Zorn E, Weller E, Wu B, Canning C, Schlossman R, Munshi NC, Anderson KC, Ritz J. Graft-versus-tumor response in patients with multiple myeloma is associated with antibody response to BCMA, a plasma-cell membrane receptor. Blood. 2005;105:3945–3950. doi: 10.1182/blood-2004-11-4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durie BG, Salmon SE. A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer. 1975;36:842–854. doi: 10.1002/1097-0142(197509)36:3<842::AID-CNCR2820360303>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 20.Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, Gertz M, Dimopoulos M, Westin J, Sonneveld P, Ludwig H, Gahrton G, Beksac M, Crowley J, Belch A, Boccadaro M, Cavo M, Turesson I, Joshua D, Vesole D, Kyle R, Alexanian R, Tricot G, Attal M, Merlini G, Powles R, Richardson P, Shimizu K, Tosi P, Morgan G, Rajkumar SV. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 21.Aarntzen EH, de Vries I, Goertz JH, Beldhuis-Valkis M, Brouwers HM, van de Rakt MW, van der Molen RG, Punt CJ, Adema GJ, Tacken PJ, Joosten I, Jacobs JF. Humoral anti-KLH responses in cancer patients treated with dendritic cell-based immunotherapy are dictated by different vaccination parameters. Cancer Immunol Immunother. 2012;61:2003–2011. doi: 10.1007/s00262-012-1263-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Vries I, Bernsen MR, Lesterhuis WJ, Scharenborg NM, Strijk SP, Gerritsen MJ, Ruiter DJ, Figdor CG, Punt CJ, Adema GJ. Immunomonitoring tumor-specific T cells in delayed-type hypersensitivity skin biopsies after dendritic cell vaccination correlates with clinical outcome. J Clin Oncol. 2005;23:5779–5787. doi: 10.1200/JCO.2005.06.478. [DOI] [PubMed] [Google Scholar]
- 23.Richardson PG, Schlossman RL, Weller E, Hideshima T, Mitsiades C, Davies F, LeBlanc R, Catley LP, Doss D, Kelly K, McKenney M, Mechlowicz J, Freeman A, Deocampo R, Rich R, Ryoo JJ, Chauhan D, Balinski K, Zeldis J, Anderson KC. Immunomodulatory drug CC-5013 overcomes drug resistance and is well tolerated in patients with relapsed multiple myeloma. Blood. 2002;100:3063–3067. doi: 10.1182/blood-2002-03-0996. [DOI] [PubMed] [Google Scholar]
- 24.Hideshima T, Mitsiades C, Akiyama M, Hayashi T, Chauhan D, Richardson P, Schlossman R, Podar K, Munshi NC, Mitsiades N, Anderson KC. Molecular mechanisms mediating antimyeloma activity of proteasome inhibitor PS-341. Blood. 2003;101:1530–1534. doi: 10.1182/blood-2002-08-2543. [DOI] [PubMed] [Google Scholar]
- 25.Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046–1060. doi: 10.1056/NEJMra1011442. [DOI] [PubMed] [Google Scholar]
- 26.Chang AE, Redman BG, Whitfield JR, Nickoloff BJ, Braun TM, Lee PP, Geiger JD, Mule JJ. A phase I trial of tumor lysate-pulsed dendritic cells in the treatment of advanced cancer. Clin Cancer Res. 2002;8:1021–1032. [PubMed] [Google Scholar]
- 27.Rosenblatt J, Vasir B, Uhl L, Blotta S, Macnamara C, Somaiya P, Wu Z, Joyce R, Levine JD, Dombagoda D, Yuan YE, Francoeur K, Fitzgerald D, Richardson P, Weller E, Anderson K, Kufe D, Munshi N, Avigan D. Vaccination with dendritic cell/tumor fusion cells results in cellular and humoral antitumor immune responses in patients with multiple myeloma. Blood. 2011;117:393–402. doi: 10.1182/blood-2010-04-277137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Vries I, Lesterhuis WJ, Scharenborg NM, Engelen LP, Ruiter DJ, Gerritsen MJ, Croockewit S, Britten CM, Torensma R, Adema GJ, Figdor CG, Punt CJ. Maturation of dendritic cells is a prerequisite for inducing immune responses in advanced melanoma patients. Clin Cancer Res. 2003;9:5091–5100. [PubMed] [Google Scholar]
- 29.Aarntzen EH, Schreibelt G, Bol K, Lesterhuis WJ, Croockewit AJ, de Wilt JH, van Rossum MM, Blokx WA, Jacobs JF, Duiveman-de Boer T, Schuurhuis DH, Mus R, Thielemans K, de Vries I, Figdor CG, Punt CJ, Adema GJ. Vaccination with mRNA-electroporated dendritic cells induces robust tumor antigen-specific CD4 + and CD8 + T cells responses in stage III and IV melanoma patients. Clin Cancer Res. 2012;18:5460–5470. doi: 10.1158/1078-0432.CCR-11-3368. [DOI] [PubMed] [Google Scholar]
- 30.Wilgenhof S, Van Nuffel AM, Corthals J, Heirman C, Tuyaerts S, Benteyn D, de Coninck A, van Riet I, Verfaillie G, Vandeloo J, Bonehill A, Thielemans K, Neyns B. Therapeutic vaccination with an autologous mRNA electroporated dendritic cell vaccine in patients with advanced melanoma. J Immunother. 2011;34:448–456. doi: 10.1097/CJI.0b013e31821dcb31. [DOI] [PubMed] [Google Scholar]
- 31.Dimopoulos MA, Richardson PG, Brandenburg N, Yu Z, Weber DM, Niesvizky R, Morgan GJ. A review of second primary malignancy in patients with relapsed or refractory multiple myeloma treated with lenalidomide. Blood. 2012;119:2764–2767. doi: 10.1182/blood-2011-08-373514. [DOI] [PubMed] [Google Scholar]
- 32.Thomas A, Mailankody S, Korde N, Kristinsson SY, Turesson I, Landgren O. Second malignancies after multiple myeloma: from 1960s to 2010s. Blood. 2012;119:2731–2737. doi: 10.1182/blood-2011-12-381426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hobo W, Maas F, Adisty N, de Witte T, Schaap N, van der Voort R, Dolstra H. siRNA silencing of PD-L1 and PD-L2 on dendritic cells augments expansion and function of minor histocompatibility antigen-specific CD8 + T cells. Blood. 2010;116:4501–4511. doi: 10.1182/blood-2010-04-278739. [DOI] [PubMed] [Google Scholar]
- 34.Hobo W, Novobrantseva TI, Fredrix H, Wong J, Milstein S, Epstein-Barash H, Liu J, Schaap N, van der Voort R, Dolstra H. Improving dendritic cell vaccine immunogenicity by silencing PD-1 ligands using siRNA-lipid nanoparticles combined with antigen mRNA electroporation. Cancer Immunol Immunother. 2013;62:285–297. doi: 10.1007/s00262-012-1334-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]