Abstract
Experimental evidence has been provided that cancer vaccines are less effective at older age than in young adults. In this study, we evaluated the possibility to recover the low effectiveness of DNA immunization against HER-2/neu increasing plasmid uptake by cells from old mice through electroporation with the aim to enhance the activation of specific immune responses. Young and old Balb/c mice received two immunizations with a pCMV-ECDTM DNA plasmid using plasmid intramuscular injection followed by electroporation (IM + E) or plasmid intramuscular injection alone (IM), and successively, they were challenged with syngeneic HER-2/neu overexpressing TUBO cells. Young mice were completely protected whereas less than 60% protection was observed in old mice after IM immunization. IM + E immunization completely protected old mice against a TUBO cell challenge. The protection was associated with increased transgene expression in the site of immunization and with the induction of both humoral and cell-mediated immunity in old mice. We conclude that the effectiveness of anticancer DNA vaccination in old ages may be improved increasing plasmid uptake and transgene expression through electroporation, suggesting the relevant role of the first steps of the immunization process in the success of cancer vaccines at older age.
Keywords: HER-2/neu, DNA vaccination, Aging, Electroporation
Introduction
In the last years, experimental evidence has been provided on the effectiveness of anticancer vaccination models based on the possibility to elicit a potent immune response and to induce immune memory against tumor antigens [1–3]. The experiments performed until now in animal models have clearly demonstrated that the efficacy of antitumor vaccination is dependent on the immunocompetence of the host [4, 5]. In fact, the rejection of tumors was related to the immune effectiveness of mice and no protection against tumor challenge was obtained in physically or chemically immunosuppressed host [5].
Over the last few years, the use of immunological measures to prevent cancer in experimental mouse models involving immunization with new vaccines against even a poor or apparently non-immunogenic tumor has yielded worse outcomes in older age than in young adults [6, 7]. The age-related reduced effectiveness of anticancer vaccination has been connected to the existence of age-related defects in the activation of specific immune responses (immunosenescence). These defects have been coupled to different mechanisms which may act at the different steps of the immunisation process and whose exact influence still remains unclear [6]. The data reported to date on the lower effectiveness of cancer vaccines in older age raise the important question on the causes involved in this defect in light of the possibility of correcting and, possibly, restoring it.
In a recent study, we demonstrated that the intramuscular DNA vaccination against HER-2/neu, a 185-kDa receptor-like tyrosine kinase that was found to be overexpressed in several types of human adenocarcinomas and especially in breast tumors, has a lower effectiveness in old than in young mice and that the reduced number of objective responses observed in aged animals was associated with an age-related impairment of cell-mediated and humoral immune responses [8].
Studying the effectiveness of different DNA delivery systems in conferring protective immunity in HER-2/neu transgenic mice, we and others have recently demonstrated that the protective immunity induced by intramuscular delivery of plasmid DNA was greatly increased by electroporation [9, 10].
The knowledge that electroporation determines a transient increase in the permeability of the plasma membrane and that plasma membrane fluidity diminishes during aging [11, 12], prompted us to investigate whether electroporation may increase the entry of DNA plasmid into cells from old mice, thus potentiating the effectiveness of intramuscular DNA immunization against HER-2/neu in old ages.
Materials and methods
Mice
Male Balb/c inbred mice were housed in plastic cages and fed with food pellets and water ad libitum. They were immunized at the age of 2 months (young) and 16 months (old). The animals were maintained at constant temperature (20 ± 1°C) and humidity (50 ± 5%) on a 12-h light/12-h dark cycle. The study was approved by the animal research ethics committee of the I.N.R.C.A.–I.R.C.C.S.
Plasmid DNA and immunization protocol
pCMV-ECDTM plasmid, encoding extracellular and transmembrane region of HER2/neu antigen under the control of the CMV early promoter/enhancer, has been kindly provided by Dr Augusto Amici from the Camerino University; pCMV empty vector was used to treat the control mice. Plasmid DNA was prepared in large scale using Giga kit (Qiagen), according to the manufacturer’s instructions. Young and old Balb/c inbred mice were randomly selected for immunization with DNA vaccine (pCMV-ECDTM, pCMV) by intramuscular injection without or with electroporation (IM or IM + E). The animals were routinely immunized by two administrations carried out at 8 and 10 weeks (young mice) or at 64 and 66 weeks (old mice) of age. For IM injection, each mouse received 100 μg of DNA through an injection into quadriceps muscle previously exposed. In selected experiments, 100, 200, or 300 μg of DNA was used. For IM + E procedure, 50 μg of DNA, dissolved in H2O containing 6 mg/ml l-glutamate and 150 mM NaCl, was given to each animal through an injection into each tibial muscle followed by three electric pulses (field strength = 200 V/cm; pulse length = 25 ms; ECM 830 field generator, BTX Division, Genetronix).
Tumor cell line
TUBO is a cloned cell line established in vitro from a lobular carcinoma that arose spontaneously in a BALB-neuT mice overexpressing the transforming rat HER-2/neu oncogene and displaying membrane class I H-2d MHC glycoproteins [17]. TUBO cells were cultured in DMEM (Life Technologies, MI, Italy) supplemented with 20% FBS (Life Technologies).
In vivo evaluation of tumor growth
Two weeks after last immunization, Balb/c mice were challenged s.c. in the middle of the left flank with 0.2 ml of a single-cell suspension containing 1 × 105 tumor cells. The incidence and growth of tumors were evaluated twice weekly, and the neoplastic masses were measured with calipers in the two perpendicular diameters for 60 days. Mice with no evidence of tumor at the end of this period were classified as tumor free whereas mice with a tumor >3 mm mean diameter were classified as tumor bearers. All mice bearing neoplastic masses exceeding 10 mm mean diameter were killed for humane reasons.
Preparation and culture conditions of spleen cells
Spleen was teased through a 60-mesh sieve in Ca2+-and Mg2+-free phosphate-buffered saline (PBS, GIBCO, Gaithersburg, MD, USA) solution. Spleen cells were then fractionated on lympholyte M (Cedarlane, Canada) and mononuclear cells separated by density gradient centrifugation (500g, 20 min). Cells from the interface of the gradients were washed twice with PBS and resuspended in RPMI 1640-containing penicillin (100 U/ml) and streptomycin (100 μg/ml).
Cytotoxic assay
Splenocytes were incubated at 37°C and 5% CO2 in RPMI medium containing 10% FCS in the presence of mitomycin-treated TUBO tumor cells as stimulators (20:1 ratio stimulators: lymphocytes) for 5 days. Cytotoxic assay was performed using a fluorimetric method as previously reported [13]. Briefly, a stock solution of carboxyfluorescein diacetate (c’FDA, Molecular Probes, Oregon, USA) (20 mg/ml acetone, stored at −20°C) was diluted in PBS to give a final concentration of 75 μg/ml. TUBO tumor cells were washed twice with PBS and then labeled with c’FDA by resuspending the cells in 1 ml working solution and incubating at 37°C in a humidified, 5% CO2 incubator for 30 min. Target cells were then washed 3 times in PBS containing 1% BSA (Sigma) and resuspended in RPMI + 10% FCS at a concentration of 1 × 105/ml. 1 × 104 c’FDA-labeled tumor target cells were incubated with effector spleen cells in 200 μl total volume in 96-well, round microtiter plates (Nunc, Roskilde, Denmark). Effector: target cell ratios from 100:1 to 12.5:1 were tested in triplicate. The plates were kept at 37°C in a humidified, 5% CO2 incubator for 3 h and then centrifuged at 700g for 5 min. The supernatant was separated from the cellular fraction by rapidly inverting the plate and flicking the supernatants out. Then, 100 μl of 1% triton X100 in 0.05 M borate buffer, pH 9.0, was added to each well. The plate was kept at 4°C for 20 h to allow for solubilization and then was read for fluorescence with a 1420 VICTOR2 multilabel counter (Wallac, Turku, Finland). The percentage of specific lysis was calculated as follows:
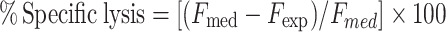 |
where F represents the fluorescence of the solubilized cells after the supernatant has been removed; med = F from target incubated in medium alone; exp = F from target incubated with effector cells.
Lytic units (LU20/107 cells) were calculated by using a computational method [14]. One LU corresponds to the number of effector cells required to produce 20% specific lysis.
CD107 cytotoxicity assay
Splenocytes were incubated at 37°C and 5% CO2 in RPMI medium containing 10% FCS in the presence of mitomycin-treated TUBO tumor cells as stimulators (20:1 ratio stimulators: lymphocytes) for 2 days. For the CD107a assays, the Ab (FITC) (BD Pharmingen) was added to the cells during the stimulation, and 1 μl/ml of Golgi plug was added to the tubes. The cells were washed and stained with mAb directed at surface phenotypic markers CD3 (PE, BD Pharmingen) and CD8 (Per CP, BD Pharmingen). The stained cells were analyzed by flow cytometry (Coulter XL flow cytometer).
In vivo cytotoxicity
In vivo cytotoxicity assay was performed as previously described [15]. Briefly, Young and old Balb/c mice were immunized with DNA vaccine encoding pCMV-ECDTM or pCMV. To generate differentially labeled target cells, splenocytes from naïve mice were incubated with either high (10 μM, CFSEhigh) or low (2 μM, CFSElow) concentrations of CFSE. The CFSEhigh cells were pulsed with the neu peptide, PDSLRDLSVF, and the CFSElow cells were pulsed with the irrelevant peptide, RPQASGVYM, and washed extensively. Both CFSEhigh (5 × 106 cells) and CFSElow (5 × 106 cells) were co-injected I.V. at a ratio of 1:1 in immunized mice, 1 week following the last immunization. Sixteen hours after injection, young and old Balb/c mice were killed by CO2, and spleens and bone marrows were removed to analyze residual CFSEhigh and CFSElow target cells remaining in recipients by flow cytometry (Coulter XL flow cytometer). Bone marrow was flushed from the femurs and tibias using a syringe with a fine needle of 25 gauge. Flushing and washing procedures were performed in Separation Buffer (PBS pH 7.2 plus 0.5% BSA and 2 mM EDTA). Percent of cytotoxicity was calculated as follows:
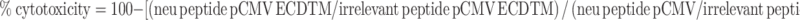 |
Cytofluorimetric evaluation of anti-rat p185neu antibodies
Serum of young and old mice immunized with pCMV-ECD-TM or pCMV were collected before TUBO cell challenge. The ability of sera to bind p185neu was evaluated by flow cytometry. 2 × 105 TUBO cells were washed twice with cold PBS supplemented with 2%BSA and 0.05% sodium azide. Cells were then stained in a standard indirect immunofluorescence procedure with 50 μl of 1:10 dilution in PBS-azide-BSA of control or immune sera. A fluorescein-conjugated rabbit anti-mouse Ig (Calbiochem) was used as second-step Ab. The cells were resuspended in Isoton II (Coulter, Hialeah, FL) and evaluated through a Coulter XL flow cytometer. The specific TUBO binding potential (Sbp) of the sera was calculated as follows: [(% positive cells with test serum) (fluorescence mean)] − [(% positive cells with control serum) (fluorescence mean)] × serum dilution, as previously described in detail [16].
RT-PCR and quantitative real-time mRNA analysis
Total RNA was isolated at 3, 6, 9, and 24 h from DNA immunization from tibial muscle using the RNeasy Fibrous Tissue Mini RNA isolation kit (Qiagen S.p.A., Milano, Italy) according to the manufacturer’s instructions. The first-strand cDNA was synthesized incubating 1 μg of RNA with deoxynucleotide triphosphate (0.5 mM), oligo dT (12.5 ng/μl), first strand buffer (1X), Moloney Murine Leukemia Virus reverse transcriptase (10 units/μl), RNase inhibitor (1 units/μl), and DTT (0.01 mM) all from Invitrogen S.R.L., in a final volume of 20 μl. The samples were incubated at 37°C for 1 h and 95°C for 10 min; subsequently, cDNA was frozen at −20°C until use. Relative quantification of mRNA expression was achieved by quantitative real-time PCR using fluorescent dye SYBR-green (iQ™ SYBR Green Supermix, BioRad, Richmond, CA, USA) during PCR amplification (iQ5 Real-time Detection System, BioRad, Richmond, CA, USA). The mouse primers used were as follows: m-ACTIN, 5′-TTCGTTGCCGGTCCACA-3′ and 5′-ACCAGCGCAGCGATATCG-3′; ECD, 5′-TCCGGCATTGCTCCGCTGAG-3′ and 5′-AAGACACTGAGGTCACGGAGACTG-3′. Each set of primers was also designed across intron/exon boundaries to detect genomic DNA contamination. Variation in gene expression in treated cells respect to the control (untreated cells) was determined using the formula: 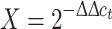 ; ΔΔc
t = δE − δC; δE = n° cycles of gene − n° cycles of house-keeping gene (m-ACTIN) in the treated cells; δC = n° cycles of DR gene − n° cycles of house-keeping gene in the control cells.
; ΔΔc
t = δE − δC; δE = n° cycles of gene − n° cycles of house-keeping gene (m-ACTIN) in the treated cells; δC = n° cycles of DR gene − n° cycles of house-keeping gene in the control cells.
Statistical analysis
Differences in tumor incidence were evaluated by the Mantel–Haenszel log-rank test. Differences in immune parameters were evaluated by parametric (Student’s t-test) or non-parametric (Mann–Whitney) tests according to the distribution of the data. Differences were considered statistically significant when P < 0.05. The statistical analysis was performed with Systat 10 (SPSS Inc) and SigmaStat software version 1.03 (Jandel Scientific, Germany).
Results
Kinetics of HER-2/neu expression in immunization site
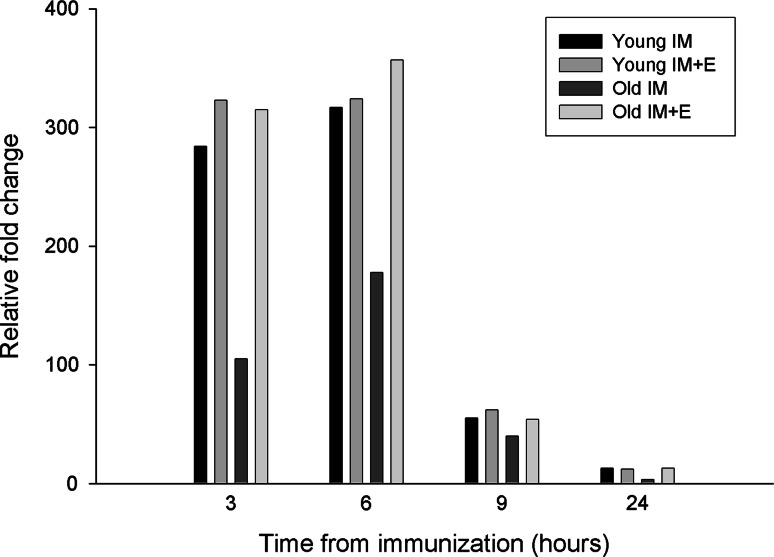
The kinetics of HER-2/neu mRNA expression after in vivo intramuscular immunization with pCMV ECDTM with or without electroporation was evaluated in young and old mice (Fig. 1). Total RNA was extracted from the immunization site of mice, retrotranscribed, and amplified through real-time RT-PCR for HER-2/neu expression. As shown in Fig. 1, both 3 and 6 h after IM immunization, HER-2/neu expression was higher in young than old mice. Afterword, the levels of the transgene progressively decreased in both aged groups. The IM + E immunization greatly increased HER-2/neu expression in old mice both at 3 and 6 h from immunization up to the levels found in young animals. No difference with intramuscular immunization alone was found in young mice at any of the times considered.
Fig. 1.
Kinetics of expression of HER-2/neu mRNA in plasmid DNA injection site. Total RNA was isolated at 3, 6, 9, and 24 h from DNA immunization from the tibial muscle and analyzed for the expression of HER-2/neu mRNA through real-time RT-PCR. Values were normalized with β-actin. Data shown are representative of one of the two independent experiments
Kinetics of tumor growth
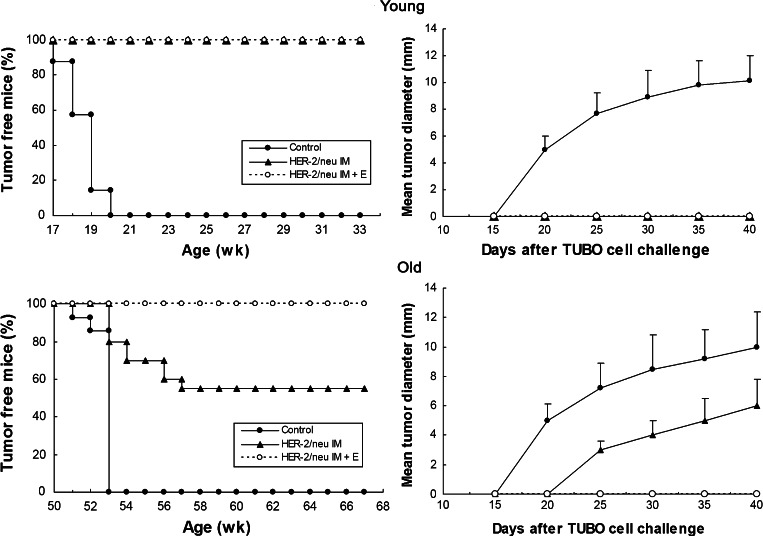
Young and old Balb/c mice received two administrations of pCMV ECDTM plasmids or pCMV empty vector. Two weeks after last immunization, mice were challenged with TUBO cells, a tumor cell line displaying membrane class I H-2d MHC glycoproteins and rat-p185neu protein. In Balb/c mice, rat-p185neu is a xenogeneic Ag that differs from mouse p185neu in less that 6% of the amino residues 13. As shown in Fig. 2 (left), a challenge with 1 × 105 TUBO cells grew progressively in all young and old Balb/c mice injected with pCMV empty vector. Young mice receiving two immunizations with pCMV ECDTM plasmid were completely protected against a TUBO challenge, in both IM and IM + E groups (Fig. 2, left). Differently, only a partial protection was observed in old mice immunized with pCMV ECDTM DNA plasmid through IM injection, being more than 40% of mice with a growing tumor at 56 days after TUBO cell challenge (Fig. 2, left). The intramuscular immunization with pCMV ECDTM followed by electroporation completely protected old mice against the challenge with TUBO cells (Fig. 2, left). As shown in Fig. 2 (right), a similar kinetics of tumor growth was observed in young and old mice. In these last, the IM immunization with pCMV ECDTM significantly reduced the mean tumor diameter at all points examined (P at least <0.05).
Fig. 2.
Effect of immunization with pCMV ECDTM plasmid on tumor incidence (left) or mean tumor diameter (right) in young and old Balb/c mice challenged with syngeneic TUBO cells. Young and old mice received two immunizations with pCMV ECDTM DNA plasmid or with pCMV and, 2 weeks ago, were challenged with TUBO cells. Eight mice for each group were used for each experiment. Data shown are representative of one of the two independent experiments
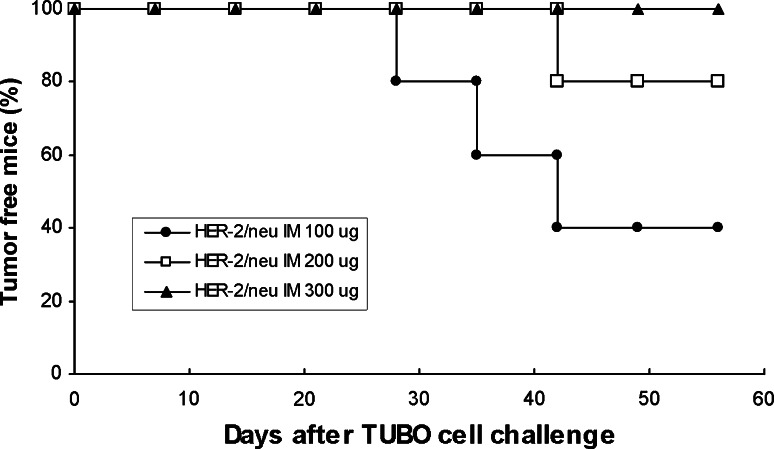
In order to evaluate whether higher concentration of IM DNA may induce stronger antitumor responses, old mice were immunized with DNA doses ranging from 100 to 300 μg. As shown in Fig. 3, increased concentrations of pCMV ECDTM plasmid IM immunization reduced the number of mice with tumors, with a 300 μg DNA concentration that protected all old mice from tumor challenge.
Fig. 3.
Effect of IM immunization with increased pCMV ECDTM plasmid concentrations on tumor incidence in old Balb/c mice challenged with syngeneic TUBO cells. Old mice received two immunizations with 100, 200, or 300 μg of pCMV ECDTM DNA plasmid and, 2 weeks ago, were challenged with TUBO cells
Humoral immunity
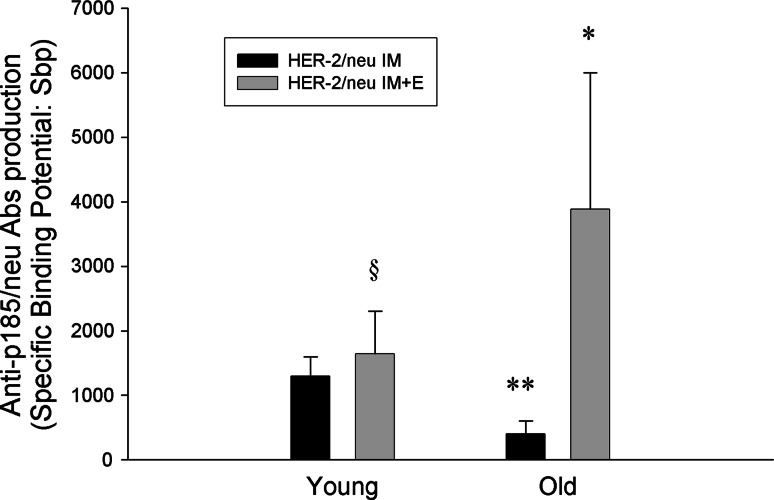
Figure 4 shows the anti-p185neu antibodies (Abs) found in the sera from young and old IM or IM + E immunized mice. Anti-p185neu Abs were detected in the sera of both aged groups. As reported in Fig. 4, Antibody production was significantly higher in pCMV ECDTM IM + E immunized old mice than in animals immunized with only IM (P = 0.004). In old mice immunized with pCMV ECDTM IM, anti-p185neu Abs were significantly lower when compared to young IM immunized mice (P < 0.05).
Fig. 4.
Presence of anti p185neu Abs in the sera of young and old mice after vaccination with pCMV ECDTM plasmid. Sera from mice immunized with pCMV ECDTM plasmid by IM or IM + E or with pCMV were collected before TUBO cell challenge. Specific p185 Sbp was evaluated by flow cytometry after indirect immunofluorescence and calculated as reported in Mat and Methods. Data shown are representative of one of the three independent experiments. *P at least <0.05 versus HER-2/neu IM old value; **P at least <0.05 versus respective young value; § P < 0.05 versus HER-2/neu IM young value
Cell-mediated immunity
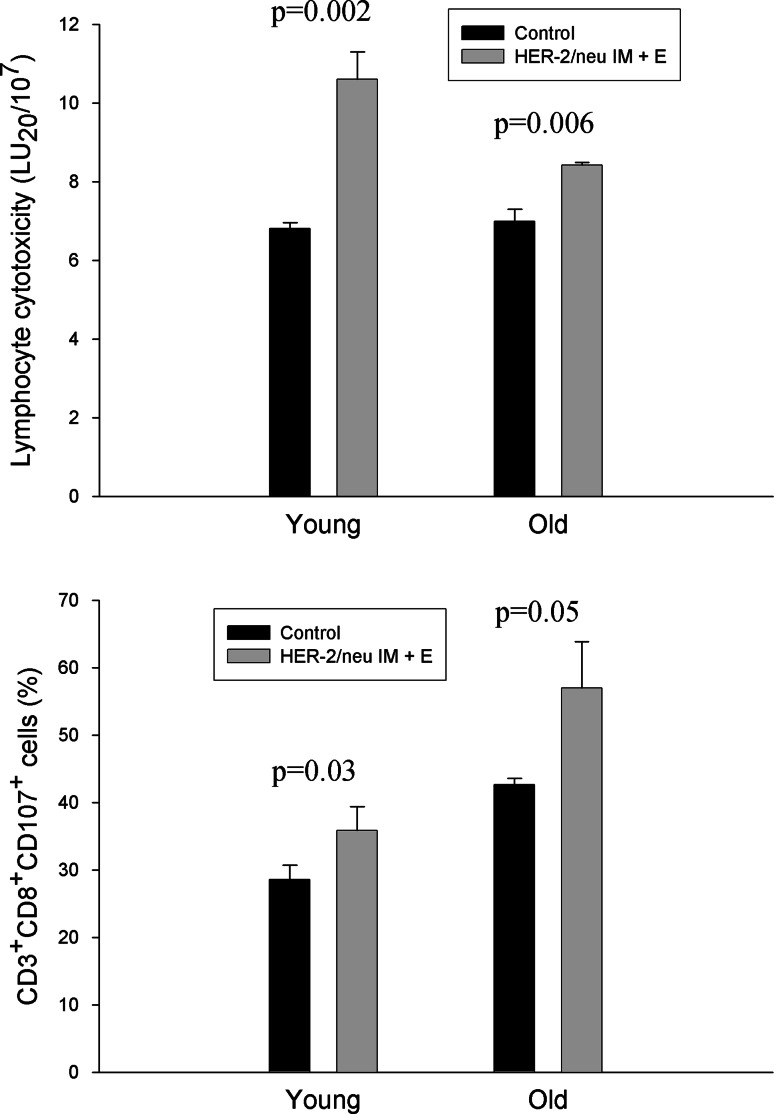
Figure 5 (top) reports the cytotoxicity of spleen lymphocytes from young and old mice immunized with pCMV ECDTM through IM or IM + E or with pCMV and in vitro primed with mitomycin-treated TUBO cells. As shown in the figure, cytotoxic activity was significantly increased in pCMV ECDTM IM + E immunized mice than in pCMV ECDTM IM or control animals, in both young and old ages (Fig. 5, top). The analysis of CD107 expression on CD8+ T cells, as a marker of cytotoxicity, also showed significantly higher levels of CD107 expression in pCMV ECDTM IM + E immunized mice than in control animals, in both young (P = 0.03) and old (P = 0.05) age (Fig. 5, bottom).
Fig. 5.
Cytotoxicity of spleen lymphocytes from young and old mice after vaccination with pCMV ECDTM plasmid. The cytotoxicity of spleen lymphocytes from mice immunized with pCMV ECDTM plasmid by IM or IM + E or with pCMV was evaluated after in vitro incubation with mytomicin-treated TUBO cells as reported in “Materials and methods.” The cytotoxicity was evaluated through an in vitro cytotoxic assay against TUBO tumor cells (top) or through the evaluation of CD107 on CD8+ T cells (bottom). Data shown are representative of one of the three independent experiments. Top *P al least < 0.05 versus control or HER-2/neu IM young values; **P at least <0.05 versus control or HER-2/neu IM old values; § P < 0.05 versus young control; bottom *P at least <0.05 versus control young value; **P at least <0.05 versus control old value
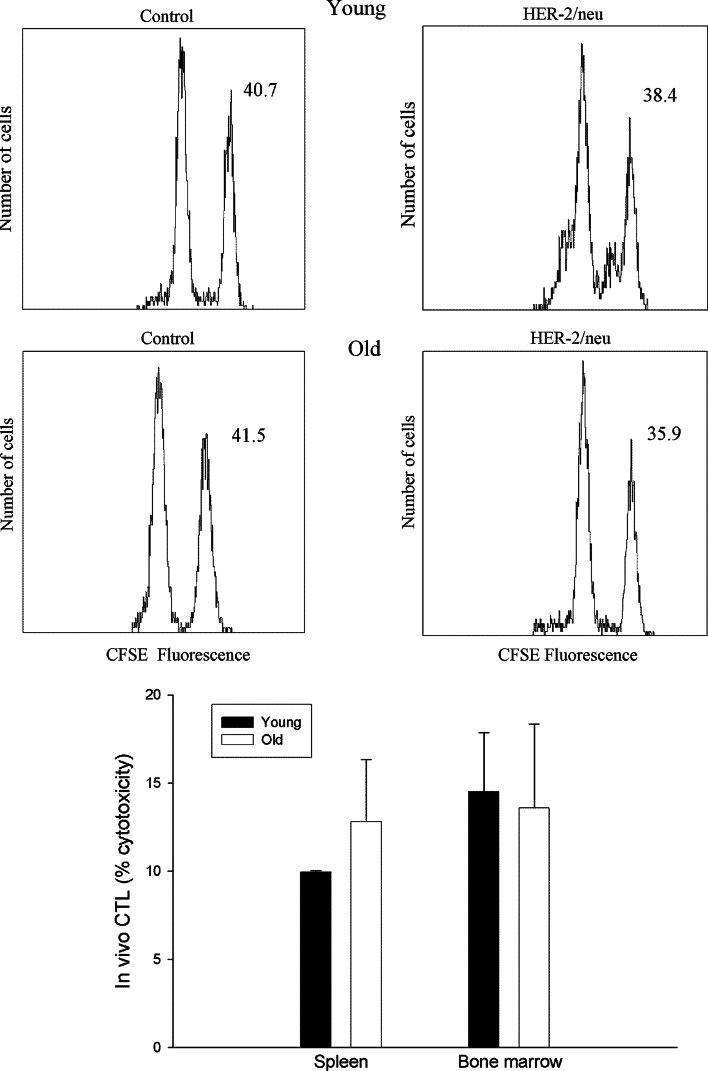
Figure 6 reports the levels of cytotoxicity found in IM + E immunized mice in vivo. As shown in Fig. 6 (top), CFSEhigh (neu peptide pulsed)-labeled cells decreased in the spleen by 7% in young mice and by 14% in old animals, whereas the levels of CFSElow-labeled cells remained relatively unaffected. Similar data were obtained in bone marrow. Figure 6 (bottom) reports the mean of in vivo cytotoxicity observed in the spleen and in bone marrow from young and old mice. As shown, significant levels of cytotoxicity were present in both spleen and bone marrow without age-dependent differences.
Fig. 6.
In vivo cytotoxicity after vaccination with pCMV ECDTM plasmid in young and old mice. Plasmid-immunized mice were injected i.v with 5 × 106 splenocytes (target cells) from naïve transgenic mice pulsed with either HER-2 immunodominant or irrelevant peptides and stained with CFSE as detailed in M&M. Sixteen hours later, the splenocytes of immunized mice were analyzed by flow cytometry. The value in each panel represents the percentage of CFSE high and CFSE low target cells remaining in the spleen in a representative sample. The histogram shows the mean ± SD of two independent experiments
Discussion
Studies conducted in immunocompetent animal models have demonstrated that vaccination with plasmid DNA represents an efficacious approach to induce antitumor immunity [1–3, 17, 18]. The induction of specific immunity obtained in these models has been strictly related to the effectiveness of the immune system, and no protection against tumor challenge has been observed in immunosuppressed host. Aging has been associated with an immune derangement, particularly evident at the level of the thymus-dependent immunity [19–21]. The age-dependent reduction in naïve T cells, the shift from a Th1 to a Th2 phenotype, the defect of antigen presentation by antigen-presenting cells (APCs) to T lymphocytes, the alteration of components of the innate immunity with potential damage to the innate–adaptive interrelationships, may determine an age-associated disadvantage with a multistep defect in which different cell populations involved in the activation of anticancer immunity are all affected [22].
A consequence of the remodeling of the immune system arising during aging, the so-called immunosenescence, is that vaccination models proved efficacious in young adult age may not be properly efficient in old ages. In effect, the vaccination with tumor cells engineered for the production of IL-2 has been shown to induce a lower protective immunity in old mice in comparison with young animals [23]. Furthermore, DNA vaccination with a HER-2/neu DNA plasmid demonstrated a lower efficacy in inducing protective immunity against a lethal challenge with syngeneic tumor cells overexpressing HER-2/neu in old in comparison with young mice [8]. The lower objective response was related to the reduced immune activation determined by the DNA vaccination in old animals. In fact, the antibody production and the proliferative capacity of lymphocytes were both lower in old than in young mice, and the activity of cytotoxic T lymphocytes was not found in old animals [8]. Other experimental models confirmed this evidence [7, 24].
In this study, we demonstrated the possibility to recover the low effectiveness of DNA immunization against HER-2/neu increasing plasmid uptake by cells from old mice through electroporation, since in the electroporated group, 100% of old mice was able to reject a subsequent challenge with syngeneic TUBO tumor cells. The effectiveness of this immunization approach was associated with the induction of both humoral and cell-mediated protective immunity. The moderate CTL response, evidenced by both lymphocyte cytotoxicity and CD3CD8CD107 response and the much stronger antibody response in IM + E than in IM-vaccinated mice present in old ages, clearly suggests that neu-specific antibodies may play an important role against tumor growth in advanced age. In young ages, the CTL response seems to represent the main immune component-mediating tumor protection.
The results reported in this study demonstrate that the defect in immunization observed in old age may be related, at least in part, to the impaired effectiveness in the first steps of the immunization procedure, with a reduced expression of the transgene and the consequent impaired presentation of tumor antigens by antigen-presenting cells.
Besides demonstrating that the lower efficacy of immunization at old age is not an irreversible phenomenon, these data suggest that different factors may be involved in the age-related defect in memory acquisition after administration of the vaccine. We previously demonstrated that experimental approaches aimed at rejuvenating T-cell compartment through grafting a young thymus or the adoptive transfer of young memory T lymphocytes did not confer tumor specific immune memory in old mice [23]. These data show that T cells per se do not seem to be the only cause of immune memory deficiency in old age and other steps of the immunization process, such as antigen internalization and presentation by antigen-presenting cells, might be involved.
Since electroporation determines a transient increase in the permeability of the plasma membrane, it is possible that the complete protection obtained in old mice after immunization with DNA plasmids using this technique was dependent on the better uptake of plasmids into antigen-presenting cells. This evidence is supported by the fact that plasma membrane fluidity diminishes during aging [11, 12], that the defect is reversible and that its correction ameliorates various immune functions impaired in old age [25, 26]. The relevant role of defects in the antigen presentation step in the age-related impaired immunization is further suggested by the studies of the Lustgarten group, which demonstrated that dendritic cell-based vaccination plus rIL-2 protected 60% of the young mice from challenge with syngeneic TRAMP-C2 tumor cells, while only a minimal effect was observed in the old mice [27]. However, when coadministered with anti-OX40 or anti-4-1BB mAbs, a vigorous antitumor response in both young (85–90%) and old (70–75%) mice was observed. Furthermore, administration of a syngeneic pre-B lymphoma cells line (BM-185) engineered to express the costimulatory molecule CD80 together with agonists of the costimulatory molecule OX40, restored, or improved the lower memory response against tumor cells, suggesting that deficiencies in long-lasting memory responses may be secondary to ineffective cross-presentation of tumor antigens by endogenous antigen-presenting cells [24].
In conclusion, we demonstrate that the effectiveness of anticancer DNA vaccination in old ages may be improved increasing plasmid uptake and transgene expression through electroporation. This evidence shows that the reduced efficiency of cancer immunization in aged mice does not represent an intrinsic and irreversible phenomenon and suggests for the relevant role of the first steps of the immunization process in the success of cancer vaccines at older age.
References
- 1.Finn OJ, Forni G. Prophylactic cancer vaccines. Curr Opin Immunol. 2002;14:172–177. doi: 10.1016/S0952-7915(02)00317-5. [DOI] [PubMed] [Google Scholar]
- 2.Quaglino E, Rovero S, Cavallo F, Musiani P, Amici A, Nicoletti G, et al. Immunological prevention of spontaneous tumors: a new prospect? Immunol Lett. 2002;80:75–79. doi: 10.1016/S0165-2478(01)00323-6. [DOI] [PubMed] [Google Scholar]
- 3.Cavallo F, Forni G. Recent advances in cancer immunotherapy with an emphasis on vaccines. Exp Rev Vaccines. 2009;8:25–28. doi: 10.1586/14760584.8.1.25. [DOI] [PubMed] [Google Scholar]
- 4.Colombo MP, Forni G. Cytokine gene transfer in tumor inhibition and tumor therapy: where are we now? Immunol Today. 1994;15:48–51. doi: 10.1016/0167-5699(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 5.Cavallo F, Signorelli P, Giovarelli M, Musiani P, Modesti A, Brunda MJ, et al. Antitumor efficacy of adenocarcinoma cells engineered to produce interleukin 12 (IL-12) or other cytokines compared with exogenous IL-12. J Natl Cancer Inst. 1997;89:1049–1058. doi: 10.1093/jnci/89.14.1049. [DOI] [PubMed] [Google Scholar]
- 6.Provinciali M, Smorlesi A. Immunoprevention and immunotherapy of cancer in aging. Cancer Immunol Immunother. 2005;54:93–106. doi: 10.1007/s00262-004-0539-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gravekamp C. Cancer vaccines in old age. Exp Gerontol. 2007;42:441–450. doi: 10.1016/j.exger.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Provinciali M, Smorlesi A, Donnini A, Bartozzi B, Amici A. Low effectiveness of DNA vaccination against HER-2/neu in ageing. Vaccine. 2003;21:843–848. doi: 10.1016/S0264-410X(02)00530-3. [DOI] [PubMed] [Google Scholar]
- 9.Quaglino E, Iezzi M, Mastini C, Amici A, Pericle F, Di Carlo E, et al. Electroporated DNA vaccine clears away multifocal mammary carcinomas in Her-2/neu transgenic mice. Cancer Res. 2004;64:2858–2863. doi: 10.1158/0008-5472.CAN-03-2962. [DOI] [PubMed] [Google Scholar]
- 10.Smorlesi A, Papalini F, Amici A, Orlando F, Pierpaoli S, Mancini C, et al. Evaluation of different plasmid DNA delivery systems for immunization against HER2/neu in a transgenic murine model of mammary carcinoma. Vaccine. 2006;24:1766–1775. doi: 10.1016/j.vaccine.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 11.Rivnay B, Globerson A, Shinitzky M. Viscosity of lymphocyte plasma membrane in aging mice and its possible relation to serum cholesterol. Mech Ageing Dev. 1979;101:71–79. doi: 10.1016/0047-6374(79)90071-X. [DOI] [PubMed] [Google Scholar]
- 12.Rivnay B, Bergman S, Shinitzky M, Globerson A. Correlation between membrane viscosity, serum cholesterol, lymphocyte activation and aging in man. Mech Ageing Dev. 1980;12:119–126. doi: 10.1016/0047-6374(80)90088-3. [DOI] [PubMed] [Google Scholar]
- 13.Provinciali M, Di Stefano G, Fabris N. Optimization of cytotoxic assay by target cell retention of the fluorescent dye carboxyfluorescein diacetate (CFDA) and comparison with conventional 51CR release assay. J Immunol Meth. 1992;155:19–24. doi: 10.1016/0022-1759(92)90266-V. [DOI] [PubMed] [Google Scholar]
- 14.Bryant J, Day R, Whiteside T, Herberman RB. Calculation of lytic units for the expression of cell-mediated cytotoxicity. J Immunol Methods. 1992;146:91–96. doi: 10.1016/0022-1759(92)90052-U. [DOI] [PubMed] [Google Scholar]
- 15.Barber DL, Wherry EJ, Ahmed R. Cutting edge: rapid in vivo killing by memory CD8 T cells. J Immunol. 2003;171:27–31. doi: 10.4049/jimmunol.171.1.27. [DOI] [PubMed] [Google Scholar]
- 16.Giovarelli M, Musiani P, Modesti A, Dellabona P, Casorati G, Allione A, et al. Local release of IL-10 by transfected mouse mammary adenocarcinoma cells does not suppress but enhances antitumor reaction and elicits a strong cytotoxic lymphocyte and antibody-dependent immune memory. J Immunol. 1995;155:3112–3123. [PubMed] [Google Scholar]
- 17.Rovero S, Amici A, Di Carlo E, Bei R, Nanni P, Quaglino E, et al. DNA vaccination against rat her-2/Neu p185 more effectively inhibits carcinogenesis than transplantable carcinomas in transgenic BALB/c mice. J Immunol. 2000;165:5133–5142. doi: 10.4049/jimmunol.165.9.5133. [DOI] [PubMed] [Google Scholar]
- 18.Amici A, Smorlesi A, Noce G, Santoni G, Cappelletti P, Capparuccia L, et al. DNA vaccination with full-length or truncated neu induces protective immunity against the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Gene Ther. 2000;7:703–706. doi: 10.1038/sj.gt.3301151. [DOI] [PubMed] [Google Scholar]
- 19.George AJT, Ritter MA. Thymic involution with ageing: obsolescence or good housekeeping? Immunol. Today. 1996;17:267–272. doi: 10.1016/0167-5699(96)80543-3. [DOI] [PubMed] [Google Scholar]
- 20.Fabris N, Mocchegiani E, Provinciali M. Plasticity of neuro-endocrine-thymus interactions during ageing. Exp Gerontol. 1997;32:415–430. doi: 10.1016/S0531-5565(96)00166-0. [DOI] [PubMed] [Google Scholar]
- 21.Pawelec G, Solana R. Immunosenescence. Immunol Today. 1997;18:514–516. doi: 10.1016/S0167-5699(97)01145-6. [DOI] [PubMed] [Google Scholar]
- 22.Provinciali M. Immunosenescence and cancer vaccines. Cancer Immunol Immunother. 2009;58:1959–1967. doi: 10.1007/s00262-009-0665-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Provinciali M, Argentati K, Tibaldi A. Efficacy of cancer gene therapy in ageing: adenocarcinoma cells engineered to release IL-2 are rejected but do not induce tumor specific immune memory in old mice. Gene Ther. 2000;7:624–632. doi: 10.1038/sj.gt.3301131. [DOI] [PubMed] [Google Scholar]
- 24.Lustgarten J, Dominguez A, Thoman M. Aged mice develop protective antitumor immune responses with appropriate costimulation. J Immunol. 2004;173:4510–4515. doi: 10.4049/jimmunol.173.7.4510. [DOI] [PubMed] [Google Scholar]
- 25.Chapman HA, Jr, Hibbs JB., Jr Modulation of macrophage tumoricidal capability by components of normal serum: a central role for lipid. Science. 1977;197:282–295. doi: 10.1126/science.195338. [DOI] [PubMed] [Google Scholar]
- 26.Provinciali M, Fabris N, Pieri C. Improvement of Natural Killer cell activity by in vitro active lipids (AL721) administration in old mice. Mech Ageing Dev. 1990;52:245–254. doi: 10.1016/0047-6374(90)90128-3. [DOI] [PubMed] [Google Scholar]
- 27.Sharma S, Dominguez A, Lustgarten J. Aging affect the anti-tumor potential of dendritic cell vaccination, but it can be overcome by co-stimulation with anti-OX40 or anti-4–1BB. Exp Gerontol. 2006;41:78–84. doi: 10.1016/j.exger.2005.10.002. [DOI] [PubMed] [Google Scholar]