Dear Editors,
We read with great interest the focused research review article by Weide et al. on emerging intralesional treatments for metastatic or unresectable melanoma [1]. The approval of talimogene laherparepvec (T-VEC) has significantly broadened the arsenal of loco-regional approaches for patients with cutaneous or nodal metastases. In addition, novel agents such as T-VEC or Daromun may be accompanied by systemic and abscopal effects of uninjected tumor lesions, offering advantages over purely ablative techniques such as cryo- or electrochemotherapy. However, little experience and specific recommendations exist regarding the application of intratumoral agents for mucosal sites.
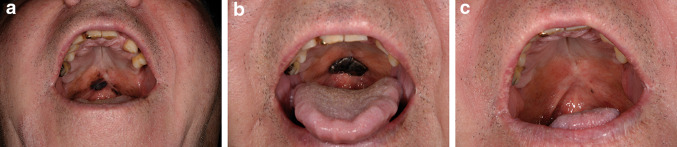
We report a 71-year-old male who presented with a large, ill-defined, protruding black mass of the oral cavity (Fig. 1a). Several biopsies confirmed nodular mucosal melanoma with ulceration and a Breslow index of at least 2.4 mm. The tumor was heavily pigmented with only little lymphocytic infiltration and virtually no membranous expression of PD-L1 (<1%). No regional lymph node or distant metastases were present at primary diagnosis, but the tumor was deemed unresectable by a multidisciplinary cancer care team due to infiltration of the osseous palate. Molecular analyses from the primary tumor revealed a wild-type situation for BRAF, NRAS, and KIT (exons 11, 13, 17). Despite the lack of PD-L1 expression, we initiated systemic treatment with nivolumab at 3 mg/kg body weight biweekly. The tumor was initially stable in size but progressed in the further disease course (Fig. 1b). Thus, nivolumab was discontinued after 11 treatment cycles. As next treatment option, we considered injection of T-VEC. Patients with primary mucosal melanoma were excluded from the pivotal trial and physicians are advised to avoid any contact of T-VEC with mucous membranes during the application procedure. Herpes virus type 1 shows a strong tropism for mucous epithelia of the oral cavity and exposure of T-VEC may result in uncontrolled herpes infection, even though this oncolytic virus cannot replicate in healthy cells due to a lack of the infected cell protein 34.5 (ICP34.5) and was safe in cutaneous melanoma of the head and neck in the OPTiM analysis [2]. We finally refrained from T-VEC and choose interleukin-2 (IL-2) as intralesional application in off-label use. Due to its exophytic growth pattern, a part of the mass was ablated with superficial shave excision prior to IL-2 injection to better reach the base of the tumor. Subsequently, the patient received intratumoral applications of 3–6 million international units IL-2 (Proleukin®) per day three times a week for 28 days. The administration dose, frequency, and duration of treatment were determined in relation to the tumor size, specifically following the recommendations of a phase 2 trial in metastasized cutaneous melanoma [3]. The injections were well tolerated. Common adverse events of intralesional IL-2 such as fever, nausea, fatigue, or dizziness were not observed, although the injections were reported painful by the patient. There was a striking response with shrinkage of the tumor to only a small black patch of the hard palate, starting 3 weeks after treatment was initiated. The surrounding area showed discrete hypopigmentation, reminiscent of the typical vitiligo-like areas reported in adjacent skin after IL-2 therapy (Fig. 1c). These effects were stable over a follow-up period of 6 months with no evidence of local tumor growth or distant metastases.
Fig. 1.
a Patient initially presented with an ill-defined black plaque of the hard palate. b Tumor showed exophytic growth after 11 cycles of nivolumab for 6 months. c 3 months later, only a small pigmented patch is visible after superficial ablation and intralesional IL-2 injections three times per week for 28 days
This unique case demonstrates the value of intratumoral agents for unresectable mucosal melanoma, even after systemic checkpoint blockade has failed, as complete resection may often be impossible for functional or cosmetic reasons. Although systemic immune checkpoint blockade is approved for mucosal melanoma, it has shown only limited efficacy in this subgroup and is accompanied by high treatment-related costs. Here, intralesional IL-2 may represent a tolerable, effective, and cost-efficient therapy option. The exact mode of action of IL-2 is not understood. Compared to systemic application, intralesional injection achieves a high local dosage with little toxicity [3]. IL-2 may induce lymphokine-activated killer cells which exert cytotoxic effects on tumor cells irrespective of immune checkpoints. This hypothesis is supported by our findings, because the tumor showed no expression of PD-L1 and only little lymphocytic infiltration.
Altogether, we challenge the application of T-VEC and other intralesional agents for mucosal sites or muco-cutaneous transition zones and propose that the potential of intratumoral treatments is not yet fully exploited. Such locations are of high unmet need and should be taken into account when novel agents are developed and launched to the market.
Markus V. Heppt
Ilana Goldscheider
Julia K. Tietze
Carola Berking
Compliance with ethical standards
Conflict of interest
Carola Berking speaker’s honoraria from Bristol-Myers Squibb (BMS), Merck Sharp & Dohme (MSD), Novartis, and Roche, consultant’s honoraria from Amgen, AstraZeneca, BMS, MSD, Novartis, Pierre Fabre, and Roche and travel support from Amgen, BMS, MSD, and Roche; Markus V. Heppt speaker’s honoraria from Roche, Novartis, BMS, MSD and travel support from BMS; Julia K. Tietze speaker’s honoraria from BMS, MSD, Novartis, Roche, Almirral, Amgen, travel support from BMS. Ilana Goldscheider declares that she has no conflict of interest.
Informed consent
Informed consent was obtained from all individual participants included in the study. Additional informed consent was obtained from all individual participants for whom identifying information is included in this article.
References
- 1.Weide B, Neri D, Elia G. Intralesional treatment of metastatic melanoma: a review of therapeutic options. Cancer Immunol Immunother. 2017;66(5):647–656. doi: 10.1007/s00262-016-1952-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andtbacka RH, Agarwala SS, Ollila DW, et al. Cutaneous head and neck melanoma in OPTiM, a randomized phase 3 trial of talimogene laherparepvec versus granulocyte-macrophage colony-stimulating factor for the treatment of unresected stage IIIB/IIIC/IV melanoma. Head Neck. 2016;38:1752–1758. doi: 10.1002/hed.24522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weide B, Derhovanessian E, Pflugfelder A, Eigentler TK, Radny P, Zelba H, Pfohler C, Pawelec G, Garbe C. High response rate after intratumoral treatment with interleukin-2: results from a phase 2 study in 51 patients with metastasized melanoma. Cancer. 2010;116:4139–4146. doi: 10.1002/cncr.25156. [DOI] [PubMed] [Google Scholar]