Abstract
Anti-PD-1 antibody treatment is approved in advanced melanoma and provides median overall survival over 24 months. The main treatment-related side effects are immune-related adverse events, which include rash, pruritus, vitiligo, thyroiditis, diarrhoea, hepatitis and pneumonitis. We report a case of autoimmune diabetes related to nivolumab treatment. A 73-year-old man was treated in second line with nivolumab at 3 mg/kg every two weeks for metastatic melanoma. At 6 weeks of treatment, he displayed diabetic ketoacidosis. Nivolumab was withheld 3.5 weeks and insulin therapy was initiated, enabling a normalization of glycaemia and the disappearance of symptoms. Laboratory investigations demonstrated the presence of islet cell autoantibodies, while C-peptide was undetectable. Retrospective explorations on serum banked at week 0 and 3 months before the start of nivolumab, already showed the presence of autoantibodies, but normal insulin, C-peptide secretion and glycaemia. Partial response was obtained at month 3, and nivolumab was then resumed at the same dose. The clinical context and biological investigations before, at and after nivolumab initiation suggest the autoimmune origin of this diabetes, most likely induced by anti-PD-1 antibody in a predisposed patient. The role of PD-1/PD-L1 binding is well known in the pathogenesis of type 1 diabetes. Therefore, this rare side effect can be expected in a context of anti-PD-1 treatment. Glycaemia should be monitored during PD-1/PD-L1 blockade. The presence of autoantibodies before treatment could identify individuals at risk of developing diabetes, but systematic titration may not be relevant considering the rarity of this side effect.
Keywords: Melanoma, Anti-PD-1 antibody, Autoimmune diabetes, Adverse events
Introduction
Nivolumab is an immune-checkpoint inhibitor antibody (anti-PD-1 antibody) that selectively blocks the interaction of the PD-1 receptor, on the T cells, with its two known programmed death ligands, PD-L1 and PD-L2, present on the surface of the tumour cells and immune cells in the tumour micro-environment. It thus restores T cell activation and proliferation and consequently induces an anti-tumour immune response. This induces a decrease in peripheral immune tolerance, which leads to T lymphocyte autoimmune clone activation. Nivolumab is associated with significant improvement in overall survival compared to the former first-line chemotherapy using dacarbazine [1] and was approved by the Food and Drug Administration for the treatment of advanced melanoma in September 2014 and by European Medicines Agency (EMA) in Europe in June 2015. The main side effects of anti-PD-1 therapy are immune-related and include rash, pruritus, vitiligo, thyroiditis, diarrhoea, hepatitis and pneumonitis. Cases of autoimmune diabetes induced by immunotherapy are infrequent and poorly described. We describe islet beta-cell antibody positivity, highlighted retrospectively, in a new case of autoimmune diabetes induced by nivolumab.
Case report
In 2011, a 73-year-old man, with a body mass index of 28 kg/m2, a history of dyslipidemia, and no personal or family history of diabetes, underwent surgical excision of a 2.65-mm-thick, ulcerated, BRAF-mutated, cutaneous melanoma on the back, with negative sentinel lymph nodes. He received low-dose alpha interferon (IFNα) adjuvant therapy for 10 months until the occurrence of Graves’ disease, treated with carbimazole for 1 year. In July 2015, he developed stage IV M1c metastatic melanoma and was treated first line with the vemurafenib (BRAF inhibitor) and cobimetinib (MEK inhibitor) combination. At 3 months, the disease had progressed per RECIST, and a second-line treatment with nivolumab 3 mg/kg every two weeks was introduced. At 6 weeks of nivolumab, before the fourth infusion, the patient suddenly complained of abdominal pain, vomiting and severe asthenia associated with a polyuria–polydipsia syndrome. He was admitted to the emergency unit of the hospital. The initial biological investigation evidenced the following: glycaemia: 27.78 mmol/l, urinary dipstick test: 3 crosses of glucose and ketone, creatinine: 177 μmol/l (baseline: 90 μmol/l), bicarbonate: 18 mmol/l and glycated haemoglobin (HbA1c): 8.8% (normal range 4–6%). These data were consistent with type 1 diabetes onset with acute functional renal failure. Insulin therapy was initiated with gradually increasing doses, providing a normalization of glycaemia and clinical features. Extended biological investigations revealed anti-glutamic acid decarboxylase antibody (GADA) > 2000 IU/L, zinc transporter 8 antibody (ZnT8A) at 802U/ml, (protein phosphatase-like) and insulinoma antigen-2 antibody (IA2A) at 27 U/ml. C-peptide was undetectable. Retrospective investigations based on frozen serum (week 0 (T0) and 3 months before starting nivolumab treatment (T-3 m)) already showed positivity for autoantibodies (Fig. 1). At this time, insulin and C-peptide concentrations and glycaemia were normal (Fig. 2). Nivolumab was resumed at the same dose after three and a half weeks without affecting glycaemia. Tumour assessment 3 months after nivolumab introduction showed complete metabolic response on positron emission tomography/computed tomography).
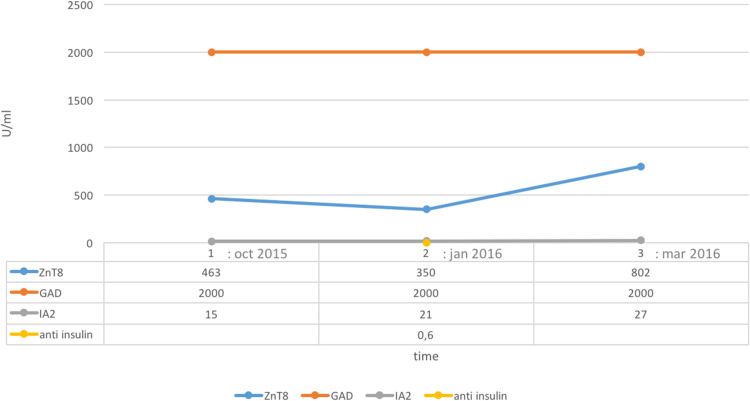
Fig. 1.
Autoantibody evolution: before, at the time of and after nivolumab treatment. These data show autoantibody presence before anti-PD-1 treatment and symptom appearance. IA2 insulinoma antigen-2, ZnT8 zinc transporter 8, GAD glutamic acid decarboxylase
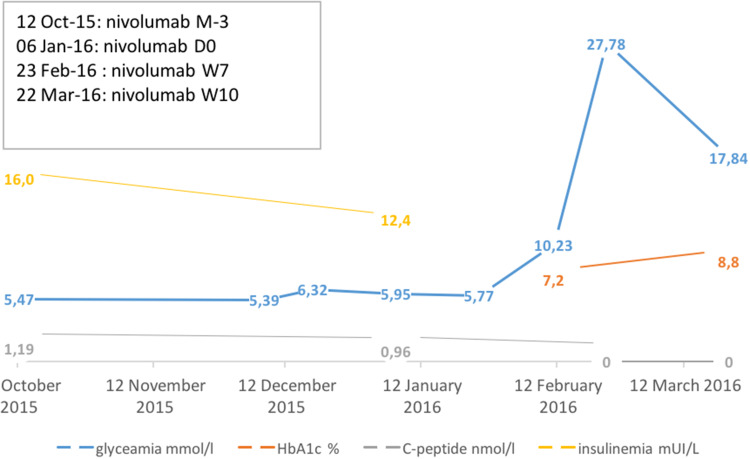
Fig. 2.
Glycaemia, HbA1c, C-peptide level and insulinemia evolution over time. These data describe biological and kinetic parameters of diabetes onset. M month, W week, D day
Discussion
The presence of islet beta-cell-related autoantibodies at diabetes onset, possibly reinforced by their triple positivity (although no data are available in this type of population), supports the diagnosis of autoimmune type 1A diabetes. The patient had already displayed autoimmune hyperthyroidism under IFNα therapy, which could have triggered the appearance of beta-cell antibodies. However, the occurrence of ketoacidosis in a patient presenting with hyperglycaemic symptoms for less than 2 weeks and the moderate rise in HbA1c level, indicate recent glycaemic failure, as observed in fulminant diabetes. Thus, diabetes onset in this patient could be related to treatment with anti-PD-1 antibody in a predisposed subject.
Blockade of PD-1 or its ligand, PD-L1, rapidly precipitates diabetes in pre-diabetic non-obese diabetic (NOD) mice [2]. In addition, selective PD-L1 deficiency in pancreatic cells and deficiency or blockade of PD-1 on T cells activate specific CD8 T cells, leading to diabetes in wild-type mice [3]. The rapid onset of diabetes in our patient was probably due to an autoaggressive effector CD8+ T cell clone that was suddenly set free when PD-1 was blocked. Indeed, PD-1 binding to PD-L1 can down-regulate the diabetogenic potential of specific CD8 T cells [3]. In addition, Indira Guleria et al. [4] showed that CD8+ T cells accumulate in the pancreas of PD-L1/PD-L2-deficient NOD mice compared with wild-type NOD controls, inducing an autoimmune response against pancreatic beta-cells and rapid destruction of beta islet cells [4].
Twenty-four [5–19] cases of diabetes have already been reported as a side effect of anti-PD-1/PD-L1 antibody treatment (Table 1). Of these cases, only one was related to anti-PD-L1 therapy [10], seven (29%) patients were treated with pembrolizumab [5, 6, 8, 9, 14, 15, 18], 13 (54%) patients were treated with nivolumab [8, 10–14, 17, 19], and for one case, the specific anti-PD-1 treatment was not named [7]. Two patients were treated with combined therapy (nivolumab associated with ipilimumab) [8, 16].
Table 1.
Literature review
| Authors | Age (year-old) | Gender | Neoplasia | IFN adjuvant therapy | Line | Treatment | Other immune-related side effect | Time to onset weeks | Symptoms | HbA1c at diagnosis (%) | Glycaemia (mmol/l) | Ketoacidosis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hansen et al. [5] | 58 | Man | BRAF V600E-mutated melanoma | Yes | 4th after vemurafenib, high-dose IL2 and ipilimumab | Pembrolizumab |
Hypothyroidism Vitiligo Fatigue |
48 | Fungal inguinal rash, fatigue, weight loss, polydipsia, polyuria no abdominal pain or symptoms of pancreatic enzyme deficiency | 9.7 | 22.22 | Yes |
| Martin-Liberal et al. [6] | 54 | Woman | BRAF WT melanoma | No | 2nd after ipilimumab | Pembrolizumab | N/A | 9 | Lethargy, vomiting, polydipsia and polyuria | N/A | N/A | Yes |
| Mellati et al. [7] | 70 | Man | Adenocarcinoma of the lung | No | N/A | Anti-PDL-1 (name unknown) | no | 15 | N/A | 9.8 | 22.33 | Yes |
| 66 | Woman | Sarcomatoid squamous cell carcinoma of the jaw | No | N/A | Anti-PD-1 (name unknown) | Hypothyroidism | 7 | N/A | 9.4 | 41.77 | Yes | |
| Hughes et al. [8]–– | 55 | Woman | Melanoma | N/A | 1st | Nivolumab + ipilimumab | Autoimmune thyroid disease | 20 | N/A | 6.9 | 27.78 | Yes |
| 83 | Woman | Non-small cell lung cancer | N/A | N/A | Nivolumab | no | 4 | N/A | 7.7 | 16.67 | Yes | |
| 63 | Man | Renal cell carcinoma with pancreatic metastasis | N/A | 4th after Proleukin, bevacizumab and interferon | Nivolumab | no | 16 | N/A | 8.2 | 11.10 | No | |
| 58 | Man | Small cell lung cancer | N/A | 3rd after Carboplatin, etoposide, paclitaxel | Nivolumab | no | 1 | N/A | 9.7 | 39.89 | Yes | |
| 64 | Woman | Melanoma | N/A | 1st | Pembrolizumab | Autoimmune thyroid disease, psoriasis | 4 | N/A | 7.4 | 38.00 | No | |
| Gaudy et al. [9] | 44 | Woman | Melanoma | N/A | N/A | Pembrolizumab | N/A | 8 | Vomiting and confusion, with polyuria, polydipsia and a very recent weight loss (15 days) | 6.85 | 50.45 | Yes |
| Munakata et al. [10] | 72 | Man | Classic Hodgkin’s lymphoma | No | 3rd after six cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) and brentuximab vedotin monotherapy as the second-line treatment | Nivolumab | N/A | 10 | Slight thirst, polyuria and general fatigue | 7.3 | 20.81 | No |
| Teramoto et al. [11] | 63 | Woman | Vulvar melanoma | No | 2nd line after dacarbazine | Nivolumab | N/A | 30 | General fatigue, polyuria and polydipsia | 8.9 | 36.69 | Yes |
| Miyoshi et al. [12] | 66 | Woman | Melanoma | No | Adjuvant therapy | Nivolumab | N/A | 15 | Diarrhoea and weight loss, anorexia, nausea, vomiting | 7.3 | 29.47 | Yes |
| Okamoto et al. [13] | 55 | Woman | Melanoma | No | 4th after dacarbazine, nimustine, cisplatin | Nivolumab | N/A | 48 | N/A | 7 | 32.19 | yes |
| Hofmann et al. [14] | 70 | Woman | Melanoma | No | 1st | Nivolumab | Hyperthyroidism | N/A | N/A | N/A | N/A | Yes |
| 78 | Woman | Melanoma | No | 2nd line after ipilimumab | Nivolumab | N/A | 2 | Vomiting, diarrhoea | N/A | N/A | Yes | |
| 58 | Woman | Melanoma | No | 2nd line after ipilimumab | Pembrolizumab | N/A | 3 | Increased thirst and a persistent urge to urinate | N/A | N/A | Yes | |
| 40 | Man | Melanoma | No | 4th after dacarbazine; polychemotherapy; ipilimumab | Nivolumab | Hypothyroidism | 6 | N/A | N/A | N/A | Yes | |
| Aleksova et al. [15] | 60 | Man | Melanoma | N/A | 2nd after ipilimumab | Pembrolizumab | N/A | 5 | N/A | N/A | 27 | Yes |
| Lowe et al. [16] | 54 | Man | Melanoma | No | 1st | Nivolumab + ipilimumab | Autoimmune thyroiditis, hepatitis, colitis and adrenal insufficiency | 31 | Weakness, myalgia, nausea and vomiting | N/A | N/A | Yes |
| Usui et al. [17] | 31 | Man | Non-small cell lung cancer | No | 3rd | Nivolumab | N/A | 2 | Fatigue and nausea | 6.4 | 38.39 | Yes |
| 62 | Woman | Non-small cell lung cancer | No | 3rd | Nivolumab | N/A | 10 | Thirst and polyuria | 6.5 | 11.11 | N/A | |
| Chae et al. [18] | 76 | Man | Adenocarcinoma of the lung | No | 1st | Pembrolizumab in combination with systemic chemotherapy (carboplatin, and nab-paclitaxel) | N/A | 3 | Asymptomatic | N/A | 33.33 | No |
| Ishikawa et al. [19] | No available data | Nivolumab |
| Authors | Lipasemia (UI/L) | Autoantibodies GAD/IA2/ZnT8/anti Insulin | C-peptide (ng/ml) N > 0.5 | HLA | Anti-PD1 therapy management | Toxicity evolution |
|---|---|---|---|---|---|---|
| Hansen et al. [5] | N | +/−/−/− | N/A | N/A | Pembrolizumab maintained with no rechallenge due to disease progression |
Subcutaneous insulin regimen administered enabling glycaemia control Reversible diabetes after anti-PD1 discontinuation: by day 54 after onset of insulin-dependent diabetes, the patient was able to discontinue insulin with glycaemic, C-Peptide and HbA1C level normalization |
| Martin-Liberal et al. [6] | N/A | +/−/−/+ | N/A | DRB1*04, DQB1*0302 (HLA A2 DR4 DQ8) |
Pembrolizumab was not discontinued At the time of writing, two further cycles of pembrolizumab had been given without further toxicity and without changes in insulin requirements |
Intravenous insulin initiated and then, switched to a subcutaneous regimen, providing satisfactory glucose levels after a 3-day admission Toxicity non-resolved |
| Mellati et al. [7] | N/A | −/N/A/N/A/− | 0.3 | N/A |
No information about anti-PD-1 management Died from his advanced cancer 7 months later |
Intravenous insulin initiated and then switched to a subcutaneous regimen, providing satisfactory glucose levels after a 3-day admission Toxicity non-resolved |
| N/A | +/+/−/−/+ | <0.1 | DR3-DQ2/DR4-DQ8 |
Evolution not reported Toxicity non-resolved |
||
| Hughes et al. [8]–– | N/A | −/−/−/− | <0.1 | A2.11, DR41 | Insulin treatment initiated but no data about anti-PD-1 management | |
| N/A | +/−/N/A/− | <0.1 | A2.11, DR41 | |||
| N/A | +/N/A/N/A/+ | 1.3 | A2.11, DR41 | |||
| N/A | +/N/A/N/A/− | <0.1 | A2.11 | |||
| N/A | −/N/A/N/A/− | 0.5 | DR41 | |||
| Gaudy et al. [9] | NCS increase | −/−/N/A/N/A | <0.1 | N/A | Pembrolizumab was stopped and reintroduced with no further adverse event |
Insulin therapy was required to control glycaemia Toxicity non-resolved |
| Munakata et al. [10] | Increased with diffuse pancreatic inflammation (MRI) | −/−/−/N/A | N/A | HLA-B*4002 | Nivolumab was re-administered after glycaemia control without further hyperglycaemia |
Long-term insulin replacement therapy was necessary It was treated 4 months more without recovery of endogenous insulin secretion Toxicity non-resolved |
| Teramoto et al. [11] | N/A | −/−/−/N/A | <0.1 | N/A | Treatment stopped 6 weeks before diabetes appearance because of disease progression then, type 1 fulminant diabetes |
Insulin therapy was started, enabling glycaemia control Toxicity non-resolved |
| Miyoshi et al. [12] | NCS increase | −/−/−/N/A | 0.23 | N/A | Nivolumab administration was continued (two doses). |
Diabetes was controlled with basal-bolus insulin therapy Toxicity non-resolved |
| Okamoto et al. [13] | N | −/−/−/− | 1 | DRB1*04:05-DQB1*04:01 | Nivolumab treatment was resumed 1 month after the patient’s referral, and no further side effects were observed |
Diabetes required insulin therapy Toxicity non-resolved |
| Hofmann et al. [14] | N/A | −/−/N/A/N/A | <0.1 | N/A | Nivolumab was continued with good tumour response |
Insulin therapy was needed to control glycaemia Toxicity non-resolved |
| N/A | +/−/N/A/N/A | Low | N/A | No information about nivolumab management |
Diabetes control with insulin therapy Toxicity non-resolved |
|
| N/A | +/−/N/A/N/A | Low | N/A | 2nd infusion of pembrolizumab was maintained until glycaemia normalization |
Insulin therapy was administered Toxicity non-resolved |
|
| N/A | N/A/N/A/N/A/N/A | N/A | N/A | N/A | ||
| Aleksova et al. [15] | N/A | −/N/A/−/N/A | Low | N/A | No information about pembrolizumab management |
Standard immunosuppression for irAEs was started using prednisolone in an attempt to salvage β-cell function but was unsuccessful Toxicity non-resolved |
| Lowe et al. [16] | N/A | +(undetectable 1 month prior)/N/A/N/A/N/A | <0.1 | Patient was removed from the study and placed on surveillance | Intravenous fluids, insulin and methylprednisolone were initiated first (due to concomitant adrenal insufficiency). After immunotherapy discontinuation, continued insulin regimen with undetectable C-peptide level | |
| Usui et al. [17] | N/A | +/N/A/N/A/N/A | <0.1 |
DRB1* 04:05-DQB1*04:01 |
Nivolumab was stopped during hyperglycaemia period | Insulin infusion but no data about evolution |
| N/A | −/N/A/N/A/N/A | N/A | DRB1*09:01-DQB1* 03:03 | Insulin therapy | ||
| Chae et al. [18] | N/A | +/+/N/A/N/A | 0.81 | Not performed | Following rapid correction and control with insulin, it was thought safe to proceed with continued pembrolizumab treatment was seen in the patient’s glycaemic control |
Empirical trial with prednisone at 10 mg per day, stopped after 25 days when no improvement Glycaemia was controlled with insulin therapy |
| Ishikawa et al. [19] | No available data |
N normal, N/A no available, NCS not clinically significant, − negative result, + positive result, IA2 insulinoma antigen-2, ZnT8 zinc transporter 8, GAD glutamic acid decarboxylase
These 24 cases of diabetes related to anti-PD-1/PD-L1 therapy occurred between 1 week and 12 months after initiation (median 8.5 weeks). At diabetes onset, ketoacidosis was reported in 18 (75%) out of the 24 patients [5–9, 11–17]. All these patients had moderate increases in HbA1c level. Ten (42%) patients presented abnormally low HbA1c, under 8.7%, contrasting with their hyperglycaemia [8–10, 12, 13, 17], alongside low or undetectable C-peptide level, which reflects rapid onset as described in fulminant diabetes.
Fulminant diabetes is a subtype of type 1 diabetes discovered in Japan [20] and has been reported in Asia, Polynesia but rather rarely in Europe [21]. It is defined as diabetes in which the process of beta-cell destruction and the progression of hyperglycaemia and ketoacidosis are extremely rapid. The pathogenesis of this disease remains to be clarified, but the involvement of human leucocyte antigen (HLA DRB1*04:05-DQB1*04:01) genes and viruses has been suggested. The hyperglycaemic symptoms last a few days. There is a high prevalence of preceding common-cold-like and gastrointestinal symptoms and an unexpectedly near-normal or moderately increased level of HbA1c, contrasting with the very high plasma glucose levels associated with ketoacidosis. C-peptide concentrations and type 1 diabetes autoantibody are usually not detectable [22]. However, in most cases, islet T lymphocyte infiltration has been observed. In our patient, the short duration of symptoms, abdominal pain, the modest rise in HbA1c level in the presence of ketoacidosis and undetectable C-peptide (while normal a few weeks before) are in favour of fulminant diabetes despite type 1 autoantibody positivity.
In contrast, 6 out of the 24 reported patients [5, 7, 8, 11] had slower onset and higher HbA1c at diagnosis. For eight patients, no information was available on the means by which diabetes was diagnosed.
Five reported cases presented at least one other immune-related side effect [5, 7, 8, 16]: all presented thyroiditis, and three presented other side effects—psoriasis [8] for one, vitiligo [5] for another and the third, who was under combination therapy, presented multiple immune-related side effects including autoimmune hepatitis, colitis and adrenal insufficiency.
Autoantibodies were positive for only 11/22 (50%) [5–8, 14, 16–18] of the patients tested. Among the autoantibody-negative patients [7–15, 17], five were Japanese [10–13, 17], a population prone to fulminant diabetes. As suspected by Usui et al. [17], we also found that the time lapse from the start of treatment with anti-PD-1/PD-L1 antibodies to the onset of autoimmune diabetes appeared to be related to the presence or absence of GADA. The median time lapse from immunotherapy initiation to diabetes onset was, respectively, 3 weeks in the presence of GADA versus 12.5 weeks without (data from the 24 patients).
Here, we present a retrospective investigation study showing the positivity of autoantibodies in a case of autoimmune diabetes appearing after anti-PD-1 treatment, possibly previously triggered by IFNα therapy. As observed in children suffering from autoimmune diabetes, this could reflect subclinical disease before diabetes onset (beta-cell destruction by a specific TCD8+ cell clone without insulinopenia), but these antibodies have no pathogenic role. In some cases, they could provide a predictive factor for disease onset prior to the onset of symptoms and a prognostic factor for the course and control of the diabetes [23]. Additionally, the time from immunotherapy initiation and autoimmune diabetes development could be related to the presence of the autoantibodies. Thus, the predictive value of autoantibody positivity in patients exposed to anti-PD-1 agents for development of diabetes requires further study.
In the case reported by Lowe et al. [16] involving combination therapy (nivolumab plus ipilimumab), antibodies measured retrospectively at the start of immunotherapy were negative and appeared with the diabetes symptoms and glycaemia increase. In fact, data from the literature on NOD mice suggest that in the absence of negative co-stimulation by the PD-1/PD-L1 pathway, CTLA-4 possibly maintains the self-tolerance through IL-2, which stimulates T regulator cells [24]. CTLA-4 expression was also reported in one study to be significantly lower in patients with fulminant type 1 diabetes [25]. The rapid development of this autoimmune toxicity resulting from two immune-checkpoint blockades (CTLA-4 and PD-1) could sidestep the first phase when antibodies are present with subclinical disease because of sudden specific T cell clone activation.
The development of combination immunotherapies coupling an anti-PD-1 antibody with an anti-CTLA-4 antibody, could induce a greater prevalence of this immune side effect. A product characteristic study including the Bristol–Myers Squibb (BMS)067, BMS069 and CA209004-8 cohorts (n = 448 patients), based on combined immunotherapies, i.e. anti-PD-1 with anti-CTLA-4 antibodies, found one case of diabetes with ketoacidosis (0.223%). This observation is not very different from those reported for 1728 patients treated with nivolumab alone, where one case of diabetes and two cases of diabetes with ketoacidosis were described (0.173%). Additional investigation is needed to identify which patients are at increased risk of developing this toxicity under checkpoint inhibitor immunotherapy.
Concerning the management of anti-PD-1 therapy in the 24 cases reported in this literature review, treatment was temporarily discontinued in six (25%) patients [9–11, 13, 14, 17], as a result of symptom severity (until glycaemia was controlled) and resumed without effect on glycaemia, as in our patient’s case. In three (12.5%) cases, anti-PD-1 therapy appears to have been continued alongside glycaemia control by insulin therapy [6, 12, 14, 18]. There was no information on this aspect for seven patients. All patients across the literature review remained under insulin therapy except for one Caucasian patient [5], whose treatment was permanently stopped due to progression of metastatic disease with subsequent decrease in glycaemia and insulin requirements, an increase in C-peptide levels and normalization of HbA1c. Insulin was gradually tapered down and then stopped, suggesting a reversible phenomenon. In one case, glucocorticoid treatment was started to reverse beta islet destruction, but was unsuccessful [15].
Conclusion
We describe a new case of autoimmune diabetes related to anti-PD-1 therapy with retrospective biological analyses, accompanied by a literature review enabling a characterization of autoimmune diabetes resulting from treatment toxicity. It also provided information on management of this rare but increasingly reported immune-related side effect. The potential severity of this complication, with frequent onset of fulminant diabetes, should motivate glycaemia monitoring during immunotherapy treatment. The question of whether autoantibodies should be screened for to identify high-risk subjects at treatment initiation needs to be evaluated.
Author Contributions
Marie-Léa Gauci, Philippe Boudou, Céleste Lebbé and Jean-François Gautier had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors made substantial contributions to study conception, and subsequent acquisition, analysis and interpretation of data. All authors made substantial scientific and intellectual contributions to the drafting and rewriting of the initial and revised manuscript.
Funding sources
This research did not receive any specific grant from any funding agencies in the public, commercial or not-for-profit sectors.
Abbreviations
- BMS
Bristol–Myers Squibb
- BRAF
Murine sarcoma viral oncogene homolog B1
- GADA
Glutamic acid decarboxylase antibody
- HbA1c
Glycated haemoglobin Insulinoma antigen-2 antibody
- IA2A
Insulinoma antigen-2 antibody
- MEK
Mitogen activated protein kinase kinase
- NOD
Non-obese diabetic
- ZnT8A
Zinc transporter 8 antibody
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Informed consent was obtained from the participant in the study.
Footnotes
Philippe Boudou, Céleste Lebbé and Jean-François Gautier are co-last authors.
References
- 1.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 2.Wang J, Yoshida T, Nakaki F, et al. Establishment of NOD-Pdcd1−/− mice as an efficient animal model of type I diabetes. Proc Natl Acad Sci USA. 2005;102:11823–11828. doi: 10.1073/pnas.0505497102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rajasalu T, Brosi H, Schuster C, et al. Deficiency in B7–H1 (PD-L1)/PD-1 coinhibition triggers pancreatic β-cell destruction by insulin-specific, murine CD8 T-cells. Diabetes. 2010;59:1966–1973. doi: 10.2337/db09-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guleria I, Gubbels Bupp M, Dada S, et al. Mechanisms of PDL1-mediated regulation of autoimmune diabetes. Clin Immunol. 2007;125:16–25. doi: 10.1016/j.clim.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Hansen E, Sahasrabudhe D, Sievert L. A case report of insulin-dependent diabetes as immune-related toxicity of pembrolizumab: presentation, management and outcome. Cancer Immunol Immunother. 2016;65:765–767. doi: 10.1007/s00262-016-1835-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin-Liberal J, Furness AJS, Joshi K, et al. Anti-programmed cell death-1 therapy and insulin-dependent diabetes: a case report. Cancer Immunol Immunother. 2015;64:765–767. doi: 10.1007/s00262-015-1689-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mellati M, Eaton KD, Brooks-Worrell BM, et al. Anti-PD-1 and anti-PDL-1 monoclonal antibodies causing type 1 diabetes. Diabetes Care. 2015;38:e137–e138. doi: 10.2337/dc15-0889. [DOI] [PubMed] [Google Scholar]
- 8.Hughes J, Vudattu N, Sznol M, et al. Precipitation of autoimmune diabetes with anti-PD-1 immunotherapy. Diabetes Care. 2015;38:e55–e57. doi: 10.2337/dc15-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaudy C, Clévy C, Monestier S, et al. Anti-PD1 pembrolizumab can induce exceptional fulminant type 1 diabetes. Diabetes Care. 2015;38:e182–e183. doi: 10.2337/dc15-1331. [DOI] [PubMed] [Google Scholar]
- 10.Munakata W, Ohashi K, Yamauchi N, Tobinai K. Fulminant type I diabetes mellitus associated with nivolumab in a patient with relapsed classical Hodgkin lymphoma. Int J Hematol. 2017;105:383–386. doi: 10.1007/s12185-016-2101-4. [DOI] [PubMed] [Google Scholar]
- 11.Teramoto Y, Nakamura Y, Asami Y, et al. Case of type 1 diabetes associated with less-dose nivolumab therapy in a melanoma patient. J Dermatol. 2017;44:605–606. doi: 10.1111/1346-8138.13486. [DOI] [PubMed] [Google Scholar]
- 12.Miyoshi Y, Ogawa O, Oyama Y. Nivolumab, an anti-programmed cell death-1 antibody, induces fulminant type 1 diabetes. Tohoku J Exp Med. 2016;239:155–158. doi: 10.1620/tjem.239.155. [DOI] [PubMed] [Google Scholar]
- 13.Okamoto M, Okamoto M, Gotoh K, et al. Fulminant type 1 diabetes mellitus with anti-programmed cell death-1 therapy. J Diabetes Investig. 2016;7:915–918. doi: 10.1111/jdi.12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hofmann L, Forschner A, Loquai C, et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur J Cancer. 2016;60:190–199. doi: 10.1016/j.ejca.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 15.Aleksova J, Lau PKH, Soldatos G, McArthur G. Glucocorticoids did not reverse type 1 diabetes mellitus secondary to pembrolizumab in a patient with metastatic melanoma. BMJ Case Rep. 2016;2016:2016217454. doi: 10.1136/bcr-2016-217454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lowe JR, Perry DJ, Salama AKS, et al. Genetic risk analysis of a patient with fulminant autoimmune type 1 diabetes mellitus secondary to combination ipilimumab and nivolumab immunotherapy. J Immunother Cancer. 2016;4:89. doi: 10.1186/s40425-016-0196-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Usui Y, Udagawa H, Matsumoto S, et al. Association of serum anti-GAD antibody and HLA haplotypes with type 1 diabetes mellitus triggered by nivolumab in patients with non-small cell lung cancer. J Thorac Oncol. 2017;12:e41–e43. doi: 10.1016/j.jtho.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 18.Chae YK, Chiec L, Mohindra N, et al. A case of pembrolizumab-induced type-1 diabetes mellitus and discussion of immune checkpoint inhibitor-induced type 1 diabetes. Cancer Immunol Immunother. 2017;66:25–32. doi: 10.1007/s00262-016-1913-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishikawa K, Shono-Saito T, Yamate T, et al. A case of fulminant type 1 diabetes mellitus, with a precipitous decrease in pancreatic volume, induced by nivolumab for malignant melanoma: analysis of HLA and CTLA-4 polymorphisms. Eur J Dermatol. 2017;27:184–185. doi: 10.1684/ejd.2016.2923. [DOI] [PubMed] [Google Scholar]
- 20.Imagawa A, Hanafusa T, Miyagawa JI, et al. A novel subtype of type 1 diabetes mellitus characterized by a rapid onset and an absence of diabetes-related antibodies. N Engl J Med. 2000;342:301–307. doi: 10.1056/NEJM200002033420501. [DOI] [PubMed] [Google Scholar]
- 21.Moreau C, Drui D, Arnault-Ouary G, et al. Fulminant type 1 diabetes in Caucasians: a report of three cases. Diabetes Metab. 2008;34:529–532. doi: 10.1016/j.diabet.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Imagawa A, Hanafusa T, Awata T, et al. Report of the committee of the Japan Diabetes Society on the research of fulminant and acute-onset type 1 diabetes mellitus: new diagnostic criteria of fulminant type 1 diabetes mellitus (2012) J Diabetes Investig. 2012;3:536–539. doi: 10.1111/jdi.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lampasona V, Liberati D. Islet autoantibodies. Curr Diab Rep. 2016;16:53. doi: 10.1007/s11892-016-0738-2. [DOI] [PubMed] [Google Scholar]
- 24.Kochupurakkal NM, Kruger AJ, Tripathi S, et al. Blockade of the programmed death-1 (PD1) pathway undermines potent genetic protection from type 1 diabetes. PLoS ONE. 2014;9:e89561. doi: 10.1371/journal.pone.0089561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujisawa R, Haseda F, Tsutsumi C, et al. Low programmed cell death-1 (PD-1) expression in peripheral CD4(+) T cells in Japanese patients with autoimmune type 1 diabetes. Clin Exp Immunol. 2015;180:452–457. doi: 10.1111/cei.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]