Abstract
Posttranslational modifications regulate the function and stability of proteins, and the immune system is able to recognize some of these modifications. Therefore, the presence of posttranslational modifications increases the diversity of potential immune responses to a determinant antigen. The stimulation of tumor-specific CD4+ helper T lymphocytes (HTLs) is considered important for the production of anti-tumor antibodies by B cells and for the generation and persistence of CD8+ cytotoxic T lymphocytes, and in some instances, HTLs can directly reduce tumor cell growth. Identification of MHC class II-restricted peptide epitopes from tumor-associated antigens including those generated from posttranslational protein modifications should enable the improvement of peptide-based cancer immunotherapy. We describe here an MHC class II binding peptide from the tumor protein p53, which possesses an acetylated lysine at position 120 (p53110-124/AcK120) that is effective in eliciting CD4+ T cell responses specific for the acetylated peptide. Most importantly, the acetylated peptide-reactive CD4 HTLs recognized the corresponding naturally processed posttranslational modified epitope presented by either dendritic cells loaded with tumor cell lysates or directly on tumors expressing p53 and the restricting MHC class II molecules. Treatment of tumor cells with a histone deacetylase inhibitor augmented their recognition by the p53110-124/AcK120-reactive CD4+ T cells. These findings prove that the epitope p53110-124/AcK120 is immunogenic for anti-tumor responses and is likely to be useful for cancer immunotherapy.
Keywords: CD4 T cell, p53, Posttranslational modification, Acetylated lysine, Epitope, Immunotherapy
Introduction
Posttranslational modifications are the outcome of modulated enzymatic action and are critical for regulating cell metabolism and gene expression. Conventional posttranslational protein modifications can be amino acid alterations including acetylation, phosphorylation, methylation, glycosylation and ubiquitination. The mode of modification can be controlled by malignant transformation, inflammation, infection and cell death [1–4]. Presentation of peptides containing posttranslational modifications by MHC molecules may induce tumor-reactive T cells and could be a useful strategy for developing cancer immunotherapy. Recently, MHC class I and class II-restricted tumor-associated phosphopeptides that function as T cell epitopes have been described, indicating that posttranslationally modulated peptides may provide a new class of targets for tumor immunotherapy [5–9].
The acetylation of lysine residues in non-nuclear proteins and histones plays a key function in transcriptional regulation and cellular metabolism processes [10–12]. Lysine acetylation is regulated by acetyltransferases and histone deacetylases (HDACs) and can be enhanced by HDAC inhibitors. Most importantly, lysine acetylation determines the function of various tumor-associated proteins including p53, c-myc and survivin [13–17], and therefore, targeting protein acetylation constitutes a promising strategy for cancer treatment.
Because many tumors overexpress p53, wild-type (wt) sequence peptide epitopes derived from this tumor suppressor protein have been considered as attractive candidates for broadly applicable cancer vaccines designed to induce tumor-reactive CD8 and CD4 T cell responses. In the case of CD4 T helper cells, so far one T cell epitope, wt p53110–124 has been described as being restricted by HLA-DR4 [18]. Coincidentally, the lysine at position 120 (K120) in this peptide is one of the residues of p53 that can be acetylated [17]. Therefore, it is possible that some CD4 HTLs may specifically recognize the acetylated version of this peptide (p53110–124/AcK120) providing that the acetylation does not eliminate the capability of the peptide to bind to MHC class II. Herein, we demonstrate that peptide p53110-124/AcK120 was able to generate acetylated peptide-specific, tumor-reactive CD4+ T cell responses and that exposing the tumor cells to HDAC inhibitors enhanced these responses. These results indicate that the development of T cell-based immunotherapy for cancer could be improved by the use of tumor-associated acetylated peptides arising from posttranslational protein modification.
Materials and methods
Cell lines
L cells mouse fibroblasts expressing transfected HLA class II molecules were obtained from Dr. R. Karr (Karr Pharma, St. Louis, MO) and Dr. T. Sasazuki (International Medical Center of Japan, Tokyo, Japan). The human colon adenocarcinoma cell lines, WiDr and HT29 were obtained from the American Type Culture Collection (ATCC, Manassas, VA). WiDr and HT29 accumulate mutant p53 (R273H) molecules. PC3 human prostate cancer cell was purchased from ATCC. The human hypopharyngeal squamous cell carcinoma (SCC) cell line, HPC-92Y was kindly provided by Dr. Synsuke Yanoma (Yokohama Tsurugamine Hospital, Yokohama, Japan). HLA typing was performed serologically using HLA tissue-typing trays (One Lambda, CA).
Synthetic peptides
Synthetic peptides were purchased from Hokkaido System Science (Sapporo, Japan). The wt p53110–124 (RLGFLHSGTAKSVTC) and p53110-12/AcK120 (referred simply as p53AcK120) peptides were used throughout this work. Tetanus toxoid (TT830–843; QYIKANSKFIGITE) peptide was used as a universal epitope–peptide control that can bind to multiple HLA class II molecules [19].
In vitro stimulation of antigen-specific CD4+ helper T cells with synthetic peptides
The method utilized for the generation of p53-reactive CD4+ T cell lines using peptide-induced lymphocytes from fresh peripheral blood derived from healthy individuals was described previously [20]. In brief, CD4 T cells were initially activated with peptide-pulsed autologous dendritic cells (DCs) and subsequent repeated stimulation with γ-irradiated autologous peripheral blood mononuclear cells (PBMCs) and peptide. Furthermore, CD4 T cell clones were obtained by limiting dilution to examine the responses against antigens. All blood materials were acquired after the appropriate informed consent.
Measurement of antigen-specific responses with CD4+ T cell clones
Assessment of CD4 T cell responses to various antigens was performed as previously described [21]. To augment acetylation of p53 in tumor cells, the tumor cells were treated with 400 nM HDAC inhibitor, trichostatin A (TSA, Sigma) during the last 18 h of IFN-γ treatment.
Western blot analyses
TSA-treated and untreated tumor cells were lysed in LDS sample buffer (Invitrogen), and the lysates were submitted to electrophoresis in NuPAGE bis–Tris SDS-PAGE gels (Invitrogen). Antibodies used to detect specific expression were: mouse anti-human p53 mAb (DO-1) (1:1,000 in blocker; Santa Cruz, CA), mouse anti-human acetylated p53 Lys120 mAb (10E5) (1:1,000 in blocker, Abnova, Taiwan) and mouse anti-β-actin (C4) (Santa Cruz Biotechnology, Santa Cruz, CA).
Measurement of peptide-specific responses in cancer patients
Peripheral blood lymphocytes were separated from fresh heparinized blood by gradient centrifugation and cultured with peptides in 96-well plates as described [21]. Lymphokine production (IFN-γ) by PBMCs obtained from head and neck squamous cell carcinoma (HNSCC) and breast cancer patients was evaluated by ELISA (BD). The institutional ethics committee gave approval of the study protocol (approval number #1571).
Results
In vitro induction of p53AcK120-specific CD4+ T cell responses
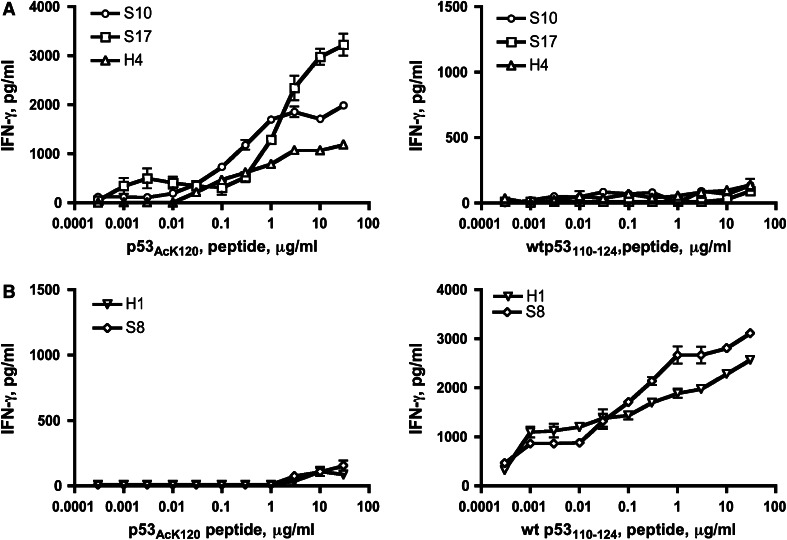
The non-mutated peptide wt p53110–124 was previously reported as a naturally processed epitope recognized by human CD4+ helper T cells via HLA-DR4 allele [18]. By chance, the lysine residue at position 120 (K120) is an acetylation site in the p53 DNA binding domain. If such acetylation does not prevent the peptide to bind to MHC class II molecules, we predicted that it could be feasible to generate CD4 T cells specific for the acetylated version of this peptide. Thus, we examined this possibility using an in vitro immunization procedure. Purified CD4+ T cells obtained from two healthy donors (donor 1: HLA-DR4, DR9, DR53 and donor 2: HLA-DR4, DR15, DR53) were repeatedly stimulated in vitro with the acetylated version of the peptide (from hereinafter referred to as p53AcK120) or non-acetylated wt p53110–124 peptides that were pulsed onto autologous DCs. After 2–3 rounds of stimulation, the CD4 microcultures were tested for their production of IFN-γ upon stimulation with peptide-loaded autologous PBMCs. Positive microcultures exhibiting at least a threefold increase in cytokine production to peptide stimulation compared to the absence of peptide were expanded, and T cell clones were isolated. As shown in Fig. 1a, p53AcK120 was able to elicit acetylated peptide-specific T cell responses (clone H4 from donor 1 and clones S10 and S17 from donor 2). The T cells responded to the acetylated peptide in a concentration-dependent manner (Fig. 1a, left panel) and did not cross-react with the non-acetylated p53110–124 peptide (Fig. 1a, right panel). Simultaneously, additional CD4 T cell clones were established using the non-acetylated p53110–124 peptide (clone H1 from donor 1 and S8 from donor 2). These T cell clones were unable to react with p53AcK120 even at high antigen concentrations (Fig. 1b, left panel), while they responded well to the non-acetylated peptide (Fig. 1b, right panel). These results demonstrate that CD4+ T lymphocytes capable of distinguishing the acetylated and non-acetylated K120 can be effectively generated.
Fig. 1.
Induction of CD4 T cell responses using non-acetylated wt p53110–124 and the peptide carrying acetylated lysine residue at position 120 (p53AcK120). a CD4 T cell clones elicited by acetylated peptide p53AcK120 (clone S10, S17 and H4) were assessed for their capability to recognize autologous PBMC as APCs in the presence of various concentration of acetylated peptide p53AcK120 (left) and non-acetylated wt p53110–124 (right), respectively. B Results with CD4 T cell clones obtained with induction of non-acetylated wt p53110–124 (clone H1 and S8) and their responses against acetylated peptide p53AcK120 (left) and non-acetylated wt p53110–124 (right) were determined, respectively
MHC class II restriction analysis of peptide p53AcK120 -reactive CD4+ T cells
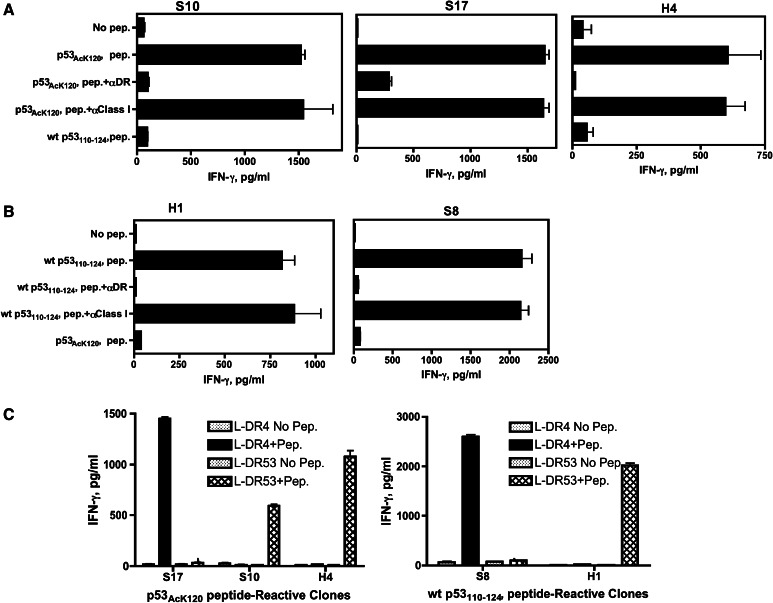
To define which HLA class II molecule presented the acetylated and non-acetylated p53 peptides to the CD4+ T cell clones, we used anti-HLA mAbs to inhibit T cell response to peptide-pulsed autologous PBMCs and a panel of mouse fibroblasts (L cells) transfected with individual HLA-DR as APCs. As shown in Fig. 2a, b, the antigen response of all CD4+ T cell clones was substantially repressed by anti-HLA-DR mAb L243 but not by mAbs to HLA class I (W6/32), indicating that both acetylated and non-acetylated p53 peptides were presented to the CD4+ T cell clones by MHC class II molecules. Furthermore, these results imply that all clones recognized their peptide in the context of HLA-DR because the L243 mAb is specific for HLA-DR and does not recognize the other MHC class II molecules (HLA-DQ, HLA-DP).
Fig. 2.
MHC restriction analysis of p53-reactive CD4+ T cell clones. a Peptide-induced T cell responses from p53AcK120-reactive CD4+ T cell clones and (b) wt p53110–124-reactive CD4+ T cell clones were evaluated by antibody blocking using anti-DR mAb L243 or anti-HLA class I mAb W6/32 (negative control) using autologous PBMCs as APCs. c CD4 T cell responses of the p53AcK120-reactive (left) and non-acetylated wt p53110–124-reactive (right) clones were also evaluated using L-cell transfected with individual HLA-DR genes as APCs to determine the restricting HLA class II alleles
When L-cell transfectants were used as APCs, the results indicated that the p53AcK120-reactive CD4+ T cell clone S17 recognized peptide in the context of HLA-DR4 while clones S10 and H4 were restricted by HLA-DR53 (Fig. 2c, left panel). In the case of the non-acetylated p53110–124-reactive CD4+ T cell clones, the restriction elements of clone S8 and H1 were HLA-DR4 and HLA-DR53, respectively (Fig. 2c, right panel). Thus, both the acetylated and non-acetylated p53110–124 peptides behave as promiscuous epitopes since they can be presented to CD4+ T lymphocytes by plural HLA class II molecules.
Peptide p53AcK120 and non-acetylated wt p53110–124-reactive CD4+ T cell clones recognize naturally processed antigen on MHC class II molecules of tumor cells
Next, we assessed the capacity of the CD4+ T cell clones to react with tumor cells expressing p53 and HLA-DR. Colon carcinoma cell lines, WiDr (HLA-DR4, HLA-DR7, HLA-DR53) and HT29 (HLA-DR4, HLA-DR7, HLA-DR53), PC3 prostate tumor cell line (HLA-DR7, HLA-DR13, HLA-DR53) and SCC cell line HPC92Y (HLA-DR4, HLA-DR9, HLA-DR53), were used as APCs after treatment with IFN-γ (to enhance expression of HLA-DR, data not shown). Prior to performing the tumor recognition assays, the expression levels of p53 and acetylated p53 at K120 were examined by Western blots. As shown in Fig. 3a, expression of total p53 protein and p53-AcK120 was observed in WiDr and HT29 but not in PC3 and HPC92Y, indicating that the WiDr and HT29 cells could be used as relevant APCs and that the PC3 and HPC92Y cells could serve as negative controls in the CD4 T cell recognition assays. The results presented in Fig. 3b, c indicate that all p53AcK120 and p53110–124-specific CD4+ T cell clones could recognize antigen directly on the matched DR+, p53+ WiDr and HT29 cells but not on the matched DR+, p53-negative PC3 and HPC92Y cells (negative controls). Moreover, the reactivity against tumor cells was abrogated by blocking anti-HLA-DR mAb, illustrating that anti-tumor T cell responses was mediated through interaction of T cell receptors with the HLA-DR molecules on the tumor cells.
Fig. 3.
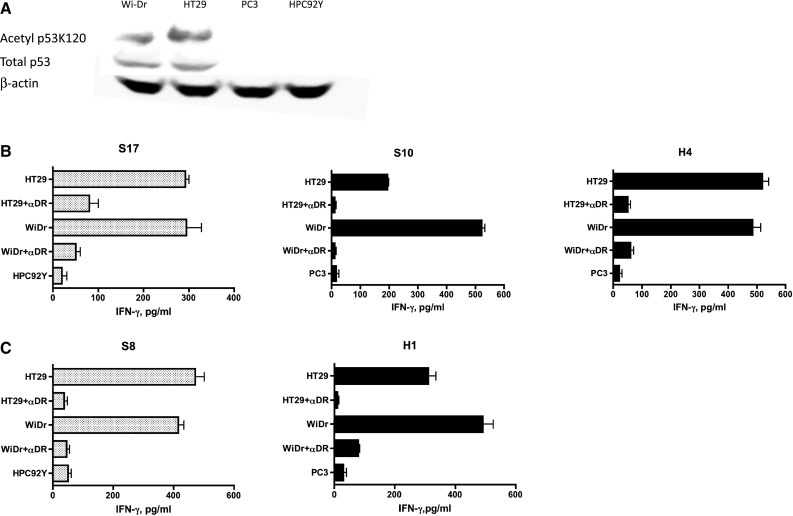
Assessment of naturally processed p53 antigen expressed in tumor cells by acetylated or non-acetylated wt p53110–124-reactive CD4+ T cell clones. a Expression of total p53 protein and p53 carrying acetylated K120 in tumor cells was examined by Western blot analysis. p53 protein and actin protein were detected in 53 and 42 kDa, respectively. b p53AcK120-reactive CD4+ T cell clones, S17, S10 and H4 and (C) wt p53110–124-reactive CD4+ T cell clones, S8 and H1 were tested for their capacity to recognize antigen directly on HLA-DR4+/DR53+/p53+ tumor cells (HT29, WiDr), DR4+/p53− HPC92Y cells or DR53+/p53− PC3 tumor cells. L243 anti-HLA-DR monoclonal antibody was used to attenuate T cell response to tumor cells
Having found that p53AcK120-reactive CD4+ T cell clones could recognize naturally processed antigen on MHC class II molecules of p53 expressing tumor cells, we studied whether the T cell recognition could be enhanced by increasing the levels of acetylated p53. The acetylation of K120 was enhanced by treatment with the inhibitor of histone deacetylase TSA, but this treatment did not significantly alter the total p53 levels and did not induce p53 expression in PC3 and HPC92Y (Fig. 4a). As predicted, higher responses of the p53AcK120-reactive CD4+ T cell clones, S17, S10 and H4 were observed toward the TSA-treated tumor cells as compared to the untreated cells (Fig. 4b). As it would be predicted, in the case of the non-acetylated wt p53110–124-reactive CD4 T cell clones S8 and H1, the responses were not affected by treatment of tumor cells with TSA (Fig. 4c). Overall, these results indicate that both the acetylated and non-acetylated p53 T cell epitopes are endogenously naturally processed through the MHC class II antigen processing machinery and can be directly presented to CD4 T cells by MHC class II molecules on tumor cells.
Fig. 4.
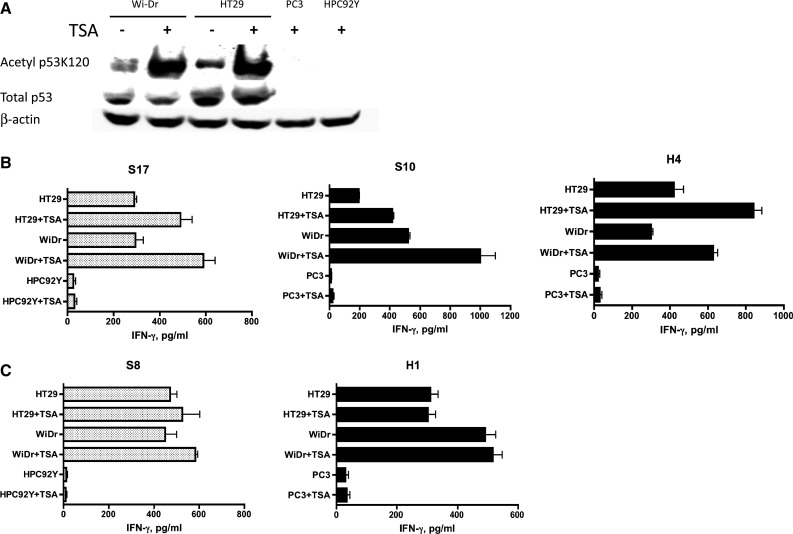
Increase in acetylated peptide-specific CD4 T cell responses against tumor cells by up-regulation of K120 acetylation in p53 protein. a HDAC inhibitor TSA increases expression of acetylated p53 protein. Expression of total p53 protein and p53 carrying acetylated K120 in the presence or absence of TSA was examined by Western blot analysis. b p53AcK120-reactive CD4+ T cell clones, S17, S10 and H4 and (c) non-acetylated wt p53110–124-reactive CD4+ T cell clones, S8 and H1 were examined for their capacity to recognize antigen directly on tumor cells after treatment with TSA
Recognition of naturally processed acetylated p53 epitope antigen presented by autologous DCs
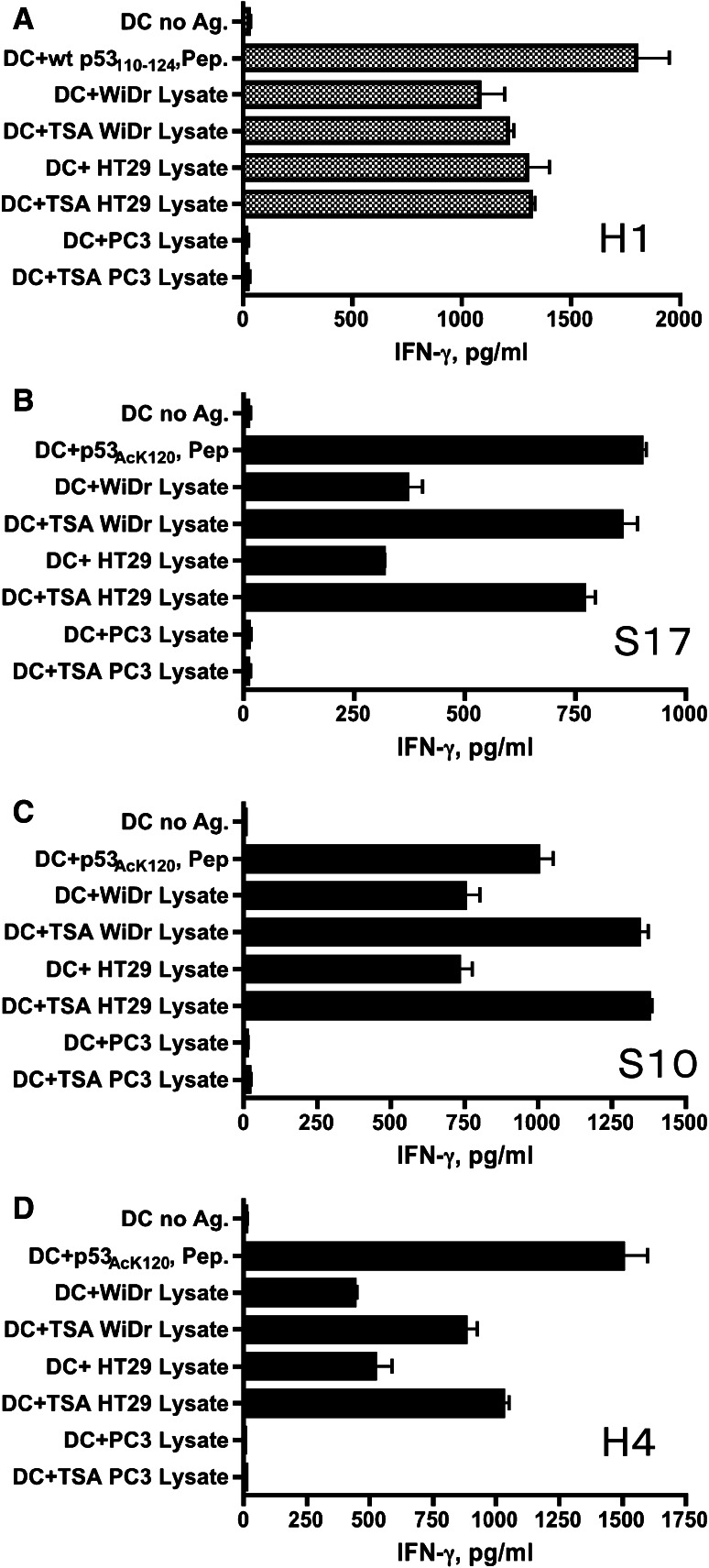
Professional APCs such as DCs are able to capture and process antigens derived from dead tumor cells and present them to CD4 helper and CD8 cytotoxic T cells. Thus, we evaluated whether DCs pulsed with p53 + tumor cell lysates (WiDr, HT29) and present the acetylated p53 epitope–peptide to antigen-specific CD4+ T cell clones. The results show that p53AcK120-specific CD4+ T cell clones S17, S10 and H4 and the non-acetylated wt p53110–124-specific CD4+ T cell clone H1 were capable of recognizing antigen prepared from p53 expressing colon carcinoma cell lysates (WiDr, HT29) but not reacting with unpulsed DCs or DCs pulsed with p53-negative PC3 cell lysate (Fig. 5). We also observed that the acetylated peptide-specific CD4 T cell responses to the tumor lysates could be substantially enhanced by the use of lysates from TSA-treated tumors. The enhancement by adding TSA-treated tumor lysates appears to be antigen-specific because no effects are observed in the same experiments using non-acetylated wt p53110–124-specific CD4+ T cell clone (H1). These results indicate that acetylated p53 epitope arises from a consequence of antigen processing through exogenous/endosomal pathway in the APCs.
Fig. 5.
Non-acetylated wt p53110–124 and p53AcK120-reactive CD4+ T cells react with naturally processed exogenous antigen presented by autologous DCs. a The non-acetylated wt p53110–124-reactive CD4+ T cell clone H1 and (b–d) p53AcK120-reactive CD4+ T cell clones, S17, S10 and H4 were able to recognize the P53+ colon carcinoma cell lysates (WiDr, HT29) but not p53-negative PC3 lysate presented by autologous DCs. In addition, the responses of these CD4+ T cell clones to naturally processed antigen derived from TSA-treated tumor cell lysates were examined
Recognition of acetylated p53 peptide-epitope by PBMCs from cancer patients
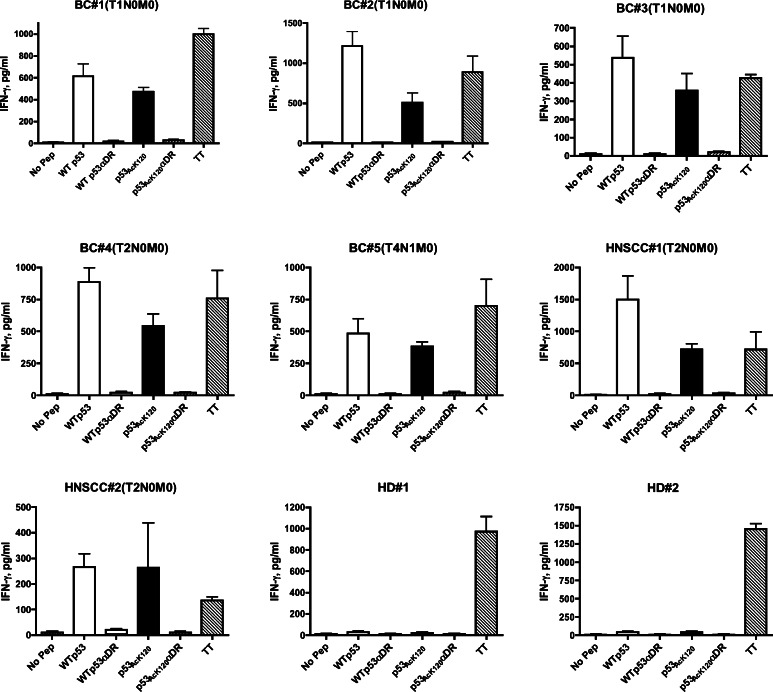
Determining the presence of peripheral blood lymphocytes specific for p53 peptides in the PBMCs of cancer patients could be important for evaluating the potential use of these peptides as cancer vaccines. To examine the responses of CD4 T cells from cancer patients to acetylated peptide, we stimulated PBMCs from five breast cancer and two HNSCC patients with the peptides in short-term cultures. Because available volumes of blood from these patients were very small, we were unable to produce T cell lines or clones to perform more detailed studies or HLA restriction analyses. Tetanus toxoid peptide, TT830–843, was used as a positive control since this peptide usually generates robust CD4 T cell responses in the vast majority of people regardless of their HLA-DR alleles. Seven days after the second p53 peptide stimulation, production of IFN-γ from microculture supernatants was measured. In most instances, substantial T cell responses to both wild-type and acetylated p53 peptides were observed in the breast and HNSCC patients, while healthy volunteers did not react with the p53 peptides (Fig. 6). Moreover, these responses were suppressed by anti-HLA-DR mAb indicating that CD4 T cell responses were mediated via T cell receptor and HLA-DR molecule complex.
Fig. 6.
Assessment of T cell responses to the non-acetylated wt p53110–124 and acetylated p53AcK120 epitopes in cancer patients. PBMCs from five breast cancer patients (BC#1-#5), two HNSCC patients (HNSCC#1, #2) and two healthy donors (HD#1, #2) were stimulated with peptides, and T cell responses were measured as described in “Materials and methods”
Discussion
Here, we have presented evidence of the existence of CD4 T cells capable of distinguishing a posttranslational modified residue, acetyl lysine in the p53 tumor suppressor protein. Posttranslational modified MHC-associated peptides through glycosylation, citrullination, cysteinylation, phosphorylation and acetylation have been described in the literature [22, 23]. However, the previous studies differ from our study because they mostly focus on CD8 T cell epitopes and although some T cell peptide–epitopes bearing N-terminal protein acetylation have been described [24, 25], to our best knowledge, this is the first example of tumor-reactive CD4+ T cells specific for peptide–epitope bearing acetylated lysine residue. Since deregulation of protein lysine acetylation is a characteristic of tumor metabolism [1], identifying MHC-bound lysine-acetylated self-peptides associated with malignant transformation could be of great value. What could be the advantage in utilizing posttranslational modified peptides to generate T cell therapy for cancer? One could speculate that the modified peptides may have the ability to elicit T cell responses of higher specificity than native peptides because peptides bearing posttranslational modifications would be less frequently found. Furthermore, if the posttranslational modified proteins are not present in the thymus and modifications occur after thymic development, posttranslational modifications raise the possibility of the formation of a new class epitopes, which would be less susceptible to immune tolerance.
p53 is a short-lived protein and is retained at low levels under normal physiological conditions [26]. However, high levels of p53 can be induced by posttranslational modifications in response to myriad types of stress. The most commonly reported posttranslational modifications of p53 include phosphorylation of serine/threonine and acetylation of lysine residues. The occurrence of highly mutated p53 in many different tumor types indicates the importance of p53 in cancer development [27]. Mutant p53 proteins generally exhibit striking acetylation and phosphorylation at sites that are well known to lead wild-type p53 into steady state, and so could accelerate the accumulation of dysfunctional mutant p53 in nucleus, where it can behave as an oncogene [28–32]. In general, HDAC inhibitors induce accumulation of hyperacetylated nucleosome core histones and can increase acetylation of lysine residues in p53 and therefore would augment the recognition of acetylated lysine-specific CD4+ T cells (Figs. 4 and 5). In addition, HDAC inhibitors have been introduced as potential treatments for cancer such as hematological malignancies [33, 34]. HDAC inhibitors have also been reported to augment the immune responses by enhancing expression of MHC molecules and costimulatory molecules [35–37]. However, the expression of MHC class II molecules on the tumor cells used in our experiments was not enhanced by HDAC inhibitor (TSA) treatment (data not presented). Therefore, we conclude that the acetylation of p53 by the HDAC inhibitor was responsible for increasing T cell responses in our studies. Accordingly, our results underline the possibility of the combined use of HDAC inhibitors and immunotherapy using epitope–peptides bearing acetylated lysine. It should be noted that both the Wi-DR and HT29 tumor cell lines used in the present studies have mutant p53 genes resulting in accumulation of mutant p53 protein, which could affect the overall levels of the p53AcK120 epitope expressed by the tumor cells. At present, we do not know whether tumors expressing wt p53-treated or not with HDAC inhibitors would be recognized by p53AcK120-reactive CD4 T cells.
In this study, we selected peptide p53110–124 carrying acetylated K120 to elicit CD4 T helper responses because the non-acetylated peptide was previously described as an HLA-DR4-restricted helper T cell epitope [18]. Several additional acetylated lysine residues in p53 have been described (K164, K320, K373 and K382 [17]), which if capable of binding to MHC, could also function as T cell epitopes. It has been previously demonstrated that p53-derived peptide, p53153–166 was efficient in stimulating CD4 T cell responses in the context of HLA-DP5 [38]. Therefore, the possibility that epitope p53153–166 bearing AcK164 may stimulate CD4+ T cells for the acetylated form of the peptide could be evaluated.
In the present study, we described that the p53110–124/AcK120 epitope was presented by HLA-DR4 molecules, which we identified serologically using tissue-typing trays. However, it is known that the DR4 serotype consists of several allelic variants (e.g., DRB1*0401, DRB1*0405, DRB1*0414, etc.), and the distribution of these subtypes varies with ethnicity. Moreover, it may be that the allelic variant specificity influences the peptide binding capacity to the HLA class II molecules. Hence, further investigation is needed to define the precise HLA-DR4 subtypes capable of presenting the p53110–124/AcK120 epitope.
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number 24791735 and 25460430. Esteban Celis is supported by NIH Grants R01CA136828 and R01CA157303.
Conflict of interest
None.
Footnotes
Takumi Kumai and Kei Ishibashi have first authorship.
Contributor Information
Esteban Celis, Phone: +1-706-7215668, Email: ecelis@gru.edu.
Hiroya Kobayashi, Phone: +81-166-682381, FAX: +81-166-682389, Email: hiroya@asahikawa-med.ac.jp.
References
- 1.Krueger KE, Srivastava S. Posttranslational protein modifications: current implications for cancer detection, prevention, and therapeutics. Mol Cell Proteomics. 2006;5:1799–1810. doi: 10.1074/mcp.R600009-MCP200. [DOI] [PubMed] [Google Scholar]
- 2.Atassi MZ, Casali P. Molecular mechanisms of autoimmunity. Autoimmunity. 2008;41:123–132. doi: 10.1080/08916930801929021. [DOI] [PubMed] [Google Scholar]
- 3.Hetzer C, Dormeyer W, Schnolzer M, Ott M. Decoding tat: the biology of HIV Tat posttranslational modifications. Microbe Infect. 2005;7:1364–1369. doi: 10.1016/j.micinf.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Anderton SM. Post-translational modifications of self antigens: implications for autoimmunity. Curr Opin Immunol. 2004;16:753–758. doi: 10.1016/j.coi.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Zarling AL, Ficarro SB, White FM, Shabanowitz J, Hunt DF, Engelhard VH. Phosphorylated peptides are naturally processed and presented by major histocompatibility complex class I molecules in vivo. J Exp Med. 2000;192:1755–1762. doi: 10.1084/jem.192.12.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zarling AL, Polefrone JM, Evans AM, Mikesh LM, Shabanowitz J, Lewis ST, Engelhard VH, Hunt DF. Identification of class I MHC-associated phosphopeptides as targets for cancer immunotherapy. Proc Natl Acad Sci USA. 2006;103:14889–14894. doi: 10.1073/pnas.0604045103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Depontieu FR, Qian J, Zarling AL, et al. Identification of tumor-associated, MHC class II-restricted phosphopeptides as targets for immunotherapy. Proc Natl Acad Sci USA. 2009;106:12073–12078. doi: 10.1073/pnas.0903852106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y, Depontieu FR, Sidney J, Salay TM, Engelhard VH, Hunt DF, Sette A, Topalian SL, Mariuzza RA. Structural basis for the presentation of tumor-associated MHC class II-restricted phosphopeptides to CD4 + T cells. J Mol Biol. 2010;399:596–603. doi: 10.1016/j.jmb.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cobbold M, De La Pena H, Norris A, et al. MHC class I-associated phosphopeptides are the targets of memory-like immunity in leukemia. Sci Transl Med. 2013;5:203ra125. doi: 10.1126/scitranslmed.3006061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee KK, Workman JL. Histone acetyltransferase complexes: one size doesn’t fit all. Nat Rev Mol Cell Biol. 2007;8:284–295. doi: 10.1038/nrm2145. [DOI] [PubMed] [Google Scholar]
- 11.Shahbazian MD, Grunstein M. Functions of site-specific histone acetylation and deacetylation. Annu Rev Biochem. 2007;76:75–100. doi: 10.1146/annurev.biochem.76.052705.162114. [DOI] [PubMed] [Google Scholar]
- 12.Yang XJ, Seto E. Lysine acetylation: codified crosstalk with other posttranslational modifications. Mol Cell. 2008;31:449–461. doi: 10.1016/j.molcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prives C, Manley JL. Why is p53 acetylated? Cell. 2001;107:815–818. doi: 10.1016/S0092-8674(01)00619-5. [DOI] [PubMed] [Google Scholar]
- 14.Patel JH, Du Y, Ard PG, et al. The c-MYC oncoprotein is a substrate of the acetyltransferases hGCN5/PCAF and TIP60. Mol Cell Biol. 2004;24:10826–10834. doi: 10.1128/MCB.24.24.10826-10834.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Holloway MP, Ma L, Cooper ZA, Riolo M, Samkari A, Elenitoba-Johnson KS, Chin YE, Altura RA. Acetylation directs survivin nuclear localization to repress STAT3 oncogenic activity. J Biol Chem. 2010;285:36129–36137. doi: 10.1074/jbc.M110.152777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dai C, Gu W. p53 post-translational modification: deregulated in tumorigenesis. Trend Mol Med. 2010;16:528–536. doi: 10.1016/j.molmed.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chikamatsu K, Albers A, Stanson J, Kwok WW, Appella E, Whiteside TL, DeLeo AB. P53(110-124)-specific human CD4 + T-helper cells enhance in vitro generation and antitumor function of tumor-reactive CD8 + T cells. Cancer Res. 2003;63:3675–3681. [PubMed] [Google Scholar]
- 19.Panina-Bordignon P, Tan A, Termijtelen A, Demotz S, Corradin G, Lanzavecchia A. Universally immunogenic T cell epitopes: promiscuous binding to human MHC class II and promiscuous recognition by T cells. Eur J Immunol. 1989;19:2237–2242. doi: 10.1002/eji.1830191209. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi H, Wood M, Song Y, Appella E, Celis E. Defining promiscuous MHC class II helper T-cell epitopes for the HER2/neu tumor antigen. Cancer Res. 2000;60:5228–5236. [PubMed] [Google Scholar]
- 21.Kumai T, Matsuda Y, Oikawa K, Aoki N, Kimura S, Harabuchi Y, Celis E, Kobayashi H. EGFR inhibitors augment antitumour helper T-cell responses of HER family-specific immunotherapy. Br J Cancer. 2013;109:2155–2166. doi: 10.1038/bjc.2013.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engelhard VH, Altrich-Vanlith M, Ostankovitch M, Zarling AL. Post-translational modifications of naturally processed MHC-binding epitopes. Curr Opin Immunol. 2006;18:92–97. doi: 10.1016/j.coi.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 23.Petersen J, Purcell AW, Rossjohn J. Post-translationally modified T cell epitopes: immune recognition and immunotherapy. J Mol Med (Berl) 2009;87:1045–1051. doi: 10.1007/s00109-009-0526-4. [DOI] [PubMed] [Google Scholar]
- 24.Zamvil SS, Mitchell DJ, Moore AC, Kitamura K, Steinman L, Rothbard JB. T-cell epitope of the autoantigen myelin basic protein that induces encephalomyelitis. Nature. 1986;324:258–260. doi: 10.1038/324258a0. [DOI] [PubMed] [Google Scholar]
- 25.Yague J, Alvarez I, Rognan D, Ramos M, Vazquez J, de Castro JA. An N-acetylated natural ligand of human histocompatibility leukocyte antigen (HLA)-B39. Classical major histocompatibility complex class I proteins bind peptides with a blocked NH(2) terminus in vivo. J Exp Med. 2000;191:2083–2092. doi: 10.1084/jem.191.12.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/S0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 27.Olivier M, Hussain SP, Caron de Fromentel C, Hainaut P, Harris CC (2004) TP53 mutation spectra and load: a tool for generating hypotheses on the etiology of cancer. IARC Sci Publ, 247–270 [PubMed]
- 28.Ullrich SJ, Sakaguchi K, Lees-Miller SP, Fiscella M, Mercer WE, Anderson CW, Appella E. Phosphorylation at Ser-15 and Ser-392 in mutant p53 molecules from human tumors is altered compared to wild-type p53. Proc Natl Acad Sci USA. 1993;90:5954–5958. doi: 10.1073/pnas.90.13.5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pearson M, Carbone R, Sebastiani C, et al. PML regulates p53 acetylation and premature senescence induced by oncogenic Ras. Nature. 2000;406:207–210. doi: 10.1038/35021000. [DOI] [PubMed] [Google Scholar]
- 30.Minamoto T, Buschmann T, Habelhah H, Matusevich E, Tahara H, Boerresen-Dale AL, Harris C, Sidransky D, Ronai Z. Distinct pattern of p53 phosphorylation in human tumors. Oncogene. 2001;20:3341–3347. doi: 10.1038/sj.onc.1204458. [DOI] [PubMed] [Google Scholar]
- 31.Langley E, Pearson M, Faretta M, Bauer UM, Frye RA, Minucci S, Pelicci PG, Kouzarides T. Human SIR2 deacetylates p53 and antagonizes PML/p53-induced cellular senescence. EMBO J. 2002;21:2383–2396. doi: 10.1093/emboj/21.10.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melnikova VO, Santamaria AB, Bolshakov SV, Ananthaswamy HN. Mutant p53 is constitutively phosphorylated at Serine 15 in UV-induced mouse skin tumors: involvement of ERK1/2 MAP kinase. Oncogene. 2003;22:5958–5966. doi: 10.1038/sj.onc.1206595. [DOI] [PubMed] [Google Scholar]
- 33.Johnstone RW. Histone-deacetylase inhibitors: novel drugs for the treatment of cancer. Nat Rev Drug Discov. 2002;1:287–299. doi: 10.1038/nrd772. [DOI] [PubMed] [Google Scholar]
- 34.Zhu WG, Otterson GA. The interaction of histone deacetylase inhibitors and DNA methyltransferase inhibitors in the treatment of human cancer cells. Curr Med Chem Anticancer Agent. 2003;3:187–199. doi: 10.2174/1568011033482440. [DOI] [PubMed] [Google Scholar]
- 35.Magner WJ, Kazim AL, Stewart C, Romano MA, Catalano G, Grande C, Keiser N, Santaniello F, Tomasi TB. Activation of MHC class I, II, and CD40 gene expression by histone deacetylase inhibitors. J Immunol. 2000;165:7017–7024. doi: 10.4049/jimmunol.165.12.7017. [DOI] [PubMed] [Google Scholar]
- 36.Manning J, Indrova M, Lubyova B, et al. Induction of MHC class I molecule cell surface expression and epigenetic activation of antigen-processing machinery components in a murine model for human papilloma virus 16-associated tumours. Immunology. 2008;123:218–227. doi: 10.1111/j.1365-2567.2007.02689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khan AN, Magner WJ, Tomasi TB. An epigenetic vaccine model active in the prevention and treatment of melanoma. J Transl Med. 2007;5:64. doi: 10.1186/1479-5876-5-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fujita H, Senju S, Yokomizo H, Saya H, Ogawa M, Matsushita S, Nishimura Y. Evidence that HLA class II-restricted human CD4 + T cells specific to p53 self peptides respond to p53 proteins of both wild and mutant forms. Eur J Immunol. 1998;28:305–316. doi: 10.1002/(SICI)1521-4141(199801)28:01<305::AID-IMMU305>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]