Abstract
Bystander immune activation by chemotherapy has recently gained extensive interest and provided support for the clinical use of chemotherapeutic agents in combination with immune enhancers. The CD40 ligand (CD40L; CD154) is a potent regulator of the anti-tumor immune response and recombinant adenovirus (RAd)-mediated CD40L gene therapy has been effective in various cancer models and in man. In this study we have assessed the combined effect of local RAd-CD40L and 5-fluorouracil (5-FU) administration on a syngeneic MB49 mouse bladder tumor model. Whereas MB49 cells implanted into immunocompetent mice responded poorly to RAd-CD40L or 5-FU alone, administration of both agents dramatically decreased tumor growth, increased survival of the mice and induced systemic MB49-specific immunity. This combination treatment was ineffective in athymic nude mice, highlighting an important role for T cell mediated anti-tumor immunity for full efficacy. 5-FU up-regulated the expression of Fas and immunogenic cell death markers in MB49 cells and cytotoxic T lymphocytes from mice receiving RAd-CD40L immunotherapy efficiently lysed 5-FU treated MB49 cells in a Fas ligand-dependent manner. Furthermore, local RAd-CD40L and 5-FU administration induced a shift of myeloid-derived suppressor cell phenotype into a less suppressive population. Collectively, these data suggest that RAd-CD40L gene therapy is a promising adjuvant treatment to 5-FU for the management of bladder cancer.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-013-1507-6) contains supplementary material, which is available to authorized users.
Keywords: CD40L, 5-Fluorouracil, Immunotherapy, Chemotherapy, Urinary bladder cancer
Introduction
The TNF family receptor CD40, and its cognate ligand, CD40L (CD154), have long been recognized as major regulators of anti-tumor immunity (reviewed in [1, 2]). Patients with mutations in the CD40L gene develop a severe immune deficiency called hyper-IgM syndrome which associates with enhanced susceptibility to malignancy [3, 4]. Triggering of CD40 on antigen-presenting cells represents a crucial step in the anti-tumor immune response. Thus, engagement of CD40 expressed on dendritic cells (DCs) stimulates the production of cytokines and costimulatory molecules which prime T-helper cells and empower the activation of cytotoxic T lymphocytes (CTL) against the tumor [5, 6]. Mounting experimental evidence demonstrates that CD40 agonists could be exploited for immunotherapy of lymphoid and solid malignancies by substituting the T cell help that is physiologically provided by CD40L-expressing activated CD4+ T lymphocytes. In line with this concept, recombinant adenovirus (RAd)-mediated delivery of murine CD40L to syngeneic mouse models of CD40-negative colorectal, lung or bladder carcinomas results in sustained tumor regression [7–12].
However, the widespread expression of CD40 in malignant cells [13–16] suggests additional therapeutic opportunities. Indeed, we and others have demonstrated that engagement of CD40 on human carcinomas results in growth retardation and sensitization to apoptosis in vitro [15–19] and in xenotransplanted mouse models lacking T cells [15, 16, 20]. Moreover, the activation of CD40 in carcinomas directly enhances their immunogenicity in vitro by increasing their antigen presentation and recognition by specific CTLs [13, 21]. Recent studies have utilized replication-proficient (oncolytic) adenoviruses expressing CD40L to further increase the eradication of solid tumors by exploiting the direct cytotoxic effects of CD40 engagement on malignant cells and the enhanced release of tumor antigens and generation of danger signals in the tumor micro milieu which stimulate an anti-tumor immune reaction [22, 23]. Therefore, the CD40 pathway provides an important opportunity for cancer therapy through its multiple effects on tumor cell growth, apoptosis, and immune recognition. This concept has led to the clinical evaluation of CD40 agonists for the treatment of lymphomas and carcinomas which confirmed that CD40 therapies are well tolerated, show minimal side effects and are associated with tumor regression [24–27].
Given the substantial heterogeneity which typifies the genomes of malignant cells within a tumor [28], combination of different agents is expected to yield enhanced clinical efficacy than single agent therapies. We reasoned that the therapeutic benefit of CD40L administration should be evaluated in combination with established chemotherapeutics for two reasons: First, chemotherapy represents the backbone of current strategies for the treatment of cancer and is likely to continue to play a major role in the oncological armamentarium of the future. Second, CD40L exerts powerful effects on tumor microenvironment [13, 26, 29, 30] and it is therefore reasonable to anticipate that more effective therapy will be achieved by targeting both malignant cells and their microenvironment. In this study we evaluate this concept and demonstrate that combined treatment of tumor-bearing syngeneic mouse models with the chemotherapeutic agent 5-fluorouracil (5-FU) and adenovirus expressing CD40L confers profound anti-tumor properties compared to each agent alone.
Materials and methods
Cell lines and immunogenic marker detection
The mouse bladder carcinoma cell line MB49, derived from C57BL/6 mice, was cultured in DMEM supplemented with 10 % fetal bovine serum, 1 % penicillin/streptomycin and 0.1 % sodium pyruvate. Medium and supplements were purchased from Invitrogen, Paisley, Scotland. The MB49 cell line was a gift from Dr. K. Esuvaranathan (National University Hospital, Singapore). ATP and HMGB1 release was measured in culture supernatants from 5-FU-treated and untreated cells using an ATP Determination Kit (A22066, Molecular Probes; Invitrogen) and HMGB1-specific ELISA (ST51011; IBL International) respectively.
Production of recombinant adenovirus
Vectors were constructed and produced as has been described previously [29]. Briefly, two replication-deficient, E1/E3-deleted, human adenoviruses type 5 were produced using the AdEasy system. The RAd-CD40L virus expresses the murine CD40L molecule under the CMV promoter and the RAdMock is a non-coding control virus. Adenoviruses were produced by four rounds of infection of 911 cells. Purification was done in two steps, first by a discontinuous Cesium Chloride (CsCl) gradient and secondly by removing the CsCl by desalting. Virus titers were determined by a fluorescence forming unit (ffu) assay where 911 cells were plated on collagen-coated 35 mM plates with grids (Sarstedt, Nümbrecht, Germany) and infected with the purified virus in different dilutions. The cells were cultured for 48 h and, after a washing with PBS, fixed in 4 % paraformaldehyde. The cells were washed with PBS and then incubated with a mouse monoclonal anti-adenovirus antibody (Chemicon, Temecula, CA, USA) for 1 h at room temperature, washed with PBS and then incubated with a secondary FITC-labeled polyclonal rabbit anti-mouse antibody (DakoCytomation, Glostrup, Denmark) for 1 h at room temperature. The cells were washed once in PBS and then green cells were counted by using a microscope with a fluorescence illuminator (Olympus, CK40).
Animal models
Female C57BL/6 mice were anesthetized and catheterized using INSYTE.w24Gx3/4 catheters (BD Biosciences, San Diego, CA, USA). The bladders were administered polycathon poly-l-lysine (Sigma, St. Louis, MO, USA) to enhance tumor uptake [31]. 2 × 105 MB49 cells per mouse were implanted into the bladder. The mice received three local intravesical treatments of RAd-CD40L or RAdMock (1 × 108 ffu), 5-FU (50 mg/kg), or the combination of RAd-CD40L and 5-FU on day 1, 6 and 12 after tumor instillation. Prior vector instillation the bladders were prewashed three times with the transduction enhancer Cloropactin WCS-90 (0.1 % solution) (United-Guardian, Inc., Hauppauge, NY, USA) and one PBS wash [31]. Cured mice were challenged with MB49 subcutaneous tumor (1 × 106 cells) and a lateral irrelevant B16 tumor (1 × 106 cells). Athymic C57BL/6Nu/Nu mice were injected subcutaneously with MB49 cells (2 × 105 cells). C57BL/6 and non-functional FasL-expressing (gld) mice (JAX Labs) were injected s.c with 1 × 106 cells and when tumors reached 45–50 mm2 in size (days 7–8), RAd-CD40L (1 × 108 ffu), control virus (1 × 108 ffu), 5-FU (50 mg/kg) and control virus or the combination of 5-FU and RAd-CD40L were administered intratumorally in 100 μl PBS followed by 2 additional injections on days 11 and 15. Tumor size was monitored two to three times a week using a digital caliper. The experiment was ended when tumors in control animals were larger than 150 mm2. Animal experiments were approved by the respective regional ethical committees in Uppsala, Sweden, and Heraklion, Greece. To study tumor-infiltrating T cells, tumor biopsies were taken for in vitro analysis on day one and three after final treatment. The tumor was mashed by using the backside of a syringe in PBS and all cells were collected in FACS tubes. Large, sticky tumor cells were decanted and the remaining cells were washed in PBS and used for flow cytometry analyses.
Flow cytometry
Cells were analyzed by flow cytometry using fluorescently-labeled antibodies against CD107a and CD3 (Nordic Biosite, Stockholm, Sweden). Cell suspensions were incubated with antibodies for 10–15 min at room-temperature and then washed with 2 ml PBS, and centrifuged at 1,500 rpm for 5 min. The supernatant was decanted and the cells were resuspended in 250 μl of 1 % paraformaldehyde in PBS. Cells were stored at 4 °C before analysis by flow cytometry (FACSCalibur, BD Biosciences, San Diego, CA, USA). Irrelevant antibodies were used to define background fluorescence. Cells from spleen and tumor from the subcutaneously tumor-bearing mice were stained with fluorescently-labeled antibodies against CD11b, Gr-1, B220, CD11c and CD8 (Biolegend). Cell suspensions were incubated with antibodies for 30 min in 4 °C, washed twice with PBA (1 % BSA in PBS) and then analyzed by flow cytometry (FACSCanto II, BD Biosciences, San Diego, CA, USA). MB49 cells that were treated with 5-FU, CD40L, the combination of 5-FU and CD40L, or left untreated were evaluated by flow cytometry for the expression of B7.1, MHCI, Fas and CD40L using fluorescently-labeled antibodies against the mentioned markers. Data analysis was performed using FlowJo software (Treestar, Ashland, OR, USA).
Isolation of splenocytes and CTL assays
Splenocytes were isolated from tumor-bearing mice as previously described [32]. Approximately 8 × 106 splenocytes were re-stimulated with 2 × 106 irradiated MB49 cells for 5 days. Viable splenocytes (effectors) were then co-cultured with 51Cr-labelled MB49 cells (targets) at different effector: target ratio for 6 h and 51Cr release was assessed as previously described [13].
Statistical analyses
All statistical analyses were performed using Graph Pad Prism 4 (Graph Pad Software Inc., San Diego, CA, USA). p values smaller than 0.05 were considered significant.
Results
Adenovirus-mediated delivery of CD40L enhances the efficacy of chemotherapy in experimental bladder cancer and induces systemic immunity
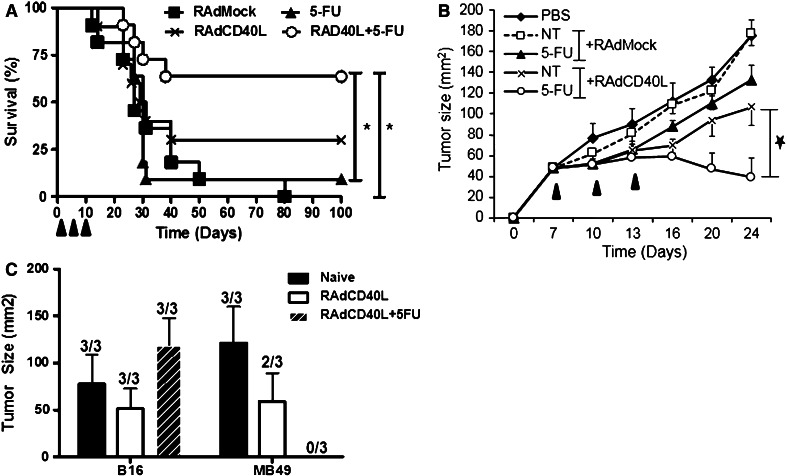
An orthotopic experimental bladder cancer model was used to evaluate the anti-tumor efficacy of chemotherapy and immunotherapy. MB49 tumor cells were instilled into the bladder of C57BL/6 mice via urethral catheterization. RAd-CD40L, control RAdMock virus, 5-FU and the combination of RAd-CD40L and 5-FU were administered in the bladder cavity at three time points (Fig. 1a). The combination therapy significantly increased survival of tumor-bearing mice compared to RAdMock (p = 0.0035) or 5-FU alone (p = 0.0097) as demonstrated by testing the groups in a survival log rank test (Fig. 1a). Furthermore, whereas RAd-CD40L alone cured 32 % of large orthotopic tumors, the combination of RAd-CD40L and 5-FU succeeded in a significant 68 % cure rate. Treatment with 5-FU or RAdMock alone had little or no effect on animal survival (16 and 0 % respectively, Fig. 1a). The RAd-CD40L/5-FU combination treatment was also found to exert a significant inhibitory effect on MB49 tumors growing subcutaneously (Fig. 1b).
Fig. 1.
RAd-CD40L and 5-FU combination treatment dramatically increases survival of immunocompetent mice bearing MB49 bladder tumors compared to either agent alone. a MB49 cells were orthotopically grown in C57BL/6 mice and treated on day 1, 7 and 14 with RAdMock, RAd-CD40L, 5-FU or the combination of RAd-CD40L and 5-FU (n = 11/group). The combination therapy cured 68 % of large orthotopic tumors (p = 0.0035) while RAd-CD40L alone cured 32 %. The experiment was repeated three times with similar results. b MB49 tumors growing subcutaneously respond only to 5-FU and RAd-CD40L combination therapy but not to each treatment alone (n = 9 mice/group). The time points of application of treatment are shown by filled triangles. c Cured mice from (a) were challenged subcutaneously with MB49 tumor and lateral unrelated B16 melanoma cell (n = 3/group). Statistical differences were evaluated using the survival log rank test
Mice that were cured of orthotopic MB49 bladder tumors by RAd-CD40L or the combination therapy were challenged subcutaneously with MB49 and lateral unrelated B16-F10 melanoma cells. All mice developed B16-F10 tumors. MB49 tumors developed in naïve and most RAd-CD40L-treated animals but not in mice treated with RAd-CD40L in combination with 5-FU (Fig. 1c). These findings demonstrate that the combination treatment evokes robust systemic MB49-specific immunity.
Reduced efficacy of RAd-CD40L and 5-FU combination treatment in immunodeficient mice
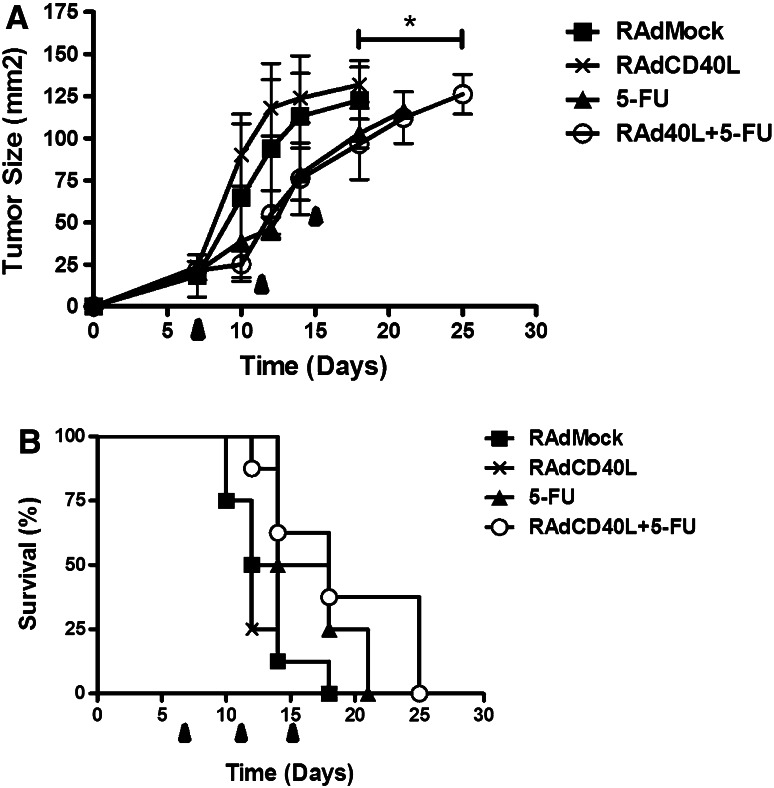
The effect of RAd-CD40L and 5-FU combination therapy was tested in MB49 tumors growing subcutaneously in immunodeficient athymic mice to define the role of functional T cells. MB49 tumors grew aggressively and were not significantly affected by RAd-CD40L treatment (Fig. 2a). Administration of 5-FU with or without RAd-CD40L had modest effects on tumor growth (p = 0.0188 and p = 0.0172 respectively, Fig. 2a) and animal survival (Fig. 2b). This contrasts with the effect of 5-FU and RAd-CD40L on MB49 growth in immunocompetent mice (see Fig. 1) suggesting that whereas the direct cytotoxic effects of 5-FU contribute to the control of tumor growth, functional T cell-mediated anti-tumor immunity is primarily responsible for effective RAd-CD40L/5-FU combination therapy.
Fig. 2.
Reduced efficacy of RAd-CD40L and 5-FU combination treatment in immunodeficient mice. RAd-CD40L and 5-FU were evaluated as single or combination therapy on MB49 tumors growing in athymic C57BL/6 Nu/Nu mice (n = 8 mice/group). Treatments were given at day 8, 11 and 15. a Tumor size at different time points is shown. Error bars represent SEM values. b Survival of treated mice. Statistical evaluation was performed using the survival log rank test: Combination treated mice were different from RAdMock (p = 0.0172), RAd-CD40L (p = 0.0083) but not from 5-FU alone. 5-FU alone was different from RAdMock (p = 0.0188)
5-FU upregulates functional Fas/CD95 and immunogenic cell death markers in MB49 cells
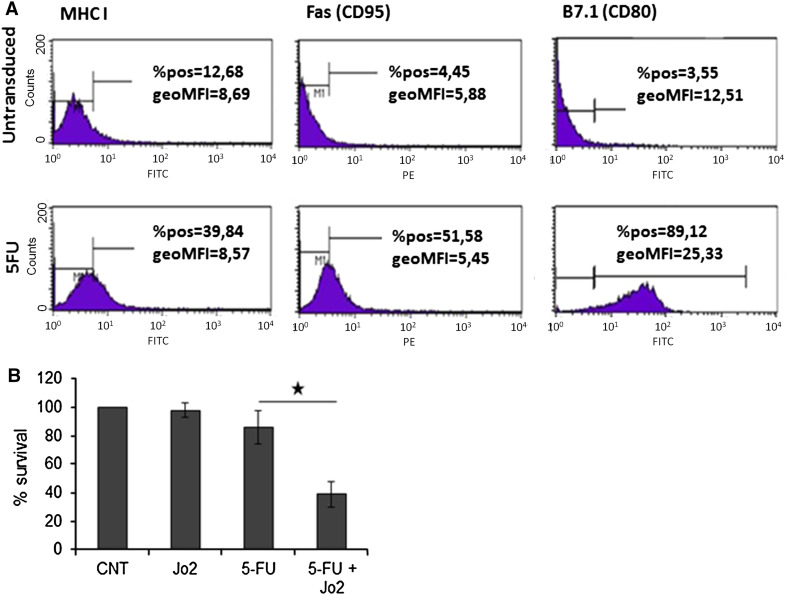
Chemotherapeutic agents have been reported to increase the immunogenicity of malignant cells. To determine if 5-FU could influence the expression of cell surface molecules associated with susceptibility to immune attack, MB49 mouse bladder carcinoma cells were exposed to a low concentration of 5-FU which did not confer significant cytotoxic activity. MHCI, Fas (CD95), and B7.1 (CD80) levels were assessed by flow cytometry. As shown in Fig. 3a, all 3 proteins were induced within 48 h of drug treatment. In contrast, RAd-CD40L had marginal effects on these cell surface markers presumably because of the low percentage CD40 expression in MB49 cells (data not shown). Indeed, we have previously shown that the up-regulation of MHCI, Fas and B7.1 by CD40 agonists critically depends on the levels of CD40 expressed on the surface of human carcinoma cells [13].
Fig. 3.
The chemotherapeutic agent 5-FU upregulates functional Fas receptor in MB49 bladder carcinoma cells. a MB49 cells were cultured in the presence of 50 μM 5-FU for 48 h and Fas expression was assessed by flow cytometry. No staining was detected in the isotype-matched control groups. b MB49 cells were exposed to 5-FU as in (a) followed by treatment with the agonistic anti-Fas mAb Jo2 (1 μg/ml). Forty-eight hours later survival was assessed by MTT conversion assays. Data shown are representative of 4 independent experiments each performed in triplicates. The mean (±SD) from one experiment is shown
Experiments were also conducted to determine if the observed upregulation of the death receptor Fas in 5-FU-treated cells was functionally important. To this end, MB49 cells were exposed to 50 μM 5-FU for 48 h and then cultured in the presence or absence of the agonistic Fas mAb Jo2 for an additional 24 h period. Assessment of survival using MTT conversion assays demonstrated that pre-treatment with 5-FU had a profound effect on Fas-mediated cytotoxicity whereas Jo2 or 5-FU alone had little effect (Fig. 3b).
Apoptotic cells are known to trigger an immune response mediated by the release of damage-associated molecular patterns (DAMPs) which can be recognised by patern recognition receptors in immune cells. Secreted ATP and released high mobility group protein B1 (HMGB1) represent in vitro measurable indicators of immunogenic cell death [33]. We found significant enhancement of ATP and HMGB1 levels in the supernatants of MB49 cells treated with 5-FU which was most prevalent at 48 h post-treatment (Supplementary Figures 1A and 1B). We conclude that low, clinically-relevant concentrations of 5-FU upregulate functional Fas/CD95 and immunogenic cell death markers in MB49 cells.
CTLs from mice receiving RAd-CD40L immunotherapy lyse 5-FU treated MB49 cells more efficiently than untreated tumor cells in a Fas ligand-dependent manner
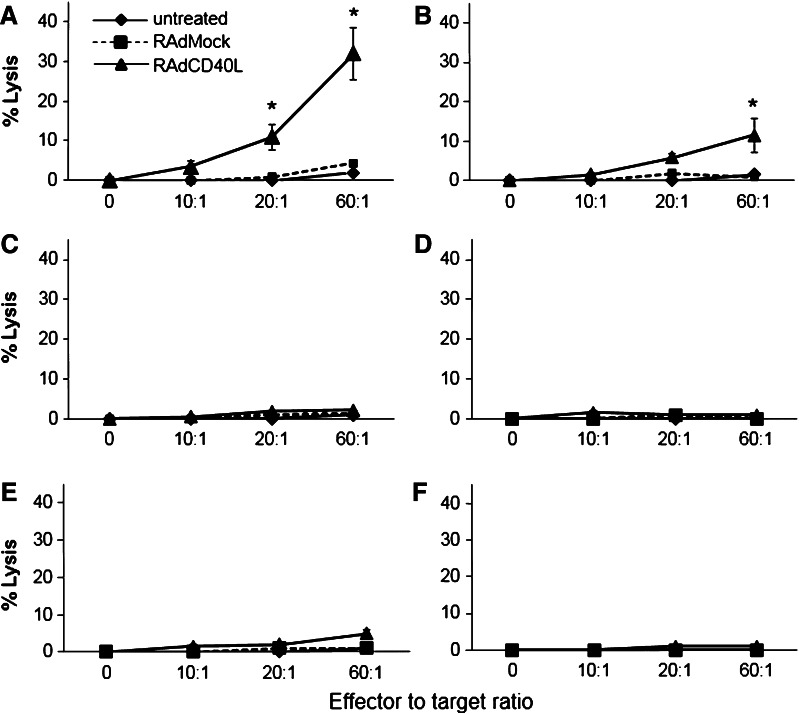
The aforementioned findings prompted us to examine whether exposure of MB49 cells to 5-FU would increase their susceptibility to tumor-specific CTLs following RAd-CD40L therapy. Splenocytes from C57BL/6 mice receiving intratumoral injections of RAd-CD40L or control virus were isolated, re-stimulated in vitro with irradiated MB49 cells and mixed with 51Cr labeled MB49 cells. Measurement of 51Cr release demonstrated that splenocytes from RAd-CD40L-treated mice lysed MB49 cells exposed to 5-FU more efficiently than untreated cultures (Fig. 4a, b). In contrast, splenocytes from control virus-treated mice failed to kill MB49 cells irrespective of drug treatment. Additionally, no CTL response was observed against the unrelated syngeneic control cell line B16-F10 (Fig. 4c, d).
Fig. 4.
CTLs from mice receiving RAd-CD40L immunotherapy lyse 5-FU treated MB49 cells more efficiently than untreated tumor cells in a Fas ligand-dependent manner. Ten days following injection of RAd-CD40L or RAdMock into established MB49 tumors, splenocytes were harvested, stimulated with irradiated MB49 cells for 5 days in culture and live cells (effectors) were evaluated for cytotoxicity against 51Cr-labelled MB49 cells which were either pre-treated for 48 h with 50 μg/ml 5-FU or cultured untreated. B16-F10 melanoma cells were used as control target cell line. The percentage lysis (51Cr release) is shown from representative experiments. Splenocytes from PBS, RAd-CD40L or RAdMock treated C57BL/6 mice were used as effectors against (a) MB49 target cells treated with 5-FU (*p < 0.005 of RAd-CD40L vs. RAdMock, assessed by t test); b untreated MB49 cells (*p < 0.005 of RAd-CD40L vs. RAdMock, assessed by t test); c B16 cells pre-treated with 5-FU; d control B16 cultures. Splenocytes from PBS, RAd-CD40L or RAdMock treated gld mice were used as effectors against e MB49 target cells pretreated with 5-FU; f B16 cells pretreated with 5-FU
To assess the impact of Fas: FasL interactions on this CD40-mediated effect, MB49 tumors were established in mice lacking functional FasL and administered RAd-CD40L or control virus. Splenocytes were assessed for MB49 or B16-F10 cytolytic activity as described above for C57BL/6 mice. The results showed that cytotoxicity was significantly attenuated regardless of 5-FU treatment (Fig. 4e, f).
To examine the in vivo relevance of FasL to the synergistic effect of 5-FU and RAd-CD40L immunotherapy, MB49 tumor-bearing gld mice were given intratumoral RAd-CD40L, RAdMock and 5-FU in all possible combinations and tumor size was assessed over time. Unlike C57BL/6 (wild-type FasL) mice (Fig. 1b), the combination of 5-FU and RAd-CD40L failed to induce tumor regression in mice lacking functional FasL (Supplementary Figure 2).
5-FU and CD40L combination treatment influences the phenotype of myeloid-derived suppressor cells
Myeloid-derived suppressor cells (MDSCs), phenotypically defined as CD11b+/B220−/Gr-1+, have been identified as a heterogeneous population of immature myeloid cells with the ability to regulate T cell activation in mice and humans [34]. In particular, CD11b+/Gr-1int MDSCs functions as suppressors of CD8+ T cells whereas CD11b+/Gr-1high MDSCs associate with poor immunosuppressive properties [35].
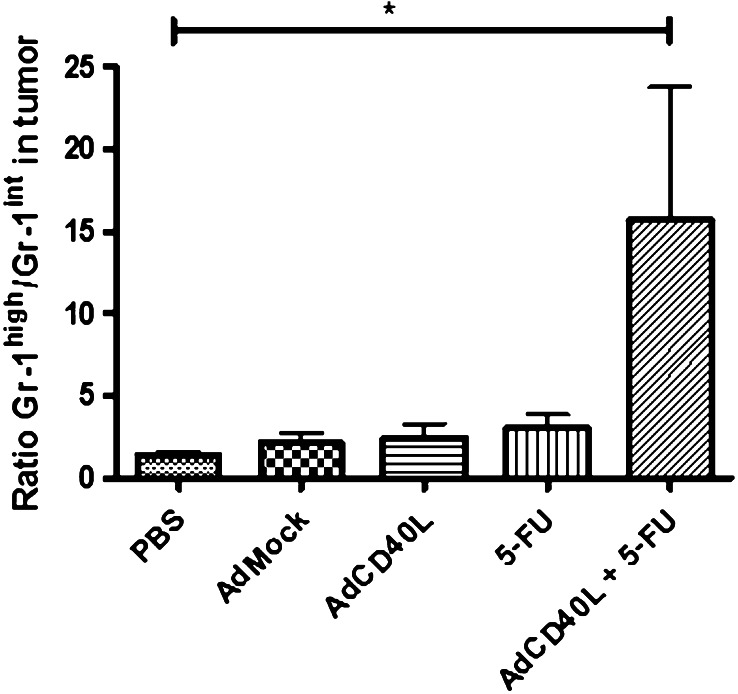
We assessed by flow cytometry the effect of RAd-CD40L, RAdMock, 5-FU, the combination of 5-FU and RAd-CD40L or vehicle control (PBS) on the number of MDSCs in the s.c MB49 model. Forty-eight hours after treatment, the spleen and tumor were excised and isolated cells were analyzed for the expression of CD11b, B220 and Gr-1 by flow cytometry. The results showed that the ratio of CD11b+/Gr-1high to CD11b+Gr-1int cells was increased in the combination treated mice compared to the other animal groups (Fig. 5).
Fig. 5.
CD40L and 5-FU combination treatment influences the phenotype of myeloid-derived suppressor cells. MB49 bladder cancer cells were grown subcutaneously on the right flank of female C57BL/6 mice (n = 5/group) and when tumor was palpable one treatment of PBS, RAdMock or RAd-CD40L or the combination of 5-FU + RAd-CD40L was given. 48 h after treatment the tumors were collected and the presence of myeloid-derived suppressor cells (MDSCs) was investigated. The ratio of CD11b+Gr-1high vs. CD11b+Gr-1int MDSC subpopulations is shown. Statistically significant difference was found between the combination and any other treatment group, as determined by the non-parametric Kruskal–Wallis test and Dunn’s post test. *p < 0.05
Discussion
Given the significant molecular heterogeneity which characterizes the malignant cells within a tumor [28], single agents are unlikely to be successful in cancer patient therapy. Indeed, the clinical experience accumulated the last 30 years of cancer management suggests that combination of different agents offers the best possible therapeutic efficacy. Despite this, chemotherapy and immunotherapy have remained largely unexplored as combination modality because of concerns that leukopenia caused by chemotherapy would eliminate tumor-targeting lymphocytes. However, more recent studies show that the efficacy of at least some chemotherapeutic agents partly depends on immune parameters [36, 37] (reviewed in [38, 39]). Of note, Nowak et al. [40] have demonstrated that CD40 signals cooperate with gemcitabine to suppress tumor growth in experimental animals, providing evidence that chemotherapy has the capacity to augment CD40-mediated antitumor immunity.
CD40 stimulation strategies with recombinant soluble CD40L protein, CD40L-expressing tumor vaccines, oncolytic viruses expressing CD40L and anti-CD40 monoclonal antibodies have been evaluated in end-stage cancer patients with encouraging results [23, 24, 26, 27] (reviewed in [2]). We have also previously shown that RAd-CD40L gene therapy inhibits experimental bladder tumors [8, 41] and its local administration in patients with bladder cancer is safe, stimulates T helper 1 immunity, suppresses regulatory T cells, and confers antitumor activity [25]. Collectively, these observations highlight the need to further explore the CD40 signaling pathway for the management of bladder cancer.
The current study demonstrates the feasibility of using 5-FU as adjuvant to CD40L immunogene therapy. In an orthotopic MB49 bladder tumor model, a survival rate of 68 % was achieved using the chemotherapy/immunotherapy combination treatment and systemic MB49-specific immunity was demonstrated (Fig. 1). In contrast, administration of either agent alone failed to substantially suppress tumor growth. The anti-tumor immune effects of CD40 agonists largely depend on antigen-presenting cell-mediated T cell priming and activation of CTLs [5, 6]. The failure of RAd-CD40L alone to efficiently suppress MB49 tumors (Fig. 1) is likely the consequence of the low CD40 levels expressed in these cells and their concomitant failure to up-regulate cell surface markers necessary for CTL recognition and killing.
Herein, we have identified Fas as an important component of the MB49 tumor response to RAd-CD40L and 5-FU combination treatment. Fas is absent in MB49 cells but is significantly up-regulated by low, clinically relevant doses of 5-FU and renders them susceptible to killing by Fas agonists (Fig. 3). Moreover, 5-FU was found to dramatically enhance the susceptibility of MB49 cells to CTLs activated by in vivo RAd-CD40L administration in a Fas-dependent manner (Fig. 4). The importance of T lymphocytes in the observed synergistic anti-tumor effect of RAd-CD40L and 5-FU combination treatment is further highlighted by its failure to suppress MB49 tumors growing in immunodeficient athymic mice (Fig. 2).
Immunotherapy is hampered by the accumulation of MDSCs in the tumor microenvironment. CD11b+Gr-1int MDSCs efficiently inhibit both activation and effector function of T cells in the mouse. It has recently been shown that 5-FU can selectively target MDSCs [42]. We have observed that local administration of 5-FU decreases the number of immunosuppressive CD11b+Gr-1int MDSCs in the combination treated mice and leads to a relative increase in CD11b+/Gr-1high MDSCs (Fig. 5) which are characterized by reduced immunosuppressive properties [35].
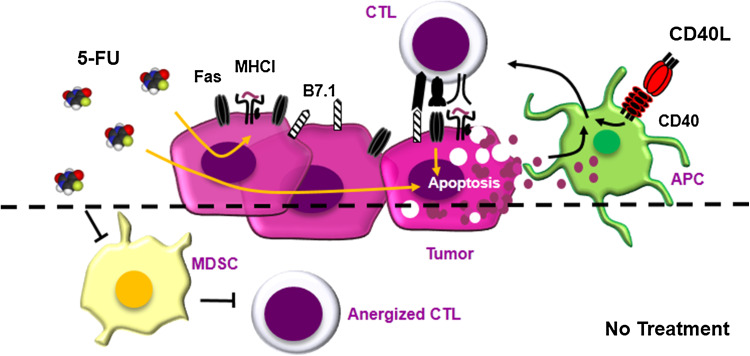
Collectively, the aforementioned data suggest that the enhanced anti-tumor efficacy of 5-FU and RAd-CD40L combination treatment largely depends on the 5-FU-mediated up-regulation of Fas on tumor cells and the indirect effects of RAd-CD40L on CTL activation (Fig. 6). In addition, we have observed that 5-FU enhances the cell surface expression of B7.1 and MHCI and the secretion of immunogenic cell death markers ATP and HMGB1. The down-regulation of B7.1, MHCI and Fas represents common immune escape mechanisms (reviewed in [43]) and the synergistic effect of 5-FU and RAd-CD40L could thus be partly attributed to the fact that the tumor becomes more immunogenic. This treatment may additionally ‘normalize’ the tumor microenvironment by targeting MDSCs thereby further increasing the susceptibility of tumor cells to CTL lysis (Fig. 6). Enhanced B7.1 expression also associates with NK cell cytotoxicity [44] raising the possibility that NK cells, which can be activated by cytokines released by T lymphocytes, may also contribute to the anti-tumor effect of the combination treatment.
Fig. 6.
Schematic representation of the proposed effects of RAd-CD40L and 5-FU combination treatment on experimental bladder cancer growth. On the basis of the data shown in this paper we propose that the enhanced anti-tumor efficacy of local 5-FU and RAd-CD40L combination treatment largely depends on the enhanced immunogenicity of 5-FU-treated tumor cells which critically involves the up-regulation of Fas. Other molecules involved in CTL recognition such as MHC class I and B7.1, as well as ATP and HMGB1 release may contribute to the enhanced immunogenicity of drug-treated tumors. This is coupled with the indirect effects of RAd-CD40L on CTL activation via the stimulation of host antigen-presenting cells (APC). Tumor cells thus become susceptible to lysis by activated FasL-expressing CTLs. This treatment may additionally ‘normalize’ the tumor microenvironment by targeting MDSCs and perhaps other immune cell types thereby further increasing the susceptibility of the malignant cells to CTLs
CD40 is over-expressed in 78 % of primary human bladder tumors [45] and the in vitro stimulation of CD40-positive human bladder cancer cell lines with CD40 agonists has been shown to confer direct anti-proliferative effects [17, 18, 46], up-regulation of functional Fas expression [17] and augmented response to 5-FU-induced killing [41]. These reported findings coupled with the data shown in this paper underscore the potential of using 5-FU and RAd-CD40L gene therapy for the management of bladder carcinoma and perhaps additional types of human cancer.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors thank Berith Nilsson at Uppsala University for technical assistance with viral vector production and Marina Ioannou at IMBB-FORTH for cell culture assistance. This work was supported by the European Commission FP6 program Apotherapy (EC contract number 037344) to Aristides Eliopoulos and Angelica Loskog, the EC FP7 programmes INFLA-CARE (EC contract number 223151) and ‘Translational Potential’ (TransPOT; EC contract number 285948) to Aristides Eliopoulos, and by a Swedish Research Council grant to Angelica Loskog. Aristides Eliopoulos also acknowledges co-funding of this research by the General Secretariat of Research and Technology of Greece through the Operational Program Competitiveness and Entrepreneurship (OPC II), NSRF 2007-2013, action “SYNERGASIA 2011”, Project THERA-CAN (contract number 11ΣΥΝ_1_485).
Conflict of interest
The authors have no conflicts of interest to declare except from Angelica Loskog who is the CEO of Lokon Pharma AB, a scientific advisor at NEXTTOBE AB and has a royalty agreement with Alligator Bioscience AB.
References
- 1.van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukoc Biol. 2000;67:2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- 2.Loskog AS, Eliopoulos AG. The Janus faces of CD40 in cancer. Semin Immunol. 2009;21:301–307. doi: 10.1016/j.smim.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Callard RE, Armitage RJ, Fanslow WC, Spriggs MK. CD40 ligand and its role in X-linked hyper-IgM syndrome. Immunol Today. 1993;14:559–564. doi: 10.1016/0167-5699(93)90188-Q. [DOI] [PubMed] [Google Scholar]
- 4.Hayward AR, Levy J, Facchetti F, Notarangelo L, Ochs HD, Etzioni A, Bonnefoy JY, Cosyns M, Weinberg A. Cholangiopathy and tumors of the pancreas, liver, and biliary tree in boys with X-linked immunodeficiency with hyper-IgM. J Immunol. 1997;158:977–983. [PubMed] [Google Scholar]
- 5.Mackey MF, Gunn JR, Maliszewsky C, Kikutani H, Noelle RJ, Barth RJ., Jr Dendritic cells require maturation via CD40 to generate protective antitumor immunity. J Immunol. 1998;161:2094–2098. [PubMed] [Google Scholar]
- 6.French RR, Chan HT, Tutt AL, Glennie MJ. CD40 antibody evokes a cytotoxic T-cell response that eradicates lymphoma and bypasses T-cell help. Nat Med. 1999;5:548–553. doi: 10.1038/5505. [DOI] [PubMed] [Google Scholar]
- 7.Kikuchi T, Crystal RG. Anti-tumor immunity induced by in vivo adenovirus vector-mediated expression of CD40 ligand in tumor cells. Hum Gene Ther. 1999;10:1375–1387. doi: 10.1089/10430349950018049. [DOI] [PubMed] [Google Scholar]
- 8.Loskog A, Bjorkland A, Brown MP, Korsgren O, Malmstrom PU, Totterman TH. Potent antitumor effects of CD154 transduced tumor cells in experimental bladder cancer. J Urol. 2001;166:1093–1097. doi: 10.1016/S0022-5347(05)65928-9. [DOI] [PubMed] [Google Scholar]
- 9.Noguchi M, Imaizumi K, Kawabe T, et al. Induction of antitumor immunity by transduction of CD40 ligand gene and interferon-gamma gene into lung cancer. Cancer Gene Ther. 2001;8:421–429. doi: 10.1038/sj.cgt.7700320. [DOI] [PubMed] [Google Scholar]
- 10.Todryk SM, Tutt AL, Green MH, Smallwood JA, Halanek N, Dalgleish AG, Glennie MJ. CD40 ligation for immunotherapy of solid tumours. J Immunol Methods. 2001;248:139–147. doi: 10.1016/S0022-1759(00)00349-5. [DOI] [PubMed] [Google Scholar]
- 11.Dzojic H, Loskog A, Totterman TH, Essand M. Adenovirus-mediated CD40 ligand therapy induces tumor cell apoptosis and systemic immunity in the TRAMP-C2 mouse prostate cancer model. Prostate. 2006;66:831–838. doi: 10.1002/pros.20344. [DOI] [PubMed] [Google Scholar]
- 12.Sun Y, Peng D, Lecanda J, Schmitz V, Barajas M, Qian C, Prieto J. In vivo gene transfer of CD40 ligand into colon cancer cells induces local production of cytokines and chemokines, tumor eradication and protective antitumor immunity. Gene Ther. 2000;7:1467–1476. doi: 10.1038/sj.gt.3301264. [DOI] [PubMed] [Google Scholar]
- 13.Buhtoiarov IN, Lum H, Berke G, Paulnock DM, Sondel PM, Rakhmilevich AL. CD40 ligation activates murine macrophages via an IFN-gamma-dependent mechanism resulting in tumor cell destruction in vitro. J Immunol. 2005;174:6013–6022. doi: 10.4049/jimmunol.174.10.6013. [DOI] [PubMed] [Google Scholar]
- 14.Sabel MS, Yamada M, Kawaguchi Y, Chen FA, Takita H, Bankert RB. CD40 expression on human lung cancer correlates with metastatic spread. Cancer Immunol Immunother. 2000;49:101–108. doi: 10.1007/s002620050608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirano A, Longo DL, Taub DD, et al. Inhibition of human breast carcinoma growth by a soluble recombinant human CD40 ligand. Blood. 1999;93:2999–3007. [PubMed] [Google Scholar]
- 16.Tong AW, Papayoti MH, Netto G, Armstrong DT, Ordonez G, Lawson JM, Stone MJ. Growth-inhibitory effects of CD40 ligand (CD154) and its endogenous expression in human breast cancer. Clin Cancer Res. 2001;7:691–703. [PubMed] [Google Scholar]
- 17.Eliopoulos AG, Dawson CW, Mosialos G, et al. CD40-induced growth inhibition in epithelial cells is mimicked by Epstein-Barr Virus-encoded LMP1: involvement of TRAF3 as a common mediator. Oncogene. 1996;13:2243–2254. [PubMed] [Google Scholar]
- 18.Knox PG, Davies CC, Ioannou M, Eliopoulos AG. The death domain kinase RIP1 links the immunoregulatory CD40 receptor to apoptotic signaling in carcinomas. J Cell Biol. 2011;192:391–399. doi: 10.1083/jcb.201003087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Afford SC, Randhawa S, Eliopoulos AG, Hubscher SG, Young LS, Adams DH. CD40 activation induces apoptosis in cultured human hepatocytes via induction of cell surface fas ligand expression and amplifies fas-mediated hepatocyte death during allograft rejection. J Exp Med. 1999;189:441–446. doi: 10.1084/jem.189.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghamande S, Hylander BL, Oflazoglu E, Lele S, Fanslow W, Repasky EA. Recombinant CD40 ligand therapy has significant antitumor effects on CD40-positive ovarian tumor xenografts grown in SCID mice and demonstrates an augmented effect with cisplatin. Cancer Res. 2001;61:7556–7562. [PubMed] [Google Scholar]
- 21.Moschonas A, Kouraki M, Knox PG, Thymiakou E, Kardassis D, Eliopoulos AG. CD40 induces antigen transporter and immunoproteasome gene expression in carcinomas via the coordinated action of NF-kappaB and of NF-kappaB-mediated de novo synthesis of IRF-1. Mol Cell Biol. 2008;28:6208–6222. doi: 10.1128/MCB.00611-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomes EM, Rodrigues MS, Phadke AP, et al. Antitumor activity of an oncolytic adenoviral-CD40 ligand (CD154) transgene construct in human breast cancer cells. Clin Cancer Res. 2009;15:1317–1325. doi: 10.1158/1078-0432.CCR-08-1360. [DOI] [PubMed] [Google Scholar]
- 23.Diaconu I, Cerullo V, Hirvinen ML, et al. Immune response is an important aspect of the antitumor effect produced by a CD40L-encoding oncolytic adenovirus. Cancer Res. 2012;72:2327–2338. doi: 10.1158/0008-5472.CAN-11-2975. [DOI] [PubMed] [Google Scholar]
- 24.Vonderheide RH, Butler MO, Liu JF, Battle TE, Hirano N, Gribben JG, Frank DA, Schultze JL, Nadler LM. CD40 activation of carcinoma cells increases expression of adhesion and major histocompatibility molecules but fails to induce either CD80/CD86 expression or T cell alloreactivity. Int J Oncol. 2001;19:791–798. doi: 10.3892/ijo.19.4.791. [DOI] [PubMed] [Google Scholar]
- 25.Malmstrom PU, Loskog AS, Lindqvist CA, Mangsbo SM, Fransson M, Wanders A, Gardmark T, Totterman TH. AdCD40L immunogene therapy for bladder carcinoma: the first phase I/IIa trial. Clin Cancer Res. 2010;16:3279–3287. doi: 10.1158/1078-0432.CCR-10-0385. [DOI] [PubMed] [Google Scholar]
- 26.Beatty GL, Chiorean EG, Fishman MP, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pesonen S, Diaconu I, Kangasniemi L, et al. Oncolytic immunotherapy of advanced solid tumors with a CD40L-expressing replicating adenovirus: assessment of safety and immunologic responses in patients. Cancer Res. 2012;72:1621–1631. doi: 10.1158/0008-5472.CAN-11-3001. [DOI] [PubMed] [Google Scholar]
- 28.Folkman J, Hahnfeldt P, Hlatky L. Cancer: looking outside the genome. Nat Rev Mol Cell Biol. 2000;1:76–79. doi: 10.1038/35036100. [DOI] [PubMed] [Google Scholar]
- 29.Loskog A, Dzojic H, Vikman S, Ninalga C, Essand M, Korsgren O, Totterman TH. Adenovirus CD40 ligand gene therapy counteracts immune escape mechanisms in the tumor microenvironment. J Immunol. 2004;172:7200–7205. doi: 10.4049/jimmunol.172.11.7200. [DOI] [PubMed] [Google Scholar]
- 30.Jackaman C, Nelson DJ. Intratumoral interleukin-2/agonist CD40 antibody drives CD4+ -independent resolution of treated-tumors and CD4+ -dependent systemic and memory responses. Cancer Immunol Immunother. 2012;61:549–560. doi: 10.1007/s00262-011-1120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loskog AS, Fransson ME, Totterman TT. AdCD40L gene therapy counteracts T regulatory cells and cures aggressive tumors in an orthotopic bladder cancer model. Clin Cancer Res. 2005;11:8816–8821. doi: 10.1158/1078-0432.CCR-05-1817. [DOI] [PubMed] [Google Scholar]
- 32.Eliopoulos AG, Wang CC, Dumitru CD, Tsichlis PN. Tpl2 transduces CD40 and TNF signals that activate ERK and regulates IgE induction by CD40. EMBO J. 2003;22:3855–3864. doi: 10.1093/emboj/cdg386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hannani D, Sistigu A, Kepp O, Galluzzi L, Kroemer G, Zitvogel L. Prerequisites for the antitumor vaccine-like effect of chemotherapy and radiotherapy. Cancer J. 2011;17:351–358. doi: 10.1097/PPO.0b013e3182325d4d. [DOI] [PubMed] [Google Scholar]
- 34.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dolcetti L, Peranzoni E, Ugel S, et al. Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur J Immunol. 2010;40:22–35. doi: 10.1002/eji.200939903. [DOI] [PubMed] [Google Scholar]
- 36.Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 37.Tesniere A, Schlemmer F, Boige V, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29:482–491. doi: 10.1038/onc.2009.356. [DOI] [PubMed] [Google Scholar]
- 38.Ramakrishnan R, Gabrilovich DI. Novel mechanism of synergistic effects of conventional chemotherapy and immune therapy of cancer. Cancer Immunol Immunother. 2013;62:405–410. doi: 10.1007/s00262-012-1390-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol. 2011;8:151–160. doi: 10.1038/nrclinonc.2010.223. [DOI] [PubMed] [Google Scholar]
- 40.Nowak AK, Robinson BW, Lake RA. Synergy between chemotherapy and immunotherapy in the treatment of established murine solid tumors. Cancer Res. 2003;63:4490–4496. [PubMed] [Google Scholar]
- 41.Vardouli L, Lindqvist C, Vlahou K, Loskog AS, Eliopoulos AG. Adenovirus delivery of human CD40 ligand gene confers direct therapeutic effects on carcinomas. Cancer Gene Ther. 2009;16:848–860. doi: 10.1038/cgt.2009.31. [DOI] [PubMed] [Google Scholar]
- 42.Vincent J, Mignot G, Chalmin F, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052–3061. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 43.Stewart TJ, Abrams SI. How tumours escape mass destruction. Oncogene. 2008;27:5894–5903. doi: 10.1038/onc.2008.268. [DOI] [PubMed] [Google Scholar]
- 44.Yeh KY, Pulaski BA, Woods ML, McAdam AJ, Gaspari AA, Frelinger JG, Lord EM. B7-1 enhances natural killer cell-mediated cytotoxicity and inhibits tumor growth of a poorly immunogenic murine carcinoma. Cell Immunol. 1995;165:217–224. doi: 10.1006/cimm.1995.1208. [DOI] [PubMed] [Google Scholar]
- 45.Cooke PW, James ND, Ganesan R, Wallace M, Burton A, Young LS. CD40 expression in bladder cancer. J Pathol. 1999;188:38–43. doi: 10.1002/(SICI)1096-9896(199905)188:1<38::AID-PATH315>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 46.Bugajska U, Georgopoulos NT, Southgate J, Johnson PW, Graber P, Gordon J, Selby PJ, Trejdosiewicz LK. The effects of malignant transformation on susceptibility of human urothelial cells to CD40-mediated apoptosis. J Natl Cancer Inst. 2002;94:1381–1395. doi: 10.1093/jnci/94.18.1381. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.