Abstract
Medulloblastoma, a primitive neuro-ectodermal tumor that arises in the posterior fossa, is the most common malignant brain tumor occurring in childhood. Even though 60–70% of children with medulloblastoma will be cured with intensive multimodal therapy, including surgery, radiotherapy, and chemotherapy, a significant proportion of surviving patients may suffer from long-term treatment-related sequelae. Therapeutic success is limited especially in younger children by radiotherapy-induced neurocognitive longterm deficits. In order to avoid or delay craniospinal radiotherapy, high-dose chemotherapy followed by autologous stem cell transplantation (HSCT) has become an established treatment modality. Data on the host immunologic environment in medulloblastoma patients are rare, notably data on cytokine expression and immune reconstitution in patients with medulloblastoma undergoing HSCT are lacking. In this present study, we therefore decided to prospectively assess immune function following 24 consecutive autologous HSCT in 17 children with medulloblastoma treated according to the German-Austrian-Swiss HIT-2000-protocol. TH1 predominance was found to be the most important factor for probability of survival. Already before HSCT, survivors showed higher IFNγ levels in sera as well as higher numbers of IFNγ-positive T-cells. After transplantation, this effect was even more pronounced. Patients with higher numbers of IFNγ- and TNFα-positive T-cells had a more favorable outcome at all analyzed time points. In addition, patients in complete remission (CR) before transplantation, known to have a better prognosis a priori, showed higher expression of IFNγ in T-cells. Taken together, this is the first report to demonstrate that high expression of IFNγ and TNFα in T-cells of medulloblastoma patients in the early post-transplant period correlates with a better prognosis. Our data point toward a potentially important influence of TH1-cytokine expression before and after transplantation on the survival of pediatric medulloblastoma patients.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-011-0981-y) contains supplementary material, which is available to authorized users.
Keywords: Pediatric medulloblastoma, High-dose chemotherapy, T cell reconstitution, IFNγ and TNFα, TH1 predominance, Prognostic factors
Introduction
Medulloblastoma represents the most common malignant central nervous system tumor constituting more than 20% of all pediatric brain tumors [1]. Even though 60–70% of children with non-metastasized medulloblastoma may be cured by aggressive multimodal therapy, including surgery, radiotherapy, and chemotherapy, a significant proportion of surviving patients may suffer from long-term treatment-related complications, especially from leukoencephalopathy and cognitive deficits [2, 3]. In particular, in younger children the increased susceptibility of the immature brain to radiotherapy-induced cognitive deficits represents a major restriction for therapy. These deficits may even increase for years after radiotherapy [4]. This has set age limits on the use of radiotherapy especially in young children [5].
In order to delay or to even replace craniospinal radiotherapy, high-dose chemotherapy has been pioneered as a therapeutic option [6–8]. High-dose chemotherapy followed by autologous stem cell transplantation (HSCT) is designed to eliminate higher numbers of chemosensitive residual tumor cells. The efficacy of this treatment modality is based on the chemosensitivity of the malignant tumor cells with a steep dose–response curve as well as the choice of drugs for which bone marrow impairment is the dose-limiting toxicity [9]. To rescue the hematopoietic system, patients receive cryopreserved autologous hematopoietic stem cells. HSCT has become an established treatment for distinct pediatric tumors [10–13]. We and others have been able to demonstrate, that it can be effective [14, 15], even in relapsed patients with extraneural metastatic medulloblastoma [14].
However, a great number of metastatic or recurrent childhood medulloblastoma have become chemoresistant and results of the most recent relapse trial within the German-Austrian-Swiss HIT-brain tumor network (HIT-Rez-97) have been disappointing [16]. Relapse rates are known to be high during the first year after completion of therapy [17, 18]. These relapses have been attributed mainly to the failure of high-dose chemotherapy to eradicate minimal residual disease. Potential mechanisms include resistance to apoptosis [19], down-regulation of death receptors, presence of decoy receptors, and loss of downstream death-signaling pathway elements [20–22]. In medulloblastoma cell lines, resistance toward TRAIL-induced apoptosis was shown to correlate with loss of caspase-8 mRNA [21]. This loss of apoptosis induction could be reversed in vitro by IFNγ. IFNγ-mediated restoration of caspase-8 expression resulted in the restoration of sensitivity to TRAIL-induced apoptosis and in an increased response to chemotherapy and radiotherapy [12, 19, 23]. IFNγ, a pleiotropic cytokine, produced mainly by T- and NK-cells, is known to be involved in antiviral responses, immune surveillance, inhibiting cellular proliferation, and in tumor suppression [24–26]. However, in contrast to the in vitro findings, data on the potential impact of cytokine expression in medulloblastoma patients are rare.
Prognostic factors in childhood medulloblastoma have been defined based on clinical criteria, and only recently the knowledge of molecular markers has increased [6, 27]. Outcome of patients <4 years of age, with metastatic disease, brainstem, or fourth ventricular floor involvement is inferior [28]. Between 20 and 40% of patients who meet the current clinical standard risk criteria (non-metastatic disease, no post-operative residual tumor) will experience tumor relapse, but cannot be identified in advance. Most recently, molecular markers and gene expression profiles have been investigated by our group and others [27, 29, 30]. Pfister et al. identified genomic aberrations such as genomic amplification of MYC/MYCN or the gain of 6q and 17q, as powerful independent markers for disease progression and reduced survival [27]. Furthermore, c-myc and trkC have been identified as promising markers in paraffin embedded tissue samples [20, 29]. However, the optimal combination of clinical and biologic markers for disease stratification has not been established yet.
For the further improvement of multimodal therapy, a better understanding of the biologic mechanisms of host tumor interaction in medulloblastoma is of utmost importance. In the present study, we therefore focused on one important aspect of the host environment; we prospectively analyzed immune function in 17 consecutive children with medulloblastoma after 24 autologous HSCT. All patients were treated according to the German-Austrian-Swiss HIT-2000-protocol and transplanted during the period from June 01, 2002 until December 31, 2006. As surrogate parameters of immune function, four-color flow cytometry, intracellular cytokine staining, and measurement of serum cytokine concentrations were performed. We asked whether distinct parameters of immune reconstitution, especially in cytokine expression, might be predictive for the prognosis in these high-risk children.
Our results confirmed for the first time that high IFNγ and TNFα production in T-cells at distinct time points in the immediate post-HSCT time period are in fact important predictive factors for long-term disease-free survival. These novel observations underline the importance of cytokine expression for tumor surveillance in childhood medulloblastoma.
Materials and methods
Patients
We prospectively studied T-/B- and NK-cell reconstitution as well as intracellular cytokine expression in T-cells in consecutive pediatric patients with medulloblastoma/supratentorial PNET or pineoblastoma undergoing autologous peripheral hematopoietic stem cell transplantation with unmanipulated grafts at the University Children Hospital of Wuerzburg between June 01, 2002 and December 31, 2006, at the following predefined time points: before stem cell transplantation, at the time of engraftment, and on days +30, +60, +100, +200, +365, 15 months and 2 years after transplantation. In total, 185 samples from 24 transplantations (17 patients) were analyzed. Pre-transplant chemotherapy and conditioning regimes were uniformly administered according to the HIT 2000 protocol that includes pediatric patients of defined histology (medulloblastoma of all subtypes, including the rare supratentorial PNET or pineoblastoma) and age (<21 years). Patients who received different treatment schedules were excluded from this study. Following the second to fourth chemotherapy cycle, all patients underwent leukapheresis for peripheral blood stem cell collection. Stem cells were mobilized by G-CSF.
This study was approved by the Human Subjects Committee of the University of Wuerzburg (Study Nr. 133/04) and was registered at ClinicalTrials.gov as NCT00231712. Patients and guardians participating in this study gave informed consent according to institutional guidelines in accordance with the Declaration of Helsinki.
Antibodies and reagents
Antibodies to the surface epitopes CD3 (clone UCHT1), CD4 (RPA-T4), CD8 (RPA-T8), CD16 (3G8)/56 (B159), CD19 (HIB19), CD45RA (HI100), CD45RO (UCHL1), TGFβ (TB21), TNFα (Mab11), IFNγ (B27), IL2 (MQ1-17H12), and IL4 (MP4-25D2) were all purchased from Becton–Dickinson (Heidelberg, Germany).
Unstimulated sera were measured for cytokines in Opteia-ELISA Kits (IL15, IL13, IFNγ, IL4; Becton–Dickinson: Heidelberg, Germany) and in Biosource ELISA kits (IL7, TGFβ; Biosource: Camarillo, USA) according to the manufacturer’s instructions.
Intracellular cytokine staining
Initially, 1 × 106 leukocytes in heparinized peripheral blood (min. 100 μl) were added to 1,000 μl RPMI-1640 medium supplemented with 10% FCS, 2 mmol/l l-glutamine, 100 U/L penicillin, 1 mM/L sodium pyruvate, and 100 μg/L streptomycin (Gibco, Eggenstein, Germany). Diluted peripheral blood cells were incubated with Phorbol-myristate acetate (PMA, 10 ng/ml) and ionomycin (1 nmol/ml) and cultured for 20 h at 37°C and 5% CO2. In order to block cytokine secretion, Brefeldin (10 μg/ml, Sigma, Taufkirchen, Germany) was added for at least 16 h. Intracellular cytokine staining was performed as described previously [31, 32]. After incubation, cells were stained for surface markers using standard procedures. Subsequently, cells were fixed with 4% paraformaldehyde, permeabilized with 0.3% saponin (Riedel–de–Haen, Deisenhofen, Germany), stained intracellularly, washed twice in 0.1% saponin, and analyzed on a FACS Calibur® using CellQuest® software (BD, Heidelberg, Germany).
In order to ensure optimal culture conditions, mitogen concentrations (PMA 1–50 ng/ml and ionomycin 0.1–5 nmol/ml) as well as culture periods (4, 6, 8, 10, 20, 24, 36, and 48 h) had been titrated with PBMCs from healthy individuals. Best results for all cytokines were obtained with 10 ng/ml PMA and 1 nmol/ml ionomycin after 20 h, which were used for all subsequent assays.
Statistical analysis
Student’s paired t-test for mean differences was used to analyze data for levels of statistical significance among two groups (survival vs. non-survival), Kruskal–Wallis Test were used among three age groups (0–4; 5–12; >12 years). Correlation between surface isotope expression and cytokine production was assessed using the Pearson correlation coefficient. Kaplan–Meier Plots were used for survival probability. Statistical correlations between parameters were analyzed using a bivariate fit model (JMP 5.0 software, SAS, Cary, NC, USA). Additional multivariate analysis was performed against conventional outcome predictors (age, gender, disease status, and stem cell dosage) between multiple groups by using repeated measures analysis of variance (ANOVA) and Bonferroni’s multiple comparison test. In all statistical applications, a P value of <0.05 was considered to indicate a statistical significant difference between the groups.
Results
Engraftment
Following high-dose chemotherapy, a median of 4.6 × 106 CD34+/kg body weight (range: 2–48 × 106 CD34+) non-manipulated peripheral blood stem cells were transplanted. All patients received G-CSF until granulocyte recovery. All patients showed engraftment after transplantation with a median time to engraftment of 11 ± 5 days (range: 8–14 days).
The median patient age in this study was 6.9 years (range 12 months-16 years; 11 male, 6 female). Patients’ follow-up was finally evaluated on May 01, 2010, with a median follow-up of 4.3 years (range 106–3,285 days). At this time point, 8 of 17 patients were still alive (Table 1). Four of these were in complete remission, four with stable disease. 9 patients died of disease progression. There was no transplant related mortality (0/24).
Table 1.
Patient characteristics

Table depicts the data of the investigated patients: age at transplantation, histology, chemotherapy induction protocol, disease status at transplant, type of conditioning regime, number of CD34-positive stem cells contained in the graft/kg body weight, day of engraftment after transplant and current disease status. Seven patients received two consecutive transplants. Out of 17 patients, 8 are currently alive; of these 4 patients are in CR, 4 are alive with disease; these patients are shaded in gray. 9 patients died of progressive disease. There was no transplant related mortality
CPM cyclophosphamid, CBCDA carboplatin, L-PAM melphalan, TTP thiotepa, VP-16 Etoposid, autoHSCT autologous stem cell transplantation, CR complete remission, PR partial response, VGPR very good partial response, SD stable disease, PD progressive disease, DOD dead of disease, ith intrathekal, engraftment first of three consecutive days with absolute neutrophile count of >500/μl
Cytokine production before transplantation: comparison of survivors vs. non-survivors
As a first step, immune parameters immediately before the start of the conditioning were investigated (Table 2). The obtained data were retrospectively analyzed for differences in survivors vs. non-survivors.
Table 2.
Data obtained by FACS analysis after intracellular cytokine staining before high-dose chemotherapy
| Lymphoid subpopulation | Before transplantation in % (alive vs. death) | P values |
|---|---|---|
| CD3 IFNγ | 30 ± 5 vs. 17 ± 3 | NS (0.05) |
| CD8 IFNγ | 17 ± 5 vs. 3 ± 2 | 0.02 |
| CD3 CD45RO IFNγ | 19 ± 3 vs. 5 ± 1 | 0.007 |
Patients who were alive on May 01, 2010 were compared to patients who had died of progressive disease. The data depict the percentage of cytokine-positive cells in the respective T-cell subpopulation. The survivors show higher expressions of IFNγ, especially in CD45RO-positive cells, than non-survivors measured at the time point before transplantation
Patients with a favorable outcome (survivors) showed higher production of IFNγ in distinct T-cell subsets: we could demonstrate that CD8+ cytotoxic T-cells (17 ± 5 vs. 3 + 2% IFNγ+/CD8+, P = 0.02) and even more pronounced memory T-cells (19 ± 3 vs. 5 + 1% IFNγ+/CD45R0+, P = 0.007) showed increased IFNγ production (Table 2). The described correlations could be confirmed by analyzing both relative percentages of IFNγ-positive cells and absolute counts of IFNγ-positive cells within the above-mentioned T-cell subpopulation. Importantly, age-dependent influences of cytokine expression profiles could be excluded in our cohort (data not shown). In contrast, analysis of additional intracellular cytokines (IL2, IL4, IL5, IL10 as well as TNFα) before transplantation did not show any statistical significant differences between survivors and non-survivors (data not shown).
With respect to cytokine secretion in unstimulated sera before transplantation, we found a tendency toward higher IFNγ levels in surviving children (P = 0.04, supplementary online Figure S1B).
Cytokine production after transplantation
All patients: immunoreconstitution after autologous SCT
We analyzed the cytokine expression at predefined time points after autologous stem cell transplantations (engraftment, days +30, +60, +100, +200, +365 and 15 months). Here, we observed that IL4, TGFβ and IFNγ secretion in unstimulated sera showed a peak at the time of engraftment and thereafter returned to pre-transplant levels during the first-year post-transplantation (data not shown).
Using intracellular cytokine staining, we found increasing values of IFNγ and TNFα expression in all T-cell subpopulations until day +200 (Fig. 1 depicts a representative example (patient # 3). Thereafter, IFNγ and TNFα declined and returned to pre-transplant levels (Table 3). In contrast, TH2-cytokine expression (IL4, IL5 and IL10) remained stable during the observation period. IL2 production in T-cells peaked at engraftment and returned to pre-transplant levels at one-year post-SCT.
Fig. 1.
Increasing TH1-cytokine expressions until day ± 200 in one representative patient (# 3). Figure 1 depicts representative results obtained by FACS analysis of PBMC from patient number # 3. Cells were stimulated with PMA and ionomycin, as described in the section of “Materials and methods”. Gating was performed on lymphocytes by scatter characteristics and CD3+ cells. The expression of IFNγ (upper lane) and TNFα (lower lane) in CD3-positive T-cells before (left column), 60 days (middle column) and 200 days (right column) post-transplant is shown
Table 3.
Immune reconstitution after autologous stem cell transplantation
| Mean values | Before autoHSCT | Engraftment | Day +30 | Day +60 | Day +100 | Day +365 | >2 years | Normal range |
|---|---|---|---|---|---|---|---|---|
| CD16/56/μl | 160 ± 125 | 100 ± 70 | 232 ± 250 | 197 ± 78 | 172 ± 75 | 152 ± 100 | 260 ± 73 | 300–600 |
| CD3/μl | 845 ± 498 | 354 ± 427 | 550 ± 253 | 456 ± 278 | 387 ± 142 | 837 ± 24 | 1,147 ± 335 | 1,800–3,600 |
| CD4/μl | 507 ± 352 | 175 ± 279 | 224 ± 183 | 249 ± 112 | 135 ± 90 | 422 ± 124 | 620 ± 156 | 800–1,800 |
| CD8/μl | 290 ± 140 | 89 ± 124 | 185 ± 81 | 224 ± 96 | 234 ± 112 | 322 ± 43 | 348 ± 43 | 800–1,500 |
| CD3CD45RA% | 52 ± 11 | 29 ± 16 | 28 ± 9 | 36 ± 8 | 36 ± 7 | 45 ± 8 | 44 ± 8 | 60–70 |
| CD3CD45RO% | 33 ± 12 | 53 ± 15 | 61 ± 10 | 53 ± 13 | 53 ± 7 | 43 ± 9 | 38 ± 8 | 21–40 |
| CD19/μl | 278 ± 194 | 62 ± 86 | 101 ± 124 | 146 ± 113 | 199 ± 129 | 148 ± 48 | 462 ± 144 | 500–1,300 |
| IgG mg/dl | 203 ± 64 | 193 ± 35 | 186 ± 25 | 197 ± 53 | 127 ± 88 | 200 ± 78 | 250 ± 134 | 570–1,500 |
| CD3+IL2% | 21 ± 9 | 50 ± 20 | 35 ± 9 | 35 ± 5 | 30 ± 4 | 20 ± 10 | 27 ± 9 | 33–52 |
| CD3+IFNγ% | 23 ± 6 | 21 ± 10 | 47 ± 13 | 52 ± 14 | 60 ± 5 | 60 ± 15 | 26 ± 5 | 15–30 |
| CD3+TNFα% | 23 ± 9 | 25 ± 5 | 38 ± 11 | 37 ± 12 | 55 ± 6 | 40 ± 15 | 31 ± 11 | 16–29 |
| CD3+IL4% | 0.5 ± 0.5 | 2 ± 0.5 | 0.5 ± 0.3 | 1 ± 0.5 | 1 ± 0.5 | 0.6 ± 0.4 | 1 ± 0 | 0.5–2 |
Lymphocyte reconstitution and cytokine expression in CD3+ T-cells before transplantation, at engraftment, on days +30, +100, +365 and >2 years post-transplantation. Cytokine production was determined by FACS after stimulation with PMA and ionomycin as described above. Percentages of cytokine-positive cells in CD3+ T-cells as mean ± SD are shown
Comparison of survivors vs. non-survivors
When comparing cytokine production after transplantation in patients who survived vs. patients who died, survivors—as in pre-transplant analysis—showed higher intracellular TH1 cytokine expression, especially high numbers of IFNγ-positive T-cells (day +100: CD3+/IFNγ+: 69 ± 9 vs. 31 ± 8%; P < 0.0001, Table 4 and supplementary online Figure S1A) and TNFα-positive T-cells (day +100: CD3+/TNFα+: 62 ± 5 vs. 29 ± 8; P < 0.001, Table 4) were found to be linked with a favorable outcome at all measured time points post-transplantation. As has been shown in the pre-transplant setting, the main contributing subpopulation for high production of IFNγ and TNFα in T-cells were cytotoxic (CD8+) and of IFNγ in memory T-cells (CD45R0+) (Table 4). Findings could be confirmed by similar correlations of the absolute counts of IFNγ-positive cells (data not shown). Most of the patients showed a consistency of IFNγ expression prior to and after transplantation.
Table 4.
Data obtained by FACS analysis after intracellular cytokine staining on day +100 post-transplant
| Lymphoid subpopulation | Day +100 in % (alive vs. death) | P values |
|---|---|---|
| CD3 IFNγ | 69 ± 9 vs. 31 ± 8 | 0.0004 |
| CD8 IFNγ | 50 ± 7 vs. 14 ± 5 | <0.0001 |
| CD3 CD45RO IFNγ | 89 ± 10 vs. 34 ± 6 | 0.001 |
| CD3 CD45RA IFNγ | 76 ± 12 vs. 27 ± 5 | 0.04 |
| CD3 TNFα | 62 ± 5 vs. 29 ± 8 | 0.001 |
| CD8 TNFα | 45 ± 16 vs. 15 ± 8 | 0.004 |
| CD3 CD45RO TNFα | 62 ± 6 vs. 40 ± 11 | NS (0.1) |
| CD3 CD45RA TNFα | 29 ± 9 vs. 7 ± 5 | 0.008 |
Patients who were alive on May 01, 2010 to patients who had died of progressive disease were compared. The data depict the percentage of cytokine-positive cells in the respective T-cell subpopulation. The survivors showed higher expressions of IFNγ and TNFα, especially in CD8-positive and CD45RO-positive cells than non-survivors on day +100 post-transplantation
Besides the impact of cytokine expression in T-cells, additional parameters of immune reconstitution were analyzed. In the group of surviving medulloblastoma patients, higher T- and B-cells counts (day +100: CD45RO+: P < 0.02, CD8+: P < 0.004 and CD19+: P < 0.03) were found at all analyzed post-transplant time points (data not shown). Furthermore, we analyzed T-cell receptor diversity as previously described by our group [33] and could not find any significant differences (data not shown). In addition, analysis of thymic output post-transplant (TREC analysis) showed well-known age-dependent expression [34], but no correlation with prognosis (data not shown).
Impact of remission status on cytokine expression
We then analyzed the potential impact of remission status before SCT on cytokine expression after HSCT. Patients in CR before transplantation are known to have a better prognosis a priori [17, 35]. No differences were found in post-transplant cytokine sera levels between CR and Non-CR patients. However, looking at intracellular cytokine expression of T-cells after transplantation, higher IFNγ expression (especially in CD8+IFNγ+ T-cells: P < 0.02; day + 100 and in CD3+ CD45RO+ IFNγ+: P < 0.007 day + 100; Table 5) was found in CR patients at the time of transplantation and at all predefined time points until day +200 when compared to non-CR patients. All other cytokines tested, namely IL2, IL4, IL5, IL10 and TNFα, did not show any correlation with outcome.
Table 5.
Data obtained by FACS analysis after intracellular cytokine staining at day +100 post-transplant
| Lymphoid subpopulation | Day +100 in % (CR vs. Non-CR before HSCT) | P values |
|---|---|---|
| CD3 IFNγ | 60 ± 7 vs. 40 ± 5 | NS (0.05) |
| CD8 IFNγ | 46 ± 7 vs. 20 ± 6 | 0.02 |
| CD3 CD45RO IFNγ | 82 ± 8 vs. 40 ± 5 | 0.007 |
IFNγ production in T-cells in patients who were in CR pre-SCT vs. patients who had detectable disease before transplantation was compared. The data depict the percentage of cytokine-positive cells in the respective T-cell subpopulation. Patients who were in CR pre-transplantation showed higher expressions of IFNγ, especially in CD8-positive and CD45RO-positive cells, on day +100 post-transplantation
Comparison of IFNγ high and low producers
Finally, we performed a Kaplan–Meier analysis for patients with high IFNγ production (>40% IFNγ+/CD3+ cells) vs. patients with low IFNγ production (<40% IFNγ+/CD3+) in T-cells measured at 60 days after SCT. This cutoff was selected based on previous data in adult patients [36, 37]. High production of IFNγ correlated significantly with a better survival post-transplant (55% for high producers vs. 15% for low producers, P < 0.0001; Fig. 2). Similar results were obtained in relation to the IFNγ production prior to transplantation and at day +30 post-HSCT (data not shown). We excluded patient age as a confounding factor by multivariate analysis (data not shown).
Fig. 2.
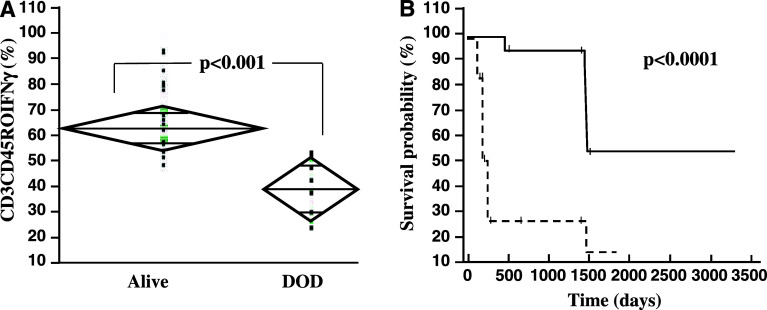
IFNγ expression and survival. a Data are given as boxplots of percentage of IFNγ+ cells in the CD45RO+CD3+-fraction on day +200. Survivors showed higher percentages of IFNγ producing memory T-cells. DOD death of disease. b Kaplan–Maier analysis for patients with higher vs. lower production of IFNγ in T-cells. At day + 60 post-transplantation, PBMC of patients (n = 17) were stimulated and determination of IFNγ-positive cells was done by FACS analysis. Results were defined as IFNγ high producers (9/17) if more than 40% of T-cells showed IFNγ expression and as low producers (8/17) if less than 40% of T-cells were IFNγ positive. 1,500 days post-transplantation survival was 55% in IFNγ high producers vs. 15% in IFNγ low producers (P < 0.0001)
Discussion
High-dose chemotherapy followed by autologous hematopoietic stem cell transplantation (auto HSCT) has become an effective and established treatment for certain malignant diseases [10, 12, 38]. Historically, neurosurgical operation, radiation therapy, and adjuvant chemotherapy have been established as standard therapy for medulloblastoma in childhood. Cooperative multicenter trials have improved prognosis over the last 15 years [6]. In an attempt to delay or to even substitute craniospinal irradiation notably in young children, therapy protocols have been amended to include HSCT in favor of a better overall survival with less neurocognitive deficiencies when compared to conventional craniolspinal radiation [8].
While immune function following allogeneic transplantation has been extensively studied in recent years, few data on immune reconstitution following autologous transplantation for solid pediatric tumors exist. Most published studies on this issue comprised mixed cohorts of children with different underlying diseases, prior therapies and various conditioning regimes [37, 39–42]. In this prospective study, we therefore decided to analyze a homogeneous pediatric cohort of 17 medulloblastoma/PNET patients with uniform stratified treatment according to the HIT-2000 protocol.
Besides the reconstitution of adequate lymphocyte counts, a key element in the recovery of immune competence is an appropriate T-cell function as measured by cytokine production. Recent data underline the importance of cytokine expression in tumor surveillance in different tumor entities including lymphoma, solid tumors, and brain tumors [43]. To the best of the authors’ knowledge, this is the first report to demonstrate data on cytokine expression after HSCT for pediatric medulloblastoma. Most importantly, we could demonstrate for the first time that children with favorable clinical course showed TH1 predominance in cytokine production. Interestingly, already before HSCT higher IFNγ production was found in the group of survivors. On follow-up post-transplant, the differences in IFNγ production between non-survivors and survivors were found to be even more pronounced. High absolute IFNγ levels as well as high relative expression of IFNγ in different T-cells were found in patients with favorable outcome (Table 4). As IFNγ expression is known to increase with age [44–46], we investigated potential age-dependent influences and could exclude patient age as a confounding factor. Furthermore, other conventional outcome predictors, such as gender, disease status, and stem cell dose, could be excluded by multivariate analysis (data not shown). The relatively small patient number certainly represents one of the limitations of the present study; therefore, results will have to be confirmed in a larger multicenter cohort in the future.
In other tumor entities, TH1 predominance has been characterized as a favorable prognostic indicator, for example in adult lymphoma, where TH1-cytokine predominance (mainly IFN expression) has been associated with good prognosis before and during therapy [47]. No data exist so far for pediatric brain tumors. Even though we did not find higher levels of TH2-cytokines and could therefore not directly demonstrate a TH2 shift [47], we could clearly demonstrate significantly lower pre- and post-transplant IFNγ (and TNFα) values in patients who later developed relapse (Fig. 2/Table 4).
In recent years, the importance of IFNγ in tumor surveillance has been intensively studied. IFNγ has been shown to enhance antigen presentation by up-regulation of MHC expression [48, 49]. In addition, IFNγ is responsible for the induction of costimulatory molecules on antigen-presenting cells [49]. Furthermore, IFNγ promotes TH1 development, in part by enhancing IL12 secretion by macrophages and partly by up-regulation of functional IL12-R on CD4+T-cells, rendering them to be more responsive to IL12. There is increasing evidence that IL12 production of antigen-presenting cells and IFNγ production of T-cells are correlated with the induction of anti-tumor immunity [48, 49]. However, in the current study we did not analyze IL12 expression. This specific question will be the subject of future studies.
Besides its essential role for the induction of anti-tumor responses, it has been suggested that IFNγ may mediate a direct physiological effect on the development of the central nervous system [50, 51]. Transgenic mice with ectopical expression of IFNγ in the CNS showed hypomyelination and abnormal cerebellar development [52, 53]. More recently, Lin et al. demonstrated in a mouse model that IFNγ expression in a very narrow developmental window of perinatal period can induce medulloblastoma as well as Atypical Teratoid Rabdoid Tumors [54]. Interestingly, once the tumor was established, further expression of IFNγ induced host immunologic response with lymphocytic infiltration and tumor apoptosis [54]. Finally, in medulloblastoma tissue itself it could be demonstrated that mRNA expression of IFNγ-R2 is significantly lower compared to normal brain tissue. Therefore, reduced IFNγ signaling, induced by either receptor down-regulation or reduced IFNγ expression, seems to play an important role in medulloblastoma pathobiology [19].
Furthermore, in medulloblastoma cell lines, IFNγ is known to increase chemosensitivity, cytotoxicity and induction of apoptosis [20, 23]. In this context, caspase-8 activity has to be discussed as an important element in the biologic mechanisms. Demethylating agents (for example valproic acid) and additional IFNγ are discussed to be contributing factors for increasing caspase-8 activity by transcriptional activation through the Stat-1/IRF1-dependent pathway. Sensitization to death receptor-induced apoptosis as well as increased sensitivity to chemotherapy and radiotherapy is a result of higher caspase-8 levels in in vitro experiments [19, 23]. Inactivation of caspase-8 to escape apoptosis might therefore be an immunologic cancer escape mechanism, which has already been described for certain tumors, including neuroblastoma, medulloblastoma, Ewing sarcoma, and melanoma [20, 23]. In this context, it is important to note that loss of caspase-8 activity correlates with inferior survival outcome in medulloblastoma [55], suggesting that up-regulation of caspase-8 activity may represent an interesting approach for future therapy options. Taken together, biologically based new treatment approaches, especially focusing on IFNγ, may turn out to become critical contributing factors for improving the prognosis of high-risk medulloblastoma patients.
The focus of the present study was the analysis of cytokine expression and secretion as an important factor of tumor–host interaction during the course of HSCT. Future studies will analyze the cytokine expression and receptor status in situ within tumor tissues to address the question whether the tumor itself may influence immunologic surveillance and IFNγ expression. Alternatively, genetic predispositions, such as polymorphisms of cytokine genes or cytokine receptors, as has been demonstrated in certain malignancies [56–58], have to be discussed and will be the focus of future studies.
In summary, this is the first report to demonstrate that IFNγ expression in T-cells is associated with prognosis in pediatric medulloblastoma patients. This interesting finding may be of importance for future risk stratification as well as for novel immunotherapy approaches in pediatric medulloblastoma. IFNγ may represent a novel promising window of opportunity for medulloblastoma patients in an effort to overcome primary or secondary therapy resistance.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We would like to thank Dr. Danny Douek, NIH, Bethesda, USA, for providing an aliquot of the TREC standard plasmid. V. Wiegering is the recipient of a junior investigator prize awarded by the South German Society of Pediatrics (2007). This work was supported by program project grant (Z-2/7-28.06.04.) of IZKF Wuerzburg, by a grant from the Tour of Hope Foundation (2006–2007) and in part by program project grant Bay-Immunet (2009). The German-Austrian-Swiss HIT 2000 study (PI: S. Rutkowski) is supported by the Deutsche Kinderkrebsstiftung. We thank the staff of the BMT and Oncology unit and the Outpatient Department, as well as the technical staff of the stem cell processing unit at the University Children’s Hospital, Wuerzburg, for their excellent and dedicated patient care.
References
- 1.Gurney JG, van Wijngaarden E. Extremely low frequency electromagnetic fields (EMF) and brain cancer in adults and children: review and comment. Neuro Oncol. 1999;1(3):212–220. doi: 10.1093/neuonc/1.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rood BR, Macdonald TJ, Packer RJ. Current treatment of medulloblastoma: recent advances and future challenges. Semin Oncol. 2004;31(5):666–675. doi: 10.1053/j.seminoncol.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Rutkowski S, Bode U, Deinlein F, Ottensmeier H, Warmuth-Metz M, Soerensen N, Graf N, Emser A, Pietsch T, Wolff JE, Kortmann RD, Kuehl J. Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med. 2005;352(10):978–986. doi: 10.1056/NEJMoa042176. [DOI] [PubMed] [Google Scholar]
- 4.Ottensmeier H, Frahsek S, Faldum A, Rutkowski S. Longterm neuropsychological follow up of young children with medulloblastoma and ependymoma treated in the trials HIT-SKK 87/92 and HIT2000. Neuro Oncol. 2010;12(6):ii13. [Google Scholar]
- 5.Palmer SL, Goloubeva O, Reddick WE, Glass JO, Gajjar A, Kun L, Merchant TE, Mulhern RK. Patterns of intellectual development among survivors of pediatric medulloblastoma: a longitudinal analysis. J Clin Oncol. 2001;19(8):2302–2308. doi: 10.1200/JCO.2001.19.8.2302. [DOI] [PubMed] [Google Scholar]
- 6.Rutkowski S. Current treatment approaches to early childhood medulloblastoma. Expert Rev Neurother. 2006;6(8):1211–1221. doi: 10.1586/14737175.6.8.1211. [DOI] [PubMed] [Google Scholar]
- 7.Warren KE, Packer RJ. Current approaches to CNS tumors in infants and very young children. Expert Rev Neurother. 2004;4(4):681–690. doi: 10.1586/14737175.4.4.681. [DOI] [PubMed] [Google Scholar]
- 8.Grill J, Sainte-Rose C, Jouvet A, Gentet JC, Lejars O, Frappaz D, Doz F, Rialland X, Pichon F, Bertozzi AI, Chastagner P, Couanet D, Habrand JL, Raquin MA, Le Deley MC, Kalifa C. Treatment of medulloblastoma with postoperative chemotherapy alone: an SFOP prospective trial in young children. Lancet Oncol. 2005;6(8):573–580. doi: 10.1016/S1470-2045(05)70252-7. [DOI] [PubMed] [Google Scholar]
- 9.Wolff JE, Finlay JL. High-dose chemotherapy in childhood brain tumors. Onkologie. 2004;27(3):239–245. doi: 10.1159/000077973. [DOI] [PubMed] [Google Scholar]
- 10.Dunkel IJ, Finlay JL. High-dose chemotherapy with autologous stem cell rescue for brain tumors. Crit Rev Oncol Hematol. 2002;41(2):197–204. doi: 10.1016/S1040-8428(01)00156-1. [DOI] [PubMed] [Google Scholar]
- 11.Mackall CL, Fleisher TA, Brown MR, Magrath IT, Shad AT, Horowitz ME, Wexler LH, Adde MA, McClure LL, Gress RE. Lymphocyte depletion during treatment with intensive chemotherapy for cancer. Blood. 1994;84(7):2221–2228. [PubMed] [Google Scholar]
- 12.Perez-Martinez A, Quintero V, Vicent MG, Sevilla J, Diaz MA, Madero L. High-dose chemotherapy with autologous stem cell rescue as first line of treatment in young children with medulloblastoma and supratentorial primitive neuroectodermal tumors. J Neurooncol. 2004;67(1–2):101–106. doi: 10.1023/B:NEON.0000021774.79094.25. [DOI] [PubMed] [Google Scholar]
- 13.Berthold F, Boos J, Burdach S, Erttmann R, Henze G, Hermann J, Klingebiel T, Kremens B, Schilling FH, Schrappe M, Simon T, Hero B. Myeloablative megatherapy with autologous stem-cell rescue versus oral maintenance chemotherapy as consolidation treatment in patients with high-risk neuroblastoma: a randomised controlled trial. Lancet Oncol. 2005;6(9):649–658. doi: 10.1016/S1470-2045(05)70291-6. [DOI] [PubMed] [Google Scholar]
- 14.Leo E, Schlegel PG, Lindemann A. Chemotherapeutic induction of long-term remission in metastatic medulloblastoma. J Neurooncol. 1997;32(2):149–154. doi: 10.1023/A:1005721510659. [DOI] [PubMed] [Google Scholar]
- 15.Gajjar A, Chintagumpala M, Ashley D, Kellie S, Kun LE, Merchant TE, Woo S, Wheeler G, Ahern V, Krasin MJ, Fouladi M, Broniscer A, Krance R, Hale GA, Stewart CF, Dauser R, Sanford RA, Fuller C, Lau C, Boyett JM, Wallace D, Gilbertson RJ. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7(10):813–820. doi: 10.1016/S1470-2045(06)70867-1. [DOI] [PubMed] [Google Scholar]
- 16.Fleischhack G, Popping K, Hasan C, Utsch B, Juttner J, Bode U (1998) High dose chemotherapy with thiotepa, carboplatin, VP16 and autologous stem cell transplantation in treatment of malignant brain tumors with poor prognosis. Results of a mono-center pilot study. Klin Padiatr 210(4):248–255. doi:10.1055/s-2008-1043887 [DOI] [PubMed]
- 17.Zeltzer PM, Boyett JM, Finlay JL, Albright AL, Rorke LB, Milstein JM, Allen JC, Stevens KR, Stanley P, Li H, Wisoff JH, Geyer JR, McGuire-Cullen P, Stehbens JA, Shurin SB, Packer RJ. Metastasis stage, adjuvant treatment, and residual tumor are prognostic factors for medulloblastoma in children: conclusions from the Children’s Cancer Group 921 randomized phase III study. J Clin Oncol. 1999;17(3):832–845. doi: 10.1200/JCO.1999.17.3.832. [DOI] [PubMed] [Google Scholar]
- 18.Dunkel IJ, Boyett JM, Yates A, Rosenblum M, Garvin JH, Jr, Bostrom BC, Goldman S, Sender LS, Gardner SL, Li H, Allen JC, Finlay JL. High-dose carboplatin, thiotepa, and etoposide with autologous stem-cell rescue for patients with recurrent medulloblastoma. Children’s Cancer Group. J Clin Oncol. 1998;16(1):222–228. doi: 10.1200/JCO.1998.16.1.222. [DOI] [PubMed] [Google Scholar]
- 19.Meister N, Shalaby T, von Bueren AO, Rivera P, Patti R, Oehler C, Pruschy M, Grotzer MA. Interferon-gamma mediated up-regulation of caspase-8 sensitizes medulloblastoma cells to radio- and chemotherapy. Eur J Cancer. 2007;43(12):1833–1841. doi: 10.1016/j.ejca.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 20.Grotzer MA, Eggert A, Zuzak TJ, Janss AJ, Marwaha S, Wiewrodt BR, Ikegaki N, Brodeur GM, Phillips PC. Resistance to TRAIL-induced apoptosis in primitive neuroectodermal brain tumor cells correlates with a loss of caspase-8 expression. Oncogene. 2000;19(40):4604–4610. doi: 10.1038/sj.onc.1203816. [DOI] [PubMed] [Google Scholar]
- 21.Zuzak TJ, Steinhoff DF, Sutton LN, Phillips PC, Eggert A, Grotzer MA. Loss of caspase-8 mRNA expression is common in childhood primitive neuroectodermal brain tumour/medulloblastoma. Eur J Cancer. 2002;38(1):83–91. doi: 10.1016/S0959-8049(01)00355-0. [DOI] [PubMed] [Google Scholar]
- 22.Reed JC. Caspases and cytokines: roles in inflammation and autoimmunity. Adv Immunol. 1999;73:265–299. doi: 10.1016/S0065-2776(08)60788-9. [DOI] [PubMed] [Google Scholar]
- 23.Fulda S, Debatin KM. 5-Aza-2’-deoxycytidine and IFN-gamma cooperate to sensitize for TRAIL-induced apoptosis by upregulating caspase-8. Oncogene. 2006;25(37):5125–5133. doi: 10.1038/sj.onc.1209518. [DOI] [PubMed] [Google Scholar]
- 24.Bach EA, Aguet M, Schreiber RD. The IFN gamma receptor: a paradigm for cytokine receptor signaling. Annu Rev Immunol. 1997;15:563–591. doi: 10.1146/annurev.immunol.15.1.563. [DOI] [PubMed] [Google Scholar]
- 25.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 26.Ikeda H, Old LJ, Schreiber RD. The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002;13(2):95–109. doi: 10.1016/S1359-6101(01)00038-7. [DOI] [PubMed] [Google Scholar]
- 27.Pfister S, Remke M, Benner A, Mendrzyk F, Toedt G, Felsberg J, Wittmann A, Devens F, Gerber NU, Joos S, Kulozik A, Reifenberger G, Rutkowski S, Wiestler OD, Radlwimmer B, Scheurlen W, Lichter P, Korshunov A. Outcome prediction in pediatric medulloblastoma based on DNA copy-number aberrations of chromosomes 6q and 17q and the MYC and MYCN loci. J Clin Oncol. 2009;27(10):1627–1636. doi: 10.1200/JCO.2008.17.9432. [DOI] [PubMed] [Google Scholar]
- 28.Packer RJ (2005) Medulloblastoma. J Neurosurg 103(4 Suppl):299–300. doi:10.3171/ped.2005.103.4.0299 (discussion 300–291) [DOI] [PubMed]
- 29.Rutkowski S, von Bueren A, von Hoff K, Hartmann W, Shalaby T, Deinlein F, Warmuth-Metz M, Soerensen N, Emser A, Bode U, Mittler U, Urban C, Benesch M, Kortmann RD, Schlegel PG, Kuehl J, Pietsch T, Grotzer M. Prognostic relevance of clinical and biological risk factors in childhood medulloblastoma: results of patients treated in the prospective multicenter trial HIT’91. Clin Cancer Res. 2007;13(9):2651–2657. doi: 10.1158/1078-0432.CCR-06-1779. [DOI] [PubMed] [Google Scholar]
- 30.Shalaby T, von Bueren AO, Hurlimann ML, Fiaschetti G, Castelletti D, Masayuki T, Nagasawa K, Arcaro A, Jelesarov I, Shin-ya K, Grotzer M (2010) Disabling c-Myc in childhood medulloblastoma and atypical teratoid/rhabdoid tumor cells by the potent G-quadruplex interactive agent S2T1-6OTD. Mol Cancer Ther 9(1):167–179. doi:10.1158/1535-7163.MCT-09-0586 [DOI] [PubMed]
- 31.Eyrich M, Wiegering V, Lim A, Schrauder A, Winkler B, Schlegel PG. Immune function in children under chemotherapy for standard risk acute lymphoblastic leukaemia - a prospective study of 20 paediatric patients. Br J Haematol. 2009;147(3):360–370. doi: 10.1111/j.1365-2141.2009.07862.x. [DOI] [PubMed] [Google Scholar]
- 32.Mascher B, Schlenke P, Seyfarth M. Expression and kinetics of cytokines determined by intracellular staining using flow cytometry. J Immunol Methods. 1999;223(1):115–121. doi: 10.1016/S0022-1759(98)00200-2. [DOI] [PubMed] [Google Scholar]
- 33.Eyrich M, Croner T, Leiler C, Lang P, Bader P, Klingebiel T, Niethammer D, Schlegel PG. Distinct contributions of CD4(+) and CD8(+) naive and memory T-cell subsets to overall T-cell-receptor repertoire complexity following transplantation of T-cell-depleted CD34-selected hematopoietic progenitor cells from unrelated donors. Blood. 2002;100(5):1915–1918. doi: 10.1182/blood-2001-11-0005. [DOI] [PubMed] [Google Scholar]
- 34.Eyrich M, Wollny G, Tzaribaschev N, Dietz K, Brugger D, Bader P, Lang P, Schilbach K, Winkler B, Niethammer D, Schlegel PG. Onset of thymic recovery and plateau of thymic output are differentially regulated after stem cell transplantation in children. Biol Blood Marrow Transplant. 2005;11(3):194–205. doi: 10.1016/j.bbmt.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Sung KW, Yoo KH, Cho EJ, Koo HH, Lim do H, Shin HJ, Ahn SD, Ra YS, Choi ES, Ghim TT. High-dose chemotherapy and autologous stem cell rescue in children with newly diagnosed high-risk or relapsed medulloblastoma or supratentorial primitive neuroectodermal tumor. Pediatr Blood Cancer. 2007;48(4):408–415. doi: 10.1002/pbc.21064. [DOI] [PubMed] [Google Scholar]
- 36.Guo H, Qiao Z, Zhu L, Wang H, Su L, Lu Y, Cui Y, Jiang B, Zhu Q, Xu L. Th1/Th2 cytokine profiles and their relationship to clinical features in patients following nonmyeloablative allogeneic stem cell transplantation. Am J Hematol. 2004;75(2):78–83. doi: 10.1002/ajh.10443. [DOI] [PubMed] [Google Scholar]
- 37.Mitra DK, Singh HP, Singh M, Alwadi A, Kochupillai V, Raina V, Kumar L, Mehra NK. Reconstitution of naive T cells and type 1 function after autologous peripheral stem cell transplantation: impact on the relapse of original cancer. Transplantation. 2002;73(8):1336–1339. doi: 10.1097/00007890-200204270-00025. [DOI] [PubMed] [Google Scholar]
- 38.Mackall CL, Stein D, Fleisher TA, Brown MR, Hakim FT, Bare CV, Leitman SF, Read EJ, Carter CS, Wexler LH, Gress RE. Prolonged CD4 depletion after sequential autologous peripheral blood progenitor cell infusions in children and young adults. Blood. 2000;96(2):754–762. [PubMed] [Google Scholar]
- 39.Guillaume T, Rubinstein DB, Symann M. Immune reconstitution and immunotherapy after autologous hematopoietic stem cell transplantation. Blood. 1998;92(5):1471–1490. [PubMed] [Google Scholar]
- 40.Hoepfner S, Haut PR, O’Gorman M, Kletzel M. Rapid immune reconstitution following autologous hematopoietic stem cell transplantation in children: a single institution experience. Bone Marrow Transplant. 2003;31(4):285–290. doi: 10.1038/sj.bmt.1703831. [DOI] [PubMed] [Google Scholar]
- 41.Kalwak K, Gorczynska E, Toporski J, Turkiewicz D, Slociak M, Ussowicz M, Latos-Grazynska E, Krol M, Boguslawska-Jaworska J, Chybicka A. Immune reconstitution after haematopoietic cell transplantation in children: immunophenotype analysis with regard to factors affecting the speed of recovery. Br J Haematol. 2002;118(1):74–89. doi: 10.1046/j.1365-2141.2002.03560.x. [DOI] [PubMed] [Google Scholar]
- 42.Reimer P, Kunzmann V, Wilhelm M, Weissbrich B, Kraemer D, Berghammer H, Weissinger F. Cellular and humoral immune reconstitution after autologous peripheral blood stem cell transplantation (PBSCT) Ann Hematol. 2003;82(5):263–270. doi: 10.1007/s00277-003-0630-4. [DOI] [PubMed] [Google Scholar]
- 43.Bronte V, Mocellin S. Suppressive influences in the immune response to cancer. J Immunother. 2009;32(1):1–11. doi: 10.1097/CJI.0b013e3181837276. [DOI] [PubMed] [Google Scholar]
- 44.Chipeta J, Komada Y, Zhang XL, Deguchi T, Sugiyama K, Azuma E, Sakurai M. CD4 + and CD8 + cell cytokine profiles in neonates, older children, and adults: increasing T helper type 1 and T cytotoxic type 1 cell populations with age. Cell Immunol. 1998;183(2):149–156. doi: 10.1006/cimm.1998.1244. [DOI] [PubMed] [Google Scholar]
- 45.Hoffmann F, Albert MH, Arenz S, Bidlingmaier C, Berkowicz N, Sedlaczek S, Till H, Pawlita I, Renner ED, Weiss M, Belohradsky BH. Intracellular T-cell cytokine levels are age-dependent in healthy children and adults. Eur Cytokine Netw. 2005;16(4):283–288. [PubMed] [Google Scholar]
- 46.Wiegering V, Eyrich M, Wunder C, Gunther H, Schlegel PG, Winkler B. Age-related changes in intracellular cytokine expression in healthy children. Eur Cytokine Netw. 2009;20(2):75–80. doi: 10.1684/ecn.2009.0149. [DOI] [PubMed] [Google Scholar]
- 47.Lee PP, Zeng D, McCaulay AE, Chen YF, Geiler C, Umetsu DT, Chao NJ. T helper 2-dominant antilymphoma immune response is associated with fatal outcome. Blood. 1997;90(4):1611–1617. [PubMed] [Google Scholar]
- 48.Dredge K, Marriott JB, Todryk SM, Dalgleish AG. Adjuvants and the promotion of Th1-type cytokines in tumour immunotherapy. Cancer Immunol Immunother. 2002;51(10):521–531. doi: 10.1007/s00262-002-0309-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santana MA, Rosenstein Y. What it takes to become an effector T cell: the process, the cells involved, and the mechanisms. J Cell Physiol. 2003;195(3):392–401. doi: 10.1002/jcp.10258. [DOI] [PubMed] [Google Scholar]
- 50.Popko B, Baerwald KD. Oligodendroglial response to the immune cytokine interferon gamma. Neurochem Res. 1999;24(2):331–338. doi: 10.1023/A:1022586726510. [DOI] [PubMed] [Google Scholar]
- 51.Sredni-Kenigsbuch D. TH1/TH2 cytokines in the central nervous system. Int J Neurosci. 2002;112(6):665–703. doi: 10.1080/00207450290025725. [DOI] [PubMed] [Google Scholar]
- 52.Corbin JG, Kelly D, Rath EM, Baerwald KD, Suzuki K, Popko B. Targeted CNS expression of interferon-gamma in transgenic mice leads to hypomyelination, reactive gliosis, and abnormal cerebellar development. Mol Cell Neurosci. 1996;7(5):354–370. doi: 10.1006/mcne.1996.0026. [DOI] [PubMed] [Google Scholar]
- 53.LaFerla FM, Sugarman MC, Lane TE, Leissring MA. Regional hypomyelination and dysplasia in transgenic mice with astrocyte-directed expression of interferon-gamma. J Mol Neurosci. 2000;15(1):45–59. doi: 10.1385/JMN:15:1:45. [DOI] [PubMed] [Google Scholar]
- 54.Lin W, Kemper A, McCarthy KD, Pytel P, Wang JP, Campbell IL, Utset MF, Popko B. Interferon-gamma induced medulloblastoma in the developing cerebellum. J Neurosci. 2004;24(45):10074–10083. doi: 10.1523/JNEUROSCI.2604-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pingoud-Meier C, Lang D, Janss AJ, Rorke LB, Phillips PC, Shalaby T, Grotzer MA. Loss of caspase-8 protein expression correlates with unfavorable survival outcome in childhood medulloblastoma. Clin Cancer Res. 2003;9(17):6401–6409. [PubMed] [Google Scholar]
- 56.Cloppenborg T, Stanulla M, Zimmermann M, Schrappe M, Welte K, Klein C. Immunosurveillance of childhood ALL: polymorphic interferon-gamma alleles are associated with age at diagnosis and clinical risk groups. Leukemia. 2005;19(1):44–48. doi: 10.1038/sj.leu.2403553. [DOI] [PubMed] [Google Scholar]
- 57.Dai L, Gast A, Horska A, Schrappe M, Bartram CR, Hemminki K, Kumar R, Bermejo JL. A case-control study of childhood acute lymphoblastic leukaemia and polymorphisms in the TGF-beta and receptor genes. Pediatr Blood Cancer. 2009;52(7):819–823. doi: 10.1002/pbc.21971. [DOI] [PubMed] [Google Scholar]
- 58.Seidemann K, Zimmermann M, Book M, Meyer U, Burkhardt B, Welte K, Reiter A, Stanulla M. Tumor necrosis factor and lymphotoxin alfa genetic polymorphisms and outcome in pediatric patients with non-Hodgkin’s lymphoma: results from Berlin-Frankfurt-Munster Trial NHL-BFM 95. J Clin Oncol. 2005;23(33):8414–8421. doi: 10.1200/JCO.2005.01.2179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.