Abstract
Background
Cerebellar degeneration-related protein 2 (CDR2) has been presumed to be the main antigen for the onconeural antibody Yo, which is strongly associated with ovarian cancer and paraneoplastic cerebellar degeneration (PCD). Recent data show that Yo antibodies also target the CDR2-like protein (CDR2L). We, therefore, examined the expression of CDR2 and CDR2L in ovarian cancer tissue from patients with and without Yo antibodies and from various other cancerous and normal human tissues.
Methods
Ovarian cancer tissue and serum samples from 16 patients were included in the study (four with anti-Yo and PCD, two with anti-Yo without PCD, five with only CDR2L antibodies, and five without onconeural antibodies). Clinical data were available for all patients. The human tissues were examined by western blot and immunohistochemistry using rabbit CDR2 and CDR2L antibodies.
Results
Ovarian cancers from all 16 patients expressed CDR2 and CDR2L proteins. Both proteins were also present in normal and cancer tissue from mammary tissue, kidney, ovary, prostate, and testis.
Conclusion
CDR2L is present in ovarian cancers from patients with and without Yo antibodies as was shown previously for CDR2. In addition, both CDR2 and CDR2L proteins are more widely expressed than previously thought, both in normal and cancerous tissues.
Keywords: Cerebellar degeneration-related protein 2, Cerebellar degeneration-related protein 2-like, Paraneoplastic cerebellar degeneration, Ovarian cancer, Yo antibody
Introduction
Paraneoplastic neurological syndromes (PNS) are immune-mediated diseases characterized by antibody production and activation of cytotoxic T cells against onconeural antigens expressed in cancer that also target identical proteins in the central nervous system [1]. Paraneoplastic cerebellar degeneration (PCD) is one of the most common of these syndromes; it is strongly associated with ovarian and breast cancer [2, 3]. In PCD, expression of onconeural antigens in the associated ovarian cancer is a common finding and a prerequisite for the immune response, but this only rarely leads to antibody production, as onconeural antibodies are infrequent in ovarian cancer patients in general [4].
Anti-Yo is the predominant onconeural antibody in the serum and cerebrospinal fluid of patients with PCD and ovarian cancer [5]. Three cerebellar antigens have been described for anti-Yo, namely the cerebellar degeneration-related protein 2 (CDR2), CDR2-like (CDR2L), and cerebellar degeneration-related protein 1 (CDR1) [6–8]. CDR2L is named CDR2-like due to 45% sequence identity with CDR2 (sequence from www.UniProt.org, aligned in www.blast.ncbi.nlm.nih.gov, BLOSUM62). Because the CDR2 mRNA was the only CDR mRNA found to be expressed in PCD-associated tumors, the CDR2 protein has been presumed to be the only Yo antigen expressed in ovarian cancers and the main target for Yo antibodies in Purkinje cells in the cerebellum [9]. However, two recent studies have suggested that sera from patients with PCD react with both CDR2 and CDR2L proteins and a pathogenic role for CDR2L antibodies in PCD has been hypothesized [10, 11].
CDR2 protein is down-regulated in rat brain cortex after birth and is only present at low levels in adult rat cortex [12]. This has also been shown for CDR2 and CDR2L at the mRNA level in the human cerebellum (Atlas of the developing human brain, www.brainspan.org), where CDR2 mRNA levels are shown to decrease and CDR2L mRNA to increase after birth. Therefore, the immune response against the CDR2L protein may be of greater clinical importance in PCD than that against CDR2, since the latter protein seems to be expressed only in low levels in the adult cerebellum.
Since both CDR2 and CDR2L antibodies are probably involved in the pathogenesis of PCD, it was expected that both proteins should be present in PCD-associated tumors. The expression of the CDR2 protein in human tissue has previously been described [13]. However, CDR2L protein in human cancers and tissues has not been studied so far. In this study, we examined ovarian cancers for expression of CDR2 and CDR2L proteins. The tumors were from patients with Yo antibodies, from patients whose sera contained antibodies against only the CDR2L and not the CDR2 protein, and from control patients without onconeural antibodies. Non-ovarian cancers and normal human tissue were also analyzed.
Materials and methods
Patient selection and human samples
The Research Biobank at the Neurological Research Laboratory, Haukeland University Hospital, consists of serum and cerebrospinal fluid samples containing onconeural antibodies or autoantibodies associated with diseases of the central nervous system from approximately 400 patients and an equivalent number of controls. The samples are sent to the laboratory for diagnostic purposes, and positive samples and controls have been consecutively included in the Research Biobank since 1995. The samples have been analyzed by immune blot, immunohistochemistry, and, for some antibodies, with a sensitive radioimmunoassay (RIA) using recombinant onconeural antigen produced by coupled in vitro transcription/translation. The latter technique has been used in screening for onconeural antibodies in cohorts of cancer patients from several cancer biobanks [4, 14, 15], and antibody positive samples from these studies are also included in the biobank. We screened the Research Biobank for Yo-positive patients and patients with only CDR2 or only CDR2L antibodies detected by RIA. The data were matched with the Bergen Gynecologic Cancer Biobank, a biobank consisting of blood and cancer samples from approximately 4000 patients who have undergone gynecological cancer surgery at the Department of Gynaecology and Obstetrics, Haukeland University Hospital since 2001. Formalin-fixed and paraffin-embedded ovarian cancer tissues from the same patients were also present in the Diagnostic Biobank, Department of Pathology, Haukeland University Hospital. Matching of data was done by the use of the patients’ national identification numbers.
The inclusion criteria for the study patients were the presence of serum Yo antibodies confirmed by a commercial immunoblot (PNS 11 Line Assay, Ravo Diagnostika GmbH, #PNS11-003/48) containing recombinant CDR2 protein or antibodies against CDR2 or CDR2L detected by RIA and available ovarian cancer tissue. Seronegative control patients were randomly selected from ovarian cancer patient samples in the Bergen Gynecologic Cancer Biobank whose sera had already been tested for onconeural antibodies. A total of 17 patients were initially included in the study and divided into three groups depending on antibody status: Yo antibody (n = 6, Yo1-6), CDR2L antibody (n = 5, CDR2L1-5), and seronegative control patients (n = 6, Control1-6). Fresh-frozen ovarian cancer tissue, sections from formalin-fixed and paraffin-embedded tissue and serum were included for every study patient. Clinical data regarding neurological symptoms and ovarian cancer were obtained from the patients’ medical records. One control patient was excluded when review of the pathological specimen showed a colon cancer, and we, therefore, included 16 patients in this study. Ovarian cancer characteristics, including CDR2 expression, were published earlier for nine of the 16 patients [4, 13]. The presence of CDR2L antibodies in the sera of patients with ovarian cancer was reported previously for five of the 16 patients [10].
All available slides from the ovarian cancer specimens were examined by two pathologists, and the tumors were classified according to the World Health Organization criteria [16]. The ovarian cancers were staged histologically according to criteria set by the International Federation of Gynecology and Obstetrics (FIGO) [17]. Sections of the primary ovarian tumors were available in all cases but one, as the tumor tissue available from patient Yo1 in the Bergen Gynecologic Cancer Biobank represented a metastasis. Cerebellar tissue, used for positive control sections, was obtained from autopsy at the Department of Pathology of a patient without neurological disease.
Sera from all 16 patients were obtained from The Research Biobank at the Neurological Research Laboratory. For three out of four patients with PCD, we chose sera collected in the early phase of the neurological disease, which coincided with cancer surgery. In Yo4 the development of PCD was related to recurrence of ovarian cancer and serum was obtained at this time point. For all other patients we analyzed sera from the period of cancer surgery.
Ethics statement
The Regional Committee for Health and Medical Research Ethics approved the study (2014/1066) and informed consent was obtained from living patients included in the study.
Onconeural antibodies and antibodies against CDR2 and CDR2L proteins
At inclusion, sera from all patients had already been evaluated for a panel of the well-characterized onconeural antibodies by immunoblot and tested on sections of rat cerebellar tissue by immunofluorescent staining. Both tests were repeated for all sera. In the immunoblot assay, Yo antibodies are detected by recombinant CDR2 protein. The RIA test was used for the detection of specific antibodies against CDR2 and CDR2L proteins in the patient sera before inclusion [4, 10], but had not been performed on sera from Yo5 and Yo6.
Immunohistochemistry
The preparation and immunofluorescent staining of rat cerebellum sections incubated with patient sera and CDR2 and CDR2L rabbit antibodies have been described previously [11]. Heat-induced antigen retrieval was performed in a pressure cooker in Diva Decloaker buffer solution (Biocare Medical, #DV2004MX). Sections were treated with patient sera (1:500), CDR2 (Sigma-Aldrich, 1:200, # HPA023870), or CDR2L antibody (Proteintech, 1:200, #14563-1-AP). The slices were mounted with Shandon Immu-Mount (Thermo Fisher Scientific, #9990402).
The immune staining was performed on sections from formalin-fixed and paraffin-embedded human tissue. The slides were incubated with antibodies against CDR2 (Sigma-Aldrich, 1:200, #HPA023870) and CDR2L (Aviva Systems Biology, 1:1500, #ARP39113_P050). The staining was performed at the Department of Pathology using an automated slide staining system (Ventana, #N750-BMKU-FS 05342716001) following standard procedures. Heat-induced antigen retrieval (Ventana, Ultra Cell Conditioning, 64 min, #950-224), was followed by incubation with primary antibody (32 min), followed by incubation with a secondary antibody (Ventana, Ultra View Universal DAB, #760-500), hematoxylin staining (Ventana, Hematoxylin II, #790-2208), and automated coverslipping (Sakura, Tissue-Tek SCA, #4764; Sakura, Tissue-Tek Coverslipping film, #4770).
Western blot analysis
We used the full-length human cDNA for CDR2 and CDR2L cloned into vector #PS100001, containing a C-terminal myc-DDK-tag (www.origene.com). A coupled in vitro transcription translation rabbit reticulocyte system (TnT Coupled Reticulocyte Lysate Systems, Promega, #L4610) was used to synthesize recombinant CDR2 and CDR2L. Lysates of fresh frozen ovarian cancer tissue were prepared at the Proteomics Unit at the University of Bergen (www.biochem.mpg.de/226356/FASP). The same protocol was used for the preparation of human cerebellar lysate from autopsy of a patient without neurological disease. Lysates from the following human tissues were obtained from ProSci, Inc. (www.prosci-inc.com): mammary tissue, mammary cancer, kidney, kidney cancer, ovary, ovarian cancer, prostate, prostate cancer, testis, and testicular cancer. The cerebellum lysates were included as controls. Proteins from lysates (50 μg of the patient samples, 20 μg of other human lysates) were separated on 10% SDS-PAGE gels (Bio-Rad, Mini-Protean TGX gels, #4561035). The proteins were transferred onto a nitrocellulose membrane (Bio-Rad, 0.45 μm pore size, #162-0115) and incubated overnight (4 °C) with patient sera or CDR antibodies diluted in 1% BSA 0,1% Tween 20 in PBS (patient sera (1:1000–1:2000), CDR2 (Sigma-Aldrich, 1:250–1:1000, #HPA023870) and CDR2L antibody (Sigma-Aldrich, 1:250, #HPA022015 or Proteintech, 1:1000, #14563-1-AP)). For quantification, protein levels from ovarian cancer samples were normalized to β-tubulin (Sigma-Aldrich, 1:500, #T4026). Cross-reactivity of all CDR2 and CDR2L rabbit antibodies used in this study was tested in western blots with recombinant CDR2L and CDR2 and did not occur. The blots were developed using horseradish peroxidase antibody (Dako, Rabbit Anti-human IgG-HRP, 1:1000, #P0214; Dako, Swine Anti-rabbit IgG-HRP, 1:1000, #P0217; Dako, Goat Anti-mouse IgG-HRP, 1:1000, #P0447) and the Pierce ECL chemiluminescence system (Thermo Fisher Scientific, #32106). ChemiDoc XRS + (Bio-Rad, #1708265) was used for gel imaging. All western blots with human samples were repeated more than three times with reproducible results.
Imaging
Rat cerebellar sections stained with patient sera were evaluated on a Leica DMRB with CoolLED pE-300-W LED illumination, and sections of ovarian cancer tissue treated with CDR2 and CDR2L antibodies were imaged on a Leica DMLS. Fiji/FigureJ and Adobe Illustrator CS were used for assembling microscopy images and western blots.
Results
Patient characteristics
The ovarian cancer patients were between 54 and 78 years (median 67 years) at cancer diagnosis. Four out of six anti-Yo-positive patients had typical features of PCD with severe ataxia as the predominant clinical presentation. The remaining patients had no neurological symptoms. Fourteen of the 16 patients had high-grade serous epithelial carcinomas (HGSC), one had a clear cell-carcinoma, and the remaining patient had an ovarian carcinosarcoma (Table 1). Two patients were diagnosed with localized ovarian cancer in FIGO stage 1, whereas the remaining patients had disseminated disease with cancers in stage 3 or 4.
Table 1.
Clinical characteristics of 16 patients with ovarian cancer
| Patient | Age at cancer | PNS | Histology | FIGO | Testing of patients sera | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| IB | IF | RIA | RIA | WB | WB | |||||
| CDR2 | CDR2/2L | CDR2 | CDR2L | CDR2 | CDR2L | |||||
| Yo1 | 60 | PCD | HGSC | 3A | + | + | + | + | + | + |
| Yo2 | 78 | None | HGSC | 3B | + | + | + | + | − | − |
| Yo3 | 58 | None | HGSC | 4 | + | + | + | + | + | + |
| Yo4 | 76 | PCD | HGSC | 3C | + | + | + | + | + | + |
| Yo5 | 78 | PCD | HGSC | 3C | + | + | ND | ND | + | + |
| Yo6 | 65 | PCD | HGSC | 3C | + | + | ND | ND | + | + |
| CDR2L1 | 74 | None | Carcinosarcoma | 3C | − | − | − | + | − | − |
| CDR2L2 | 72 | None | HGSC | 3C | − | − | − | + | − | − |
| CDR2L3 | 57 | None | HGSC | 4 | −a | − | − | + | − | − |
| CDR2L4 | 54 | None | Clear cell-carcinoma | 1A | − | − | − | + | − | − |
| CDR2L5 | 69 | None | HGSC | 3C | − | − | − | + | − | − |
| Control1 | 56 | None | HGSC | 3C | − | − | − | − | − | − |
| Control2 | 78 | None | HGSC | 4 | − | − | − | − | − | − |
| Control3 | 69 | None | HGSC | 1A | − | − | − | − | − | − |
| Control4 | 64 | None | HGSC | 3C | − | − | − | − | − | − |
| Control5 | 57 | None | HGSC | 3C | − | − | − | − | − | − |
IB immunoblot, IF immunofluorescence, ND no data, WB western blot
Ovarian cancer characteristics, including CDR2 expression, have been published earlier for patients Yo1-3, CDR2L4-5, Control1-2 and Control4-5, and data on CDR2L antibodies detected with different techniques have been published earlier for patient CDR2L1-5 [10, 13, 14]
a Zic4 antibody
Onconeural antibodies and antibodies against CDR proteins
Testing with immunoblot confirmed the presence of anti-Yo (anti-CDR2) in the six patients included as Yo-positive (Table 1, Fig. 1). All of the Yo-positive sera showed staining of cytoplasm in Purkinje cells of rat cerebellar sections (Fig. 1a) recognized as the typical staining pattern for Yo-positive sera and coinciding with rabbit CDR2L antibody staining. Incubation of sections with rabbit CDR2 antibody gave weak cytoplasmic Purkinje cell staining, and only anti-human or anti-rabbit secondary antibody showed no staining. In western blot with recombinant CDR2 and CDR2L protein, five out of six Yo-positive sera gave bands corresponding to the recombinant protein control with rabbit CDR2 antibody and CDR2L (Proteintech) antibody (Fig. 1b). Serum sample from patient Yo2 that contained markedly lower CDR2 and CDR2L antibody levels based on RIA, showed a weaker anti-CDR2 band in the immunoblot than did sera from the remaining Yo-positive patients and did not react with recombinant CDR2 and CDR2L proteins in western blot. Control lanes containing reticulocyte lysate without recombinant protein incubated with patient sera were negative for all patients. No bands were observed in lanes with only anti-rabbit or anti-human secondary antibody.
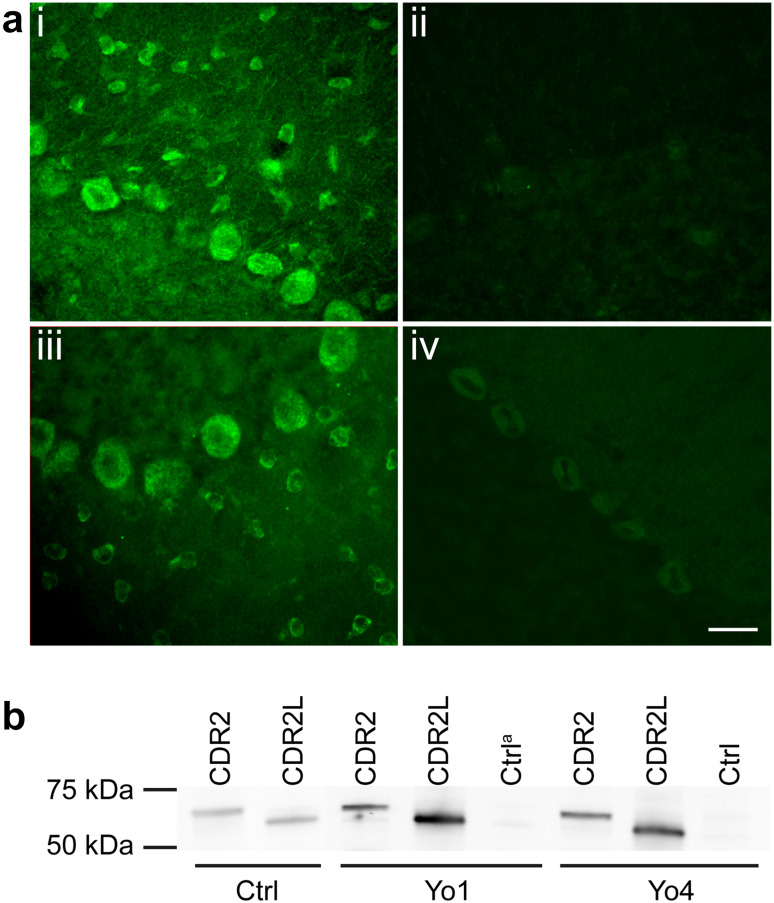
Fig. 1.
CDR2 and CDR2L antibodies in sera from Yo-positive patients. a shows sections of rat cerebellum with immunofluorescent staining of patient sera and rabbit CDR2 and CDR2L antibodies. Serum from Yo6 (i) stains the cytoplasm of Purkinje, stellate, and basket cells, similar to the rabbit CDR2L antibody staining (iii). Serum from CDR2L4 (ii) does not stain the sections, whereas the rabbit CDR2 antibody (iv) shows weak cytoplasmic Purkinje cell staining. Scale bar 50 μm. b western blot of recombinant CDR2 and CDR2L protein incubated with patient sera and rabbit CDR2 antibody and CDR2L antibody (Proteintech). Sera from Yo1 and Yo4 show bands coinciding with rabbit CDR2 and CDR2L antibodies of around 62 and 58 kDa, respectively, representing antibodies against CDR2 and CDR2L proteins in the patients sera. Control lanes with reticulocyte lysate without recombinant proteins are negative. aCtrl: control lane
All patients with only CDR2L antibodies detected by RIA were seronegative for Yo antibodies in immunoblot (anti-CDR2), but serum from patient CDR2L3 also contained Zic4 antibodies (Table 1). None of the sera stained rat cerebellar sections (Fig. 1a), and there were no detectable bands in western blot of recombinant CDR2 and CDR2L protein. No onconeural antibodies were detected in sera from the negative control patients with ovarian cancer when investigated by immunoblot, immunofluorescence, or western blot (data not shown).
Expression of CDR2 and CDR2L in ovarian cancer
Tissue from ovarian cancers from all 16 patients expressed both CDR2 and CDR2L proteins when evaluated by immunohistochemistry and western blot using specific rabbit antibodies (Fig. 2). The staining intensity and binding pattern varied, however. The CDR2 and CDR2L antibodies gave a primarily cytoplasmic staining of the cancer cells (Fig. 2a), whereas the CDR2 antibody also showed nuclear staining in mitotic cancer cells. CDR2 and CDR2L were also present in the cytoplasm of normal endothelial, stromal, and epithelial cells in the ovarian sections (data not shown). In cerebellar sections, serving as positive controls, the rabbit CDR2L antibody stained strongly the cytoplasm of Purkinje cells and molecular layer interneurons (Fig. 2a), whereas the rabbit CDR2 antibody showed similar but weak staining (data not shown). Control sections of ovarian cancer with only secondary anti-rabbit antibodies did not show any staining.
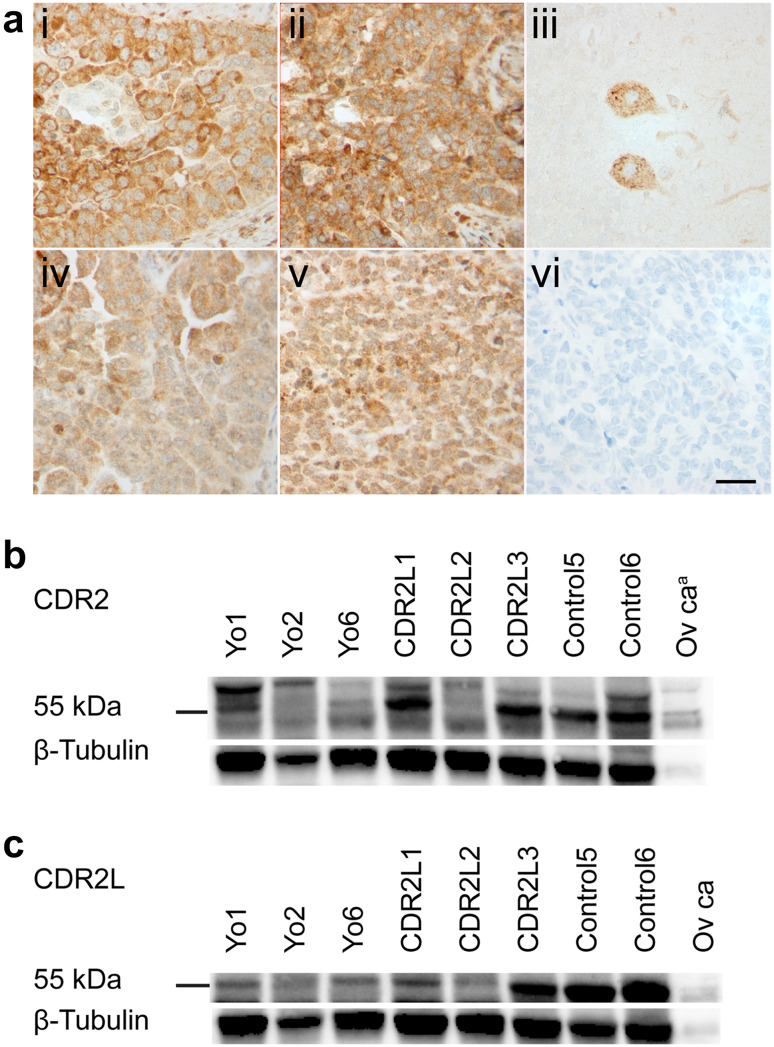
Fig. 2.
Expression of CDR2 and CDR2L proteins in human ovarian cancer. a shows formalin-fixed and paraffin-embedded sections of ovarian HGSC and cerebellum with immunostaining with rabbit CDR2 and CDR2L antibodies. Ovarian cancer sections from Yo4 (i) and CDR2L3 (ii) show strong CDR2 cytoplasmic staining of cancer cells and similar, but less intense, CDR2L staining (iv, v). In cerebellar sections, Purkinje cell cytoplasm shows CDR2L antibody staining (iii) and ovarian cancer section incubated with only secondary antibody shows no staining (vi). Scale bar 50 µm. b, c, western blots of human ovarian cancer lysates (50 μg protein) from patients Yo1-2, Yo6, CDR2L1-3, and Control5-6 were incubated with rabbit CDR2 antibody and CDR2L antibody (Sigma-Aldrich), respectively. The control lane to the right in both blots represents purchased ovarian cancer lysate (20 μg protein). In b, CDR2 protein presents as a double band around 55 kDa and an additional band at around 60 kDa in all samples. In c CDR2L presents as one band around 55 kDa in all lanes. Western blots were normalized to β-tubulin. aPurchased ovarian cancer lysate
Western blots of ovarian cancer lysates treated with CDR2 antibody showed a double band around 55 kilodaltons (kDa) and a single band around 60 kDa (Fig. 2b), and these bands were seen both in patient samples and a purchased control ovarian cancer lysate. Cerebellum lysate, included as a control, also showed a double band but with approximately 3 kDa higher molecular weight (data not shown). Western blots of ovarian cancer lysates treated with CDR2L antibody (Sigma-Aldrich) showed a band around 55 kDa, identical to the bands in control lysates from ovarian cancer (Fig. 2c) and cerebellum (data not shown). Another CDR2L antibody (Proteintech) also gave a band around 55 kDa, but this was accompanied by a band at around 60 kDa, very similar to CDR2.
Recombinant CDR2 and CDR2L proteins were included as positive controls and presented as a double band at around 62 kDa and a single band at around 58 kDa, respectively (data not shown). A myc-DDK-tag of 31 amino acids, of which 30% are acidic, may explain the discrepancy in molecular weight between the cancer samples and the recombinant control. No bands were observed in lanes with only anti-rabbit secondary antibody.
Expression of CDR2 and CDR2L in other tissues
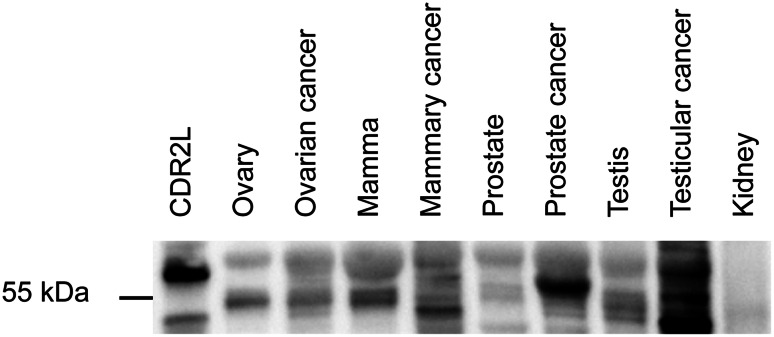
We also performed western blots of human lysates from mammary tissue, mammary cancer, kidney, kidney cancer, ovary, ovarian cancer, prostate, prostate cancer, testis, and testicular cancer with rabbit CDR2 and CDR2L antibodies. For all antibodies, the results described for ovarian cancer samples were reproduced: A single or double band at around 55 kDa and an additional band at around 60 kDa were observed in all tissues [shown in Fig. 3 for CDR2L (Sigma-Aldrich)]. We observed strong CDR2 and CDR2L bands in testicular and prostate cancer tissue and in these lysates the proteins were more abundant in the cancer than in the corresponding normal tissue. Kidney and kidney cancer showed faint bands for both proteins. No bands were observed in lanes with only anti-rabbit secondary antibodies.
Fig. 3.
Expression of CDR2L protein in various human tissues. Western blot of lysates from various human tissues (20 μg protein) incubated with rabbit CDR2L antibody (Sigma-Aldrich). CDR2L protein presents as a single or double band around 55 kDa and an additional band around 60 kDa in all samples (very faint in normal kidney lysate). Recombinant CDR2L in the lane to the left serves as positive control and shows a band around 58 kDa
Discussion
We found that CDR2 and CDR2L proteins were expressed at various levels in all ovarian cancer samples tested independently of the absence or presence of serum Yo antibodies (anti-CDR2 and CDR2L antibodies) or isolated CDR2L antibodies. With regard to the CDR2 protein, our results confirm those of previous studies, as CDR expression was found in ovarian cancers from patients with Yo antibodies [18], in patients without Yo antibodies, and in other cancer tissues [13, 19]. The expression of CDR2L has not been analyzed previously.
The widespread expression of CDR2 in various tumors has led to the hypothesis that CDR2 plays a role in tumorigenesis [19, 20], probably through the interaction with c-myc [21]. We observed cytoplasmic localization of both CDR2 and CDR2L, with additional nuclear CDR2 staining in mitotic cancer cells, which could correspond to peaked CDR2 levels during mitosis. The function of CDR2L in ovarian cancer is unknown. However, the function of CDR2L in Purkinje cells has been studied, and there is evidence that CDR2L is involved in maintaining calcium homeostasis by interacting with proteins critical to calcium regulation [11]. We found that both the CDR2 and CDR2L proteins were expressed in several cancers, and the protein levels seemed particularly high in prostate and testicular cancer tissue. The colon cancer sample, which was excluded from the ovarian cancer cohort, also showed CDR2 and CDR2L staining (data not shown). As both CDR2 and CDR2L proteins are expressed in malignancies as colon and prostate cancers, which are common cancers, it is surprising that Yo antibodies and PCD are seldom associated with these diseases. Yo antibodies have been reported in two cases with prostate cancer [22, 23], but PNS are generally rarely described in prostatic malignancies [24]. The strong association between ovarian cancer and Yo antibodies remains to be elucidated, as CDR2 and CDR2L are widely distributed in various cancers.
The widespread expression of CDR2 and CDR2L raises the question of why PCD associated with anti-Yo antibodies is so infrequent, when most ovarian cancers express both CDR2 and CDR2L antigens. Most cancer patients mount anti-tumoral immune responses [25], but multiple steps are probably essential to initiate the neurological disease. HLA class, activation of dendritic cells, CD4+ lymphocytes, T cell activation, tolerance, and blood–brain barrier are components likely to participate in the pathogenesis of PCD [26–28]. The activation of dendritic cells and CD4+ lymphocytes are also key factors in the development of an immune response versus tolerance [27]. Dendritic cells are more prone to be activated by necrosis than by apoptosis of cancer cells [29]; however, we did not see any association between necrosis or low tumor differentiation levels and Yo antibodies in ovarian cancer patients. This is in line with earlier reports, but studies on tumors from Yo patients are sparse due to the limited number of available patients and tumor tissue.
In addition to several important host immunological factors, there might also be differences in the CDR proteins in tumors from patients who develop Yo antibodies and tumors of seronegative patients. In a previous study, the CDR2 cDNAs of ovarian cancers from 14 patients were sequenced (three patients with Yo antibodies, but without PCD and 11 patients without Yo antibodies), but no variants were found when compared to reference CDR2 cDNA [13]. Post-translational modifications and splicing variants could increase the immunogenicity of CDR2 and CDR2L proteins in some tumors. Our observation of double CDR2 and CDR2L bands in western blots of ovarian cancer lysates indicate different post-translational forms. In a study on the potential role of CDR2 in neurodegeneration, the upper band of a double CDR2 band in western blots of rat lysates from various organs was found to be a phosphorylated variant of the protein [12]. We did not have sufficient cancer samples to explore possible post-translational modifications of the protein in the double bands that we observed further. The rabbit CDR2L antibodies gave bands of corresponding size in ovarian cancer and cerebellum lysate. However, the CDR2 antibody gave bands at a higher molecular weight in cerebellar lysate than in ovarian cancer, and this does not correspond with equally sized proteins described in earlier publications using PCD antisera for protein detection [9]. Commercial antibodies and patient sera recognizing different epitopes in denatured proteins probably explain the difference, but PCD sera may also contain polyclonal antibodies against both CDR2 and CDR2L.
CDR2 and CDR2L proteins were also present in various normal tissues (i.e., mammary, kidney, ovary, prostate and testis tissues) by western blot and in normal endothelial, stromal, and epithelial cells in the ovary as shown by immunohistochemistry. This is in line with previous findings for the CDR2 protein [13, 20]. These observations support the hypothesis that breakage of tolerance is a crucial step in the development of PCD. Recently, it was shown that blocking CTLA4 in T cells, and thereby reducing an important inhibitory immunological signal, induced PCD in mice [30]. Since CDR2 and CDR2L proteins occur in various normal tissues, we hypothesize a central tolerance toward these proteins, as has been shown in experimental models for other onconeural proteins [31, 32]. The presence of CDR2 and CDR2L in normal tissue also raises the question of why Yo antibody-related disease is limited to the nervous system. Further studies on the function and localization of CDR2 and CDR2L proteins in various cell types are therefore needed to understand the pathogenesis of PCD.
In all patients but one, the ovarian cancer was histologically classified as a carcinoma; the one case was classified as mixed epithelial and mesenchymal (carcinosarcoma). Only two out of 16 patients had localized disease at the time of diagnosis. This corresponds well with previous data on ovarian cancer, with around 90% being of epithelial origin [33] and only 15% being confined to the primary site at the time of diagnosis [34].
In patient sera containing only anti-CDR2L, these antibodies had previously been detected with RIA, but were not confirmed with immunofluorescence and western blot in this study. The RIA index values of CDR2L antibodies were generally lower than those of co-existing CDR2 and CDR2L antibodies (data not shown), and this probably reflects lower serum levels of antibody in sera containing only CDR2L antibodies. RIA has been shown to have higher sensitivity than other laboratory modalities in detecting onconeural antibodies [4, 35, 36]. This could be explained by several factors: that radioactivity is used in detection, that the proteins are in a fluid-phase, which makes conformational epitopes available for binding, and that the patient samples are less diluted than when used in other modalities. An important point is, however, that the presence of CDR2L antibodies without concomitant CDR2 antibodies do not seem to correlate with PNS and their detection may therefore be of limited clinical value [10].
In conclusion, CDR2 and CDR2L proteins are expressed in ovarian cancers, independently of serum antibody status. CDR2 and CDR2L proteins are also expressed in other cancers and in normal tissues of various organs. Thus, our results show that CDR2 and CDR2L proteins are more widely distributed than previously described. The initiation of the immune response to these proteins must be related to several additional immunological host factors likely including breakage of tolerance.
Acknowledgements
The authors wish to commemorate late Helga Salvesen, MD Professor at the Department of Gynaecology and Obstetrics, Haukeland University Hospital, Norway and Centre for Cancer Biomarkers, Department of Clinical Science, University of Bergen, Norway, in appreciation of her great enthusiasm and knowledge. She contributed greatly to this project by including cancer samples from the Bergen Gynecologic Cancer Biobank. We also thank Miletic Hrvoje, MD PhD at the Department of Pathology, Haukeland University Hospital, Bergen and Department of Biomedicine, University of Bergen, for the reconsideration of the pathological specimens, and Manja Schubert, ScD at the Department of Neurology, Haukeland University Hospital, Bergen, for valuable advice and discussion.
Abbreviations
- CDR1
Cerebellar degeneration-related protein 1
- CDR2
Cerebellar degeneration-related protein 2
- CDR2L
Cerebellar degeneration-related protein 2-like
- CDR2L1-5
Patients with only CDR2L antibodies detected by RIA
- Control1-5
Control patients without antibodies
- FIGO
International Federation of Gynecology and Obstetrics
- HGSC
High-grade serous carcinoma
- kDa
Kilodalton
- PCD
Paraneoplastic cerebellar degeneration
- PNS
Paraneoplastic neurological syndrome
- RIA
Radioimmunoassay
- Yo1-6
Patients with Yo antibodies
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed involving human participants were approved by The Regional Committee for Health and Medical Research Ethics (2014/1066) and were in accordance with the Declaration of Helsinki (1964) and its later amendments or comparable ethical standards. Informed consent was obtained from living patients included in the study.
References
- 1.Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med. 2003;349(16):1543–1554. doi: 10.1056/NEJMra023009. [DOI] [PubMed] [Google Scholar]
- 2.Giometto B, Grisold W, Vitaliani R, Graus F, Honnorat J, Bertolini G, et al. Paraneoplastic neurologic syndrome in the PNS Euronetwork database: a European study from 20 centers. Arch Neurol. 2010;67(3):330–335. doi: 10.1001/archneurol.2009.341. [DOI] [PubMed] [Google Scholar]
- 3.Titulaer MJ, Soffietti R, Dalmau J, Gilhus NE, Giometto B, Graus F, et al. Screening for tumours in paraneoplastic syndromes: report of an EFNS task force. Eur J Neurol. 2011;18(1):19. doi: 10.1111/j.1468-1331.2010.03220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monstad SE, Storstein A, Dorum A, Knudsen A, Lonning PE, Salvesen HB, et al. Yo antibodies in ovarian and breast cancer patients detected by a sensitive immunoprecipitation technique. Clin Exp Immunol. 2006;144(1):53–58. doi: 10.1111/j.1365-2249.2006.03031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peterson K, Rosenblum MK, Kotanides H, Posner JB. Paraneoplastic cerebellar degeneration. I. A clinical analysis of 55 anti-Yo antibody-positive patients. Neurology. 1992;42(10):1931–1937. doi: 10.1212/WNL.42.10.1931. [DOI] [PubMed] [Google Scholar]
- 6.Dropcho EJ, Chen YT, Posner JB, Old LJ. Cloning of a brain protein identified by autoantibodies from a patient with paraneoplastic cerebellar degeneration. Proc Natl Acad Sci USA. 1987;84(13):4552–4556. doi: 10.1073/pnas.84.13.4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fathallah-Shaykh H, Wolf S, Wong E, Posner JB, Furneaux HM. Cloning of a leucine-zipper protein recognized by the sera of patients with antibody-associated paraneoplastic cerebellar degeneration. Proc Natl Acad Sci USA. 1991;88(8):3451–3454. doi: 10.1073/pnas.88.8.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fathallah-Shaykh H, Finizio J, Posner JB. Yo sera recognize a family of DNA binding proteins. Neurology. 1992;42(Suppl 3):415. [Google Scholar]
- 9.Corradi JP, Yang C, Darnell JC, Dalmau J, Darnell RB. A post-transcriptional regulatory mechanism restricts expression of the paraneoplastic cerebellar degeneration antigen cdr2 to immune privileged tissues. J Neurosci. 1997;17(4):1406–1415. doi: 10.1523/JNEUROSCI.17-04-01406.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eichler TW, Totland C, Haugen M, Qvale TH, Mazengia K, Storstein A, et al. CDR2L antibodies: a new player in paraneoplastic cerebellar degeneration. PLoS ONE. 2013;8(6):e66002. doi: 10.1371/journal.pone.0066002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schubert M, Panja D, Haugen M, Bramham CR, Vedeler CA. Paraneoplastic CDR2 and CDR2L antibodies affect Purkinje cell calcium homeostasis. Acta Neuropathol. 2014;128(6):835–852. doi: 10.1007/s00401-014-1351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hwang JY, Lee J, Oh CK, Kang HW, Hwang IY, Um JW, et al. Proteolytic degradation and potential role of onconeural protein CDR2 in neurodegeneration. Cell Death Dis. 2016;7(6):e2240. doi: 10.1038/cddis.2016.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Totland C, Aarskog NK, Eichler TW, Haugen M, Nostbakken JK, Monstad SE, et al. CDR2 antigen and Yo antibodies. Cancer Immunol Immunother. 2011;60(2):283–289. doi: 10.1007/s00262-010-0943-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monstad SE, Knudsen A, Salvesen HB, Aarseth JH, Vedeler CA. Onconeural antibodies in sera from patients with various types of tumours. Cancer Immunol Immunother. 2009;58(11):1795–1800. doi: 10.1007/s00262-009-0690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Storstein A, Monstad SE, Haugen M, Mazengia K, Veltman D, Lohndal E, et al. Onconeural antibodies: improved detection and clinical correlations. J Neuroimmunol. 2011;232(1–2):166–170. doi: 10.1016/j.jneuroim.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Kurman RJ, Carcangiu ML, Herrington CS, Young RH, editors. WHO classification of tumours of female reproductive organs. 4. Geneva: WHO Press; 2014. [Google Scholar]
- 17.Prat J. Oncology FCoG. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet. 2014;124(1):1–5. doi: 10.1016/j.ijgo.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Furneaux HM, Rosenblum MK, Dalmau J, Wong E, Woodruff P, Graus F, et al. Selective expression of Purkinje-cell antigens in tumor tissue from patients with paraneoplastic cerebellar degeneration. N Engl J Med. 1990;322(26):1844–1851. doi: 10.1056/NEJM199006283222604. [DOI] [PubMed] [Google Scholar]
- 19.Darnell JC, Albert ML, Darnell RB. Cdr2, a target antigen of naturally occuring human tumor immunity, is widely expressed in gynecological tumors. Cancer Res. 2000;60(8):2136–2139. [PubMed] [Google Scholar]
- 20.Balamurugan K, Luu VD, Kaufmann MR, Hofmann VS, Boysen G, Barth S, et al. Onconeuronal cerebellar degeneration-related antigen, Cdr2, is strongly expressed in papillary renal cell carcinoma and leads to attenuated hypoxic response. Oncogene. 2009;28(37):3274–3285. doi: 10.1038/onc.2009.186. [DOI] [PubMed] [Google Scholar]
- 21.O’Donovan KJ, Diedler J, Couture GC, Fak JJ, Darnell RB. The onconeural antigen cdr2 is a novel APC/C target that acts in mitosis to regulate c-myc target genes in mammalian tumor cells. PLoS ONE. 2010;5(4):e10045. doi: 10.1371/journal.pone.0010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matschke J, Kromminga A, Erbersdobler A, Lamszus K, Anders S, Kofuncu E. Paraneoplastic cerebellar degeneration and anti-Yo antibodies in a man with prostatic adenocarcinoma. J Neurol Neurosurg Psychiatry. 2007;78(7):775–777. doi: 10.1136/jnnp.2006.112961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosine N, Chretien P, Adam C, Beaudonnet G, Not A, Drai J, et al. Expression of Yo antigen in a prostatic adenocarcinoma. Can J Neurol Sci. 2017;44(2):221–223. doi: 10.1017/cjn.2016.400. [DOI] [PubMed] [Google Scholar]
- 24.Storstein A, Raspotnig M, Vitaliani R, Giometto B, Graus F, Grisold W, et al. Prostate cancer, Hu antibodies and paraneoplastic neurological syndromes. J Neurol. 2016;263(5):1001–1007. doi: 10.1007/s00415-016-8090-7. [DOI] [PubMed] [Google Scholar]
- 25.Coronella-Wood JA, Hersh EM. Naturally occurring B-cell responses to breast cancer. Cancer Immunol Immunother. 2003;52(12):715–738. doi: 10.1007/s00262-003-0409-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3(11):991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 27.Albert ML, Darnell RB. Paraneoplastic neurological degenerations: keys to tumour immunity. Nat Rev Cancer. 2004;4(1):36–44. doi: 10.1038/nrc1255. [DOI] [PubMed] [Google Scholar]
- 28.de Graaf MT, de Beukelaar JW, Haasnoot GW, Levering WH, Rogemond V, Didelot A, et al. HLA-DQ2+ individuals are susceptible to Hu-Ab associated paraneoplastic neurological syndromes. J Neuroimmunol. 2010;226(1–2):147–149. doi: 10.1016/j.jneuroim.2010.05.035. [DOI] [PubMed] [Google Scholar]
- 29.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191(3):423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yshii LM, Gebauer CM, Pignolet B, Mauré E, Quériault C, Pierau M, et al. CTLA4 blockade elicits paraneoplastic neurological disease in a mouse model. Brain. 2016;139(11):2923–2934. doi: 10.1093/brain/aww225. [DOI] [PubMed] [Google Scholar]
- 31.Blachere NE, Orange DE, Santomasso BD, Doerner J, Foo PK, Herre M, et al. T cells targeting a neuronal paraneoplastic antigen mediate tumor rejection and trigger CNS autoimmunity with humoral activation. Eur J Immunol. 2014;44(11):3240–3251. doi: 10.1002/eji.201444624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calzascia T, Loh JM, Di Berardino-Besson W, Masson F, Guillaume P, Burkhardt K, et al. Peripheral tolerance limits CNS accumulation of CD8 T cells specific for an antigen shared by tumor cells and normal astrocytes. Glia. 2008;56(15):1625–1636. doi: 10.1002/glia.20715. [DOI] [PubMed] [Google Scholar]
- 33.Prat J. New insights into ovarian cancer pathology. Ann Oncol. 2012;23(Suppl 10):x111–x117. doi: 10.1093/annonc/mds300. [DOI] [PubMed] [Google Scholar]
- 34.Bethesda MD (2016) SEER cancer stat facts: ovarian cancer. National Cancer Institute. https://seer.cancer.gov/statfacts/html/ovary.html. Accessed 5 Nov 2016
- 35.Monstad SE, Vedeler CA. An immunoprecipitation assay for the detection of onconeural antibodies. Acta Neurol Scand Suppl. 2006;183:71–72. doi: 10.1111/j.1600-0404.2006.00622.x. [DOI] [PubMed] [Google Scholar]
- 36.Storstein A, Monstad SE, Nakkestad HL, Husebye ES, Vedeler CA. Paraneoplastic antibodies against HuD detected by a sensitive radiobinding assay. J Neurol. 2004;251(2):197–203. doi: 10.1007/s00415-004-0303-9. [DOI] [PubMed] [Google Scholar]