Abstract
Autologous glioblastoma multiforme tumor cells treated with an antisense oligodeoxynucleotide (AS-ODN) targeting insulin-like growth factor receptor-1 (IGF-1R) are the basis of a vaccine with therapeutic effects on tumor recurrence in a pilot clinical trial. As a preface to continued clinical investigation of this vaccination strategy, we have studied the contribution of an optimized IGF-1R AS-ODN, designated “NOBEL”, to the induction of immunity to mouse GL261 glioma cells. The impact of NOBEL on mechanisms contributing to the development of GL261 immunity was first examined in the periphery. GL261 cells are naturally immunogenic when implanted into the flanks of congenic C57BL/6 mice, immunizing rather than forming tumors in around 50 % of these animals but causing tumors in the majority of mice lacking T and B lymphocytes. Overnight treatment with NOBEL in vitro reduces IGF-1R expression by GL261 cells but has minimal effect on cell viability and does not reduce the capacity of the cells to form tumors upon implantation. In contrast, tumors are extremely rare when GL261 cells are mixed with NOBEL at inoculation into the flanks of C57BL/6, and the recipient mice become immune to subcutaneous and intracranial challenge with untreated GL261. Adaptive immune mechanisms contribute to this effect, as immunocompromised mice fail to either fully control tumor formation or develop immunity following flank administration of the GL261/NOBEL mix. NOBEL’s structure has known immunostimulatory motifs that likely contribute to the immunogenicity of the mix, but its specificity for IGF-1R mRNA is also important as a similarly structured sense molecule is not effective.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-015-1654-z) contains supplementary material, which is available to authorized users.
Keywords: Glioma, Immunity, GL261, Vaccine, IGF-1R antisense
Introduction
Grade IV astrocytomas, or glioblastoma multiforme (GBM), are the most common primary brain neoplasm [1]. The tumors are invasive within the central nervous system (CNS) and, despite aggressive treatment generally consisting of surgical resection followed by radiation and chemotherapy, the median survival is only 18 months [2]. The inadequacy of conventional treatment for GBM has stimulated investigations of alternative therapeutic approaches, including immunotherapy. Due to the specificity of the immune response and the potential of long-term surveillance against tumor cells, the prospect of eliciting an immune response against tumor antigens is an attractive approach for cancer treatment [4]. Immunotherapies for various cancers are already approved by the FDA [5], and others are currently being assessed in clinical trials [6]. CNS tissues represent an additional challenge for immunotherapy as they are protected from circulating immune cells and factors by the blood–brain barrier (BBB) and do not have a lymphatic system. This immunological privilege may be expected to hinder an immune response to GBM [7] and, despite experiments in rodent models demonstrating brain tumor immunity [8, 9], clinical trials in human GBM have largely met with only limited success [4, 10]. The choice of immunogen is clearly an important aspect of these trials. The strategy that we are investigating in Phase 1 clinical trials uses a vaccine consisting of autologous tumor cells recovered at surgery that have been treated with an antisense oligodeoxynucleotide targeting type 1 insulin-like growth factor receptor (IGF-1R/AS-ODN) [11]. IGF-1R is a tyrosine kinase cell surface receptor that regulates cellular functions including proliferation, transformation and cell survival [12]. Involved in tumorigenesis and protection against apoptosis, IGF-1R is viewed as a promising target for antitumor therapies [13, 14] and was originally studied for its effects on the growth of tumor cells, including gliomas [15]. Tumor cells in which IGF-1R has been targeted by antisense or small interfering RNA strategies have proven to be immunogenic in both rat glioma and mouse breast cancer models [16, 17]. Studies demonstrating the immunogenicity of rat C6 glioma cells treated with IGF-1R/AS-ODN [30] led to the development of an autologous GBM vaccine investigated in a pilot clinical trial [11]. In that study, patients were immunized shortly after resection surgery by 24-h implantation in the rectus sheath of 0.1 μm porous chambers containing irradiated, IGF-1R/AS-ODN-treated autologous tumor cells. Eight out of 12 of the treated individuals showed clinical improvements consistent with the activity of immune mechanisms [11].
Tumor cells treated with IGF-1R/AS-ODN may be immunogenic as a consequence of apoptosis and antigen release. In addition, the structure of an AS-ODN may contribute to immunogenicity due to the presence of phosphorothioate linkages used to render the molecule resistant to nuclease activity [18, 19], CpG motifs [20], guanosine dinucleotide [21–23] and palindromic motifs [24]. The objective of this study was to establish the mechanisms through which a novel IGF-1R/AS-ODN (NOBEL) used in GBM clinical trials promotes glioma immunity using the mouse GL261 model.
Materials and methods
Oligodeoxynucleotides
A two codon downstream shift of the IGF-1R AS-ODN designated “DWA” used in our initial clinical trial [11] rendered “NOBEL”, a molecule with an improved growth inhibition to cytotoxicity ratio [25]. The sequence of this phosphorothioate-linked AS-ODN (Supplemental Fig. 1) is complementary to codon 2–7 of IGF-1R mRNA with its target conserved between mouse and human IGF-1R mRNA. NOBEL was synthesized by Girindus America and NOBEL-Sense by Integrated DNA Technologies.
Mice and tumor cell implantation
Male wild-type and knock-out mice on a C57BL/6 background, 8–10 weeks of age at experiment start, were obtained from either Taconic Farms Inc. (C57BL/6 and B2M−/−), the Jackson Laboratory (C57BL/6 and Rag2−/−), or from in-house breeding of mice from the Jackson Laboratory (JHD−/−). Mice were anesthetized with isoflurane and inoculated subcutaneously in the right flank with 106 GL261 cells, untreated, treated overnight or mixed with NOBEL, in 100 µl phosphate-buffered saline (PBS) or stereotactically into the right cerebral cortex (1 mm anterior to the bregma and 1 mm to the right of the midline, at a depth of 3 mm) with 105 untreated GL261 cells in 2 µl PBS. Challenge consisted of the implantation of untreated GL261 cells, either 106 in the opposite flank or 105 in the right cerebral cortex as above. Blood samples were collected from the orbital plexus of mice under anesthesia. Tumor volume (V) (mm3) was calculated as V = W 2 × L/2 where W is the width in mm and L is the length in mm as determined using a caliper. All animal breeding and experimentation was performed under protocols approved by the Institutional Animal Care and Use Committee of Thomas Jefferson University.
GL261 and NOBEL treatment
GL261, a glioma cell line originating from the brain of a C57BL/6 mouse [26, 27], was obtained from the Division of Cancer Treatment and Diagnosis Tumor Repository of the National Cancer Institute. Cells were expanded in RPMI medium containing 10 % heat-inactivated fetal bovine serum (∆FBS, Mediatech) at 37 °C in 5 % CO2. Treatment with NOBEL was conducted in serum-free Opti-MEM (Gibco). Both media were supplemented with 4 mM l-glutamine (Gibco), 50 μg/ml gentamicin (Gibco) and 0.05 mM 2-mercaptoethanol (Sigma-Aldrich). NOBEL/GL261 mix consisted of 106 GL261 cells mixed with 4 mg of NOBEL immediately prior to the injection in a volume of 100 μl PBS. For viability assessment, GL261 cells were incubated at 2 × 104 per well in flat bottom 96-well plates (Fisher Scientific) containing 100 μl of serum-free Opti-MEM and NOBEL or NOBEL-Sense for 24 h then stained with SYTOX® (0.5 mM, Invitrogen) for 15 min before analysis by flow cytometry (EasyCyte 8HT, Millipore).
Real-time quantitative reverse-transcription polymerase chain reaction (qRT-PCR) analysis of mRNA expression
Levels of IGF-1R mRNA in GL261 cells cultured with NOBEL and NOBEL-Sense for 24 h as described above were determined by qRT-PCR as previously described and normalized to expression of the housekeeping gene L13a [28]. Primers and probes were designed using the Web Primer3 program and purchased from Integrated DNA Technologies: Housekeeping ribosomal gene L13a Forward 5-TGGAAGTACCAGGCAGTGACA-3′; Reverse 5′-GCCTGTTTCCGTAACCTCAAG-3′, probe 5′-FAM-AACGGAAGGAAAAGGCCAAGATGCAC-3′; IGF-1R Forward 5′-CGACGAGTGGAGAAATCTGTG-3′, Reverse 5′-ATGAGCAGGATGTGGAGGAA-3′ and probe 5′-FAM-CATTGACATCCGCAACGACTATCAGC-BHQ1-3′.
Cell processing, staining and flow cytometry
Draining and contralateral non-draining lymph nodes from C57BL/6 mice injected with either NOBEL, NOBEL-Sense or GL261 cells mixed with NOBEL 24 or 48 h previously were mechanically disrupted and digested with collagenase (1 mg/ml) and DNase (0.02 mg/ml, both from Sigma) in PBS for 20 min at 37 °C. The cell suspension was passed through a 70-mm nylon mesh strainer and washed with PBS prior to the staining with fluorescently labeled monoclonal antibodies specific for mouse B220 (RA3-6B2) CD19 (1D3), CD86 (GL1), Gr-1 (RB6-8C5) and PDCA-1 (CD317/BST2/Tetherin) (all from BD Biosciences) and analysis by flow cytometry. For flow cytometric analysis of IGF-1R expression by NOBEL and NOBEL-Sense-treated GL261, cells were treated for 48 h in culture as described above and then stained for 1 h at 4 ºC with mouse anti-IGF-1R PE-conjugated antibody (1H7, BD Biosciences). GL261-specific antibodies in sera were detected by incubating the cells in sera from immunized and non-immune mice for 1 h followed by staining for 1 h at 4 ºC with goat anti-mouse IgG-FITC and IgM-TRITC (SouthernBiotech) and flow cytometry. Isotype controls were used for all staining. Fluorescence was assessed on a BD LSRII system (BD Bioscience) and data analysis performed using FlowJo software (Tree Star Inc.). For immunofluorescent analysis of brain tissue sections, C57BL/6 mice, either naïve or immunized by the administration of GL261 in the flank, were stereotactically implanted with 105 GL261 cells in 2 μl PBS in the right cerebral cortex, transcardially perfused 12 days later, and cortical brain tissue snap-frozen in Tissue-Tek® freezing medium (Sakura Finetek USA). Sections along the track of the implantation were prepared and stained with the nuclear DAPI stain, anti-mouse CD4 (RM4-5, BD Biosciences) as well as anti-mouse Igκ (187.1, BD Bioscience) and then with Alexa488 or Alexa 633-conjugated secondary antibodies (Invitrogen). Slides were imaged with a Nikon S1 Plus laser confocal microscope (Nikon), and montages were assembled and annotated in Photoshop (Adobe).
Cell-based ELISA
GL261 cells were plated at 2 × 104 in round-bottom 96-well plates (Nalge Nunc), incubated overnight in growth medium then washed in buffer consisting of Hanks balanced salt solution with 1 % ΔFBS and 0.05 % sodium azide (Sigma-Aldrich). Mouse sera diluted in wash buffer was added for 2 h at 37 ºC followed by 1 h at 37 ºC with alkaline phosphatase-conjugated anti-mouse IgM (1:5,000), IgG1 (1:2,000), IgG2a (1:2,000), IgG2b (1:2,000) or IgG3 (1:1,000) (all from MP Biomedicals). Secondary antibodies were detected using nitrophenyl phosphate disodium salt hexahydrate substrate (Sigma-Aldrich) read by absorbance at 405 nm using a Kinetic Reader (Bio-Tek Instruments).
Statistical analysis
Each experiment was replicated at least twice with either cumulative survival results or findings from representative studies being presented. Graphs were plotted, and statistics assessed using Prism 5.0 (GraphPad Software, Inc.) or FlowJo. For mouse experiments, all survival curves were statistically assessed with the Mantel-Cox test. For all cell experiments, the Kruskal–Wallis test followed by Dunn’s post hoc test was used to assess differences in study parameters between groups.
Results
In vitro treatment of GL261 with NOBEL IGF-1R/AS-ODN inhibits IGF-1R expression with minimal cytotoxicity
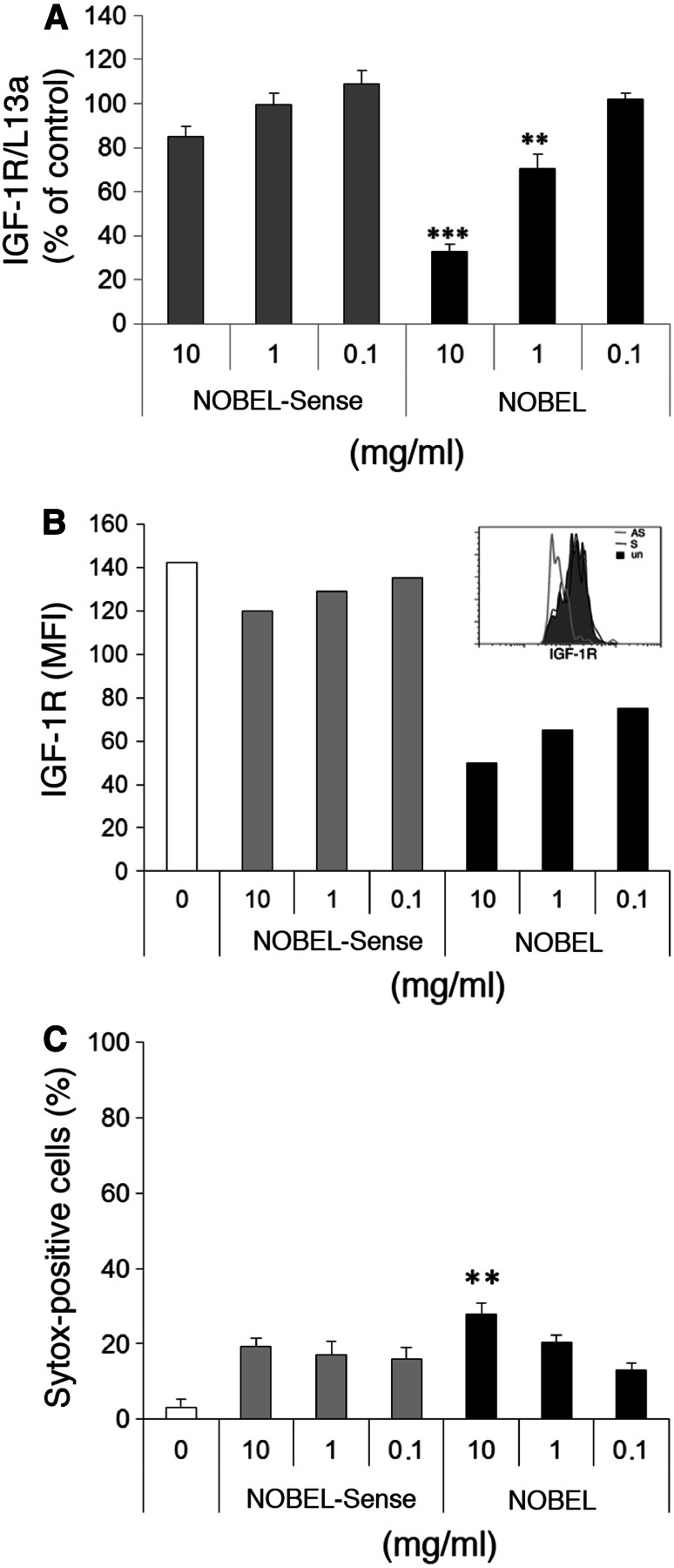
Twenty-four hour treatment of GL261 cells with NOBEL in vitro resulted in a statistically significant, dose-dependent reduction in the levels of IGF-1R mRNA at 10 mg and 1 mg per 2 × 104 cells in 200 μl, while similar treatment with NOBEL-Sense had no effect (Fig. 1a). Forty-eight hours after NOBEL addition, IGF-1R expression by the cells, assessed by flow cytometry as the mean fluorescence intensity (MFI) of bound IGF-1R-specific fluorescent antibody, was reduced at 10, 1 and 0.1 mg (Fig. 1b). NOBEL-Sense treatment had negligible effects. To determine whether the reduced expression of IGF-1R impacts cell survival, we measured GL261 cell viability with Sytox®, a nucleic acid stain that penetrates cells with compromised membranes, 24 h after treatment with NOBEL or NOBEL-Sense. A slight, but statistically significant increase in cell death over untreated cells was seen following treatment with a range of doses of either NOBEL or NOBEL-Sense (Fig. 1c). However, only 10 mg of NOBEL elicited a relatively minor impact on cell viability that was increased over the other treatment groups.
Fig. 1.
Treatment of GL261 cells with NOBEL inhibits the expression of IGF-1R with minimal cytotoxicity. a IGF-1R mRNA levels in GL261 cells determined by qRT-PCR following 24-h treatment with the indicated mg quantities of NOBEL and NOBEL-Sense. Results are shown as copy numbers of IGF-1R, normalized to L13a, compared with untreated cells. b GL261 cells, incubated with and without the indicated mg quantities of NOBEL or NOBEL-Sense for 48 h, were stained with IGF-1R-specific antibody and analyzed by flow cytometry. Results are expressed in mean fluorescence intensity (MFI) with a representative histogram of IGF-1R expression after NOBEL or NOBEL-Sense incubation at 1 mg for 48 h shown in the inset. c Cytotoxicity was determined by the Systox+ assay following 24-h incubation with and without the indicated mg quantities of NOBEL and/or NOBEL-Sense. Statistical significance was assessed by the Kruskal–Wallis test followed by Dunn’s post hoc test (**p < 0.005; ***p < 0.001)
The addition of NOBEL enhances the inherent immunogenicity of GL261 cells implanted in the flank
Initial studies revealed that approximately 28 days were required for GL261 immunity to fully develop following the administration of a single dose of NOBEL-treated GL261 cells to the flanks of C57BL/6 mice (Supplemental Fig. 2) but did not allow us to conclude whether NOBEL was acting on the GL261 cells in vitro or in vivo. To resolve this issue, we performed parallel experiments with GL261 that had been either pretreated with NOBEL and washed prior to the injection or merely mixed with NOBEL immediately before being injected into the right flanks of C57BL/6 mice. Neither overnight treatment with NOBEL nor NOBEL-Sense treatment had any effect on tumor incidence which was approximately 60 % in both groups of mice by 30 days post-implantation (Fig. 2a). However, when 4 mg of NOBEL was mixed with 106 GL261 cells prior to the injection into the flanks of congenic mice, only 8 % of mice grew tumors (Fig. 2b). Within 33 days of implantation, 55 % of mice that received untreated GL261 and 45 % of mice given GL261 mixed with NOBEL-Sense had developed tumors. Having arisen in the CNS, GL261 may be naturally immunogenic in the periphery of congenic mice, and this may be enhanced by the presence of NOBEL. As a first test of this hypothesis, mice that failed to grow tumors by 35 days after their initial inoculation were challenged in the opposite flank with 106 untreated GL261 cells. This rarely resulted in tumor development (Fig. 2a, b).
Fig. 2.
Mixing GL261 with NOBEL immediately prior to the injection is more effective than overnight treatment at inhibiting growth of the cells in the flanks of C57BL/6 mice. At day 0, C57BL/6 mice received 106 GL261 cells treated overnight with either NOBEL (n = 42) or NOBEL-Sense (n = 20) or untreated (n = 40) (a) or 106 GL261 cells mixed immediately prior to the administration with either NOBEL (n = 41) or NOBEL-Sense (n = 20) or untreated (n = 42) (b) in the right flank. Tumor incidence was monitored for 35 days. At day 35, mice that failed to develop tumors as well as nine naïve controls (PBS) were then given untreated GL261 cells in the opposite flank and monitored for an additional 45 days. The time to palpable tumor detection is expressed as a Kaplan–Meier plot of the percentage of tumor-free mice. Tumor incidence in mice that received cells treated with NOBEL significantly differed from that seen in both mice injected with untreated and NOBEL-Sense treated cells as determined by the log-rank test (***p < 0.001)
NOBEL is immunostimulatory but only effective in preventing tumor development when co-administered with GL261 cells
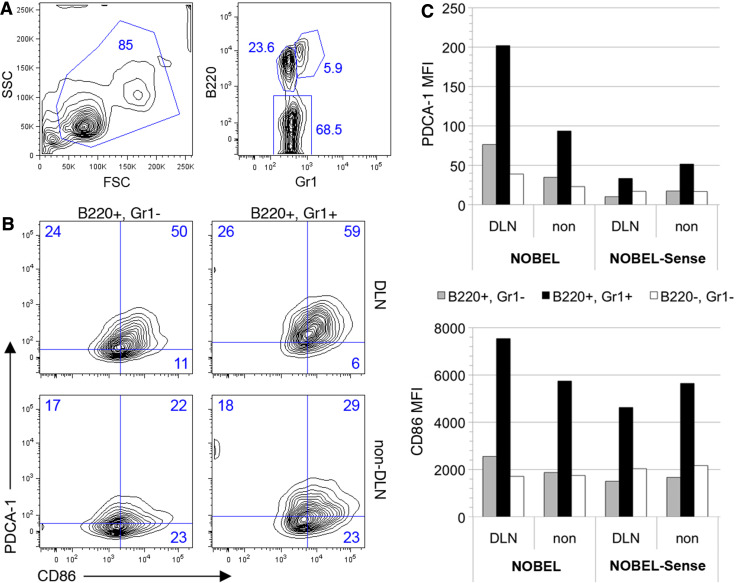
NOBEL and NOBEL-Sense both have identical structural motifs known to stimulate antigen presenting cells (APC) through Toll-like receptors and other ligands. To assess the impact of these molecules on APC activation in vivo, mice were given NOBEL or NOBEL-Sense in the flank, and cells recovered from draining and contralateral, non-draining lymph nodes 48 h later for analysis by flow cytometry (Fig. 3). B cells (B220+Gr1−; CD19+, data not shown) and plasmacytoid dendritic cells (B220+Gr1+; CD19−; data not shown) from the lymph nodes draining the NOBEL-treated flank showed elevated expression of the activation marker PDCA1 and the costimulatory antigen CD86 (Fig. 3b) which was not seen in similar cells from NOBEL-Sense-treated animals (Fig. 3c). PDCA1 was also expressed at moderately higher levels by a subset of B220− cells (predominantly T cells, with minor proportions of NK and conventional dendritic cells) (Fig. 3c). The inclusion of GL261 cells with NOBEL does not diminish this response (Supplemental Fig. 3). NOBEL evidently acts through a local effect as tumor development is unaffected when NOBEL and GL261 are administered to C57BL/6 mice in opposite flanks (Fig. 4).
Fig. 3.
NOBEL non-specifically activates cells in draining lymph node. Cells were recovered from draining and non-draining lymph node 48 h after inoculation of 4 mg NOBEL or NOBEL-Sense into the flank of C57BL/6 mice and analyzed by flow cytometry. a Flow contour plot showing the scatter characteristics and B220/Gr1 staining characteristics of lymph node cells. b Flow contour plots for PCDA-1 and CD86 expression by B220+Gr1+ and B220+Gr1− cells in draining (DLN) and non-draining lymph nodes of NOBEL-treated mice. Numbers in A and B represent the cell percentages in each gate. c Mean fluorescent index (MFI) of B220+Gr1+, B220+Gr1− and B220−Gr1− cells from the draining and non-draining lymph nodes of mice given NOBEL and NOBEL-Sense
Fig. 4.
NOBEL must be co-administered with GL261 cells to prevent tumor formation. Tumor incidence was monitored for 60 days after injection in C57BL/6 mice of either untreated GL261 cells or a mix of GL261 cells and NOBEL in the right flank, or NOBEL in left flank and untreated GL261 in right flank. The time to palpable tumor detection, expressed as the percentage of tumor-free mice, is presented as a Kaplan–Meier plot. The statistical significance of differences in tumor growth between mice receiving untreated GL261 and the other groups of mice was assessed using the log-rank test (*p < 0.05)
The reduced tumorigenicity of GL261 cells mixed with NOBEL in the flank is associated with the development of immunity to subcutaneous and intracranial challenge with untreated GL261
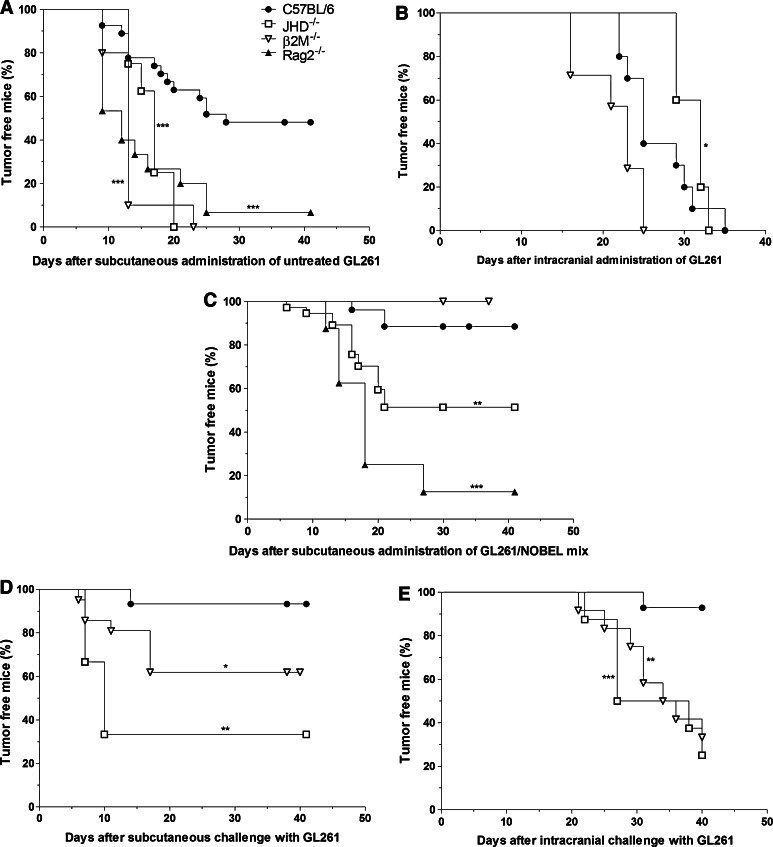
Consistent with the hypothesis that the failure of GL261 to form tumors when implanted in the flank of a proportion of C57BL/6 mice is a consequence of immune mechanisms, tumor incidence following flank injection of untreated GL261 is close to 100 % in congenic gene-knockout mice lacking both T and B cells (Rag2−/−), CD8 T cells (B2M−/−) or B cells (JHD−/−) (Fig. 5a) and in the cerebral cortex of B2M−/−, JHD−/− as well as C57BL/6 mice (Fig. 5b). The inclusion of NOBEL in a mix with GL261 upon inoculation into the flank results in reduced tumor formation in C57BL/6, B2M−/− and, to a lesser extent, in JHD−/− mice but not in the more severely immunocompromised Rag2−/− mice (Fig. 5c). To establish whether or not the antitumor effects of NOBEL are mediated by the induction of adaptive immune mechanisms, mice without palpable tumors at 30 days were challenged in either the opposite flank or intracranially with untreated GL261. A large proportion of the B2M−/− mice (40 %) and the majority of the few remaining JHD−/− mice developed tumors from GL261 cells implanted in the opposite flank, unlike similarly treated C57BL/6 mice where the incidence was less than 10 % (Fig. 5d). However, only the C57BL/6 mice were generally protected against the intracranial challenge (Fig. 5e). It should be noted here that, consistent with the clinical vaccination paradigm (11) where surgery provides immune access across the BBB, the needle stick when GL261 cells are implanted in the cortex evidently allows immune cells to enter brain tissues (Supplemental Fig. 4).
Fig. 5.
Innate immune mechanisms in B2M−/− mice are sufficient to prevent the growth of GL261 cells mixed with NOBEL but both cellular and humoral mechanisms are required to prevent flank and brain tumor formation by untreated GL261 cells. The development of tumors following the implantation of untreated GL261 into the flanks and cerebral cortices of normal and immunodeficient mice is compared in a and b. a 106 Untreated GL261 cells were implanted into the right flanks of C57BL/6 (n = 27), and congenic B2M−/− (n = 10), JHD−/− (n = 9) and Rag2−/− (n = 15) mice. b 105 untreated GL261 cells were stereotactically implanted into the right cortex of C57BL/6 (n = 10), congenic B2M−/− (n = 5) and JHD−/− (n = 7) mice. Tumor development in these mice following flank administration of a mix of NOBEL and GL261 cells is shown in c and animals that failed to develop a tumor were then challenged with untreated GL261 either in the opposite flank (d) or intracranially (e). c B2M−/− (n = 20), JHD−/− (n = 33) and C57BL/6 (n = 26) mice received 106 GL261 mixed with NOBEL prior to the implantation in the right flank. d C57BL/6 (n = 15), B2M−/− (n = 20), JHD−/− (n = 3) mice that had failed to grow a palpable tumor by 29 days after the implantation of 106 GL261 mixed with NOBEL into the right flank were given 106 untreated GL261 cells in the left flank. e C57BL/6 (n = 14), B2M−/− (n = 8), JHD−/− (n = 12) mice that had failed to grow a tumor by 30 days after the implantation of 106 GL261 mixed with NOBEL into the right flank were given 105 untreated GL261 cells in the right cerebral cortex. In a and c, mice were monitored for either 29 or 41 days post-cell implantation, and in d for 41 days post-challenge inoculation. In b and e, mice were monitored at 1–4 day intervals until clinical signs of tumor development or for 40 days. The time to palpable tumor in flank experiments or the development of severe neurological signs/morbidity in brain implantation experiments, both expressed as the percentage of tumor-free mice, is presented as a Kaplan–Meier plot. The statistical significance of differences between C57BL/6 mice and the different gene deficient mice was assessed using the Mantel-Cox test and is expressed by *p < 0.05; **p < 0.01; ***p < 0.001
C57BL/6 mice that reject GL261 produce GL261-reactive antibodies
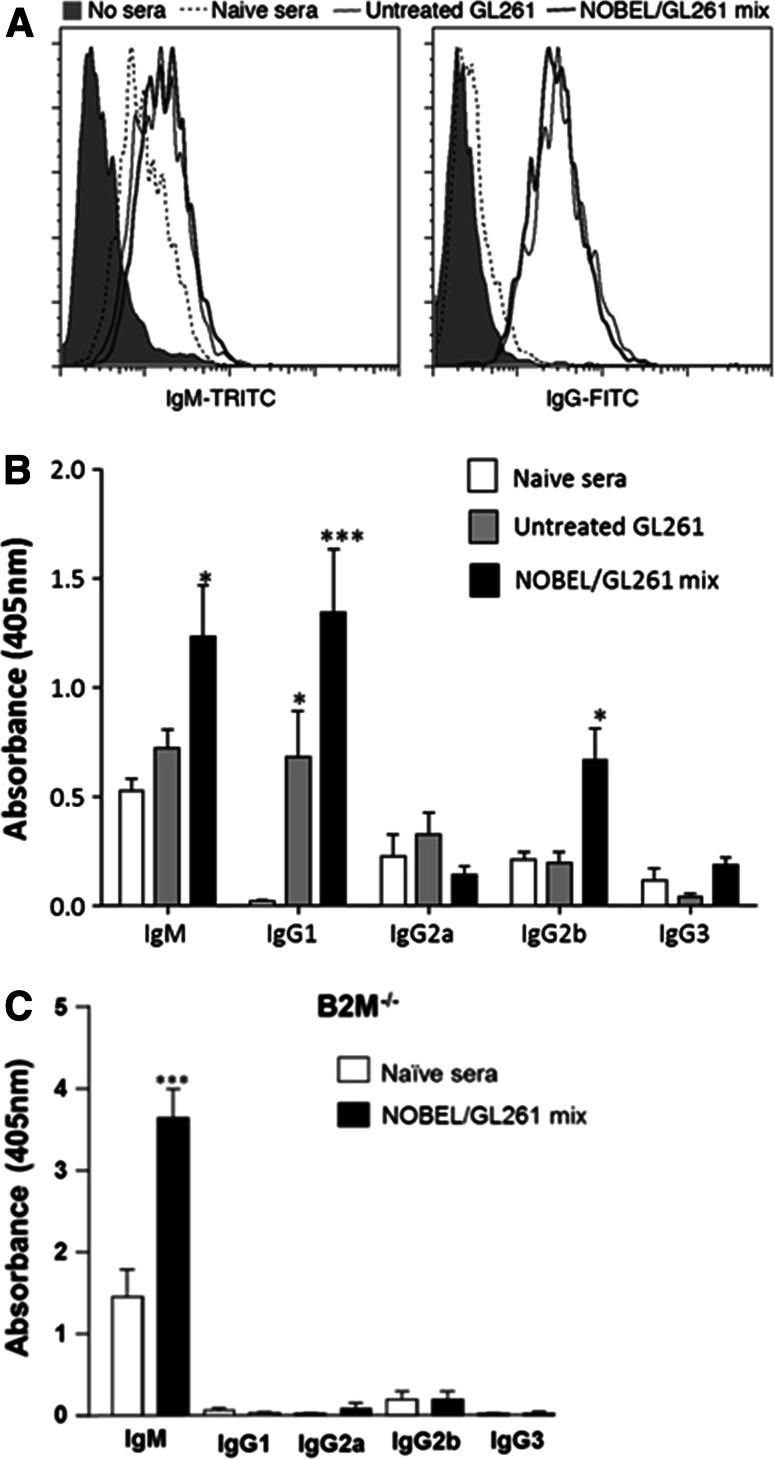
The reduced capacity of JHD−/− mice to prevent tumor development after flank injection of GL261 suggests a possible humoral contribution to glioma immunity. As an initial test of this possibility, we used flow cytometry to determine whether sera from GL261-immune mice contain antibodies reactive for the tumor cells. We found GL261-reactive IgM antibodies in sera from both naïve mice and those that had received GL261 or the GL261/NOBEL mix but tumor cell-reactive IgG antibodies only in sera from the two latter groups (Fig. 6a). To characterize this response further, we used cell-based ELISA with GL261 cells as the capture substrate (Fig. 6b). GL261-reactive IgM antibodies were found at equivalent levels in sera from naïve and GL261-inoculated C57BL/6 mice and at somewhat elevated levels in sera from mice given the GL261/NOBEL mix. GL261-reactive IgG1 antibodies, undetectable in sera from normal mice, were detected at increasing levels in sera from C57BL/6 mice that had received untreated GL261 and the GL261/NOBEL mix. An IgG2b response was only observed in sera from mice that had received the GL261/NOBEL mix. No GL261-reactive IgG2a or IgG3 antibodies were seen. Finally, we assessed serum from B2M−/− mice given the GL261/NOBEL mix 30 days previously finding levels of IgM GL261-specific antibodies that were strongly elevated over those of naïve mice but no evidence of any GL261-specific IgG antibodies (Fig. 6c).
Fig. 6.
GL261-reactive antibodies are elicited by the administration of GL261 cells in the flank, and this response is enhanced by the inclusion of NOBEL with the cells. a GL261 cells were incubated with pooled sera from naïve C57BL/6 mice or mice that had received either untreated 106 GL261 cells or the GL261/NOBEL mix 30 days prior to the sera collection and then stained with fluorescent antibodies specific for IgM and IgG prior to the analysis by flow cytometry. The results are expressed as histograms. b CELISA with isotype-specific secondary antibodies were used to characterize the GL261-reactive antibodies present in individual serum samples from untreated mice and animals that had received untreated GL261 cells or a mix of the cells and NOBEL 30 days previously. The mean and standard deviation of the optical density at 405 nm for a 1/10 dilution of sera from groups of 10 mice each are shown. c Sera from 10 B2M−/− mice prior to and 30 days following implantation with the GL261/NOBEL mix was assessed, and the results presented as described for b. The significance of differences between the results for naïve and test sera was assessed using the Kruskal–Wallis test followed by Dunn’s post hoc test (*p < 0.05; ***p < 0.001)
Discussion
The first suggestion that targeting the IGF-1 pathway may have immunostimulatory consequences for glioma cells came from the studies of Resnicoff et al. [29] showing that C6 cells transfected to express antisense for IGF-1R induce a C6-specific immune response when injected into syngeneic normal rats. Immune stimulation was thought to be a consequence of immunogens released by apoptotic cells [30, 31]. Simply treating tumor cells with IGF-1R/AS-ODN for 24 h proved to effectively reduce IGF-1R expression and cause extensive tumor cell death when the treated cells were implanted in biodiffusion chambers in vivo [29]. These studies culminated in a pilot human clinical trial involving chamber-encapsulated, IGF-1R/AS-ODN-treated autologous GBM cells as a vaccine [11]. While tumor cell apoptosis may contribute to the efficacy of IGF-1R/AS-ODN-treated autologous GBM cell vaccine, the immunomodulatory motifs within the antisense oligodeoxynucleotide may also be important. For NOBEL, these motifs include a CpG motif and a palindromic sequence, which both drive innate immune responses in a variety of immune cells when recognized by toll-like receptor 9 (TLR9) [32–34], and phosphorothioate linkages that induce B cell proliferation and increase the production of cytokines such as IFN-γ [35]. These motifs likely contribute non-specifically to glioma immunity as the intracerebral administration of a non-specific CpG–ODN several days after GL261 glioma cell implantation into mouse brain resulted in T cell infiltration into the brain, local activation of NK cells and partial protection against tumor growth [36, 37].
Not surprisingly due to their CNS origin, GL261 cells possess inherent immunogenicity when administered in the periphery, but invariably form tumors when injected intracranially in congenic C57BL/6 mice [26, 27, 38]. In this study, we have focused on understanding the impact of NOBEL treatment on the tumorigenicity and immunogenicity of GL261 cells implanted in the flank due to the relevance of the periphery to mechanisms contributing to the induction of immunity. Twenty-four hour incubation of GL261 with a concentration of NOBEL that reduces IGF-1R expression but has minimal effect on cell viability in culture has no effect on tumor formation if the antisense is washed out prior to the implantation of the cells. However, adding the same dose of NOBEL to GL261 cells at injection largely prevents tumor formation in normal mice. While structurally similar to NOBEL-Sense, only NOBEL non-specifically activates cells in the lymph nodes draining its site of administration. Plasmacytoid dendritic cells and B cells appear to be particularly sensitive to NOBEL as evidenced by their enhanced expression of PDCA-1. Consistent with a local effect that impacts both immunity and tumor cell growth, only the presence of NOBEL at the site of inoculation alters the outcome of GL261 implantation. Tumor growth is unaltered by either mixing NOBEL-Sense with GL261 cells or administering NOBEL to the flank opposite of where the tumor cells were implanted. Thus, we conclude that the specificity of NOBEL for IGF-1R contributes to its capacity to render GL261 cells less tumorigenic.
GL261 cells generally form tumors when implanted into the flanks of severely immunocompromised congenic Rag-2−/− mice yet fail to form tumors in a substantial proportion of C57BL/6 mice. This is evidently due to the induction of an immune response against the cells in the latter animals as mice that do not grow tumors upon initial GL261 implantation are protected against tumor growth when the cells are implanted in the opposite flank. The fact that the presence of NOBEL does not alter the capacity of GL261 cells to form tumors in Rag-2−/− mice indicates that the antisense is not simply cytotoxic. As tumor incidence is greatly reduced in C57BL/6 but not in Rag-2−/− mice, the addition of NOBEL to GL261 cells upon implantation in the flank evidently alters the balance between tumorigenicity and immunogenicity.
The general consensus is that CD8 T cells are the principal effectors of glioma immunity [38, 39]. However, GL261 cells generally fail to form tumors when mixed with NOBEL and inoculated into the flanks of B2M−/− mice missing both CD8 T and NKT cells. Nevertheless, when these mice are challenged with untreated GL261 in the opposite flank approximately 40 % develop a tumor and most develop a tumor when the challenge is in the cerebral cortex. These results suggest that the effects of NOBEL on the growth of GL261 cells in the flanks of B2M−/− mice do not result in the development of effective GL261-specific immunological memory and may therefore be due to innate immune mechanisms. On the other hand, 40 % of JHD−/− mice develop a tumor in the flank after receiving a mix of GL261 and NOBEL, and most of the remainder develop tumors when untreated GL261 are implanted either in the opposite flank or cerebral cortex. These results, together with the failure of B2M−/− mice to make GL261-reactive IgG, suggest that B cells and/or antibody production may be particularly important in the establishment of GL261-specific immunological memory with the capacity to prevent tumor cell growth in the brain.
The contribution of B cells to tumor immunity is poorly understood and may differ for various tumor types. For example, B cell infiltration into melanomas is often associated with poor prognosis [41, 42]. In gliomas, B cells may play a protective role either by acting as antigen presenting cells that are essential in the expansion of the tumor-specific T cells that mediate tumor regression in the brain [8] or by producing tumor-specific antibodies. Consistent with this latter possibility, we detected GL261-reactive IgG antibodies in the sera of mice that failed to grow a tumor after receiving untreated GL261. Moreover, elevated antibody levels were detected in sera from mice immunized with the GL261/NOBEL mix confirming that the presence of NOBEL enhances this aspect of GL261 immunity. Glioma-specific antibodies have been detected in rats intradermally implanted with glioma cells and in rats bearing an intracranial glioma that were treated with adenoviruses expressing immunostimulatory and antitumor molecules [43]. While antibody was only detected in long-term survivors, CD8+ cells were considered to be the principle antitumor effectors in this study since depletion of these cells led to loss of the protective effect [43]. Our results are in agreement with the concept that both B cells and CD8 T or NKT cells contribute to glioma-specific immunity when elicited by a mix of GL261 and NOBEL. However, we found that the GL261-reactive antibody response in CD8 T and NKT cell deficient B2M−/− mice receiving the GL261/NOBEL mix is limited to IgM, and it is therefore impossible to determine whether the deficit in the establishment of glioma immune memory seen in these animals is due to the lack of cellular immunity or IgG. Based on these results, we conclude that a variety of mechanisms, likely including processes mediated by GL261-reactive IgG, contribute to the natural development of immunity that prevents GL261 cells from forming tumors in the flanks of a substantial proportion of congenic mice, and that these mechanisms are enhanced by NOBEL. Consequently, through immunostimulation together with effects mediated by targeting IGF-1R, NOBEL causes GL261 cells implanted in the flank to immunize rather than form tumors in immunocompetent mice. The resulting immune response is capable of preventing tumor formation when untreated GL261 cells are implanted either subcutaneously or intracranially.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors gratefully acknowledge technical support provided by Rhonda B. Kean and Emily Bongiorno. This work was funded by the generous support of the Albert F. Stevens Foundation through a grant to David W. Andrews.
Conflict of Interest
The authors declare that they have no conflict of interest.
Abbreviations
- AS-ODN
Antisense oligodeoxynucleotide
- APC
Antigen presenting cells
- BBB
Blood–brain barrier
- B2M−/−
Beta-2 microglobulin knockout (CD8 T deficient)
- CELISA
Cell-based ELISA
- DLN
Draining lymph nodes
- ELISA
Enzyme-linked immunosorbent assay
- FBS
Fetal bovine sera
- GBM
Glioblastoma multiforme
- IGF-1R
Insulin-like growth factor 1 receptor
- JHD−/−
Antibody heavy chain knockout (B cell deficient)
- MFI
Mean fluorescence intensity
- NKT
Natural killer T cells
- PBS
Phosphate-buffered saline
- qRT-PCR
Quantitative real-time polymerase chain reaction
- Rag2−/−
Recombination activating gene 2 knockout (T and B deficient)
- TLR9
Toll-like receptor 9
Footnotes
Mélanie Morin-Brureau and Kirsten M. Hooper made equal contributions to this work.
References
- 1.Smith JS, Jenkins RB. Genetic alterations in adult diffuse glioma: occurrence, significance, and prognostic implications. Front Biosci. 2000;5:D213–D231. doi: 10.2741/Smith. [DOI] [PubMed] [Google Scholar]
- 2.Louis DN. Molecular pathology of malignant gliomas. Annu Rev Pathol. 2006;1:97–117. doi: 10.1146/annurev.pathol.1.110304.100043. [DOI] [PubMed] [Google Scholar]
- 3.Avgeropoulos NG, Batchelor TT. New treatment strategies for malignant gliomas. Oncologist. 1999;4:209–224. [PubMed] [Google Scholar]
- 4.Jackson C, Ruzevick J, Phallen J, Belcaid Z, Lim M. Challenges in immunotherapy presented by the glioblastoma multiforme microenvironment. Clin Dev Immunol. 2011;2011:732413. doi: 10.1155/2011/732413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madan RA, Gulley JL. Therapeutic cancer vaccine fulfills the promise of immunotherapy in prostate cancer. Immunotherapy. 2011;3:27–31. doi: 10.2217/imt.10.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Disis ML. Immunologic biomarkers as correlates of clinical response to cancer immunotherapy. Cancer Immunol Immunother. 2011;60:433–442. doi: 10.1007/s00262-010-0960-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pachter JS, de Vries HE, Fabry Z. The blood-brain barrier and its role in immune privilege in the central nervous system. J Neuropathol Exp Neurol. 2003;62:593–604. doi: 10.1093/jnen/62.6.593. [DOI] [PubMed] [Google Scholar]
- 8.Candolfi M, Curtin JF, Yagiz K, et al. B cells are critical to T-cell-mediated antitumor immunity induced by a combined immune-stimulatory/conditionally cytotoxic therapy for glioblastoma. Neoplasia. 2011;13:947–960. doi: 10.1593/neo.11024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tran Thang NN, Derouazi M, Philippin G, et al. Immune infiltration of spontaneous mouse astrocytomas is dominated by immunosuppressive cells from early stages of tumor development. Cancer Res. 2010;70:4829–4839. doi: 10.1158/0008-5472.CAN-09-3074. [DOI] [PubMed] [Google Scholar]
- 10.Vauleon E, Avril T, Collet B, Mosser J, Quillien V (2010) Overview of cellular immunotherapy for patients with glioblastoma. Clin Dev Immunol. Article ID 689171. doi:10.1155/2010/689171 [DOI] [PMC free article] [PubMed]
- 11.Andrews DW, Resnicoff M, Flanders AE, et al. Results of a pilot study involving the use of an antisense oligodeoxynucleotide directed against the insulin-like growth factor type I receptor in malignant astrocytomas. J Clin Oncol. 2001;19:2189–2200. doi: 10.1200/JCO.2001.19.8.2189. [DOI] [PubMed] [Google Scholar]
- 12.Hernandez-Sanchez C, Blakesley V, Kalebic T, Helman L, LeRoith D. The role of the tyrosine kinase domain of the insulin-like growth factor-I receptor in intracellular signaling, cellular proliferation, and tumorigenesis. J Biol Chem. 1995;270:29176–29181. doi: 10.1074/jbc.270.49.29176. [DOI] [PubMed] [Google Scholar]
- 13.Liu S, Jin F, Dai W, Yu Y. Antisense treatment of IGF-IR enhances chemosensitivity in squamous cell carcinomas of the head and neck. Eur J Cancer. 2010;46:1744–1751. doi: 10.1016/j.ejca.2010.03.038. [DOI] [PubMed] [Google Scholar]
- 14.Shen YM, Yang XC, Yang C, Shen JK. Enhanced therapeutic effects for human pancreatic cancer by application K-ras and IGF-IR antisense oligodeoxynucleotides. World J Gastroenterol. 2008;14:5176–5185. doi: 10.3748/wjg.14.5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Resnicoff M, Huang Z, Herbert D, Abraham D, Baserga R. A novel class of peptides that induce apoptosis and abrogate tumorigenesis in vivo. Biochem Biophys Res Commun. 1997;240:208–212. doi: 10.1006/bbrc.1997.7640. [DOI] [PubMed] [Google Scholar]
- 16.Durfort T, Tkach M, Meschaninova MI, et al. Small interfering RNA targeted to IGF-IR delays tumor growth and induces proinflammatory cytokines in a mouse breast cancer model. PLoS ONE. 2012;7:e29213. doi: 10.1371/journal.pone.0029213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schillaci R, Salatino M, Cassataro J, et al. Immunization with murine breast cancer cells treated with antisense oligodeoxynucleotides to type I insulin-like growth factor receptor induced an antitumoral effect mediated by a CD8+ response involving Fas/Fas ligand cytotoxic pathway. J Immunol. 2006;176:3426–3437. doi: 10.4049/jimmunol.176.6.3426. [DOI] [PubMed] [Google Scholar]
- 18.Stein CA. Does antisense exist? Nat Med. 1995;1:1119–1121. doi: 10.1038/nm1195-1119. [DOI] [PubMed] [Google Scholar]
- 19.Stein CA, Subasinghe C, Shinozuka K, Cohen JS. Physicochemical properties of phosphorothioate oligodeoxynucleotides. Nucleic Acids Res. 1988;16:3209–3221. doi: 10.1093/nar/16.8.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krieg AM, Yi AK, Matson S, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi N, Hong C, Klinman DM, Shirota H. Oligodeoxynucleotides expressing polyguanosine motifs promote antitumor activity through the upregulation of IL-2. J Immunol. 2013;190:1882–1889. doi: 10.4049/jimmunol.1201063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng G, Guo Z, Kiniwa Y, et al. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science. 2005;309:1380–1384. doi: 10.1126/science.1113401. [DOI] [PubMed] [Google Scholar]
- 23.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+ CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 24.Iho S, Yamamoto T, Takahashi T, Yamamoto S. Oligodeoxynucleotides containing palindrome sequences with internal 5′-CpG-3′ act directly on human NK and activated T cells to induce IFN-gamma production in vitro. J Immunol. 1999;163:3642–3652. [PubMed] [Google Scholar]
- 25.Baserga R, Resnicoff M, D’Ambrosio C, Valentinis B. The role of the IGF-I receptor in apoptosis. Vitam Horm. 1997;53:65–98. doi: 10.1016/S0083-6729(08)60704-9. [DOI] [PubMed] [Google Scholar]
- 26.Szatmari T, Lumniczky K, Desaknai S, et al. Detailed characterization of the mouse glioma 261 tumor model for experimental glioblastoma therapy. Cancer Sci. 2006;97:546–553. doi: 10.1111/j.1349-7006.2006.00208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ausman JI, Shapiro WR, Rall DP. Studies on the chemotherapy of experimental brain tumors: development of an experimental model. Cancer Res. 1970;30:2394–2400. [PubMed] [Google Scholar]
- 28.Phares TW, Kean RB, Mikheeva T, Hooper DC. Regional differences in blood-brain barrier permeability changes and inflammation in the apathogenic clearance of virus from the central nervous system. J Immunol. 2006;176:7666–7675. doi: 10.4049/jimmunol.176.12.7666. [DOI] [PubMed] [Google Scholar]
- 29.Resnicoff M, Abraham D, Yutanawiboonchai W, et al. The insulin-like growth factor I receptor protects tumor cells from apoptosis in vivo. Cancer Res. 1995;55:2463–2469. [PubMed] [Google Scholar]
- 30.Resnicoff M, Li W, Basak S, Herlyn D, Baserga R, Rubin R. Inhibition of rat C6 glioblastoma tumor growth by expression of insulin-like growth factor I receptor antisense mRNA. Cancer Immunol Immunother. 1996;42:64–68. doi: 10.1007/s002620050252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu X, Turbyville T, Fritz A, Whitesell L. Inhibition of insulin-like growth factor I receptor expression in neuroblastoma cells induces the regression of established tumors in mice. Cancer Res. 1998;58:5432–5438. [PubMed] [Google Scholar]
- 32.Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298:2199–2202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 33.Jung J, Yi AK, Zhang X, Choe J, Li L, Choi YS. Distinct response of human B cell subpopulations in recognition of an innate immune signal, CpG DNA. J Immunol. 2002;169:2368–2373. doi: 10.4049/jimmunol.169.5.2368. [DOI] [PubMed] [Google Scholar]
- 34.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–760. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 35.Samulowitz U, Weber M, Weeratna R, et al. A novel class of immune-stimulatory CpG oligodeoxynucleotides unifies high potency in type I interferon induction with preferred structural properties. Oligonucleotides. 2010;20:93–101. doi: 10.1089/oli.2009.0210. [DOI] [PubMed] [Google Scholar]
- 36.Alizadeh D, Zhang L, Brown CE, Farrukh O, Jensen MC, Badie B. Induction of anti-glioma natural killer cell response following multiple low-dose intracerebral CpG therapy. Clin Cancer Res. 2010;16:3399–3408. doi: 10.1158/1078-0432.CCR-09-3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grauer OM, Molling JW, Bennink E, et al. TLR ligands in the local treatment of established intracerebral murine gliomas. J Immunol. 2008;181:6720–6729. doi: 10.4049/jimmunol.181.10.6720. [DOI] [PubMed] [Google Scholar]
- 38.Biollaz G, Bernasconi L, Cretton C, et al. Site-specific anti-tumor immunity: differences in DC function, TGF-beta production and numbers of intratumoral Foxp3+ Treg. Eur J Immunol. 2009;39:1323–1333. doi: 10.1002/eji.200838921. [DOI] [PubMed] [Google Scholar]
- 39.Brown CE, Starr R, Martinez C, et al. Recognition and killing of brain tumor stem-like initiating cells by CD8+ cytolytic T cells. Cancer Res. 2009;69:8886–8893. doi: 10.1158/0008-5472.CAN-09-2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith KE, Fritzell S, Badn W, et al. Cure of established GL261 mouse gliomas after combined immunotherapy with GM-CSF and IFNgamma is mediated by both CD8+ and CD4+ T-cells. Int J Cancer. 2009;124:630–637. doi: 10.1002/ijc.23986. [DOI] [PubMed] [Google Scholar]
- 41.Perricone MA, Smith KA, Claussen KA, et al. Enhanced efficacy of melanoma vaccines in the absence of B lymphocytes. J Immunother. 2004;27:273–281. doi: 10.1097/00002371-200407000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Shah S, Divekar AA, Hilchey SP, et al. Increased rejection of primary tumors in mice lacking B cells: inhibition of anti-tumor CTL and TH1 cytokine responses by B cells. Int J Cancer. 2005;117:574–586. doi: 10.1002/ijc.21177. [DOI] [PubMed] [Google Scholar]
- 43.Ghulam Muhammad AK, Candolfi M, King GD, et al. Antiglioma immunological memory in response to conditional cytotoxic/immune-stimulatory gene therapy: humoral and cellular immunity lead to tumor regression. Clin Cancer Res. 2009;15:6113–6127. doi: 10.1158/1078-0432.CCR-09-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.