Abstract
Background
Antigen-derived HLA class I-restricted peptides can generate specific CD8+ T-cell responses in vivo and are therefore often used as vaccines for patients with cancer. However, only occasional objective clinical responses have been reported suggesting the necessity of CD4+ T-cell help and possibly antibodies for the induction of an effective anti-tumor immunity in vivo. The SSX2 gene encodes the cancer testis antigen (CTA) HOM-MEL-40/SSX2, which is frequently expressed in a wide spectrum of cancers. Both humoral and cellular immune responses against SSX2 have been described making SSX2 an attractive candidate for vaccine trials.
Methods
SYFPEITHI algorithm was used to predict five pentadecamer peptides with a high binding probability for six selected HLA-DRB1 subtypes (*0101, *0301, *0401, *0701, *1101, *1501) which are prevalent in the Caucasian population.
Results
Using peripheral blood cells of 13 cancer patients and 5 healthy controls, the HOM-MEL-40/SSX2-derived peptide p101-111 was identified as an epitope with dual immunogenicity for both CD4+ helper and cytotoxic CD8+ T cells. This epitope also reacted with anti-SSX2 antibodies in the serum of a patient with breast cancer. Most remarkably, SSX2/p101-111 simultaneously induced specific CD8, CD4, and antibody responses in vitro.
Conclusions
p101-111 is the first CTA-derived peptide which induces CD4+, CD8+, and B-cell responses in vitro. This triple-immunogenic peptide represents an attractive vaccine candidate for the induction of effective anti-tumor immunity.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-011-1030-6) contains supplementary material, which is available to authorized users.
Keywords: Tumor immunology, Cancer testis antigen, SSX2, CD8+ T-cell epitope, CD4+ T-cell epitope, B-cell epitope
Introduction
Cancer testis antigens (CTA) [1], also termed cancer germline antigens [2] are ideal targets for the development of cancer vaccines, because they are expressed in a broad spectrum of different human neoplasms [3], but not in normal cells except for germ cells in the testis. HOM-MEL-40 is one of the most frequently expressed CTA. The sequence of the HOM-MEL-40 gene is identical with that of SSX2, which is found in 70% of human synovial sarcomas [4]. SSX2 is expressed in a significant proportion of a broad range of human cancers [5], making SSX2 a promising candidate for vaccine development. Strategies of “reverse T-cell immunology” have allowed the identification of peptide epitopes derived from several SEREX-defined CTA that induce cytotoxic CD8+ and CD4+ T-cell responses. For SSX2, two HLA-A*0201-restricted T-cell epitopes, peptide p41-49 [6] and peptide p103-111 [7], and two HLA-DR-restricted epitopes, p103-112 [8, 9] and p37-58 [9], have been described to date.
Only occasional objective clinical responses have been reported after vaccination with CD8+-stimulating peptides suggesting the necessity of CD4+ T-cell help and possibly antibodies [10] for the induction of effective anti-tumor immunity in vivo. While combined CD4+ and CD8+ responses have been observed after vaccination with antigenic whole protein, the manufacturing of protein vaccines can be demanding and expensive. Possibly similar CD4+ and CD8+ responses might be obtained by vaccination with peptides of triple affinity for CD4+ and CD8+ T cells as well as antibodies. We now describe the identification of an SSX2-derived epitope with such a triple affinity which could serve as a valuable tool for immunotherapeutic approaches targeting HOM-MEL-40/SSX2.
Patients and methods
The study had been approved by the local ethics review committee (Ethikkommission der Aerztekammer des Saarlandes) and was done in accordance with the Declaration of Helsinki. Recombinant DNA work was done with the permission and according to the regulations of the local authorities (Regierung des Saarlandes) and after obtaining written informed consent.
Patients and healthy controls
Nine unselected breast cancer patients (BC), three patients with colon carcinoma (COL), one patient with multiple myeloma (MM), and five healthy donors (Co) were included in this study irrespective of their anti-HOM-MEL-40/SSX2 serology and HLA-DR subtype (Table 2). The patients were studied at the time of diagnosis before any therapy.
Table 2.
HLA-DR configuration, SSX2 expression, anti-SSX2 serology, and T-cell response from breast cancer (BC), colon carcinoma (COL), myeloma (MM) patients, and healthy controls (Co) analyzed in this study
| HLA-DR | SSX2 | T-cell response to | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| B1* | B3* | B4* | B5* | Expression | Serology | p98-112 | p101-115 | p101-111 | |||
| CD4+ | CD8+ | ||||||||||
| Mel-Hei | n.d. | n.d. | n.d. | n.d. | n.d. | + | + | n.d. | n.d. | n.d. | n.d. |
| BC399 | 0101 | 0701 | − | 0101 | − | + | − | + | + | + | n.d. |
| BC400 | 0701 | 1302 | 0301 | 0103 | − | + | − | − | − | − | n.d. |
| BC401 | 0403 | 1102 | 0202 | − | − | − | − | − | − | − | n.d. |
| BC403 | 1314 | 1501 | 0202 | − | 0101 | + | − | − | − | − | n.d. |
| BC404 | 0101 | 0701 | − | 0103 N | − | + | n.d. | − | − | − | n.d. |
| BC405 | 1104 | 1501 | 0202 | − | 0101 | + | n.d. | − | − | − | n.d. |
| BC410 | 1101 | 1401 | 0202 | − | − | + | n.d. | − | − | − | n.d. |
| BC418 | 0101 | 0301 | 0202 | − | − | − | − | + | + | + | n.d. |
| BC699 | 0101 | 1501 | − | − | 0101 | + | − | n.d. | n.d. | + | + |
| COL10 | 1301 | 1301 | 0202 | − | − | − | − | n.d. | n.d. | − | − |
| COL12 | 0301 | 0803 | 0101 | − | − | − | + | n.d. | n.d. | − | − |
| COL13 | 0101 | 0301 | 0202 | − | − | + | − | n.d. | n.d. | + | + |
| MM10 | 0103 | 0404 | − | 0103 | − | n.d. | n.d. | n.d. | n.d. | − | − |
| Co17 | 0701 | 1101 | 0202 | 0103 N | − | − | − | − | − | − | − |
| Co70 | n.d. | n.d. | n.d. | n.d. | n.d. | − | − | − | − | − | − |
| Co71 | n.d. | n.d. | n.d. | n.d. | n.d. | − | − | − | − | − | n.d. |
| Co72 | n.d. | n.d. | n.d. | n.d. | n.d. | − | − | − | − | − | n.d. |
| Co73 | 0801 | 1201 | 0202 | − | − | − | − | − | − | − | n.d. |
Neither DR-responding patient BC399 nor BC418 were HLA-A*0201 positive. In contrast, patients BC699, COL10-13, and MM10 expressed also HLA-A*0201. From patient Mel-Hei, only serum and tumor samples were analyzed for the existence of SSX2 epitope-specific antibodies and SSX2 expression, respectively
Cell lines
In this study, 3 continuous human tumor cell lines (BT 549, SK-MEL-37, T2) were used as HLA-A*0201+ antigen-presenting cells (APC) of which SK-MEL-37 is expressing the SSX2 antigen. The SSX2 status was regularly tested in our laboratory using the corresponding RT-PCR as described below. The HLA status of all cell lines and test person’s peripheral blood mononuclear cells (PBMC) were also checked regularly by using sequence-specific oligonucleotide (SSO)- and sequence-specific primer (SSP)-PCR, respectively at an approved external institute (www.immungenetik-kl.de). All LCL were established in our laboratory. Regularly, controls of their HLA types compared with the donor PBMC in the approved institute mentioned above, excluded any cross-contamination.
Analysis of SSX2 mRNA expression by tumors
Fresh tumor biopsies were frozen within 15 min after excision. The transcription of SSX2 mRNA was checked by RT-PCR as described previously [11].
Selection of peptides
Peptides were derived from the published HOM-MEL-40/SSX2 sequence [11]. The SYFPEITHI algorithm (www.syfpeithi.de) was used to predict SSX2-derived peptides with a high probability to bind to six DRB1 subtypes (*0101, *0301, *0401, *0701, *1101, and *1501) that are prevalent among the Caucasian population (Table 1). The 13-mer PADRE, a pan-DR-binding peptide was used as a negative control [12]. A mix of 7 pentadecamers derived from the pp65 antigen of the human CMV (p32, p117, p243, p269, p299, p510, and p524) and predicted to bind to the HLA-DR subtypes of interest was used as positive control. Peptides were synthesized following the Fmoc/tBu strategy as described [13, 14]. Purity was > 90 % as assessed by HPLC and mass spectrometry. All peptides were dissolved in a mixture of water and DMSO.
Table 1.
Peptides derived from the HOM-MEL-40/SSX2 antigen selected for this study
| Sequence | Position | Binding probability to HLA-DRB1 | |||||
|---|---|---|---|---|---|---|---|
| *0101 | *0301 | *0401 | *0701 | *1101 | *1501 | ||
KIFYVYMKRKYEAMT |
p45-59 | 18 | 8 | 16 | 10 | 24 | 0 |
KLGFKATLPPFMCNK |
p60-74 | 25 | 10 | 16 | 26 | 10 | 16 |
QMTFG RLQGISPKI M |
p98-112 | 34 | 8 | 16 | 10 | 22 | 22 |
TFGRLQGISPK |
p100-110 | NA | |||||
FG RLQGISPKI |
p101-111 | NA | |||||
G RLQGISPKI M |
p102-112 | NA | |||||
RLQGISPKI MP |
p103-113 | NA | |||||
LQGISPKIMPK |
p104-114 | NA | |||||
FG RLQGISPKI MPKK |
p101-115 | 29 | 11 | 20 | 22 | 13 | 18 |
RKQLVIYEEISDPEE |
p171-185 | 13 | 18 | 8 | 14 | 6 | 26 |
| Caucasian allele frequency (%) | 8.7 | 10 | 8 | 11.7 | 8.3 | 12.1 | |
Binding scores of the respective peptides predicted by SYFPEITHI are shown. Scores of 20 and more are considered to have a high probability of binding to the respective HLA-DR subtype. The amino acids printed in bold letters represent the sequence of the HLA-A2-restricted epitope p103-111 with a binding score for HLA-A*0201 of 23. “NA” (not available) indicates that SYFPEITHI provides MHC-II predictions only for polypeptides of a length of at least 15 amino acids
Production of recombinant proteins
SSX2 full-length protein [8, 14] and the SCP1 fragment p630-817 [15] were expressed in Escherichia coli DH5α as described earlier. Purified proteins were shown to react with the anti-SSX monoclonal antibody E3AS (by courtesy of D.R. de Bruijn, Department of Human Genetics, Radboud University Medical Centre, Nijmegen, Netherlands) or with a polyclonal anti-SCP1 antiserum (obtained by immunizing a rabbit with SCP1 fragment p630-817) by Western blot. Purity of the recombinant proteins was at least 90% as assessed by SDS PAGE. Endotoxin contamination of the proteins expressed in E. coli (particularly with regard to LPS) was excluded using the Limulus Amoebocyte Lysate (LAL) gel-clot assay according to the suppliers instructions (Charles River Laboratories, Sulzfeld, Germany).
Generation of SSX2-derived epitope-specific CD4+ T cells
PBMC and serum samples were obtained from thirteen patients with breast cancer, colon carcinoma, or myeloma, and five healthy donors and were isolated by Ficoll-Paque PLUS separation (GE Healthcare Bio-Sciences AB, Uppsala, Sweden). For in vitro priming, the unseparated PBMC were suspended in serum-free medium (Lonza, Verviers, Belgium) supplemented with 2 mM l-glutamine (Gibco, Invitrogen, Karlsruhe, Germany) and 1% penicillin/streptomycin (Gibco) and divided into two fractions. One fraction was pulsed with a pool of the five pentadecamer SSX2 peptides p45-59, p60-74, p98-112, p101-115, and p171-186. As positive control for the in vitro stimulation, the second fraction of the PBMC was pulsed with a mix of 7 peptides derived from the pp65 antigen of the human CMV. Pulsing, cultivation, and stimulation were performed as described [8].
Establishment of epitope-specific T-cell lines and clones
Responding T cells were isolated from the bulk population by IFN-γ-based magnetic cell enrichment using a cytokine-secretion assay (Miltenyi Biotec Inc., Bergisch Gladbach, Germany) and cloned by limiting-dilution culture as described [8, 16]. Specifically reacting clones were expanded by restimulation performed after 14 days under identical conditions. A summary of all T-cell lines and clones applied in this study is given under supplementary materials (SM) in table SM-1.
Analysis of the Vβ region of the T-cell receptor (TCR)
The TCR Vβ usage was assessed by RT-PCR as described [17]. Data were confirmed by subsequent sequencing of the PCR products. The respective sequences were designated according to imMunoGeneTics database (www.imgt.org).
Establishment of lymphoblastoid cell lines (LCL)
LCL were established from PBMC as described [18]. All LCL used for this study strongly expressed HLA-DR molecules as assessed by flow cytometry.
Generation of dendritic cells (DC)
Monocyte-derived DC were generated from PBMC as described [15]. The stage of DC maturation was assessed by the reactivity of the DC with a panel of monoclonal antibodies and analyzed by flow cytometry using a FACScan (Becton–Dickinson, Heidelberg, Germany). Mature DC had the phenotype CD14low, CD11c+, CD86high, HLA-DRhigh, and MHC-Ihigh.
Flow cytometric characterization of cells
For phenotypic characterization of effector cells and APC, the cells were stained according to the supplier’s instructions using the following antibodies: anti-HLA-DR/PE (clone L243), anti-hCD4/FITC and anti-hCD4/PerCP (both from clone SK3), anti-hCD8/PE (clone SK1), anti-hCD45RA (clone L48), anti-CD25/PE (clone 2A3), and anti-hCCR7/PE (clone 3D12; all obtained from Becton-Dickinson). After staining, the cells were resuspended in CellWash solution (Becton–Dickinson) and 2 to 3 × 104 cells were analyzed using a FACScan 80214 (Becton–Dickinson).
Blocking of T-cell responses
T-cell receptor/MHC-II interaction was blocked by antibodies against HLA-DR (clone L243) or HLA-DP (clone B7/21; both from Becton–Dickinson), CD4+ T cells were blocked by anti-CD4 antibody clone MT310 and CD8+ T cells by anti-CD8 antibody clone DK25 (both from DakoCytomation, Hamburg, Germany) as described [15]. None of the antibodies exhibited any cytotoxic activity at the concentrations used for the blocking experiments, as demonstrated by the absence of inhibition of MHC-I-mediated T-cell responses by L243, B7/21, MT310, or MHC-II-mediated T-cell responses by DK25.
IFN-γ ELISPOT assay
Two IFN-γ ELISPOT assays were performed on days 21 and 28, respectively, using pool-stimulated bulk T cells as effectors to demonstrate specific reactivity to autologous APC (PBMC, irradiated with 60 Gy, 5 × 104/well) pulsed with peptides (2 μg/ml) of the corresponding pool. In case of a specific response, the bulk population was cloned as described above. The resulting CD4+ T-cell clones were used for repeated assays with various allogeneic or autologous APC (irradiated LCL or DC). Assays were performed as described before [8]. All tests were run in triplicates, and mean values and standard deviations were determined. T-cell responses were scored positive if the number of IFN-γ spots induced by SSX2-stimulated T cells was significantly higher than that induced by T cells against controls (without APC, APC + PADRE, APC without effectors) with a significance of P ≤ 0.01. Spots were counted using a Bioreader-3000 Pro (Biosys, Karben, Germany). ELISPOTs were run in triplicates, and mean values and standard deviations were determined.
IL-5 ELISA
The supernatants of the IFN-γ ELISPOT assays were analyzed for IL-5 production using the DuoSet® ELISA kit for human IL-5 from R&D Systems GmbH as described in the supplier′s instructions.
Assessment of cytotoxicity
Cytotoxicity of CD8+ T cells from CTL clone #24 against peptide-pulsed and Cr51-labeled T2 target cells was assessed using a chrome release assay as described before [7]. For the Cr51-release assay, 1 × 103 T2 cells were seated in each well. Effector and target cells were coincubated for 4 h at a ratio of 10:1. Cytotoxicity of the bulk T cells after in vitro stimulation with p101-111 against the SSX2+ melanoma cell line SK-MEL-37 and the SSX2− breast cancer cell line BT 549 (both lines in RPMI 1640 [Gibco], 10% FCS, L-Glu 2 mM, Pen/Strept) was assessed using an assay based on the release of Lactate dehydrogenase out of the target cells according to the suppliers instructions (Roche Diagnostics, Mannheim, Germany). In the LDH assay, 1 × 104 target cells were seated in each well. Effector and target cells were coincubated for 4 h at a ratio of 10:1 (→ 1 × 105 CD8+ bulk T cells).
Detection of serum antibodies against SSX2 and determination of B-cell epitopes
Serum antibodies against HOM-MEL-40/SSX2 were assessed semiquantitatively by RAYS as described [19]. Unlike the matrix-bound synthesized decamer peptides used in previous studies [20], in this study, B-cell epitopes of the SSX2 antigen were determined by ELISA using recombinant overlapping SSX2 fragments of different length. These fragments were amplified and recombinantly expressed in HEK293 cells under control of a CMV promoter introducing a FLAG tag at the C-terminus of the fragment. Total cell extract was prepared and coated indirectly to Nunc maxisorb plates (Nunc, Langenselbold, Germany) using anti-FLAG mAb (Sigma–Aldrich Chemie, Munich, Germany). Serum samples were diluted 1:500, and supernatants were diluted as indicated.
Statistical analysis
All tests were repeated at least twice. The significance of the ELISPOT results was determined by a 2-sided Student’s t-test.
Results
Identification of HOM-MEL-40/SSX2-derived epitopes stimulating CD4+ T cells
Besides the 3 known peptides p45-59, p60-74, and p171-185 [8], SYFPEITHI predicted 2 additional pentadecamer peptides (p98-112 and p101-115) to have a strong promiscuous binding to the six DRB1 subtypes (*0101, *0301, *0401, *0701, *1101, and *1501) that are prevalent in the Caucasian population (Table 1). Although p98-112 and p101-115 share 12/15 amino acids, they have different binding probabilities (Table 1) to the six DRB1 subtypes represented in SYFPEITHI. Moreover, the overlapping region of both peptides which spans p101-112 also comprises the HLA-A*0201-restricted MHC-I epitope p103-111 [7].
Priming of bulk cultures from patients BC399 and BC418 with a peptide pool consisting of p45-59, p60-74, p171-185, p98-112, and p101-115 induced a significant (P < 0.001) and specific T-cell response against both p98-112 and p101-115 (Fig. 1a, b). The reactivity against both peptides increased after each additional restimulation with the peptides. To check for potential cross-reactivity between peptide p98-112 and p101-115, the in vitro stimulations were repeated using each peptide separately. Priming with either peptide induced T cells reacting with both p98-112 and p101-115 (Fig. 1c). In order to test whether these p98-112- and p101-115-reactive T cells also cross-react with the HLA-A2-binding CTL epitope p103-111, autologous HLA-A2− PBMC were pulsed with peptide p103-111 and used as APC for the stimulation of bulk T cells from patient BC399. As shown in Fig. 1a, there was no cross-reaction against the HLA-A2-restricted peptide p103-111, demonstrating that the HLA-A2-binding epitope did not fit into any MHC molecules responsible for the primary T-cell response.
Fig. 1.
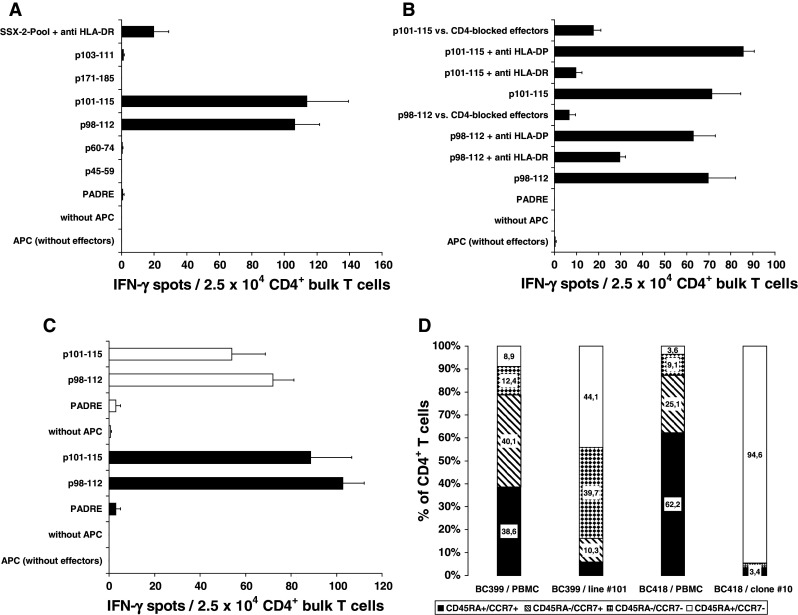
CD4+ T-cell responses against HOM-MEL-40/SSX2-derived peptides and characterization of the CD4+ effector T cells. T cells from patients BC399 (a) and BC418 (b) responded after priming with the peptide pool to challenge with both p98-112 and p101-15. The response of T-cells from both patients against both peptides was blocked by anti-HLA-DR, but not by anti-HLA-DP. T cells from patient BC418 were also blocked by anti-CD4. c Response of bulk T cells from patient BC399 using single peptide p98-112 or p101-115. Filled bars represent the IFN-γ response of p98-112 primed bulk T cells when challenged with p98-112 (P = 0.003) and p101-115 (P < 0.02), respectively. Empty bars represent the response of T cells primed with p101-115 and challenged with p98-112 (P < 0.007) and p101-115 (P = 0.04), respectively. Y axes display the different APC settings used to challenge T cells during IFN-γ ELISPOT. d During stimulation with peptide p101-115 (patient BC399) and p98-112 (patient BC418), respectively, effector memory T cells of both patients increased considerably. CD4+ T cells of clone #10 all belong to the effector memory type
Demonstration of the DR restriction of the CD4+-mediated TH1 response to p98-112 and p101-115
Bulk T cells, T-cell lines, and T-cell clones responding to p98-112 or p101-115 did not release IL-5 into the supernatant (detection limit of the IL-5 ELISA < 25 pg/ml), whether stimulated with the entire peptide pool or with single peptides. The fact that the CD4+ T cells produced IFN-γ, but not IL-5 upon stimulation with p98-112 and p101-115 indicates that these T cells belong to the TH1 subset. The HLA-DR restriction of the TH1 response against p98-112 and p101-115 from patients BC399 and BC418 was demonstrated by blocking the reaction with anti-HLA-DR. While pre-incubation of the APC with anti-DR-antibody clone L243 blocked the reaction, pre-incubation with anti-DP-antibody clone B7/21 left the T-cell response unchanged (Fig. 1a, b). The response of effector cells derived from patient BC418 to both peptides was mediated by CD4+ T cells because pre-incubation of the effector cells with anti-CD4-antibody clone MT310 significantly (P = 0.028 vs. p98-112 and P = 0.03 vs. p101-115) inhibited the reaction (Fig. 1b).
Establishment and characterization of CD4+ T-cell lines and clones with affinity to p98-112 and p101-115
The p98-112- and p101-115-reactive T cells were enriched by MACS separation utilizing their IFN-γ secretion upon restimulation with the corresponding peptide. The IFN-γ positive MACS fractions were submitted to limited-dilution culture, and after 2 weeks, the outgrowing clones and lines were expanded and characterized. From patient BC399, two T-cell lines could be established: line #98 derived from the bulk culture after p98-112 priming and line #101 from bulk T cells after priming with p101-115. T-cell lines #98 as well as line #101 were shown to recognize both p98-112 and p101-115. The proportion of CD4+ T cells in both lines increased to more than 80%. According to the differentiation scheme suggested by Lanzavecchia's group [21]—naïve T cells express CD45RA and CCR7, central memory T cells are negative for CD45RA, but positive for CCR7, and effector memory cells do not express CCR7 anymore, irrespective of their CD45RA expression—the fraction of effector memory T cells increased, while the proportion of naïve CD4+ T cells decreased over the course of stimulations with peptide p101-115, as shown by loss of CCR7 (Fig. 1d). From patient BC418, different clones were established from the bulk population after stimulation with p98-112. In contrast to the T-cell lines derived from patient BC399, CD4+ T cells from clone #10 derived from patient BC418 made up >98% of the respective population. All clones were shown to respond specifically to both p98-112 and p101-115. Clone #10 was chosen for further investigations. Flow cytometry of the CD4+ cells of clone #10 showed that these cells expressed neither GITR, nor CTLA-4, nor CCR7 assigning them to the non-regulatory effector memory T-helper cells [21] (Fig. 1d).
High-resolution dissection of the DR restriction
Using allogeneic APC with a known 4-digit HLA-DR type, the DR restriction of a T-cell response can be narrowed down to a 4-digit resolution. To determine the HLA-DR restriction of the responses to p98-112 and p101-115, LCL or tumor cell lines with a known DR configuration were pulsed with these peptides and used as APC in IFN-γ ELISPOT assays. Peptide-pulsed APC were used to stimulate p98-112- or p101-115-specific T cells derived from the T-cell lines #98 and #101, and from clone #10, respectively, and the observed reactions were blocked with anti-HLA-DR antibody L243. As shown in Fig. 2a, T cells from both BC399-derived T-cell lines #98 and #101 responded to SK-MEL-37 cells pulsed with either p98-112 or p101-115 in a DR-restricted way (P < 0.05). As BC399 shares only DRB1*0101 with SK-MEL-37, the T-cell response was definitely restricted to this subtype. Identical results were obtained using LCL from BC418 as well as Co149 as APC (Table SM-2). Restriction to HLA-DRB1*0701 or DRB4*0101 could be excluded due the fact that cells from LCL BC355 pulsed with SSX2/p98-112 did not induce a T-cell response by the two lines #98 and #101 (Table SM-2). In addition to DRB1*0101, cells from patient BC399 also expressed MHC-II molecules of the DRB1*0701 subtype (Table 2). Therefore, T cells from both T-cell lines (#98 and #101) were tested against LCL from donor Co17 and Co89 which share only DRB1*0701 with BC399. T cells from lines #98 and #101 responded in a DR-restricted fashion (P = 0.006 for line #98 and P = 0.002 for line #101) only to a challenge with peptide p101-115, but not with p98-112 in the context of HLA-DRB1*0701 (Fig. 2b with LCL Co89 as APC, Tables SM-2 and SM-3), confirming that each T-cell line derived from patient BC399 harbored at least two separate T-cell clones with a different DR restriction. Furthermore, these results confirm the SYFPEITHI algorithm, which had predicted a much better binding of p101-115 to HLA-DRB1*0701 than p98-112 (Table 1). To dissect the DR restriction of clone #10 which was derived from patient BC418 after in vitro simulation with p98-112, LCL from BC399, PC 1, Co89, AlaBM, and Co125 were pulsed with peptide p98-112 or p101-115, respectively. As shown in Fig. 2c, each peptide induced IFN-γ secretion in more than 50 % of the CD4+ T cells from clone #10 in a DR-restricted way only if the peptides were presented by LCL from BC399 and Co89 who share the DRB1*0101 subtype with patient BC418 (P < 0.01). Restriction by HLA-DRB3*0202 was excluded because cells from clone #10 cells did not react against LCL from Co89 pulsed with p98-112 or p101-115. Blood donor Co89 and patient BC418 have only the DRB3 subtype *0202 in common. None of the clones derived from patient BC418 showed a p98-112- or p101-115-specific response restricted to DRB1*0301, which is in line with the low binding probability of both peptides to this MHC-II subtype predicted by SYFPEITHI (Table 1). The conclusion from these results is that the T-cell response of the BC418-derived clone #10 is also restricted by HLA-DRB1*0101 (Table SM-4).
Fig. 2.
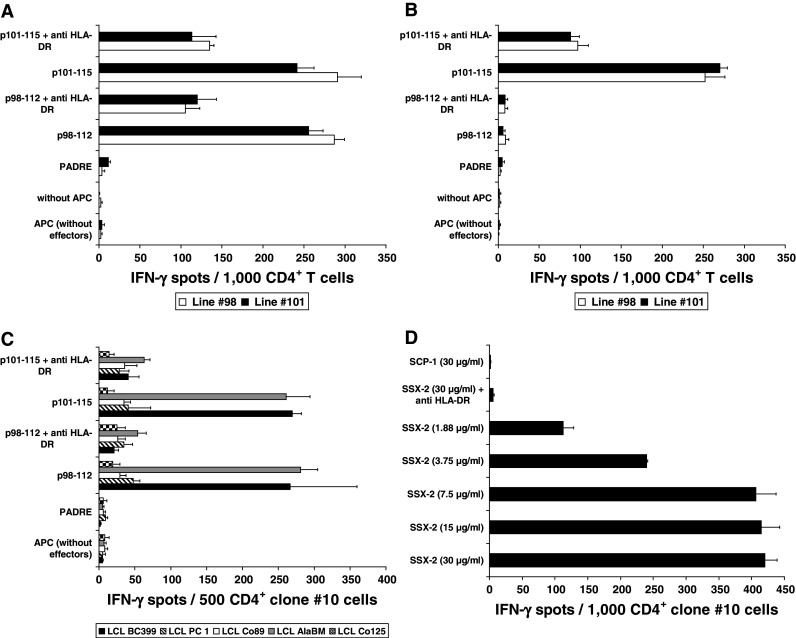
Dissection of the HLA-DR restriction of the T-cell response against SSX2-derived peptides p98-112 and p101-115 and natural processing and presentation of epitope p98-112. a Restriction of the T-cell response against p98-112 and p101-115 using the melanoma cell line SK-MEL-37 as APC. This cell line shares only DRB1*0101 with the BC399-derived T cells from line #98 and #101. DR restriction was demonstrated by blocking the response of both T-cell lines against APC pre-incubated with the anti-DR antibody clone L243. b DRB1*0701 restriction of the response from T cells of the BC399-lines #98 and #101 against LCL Co89 pulsed with peptide p101-115. *0701 is the only DRB1 subtype shared by Co89 and patient BC399. c Dissection of the restriction of the T-cell response of clone #10 from patient BC418: a response after stimulation with p98-112 or p101-115 only was observed when these peptides were presented by LCL from BC399 and AlaBM (P < 0.01) proving the restriction of these T cells to HLA-DRB1*0101, which is the only DR molecule shared by effector cells and APC. There was no response if both peptides were presented on LCL from PC 1, Co89, or Co125 excluding a restriction to HLA-DRB1*0301 or DRB3*0202. Y-axes of the A, B, and C panels display the different APC settings used to challenge T cells during IFN-γ ELISPOT. d Loading of autologous DC (1 × 104/well) with increasing concentrations of SSX2 whole protein induced a concentration-dependent response of clone #10 CD4+ T cells, which was blocked by anti-HLA-DR. The control antigen (SCP1 fragment p630-817) was not recognized (P < 0.001). Y axis shows the different DC settings used to challenge T cells during IFN-γ ELISPOT
Natural processing and presentation of the different HOM-MEL-40/SSX2-derived epitopes
The natural processing and presentation of p98-112 was demonstrated by adding recombinant HOM-MEL-40/SSX2 full-length antigen to the culture medium used to generate DC. An antigen fragment spanning the sequence from position p630-817 of the CTA HOM-TES-14/SCP1 served as negative control. Figure 2d shows the specific response of T cells from patient BC418 derived clone #10 against full-length SSX2, processed and presented by autologous DC using the class-II pathway. The response correlated with the concentration of the SSX2 protein in the DC culture medium. The reactivity against the processed epitope could be blocked by pre-incubation of the APC with anti-DR antibody, proving once more the HLA-DR restriction of the SSX2 epitope p98-112.
Determination of the CD4+ T-cell stimulating core epitope shared by the peptides p98-112 and p101-115
The results described so far made it very likely that both HOM-MEL-40/SSX2-derived epitopes p98-112 and p101-115 share a common core epitope responsible for their immunogenicity. To define this epitope more precisely, the five undecamer peptides p100-110, p101-111, p102-112, p103-113, and p104-114 were synthesized and used for pulsing allogeneic APC. As shown in Fig. 3a, T cells from the patient BC410 derived clone #10 responded to p98-112, which was used for primary stimulation, as well as to p101-115 and to the undecamer peptide p101-111 (P = 0.0004 vs. negative control). The responses to these three peptides were of a similar strength. In the context of HLA-DRB1*0101, the T-cell response could be maintained in culture with peptide concentrations as low as 5 ng/ml used for APC pulsing (data not shown). The core epitope p101-111 showed the same titration curve as the longer peptides. The second undecamer p102-112 which also spans the sequence shared by p98-112 and p101-115 induced an IFN-γ release of the CD4+ cells from clone #10 (with P = 0.04 vs. negative control) which was only one tenth of the release induced by p101-111, while the other undecamers did not induce a measurable T-cell response (Fig. 3a). Taken together, these results confirm the undecamer p101-111 as the core immunogenic epitope responsible for the T-cell responses induced by both p98-112 and p101-115. In contrast, the previously described HLA-A*0201-restricted SSX2 nonamer epitope p103-111 [7], which is also located within the sequence shared by both pentadecamer peptides, did not stimulate HLA-DR-restricted responses (Fig. 1a).
Fig. 3.
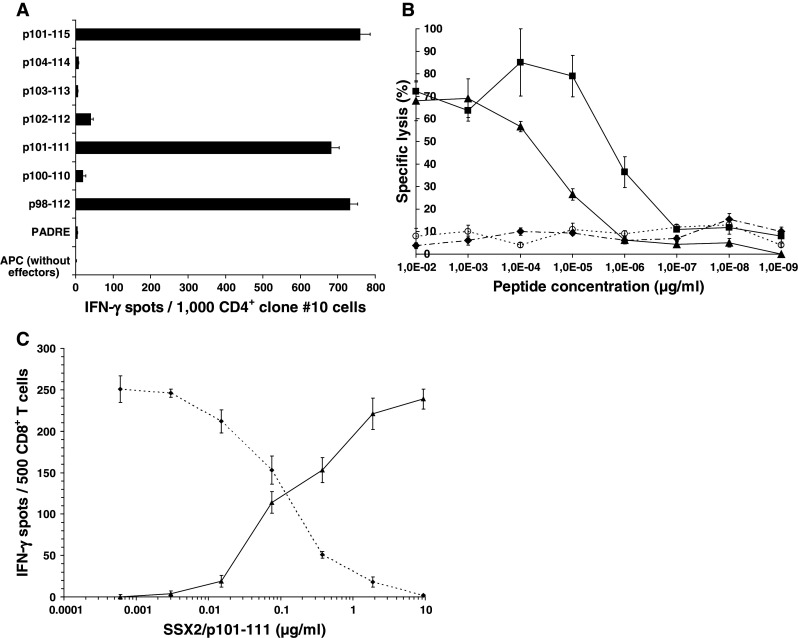
Determination of peptide p101-111 as immunogenic core of the two peptides p98-112 and p101-115 and demonstration of the cytotoxic T-cell response against the core epitope p101-111. a After priming with peptide p98-112 CD4+ T cells of clone #10 (BC418) responded equally in IFN-γ ELISPOT when challenged with the pentadecamers p98-112 and p101-115 (P < 0.001) presented by allogeneic APC (3 × 104 LCL BC399/well). Peptide p101-111 was the only undecamer peptide to induce a response by clone #10 T cells (P < 0.001). Y axis displays the different APC settings used during IFN-γ ELISPOT. b HLA-A*0201-restricted CD8+ T cells from clone #24, which were generated by in vitro priming with the SSX2-derived nonamer p103-111, were used as effector cells in a CR51 release assay against HLA-A*0201+ T2 cells pulsed with decreasing concentrations of peptide p101-111 (triangles) or p103-111 (squares) by an E/T ratio of 10:1. Specific lysis of peptide-pulsed targets induced by clone #24 CD8+ T cells demonstrated a strong and dose-dependent cytotoxic T-cell response in an HLA-A*0201-restricted way. In contrast, the CTL epitope p41-49 (diamonds/dashed line), also derived from SSX2, as well as the DRB1*0101-restricted pentadecamer p98-112 (empty circles/spotted line) did not cause any cytotoxicity in the context of HLA-A*0201. c Further evidence for the HLA-A*0201 restriction of the CD8+ T cells after SSX2/p101-111 stimulation was shown by displacing an HLA-A*0201 reference peptide out of its MHC molecules, leading to modified antigen presenting functions of the APC and to a different induction pattern for T-cell responses finally. Increasing concentrations of the triple epitope resulted in a decreasing response of the HLA-A*0201-restricted IMP-specific clone #46 (diamonds/dashed line) and in a similarly increasing activity of the SSX2/p101-111 specific clone #9 (triangles/continuous line). Read out by ELISPOT against T2 cells pulsed with 0.1 μg/ml of the A*0201 reference peptide IMP/p58-66 either alone (1st concentration) or concurrently with increasing concentrations of SSX2/p101-111 (2nd-7th concentration). Both clones were run in separate experiments using T2 cells prepared in the same way
Demonstration of the cytotoxic and HLA-A*0201-restricted activity of CD8+ T cells stimulated by p101-111
We had previously demonstrated that the SSX2-derived nonamer epitope p103-111 causes a strong response of cytotoxic T lymphocytes (CTL) when presented in the context of HLA-A*0201, the most common MHC-I molecule among the Caucasian population [7]. In contrast, the nonamer p103-111 was not recognized in the context of either HLA-DRB1*0101 or DRB1*0701 as assessed by IFN-γ ELISPOT (Fig. 1a). Since this A*0201-restricted epitope is located inside the DR-restricted epitope p101-111, we investigated whether the undecamer p101-111 was able to induce a CTL response restricted to HLA-A*0201. To this end, the T cells of the p103-111 responsive CTL clone #24 were used as effector cells in a Cr51 release assay. As shown in Fig. 3b, peptides p101-111 and p103-111 induced killing of the APC by the CD8+ T cells from clone #24 in the context of HLA-A*0201, while neither the DR-restricted pentadecamer peptide p98-112 nor the HLA-A*0201-restricted nonamer p41-49, did. Thus, p101-111 is a peptide with dual MHC-I- and MHC-II-restricted affinity: as a single peptide, it has the capacity to induce both CD8+-mediated cytotoxicity and CD4+-mediated T-cell help for the immune response against HOM-MEL-40/SSX2+ tumors. HLA-A*0201 restriction of this cytotoxic T-cell response was proven by displacing the HLA-A*0201 reference peptide p58-66 derived from the matrix protein of the human influenza virus out of its MHC pocket by SSX2/p101-111. To this end, HLA-A*0201+ T2 cells were pulsed with IMP peptide at a constant concentration of 0.1 μg/ml IMP peptide either exclusively (1st concentration) or simultaneously with increasing concentrations of SSX2/p101-111 (2nd to 7th concentration). The respective 7 T2 fractions were used as APC in an IFN-γ ELISPOT against CD8+ CTLs either from clone #46 specifically responding to IMP/p58-66 (derived from a former study) or T cells of the SSX2/p101-111-specific clone #9 (derived from BC699). As shown in Fig. 3c, increasing concentrations of SSX2/p101-111 resulted in decreasing numbers of IFN-γ spots by the IMP-specific T-cell clone #46, while the number of IFN-γ spots caused by the SSX2/p101-111-specific clone #9 increased up to its maximum level. At this concentration, the SSX2 peptide p101-111 had completely displaced the HLA-A*0201-binding IMP peptide p58-66. This experiment was performed using another SSX2/p101-111-specific CD8+ clone #86 (derived from BC699) yielding identical results. HLA-A2 restriction had been additionally proven by blocking the response of all applied clones using HLA-A2-specific antibody BB7.2 to label the APC (data not shown).
Identification of SSX2 p101-111 as a B-cell epitope
To check whether p101-111 also represents a B-cell epitope, anti-SSX2 antibody-containing sera of patients with SSX2+ breast cancers which had been identified in a former study [22] were tested for reactivity with p101-111. Coating overlapping SSX2 fragments for ELISA, p101-111 showed a strong reactivity with three of these sera. As an example, the ELISA reactivity of one of the positive sera (Mel-Hei; Tab. 2) is shown in Fig. 4a. The analysis of the binding pattern of the SSX2-specific antibodies from this positive serum demonstrated that the amino acid sequence between position 101 and 110 was recognized by these antibodies and thus the SSX2-derived T-cell epitope p101-111 presents also an antibody-binding epitope. In contrast, none of the tested negative sera gave a significant signal (Fig. 4a). The spontaneous serum antibodies from patient COL12 (Table 2) were targeted on another epitope inside the SSX2 sequence.
Fig. 4.
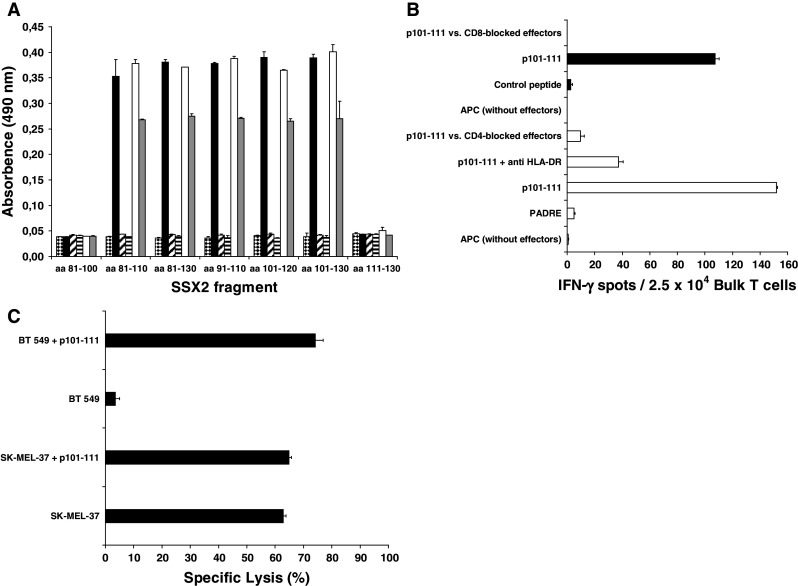
Simultaneous induction of a response by T-helper cells and by cytotoxic T cells as well as antibody production after in vitro stimulation with the SSX2-derived epitope p101-111. a After 35 days of in vitro stimulation of PBMC derived from patient BC699 with p101-111, the supernatant was analyzed by ELISA for antibodies binding to recombinantly expressed SSX2 fragments spanning the amino acids sequences as indicated on the X axis. This supernatant contained antibodies binding concentration dependent to SSX2 101-110 (empty [1:5-diluted] and gray [1:10-diluted] bars) and showed the same binding pattern (P < 0.01) as the positive serum derived from a melanoma cancer patient (black bars). At the beginning of the stimulation, such antibodies had not been present (horizontal stripes). A parallel stimulation using CMV-derived control peptides did not induce antibodies with affinity to any of the SSX2 fragments (striped bars [1:10]). SSX2− control serum also induced no significant ELISA signals (checkered bars). Only antibodies of the IgG class were analyzed. b PBMC from the HLA-A*0201, HLA-DRB1*0101 patient BC699 who had an SSX2-expressing tumor, were stimulated in vitro for 28 days with the epitope p101-111. The bulk lymphocytes developed simultaneously a strong T-cell response mediated by CD4+ T-helper cells as wells as by CD8+ cytotoxic T cells as shown by IFN-γ- release in the ELISPOT assay. Empty bars represent the response of 2.5 × 104 CD4+ bulk T cells/well against autologous PBMC (5 × 104/well) pulsed and labeled with the indicated peptide (4 μg/ml) and antibodies, respectively. Black bars represent the response of 2.5 × 104 CD8+ bulk T cells/well against T2 cells (3 × 104/well) pulsed and labeled with the indicated peptide (4 μg/ml) and antibodies, respectively. Y axis shows the different APC conformations. c After 35 days of in vitro stimulation with the undecamer peptide p101-111, the CD8+ T-cell fraction of the bulk culture of BC699 had developed a strong cytotoxic activity as assessed by LDH release assay. Lysis of cells of the HLA-A*0201+ and SSX2+ melanoma cell line SK-MEL-37 without external peptide pulsing demonstrated that epitope p101-111 is processed and presented via the MHC-I pathway. The HLA-A*0201+, but SSX2− line BT 549 caused lysis only after external pulsing with this peptide. Y axis displays the different APC conformations
Simultaneous induction of a response by T-helper and cytotoxic T cells as well as antibody production in vitro after stimulation with the HOM-MEL-40/SSX2-derived epitope p101-111
To investigate whether the SSX2-derived peptide p101-111 can prime CD4+, CD8+ T cells, and B cells simultaneously in vitro, unsorted PBMC derived from the patients BC699, COL10, COL12, COL13, and MM10 (for characters see Table 2) were stimulated in vitro with SSX2/p101-111 according to the protocol described above for CD4+ T cells. Using a precursor assay as described before [18], no spontaneous activity of precursor T cells specific for SSX2/p101-111 was observed. After 28 days of bulk culture, a strong and specific T-cell response of the CD4+ bulk fraction against p101-111 pulsed onto autologous PBMC was induced in case of BC699 (Fig. 4b) and COL13. The CD4+ nature of this response was demonstrated by blocking the IFN-γ secretion after the effector cells had been incubated with anti-CD4 antibody. DR restriction of this response was demonstrated by the fact that the IFN-γ release was significantly reduced after blocking APC with the anti-DR antibody clone L243. Subtype-specific DR restriction of the bulk-derived CD4+ T-cell response was determined by using allogeneic p101-111-pulsed LCL from patient BC399 as APC. These APC induced a strong, specific, and DR-restricted IFN-γ release by the CD4+ fraction of the bulk T cells after 28 days of in vitro stimulation with p101-111. Because the bulk effector cells from patient BC699 and the APC from patient BC399 share only HLA-DRB1*0101 (Tab. 2), the presentation of SSX2/p101-111 by this MHC-II subtype could be once more confirmed. The same response pattern resulted from the COL13 stimulation. This also applied for the DR restriction of the bulk CD4+ T cells. Quantitatively, the bulk response of the CD4+ T cells (from COL13) resulted in a 20% lower number of IFN-γ spots (data not shown). The CD8+ T-cell fraction in the bulk culture of BC699 responded also to a challenge with the HOM-MEL-40/SSX2-derived epitope p101-111 (Fig. 4b). Incubating the bulk effector cells with anti-CD8 antibody caused a complete reduction of the IFN-γ secretion by the corresponding T cells. In this case, peptide-pulsed T2 cells were used as APC, which share the HLA-A*0201 molecule with the effector cells from BC699. Moreover, at day 35, the cytotoxicity of the bulk T cells was tested. The CD8+ T cells derived form the bulk culture after in vitro stimulation with SSX2/p101-111 caused the lysis of > 60 % of the SK-MEL-37 cells used as SSX2-expressing targets. The lysis of cells of this HLA-A*0201+ cell line without external peptide pulsing demonstrated that the antigen-derived epitope p101-111 is processed and presented by MHC-I molecules. In contrast, the HLA-A*0201+, but SSX2− cell line BT 549 caused lysis mediated by the p101-111-specific CTL from patient BC699 only after additional pulsing with this peptide (Fig. 4c). All T-cell clones derived from the p101-111-responding bulk population displayed the effector memory phenotype according to Lanzavecchia’s scheme. Comparable to the situation of the CD4+ bulk response, the CD8+ bulk T cells of the COL13 stimulation also showed a similar, but 10% weaker response as observed above with BC699: 95 IFN-γ spots against p101-111-pulsed T2 cells, reduction to 10 spots after anti-CD8 antibody labeling of the bulk cells used for ELISPOT, and no response against an A2-binding control peptide. Finally, because peptide p101-111 represents a target epitope for serum antibodies with affinity for SSX2, the supernatant of the bulk culture after 35 days of stimulation with p101-111 was screened for anti-SSX2 antibodies. An ELISA using overlapping peptides covering the relevant SSX2 region as a coat revealed that antibodies specific for SSX2/p101-111 were induced in B-cells simultaneously with the T-cell responses by stimulation with this peptide in vitro. As shown in Fig. 4a, supernatant of the in vitro stimulation of PBMC from patient BC699 with p101-111 contained antibodies with the same binding pattern as the antibodies in the anti-SSX2 positive serum of the melanoma patient Mel-Hei. Such antibodies were not detected at the beginning of the culture, demonstrating that they occurred in vitro only after stimulation with p101-111. As a negative control, supernatant of in vitro stimulation of PBMC derived from a CMV+ donor (HLA-A*0201+ and HLA-DRB1*1101+) with peptides derived from the human CMV/pp65 antigen restricted to these two MHC molecules did not contain antibodies binding to any of the SSX2 fragments used as coat for the ELISA (Fig. 4a). However, no SSX2/p101-111-specific antibodies were detected in the supernatant of the COL13 stimulation. Neither the human AB serum which was added to the X-Vivo medium used for T-cell stimulation nor the serum of patient BC699 and COL13 contained antibodies binding to any of the tested SSX2 fragments.
T-cell receptor Vβ usage of the T-cell clones and lines
One CD4+ T-helper cell clone (K27) and two CD8+ CTL clones (K30 and K86) as well as a CD4+/CD8+ T-cell pool after MACS enrichment of the IFN-γ+ T cells in response to in vitro stimulation with SSX2/p101-111 of breast cancer patient BC699 were analyzed for the expression of the Vβ region of their TCR. Each of the 3 investigated clones expressed a different Vβ type: CD4+ T-helper cell clone K27 used Vβ7-2 after stimulation with p101-111 in the context of HLA-DRB1*0101. The CD8+ CTL clones K30 used Vβ13 and K86 used Vβ 20-1 in response to SSX2/p101-111 in the context of HLA-A*0201. In contrast, the bulk population consisting of IFN-γ-pouring CD4+/CD8+ T cells in response to p101-111 expressed 16 of the 23 different Vβ subtypes. Since all the clones were derived from this bulk population, our results demonstrate that T cells responding to epitope p101-111 used a broad spectrum of Vβ subtypes.
Discussion
We set out to define an SSX2-derived peptide capable of inducing both CD8+ and CD4+ T cell responses, because the use of such peptides with dual affinity for MHC-I and MHC-II could overcome the problems inherent to exclusive CD8+ stimulating MHC-I-restricted peptides. Trying to increase the number of epitopes mediating MHC-II-restricted CD4+ cells, we ended up with two novel pentadecamer peptides (p98-112 and p101-115). In contrast to former studies [8], none of the two breast cancer patients whose CD4+ T cells showed this reactivity had anti-SSX2 antibodies in their serum. The failure to detect anti-SSX2 antibodies in the serum of these patients may be due to the prevalence of a TH1 over TH2 against SSX2 in these patients. Thus, while MHC-II-restricted T-cell responses against CTA might be more readily detected in patients with antibodies against the respective antigen in their serum, the presence of such antibodies is not a conditio sine qua non for the detection of MHC-II-restricted T-cell responses in the same patient. Moreover, SSX2 mRNA expression as determined by RT-PCR was only demonstrated in the tumor cells of patient BC399, but not in the tumor from patient BC418, in whom the detection of SSX2 might be missed because the tumor sample had been taken from an antigen-negative area of the heterogeneous tumor (Table 2). The close neighborhood of the peptides p98-112 and p101-115 with 11 overlapping amino acids and the observation that stimulation with either one of these peptides resulted in T-cell lines and T-cell clones which reacted with both p98-112 and p101-115 suggested that they have the same core peptide. This core peptide was identified as the undecamer p101-111, because the neighboring undecamers p102-112 and p100-110 induced only weak CD4+ responses or none at all. Because p101-111 comprises the nonamer peptide sequence p103-111, which had previously been shown to stimulate MHC-I (HLA-A*0201)-restricted cytotoxic CD8+ T cells, the question arose whether the peptide with the proven MHC-I restriction is capable of stimulating an MHC-II-restricted response and vice versa. Because this was indeed the case for p101-111, this peptide fulfills the criteria for a peptide with dual MHC-I and MHC-II affinity. The neighboring p102-112 induced a much weaker MHC-II-restricted response than p101-111, whereas p100-110 induced no MHC-II-restricted response at all, leaving the p101-111 undecamer as the only SSX2-derived peptide with this dual capacity. While p101-111 had this dual affinity for MHC-I and MHC-II, p103-111, a nonamer peptide with proven MHC-I-restricted capacity to stimulate cytotoxic CD8+ cells, did not induce MHC-II-restricted T-cell responses even though it shares all its 9 amino acids with p103-111. The failure of p103-111 to induce MHC-II-restricted CD4+ responses is most likely due to its insufficient length which does not allow binding to the groove of MHC-II molecules. In an additional serological analysis, reactivity of p101-111 with serum containing SSX2 antibodies was demonstrated, proving p101-111 as a peptide with triple affinity for CD4+ T-cell, CD8+ T-cell, and B-cell antigen receptors. In final in vitro stimulations with peptide p101-111, it was possible to elicit simultaneously CD8+ cytotoxic T cells and CD4+ T-helper cells responding specifically to this SSX2 epitope. Moreover, the supernatant of these stimulations contained also antibodies binding to the p101-111 epitope.
In this study, we could not detect any spontaneous SSX2/p101-111-specific precursor T cells in the peripheral blood of the patients or control persons. Otherwise, our protocol for in vitro T-cell stimulations is not optimized for priming naïve T cells (for this purpose, the use of professional APC, especially dendritic cells, would be better). So it is possible, that spontaneous T-cell responses against this SSX2 epitiope do exist, but they are so weak that they are hard to detect. In contrast, one melanoma patient (Mel-Hei) showed such spontaneous antibodies in his serum. Whether the existence of such antibodies indicates necessarily also the existence of the corresponding T-helper cells, cannot be answered by now. However, high-titered IgG responses usually implies cognate T-cell help. Irrespective of this, breast cancer patient BC699 whose PBMC were used to stimulate successfully also p101-111-specific antibodies in vitro, did not have such antibodies in her serum.
So far, only few tumor antigen-derived epitopes with dual affinity for both cytotoxic and helper T-cells have been reported [23], and to the best of our knowledge, p101-111 is the first CTA-derived peptide with this functional capability that been defined and completely characterized in vitro. In addition to its dual affinity, p101-111 was also the target epitope of anti-SSX2 antibodies in the serum of a patient with an SSX2+ breast cancer, proving that p101-111 also functions as a ligand for the B-cell antigen receptor. Thus, p101-111 besides inducing CD4+ and CD8+ responses when used as a vaccine for patients with SSX2 expressing tumors, might also induce the production of antibodies that bind to this peptide. While the functional role of antibodies against CTA has not fully been elucidated, recent studies [10] suggest that such antibodies play a role for the in vivo cross-priming of specific cytotoxic T lymphocytes after vaccination with tumor antigen. Another model to explain how CTA-specific antibodies are contributing to immune surveillance of tumors is that high levels of these antibodies form immune complexes with corresponding CTA molecules that have been released from apoptotic or necrotic tumor cells. Those immune complexes can enter efficiently APC which than will prime or stimulate T cells [24]. Both approaches underline the importance of specific antibody induction and support the use of vaccines that induce both anti-tumor specific CD4+ and CD8+ T cells as well as antibodies.
To the best of our knowledge, SSX2/p101-111 is the first peptide for which such a triple capacity has been shown and thus it is a prime candidate for the development of anti-SSX2 cancer vaccines. The clinical potency of such a multi-specific peptide has recently been shown in vivo using a peptide derived from NY-ESO-1 [25]. Since p101-111 is restricted by MHC-I and MHC-II subtypes that are prevalent in the Caucasian population and due to the low costs for manufacturing this peptide, a large proportion of patients suffering from SSX2+ tumors would qualify for a vaccine study with p101-111.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (DFG grant PF-135-6.2), by the Helmholtz-Gesellschaft Deutscher Forschungszentren e.V. (Virtuelles Institut Grant VH-VI-108), and by the Deutsche Krebshilfe (grant 109366). Frank Neumann and Boris Kubuschok were supported by HOM-FOR grant 1000/T201000071 (06/2).
References
- 1.Chen YT, Scanlan MJ, Sahin U, Tureci O, Gure AO, Tsang S, et al. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc Natl Acad Sci USA. 1997;94:1914–1918. doi: 10.1073/pnas.94.5.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schultz ES, Lethe B, Cambiaso CL, Van Snick J, Chaux P, Corthals J, et al. A MAGE-A3 peptide presented by HLA-DP4 is recognized on tumor cells by CD4+ cytolytic T lymphocytes. Cancer Res. 2000;60:6272–6275. [PubMed] [Google Scholar]
- 3.Preuss KD, Zwick C, Bormann C, Neumann F, Pfreundschuh M. Analysis of the B-cell repertoire against antigens expressed by human neoplasms. Immunol Rev. 2002;188:43–50. doi: 10.1034/j.1600-065X.2002.18805.x. [DOI] [PubMed] [Google Scholar]
- 4.Clark J, Rocques PJ, Crew AJ, Gill S, Shipley J, Chan AM, et al. Identification of novel genes, SYT and SSX, involved in the t(X;18)(p11.2;q11.2) translocation found in human synovial sarcoma. Nat Genet. 1994;7:502–508. doi: 10.1038/ng0894-502. [DOI] [PubMed] [Google Scholar]
- 5.Tureci O, Chen YT, Sahin U, Gure AO, Zwick C, Villena C, et al. Expression of SSX genes in human tumors. Int J Cancer. 1998;77:19–23. doi: 10.1002/(SICI)1097-0215(19980703)77:1<19::AID-IJC4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 6.Ayyoub M, Stevanovic S, Sahin U, Guillaume P, Servis C, Rimoldi D, et al. Proteasome-assisted identification of a SSX-2-derived epitope recognized by tumor-reactive CTL infiltrating metastatic melanoma. J Immunol. 2002;168:1717–1722. doi: 10.4049/jimmunol.168.4.1717. [DOI] [PubMed] [Google Scholar]
- 7.Wagner C, Neumann F, Kubuschok B, Regitz E, Mischo A, Stevanovic S, et al. Identification of an HLA-A*02 restricted immunogenic peptide derived from the cancer testis antigen HOM-MEL-40/SSX2. Cancer Immun. 2003;3:18. [PubMed] [Google Scholar]
- 8.Neumann F, Wagner C, Stevanovic S, Kubuschok B, Schormann C, Mischo A, et al. Identification of an HLA-DR-restricted peptide epitope with a promiscuous binding pattern derived from the cancer testis antigen HOM-MEL-40/SSX2. Int J Cancer. 2004;112:661–668. doi: 10.1002/ijc.20461. [DOI] [PubMed] [Google Scholar]
- 9.Ayyoub M, Merlo A, Hesdorffer CS, Speiser D, Rimoldi D, Cerottini JC, et al. Distinct but overlapping T helper epitopes in the 37–58 region of SSX-2. Clin Immunol. 2005;114:70–78. doi: 10.1016/j.clim.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 10.Valmori D, Souleimanian NE, Tosello V, Bhardwaj N, Adams S, O’Neill D, et al. Vaccination with NY-ESO-1 protein and CpG in Montanide induces integrated antibody/Th1 responses and CD8 T cells through cross-priming. Proc Natl Acad Sci USA. 2007;104:8947–8952. doi: 10.1073/pnas.0703395104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tureci O, Sahin U, Schobert I, Koslowski M, Schmitt H, Schild HJ, et al. The SSX-2 gene, which is involved in the t(X;18) translocation of synovial sarcomas, codes for the human tumor antigen HOM-MEL-40. Cancer Res. 1996;56:4766–4772. [PubMed] [Google Scholar]
- 12.Alexander J, Sidney J, Southwood S, Ruppert J, Oseroff C, Maewal A, et al. Development of high potency universal DR-restricted helper epitopes by modification of high affinity DR-blocking peptides. Immunity. 1994;1:751–761. doi: 10.1016/S1074-7613(94)80017-0. [DOI] [PubMed] [Google Scholar]
- 13.Lang KS, Moris A, Gouttefangeas C, Walter S, Teichgraber V, Miller M, et al. High frequency of human cytomegalovirus (HCMV)-specific CD8 + T cells detected in a healthy CMV-seropositive donor. Cell Mol Life Sci. 2002;59:1076–1080. doi: 10.1007/s00018-002-8488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen YT, Stockert E, Chen Y, Garin-Chesa P, Rettig WJ, van der Bruggen P, et al. Identification of the MAGE-1 gene product by monoclonal and polyclonal antibodies. Proc Natl Acad Sci USA. 1994;91:1004–1008. doi: 10.1073/pnas.91.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neumann F, Wagner C, Preuss KD, Kubuschok B, Schormann C, Stevanovic S, et al. Identification of an epitope derived from the cancer testis antigen HOM-TES-14/SCP1 and presented by dendritic cells to circulating CD4+ T cells. Blood. 2005;106:3105–3113. doi: 10.1182/blood-2005-04-1487. [DOI] [PubMed] [Google Scholar]
- 16.zum Buschenfelde CM, Metzger J, Hermann C, Nicklisch N, Peschel C, Bernhard H. The generation of both T killer and Th cell clones specific for the tumor-associated antigen HER2 using retrovirally transduced dendritic cells. J Immunol. 2001;167:1712–1719. doi: 10.4049/jimmunol.167.3.1712. [DOI] [PubMed] [Google Scholar]
- 17.Utz U, Banks D, Jacobson S, Biddison WE. Analysis of the T-cell receptor repertoire of human T-cell leukemia virus type 1 (HTLV-1) Tax-specific CD8 + cytotoxic T lymphocytes from patients with HTLV-1-associated disease: evidence for oligoclonal expansion. J Virol. 1996;70:843–851. doi: 10.1128/jvi.70.2.843-851.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kubuschok B, Schmits R, Hartmann F, Cochlovius C, Breit R, Konig J, et al. Use of spontaneous Epstein-Barr virus-lymphoblastoid cell lines genetically modified to express tumor antigen as cancer vaccines: mutated p21 ras oncogene in pancreatic carcinoma as a model. Hum Gene Ther. 2002;13:815–827. doi: 10.1089/10430340252898993. [DOI] [PubMed] [Google Scholar]
- 19.Mischo A, Wadle A, Watzig K, Jager D, Stockert E, Santiago D, et al. Recombinant antigen expression on yeast surface (RAYS) for the detection of serological immune responses in cancer patients. Cancer Immun. 2003;3:5. [PubMed] [Google Scholar]
- 20.Preuss KD, Regitz E, Neumann F, Pfreundschuh M. B-cell epitopes from the cancer testis antigen NY-ESO-1. Int J Cancer. 2006;118:253. doi: 10.1002/ijc.21314. [DOI] [PubMed] [Google Scholar]
- 21.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 22.Mischo A, Kubuschok B, Ertan K, Preuss KD, Romeike B, Regitz E, et al. Prospective study on the expression of cancer testis genes and antibody responses in 100 consecutive patients with primary breast cancer. Int J Cancer. 2006;118:696–703. doi: 10.1002/ijc.21352. [DOI] [PubMed] [Google Scholar]
- 23.Zeng G, Li Y, El Gamil M, Sidney J, Sette A, Wang RF, et al. Generation of NY-ESO-1-specific CD4+ and CD8+ T cells by a single peptide with dual MHC class I and class II specificities: a new strategy for vaccine design. Cancer Res. 2002;62:3630–3635. [PMC free article] [PubMed] [Google Scholar]
- 24.van den Broek M, von Boehmer L, Knuth A. Developments in cancer immunotherapy. Dig Dis. 2010;28:51–56. doi: 10.1159/000282064. [DOI] [PubMed] [Google Scholar]
- 25.Odunsi K, Qian F, Matsuzaki J, Mhawech-Fauceglia P, Andrews C, Hoffman EW, et al. Vaccination with an NY-ESO-1 peptide of HLA class I/II specificities induces integrated humoral and T cell responses in ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:12837–12842. doi: 10.1073/pnas.0703342104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


